Abstract
Background
Most previous reports have found that Enterococcus faecalis isolates do not show significant adherence to fibronectin and fibrinogen
Methods
The influence of various conditions on E. faecalis adherence to ECM proteins was evaluated using a radiolabeled adherence assay.
Results
Among conditions studied, growth in 40% serum, a biological cue with potential clinical relevance, elicited adherence of all 46 E. faecalis strains tested to fibronectin and fibrinogen, but not to elastin; the adherence levels were independent of strain source and was eliminated by treating cells with trypsin. As previously reported, serum also elicited collagen adherence. While prolonged exposure to serum during growth was needed for enhancement of adherence to fibrinogen, brief exposure (<5 min) to serum had an immediate, although partial, effect on adherence to fibronectin and collagen. Pre-treatment of bacteria with chloramphenicol did not decrease the enhanced fibronectin and collagen adherence, indicating that protein synthesis is not required for the latter effect.
Conclusion
Together, these data suggest that serum components may serve i) as host environmental stimuli to induce production of extracellular matrix protein binding adhesin(s), as previously seen with collagen adherence, and also ii) as activators of adherence, perhaps by forming bridges between ECM proteins and adhesins.
Keywords: Enterococcus faecalis, extracellular matrix, adherence, collagen, fibrinogen, fibronectin, elastin, serum induction, MSCRAMMs
INTRODUCTION
Enterococcus faecalis, a mammalian gut commensal and facultative pathogen with an intermediate level of virulence, causes many infections including nosocomial bacteremia, urinary tract infections, and endocarditis [1]. Similar to other gram-positive pathogens, the first step in the infection process of E. faecalis is thought to begin with bacterial adhesion to extracellular matrix (ECM) of damaged tissue [2, 3]. The ECM of mammalian tissues is largely composed of glycoproteins (e.g., collagens, laminin, fibronectin (Fn), lactoferrin, and fibrinogen (Fg)) and proteoglycans. In normal tissues, the ECM is covered by epithelial or endothelial cells and trauma that damages host tissue exposes the ECM, thus allowing microbial colonization and infection. The published E. faecalis genome [4] predicts that E. faecalis possesses a number of cell wall anchored surface proteins with characteristic immunoglobulin-like folds, which are referred to as putative MSCRAMMs (microbial surface components recognizing adhesive matrix molecules [5]), that could promote colonization. MSCRAMMs of other gram-positive bacteria have been shown to play a major role in adherence and colonization in animal models [2, 3], and it is likely that the same is true for E. faecalis.
During the past two decades, variable results have been reported from E. faecalis adherence studies using different strains grown in routine laboratory media [6-15]. However, it is difficult to directly compare these studies because the results have been variably expressed as binding of ECM proteins to enterococci and vice versa, and because a wide range of techniques have been used (e.g., a crude particle agglutination method, standard radiolabeled or spectrophotometric bacterial adherence methods, and a microscopic method). Our studies, as well as those of several others, have categorized E. faecalis as conditional adherent to collagens and laminin [12, 16, 17] and as non-adherent to both Fg [10, 12] and Fn [7, 12, 15]. Seemingly in contrast to other reports, Tomita and Ike [10] showed low level adherence of several E. faecalis clinical isolates to collagens, laminin, and FN using a more sensitive microscopic method and a lower cut off compared to the cut off used in standard adherence studies that assess the percent of bacteria bound. Our subsequent genetic analyses demonstrated that Ace (an adhesin to collagen from E. faecalis) mediates the conditional (i.e., after growth at 46°C, in serum or in collagen) adherence of E. faecalis to collagen type I (CI), collagen type IV (CIV), and laminin [17, 18], in addition to dentin, a stabilized form of collagen [19].
It is known that pathogenic bacteria can alter expression of surface adhesins and their adherence upon exposure to host factors during infection. Increased surface proteins of oral streptococci upon exposure to ECM proteins [20], increased adherence of Proteus mirabilis to human urinary tract cell line after growth in urine [21], and increased adhesive ability of E. faecalis strains to heart cells [22] as well as to collagens [16, 17] after growth in serum, are examples of this phenomenon. The present study was aimed at examining the adherence of diverse E. faecalis strains to ECM proteins after in vitro growth under conditions that may mimic physiological ones. Our results show that serum enhances E. faecalis adherence to ECM proteins and provide evidence that the serum enhancement may occur via two different mechanisms.
MATERIALS AND METHODS
Bacterial strains
The E. faecalis isolates used in this study include OG1RF [23], 14 endocarditis isolates, 12 other clinical isolates (isolated from bone, catheters, urine, and wounds), 13 community-derived fecal isolates, and six animal isolates. These isolates were recovered over 27 years from broad geographic regions (United States, Thailand, China, Argentina, and Spain). The majority of these isolates were typed previously and known to be distinct by pulsed-field gel electrophoresis (PFGE). Two endocarditis strains TX0052 and MC02152 [17, 24], and OG1RF [23] with clearly different PFGE patterns were chosen for detailed analyses.
Growth conditions
E. faecalis cells were grown in Brain Heart Infusion (BHI) (Difco) at 37°C for routine purposes. For exploratory adherence studies, OG1RF cells were grown in BHI with or without different concentrations of sugars (0.2 to 1% glucose or 0.5 to 2% sucrose), bile salts (0.005 to 0.01% (wt/vol) of 1:1 mixture of sodium cholate and sodium deoxycholate; Sigma) or heat-inactivated horse serum (10 to 80%; Sigma) either at aerobic conditions or in 5% CO2 and also in different laboratory media (Todd-Hewitt (Difco), tryptic soy (Difco) with 0.25% glucose, and complete synthetic medium [23]). For subsequent assays of this study, cells were grown either in BHI or in BHI plus 40% horse serum (BHIS).
