Summary
Force-producing convergence (narrowing) and extension (lengthening) of tissues by active intercalation of cells along the axis of convergence play a major role in axial morphogenesis during development of both vertebrate and invertebrate embryos, and failure of these processes in human embryos leads to embryonic defects including spina bifida and anencephaly. Here we use Xenopus laevis, a system in which the polarized cell motility that drives this active cell intercalation has been related to development of forces that close the blastopore and elongate the body axis, to examine the role of myosin IIB in convergence and extension. We find that myosin IIB is localized in the cortex of intercalating cells, and that morpholino knockdown of this myosin isoform shows that it is essential for maintenance of a stereotypical, cortical actin cytoskeleton that we visualize with time-lapse fluorescent confocal microscopy. We show that this actin network consists of foci or nodes connected by cables and is polarized relative to the embryonic axis, preferentially cyclically shortening and lengthening parallel to the axis of cell polarization, elongation, and intercalation, and also parallel to the axis of convergence forces during gastrulation. MHC-B-depletion results in disruption of this polarized cytoskeleton, loss of the polarized protrusive activity characteristic of intercalating cells, eventual loss of cell-cell and cell matrix adhesion, and dose-dependent failure of blastopore closure, arguably because of failure to develop convergence forces parallel to the myosin IIB-dependent dynamics of the actin cytoskeleton. These findings bridge the gap between a molecular-scale motor protein and tissue-scale embryonic morphogenesis.
Keywords: Morphogenesis, Xenopus, Myosin, Mesoderm, Notochord
Introduction
The tissue movements of convergence (narrowing) and extension (lengthening), often referred to as convergent extension (CE), function in many aspects of vertebrate and invertebrate morphogenesis (Keller, 2002; Keller et al., 2003; Myers et al., 2002; Zallen, 2007). Any example of CE in these diverse systems could either be due to passive stretching of the tissue due to external forces or could be active and autonomously driven by internal force-generating processes (Keller et al., 2000). CE of the Drosophila germband, an epithelial sheet of cells, occurs by intercalation of cells to form a narrower, longer array in a process involving apical junctional remodeling, either between pairs of neighboring cells or between multiple cells in rosettes, and is dependent on polarized localization of myosin II in these junctions (Bertet et al., 2004; Blankenship et al., 2006; Zallen and Wieschaus, 2004). CE of the dorsal axial and paraxial mesoderm of vertebrates, a mesenchymal tissue that therefore lacks apical junctions, also occurs by polarized intercalation of cells to form a longer, narrower array, and this process depends on a characteristic polarized protrusive activity (Glickman et al., 2003; Keller et al., 2000; Sepich et al., 2000), for which the vertebrate non-canonical Wnt/planar cell polarity (PCP) pathway is essential (Goto and Keller, 2002; Heisenberg et al., 2000; Tada and Smith, 2000). In both epithelial (Iwaki et al., 2001; Lengyel and Iwaki, 2002; Zallen and Wieschaus, 2004) and mesenchymal systems (Ninomiya et al., 2004) patterning cues in the future axis of extension are necessary for intercalation of cells in the axis of convergence. It is not known in any system how the forces that drive the active intercalation of cells, and thus convergent extension, are actually generated and transmitted from the molecular to the cellular and tissue level. Here we examine the function of conventional myosins in this process as it occurs in the dorsal mesoderm of Xenopus laevis, a system in which the cellular and tissue level biomechanics are at least partially understood.
In Xenopus, active cell intercalation during CE is accompanied by a characteristic suite of polarized cell behavior (MIB: mediolateral intercalation behavior) (Shih and Keller, 1992a), which is thought to exert traction on adjacent cells and pull them between one another. As the cells actively wedge between one another, they force the elongation of the tissue (Keller et al., 1992). At the tissue level the axis of mediolateral intercalation, and thus the axis of tissue convergence, describes an arc across the dorsal lip of the blastopore. These convergence forces are generated by this polarized, oriented cell behavior, and are associated with development of circumblastoporal cell intercalation leading to shortening of these arcs of hoop stress around the blastopore to squeeze the blastopore shut, as well as drive an orthogonal anterior-posterior axial extension (Keller et al., 2000; Shih and Keller, 1992a; Shih and Keller, 1992b). These arcs of tension must run through cells and, at least at the notochord-somite boundary, extracellular matrix, but the molecular mechanisms and subcellular functional elements that support this tension or its generation have not been identified. Disruption of the mechanical continuity of these arcs of convergence will block the development of these circumblastoporal forces and thus blastopore closure (Keller, 1981; Schectman, 1942; Skoglund and Keller, 2007). Here we report that myosin IIB is essential for the organization of a previously undescribed cortical actin cytoskeletal network underlying development of these circumblastoporal convergence forces.
Cytoskeletal (non-muscle) myosin II complexes are molecular motors that can both bind to and crosslink actin filaments into higher order structures, as well as translate chemical energy into force production inside the cell by coupling ATP hydrolysis to movement along an actin filament (Geeves and Holmes, 2005; Rayment and Holden, 1994). Vertebrates have three myosin II’s (A, B and C), characterized by distinct heavy chain isoforms (MHC-A, –B, and –C) (Berg et al., 2001; Golomb et al., 2004). Myosins are hexamers composed of pairs of myosin heavy chains along with two regulatory and two essential light chains to form a bivalent actin-binding unit, which further aggregates into higher order bipolar filaments to facilitate actin crosslinking and thereby regulate cytoskeletal architecture in the cell (Landsverk and Epstein, 2005). Myosin IIA and IIB exhibit distinct biochemical rates for a conserved set of activities, including on/off rates for actin binding, rates of actin-dependent ATP hydryolysis and duty cycle characteristics governing the balance between cytoskeletal assembly activity and force production (Geeves and Holmes, 1999; Holmes and Geeves, 2000; Kelley et al., 1996). They can be differentially located even when expressed in the same cell (Kelley et al., 1996; Kolega, 1998; Maupin et al., 1994; Rochlin et al., 1995), suggesting each isoform has specific roles.
We have investigated the role of myosin IIB in convergence and extension. In Xenopus, myosin IIB is the best candidate to be involved in CE, because MHC-B mRNA is expressed in dorsal mesoderm and up- regulated by activin in animal cap experiments (Bhatia-Dey et al., 1993; Bhatia-Dey et al., 1998; Kelley et al., 1996), and thus is expressed at the right time and place for involvement in CE of presumptive mesoderm. We find that myosin IIB is required for normal blastopore closure by operating in the process of CE at the dorsal lip. We show that myosin IIB organizes a cortical actin network in intercalating cells, that this cytoskeletal structure is polarized with respect to the embryonic axis and that mis-regulation of this network likely underlies the range of phenotypes observed in MHC-B depleted cells, including defects in expression of polarized cell motility and reduced cell-cell and cell-matrix adhesion.
Materials and Methods
Embryos and manipulations
Both pigmented and albino Xenopus embryos were generated by inducton of egg laying and in vitro fertilization (Kintner and Melton, 1987) and staged (Nieuwkoop and Faber, 1967) by conventional means. In situ hybridizations were performed as described (Skoglund et al., 2006), using restriction enzyme Xho I and polymerase SP6 to make the brachyury probe from the plasmid pXBRA (Amaya et al., 1993). Explants to visualize notochordal cell behavior were cut as described in Figure 2C.
Figure 2.
