SUMMARY
The accumulation of LDL-derived cholesterol in the artery wall is the initiating event that causes atherosclerosis. However, the mechanisms that lead to the initiation of atherosclerosis are still poorly understood. Here, by using endothelial cell-specific transgenesis of the caveolin-1 (Cav-1) gene in mice, we show the critical role of Cav-1 in promoting atherogenesis. Mice were generated lacking Cav-1 and apoE but expressing endothelial-specific Cav-1 in the double knockout background. Genetic ablation of Cav-1 on an apoE knockout background inhibits the progression of atherosclerosis while re-expression of Cav-1 in the endothelium promotes lesion expansion. Mechanistically, the loss of Cav-1 reduces LDL infiltration into the artery wall, promotes nitric oxide production and reduces the expression of leukocyte adhesion molecules, effects completely reversed in transgenic mice. In summary, this unique model provides physiological evidence supporting the important role of endothelial Cav-1 expression in regulating the entry of LDL into the vessel wall and the initiation of atherosclerosis.
INTRODUCTION
Atherosclerosis is a chronic inflammatory process involving complex interactions of modified lipoproteins, monocyte-derived macrophages or foam cells, T lymphocytes, endothelial cells, smooth muscle cells, and fibroblasts (Glass and Witztum, 2001). The accumulation of LDL-derived cholesterol in the artery wall is the initial event that leads to atherosclerosis, yet the mechanisms involved in LDL trafficking through the endothelium remain unclear (Vasile et al., 1983). Vascular permeability to LDL can occur via either a transcellular or a paracellular pathway (Frank et al., 2004). One potential transcellular pathway may occur through caveolae since caveolae are the prominent plasmalemmal vesicle found in the vascular endothelium in vivo. Caveolae are 50- to 100-nm flask-shaped invaginations of plasma membrane and caveolin-1 (Cav-1) is essential for caveolae biogenesis in endothelium, thus orchestrating Cav-1 dependent intracellular trafficking and signal transduction (Liu et al., 2002).
The role of Cav-1 and its family members, Cav-2 and Cav-3, have been studied through the generation of various knockout mice. Cav-1 knockout mice exhibit a complete absence of caveolae in all Cav-1 expressing tissues while mice lacking Cav-3 are devoid of caveolae in muscle tissues (Drab et al., 2001; Galbiati et al., 2001; Razani et al., 2001). The putative functions of caveolae include cholesterol transport, endocytosis, transcytosis and signal transduction. Physiologically, the loss of caveolae results in impairment of cholesterol homeostasis, insulin sensitivity, nitric oxide production, calcium signalling and defects cardiopulmonary and vascular function (Cohen et al., 2003; Frank et al., 2006; Razani et al., 2001). Interestingly, mice deficient in Cav-1 exhibit resistance to atherosclerosis despite a marked proatherogenic lipid profile suggesting that Cav-1 determines the athero-susceptibility of the vessel wall. However, the precise mechanisms to explain this paradox remain uncertain (Frank et al., 2004).
Recent findings have shown a complex role of Cav-1 during the progression of atherosclerosis depending on the cell types examined (Frank et al., 2004b). In endothelial cells, Cav-1 and caveolae may play a proatherogenic role by promoting transcytosis of LDL-cholesterol particles from the blood to the subendothelial space and by tonically inhibiting endothelial nitric oxide synthase (eNOS) (Frank et al., 2004b; García-Cardeña et al., 1996). In contrast, in vascular smooth muscle cells (VSMC), the ability of Cav-1 to negatively regulate cell proliferation and migration (neointimal hyperplasia) may have an anti-atherogenic effect (Hassan et al., 2004; Hassan et al., 2006). Recently, there is evidence that proliferation of VSMC in human atherosclerosis is accompanied by a reduction in Cav-1 expression (Schwencke et al., 2005), effects also seen after arterial injury in rabbits (Peterson et al., 2003). Finally, there is evidence that Cav-1 in monocyte/macrophages influence phagocytosis (Li et al., 2005) and intracellular cholesterol metabolism (Frank et al., 2006), both pathways that may influence atherogenesis. Thus, Cav-1 has the potential to modulate all three major cell types involved in atherogenesis. Here, we show using a genetic model that endothelial caveolae are essential for the progression of atherosclerosis. Mechanistically, endothelial Cav-1 and caveolae are critical for LDL infiltration into the arterial wall, nitric oxide (NO) production and macrophage accumulation, all events necessary for atherogenesis. In addition, endothelial Cav-1 influences the hepatic clearance of triglycerides by regulating the size of liver endothelial cell fenestrations.