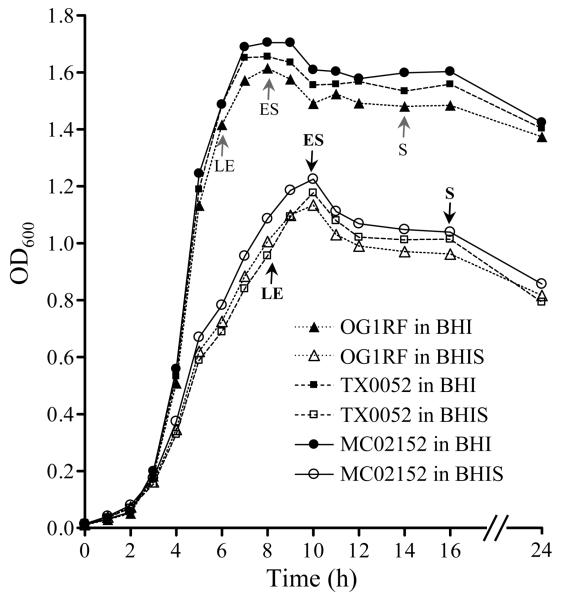
Growth curves of E. faecalis were generated using conditions that were used for adherence assays. In brief, bacteria were inoculated into BHI or BHIS (with starting inoculum of OD600= 0.01 to 0.015 or ∼ 1 × 107 cfu), mixed thoroughly by rigorous vortexing, dispensed into 5 ml aliquots and incubated at 37°C with shaking in an orbital shaker. At 1 h intervals, OD600 was measured from a single tube which was then discarded. cfu determinations were made on BHI agar at 0, 6, 8, 10, and 16 h.
ECM proteins and adherence assay
To test adherence of E. faecalis to immobilized proteins, Immulon-1B-Removawells (Dynatech Laboratories) were coated overnight at 4°C with bovine CI (Cohesion Technologies), human CIV (Sigma), human plasma Fn (Enzyme Research Laboratories), human cellular Fn (Sigma), and Fn-depleted human Fg (Enzyme Research Laboratories), human elastin (En, Elastin products company) and bovine serum albumin (BSA). Adherence was carried out by a previously described assay [18] except that 35S-labeled bacteria were resuspended in PBS with 0.5% Tween-80, 0.1% BSA (PBSTB) to minimize clumping. BHIS grown OG1RF cells were tested for adherence to each ECM protein with incubation in ECM-coated wells initially ranging from 1 to 4 h; since adherence peaked at 2 h and was not enhanced by further incubation, 2 h incubations were subsequently used. Using BHI-grown OG1RF, we also tested the effect of 0.1, 1, and, 10 mM cations (Ca++, Mg++, and Mn++) at the adherence step. For the cation effect studies, PBS was substituted with 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7·5). Isolates were classified as adhering to ECM proteins if ≥5% of total labeled cells adhered [18].
Trypsin treatment of bacteria
For proteolytic treatment to remove surface proteins, 108 cells of labeled bacteria in PBS were incubated with 1 mg of trypsin for 1 h at 37°C. The suspensions were washed three times in PBS for use in adherence assays.
Serum exposure studies
To test the effect of short periods of exposure to serum on E. faecalis adherence, bacteria were 35S-labeled by growing in BHI for 8 h, then washed in PBS; cell densities were adjusted to OD600 = 0.5 in PBS followed by incubation for 3 to 5 min at 37°C with an equal volume of pre-warmed serum, followed by centrifugation at 4°C. Before adding serum-exposed cells to ECM coated wells, bacteria were washed in PBS and cell densities were adjusted in PBSTB.
For experiments to test the effects of bacterial protein synthesis inhibition on these serum exposure phenotypes, BHI-grown 35S-labeled bacteria processed and adjusted to OD600 = 0.5 in PBS as above, were treated with 1× and 2× the MIC of chloramphenicol at 37°C for 30 min prior to the addition of an equal volume of pre-warmed serum [25, 26]. The chloramphenicol MICs for the three tested strains (determined using CSLI [27] recommendations) were 4 μg/ml for OG1RF and 8 μg/ml for both TX0052 and MC02152
Colony hybridization
Colony lysate blots containing denatured E. faecalis genomic DNA were prepared as previously described [28] and hybridized with probes representing 8 genes that encode predicted MSCRAMMs [5]. Probe details and primers used for amplification are listed in Table 1.
Table 1.
Details of primers used for generating MSCRAMM encoding gene probes
| Genea | Amplicon size (bp) |
Sequence (5′→3′) | Reference |
|---|---|---|---|
| ef0089 | 1179 | F: CGACAGAATCAACGGCAATCACGAG R: CTGCTTGGTCTTTTGGAATGGTTTGT |
5, 29 |
| ef1269 | 707 | F: CGATGTAGCAAATAAAACGGTCACG R: CTAAACGCTGGCCTGCTTCATCTTCT |
5, 29 |
| ef1824 | 1038 | F: CGATATGCGGTTAGGGTGGTTCTT R: TTTGGATTGTTGTTTGGATTCGTCATTG |
5, 29 |
| ef1896 | 759 | F: AAACAGTGACGGTTGAATTAGATTTAG R: TAGCACCTGATTCTTTATCAACTTTTT |
This study |
| ef2244 | 1199 | F: AGAGATTGCTTGGGCGGGCTTATTT R: ATTCATTTGCTTTTGCTCGTTCATTTA |
5, 29 |
| ef2347 | 734 | F: AGGTGTTAGTATTCCAACAGAAGTGAC R: TAGTTACCTTTGTACCATGGG |
This study |
| ef2505 | 766 | F: AGTTATCGAACTCAACACCATCTTTAC R: ACTTGAGGTTCCAAGTTATCTTGTTTT |
This study |
|
ef3023 (hylA) |
1045 | F: CTAACTTAACAGATATTTCAATCAC R: GCATCTGTCGTACCAGTAATGCCAC |
5, 29 |
All gene probes were amplified from V583.