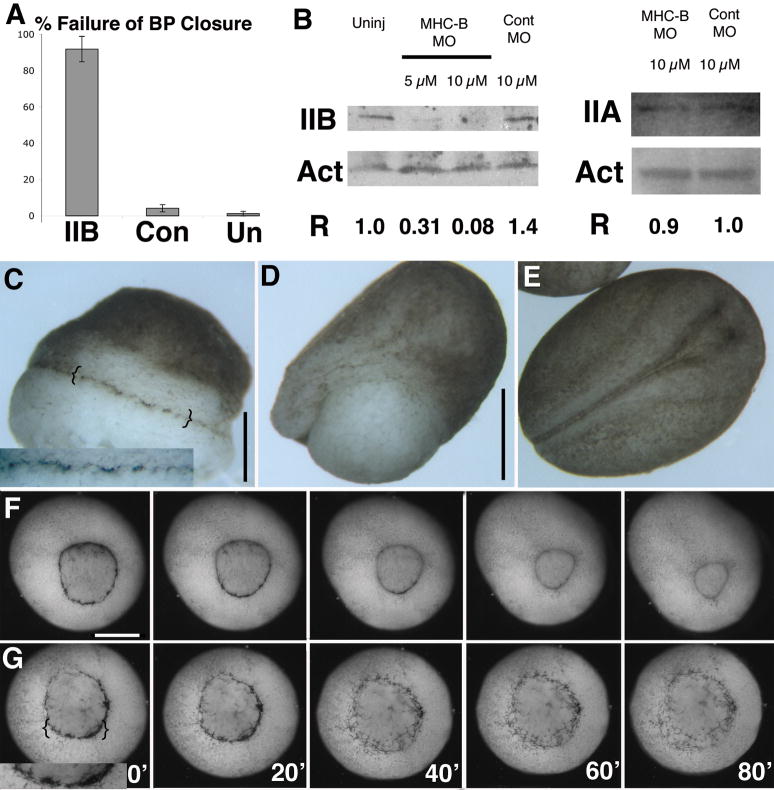
Percent of embryos failing to close the blastopore is plotted for 10 μM IIBMO (n = 74), 10 μM control (n = 72) and uninjected (n = 84) embryos, with SEM (standard error of the mean)(A). Western blots showing MHC-B (IIB), MHC-A (IIA) and actin (Act) levels in uninjected, 5 μM and10 μM morpholino, and 10 μM control morpholino embryos. The normalized ratio of MHC-B or MHC-A to Actin (R) is also shown (B). A representative morphant embryo (C), a unilaterally (right-side)-morphant embryo (D), and a control embryo (E) at stage 19 are shown. Time-lapse movie frames show the vegetal (blastopore) view of development of a 10 μM morphant (F) and a 10 μM control embryo (G) with time in minutes shown. Insets show bottle cells. Scale bars are 500 μm, except in D at 1 mm.
Whole mount immunochemisty and Western blotting
MHC-A and MHC-B were detected in whole mount staining experiments and western blotting with isoform specific anti-peptide antibodies (Covance, Berkeley, CA). Whole mount immunochemistry followed (Skoglund et al., 2006), using rhodamine conjugated secondary (Jackson Immuno Research, West Grove, PA) for confocal imaging after dehydration and clearing as described (Skoglund et al., 2006). Western blots were performed as described (Skoglund and Keller, 2007) using stage 19 embryo extracts for MHC-B and stage 13 embryos for MHC-A, run on 5% acryamide gels and detection was with anti-rabbit HRP using Supersignal reagents (Pierce, Rockford, IL). Densitometry was performed as described (Skoglund et al., 2006).
Adhesion assays for C-cadherin and fibronectin and evaluation of surface expression of C-cadherin and Integrin α5
C-cadherin adhesion assay was as described (Chen and Gumbiner, 2006; Niessen and Gumbiner, 2002), with 4 μg/ml of purified recombinant C-Cad extracellular domain used for coating plastic as a substrate for cells, done in triplicate with more than 400 cells/condition per experiment. The fibronectin (FN) adhesion assay was similar, but substituted 20 μg/ml FN as substrate (Sigma, St. Louis, MO). Rescue of myosin IIB depletion mediated adhesion to FN was with a human myosin IIB- GFP fusion construct (Vicente-Manzanares et al., 2007), injected at 100 pg/embryo at 4 cell stage. This experiment was modified because dorsal explants were cut at early gastrulation and cultured until stage 18, requiring addition of 10 μl of 0.5 M EDTA to facilitate dissociation to single cells but allowing visualization of fluorescent cells in the adhesion assay. The biotinylation and trypsin experiments to determine cell surface amount of C-Cad was as described (Chen and Gumbiner, 2006), and the same procedure was used to determine cell surface amount of α5 using a polyclonal antibody directed against α5.
Morpholinos and injections
A morpholino-oligonucleotide (MO) directed against the start site of MHC-B (CTTCCTGCCCTGGTCTCTGTGACAT) was produced (Gene Tools, Philomath, OR.), as well as a control morpholino that varies at five nucleotides (CTTGCTCCCCTGCTCTCTCTGAGAT). Morpholino injections were in both cells at 2 cell stage or in single blastomeres at 32 cell stage targeted to the dorsal marginal zone by pigment cues (Lee and Gumbiner, 1995), with 30 picoliters – 5 nanoliters of stock morpholino at 1 mM injected to achieve desired dosage between 1–10 μM final concentration in the injected cell. Dorsal cells were co-injected with 0.5 nl of 30 ng/μl RNA in water in blastomeres at the 32-cell stage. Carboxy-terminal moesin-GFP was from the RNA expression plasmid CS107-GFP-Moe constructed by Dr. John Wallingford’s lab from the original (Litman et al., 2000). Capped RNA was made using AscI/SP6 and the mMessage mMachine (Ambion, Austin, TX). In some experiment 50 ng ruby- labelled dextran (Invitrogen, Molecular Probes, Carlsbad, CA) was co-injected at 32-cell stage. Myosins were pharmacologically targeted by injecting 50 nl of 2 mM (−)-blebbistatin (Calbiochem, San Diego, CA) in dimethylsulphoxide (DMSO-Sigma, St. Louis, MO), or DMSO alone, into the blastocoel of mid- gastrula (St. 10) embryos and observing defects in blastopore closure. Evaluating whether ectopic C-Cad expression could rescue partially MHC-IIB morphant embryos was done in triplicate by injecting 1.5 ng C-Cad RNA (Chen and Gumbiner, 2006) with or without 5 μM MHC-IIB MO, and assaying embryos for blastopore closure defects.
Imaging
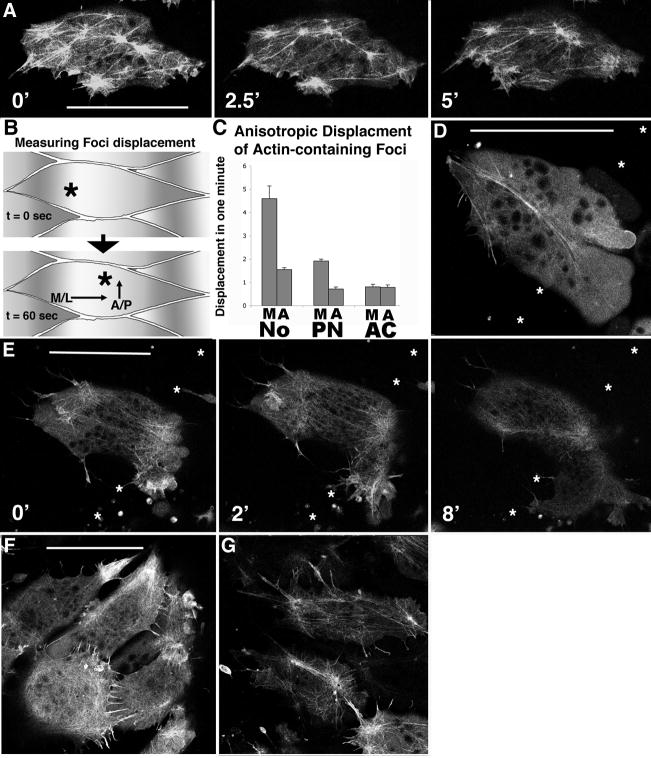
Vegetal view movies of whole embryos were done on an inverted Nikon IX70 running Metamorph software, and using a 4x objective. Still images were captured using either of two cameras mounted on a Zeiss stereoscope, a Hamamatsu color chilled CCD or a MTI CCD camera, and images processed though NIH Image (NIH), ImageJ (NIH) or Photoshop (Adobe) software. Confocal imaging was with a Nikon IX70 with a BioRad Radiance2100 system and software at the Keck Center for Cellular Imaging at UVA, using 60x and 100x 1.4 NA oil immersion lenses. Framing intervals of 15 sec to 3 min were used and image processing consisted of frame averaging and brightness and contrast adjustment as described previously (Shih and Keller, 1992a). Quantitation of the motion of actin foci was by plotting foci position from magnified confocal movies onto acetate sheets, and measuring AP and ML displacement for each one-minute interval. For notochord, ten cells with two to six foci per cell (33 foci total) were followed for five minutes, for pre-involution axial mesoderm (pre-notochord or PN) and animal cap (AC) five cells with at least 20 foci were followed. The average medio-lateral (M) and anterior-posterior (A) displacement in arbitrary units was generated for each cell, except for AC cells where it is not possible to tell A-P position so the direction of maximum excursion of the first foci examined became M, and the orthogonal direction became A, and the average of these is presented with SEM (standard error of the mean).