RESULTS AND DISCUSSION
Generation of mice lacking Cav-1 and ApoE but expressing endothelial-specific Cav-1
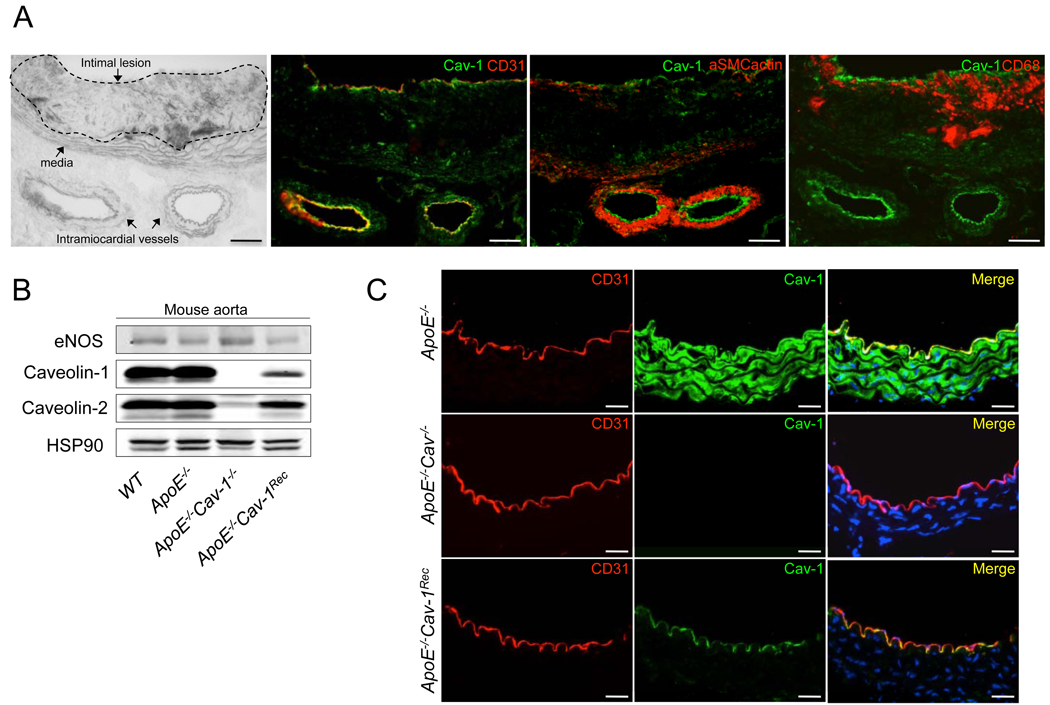
To dissect the specific role of Cav-1 during atherogenesis, we analyzed the expression of Cav-1 in atherosclerotic plaques from atherosclerosis-prone apolipoprotein E (ApoE) knockout mice. As seen in Fig. 1a, Cav-1 is highly expressed in CD31-positive endothelial cells (EC, second panel) and to a lesser extent in vascular smooth muscle cells (VSMC, third panel). Undetectable levels of Cav-1 were found in macrophages localized in atherosclerotic plaques (fourth panel). Similar patterns of Cav-1 expression pattern have been recently reported in human atherosclerotic plaques (Rodriguez-Feo et al., 2008). Since Cav-1 is highly expressed in endothelium, we directly tested the role of endothelial-specific Cav-1/caveolae by generating a mouse model, which re-expresses Cav-1 in EC in the absence of ApoE or Cav-1 (Supplementary Fig. S1). Fig. 1b documents protein expression in isolated aortae from these mice. In the double knockout mice (ApoE−/−Cav-1−/−), Cav-1 and Cav-2 proteins were both absent due to the role of Cav-1 in stabilizing Cav-2 protein levels (Parolini et al., 1999). Interestingly, in the endothelial-specific reconstituted mice (ApoE−/−Cav-1Rec), the expression of both Cav-1 and Cav-2 were restored in the endothelium, where eNOS expression was used as endothelial cell marker. Next, we examined Cav-1 reconstitution in the aortic endothelium using immunofluorescence microscopy. As shown in Fig. 1c, ApoE−/− (top panel) and ApoE−/−Cav-1Rec (bottom panel) mice had relatively equal levels of immunoreactive Cav-1 in endothelial cells as defined by CD31 staining. ApoE−/−Cav-1−/− vessels lacked endothelial Cav-1 immunoreactivity without changing the levels of CD31 (middle panel).
Fig 1. Characterization of Cav-1 expression in arteries from ApoE−/−, ApoE−/− Cav-1−/− and ApoE−/−Cav-1Recmice.