Statistics
Student's t test and Analysis of Variance (ANOVA) with Bonferronic's post test were used for comparing continuous variables and Fisher's exact test was used for categorical results. The differences were considered significant when P < 0.05.
RESULTS
Effect of serum on adherence of E. faecalis to immobilized extracellular matrix proteins
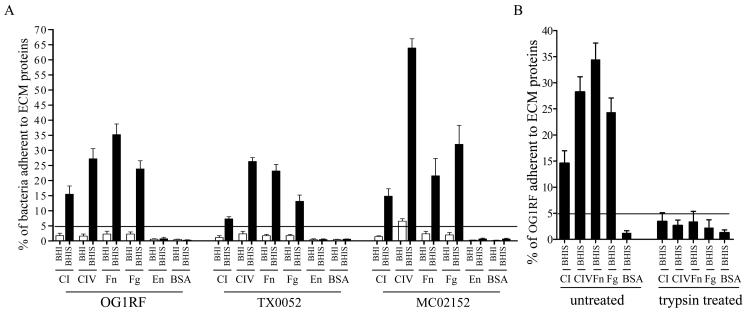
We first examined the ability of E. faecalis OG1RF [23] and two endocarditis strains to adhere to CI, CIV, Fn, Fg, En, and BSA. Similar to our earlier report for E. Faecalis [12], there was no adherence (< 5% cells adhered) after growth in BHI at 37°C to any of these proteins, except strain MC02152 with 6.5% cells adhering to CIV, consistent with our previous result for this strain [17, 24]. We next tested several growth conditions and assay parameters to identify conditions that might promote E. faecalis adherence to different ECM proteins. While the adherence results of cells harvested from different laboratory growth media supplemented with or without sugars or bile salts (see Methods) were qualitatively identical to BHI-grown OG1RF cells, growth in BHI plus 40% horse serum yielded much higher overall adherence levels (P < 0.0001) to Fn and Fg, in addition to collagens [17], but not to En and BSA (figure 1A). Supplementing different cations during the adherence step did not enhance the adherence levels of BHI-grown OG1RF cells. As shown in figure 1A, the increase in adherence of BHIS-grown cells versus BHI-grown cells to different ECM proteins ranged from 7 (TX0052 to CI) to 17 fold (OG1RF to CIV). Of note, we also tested cellular-Fn (the fibronectin form present on cell surfaces) as a substrate and found no qualitative differences in OG1RF adherence results compared with the serum form of Fn (data not shown). The serum enhanced OG1RF adherence to CI, CIV, Fn and Fg was almost completely eliminated by trypsin treatment of bacteria (figure 1B). It was also observed during these studies that E. faecalis cells grown in BHIS showed some clumping, which may explain why some standard deviation (SD) values of the mean for adherence data from independent experiments were high (e.g., SD of Fn adherence of MC02152 was 26% of its mean adherence value). However, the qualitative adherence differences between independent experiments were highly reproducible. The BHIS growth conditions that enhanced ECM protein adherence also slowed the doubling time of E. faecalis and the overnight culture density and cfu of BHIS-grown E. faecalis were reproducibly ca. two times lower than the BHI-grown E. faecalis (figure 2).
Figure 1.
Influence of growth in serum on adherence of three E. faecalis strains to immobilized ECM proteins. Panel A shows adherence of three different E. faecalis strains to ECM proteins, collagen type I (CI), collagen type IV (CIV), fibronectin (Fn), fibrinogen (Fg), elastin (En), and BSA at early-stationary phase growth in BHI broth or BHIS (BHI plus 40% serum). Panel B shows the effect of trypsin treatment on adherence of BHIS-grown OG1RF to ECM proteins. Adherence was tested in wells coated with 1 μg of ECM proteins. Bars represent the means of percent of cells adherent ± standard deviation for 8 to 12 wells. Results are representative of at least three independent experiments.
Figure 2.
Growth patterns of E. faecalis strains in BHI and BHIS. Data from BHI grown cultures were marked with solid squares, circles and triangles and BHIS were marked with open squares, circles and triangles. Time points at which adherence was tested are marked with gray (BHI) and black (BHIS) arrows. BHIS, BHI plus 40% serum; LE, late-exponential phase; ES, entry into stationary phase; S, 6 h after entry into stationary phase.