Results
Myosin heavy chain B is predominantly found in cell cortices in dorsal, axial and paraxial tissues
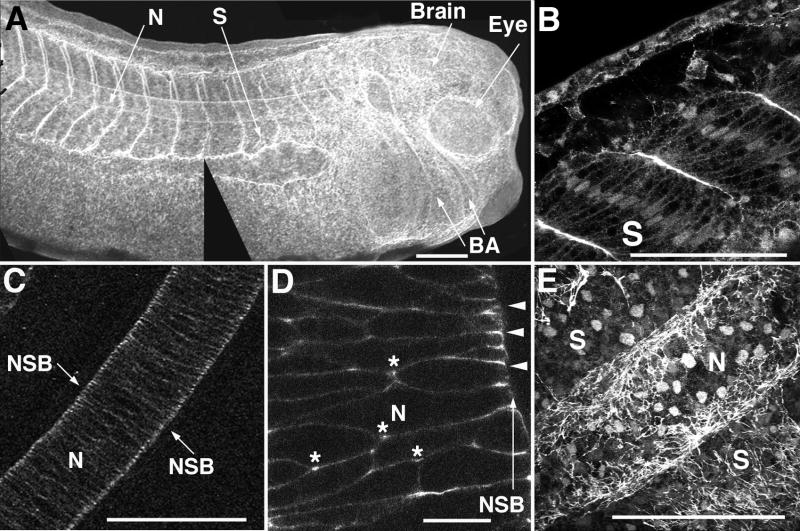
To examine the distribution of myosin IIB we used an isoform-specific antibody against myosin heavy chain B (MHC-B) to localize this protein complex in the developing embryo. We find that MHC-B protein is predominantly localized in the dorsal axis in tailbud stage embryos, where it is found in the notochord and somites, as well as in the developing eye, brain and branchial arches (Fig. 1A). In confocal section, the somites of a late neurula stage embryo are strongly outlined, indicating that MHC-B is concentrated in somitic cell cortices that face tissue boundaries (Fig. 1B), and it can also be seen in all somitic cell cortices as well as nuclei. In the deeply interdigitated notochordal cells at mid-axial level of a late neurula stage embryo MHC-B is localized in the cell cortices and therefore outlines each cell (Fig. 1C). There is also a pronounced concentration of MHC-B protein along the surfaces of the notochordal cells where they contact the notochordal-somitic boundary (NSB), an extracellular matrix rich structure that is assembled de novo during gastrulation (Shih and Keller, 1992b; Skoglund et al., 2006). In more posterior notochordal regions, where cell intercalation and CE are still in an earlier phase, MHC-B is distributed throughout the cortical region of the cells as well, but it is also distributed in a polarized fashion within the cortex, with high levels at the anterior and posterior edges of the cells where they attach to the notochordal-somitic boundary (Fig 1D, pointers), and at triple cell junctions where the large lamellipodia that lead the process of active cell intercalation are formed (Shih and Keller, 1992a) (Fig. 1D-asterisks). This pattern is consistent with a role for myosin IIB as a mechanical element in the medio-lateral arcs of tension that are postulated to accompany dorsal mesodermal CE (Keller et al., 2000). Imaging the ventral surface of a neural deep-over-mesoderm explant for MHC-B reveals staining over the basal surfaces of notochordal and somitic cells (Fig 1E), and indicates that the myosin IIB enrichment at these tissue boundaries is not due entirely to a generalized increased concentration of myosin IIB in the cortex of cells facing tissue boundaries but also includes a component of myosin IIB organized in a linear or fibrillar pattern at these tissue boundaries.
Figure 1.
MHC-B protein is localized to the dorsal axis (A), both at the surfaces of the somites (B) and in a polarized distribution in mature notochord (C). Posterior notochordal cells actively undergoing CE exhibit MHC-B localized to triple cell junctions inside the notochord (*) where invasive protrusions extend between adjacent cells during intercalation, and at cell-cell-matrix junctions at the notochord-somite boundary (pointers in D). Basal (mesodermal side) view of MHC-B staining in a deep neural-over-mesoderm explant reveals MHC-B to exhibit a fibrillar distribution over notochord and somites (E). Scale bars are 500 μm, except in D at 50 μm. N is notochord, S is somitic mesoderm and NSB is notochordal-somitic boundary.
Myosin heavy chain B “ knockdown” blocks blastopore closure and gastrulation
To examine the function of myosin IIB in the Xenopus embryo, we did a morpholino oligonucleotide (MO)-mediated knockdown of MHC-B. Morphant embryos exhibit a dramatic failure of blastopore closure and gastrulation (Figs. 2A, C), which is accompanied by a reduction of MHC-B protein but not MHC-A protein as assayed by western blot (Fig. 2B), and unilaterally morphant embryos display an asymmetric morphogenesis that produces an open blastopore on the injected side (right side, Fig. 2D). In contrast, control MO injected embryos show neither developmental defects nor a reduction in MHC-B protein levels indicating that this failure of blastopore closure phenotype is due to loss of MHC-B protein (Figs. 2A, B, E). In normal embryos, the involuting marginal zone undergoes convergence and extension, which aids its involution, squeezes the blastopore shut with a closure point about two thirds of the way across the yolk plug from the dorsal lip, and extends the marginal zone tissues across the vegetal (sub-blastoporal) endoderm, thus covering it over and forming an elongated archenteron on the dorsal side of the embryo (Fig 2F, Supplemental movie 1 (SM 1)). In contrast, morphant embryos fail to involute or otherwise internalize the marginal zone, fail to squeeze the blastopore shut and to cover over the endoderm, and fail to elongate the dorsal body axis and form an elongate archenteron (Fig. 2G, SM 2). In involuting axial and paraxial mesoderm all these processes depend on CE (Keller et al., 2000; Keller et al., 2003), and thus this phenotype is consistent with a specific failure of CE in injected embryos. Other morphogenic processes occur normally in morphant embryos, including cell division through the cleavage stages, and the apical constriction that is associated with bottle cell formation (shown by the presence of the typical pigment accumulation, insets, Figs. 2C, G) (Hardin and Keller, 1988; Keller, 1981), indicating that they do not depend on zygotic MHC-B protein. Blastopore closure can also be perturbed by acute application of the myosin II inhibitor blebbistatin to the embryo at early gastrulation stages, with 41% of embryos failing to close their blastopores and 28% exhibiting delayed blastopore closure (n=32), while DMSO alone treated embryos do not exhibit significant developmental defects (5 % delayed blastopore closure; n=40).
MHC-B MO Mediated Failure of Gastrulation Is Dose-dependent
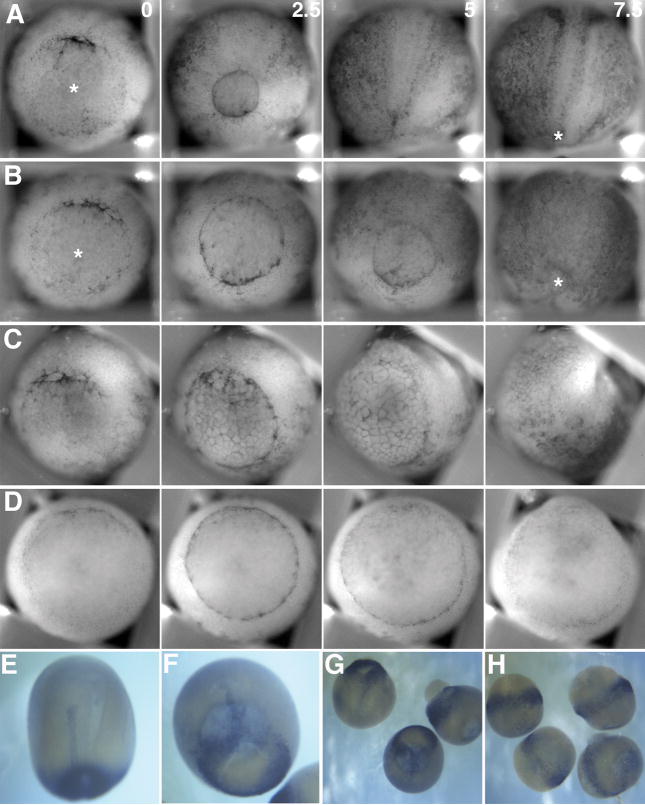
We titrated MHC-B MO injection to create a series of embryos of declining MHC-B protein levels. We found that the severity of failure of blastopore closure is dependent on the dose of morpholino injected, and thus on MHC-B levels (Fig. 2B). Unmanipulated embryos close their blastopores by 5 hours after dorsal lip formation, at the normal site for closure closer to the ventral lip than the dorsal lip (astericks, Fig. 3A). In contrast, 2.5 μM MO injected embryos exhibit blastopore closure delayed by 2.5 hours, and a more dorsally localized closure point (asterisks, Fig 3B). Increasing the MHC-B MO dose to 5 μM further delays blastopore closure, which occurs in an abnormal, symmetric fashion. In some cases it fails, and a small, symetrical blastopore remains open (Fig. 3C). At 10 μM MO, we see the highly penetrant large open blastopore phenotype characterized by a complete block of axial morphogenesis as described above (Fig. 3D). When the extent of notochordal extension is assayed by whole mount in situ hybridization for expression of brachyury (Bra), which identifies preinvolution mesoderm and postinvolution notochord, morphant embryos exhibit dose-dependent deficiencies in axial development with the length of the developing notochord being inversely related to amount of MHC-B MO injected, and thus related to levels of MHC-B protein in the embryo. The normal pattern of Bra expression in an early neurula (stage 13) embryo reveals the normal extent of notochordal development (Fig. 3E), while a 5 μM MO injected sibling embryo exhibits both delayed blastopore closure and a shorter notochord (Fig. 3F). This dose-dependent shortening of the notochord is slightly variable at 5 μM MO and these embryos often exhibit a notch or asymmetry at the dorsal lip of the blastopore (Fig. 3G), but these embryos will develop relatively normally although by tailbud stages they exhibit a distinct dorsal bend or flexure (data not shown). In contrast, embryos injected at 10μM MO have little or no apparent notochord, a large open blastopore and express brachyury in a pattern reminiscent of that seen in normal embryos at early gastrula stages (Fig. 3H). Because Bra is expressed in an abnormal pattern in 10 μM MO morphants that is similar to the normal fate map of dorsal mesoderm at the beginning of gastrulation (Keller, 1976), and because the 5 μm MO morphants exhibit Bra expression in an intermediate pattern, this suggests a MHC-B MO dose-dependent failure of normal cell movements to generate the dorsal axis at gastrulation rather than a failure of tissue specification.