(a). Cav-1 is present in EC and VSM in atherosclerotic vessels from apoE−/− mice. Representative histological analysis of cross-sections from the aoric sinus in phase contrast and co-stained with CD31 (endothelial marker), αSMC actin (vascular smooth muscle marker) and CD68 (macrophage marker). (b). Protein levels of eNOS, Cav-1 and Cav-2 from mouse aorta. Hsp90 was used as a loading control. (c). Immunostaining for Cav-1 in vessels from ApoE−/−, ApoE−/− Cav-1−/− and ApoE−/−Cav-1Rec mice showing the expression of endothelial marker CD31 (red) and Cav-1 protein (green). The scale bars represent 100 µm
Endothelial-specific Cav-1 expression is essential for the progression of atherosclerosis
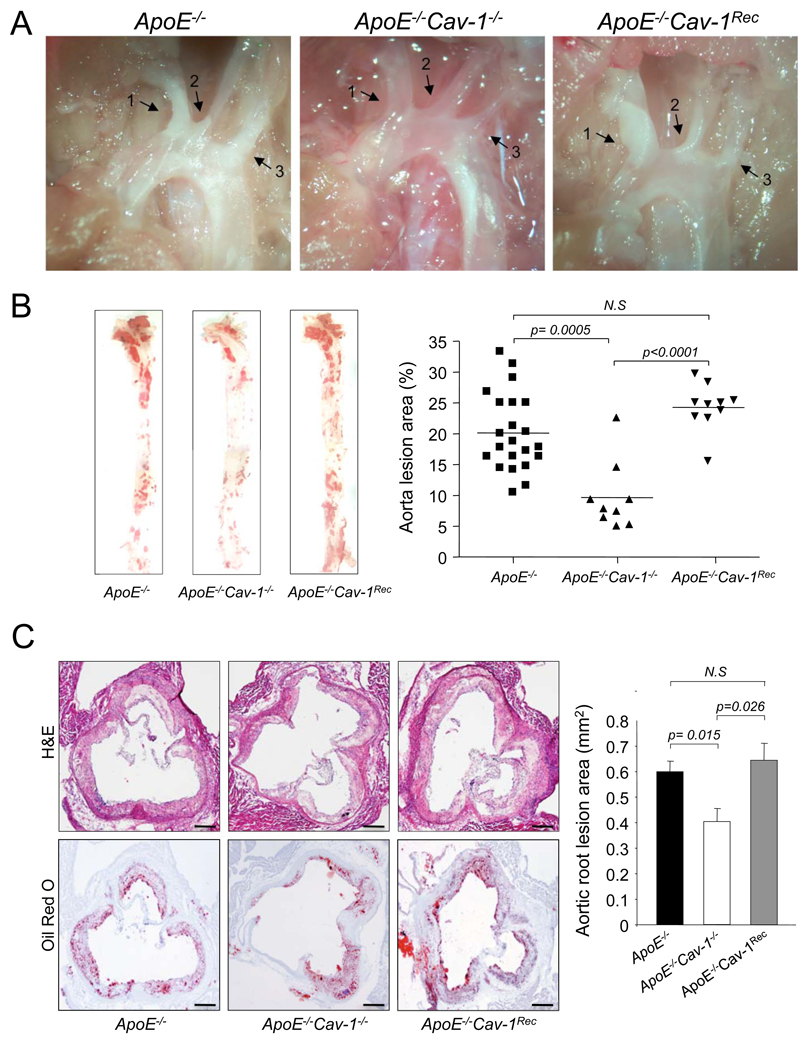
To study the functional role of endothelial Cav-1 in atherogenesis, ApoE−/−, ApoE−/−Cav-1−/− and ApoE−/−Cav-1Rec mice were fed a high-cholesterol diet for 12 weeks. As in seen in Fig. 2a, atherosclerotic plaques were clearly visible by light microscopy in the aortic arch. Interestingly, the absence of Cav-1 markedly reduces plaque formation in the carotid arteries and re-expression of Cav-1 in the endothelium restores athero-susceptibility in these sites. To quantify the extent of atherosclerosis, aortas were opened longitudinally and stained en face with Oil-Red O to visualize lipid-laden, atherosclerotic plaques. As shown in Fig. 2b, the absence of Cav-1 dramatically reduces the number and size of aortic plaques and the re-expression of Cav-1 in the endothelium increases plaque formation in the doubly mutant mice. Similar results were obtained in another cohort of mice by analyzing the lesion areas in cross sections of the aortic sinus (Fig. 2c).
Fig 2. Endothelial-specific expression of Cav-1 is critical for the progression of atherosclerosis.
(a). Representative examples of light microscopic images from ApoE−/−, ApoE−/− Cav-1−/− and ApoE−/− Cav-1Rec aortic arches. Atherosclerotic plaques are easily visualized by the white areas inside the arteries. The arrows indicate the innominate artery (1), left carotid artery (2) and left subclavian artery (3). (b). Oil red O staining of aortas from mice with the indicated genotypes. Atheroma formation was significantly reduced in ApoE−/− Cav-1−/− mice (n=9) compared to ApoE−/− mice (n=22). Re-expression of Cav-1 in the endothelium (ApoE−/− Cav-1Rec, n=10) promotes plaque formation in the doubly mutant mice. (c). Representative histological analysis of aortic sinus stained with hematoxylin and eosin, and Oil red O from the indicated genotypes (n=6). The scale bars represent 200 µm
Endothelial-specific Cav-1 expression regulates LDL infiltration and nitric oxide production
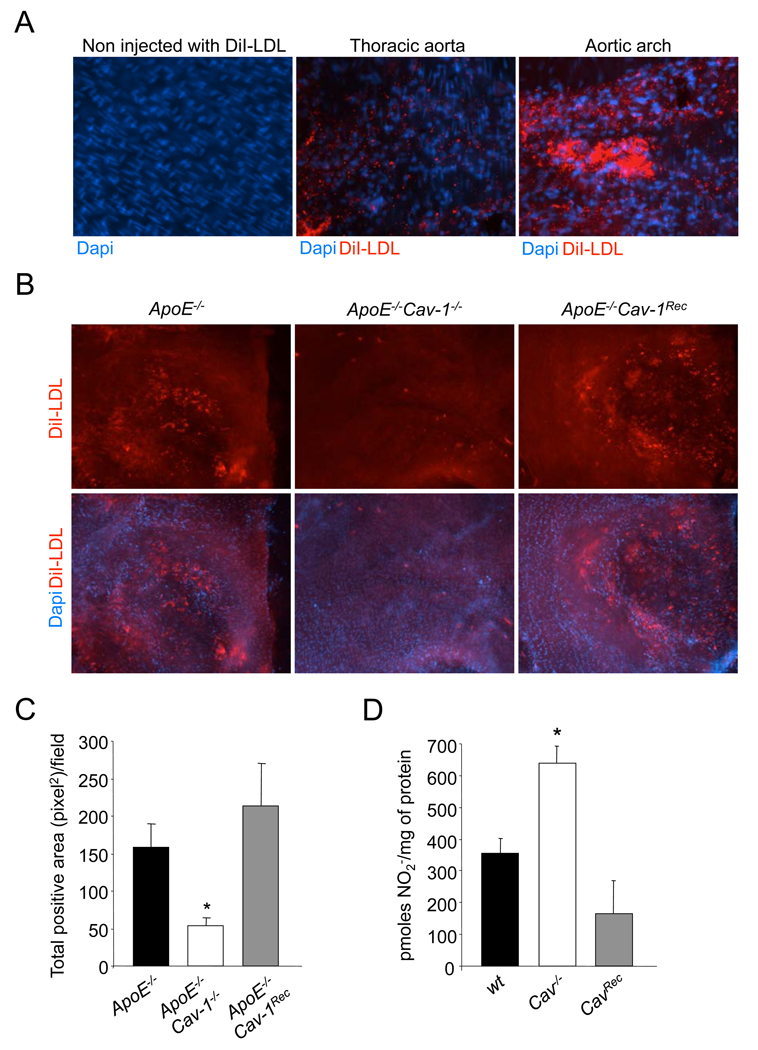