Serum elicited adherence of E. faecalis to fibronectin and fibrinogen is a general phenomenon
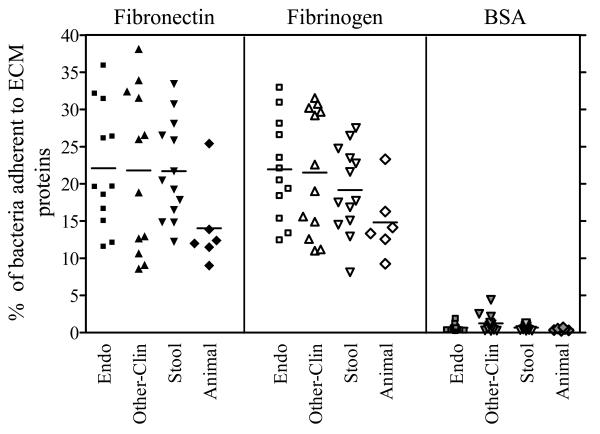
We next tested the adherence ability of 43 additional diverse E. faecalis strains grown in BHIS to immobilized fibronectin and fibrinogen. Among these, 12 strains were studied previously after growth in BHI using the same adherence assay and reported as non-adherent to Fn and Fg [12]. As shown in figure 3, all strains grown in BHIS were found to adhere to both Fn and Fg. Individual differences were observed in the percentages of adherent cells of different strains and were in the range of 9 ± 1 to 38 ± 5% for Fn and 8 ± 1 to 32 ± 3% for Fg (figure 3). However, no correlation was noted between the ability or the levels of adherence of the various strains grown in BHIS to the ECM proteins and the source (clinical isolate versus fecal isolate from healthy individuals), the host from which they had been recovered (animal versus human), or their geographical origin.
Figure 3.
Adherence of 43 additional E. faecalis isolates to immobilized fibronectin and fibrinogen after growth in BHIS (BHI plus 40% serum). Cells were grown for 10 h (to entry into stationary phase). Adherence was tested in wells coated with 1 μg of fibronectin or fibrinogen or BSA. Each data point represents the mean % of cells adherent for 9 wells. Results are from three independent experiments. Endo, strains isolated from patients with E. faecalis endocarditis; Other-Clin, strains isolated from E. faecalis non-endocarditis clinical infections; Stool, isolates from stools of community healthy volunteers; Animal, isolates from animal origin.
Influence of growth phase on E. faecalis adherence
Comparison of adherence percentages of three BHIS-grown E. faecalis strains harvested at 8 h (∼ late-exponential phase), 10 h (∼ entry into stationary phase), and 16 h (6 h after the cultures entered stationary phase) (figure 2), overall, showed positive adherence to CI, CIV, Fn, and Fg, except for a lack (≤ 5%) of CI adherence at the earliest time point with 2 strains, and no adherence to En- or BSA-coated wells (table 2). The variability in adherence percentages at different growth phases was greatest for CI (e.g., an 11-fold increase with OG1RF cells harvested at 8 h versus 16 h) while much smaller differences (less than two-fold) were seen with Fg adherence (table 2). For subsequent analyses, cells harvested at 10 h were used. Of note, uneven radiolabeling of cells harvested until mid-exponential phase (6 h) caused large variation in independent values and hence we did not attempt to compare those values.
Table 2.
ECM protein adherence percentages of BHIS-grown E. faecalis strains harvested at different stages of growth
| ECMa/Strain | OG1RF |
TX0052 |
MC02152 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| late-expob | entry-stat | stat | late-expo | entry-stat | stat | late-expo | entry-stat | stat | |
| CI | 2 ± 1c | 16 ± 1 | 22 ± 3 | 2 ± 1 | 8 ± 1 | 10 ± 2 | 9 ± 3 | 15 ± 1 | 10 ± 1 |
| CIV | 40 ± 4 | 26 ± 4 | 37 ± 5 | 33 ± 4 | 33 ± 6 | 27 ± 4 | 65 ± 5 | 78 ± 7 | 56 ± 1 |
| Fn | 18 ± 3 | 33 ± 4 | 36 ± 3 | 10 ± 1 | 30 ± 4 | 23 ± 4 | 23 ± 2 | 23 ± 6 | 21 ± 2 |
| Fg | 24 ± 2 | 25 ± 3 | 28 ± 4 | 10 ± 1 | 14 ± 3 | 13 ± 1 | 35 ± 3 | 36 ± 6 | 29 ± 2 |
| En | < 1% | < 1% | < 1% | < 1% | < 1% | < 1% | < 1% | < 1% | < 1% |
| BSA | < 1% | < 1% | < 1% | < 1% | < 1% | < 1% | < 1% | < 1% | < 1% |
ECM: extracellular matrix proteins; CI: collagen type I; CIV: collagen type IV; Fn: fibronectin; Fg: fibrinogen; En: elastin; BSA: bovine serum albumin.
late-expo: late-exponential growth phase (8 h); entry-stat: entry into stationary growth phase (10 h); stat: 6 h after the cultures entered stationary growth phase (16 h)
Values are means of % of cells adhering ± standard deviation for six wells. Results are representative of three to six independent experiments.