Figure 3.
Still images from simultaneous time-lapse videorecordings show vegetal views of control (A) and morphant embryos injected with 2.5 μM (B), 5 μM (C), and 10 μM (D) MHC-B MO. All embryos are oriented with their dorsal side up. At stage 10+−10 1/4 (t=0) bottle cells form in the dorsal lip of the blastopore of all control and morphant embryos. By control stage 12 (t=2.5) ventral bottle cells have formed in all control and morphant embryos, but blastopore closure is delayed in a dose-dependent manner. The site of blastopore closure in 2.5μM (B, t=7.5) morphant embryos is not located as ventrally as in control embryos (A, t=7.5). RNA in situ hybridizations of stage 13 embryos for brachyury expression reveals the extent of notochordal morphogenesis in a control embryo (E), and reduced notochordal morphogenesis in a 5 μM morphant (F). The 5 μM morphant embryos exhibit some variability in notochordal extension (G), but 10 μM morphant embryos have essentially no notochord extended (H).
MHC-B depleted cells show loss of polarity, and loss of adhesion
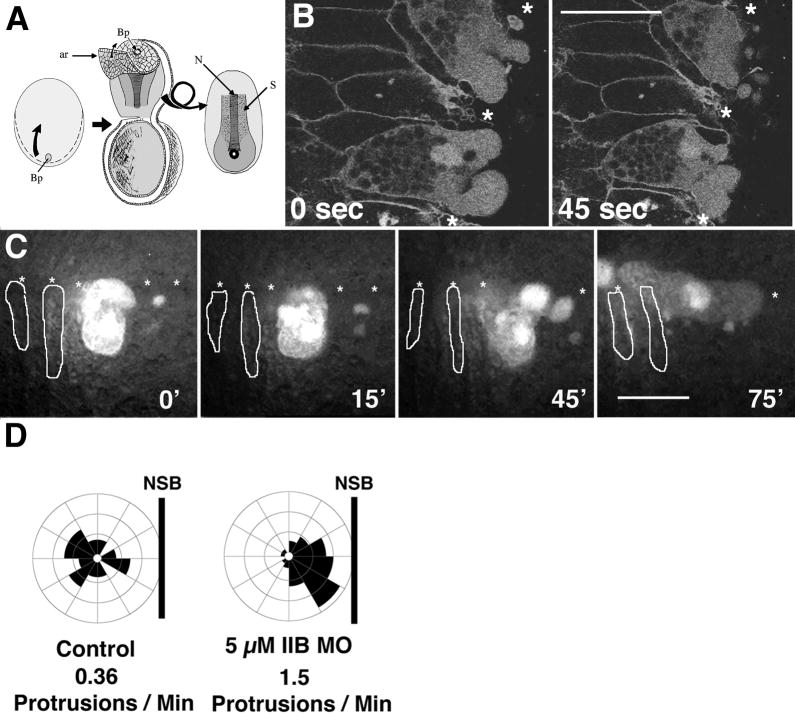
To examine the cellular response to depletion of MHC-B, we made mosaic embryos by injecting MHC-B MO into one dorsal cell at the 32-cell stage. Normally, internal notochord cells are bipolar, with large lamelliform protrusions on both medial and lateral ends. As cell intercalation proceeds, and internal cells intercalate into the boundary row of cells, they may undergo a short period of blebbing at the boundary, but they quickly become quiescent and form a flattened surface on the boundary, with protrusive activity apparently inhibited by contact with the boundary (Shih and Keller, 1992b). This “boundary capture” stabilizes and defines notochordal separation from the somitic mesoderm (Keller and Danilchik, 1988; Shih and Keller, 1992b). When embryos mosaic for 10 μM MO are examined most morphant cells are excluded from the otherwise normal embryo by late gastrula stages, apparently due to their relatively lower adhesion (see below-data not shown). Reducing the concentration of MO injected to 5 μM leads to embryos mosaic for partially MHC-B depleted cells that remain in the notochord until neurula stages, and these cells can be imaged during morphogenesis in dorsal, axial explants in which the endodermal epithelium covering the notochord is removed (Fig. 4A). In these partially MHC-B depleted cells, protrusive activity is up-regulated at the ends of cells that bound the NSB. These protrusions are abnormally large, superficially resembling blebs exhibited by dying cells. However, unlike blebs which are hemispherical, these protrusions adopt whatever shape the space around the cells allow and so they appear to be variably loboform, lamelliform, and circuitously channeled between other cells (Fig. 4B, SM 3). Partially morphant cells are eventually shed from the notochord; although they often fragment similar to myosin-null Dictyostelium cells in mechanically restricted environments (Laevsky and Knecht, 2003), they continue to express cell motile behaviors in culture for more than 5 hours after shedding indicating that they are not necrotic (data not shown). Just before being shed, the internal ends of these morphant cells are the last part of the cell to adopt this behavior, and on doing so, the cells leave the notochord and take up residence in whatever space is available to them, which is usually the groove formed at the notochordal-somitic boundary (Fig. 4C, SM 4). This phenotype represents both an increase in protrusive activity as well as an abnormal distribution of protrusive activity, and indicates a polarization defect in these cells (Fig. 4D). These behaviors suggest a loss of cortical, mechanical integrity, loss of the normal boundary-mediated constraint on protrusive activity, loss of cell adhesion to the boundary extracellular matrix, and loss of cell-cell adhesion in morphant cells.
Figure 4.
Explanting the dorsal tissues of a neurula above the blastopore (Bp) and removing the endodermal archenteron roof (ar) exposes the notochord (N) and somitic mesoderm (S) for imaging during CE (A). In such explants confocal time-lapse imaging of a notochord mosaic for cells injected with 5μM MHC-B morpholino reveals that morphant cells (labelled) in the background of a notochord expressing a membrane bound GFP (revealing cell outlines) shows the loss of regulation of polarized cell motility (B). An epi-fluorescent time-lapse sequence of these cells exhibit aberrant cell motile behaviors and eventually are excluded from the notochord (C). Control notochordal cells at or near the notochord-somite boundary (NSB) exhibit monopolar motile behaviors, with few protrusions toward the notochord-somite boundary, while 5 μM morphant cells both express increased motility, and improperly direct this motility towards the boundary (D). One unit represents six percent of the total protrusive activity directed towards that sector, and is each sector is oriented relative to the notochord-somite boundary. Asterisks represent the notochord-somite boundary, and scale bars are 50 microns.
The cortical actin cytoskeleton is polarized relative to the embryonic axis in notochordal cells
We have visualized the F-actin cytoskeleton in live notochordal cells using the Facting binding probe moesin-GFP and laser scanning confocal micrsocopy. Normal, non-morphant notochordal cells display a dynamic, basket-like network of fine, straight actin bundles organized in a hub and spoke fashion, with several hubs or foci where actin bundles meet arrayed across the cortical region of these elongate cells (Fig. 5A, SM 5). During notochordal intercalation these foci are dynamic, constantly moving within the cell on a timescale of seconds. The predominant displacement of these foci, and thus of the actin cables attached to them, is parallel to the long axis of these cells (Fig. 5B, C). Because the anisotropic behavior of these cytoskeletal elements in notochordal cells corresponds to both the axis of medio-lateral cell intercalation and the axis of convergence, this is consistent with a role for the cortical actin network in notochordal cell intercalation. Examining this cortical actin network in pre-involution mesoderm reveals that it is less robustly polarized, and in animal cap cells it is not polarized, indicating that it becomes polarized with the same developmental time-course as the expression of cell intercalation behaviors (Fig. 5C).