The above data suggest the presence of Cav-1 in endothelium is necessary for the development of atherosclerosis. Since it is well established that the transport of LDL-cholesterol from the blood into the artery wall is the initiating event that triggers atherosclerosis (Glass and Witztum, 2001), we examined the importance of EC Cav-1 in this process. Indeed, the perm-selectivity of the endothelium to albumin and radiolabeled LDL are reduced in large blood vessels, such as the aorta, in Cav-1 deficient mice (Frank et al., 2008; Schubert et al., 2002). To test this directly, we examined LDL uptake and infiltration into vessels of ApoE−/−, ApoE−/−Cav-1−/− and ApoE−/−Cav-1Rec mice, after jugular vein injections of fluorescent DiI-LDL and monitored the accumulation of LDL by en face imaging of the vessel wall. More DiI-LDL accumulated in atherosclerosis-prone sites of the aortic arch (i.e. the lesser curvature in the aortic arch) with respect to the thoracic aorta (Fig. 3a). The absence of Cav-1 caused a three-fold decrease in DiI-LDL infiltration in the aortic segments, an effect rescued by the reintroduction of Cav-1 into the endothelium (Fig. 3b and 3c). Thus, Cav-1 dependent caveolae regulates the entry of LDL into the vessel wall.
Fig 3. Endothelial-specific Cav-1 regulates LDL entry into the artery wall and the NO production in EC.
(a). Immunofluorescence analysis of Di-LDL infiltration in different aortic segments. En face fluorescence images of aortic sections from 6-week- old ApoE−/− old mice 30 min after intravenous injection with DiI-LDL. The images were captured using a 40X objective. (b). En face fluorescence images of aortic sections from 6 week old ApoE−/−, ApoE−/−Cav-1−/− and ApoE−/−Cav-1Rec old mice 30 min after intravenous injection with DiI-LDL. (c) Quantification of DiI fluorescence intensity from en face images. The data are quantified as fluorescence-positive area versus total area. The data represent the mean ± SEM; n=5 mice in each group. * Indicates p< 0.05 compared with ApoE−/−. The images were captured using a 10X objective. (d) NO release from wt, Cav-1−/− and Cav-1Rec. The nitrite accumulation was quantified for 8 h. The date represents the mean ± SEM of triplicate samples repeated in three independent experiments. * Indicates p< 0.05 compared with control.
The production of nitric oxide (NO) by vascular endothelium is important for cardiovascular homeostasis, as endogenous NO regulates many fundamental processes, (Sessa, 2004) and impaired NO bioavailability is a commonly used index of endothelial dysfunction in atherosclerosis (Busse and Fleming, 1996). Genetic deletion of endothelial NO synthase (eNOS) causes many cardiovascular phenotypes, including accelerated atherosclerosis (Kuhlencordt et al., 2001). Since endogenous eNOS activation is under the tonic inhibitory influence of Cav-1 as a negative regulator of eNOS activity (Garcia-Cardena et al., 1996) we directly examined the effect of Cav-1 on NO production, using EC from WT, Cav-1−/− and Cav-1Rec mice. As seen in Fig. 3d, the loss of Cav-1 increases NO production, while the re-expression of Cav-1 reduces the NO accumulation to the same levels as WT cells. Thus, elevated production of NO by the endothelium may also contribute to the reduced atherosclerosis observed in the doubly mutant mice.