Short exposure of E. faecalis to serum is sufficient to increase its adherence to collagens and fibronectin
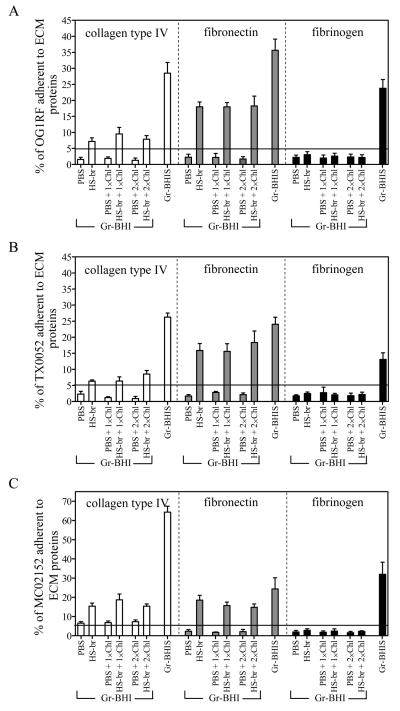
To investigate the mechanism of the serum elicited adherence to different ECM proteins, we undertook studies to determine if prolonged exposure to serum was required (as seen with serum-induced, Ace-mediated collagen adherence [17]) or if brief exposure sufficed. For the latter, adherence assays were carried out with BHI-grown entry into stationary phase (8 h) cultures that were divided into several portions and then exposed briefly to serum in the presence or absence of the protein synthesis inhibitor, chloramphenicol. As shown in figure 4A, brief exposure (< 5 min) of OG1RF to serum (HS-br) just before performing adherence led to a clear increase in adherence to collagens (4-fold to CI (data not shown) and 5-fold to CIV, P<0.001) and Fn (8-fold, P<0.001) relative to exposure to PBS, an effect that was not eliminated by pre-exposure to 1× or 2× the MIC of chloramphenicol (figure 4A). The lack of chloramphenicol effect plus the very brief exposure to serum indicate that protein synthesis is not essential for these phenotypes, and suggests that some degree of the CI, CIV, and Fn adherence phenotypes occurs via serum component deposition on E. faecalis cell surfaces. However, the percentage of cells exposed briefly to serum that adhered to collagens and Fn was always lower (2.3-fold to CI (data not shown), 3.4-fold to CIV, and 2-fold to Fn; P<0.001) compared to cells grown in BHIS (i.e., prolonged incubation) (Gr-BHIS in figure 4A), suggesting an additional effect that requires more prolonged exposure, such as protein synthesis. On the contrary, no significant differences were seen in the adherence to Fg of briefly serum-exposed OG1RF (figure 4A), En, or BSA (data not shown) compared to non-serum exposed cells. The variability of adherence to different ECM proteins, with no binding to En and no increase in adherence to Fg after brief serum exposure, indicates that clumping plays little or no role in specific adherence to immobilized ECMs, although it is possible that it may increase the number of cells present once specific adherence has occurred.
Figure 4.
Effect of brief and prolonged exposure to serum on adherence of E. faecalis cells to collagen, fibronectin, and fibrinogen. Panels A, B, and, C, show adherence percentages of OG1RF, TX0052, and MC02152, respectively, under cultured and exposure conditions labeled on the X-axis. Gr-BHI, cells grown in BHI; Gr-BHIS, cells grown in BHI plus 40% serum; PBS, cells exposed to PBS after growth in BHI; HS-br, cells exposed briefly to horse serum after growth in BHI; 1×Chl and 2×Chl, cells pretreated with chloramphenicol with concentrations corresponding to 1× and 2× the MIC prior to exposure to HS. Adherence was tested as above. Bars represent the means of percent of cells adherent ± standard deviation for 4 to 12 wells representing at least two independent experiments. Collagen type IV adherence bars are filled with white, fibronectin with grey, and fibrinogen with black. Collagen type I adherence results resembled collagen type IV and hence data are not shown. Control experiments in which cells were incubated with methanol (solvent of chloramphenicol) in PBS showed no effect in the adherence (data not shown).
As shown in figures 4B and 4C, the two distinct endocarditis strains tested also exhibited increases in their adherence after exposure to serum. As above, the increases seen with brief exposure to serum were not eliminated by chloramphenicol (figures 4B and 4C).
Distribution of MSCRAMM encoding genes in E. faecalis strains
We screened for the presence of 8 predicted MSCRAMM encoding genes [5] using high-stringency hybridization. The results with 46 strains (including 7 isolates of a previous study that tested five of these genes [5]) showed the presence of ef0089, ef1269, and ef2224 in all. The remaining five genes were variably present (table 3): 13% for ef2347; 46% for ef1824 and ef1896; 67% for ef2505, and 76% were positive for ef3023 (putative hylA gene). While ef2505 gene was noted to be enriched (20 of 26) in infection-derived isolates compared to community-derived isolates from human stools (4 of 13) (P = 0.013), there were no significant differences in the distribution of the other four genes. On the whole, there were 18 (of a possible 32) different combinations of the five variable genes (table 3); only two strains, both belonging to the BVE (for Bla+ Vanr endocarditis) clonal complex [29] (CC2 [30]), had all genes.
Table 3.