Figure 5.
Confocal microscopy of control cells injected with moesin-GFP reveals a dynamic cortical actin network in a basket-like distribution, with 3–5 foci of actin visible per cell (A). These actin-rich foci visibly move within the cell on a sub- minute timescale (astericks in A). Plotting the relative displacement of foci along the embryonic axis, both the medial-lateral direction and the anterior-posterior direction (B), reveals that the rate of displacement of these foci is greater in the medial-lateral (M) dimension that in the anterior-posterior dimension (A) for notochordal cells (No) (p<0.02 by students t-test), and this polarity is detectable in pre-notochordal cells (PN) but not in animal cap cells (AC) (C). In contrast to control cells, MHC-B injected cells reveal dramatic disruption of the cortical actin network (D). Confocal time-lapse sequence of a morphant cell exhibiting aberrant motility concomitant with cortical actin breakdown (E). Asterisks indicate notochord-somite boundary, and elapsed time is shown in minutes. Mildly morphant notochordal cells (1 μM MO) remain in notochord with intact cortical actin networks, but exhibit profuse filopodia (E) relative to control notochordal cells (F), revealing a threshold cell polarity phenotype in response to MHC-B depletion. Asterisks represent the notochord-somite boundary, and scale bars are 50 microns.
MHC-B depleted cells show dose-dependent loss of cortical actin cytoskeletal integrity
We wished to determine whether myosin IIB regulates the cortical actin network in notochordal cells. We find that notochordal cells morphant for MHC-B MO at a dose of 5 μM this cortical actin network is perturbed, indicating that the proper regulation of this network requires myosin IIB in these cells (Fig. 5D, SM 6). During this behavior, actin cables are absent or few in number, and those that are present are thickened and sinuous. Moreover, the cells appear able to make large, rapidly-extending protrusions but cannot retract them or effectively pull the cell body toward the protrusions. The transition between normal and perturbed cortical actin organization in morphant cells correlates with the loss of the normal, polarized protrusive activity of the notochord cells and the eventual exclusion of MHC-B depleted cells from the notochord in time-lapse movies (Fig. 5E, SM 7). This change in protrusive activity is accompanied by, or followed closely by disruption of the characteristic hub and cable pattern of cortical actin microfilament bundles. Instead of a foci connected by straight (and thus apparently taut, or tensioned) actin cables, there are fewer, thicker cables and they are often sinuous, unconnected to foci, and appear to be under little or no tension. These observations suggest that the normal foci-cable array of cortical actin cytoskeleton depends on myosin IIB.
Both the disruption of the cortical actin cytoskeleton and the cellular exclusion phenotype are dose dependent on MHC-B levels, because morphant notochordal cells with a low-level MHC-B depletion (1 μM MO) both remain in the notochord and have a relatively intact cortical actin cytoskeleton (Fig. 5F, G, SM 8). However, these morphant cells have an increased number of actin rich filopodial protrusions relative to normal cells, indicating that the polarized protrusive activity characterizing these intercalating cells is sensitive to MHC-B levels.
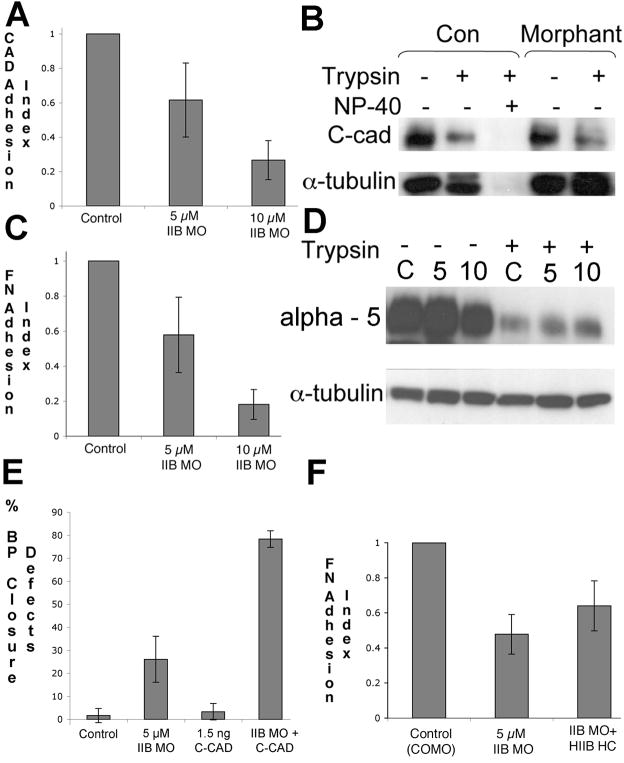
Adhesion of Morphant Cells To Cadherins and To Fibronectin Matrix Is Reduced
Perturbing myosin II function affects cell adhesion and cadherin localization in mice, Drosophila, Dictyostelium and cultured cells (Conti et al., 2004; Shewan et al., 2005; Xu et al., 1996). Because regulation of C-cadherin (C-Cad) is necessary for mesodermal CE in Xenopus embryos (Brieher and Gumbiner, 1994; Lee and Gumbiner, 1995; Zhong et al., 1999), we assayed C-Cad-mediated adhesion in MHC-B depleted cells. We found a dramatic, dose-dependent reduction in adhesion (Fig. 6A). However, morphant cells maintain their cell surface C-Cad protein levels when assayed either by trypsin digestion (Fig. 6B), or by surface biotinylation (data not shown). Because expressing exogenous C-Cad protein does not rescue the blastopore closure phenotype of partially morphant embryos, but rather makes this phenotype worse, again suggests that C-Cad protein levels are not limiting in morphant embryos (Fig. 6E). Morphant cells also exhibit a reduction in adhesion to fibronectin (Fig. 6C), indicating that integrin receptor-mediated adhesion is dependent on myosin IIB in these cells. Similar to the C-Cad, morphant cells maintain their cell surface integrin alpha-5 protein levels when assayed either by trypsin digestion (Fig. 6D), or by surface biotinylation (data not shown). Expression of a human myosin IIB heavy chain -GFP fusion rescues FN adhesion in morphant cells (Fig. 6F), indicating that the morpholino specifically targets myosin IIB heavy chain. Thus myosin IIB is necessary for both cell-cell and cell–matrix adhesion, either directly or indirectly through its role in organizing the cortical actin network since the assembly state of actin affects cadherin function (Jaffe et al., 1990).
Figure 6.
Morphant dorsal axial cells at St. 13 exhibit a dramatic dose-dependent reduction in adhesion to recombinant extracellular domain of C-cadherin (A). However, cell surface levels of C-Cad are unchanged in morphant cells, because similar levels of control and morphant cell C-Cad is available on the cell surface for trypsin digestion (B). Morphant cells also show a dramatic reduction in adhesion to fibronectin (FN) (C), and integrin α5 surface levels also are not affected by myosin IIB depletion (D). Failure of blastopore closure due to MHC-B depletion is not rescued by exogenous expression of C-Cad, consistent with the hypothesis that C-Cad levels are not limiting in morphant embryos (E). Adhesion to FN in morphant cells is rescued by co-expression of exogenous human MHC-B (F).
Discussion
MHC-B plays an important and necessary role in CE
Several observations suggest that myosin IIB is involved in CE. First, MHC-B mRNA is expressed in the dorsal mesoderm of the embryo, and it is up-regulated by activin treatment of animal caps, which also induces convergent extension (CE) in these caps (Bhatia-Dey et al., 1998; Kelley et al., 1996). Second, we find MHC-B protein distributed in a polarized fashion in notochordal cells undergoing CE. Third, we show that reducing MHC-B protein in the embryo leads to failure of gastrulation. We find that myosin IIB organizes a polarized cortical actin network in notochordal cells, and that this network is required for CE. Because this network is required for both polarized expression of cell motility and regulation of cell adhesion, these defects are likely responsible for failure of morphogenesis. This work identifies the dynamic myosin IIB-dependent cortical actin network as the cell component integrating adhesion and polarized motility during CE.