Increased vessel wall inflammation and macrophage infiltration is mediated by endothelial-specific Cav-1
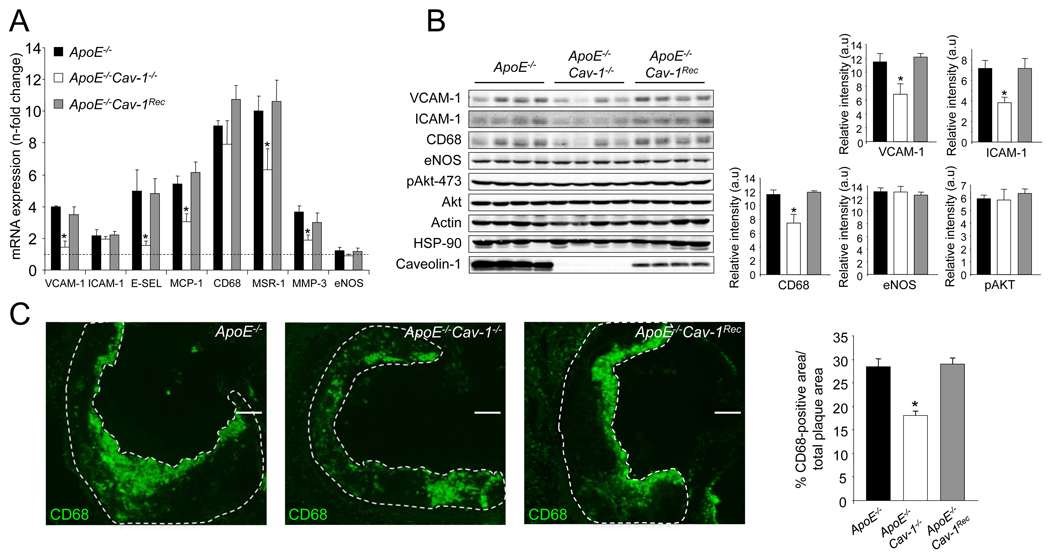
To gain insights into how Cav-1 regulates the extent of atherogenesis, we examined several additional potential mechanisms. Activation of EC by proinflammatory cytokines occurs via the expression of various leukocyte adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1), intracellular adhesion molecule 1 (ICAM-1), and E-selectin, which capture and transport circulating leukocytes to atherosclerotic lesions (Galkina and Ley, 2007). To directly examine whether the loss of Cav-1 influences adhesion molecule expression, the levels of VCAM-1 and ICAM-1 were quantified by Western blotting in EC isolated from WT, Cav-1−/− and Cav-1Rec mice after stimulation with tumor necrosis factor α (TNFα). As seen in Supplementary Fig. 2a and 2b, the absence of Cav-1 causes a slight decrease, but non significant change, in VCAM-1 and ICAM-1 expression, which is restored in EC from Cav-1Rec mice. Similar results were obtained analyzing cell surface protein expression for VCAM-1 and ICAM-1 by flow cytometry (Supplementary Fig. 2c) and a greater reduction in the surface expression of E-selectin was found in Cav-1−/− ECs (Supplementary Fig. 2c) however, the absence or presence of Cav-1 did not influence the proportional distribution of these adhesion molecules in EC using discontinuous sucrose gradients (Supplementary Fig. 2d). Next, we analyzed the expression patterns of markers of inflammation in the vessel wall of ApoE−/−, ApoE−/− Cav-1−/− and ApoE−/−Cav-1Rec mice before and after 3 months on a high cholesterol diet (i.e. fold change after diet) by quantitative polymerase chain reaction (qPCR) array technology. As seen in Supplementary Fig. S3 and Supplementary Fig. S4, the expression levels of several inflammatory molecules (VCAM-1, ICAM-1, E-selectin, P-selectin) and macrophage markers were significantly downregulated in the ApoE−/− Cav-1−/− mice compared to ApoE−/− alone and were confirmed using a different set of primers (Fig. 4a). The downregulation of these “pro-atherogenic” genes in absence of Cav-1 was reversed in the endothelial-specific reconstituted mice (ApoE−/− Cav-1Rec) and confirmed by Western blotting (Fig. 4b, quantified in the right panel). However, enhanced inflammatory and macrophage specific gene expression did not influence the levels of eNOS and Akt, important enzymes involved in the control of vascular homeostasis (Fig. 4b).
Fig 4. Loss of Cav-1 alters the expression profile of atherosclerosis-related genes and macrophage content in the artery wall.