Combinations of predicted MSCRAMM encoding genes for 46 E. faecalis strains
| gene presence (+) or absence (−) |
No. of isolates (clinical/ community fecal/ animal)a |
||||||
|---|---|---|---|---|---|---|---|
| Pattern designation |
ef0089/ ef2224/ ef1269 |
ef3023 | ef2505 | ef1824 | ef1896 | ef2347 | |
| 100% | 76% | 67% | 46% | 46% | 13% | ||
| 1 | + | + | + | + | + | + | 2b / 0 / 0 |
| 2 | + | + | + | − | + | + | 1 / 0 / 0 |
| 3 | + | + | + | + | − | + | 0 / 0 / 1 |
| 4 | + | − | + | + | + | + | 1 / 0 / 0 |
| 5 | + | + | + | + | + | − | 2 / 1 / 0 |
| 6 | + | + | − | + | + | − | 0 / 1 / 0 |
| 7 | + | + | + | + | − | − | 4 / 0 / 2 /1c |
| 8 | + | − | + | − | + | + | 1 / 0 / 0 |
| 9 | + | + | + | − | + | − | 3 / 1 / 1 |
| 10 | + | + | − | + | − | − | 2 / 2 / 0 |
| 11 | + | − | + | + | − | − | 0 / 0 / 1 |
| 12 | + | + | − | − | + | − | 1 / 2 / 0 |
| 13 | + | − | + | − | + | − | 2 / 0 / 0 |
| 14 | + | + | + | − | − | − | 2 / 2 / 0 |
| 15 | + | − | − | + | − | − | 1 / 0 / 0 |
| 16 | + | − | − | − | + | − | 0 / 2 / 0 |
| 17 | + | + | − | − | − | − | 2 / 2 / 0 |
| 18 | + | − | + | − | − | − | 2 / 0 / 1 |
This shows the number of isolates with each pattern
The fourth number designates the laboratory strain OG1RF. Of note, although OGIRF had # 7 pattern designation using high stringency hybridization, a remote homologue of ef1896 was identified in the draft genomic sequence of E. faecalis strain OG1RF generated by our collaboration with the human genome sequencing center at Baylor College of Medicine (available at http://www.hgsc.bcm.tmc.edu/projects/microbial/microbial-detail.xsp?project_id=111).
DISCUSSION
Knowledge of E. faecalis adherence to individual ECM proteins is important for our understanding of the pathogenesis of infections caused by this organism. While we recently identified an array of putative MSCRAMM encoding genes [5] in the sequenced strain of E. faecalis [4], (one of which encode the collagen adhesin, Ace [18]. and three of which make up the endocarditis and biofilm-associated pili (Ebp) structures [31]), most previous ECM protein adherence studies of E. faecalis, including ours, categorized E. faecalis as a species that is non-adherent to Fg [10, 12] and Fn [7, 12, 15]. The lack of a scorable phenotype has been a serious impediment to our efforts, and presumably those of others, to assign a functional role to these and other putative adhesins. While Tomita and Ike [10] described low level Fn adherence by counting cells in a microscopic assay, this likely reflects a more sensitive assay and lower cut off. Despite the predominance of negative results with E. faecalis, the influence of growth conditions (such as growth media, temperature, pH, and/or growth phase) and environmental cues in adherence of many bacterial species to host proteins or cells has been well-documented. Even in E. faecalis, different studies have reported an influence of growth temperature [12], serum [17], and ligand [17] on adherence to collagen and laminin; an influence of serum on aggregation substance synthesis [32] and adherence to heart cells [22, 33]; and an influence of various sugars on biofilm formation [34, 35]. Considering the significance of Fn and Fg adherence in the pathogenesis of streptococcal and staphylococcal infections [2, 36], the present study was aimed at identifying variables that promote E. faecalis adherence to Fn and Fg.
Here, we found that BHIS-grown E. faecalis cells generated highly significantly (P < 0.0001) increased adherence to Fn and Fg relative to growth in BHI. A reduction in serum-elicited bacterial adherence after protease treatment of cells indicates that these phenotypes are mediated by proteinaceous surface adhesins. We also examined the adherence of BHIS-grown bacteria harvested at different parts of growth cycle and found strain-dependent variability for CI, CIV, and Fn adherence, suggesting variable expression or variable surface presentation of putative adhesins during different phases of growth.
At least two different mechanisms for serum elicited adherences to different ECM proteins can be envisaged from the literature. One possibility is that serum components serve as signals to induce production of MSCRAMMs on the surface (e.g., increased E. faecalis Ace expression on surface [17]). A second possibility is that adhesion occurs in the presence of an intermediate serum component serving as bridge (e.g., Streptococcus pyogenes adhere to collagen via surface-bound fibronectin [37]). Our results showing a requirement for prolonged incubation (e.g., growth) of E. faecalis with serum to elicit Fg adherence suggests that Fg binding adhesins may be induced by serum components or that growth in a stress condition such as serum alters their surface presentation. On the other hand, the results showing considerable enhancement of adherence to collagens and Fn almost immediately after exposure to serum and failure to prevent this brief serum exposure mediated increase by pretreatment of cells with chloramphenicol, a protein synthesis inhibitor, suggests an indirect “bridging” mechanism for adherence to these ECM proteins. However, adherence to collagens and Fn was usually further enhanced in BHIS-grown cells, implying that collagen and Fn adherence may be influenced by both of the above proposed mechanisms.
We also demonstrated Fn and Fg adherence of 43 additional BHIS-grown E. faecalis strains from patients with endocarditis, other E. faecalis infections, stools of healthy volunteers, and animals, but found no correlation between the adherence phenotypes and strain origin, indicating that serum elicited Fn and Fg adherence is a general phenomenon of E. faecalis. We then screened for the presence of 8 predicted MSCRAMM encoding genes [5] in these strains. Overall, hybridization results showed the ubiquitous presence of three genes (ef0089, ef1269, and ef2224), similar to our earlier report with 30 exclusively clinical strains [5], and variable occurrence of the remaining five genes (13% to 76%). Only 2 strains, both members of the multilocus sequence type defined CC2 (also referred to as BVE [29]) hospital enriched clone [30] had all genes. All 46 isolates were previously shown to possess ace [29, 31, 38] and the ebp operon genes, ebpA, ebpB, and ebpC encoding endocarditis and biofilm-associated pilus [31]. Since studies of serum elicited collagen adherence with ace mutants suggested an additional CI binding protein [17] and ruled out the role of Ace in adherence to Fn and Fg (data not shown), it seems likely that the ubiquitous Ebp pili proteins [31] or the putative MSCRAMMs EF0089, EF2224, or EF1269 may be associated with these common phenotypes. Preliminary data from ongoing studies using recombinant proteins of these ORFs and constructed isogenic mutant strains also points towards this possibility (J. Sillanpää, S.R.N., B.E.M., and M. Hook, unpublished data).