In Drosophila, myosin II is also involved in the epithelial cell intercalation that occurs during germ band CE (Bertet et al., 2004; Zallen and Wieschaus, 2004). Cell intercalation in germ band extension involves remodeling of the circumapical adherens junctions during neighbor changes among pairs of cells (Bertet et al., 2004) or in multicellular rosettes of cells (Blankenship et al., 2006) whereas the cell intercalation in the Xenopus dorsal mesoderm involves deep, non-epithelial mesenchymal cells, which are attached to each other at foci and lack an apical junctional complex. In Xenopus, we find myosin IIB distributed cortically in involuting mesodermal cells, with higher medial and lateral concentrations, whereas in the Drosophila germ band myosin II is localized asymmetrically adjacent to anterior and posterior apical contacts (Bertet et al., 2004; Zallen and Wieschaus, 2004), indicating that although these two examples of CE both use myosin II they are mechanistically distinct processes.
Embryos morphant for MHC-B fail to close their blastopores in a fashion that indicates failure of CE in the involuting dorsal axial and paraxial mesoderm (Keller et al., 2000; Keller et al., 2003). Normally, convergent extension of the axial and paraxial mesoderm constricts the involuting marginal zone, which closes the blastopore, and it simultaneously elongates the body axis by extension. This occurs because the polarized protrusive activity and traction of the deep mesodermal cells, the resulting cell intercalation, and the consequent convergence, all occur along the presumptive mediolateral axis of the dorsal tissues, which in the involuting marginal zone of the embryo is described as an arc-like pattern around the blastopore (Keller et al., 1992; Keller, 1984; Shih and Keller, 1992b). Shortening these arcs by cell intercalation results in constriction of the blastopore and the resulting extension in the perpendicular direction results in axial elongation, all in one stroke (Keller et al., 2003; Keller and Shook, 2004). Morphant embryos show a dose-dependent gradation of success in constricting the blastopore, with stronger depletions failing entirely and weaker depletions showing limited closures and specific defects in dorsal, axial extension. This behavior is strong evidence for myosin IIB dependence of convergent extension. In contrast, other morphogenic processes occur normally, including cell division during cleavage and bottle cell formation, suggesting that the cellular mechanisms underlying CE in dorsal mesodermal tissue are the most dependent on myosin IIB and thus are the most sensitive its loss, consistent with a morpho-mechanical role for this myosin isoform in CE.
Cellular MHC-B morphant phenotypes show that MHC-B is essential for a specialized cortical actin cytoskeleton necessary for development of convergence forces
Protrusive activity of the type displayed in bipolar, oriented fashion by the deep mesodermal cells is invariably associated with traction forces in cell culture on deformable substrates (Beningo and Wang, 2002; Harris et al., 1980; Lo et al., 2004; Lo et al., 2000), this suggests that these cells are exerting traction on adjacent cells or on matrix and are under tension in the mediolateral axis, which is also the axis of convergence. Notochordal cell elongation and intercalation in the presence, and only in the presence of their characteristic bipolar protrusive activity further supports this contention. A long-standing question has been what generates the tensile forces at the cell level that drives cell intercalation and convergence, and what integrates these forces over long distances along the arcs of convergence. The anisotropic dynamic behavior of the cortical actin cytoskeleton described here and its perturbation in myosin IIB morphant cells suggests that this cytoskeletal architecture and associated cortical tension plays a large role in this process. First, the predominant movement of the actin nodes and thus the predominant change in length of the actin cables connecting them is in the mediolateral axis of intercalation, cell traction, and tensile force generation, suggesting that this cytoskeleton functions in these processes. These tensile forces across the involuting marginal zone rise to 1.5 μN during gastrulation (D. Shook, personal communication). Second, the sequence of defects appearing in cells partially depleted for MHC-B include loss of integrity of this cortical actin network, abnormal protrusive activity, loss of the elongate polarized morphology, apparent loss of cortical integrity, as judged by lack of ability of the cell to maintain its shape and accommodation of its shape to surrounding spaces, and finally the loss of adhesion. Third, myosin IIB is localized to the cell cortex, which is expected to be under tension along cell surfaces between protrusions (Kolega, 1986), and thus important in developing mediolateral traction or resisting the cell elongation it might produce. Myosin IIB is also increased in the cell-cell, cell-matrix junctions of cell surfaces facing the notochordal-somitic mesodermal boundary, a region of stable anchorage and suppression of protrusive activity that is required for the integrity of the boundary and for directed extension (Shih and Keller, 1992b; Skoglund et al., 2006; Skoglund and Keller, 2007). It is also increased in regions of triple cell junctions found in the interior of the notochord, an increase that probably reflects the presence of the polarized, medial and lateral protrusions thought to generate the traction for cell intercalation. Finally, the vigorous but aberrant protrusive activity, the lack of ability to constraint their shape in a mechanically resistant environment, and the tendency for fragmentation are all characteristics that myosin IIB morphant Xenopus cells have in common with myosin II null mutants in Dictyostelium, where this molecule functions principally as an actin cross linker and is important in maintenance of cortical integrity necessary for migration along mechanically restricted environments (Laevsky and Knecht, 2001; Laevsky and Knecht, 2003; Xu et al., 2001). These data combine to suggest that myosin IIB has a mechanical role in the generation or transmission of the mediolateral tensile forces thought to underlie convergent extension, and thus blastopore closure and axial elongation (Keller et al., 2000).
Myosin IIB is essential for cell-cell and cell-matrix adhesion
Severely depleted morphant cells eventually appear to lose adhesion to adjacent cells and extracellular matrix, a loss of adhesive ability that was confirmed by decreased adhesion to defined cadherin and fibronectin substrates. This suggests a role for myosin IIB in adhesion, a notion supported by the fact that myosin IIB is important for clustering E-cadherin at adhesion sites, and forming adhesions in cultured epithelial cells (Shewan et al., 2005). The important cadherin in convergent extension appears to be C-cadherin in these mesenchymal Xenopus cells (Brieher and Gumbiner, 1994; Zhong et al., 1999), and we that show myosin IIB regulates Xenopus cell-cell adhesion by a mechanism distinct from regulating surface expression of C-cadherin. The fact that both cadherin-mediated adhesion to cadherin and integrin-mediated adhesion to extracellular matrix are affected in morphant cells suggests a general and perhaps indirect effect of myosin on adhesion. This consequence of myosin IIB depletion may be mediated by loss of the actin cytoskeletal organization as it parallels the loss of adhesion that occurs on disruption of microfilaments with cytochalasin (Jaffe et al., 1990), and suggests that a normal function of the dynamic cortical actin network is to dynamically regulate cell –cell and cell matrix adhesion on notochordal cells in a manner appropriate to support CE.
Both integrin (O’Toole et al., 1994) and cadherin (Marsden and DeSimone, 2003) adhesive functions can be modulated by cytoplasmic signals, and modulation of one of these adhesion systems can affect the other (Finnemann et al., 1995; Winklbauer, 1998). Our results allow for the possibility that this interdependency could be partially mediated by local modifications of the cortical actin network, because we show that both adhesion systems depend on this network. This view is supported by the observation that Xenopus C-Cad intracellular tail interactions with p120 catenin regulate the local pattern and density of the cortical actin network in blastomeres (Tao et al., 2007).
Summary of myosin IIB function in CE
A diagram summarizes how we think myosin IIB functions in the context of what is known about CE in Xenopus (Fig. 7). The mediolateral intercalation of cells occurs as the initially un-polarized, isodiametric deep mesodermal cells become polarized to form large filo-lamelliform protrusions at their medial and lateral ends, which display repeated cycles of extension (black arrows, Fig. 7A) and shortening (grey arrows, Fig. 7A) (Keller et al., 1989; Shih and Keller, 1992a). As this type of protrusive activity is invariably associated with exertion of traction on the substrate (Beningo et al., 2002; Harris et al., 1980; Lo et al., 2004; Lo et al., 2000), which in this case is either adjacent cells or ECM, this protrusive activity is very likely to generate the traction that first elongates the cells, and then pulls them between one another, thus directly shortening the mediolateral axis of the tissue, and indirectly, by wedging the cells between one another, extending the anterior-posterior axis (large arrows, Fig. 7C). The myosin IIB-dependent dynamic cortical actin network consisting of actin cables (green, Fig. 7B) meeting at foci or nodes (purple, Fig. 7B) serves as a tensile element in the cell cortex of the cells that limits the elongation of the cell, driven by the polarized traction, and transmits the tension from one adhesion site to another inside the cell. Tension is transmited from cell to cell through adhesion sites, generating arcs of tension across the tissue fabric. The dynamic oscillation of the length of the actin cables and movement of the nodes in the mediolateral axis, which is also the axis of development of the convergence forces and suggests an active contractile role for the cortical actin network in CE. The cortical distribution of myosin IIB and the apparent lack of cortical tension in the morphant cells is consistent with involvement of this molecule in cross-linking the cortical actin cytoskeleton. In addition to maintaining cortical integrity, myosin IIB may also participate in active contraction by reeling actin filaments into the foci and thus generating the force for intercalation, but this remains to be determined by imaging these molecules and assaying their activity state in living cells. The concentration of myosin IIB in the anterior/posterior corners of the cell surfaces contacting the extracellular matrix structure at the notochord-somite boundaries (NSB) (red, Fig. 7), may reflect a mechanical reinforcement at this critical boundary. The apparent concentration at the interior, triple cell junctions (red, Fig. 7B) may reflect the same thing, although in this case the increased staining may not reflect an increase of myosin IIB per unit area of cortex, but instead it may reflect the close apposition of four cortical regions, or the presence of the molecule in the lamellipodia at these ends of the cells. In either case, it reflects a high concentration of myosin IIB per cell volume, and again may reflect a mechanical reinforcement. Myosin IIB is involved in polarized cell behavior (Lo et al., 2004) but whether it is a direct involvement of this molecule in polarization or an indirect involvement mediated by its role in organizing the actin cytoskeleton, similar to the loss of adhesion phenotype, also remains to be determined.
