Abstract
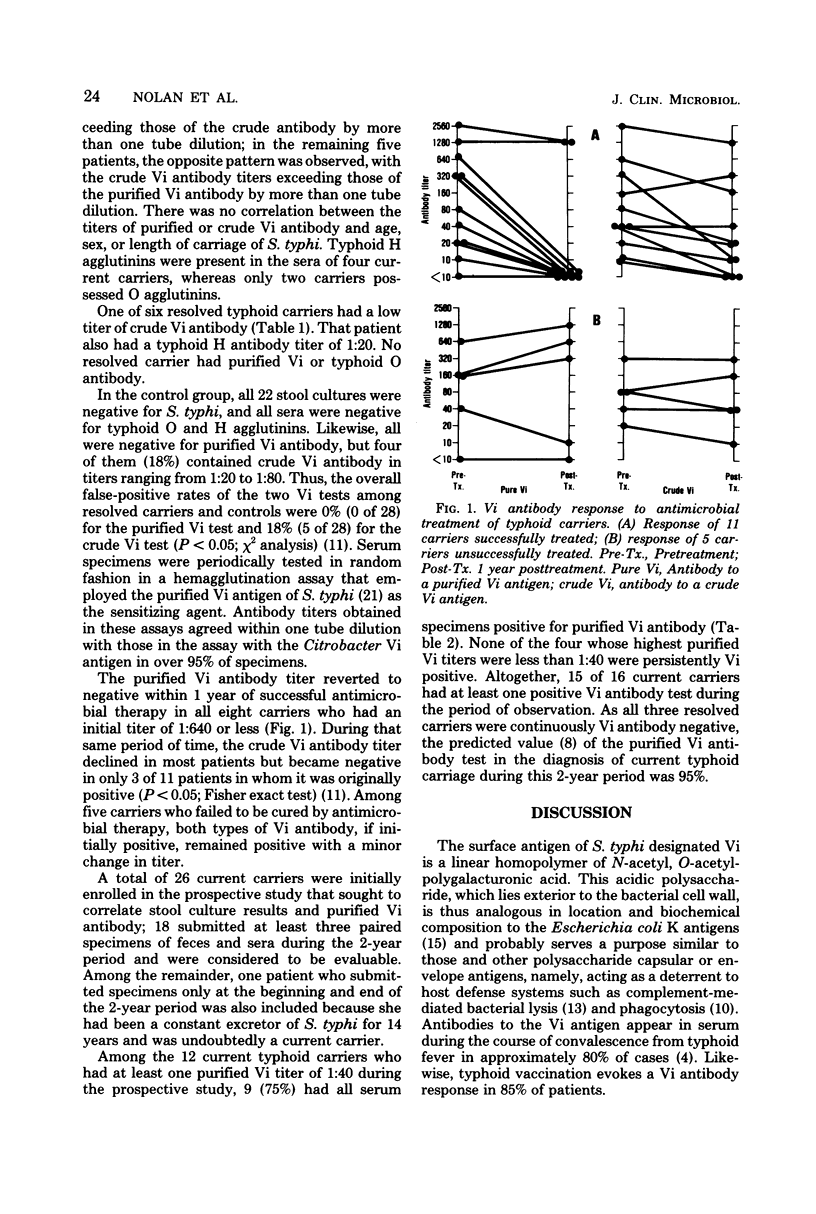
The assay for serum antibody to the Salmonella typhi capsular polysaccharide (Vi) antigen has recently been revised because of the availability of a purified, highly polymerized Vi antigen. We compared this revised Vi antibody assay to the traditional one for potential usefulness in the surveillance of chronic enteric carriers of S. typhi. The purified Vi antigen of Citrobacter freundii was incorporated into a passive hemagglutination assay for serum Vi antibody; the standard Vi antibody assay was also a hemagglutination assay that employed as the Vi antigen a crude extract of Citrobacter (Ballerup O group 29). As determined by the revised assay, Vi antibody was found in the sera of 22 (71%) of 31 current typhoid carriers, none of 6 resolved carriers, and none of 22 control subjects. According to the traditional assay, Vi antibody was present in 23 of those current carriers (74%), 1 of the resolved carriers (17%), and 4 of the control subjects (18%). The rate of false-positive Vi antibody tests among resolved carriers and control subjects was less with the revised assay (P < 0.05). Successful antimicrobial therapy resulted in a reversion to seronegativity within 1 year in 8 of 10 Vipositive carriers according to the revised assay, but in only 3 of 11 according to the standard assay (P < 0.05). During a 2-year period of observation, 15 (94%) of 16 current typhoid carriers had at least one positive purified Vi antibody test; among 12 of those patients with Vi titers of 1:40 or greater, 9 (75%) were continuously Vi positive. Thus, the revised Vi antibody assay is more specific and no less sensitive than the standard assay for the condition of current enteric carriage of S. typhi. This serological test could be of value in the surveillance of typhoid carriers by public health agencies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. Vi haemagglutination test for the identification of typhoid carriers. Guys Hosp Rep. 1970;119(2):111–117. [PubMed] [Google Scholar]
- BAKER E. E., WHITESIDE R. E., BASCH R., DEROW M. A. The VI antigens of the Enterobacteriaceae. I. Purification and chemical properties. J Immunol. 1959 Dec;83:680–696. [PubMed] [Google Scholar]