In summary, the results presented here demonstrate that adherence of E. faecalis to ECM proteins is enhanced upon exposure to serum, a biological cue with relevance to bacteria growing in vivo. This finding, coupled with our recent observation of an increase in experimental murine urinary tract infections when OG1RF cells were cultured in BHIS compared to BHI (R. Lewis, K.V. Singh and B.E.M., unpublished data) may be relevant to clinical situations in which infections are associated with bacteremia. Further analyses indicated that adherence of E. faecalis to different host proteins occurs via at least by two different mechanisms and via different surface adhesins. However, the specific adhesins on E. faecalis and the sensed host signals have yet to be determined.
Acknowledgments
We thank Kavindra V. Singh for his help and Karen Jacques-Palaz for her technical assistance. This work was supported by an NIH grant R37 AI 47923 from the Division of Microbiology and Infectious Diseases, NIAID, to B. E. Murray.
Financial support: This work was supported by an NIH grant R37 AI 47923 from the Division of Microbiology and Infectious Diseases, NIAID to BEM
Footnotes
Potential conflict of interest: None
References
- 1.Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke SR, Foster SJ. Surface adhesins of Staphylococcus aureus. Adv Microb Physiol. 2006;51:187–224. doi: 10.1016/S0065-2911(06)51004-5. [DOI] [PubMed] [Google Scholar]
- 3.Rivas JM, Speziale P, Patti JM, Hook M. MSCRAMM-targeted vaccines and immunotherapy for staphylococcal infection. Curr Opin Drug Discov Devel. 2004;7:223–7. [PubMed] [Google Scholar]
- 4.Paulsen IT, Banerjei L, Myers GS, et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003;299:2071–4. doi: 10.1126/science.1080613. [DOI] [PubMed] [Google Scholar]
- 5.Sillanpaa J, Xu Y, Nallapareddy SR, Murray BE, Hook M. A family of putative MSCRAMMs from Enterococcus faecalis. Microbiology. 2004;150:2069–78. doi: 10.1099/mic.0.27074-0. [DOI] [PubMed] [Google Scholar]
- 6.Shorrock PJ, Lambert PA. Binding of fibronectin and albumin to Enterococcus (Streptococcus) faecalis. Microb Pathog. 1989;6:61–7. doi: 10.1016/0882-4010(89)90008-9. [DOI] [PubMed] [Google Scholar]
- 7.Styriak I, Laukova A, Fallgren C, Wadstrom T. Binding of selected extracellular matrix proteins to enterococci and Streptococcus bovis of animal origin. Curr Microbiol. 1999;39:327–0335. doi: 10.1007/s002849900467. [DOI] [PubMed] [Google Scholar]
- 8.Styriak I, Laukova A, Ljungh A. Lectin-like binding and antibiotic sensitivity of enterococci from wild herbivores. Microbiol Res. 2002;157:293–303. doi: 10.1078/0944-5013-00166. [DOI] [PubMed] [Google Scholar]
- 9.Styriak I, Laukova A, Strompfova V, Ljungh A. Mode of binding of fibrinogen, fibronectin and iron-binding proteins by animal enterococci. Vet Res Commun. 2004;28:587–98. doi: 10.1023/b:verc.0000042865.63246.de. [DOI] [PubMed] [Google Scholar]
- 10.Tomita H, Ike Y. Tissue-specific adherent Enterococcus faecalis strains that show highly efficient adhesion to human bladder carcinoma T24 cells also adhere to extracellular matrix proteins. Infect Immun. 2004;72:5877–85. doi: 10.1128/IAI.72.10.5877-5885.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyriak I, Ljungh S. Binding of extracellular matrix molecules by enterococci. Curr Microbiol. 2003;46:435–42. doi: 10.1007/s00284-002-3879-2. [DOI] [PubMed] [Google Scholar]
- 12.Xiao J, Hook M, Weinstock GM, Murray BE. Conditional adherence of Enterococcus faecalis to extracellular matrix proteins. FEMS Immunol Med Microbiol. 1998;21:287–95. doi: 10.1111/j.1574-695X.1998.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 13.Yanagisawa N. Adhesive properties of bacteria isolated from patients with urinary tract infection to the urinary bladder. Nippon Hinyokika Gakkai Zasshi. 1997;88:24–34. doi: 10.5980/jpnjurol1989.88.24. [DOI] [PubMed] [Google Scholar]
- 14.Zareba TW, Pascu C, Hryniewicz W, Wadstrom T. Binding of enterococci to extracellular matrix proteins. Adv Exp Med Biol. 1997;418:721–3. doi: 10.1007/978-1-4899-1825-3_169. [DOI] [PubMed] [Google Scholar]
- 15.Zareba TW, Pascu C, Hryniewicz W, Wadstrom T. Binding of extracellular matrix proteins by enterococci. Curr Microbiol. 1997;34:6–11. doi: 10.1007/s002849900135. [DOI] [PubMed] [Google Scholar]
- 16.Love RM. Enterococcus faecalis - a mechanism for its role in endodontic failure. Int Endod J. 2001;34:399–405. doi: 10.1046/j.1365-2591.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 17.Nallapareddy SR, Murray BE. Ligand-signaled upregulation of Enterococcus faecalis ace transcription, a mechanism for modulating host-E. faecalis interaction. Infect Immun. 2006;74:4982–9. doi: 10.1128/IAI.00476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nallapareddy SR, Qin X, Weinstock GM, Hook M, Murray BE. Enterococcus faecalis adhesin, Ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect Immun. 2000;68:5218–24. doi: 10.1128/iai.68.9.5218-5224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubble TS, Hatton JF, Nallapareddy SR, Murray BE, Gillespie MJ. Influence of Enterococcus faecalis proteases and the collagen-binding protein, Ace, on adhesion to dentin. Oral Microbiol Immunol. 2003;18:121–6. doi: 10.1034/j.1399-302x.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert FB, Luther DA, Oliver SP. Induction of surface-associated proteins of Streptococcus uberis by cultivation with extracellular matrix components and bovine mammary epithelial cells. FEMS Microbiol Lett. 1997;156:161–4. doi: 10.1111/j.1574-6968.1997.tb12722.x. [DOI] [PubMed] [Google Scholar]