Figure 7.
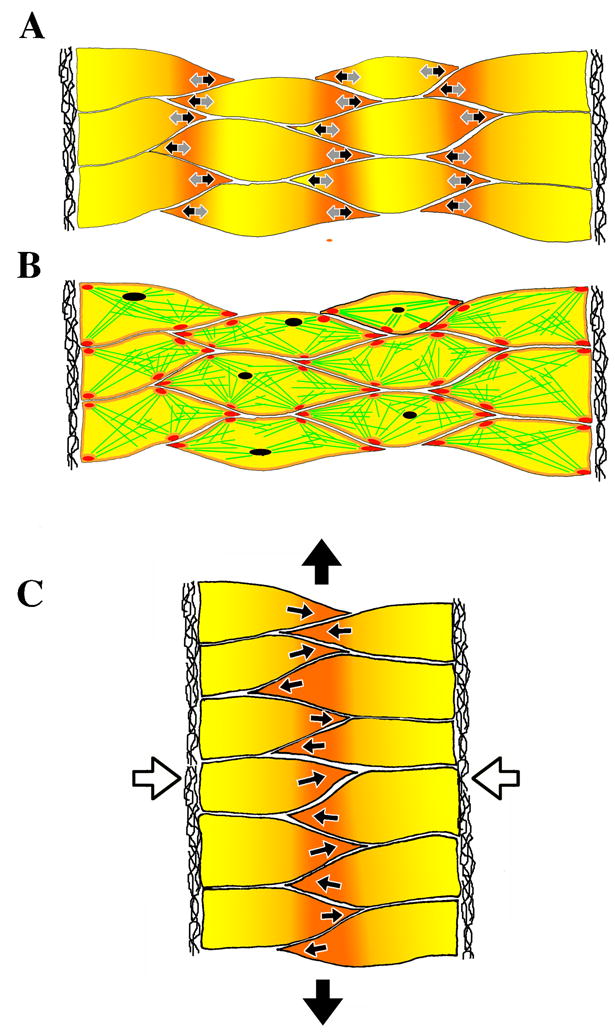
Cell intercalation in the notochord requires two distinct cell activities, cell contraction in the cell body (grey arrows in A) and polarized protrusive activity (black arrows). Contraction events are driven by the myosin IIB-dependent cortical actin network (green lines in B) organized into dynamic foci (purple), and interacting with myosin IIB at adhesion sites (red). Integrating this episodic cell shortening with polarized protrusive activity and dynamically regulated myosin IIB-dependent adhesion leads to cell intercalation (white arrows in C) and tissue level convergence and extension (black arrows). The extracellular matrix of the notochord-somite boundary is lateral in each case.
Supplementary Material
Acknowledgments
We thank Ammasi Periasamy and Ye Chen for help with confocal imaging at the Keck Center for Cellular Imaging (KCCI) at the University of Virginia. We are also grateful to Lance Davidson, Caroline Flournoy, Crystal Scott, and David Shook for stimulating discussions in the laboratory, both with reference to possible molecular mechanisms and imaging techniques. We thank Miguel Vincente-Manzanares and Rick Horowitz for the myosin IIB- GFP fusion plasmid. This work was supported by National Institutes of Health (NIH) grants HD36426-01 and HD25594-13 to Ray Keller and a predoctoral fellowship from Fundação para a Ciência e a Tecnologia (FCT) SFRH/BD/4851/2001 to Ana Rolo.
References
- Amaya E, Stein PA, Musci TJ, Kirschner MW. FGF signalling in the early specification of mesoderm in Xenopus. Development. 1993;118:477–87. doi: 10.1242/dev.118.2.477. [DOI] [PubMed] [Google Scholar]
- Beningo KA, Lo CM, Wang YL. Flexible polyacrylamide substrata for the analysis of mechanical interactions at cell-substratum adhesions. Methods Cell Biol. 2002;69:325–39. doi: 10.1016/s0091-679x(02)69021-1. [DOI] [PubMed] [Google Scholar]
- Beningo KA, Wang YL. Flexible substrata for the detection of cellular traction forces. Trends Cell Biol. 2002;12:79–84. doi: 10.1016/s0962-8924(01)02205-x. [DOI] [PubMed] [Google Scholar]
- Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol Biol Cell. 2001;12:780–94. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–71. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- Bhatia-Dey N, Adelstein RS, Dawid IB. Cloning of the cDNA encoding a myosin heavy chain B isoform of Xenopus nonmuscle myosin with an insert in the head region. Proc Natl Acad Sci U S A. 1993;90:2856–9. doi: 10.1073/pnas.90.7.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia-Dey N, Taira M, Conti MA, Nooruddin H, Adelstein RS. Differential expression of non-muscle myosin heavy chain genes during Xenopus embryogenesis. Mech Dev. 1998;78:33–6. doi: 10.1016/s0925-4773(98)00136-1. [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–70. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Brieher WM, Gumbiner BM. Regulation of C-cadherin function during activin induced morphogenesis of Xenopus animal caps. J Cell Biol. 1994;126:519–27. doi: 10.1083/jcb.126.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gumbiner BM. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J Cell Biol. 2006;174:301–13. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem. 2004;279:41263–6. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- Finnemann S, Kuhl M, Otto G, Wedlich D. Cadherin transfection of Xenopus XTC cells downregulates expression of substrate adhesion molecules. Mol Cell Biol. 1995;15:5082–91. doi: 10.1128/mcb.15.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves MA, Holmes KC. Structural mechanism of muscle contraction. Annu Rev Biochem. 1999;68:687–728. doi: 10.1146/annurev.biochem.68.1.687. [DOI] [PubMed] [Google Scholar]
- Geeves MA, Holmes KC. The molecular mechanism of muscle contraction. Adv Protein Chem. 2005;71:161–93. doi: 10.1016/S0065-3233(04)71005-0. [DOI] [PubMed] [Google Scholar]
- Glickman NS, Kimmel CB, Jones MA, Adams RJ. Shaping the zebrafish notochord. Development. 2003;130:873–87. doi: 10.1242/dev.00314. [DOI] [PubMed] [Google Scholar]
- Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG, Goldin E, Conti MA, Sellers JR, Adelstein RS. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J Biol Chem. 2004;279:2800–8. doi: 10.1074/jbc.M309981200. [DOI] [PubMed] [Google Scholar]
- Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165–81. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- Hardin J, Keller R. The behaviour and function of bottle cells during gastrulation of Xenopus laevis. Development. 1988;103:211–30. doi: 10.1242/dev.103.1.211. [DOI] [PubMed] [Google Scholar]
- Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208:177–9. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Holmes KC, Geeves MA. The structural basis of muscle contraction. Philos Trans R Soc Lond B Biol Sci. 2000;355:419–31. doi: 10.1098/rstb.2000.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki DD, Johansen KA, Singer JB, Lengyel JA. Drumstick, bowl, and lines are required for patterning and cell rearrangement in the Drosophila embryonic hindgut. Dev Biol. 2001;240:611–26. doi: 10.1006/dbio.2001.0483. [DOI] [PubMed] [Google Scholar]
- Jaffe SH, Friedlander DR, Matsuzaki F, Crossin KL, Cunningham BA, Edelman GM. Differential effects of the cytoplasmic domains of cell adhesion molecules on cell aggregation and sorting-out. Proc Natl Acad Sci U S A. 1990;87:3589–93. doi: 10.1073/pnas.87.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Keller R, Cooper MS, Danilchik M, Tibbetts P, Wilson PA. Cell intercalation during notochord development in Xenopus laevis. J Exp Zool. 1989;251:134–54. doi: 10.1002/jez.1402510204. [DOI] [PubMed] [Google Scholar]
- Keller R, Danilchik M. Regional expression, pattern and timing of convergence and extension during gastrulation of Xenopus laevis. Development. 1988;103:193–209. doi: 10.1242/dev.103.1.193. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, Skoglund P. Mechanisms of convergence and extension by cell intercalation. Philos Trans R Soc Lond B Biol Sci. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- Keller R, Shih J, Domingo C. The patterning and functioning of protrusive activity during convergence and extension of the Xenopus organiser. Dev Suppl. 1992:81–91. [PubMed] [Google Scholar]
- Keller R, Shook D. Gastrulation in Amphibians. In: Stern CD, editor. Gastrulation: From Cells to Embryo. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2004. [Google Scholar]
- Keller RE. Vital dye mapping of the gastrula and neurula of Xenopus laevis. II. Prospective areas and morphogenetic movements of the deep layer. Dev Biol. 1976;51:118–37. doi: 10.1016/0012-1606(76)90127-5. [DOI] [PubMed] [Google Scholar]
- Keller RE. An experimental analysis of the role of bottle cells and the deep marginal zone in gastrulation of Xenopus laevis. J Exp Zool. 1981;216:81–101. doi: 10.1002/jez.1402160109. [DOI] [PubMed] [Google Scholar]
- Keller RE. The cellular basis of gastrulation in Xenopus laevis: active, postinvolution convergence and extension by mediolateral interdigitation. Am Zool. 1984;24:589–603. [Google Scholar]