- Brodie J. Antibodies and the Aberdeen typhoid outbreak of 1964. I. The Widal reaction. J Hyg (Lond) 1977 Oct;79(2):161–180. doi: 10.1017/s0022172400052979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau P. Y., Chan A. C. Modified Vi tests in the screening of typhoid carriers. J Hyg (Lond) 1976 Aug;77(1):97–104. doi: 10.1017/s002217240005556x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein A. R. Clinical biostatistics XXXI. On the sensitivity, specificity, and discrimination of diagnostic tests. Clin Pharmacol Ther. 1975 Jan;17(1):104–116. doi: 10.1002/cpt1975171104. [DOI] [PubMed] [Google Scholar]
- Forrest C. R., Matthews R. N., Robertson M. J., Hanley W. P. Vi reaction in Hong Kong. Br Med J. 1967 May 20;2(5550):472–475. doi: 10.1136/bmj.2.5550.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- Nolan C. M., White P. C., Jr Treatment of typhoid carriers with amoxicillin. Correlates of successful therapy. JAMA. 1978 Jun 2;239(22):2352–2354. doi: 10.1001/jama.239.22.2352. [DOI] [PubMed] [Google Scholar]
- OSAWA E., MUSCHEL L. H. THE BACTERICIDAL ACTIONS OF O AND VI ANTIBODIES AGAINST SALMONELLA TYPHOSA. J Immunol. 1964 Feb;92:281–285. [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Ryder R. W., Blake P. A. Typhoid fever in the United States, 1975 and 1976. J Infect Dis. 1979 Jan;139(1):124–126. doi: 10.1093/infdis/139.1.124. [DOI] [PubMed] [Google Scholar]
- SCHUBERT J. H., EDWARDS P. R., RAMSEY C. H. Detection of typhoid carriers by agglutination tests. J Bacteriol. 1959 May;77(5):648–654. doi: 10.1128/jb.77.5.648-654.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. C. Chronic enteric carriers: management of personal problems. Br Med J. 1966 Sep 3;2(5513):551–555. doi: 10.1136/bmj.2.5513.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Feeley J. C. Isolation of Vi antigen and a simple method for its measurement. Appl Microbiol. 1972 Oct;24(4):628–633. doi: 10.1128/am.24.4.628-633.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Feeley J. C., Northrup R. S., Forlines M. E. Vi antigen from Salmonella typhosa and immunity against typhoid fever. I. Isolation and immunologic properties in animals. Infect Immun. 1974 Feb;9(2):348–353. doi: 10.1128/iai.9.2.348-353.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