- 21.Latta RK, Schur MJ, Tolson DL, Altman E. The effect of growth conditions on in vitro adherence, invasion, and NAF expression by Proteus mirabilis 7570. Can J Microbiol. 1998;44:896–904. doi: 10.1139/cjm-44-9-896. [DOI] [PubMed] [Google Scholar]
- 22.Guzman CA, Pruzzo C, Plate M, Guardati MC, Calegari L. Serum dependent expression of Enterococcus faecalis adhesins involved in the colonization of heart cells. Microb Pathog. 1991;11:399–409. doi: 10.1016/0882-4010(91)90036-a. [DOI] [PubMed] [Google Scholar]
- 23.Murray BE, Singh KV, Ross RP, Heath JD, Dunny GM, Weinstock GM. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J Bacteriol. 1993;175:5216–23. doi: 10.1128/jb.175.16.5216-5223.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nallapareddy SR, Singh KV, Duh RW, Weinstock GM, Murray BE. Diversity of ace, a gene encoding a microbial surface component recognizing adhesive matrix molecules, from different strains of Enterococcus faecalis and evidence for production of Ace during human infections. Infect Immun. 2000;68:5210–7. doi: 10.1128/iai.68.9.5210-5217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talbot UM, Paton AW, Paton JC. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect Immun. 1996;64:3772–7. doi: 10.1128/iai.64.9.3772-3777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanier G, Segura M, Friedl P, Lacouture S, Gottschalk M. Invasion of porcine brain microvascular endothelial cells by Streptococcus suis serotype 2. Infect Immun. 2004;72:1441–9. doi: 10.1128/IAI.72.3.1441-1449.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement: approved standard [M100-S15] CLSI; Wayne, PA: 2005. [Google Scholar]
- 28.Singh KV, Qin X, Weinstock GM, Murray BE. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J Infect Dis. 1998;178:1416–20. doi: 10.1086/314453. [DOI] [PubMed] [Google Scholar]
- 29.Nallapareddy SR, Wenxiang H, Weinstock GM, Murray BE. Molecular characterization of a widespread, pathogenic, and antibiotic resistance-receptive Enterococcus faecalis lineage and dissemination of its putative pathogenicity island. J Bacteriol. 2005;187:5709–18. doi: 10.1128/JB.187.16.5709-5718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz-Garbajosa P, Bonten MJ, Robinson DA, et al. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J Clin Microbiol. 2006;44:2220–8. doi: 10.1128/JCM.02596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nallapareddy SR, Singh KV, Sillanpaa J, et al. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest. 2006;116:2799–807. doi: 10.1172/JCI29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreft B, Marre R, Schramm U, Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992;60:25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guzman CA, Pruzzo C, LiPira G, Calegari L. Role of adherence in pathogenesis of Enterococcus faecalis urinary tract infection and endocarditis. Infect Immun. 1989;57:1834–8. doi: 10.1128/iai.57.6.1834-1838.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Creti R, Koch S, Fabretti F, Baldassarri L, Huebner J. Enterococcal colonization of the gastro-intestinal tract: role of biofilm and environmental oligosaccharides. BMC Microbiol. 2006;6:60. doi: 10.1186/1471-2180-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pillai SK, Sakoulas G, Eliopoulos GM, Moellering RC, Jr., Murray BE, Inouye RT. Effects of glucose on fsr-mediated biofilm formation in Enterococcus faecalis. J Infect Dis. 2004;190:967–70. doi: 10.1086/423139. [DOI] [PubMed] [Google Scholar]
- 36.Joh D, Wann ER, Kreikemeyer B, Speziale P, Hook M. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 1999;18:211–23. doi: 10.1016/s0945-053x(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 37.Dinkla K, Rohde M, Jansen WT, Carapetis JR, Chhatwal GS, Talay SR. Streptococcus pyogenes recruits collagen via surface-bound fibronectin: a novel colonization and immune evasion mechanism. Mol Microbiol. 2003;47:861–9. doi: 10.1046/j.1365-2958.2003.03352.x. [DOI] [PubMed] [Google Scholar]
- 38.Duh RW, Singh KV, Malathum K, Murray BE. In vitro activity of 19 antimicrobial agents against enterococci from healthy subjects and hospitalized patients and use of an ace gene probe from Enterococcus faecalis for species identification. Microb Drug Resist. 2001;7:39–46. doi: 10.1089/107662901750152765. [DOI] [PubMed] [Google Scholar]