(a) Expression profile of atherosclerosis-related genes assessed by real-time PCR. Five independent qPCR reactions were carried out for each condition with iCycler (BioRad). The n-fold change for each gene, after diet compared to before diet was calculated. The data represents the mean ±SEM of quintuplicate samples. Black bars correspond to ApoE−/−, white bars to ApoE−/−Cav-1−/− and grey bars to ApoE−/−Cav-1Rec mice. (b) Western Blot analyses (left panel) and densitometry (right panel) of VCAM-1, ICAM-1, CD68, eNOS, pAkt-473, Akt, Actin, HSP-90 and Cav-1 in aortic extracts prepared ApoE−/−, ApoE−/−Cav-1−/− and ApoE−/−Cav-1Rec mice fed a high cholesterol diet for 12 weeks. Results for four representative mice are shown for each genotype. The data represent the mean ± SEM of quadruplicate samples. (c). CD68-positive macrophages in lesions from ApoE−/−, ApoE−/−Cav-1−/− and ApoE−/−Cav-1Rec mice after 12 weeks of a high cholesterol diet were detected by CD68 staining. The data are quantified as CD68-positive area versus total lesion area (right panel). The data represent the mean ± SEM; n=5 in each group.
The enhanced expression of markers of inflammation suggested that the levels of macrophage infiltration might differ in the three strains. As seen in Fig. 4c, the loss of Cav-1 reduces macrophage accumulation (depicted by CD68-positive cells) in plaques, whereas, re-expression of Cav-1 in the endothelium increases the macrophage infiltration in the ApoE−/−Cav-1−/−. Collectively, these data demonstrate that endothelial Cav-1 influences LDL uptake, NO production and macrophage accumulation leading to an augmented inflammatory plaque phenotype and larger lesions.
Endothelial-specific Cav-1 expression reverses the dyslipidemia of the double mutant mice
Finally, we examined multiple metabolic parameters of the mice. Body weights were similar in all the groups of mice before and after a high cholesterol diet (Supplementary Fig. 5a). The absence of Cav-1 increases plasma triglyceride (TG) and cholesterol levels (Supplementary Fig. 5b) as previously reported (Razani et al., 2002). Interestingly, the re-expression of Cav-1 in the endothelium restores plasma TG to normal levels and partially lowers cholesterol levels before and after diet (Supplementary Fig. 5b and 5c). Next, lipoprotein profiles of mice fed with a normal chow (Supplementary Fig. 5d and 5e) or a high cholesterol diet (Supplementary Fig. 5f and 5g) were analyzed. ApoB containing and remnant lipoproteins (chylomicron remnants, VLDL and IDI/LDL-size fractions) were elevated in the double mutant mice (red line; ApoE−/−Cav-1−/−). Interestingly, re-expression of Cav-1 in EC (blue line) reverses this “pro-atherogenic” lipoprotein profile in mice fed normal chow or a high cholesterol diet. Since the enhanced levels of TG in Cav-1 deficient mice are not due to changes in lipoprotein lipase (LPL) and/or hepatic lipase (HL) activity (Razani et al., 2002), we further characterized the rate of liver VLDL-TG synthesis in ApoE−/−, ApoE−/−Cav-1−/− and ApoE−/−Cav-1Rec mice using Triton WR-1339 (Otway and Robinson, 1967), which inhibits LPL and therefore allowing quantification of VLDL secretion. As seen in Supplementary Fig. 6a, we did not observe any significant differences between the different groups of mice analyzed suggesting the clearance of VLDL/CM was impaired in ApoE−/−Cav-1−/− mice. Moreover, the expression of genes involved in the lipid synthesis and secretion of VLDL in the liver were also similar in the two strains (Supplementary Fig. 6b).
It is appreciated that the morphology of hepatic sinusoids are critical for the clearance of chylomicron remnants and postprandial hyperlipemia (Cogger et al., 2008; DG et al., 2007; Hilmer et al., 2005). The fenestrations of the liver sinusoid endothelium (LSEC) influence the rate of substrate transfer (including lipoproteins) between the sinusoidal blood, the space of Disse and hepatocytes (Fraser et al., 1995); thus changes in LSEC fenestrations or number has a significant impact on hepatic clearance functions. Cav-1 can be found in fenestrations of isolated LSECs (Cogger et al., 2008) and in rat liver using immunogold microscopy (Ogi et al., 2003). Similarly, we found Cav-1 in LSEC of ApoE−/− but not ApoE−/−Cav-1−/− mice with immunogold electron microscopy (Supplementary Fig. 7). To determine whether the absence or presence of Cav-1 influences SEC fenestrations, we analyzed sinusoid morphology using scanning electron microscope (SEM). As seen in Supplementary Fig. 8a, quantitative EM studies indicates that Cav-1 changes the porosity of the LSEC, indexed by a reduction in the diameter but not in the frequency of fenestrations (Supplementary Fig. 8b and 8c). The idea that Cav-1 regulates SEC porosity is supported by the correlation between sinusoid pseudocapillarization and the levels of hepatic Cav-1 (Jamieson et al., 2007). Thus, the reduced clearance of TG in ApoE−/−Cav-1−/− mice is likely due to pseudocapillarization of the SEC.