- Kelley CA, Sellers JR, Gard DL, Bui D, Adelstein RS, Baines IC. Xenopus nonmuscle myosin heavy chain isoforms have different subcellular localizations and enzymatic activities. J Cell Biol. 1996;134:675–87. doi: 10.1083/jcb.134.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner CR, Melton DA. Expression of Xenopus N-CAM RNA in ectoderm is an early response to neural induction. Development. 1987;99:311–25. doi: 10.1242/dev.99.3.311. [DOI] [PubMed] [Google Scholar]
- Kolega J. Effects of mechanical tension on protrusive activity and microfilament and intermediate filament organization in an epidermal epithelium moving in culture. J Cell Biol. 1986;102:1400–11. doi: 10.1083/jcb.102.4.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolega J. Cytoplasmic dynamics of myosin IIA and IIB: spatial ‘sorting’ of isoforms in locomoting cells. J Cell Sci. 1998;111 (Pt 15):2085–95. doi: 10.1242/jcs.111.15.2085. [DOI] [PubMed] [Google Scholar]
- Laevsky G, Knecht DA. Under-agarose folate chemotaxis of Dictyostelium discoideum amoebae in permissive and mechanically inhibited conditions. Biotechniques. 2001;311144:1140–2. 1146–9. doi: 10.2144/01315rr03. [DOI] [PubMed] [Google Scholar]
- Laevsky G, Knecht DA. Cross-linking of actin filaments by myosin II is a major contributor to cortical integrity and cell motility in restrictive environments. J Cell Sci. 2003;116:3761–70. doi: 10.1242/jcs.00684. [DOI] [PubMed] [Google Scholar]
- Landsverk ML, Epstein HF. Genetic analysis of myosin II assembly and organization in model organisms. Cell Mol Life Sci. 2005;62:2270–82. doi: 10.1007/s00018-005-5176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Gumbiner BM. Disruption of gastrulation movements in Xenopus by a dominant-negative mutant for C-cadherin. Dev Biol. 1995;171:363–73. doi: 10.1006/dbio.1995.1288. [DOI] [PubMed] [Google Scholar]
- Lengyel JA, Iwaki DD. It takes guts: the Drosophila hindgut as a model system for organogenesis. Dev Biol. 2002;243:1–19. doi: 10.1006/dbio.2002.0577. [DOI] [PubMed] [Google Scholar]
- Litman P, Amieva MR, Furthmayr H. Imaging of dynamic changes of the actin cytoskeleton in microextensions of live NIH3T3 cells with a GFP fusion of the F-actin binding domain of moesin. BMC Cell Biol. 2000;1:1. doi: 10.1186/1471-2121-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Buxton DB, Chua GC, Dembo M, Adelstein RS, Wang YL. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol Biol Cell. 2004;15:982–9. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW. Integrin-ECM interactions regulate cadherin-dependent cell adhesion and are required for convergent extension in Xenopus. Curr Biol. 2003;13:1182–91. doi: 10.1016/s0960-9822(03)00433-0. [DOI] [PubMed] [Google Scholar]
- Maupin P, Phillips CL, Adelstein RS, Pollard TD. Differential localization of myosin-II isozymes in human cultured cells and blood cells. J Cell Sci. 1994;107 (Pt 11):3077–90. doi: 10.1242/jcs.107.11.3077. [DOI] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L. Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet. 2002;18:447–55. doi: 10.1016/s0168-9525(02)02725-7. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Gumbiner BM. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J Cell Biol. 2002;156:389–399. doi: 10.1083/jcb.200108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam: North Holland Publishing; 1967. [Google Scholar]
- Ninomiya H, Elinson RP, Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature. 2004;430:364–7. doi: 10.1038/nature02620. [DOI] [PubMed] [Google Scholar]
- O’Toole TE, Katagiri Y, Faull RJ, Peter K, Tamura R, Quaranta V, Loftus JC, Shattil SJ, Ginsberg MH. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–59. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I, Holden HM. The three-dimensional structure of a molecular motor. Trends Biochem Sci. 1994;19:129–34. doi: 10.1016/0968-0004(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Rochlin MW, Itoh K, Adelstein RS, Bridgman PC. Localization of myosin II A and B isoforms in cultured neurons. J Cell Sci. 1995;108 (Pt 12):3661–70. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- Schectman The mechanics of amphibian gastrulation. I. Gastrulation-producing interactions between various regions of an anuran egg (Ityla regila) Univ Calif Publ Zool. 1942;51:1–39. [Google Scholar]
- Sepich DS, Myers DC, Short R, Topczewski J, Marlow F, Solnica-Krezel L. Role of the zebrafish trilobite locus in gastrulation movements of convergence and extension. Genesis. 2000;27:159–73. doi: 10.1002/1526-968x(200008)27:4<159::aid-gene50>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell. 2005;16:4531–42. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J, Keller R. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development. 1992a;116:901–14. doi: 10.1242/dev.116.4.901. [DOI] [PubMed] [Google Scholar]
- Shih J, Keller R. Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis. Development. 1992b;116:915–30. doi: 10.1242/dev.116.4.915. [DOI] [PubMed] [Google Scholar]
- Skoglund P, Dzamba B, Coffman CR, Harris WA, Keller R. Xenopus fibrillin is expressed in the organizer and is the earliest component of matrix at the developing notochord-somite boundary. Dev Dyn. 2006;235:1974–83. doi: 10.1002/dvdy.20818. [DOI] [PubMed] [Google Scholar]
- Skoglund P, Keller R. Xenopus fibrillin regulates directed convergence and extension. Dev Biol. 2007;301:404–16. doi: 10.1016/j.ydbio.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–38. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Tao Q, Nandadasa S, McCrea PD, Heasman J, Wylie C. G-protein-coupled signals control cortical actin assembly by controlling cadherin expression in the early Xenopus embryo. Development. 2007;134:2651–61. doi: 10.1242/dev.002824. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176:573–80. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbauer R. Conditions for fibronectin fibril formation in the early Xenopus embryo. Dev Dyn. 1998;212:335–45. doi: 10.1002/(SICI)1097-0177(199807)212:3<335::AID-AJA1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Xu XS, Kuspa A, Fuller D, Loomis WF, Knecht DA. Cell-cell adhesion prevents mutant cells lacking myosin II from penetrating aggregation streams of Dictyostelium. Dev Biol. 1996;175:218–26. doi: 10.1006/dbio.1996.0109. [DOI] [PubMed] [Google Scholar]
- Xu XS, Lee E, Chen T, Kuczmarski E, Chisholm RL, Knecht DA. During multicellular migration, myosin ii serves a structural role independent of its motor function. Dev Biol. 2001;232:255–64. doi: 10.1006/dbio.2000.0132. [DOI] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–63. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–55. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Brieher WM, Gumbiner BM. Analysis of C-cadherin regulation during tissue morphogenesis with an activating antibody. J Cell Biol. 1999;144:351–9. doi: 10.1083/jcb.144.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.