The major finding of this study is that endothelial caveolae regulate the portals of entry for LDL into the vessel wall since transgenic expression of endothelial Cav-1 reverts the typically atheroprotected ApoE−/−Cav-1−/− mouse to an atheroprone mouse. Mechanistically, we show that Cav-1 controls LDL uptake, endothelial NO levels and the expression of vascular adhesion molecules that promote macrophage infiltration. Early EM studies examining LDL transport across the endothelium documented the uptake of LDL via two routes; a saturable, clathrin mediated endocytic mechanism via the LDL- receptor and a fluid phase non-saturable transcytotic mechanism through non-coated plasmalemmal vesicles, perhaps caveolae (Simionescu and Simionescu, 1991). Although Cav-1 is expressed in several cell types (EC, macrophages, vascular smooth muscle cells (VSMCs) and fibroblasts), implicated in the pathogenesis of atherosclerosis, Cav-1 in EC is sufficient to promote lesion development. Thus our findings provide an important in vivo demonstration that endothelial cell Cav-1 regulates the entry of LDL to promote atherogenesis.
EXPERIMENTAL PROCEDURES
Materials and additional methods are available in supplemental experimental procedures.
Animal procedures
Cav-1−/− mice were generated as previously described (Drab et al., 2001). Cav1−/− mice that have been backcrossed 6 generations onto a C57BL/6 background were crossed with ApoE-deficient mice, also on the C57BL/6 background, to generate mice heterozygous at both loci. These ApoE+/− Cav1+/− mice were crossed a second time with ApoE−/− mice. The ApoE/− Cav1−/+ progeny from this round of breeding were then inter-crossed to produce ApoE−/− Cav1−/− and ApoE/− Cav1+/+ littermates that were used as controls for all studies. Endothelial-specific Cav-1 TG mice carrying canine Cav-1 transgene under the preproendothelin-1 promoter were crossed 6 generations with F6 generation Cav-1−/−, as reported previously (Yu et al., 2006). Accelerated atherosclerosis was induced by feeding the mice for 14 weeks with a high-fat Western-type diet containing 1.25% cholesterol (ResearchDiets, D12108). The Institutional Animal Care Use Committee of Yale University approved all the experiments.
Atherosclerotic lesion analysis
After 12 weeks of being fed a Western-type diet, mice were anesthetized and euthanized. Mouse hearts were perfused using 10 ml of PBS (Invitrogen) followed by 10 ml of 4% Para-formaldehyde (PFA). After incubation in 4% PFA overnight, the adventitia was thoroughly cleaned under a dissecting microscope, and the aorta was cut open longitudinally and pinned on to a silicone plate. To calculate the lesion area, aortas were stained with Oil Red O (Sigma) before the analysis. Oil Red stock solution (35ml; 0.2% weight/volume in methanol) was mixed with 10 ml of 1 M NaOH and filtered. Aortas were briefly rinsed with 78% methanol, incubated in Oil Red O solution for 50 min, then destained in 78% methanol for 5 min and mounted on microscopic slides using aqueous mounting medium (Stephens Scientific). Plaques were analyzed under the Nikon SMZ 1000 microscope, connected to a Kodak DC290 digital camera. The images were analyzed using Adobe Photoshop 6.0 (Adobe) and the lesions quantified using the IMAGE J (NIH) program. For other images, 10-(µm thick cryosections of the proximal aorta were serially sectioned and stained with hematoxylin/eosin for quantifications of the lesion areas using IMAGE J (NIH) program. Aortic lesion size of each animal was obtained by averaging lesion areas in six sections from the same mouse.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants R01 HL64793, R01 HL61371, R01 HL57665, P01 Hl70295 and Contract No. N01-HV-28186 (NHLBI-Yale Proteomics Contract) from the National Institutes of Health to W.C. Sessa, Scientist Development Grants from American Heart Association (to C. Fernández-Hernando and to Y.Suárez) and in part by a grant from Ministerio de Educación y Ciencia (SAF2005-07308), Spain. CIBER Fisiología Obesidad y Nutrición is an initiative of the Instituto de Salud Carlos III.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Busse R, Fleming I. Endothelial dysfunction in atherosclerosis. J Vasc Res. 1996;33:181–194. doi: 10.1159/000159147. [DOI] [PubMed] [Google Scholar]
- Cogger VC, Arias IM, Warren A, McMahon AC, Kiss DL, Avery VM, Le Couteur DG. The response of fenestrations, actin, and caveolin-1 to vascular endothelial growth factor in SK Hep1 cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G137–G145. doi: 10.1152/ajpgi.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol. 2003;285:C222–C235. doi: 10.1152/ajpcell.00006.2003. [DOI] [PubMed] [Google Scholar]
- DG LEC, Cogger VC, McCuskey RS, R DEC, Smedsrod B, Sorensen KK, Warren A, Fraser R. Age-related changes in the liver sinusoidal endothelium: a mechanism for dyslipidemia. Ann N Y Acad Sci. 2007;1114:79–87. doi: 10.1196/annals.1396.003. [DOI] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Frank PG, Cheung MW, Pavlides S, Llaverias G, Park DS, Lisanti MP. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am J Physiol Heart Circ Physiol. 2006;291:H677–H686. doi: 10.1152/ajpheart.01092.2005. [DOI] [PubMed] [Google Scholar]
- Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004a;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- Frank PG, Lisanti MP. Caveolin-1 and caveolae in atherosclerosis: differential roles in fatty streak formation and neointimal hyperplasia. Curr Opin Lipidol. 2004b;15:523–529. doi: 10.1097/00041433-200410000-00005. [DOI] [PubMed] [Google Scholar]
- Frank PG, Pavlides S, Cheung MW, Daumer K, Lisanti MP. Role of caveolin-1 in the regulation of lipoprotein metabolism. Am J Physiol Cell Physiol. 2008;295:C242–C248. doi: 10.1152/ajpcell.00185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser R, Dobbs BR, Rogers GW. Lipoproteins and the liver sieve: the role of the fenestrated sinusoidal endothelium in lipoprotein metabolism, atherosclerosis, and cirrhosis. Hepatology. 1995;21:863–874. [PubMed] [Google Scholar]
- Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H, Jr, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem. 2001;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem. 1996;271:27237–27240. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Hassan GS, Jasmin JF, Schubert W, Frank PG, Lisanti MP. Caveolin-1 deficiency stimulates neointima formation during vascular injury. Biochemistry. 2004;43:8312–8321. doi: 10.1021/bi049609t. [DOI] [PubMed] [Google Scholar]
- Hassan GS, Williams TM, Frank PG, Lisanti MP. Caveolin-1 deficient aortic smooth muscle cells show cell autonomous abnormalities in proliferation, migration, and endothelin-based signal transduction. Am J Physiol Heart Circ Physiol. 2006;290:H2393–H2401. doi: 10.1152/ajpheart.01161.2005. [DOI] [PubMed] [Google Scholar]
- Hilmer SN, Cogger VC, Fraser R, McLean AJ, Sullivan D, Le Couteur DG. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005;42:1349–1354. doi: 10.1002/hep.20937. [DOI] [PubMed] [Google Scholar]
- Jamieson HA, Cogger VC, Twigg SM, McLennan SV, Warren A, Cheluvappa R, Hilmer SN, Fraser R, de Cabo R, Le Couteur DG. Alterations in liver sinusoidal endothelium in a baboon model of type 1 diabetes. Diabetologia. 2007;50:1969–1976. doi: 10.1007/s00125-007-0739-4. [DOI] [PubMed] [Google Scholar]
- Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- Li J, Scherl A, Medina F, Frank PG, Kitsis RN, Tanowitz HB, Sotgia F, Lisanti MP. Impaired phagocytosis in caveolin-1 deficient macrophages. Cell Cycle. 2005;4:1599–1607. doi: 10.4161/cc.4.11.2117. [DOI] [PubMed] [Google Scholar]
- Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002;277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- Ogi M, Yokomori H, Oda M, Yoshimura K, Nomura M, Ohshima S, Akita M, Toda K, Ishii H. Distribution and localization of caveolin-1 in sinusoidal cells in rat liver. Med Electron Microsc. 2003;36:33–40. doi: 10.1007/s007950300004. [DOI] [PubMed] [Google Scholar]
- Otway S, Robinson DS. The use of a non-ionic detergent (Triton WR 1339) to determine rates of triglyceride entry into the circulation of the rat under different physiological conditions. J Physiol. 1967;190:321–332. doi: 10.1113/jphysiol.1967.sp008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini I, Sargiacomo M, Galbiati F, Rizzo G, Grignani F, Engelman JA, Okamoto T, Ikezu T, Scherer PE, Mora R, Rodriguez-Boulan E, Peschle C, Lisanti MP. Expression of caveolin-1 is required for the transport of caveolin-2 to the plasma membrane. Retention of caveolin-2 at the level of the golgi complex. J Biol Chem. 1999;274:25718–25725. doi: 10.1074/jbc.274.36.25718. [DOI] [PubMed] [Google Scholar]
- Peterson TE, Guicciardi ME, Gulati R, Kleppe LS, Mueske CS, Mookadam M, Sowa G, Gores GJ, Sessa WC, Simari RD. Caveolin-1 can regulate vascular smooth muscle cell fate by switching platelet-derived growth factor signalling from a proliferative to an apoptotic pathway. Arterioscler Thromb Vasc Biol. 2003;23:1521–1527. doi: 10.1161/01.ATV.0000081743.35125.05. [DOI] [PubMed] [Google Scholar]
- Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, Lisanti MP. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277:8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Feo JA, Hellings WE, Moll FL, De Vries JP, van Middelaar BJ, Algra A, Sluijter J, Velema E, van der Broek T, Sessa WC, De Kleijn DP, Pasterkamp G. Caveolin-1 influences vascular protease activity and is a potential stabilizing factor in human atherosclerotic disease. PLoS ONE. 2008;3:e2612. doi: 10.1371/journal.pone.0002612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- Schwencke C, Schmeisser A, Walter C, Wachter R, Pennach S, Weck B, Braun-Dullaeus RC, Kasper M, Strasser RH. Decreased caveolin-1 in atheroma: loss of antiproliferative control of vascular smooth muscle cells in atherosclerosis. Cardiovasc Res. 2005;68:128–135. doi: 10.1016/j.cardiores.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Simionescu M, Simionescu N. Endothelial transport of macromolecules: transcytosis and endocytosis. A look from cell biology. Cell Biol Rev. 1991;25:1–78. [PubMed] [Google Scholar]
- Vasile E, Simionescu M, Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. J Cell Biol. 1983;96:1677–1689. doi: 10.1083/jcb.96.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.