Abstract
Animals assess food availability in their environment by sensory perception and respond to the absence of food by changing hormone and neurotransmitter signals. However, it is largely unknown how the absence of food is perceived at the level of functional neurocircuitry. In Caenorhabditis elegans, octopamine is released from the RIC neurons in the absence of food and activates the cyclic AMP response element binding protein in the cholinergic SIA neurons. In contrast, dopamine is released from dopaminergic neurons only in the presence of food. Here, we show that dopamine suppresses octopamine signalling through two D2-like dopamine receptors and the G protein Gi/o. The D2-like receptors work in both the octopaminergic neurons and the octopamine-responding SIA neurons, suggesting that dopamine suppresses octopamine release as well as octopamine-mediated downstream signalling. Our results show that C. elegans detects the absence of food by using a small neural circuit composed of three neuron types in which octopaminergic signalling is activated by the cessation of dopamine signalling.
Keywords: C. elegans, CREB, dopamine, G protein-coupled receptor, octopamine
Introduction
Animals respond to the absence of food by modulating many physiological responses such as metabolism (Kahn and Flier, 2000; Finn and Dice, 2006), life span (Kaeberlein et al, 2006; Lee et al, 2006; Mair and Dillin, 2008), and behaviours including feeding (Ramos et al, 2005) and learning (Giles et al, 2006). Food deprivation mediates these adaptive responses using hormone and neurotransmitter signals. Although decreased food intake leads to decreased nutrition, which triggers starvation-mediated signals, animals can also assess food availability independently of ingestion. Food in the environment can be directly detected using sensory modalities such as smell, taste, or touch, and the presence of the perception of food or its absence also induces specific responses without food intake (Hajnal and Norgren, 2004; Hajnal et al, 2004; Robertson, 2006). Specifically, the perception of food alters the release of neurotransmitters such as dopamine and noradrenaline, which have important functions in food reward and feeding regulation in mammals. However, the way in which the absence of food is perceived and is translated into altered neurotransmitter signalling remains largely unknown. The complexity of the mammalian brain makes it difficult to analyse how different neurotransmitter signals interact to regulate this process. Here, we analyse a food-regulated amine neurotransmitter signalling pathway in Caenorhabditis elegans to identify and characterize mechanisms involved in this regulation.
C. elegans is particularly suitable for understanding the molecular basis of neural signalling because of its simple and well-described nervous system (White et al, 1986). C. elegans responds differently to the presence or absence of food (bacteria), and bioamines have important functions in the response to these conditions. For example, exogenous application of octopamine, the invertebrate equivalent of noradrenaline (Roeder, 1999), induces behavioural changes that are similar to those observed during starvation (Horvitz et al, 1982). Furthermore, endogenous octopamine is known to be required to mediate a starvation-induced response in specific neurons (Suo et al, 2006). Using a cre∷gfp reporter (Kimura et al, 2002), we earlier found that cyclic AMP response element binding protein (CREB) is activated in the four cholinergic SIA neurons when food is absent. Octopamine, released from a pair of the octopaminergic RIC neurons in the absence of food, humorally activates the octopamine receptor SER-3 in the SIA neurons. SER-3 then induces CREB activation through the G protein Gq, whereas Gi/o suppresses this signal. Although the physiological function of this CREB activation has not yet been shown, cre∷gfp serves as a reporter for food response and was used to demonstrate that octopamine signalling is activated in the absence of food. It is reported that octopamine signalling works downstream of the DAF-7 TGFβ signalling pathway in C. elegans (Greer et al, 2008). However, it is unknown whether any other signalling pathway regulates octopamine signalling in this animal.
Dopamine is known to be involved in food sensing in C. elegans (Sawin et al, 2000; Kindt et al, 2007). There are eight dopaminergic neurons in C. elegans hermaphrodites that have sensory endings exposed to the external milieu and directly sense the presence of food by mechanosensation. This sensation is believed to activate dopamine release from these neurons and the released dopamine subsequently reduces the rate of animal locomotion and the frequency of locomotory reversals (Sawin et al, 2000; Hills et al, 2004; Sanyal et al, 2004; Kindt et al, 2007). Dopamine is known to act humorally and hence a direct synaptic connection is not necessary for dopamine signalling (Sawin et al, 2000; Chase et al, 2004; Sanyal et al, 2004). It is nonetheless interesting that the dopaminergic CEP neurons are known to be pre-synaptic to both the octopaminergic RIC neurons and the SIA neurons (White et al, 1986), the latter exhibiting starvation-mediated CREB activation (Suo et al, 2006).
Given that octopamine and dopamine signals are activated in the absence or presence of food, respectively, and that the dopaminergic neurons are in a suitable location to control the neurons involved in the octopamine signalling, we hypothesized that the dopamine signalling interacts with the octopamine signalling to regulate food response. Here, we show that dopamine signalling suppresses octopamine signalling through a mechanism that requires two D2-like dopamine receptors, DOP-2 and DOP-3, located on both the SIA neurons and RIC neurons. These results reveal a signalling mechanism involving two antagonistically acting bioamines working in a small neural network comprising three neuron types to regulate the response to food or its absence in C. elegans.
Results
Exogenous dopamine suppresses exogenous octopamine-mediated CREB activation
In C. elegans animals transgenic for a cre∷gfp reporter (the fusion of the cyclic AMP response element (CRE) and green fluorescent protein (GFP)), GFP is expressed in cells in which CREB is activated (Kimura et al, 2002). Using this reporter system, we found that the absence of food induces CRE-mediated GFP expression in the SIA neurons (Suo et al, 2006). This response requires the CREB homologue CRH-1, suggesting that CRE-mediated GFP expression in the absence of food is indicative of CREB activation (Suo et al, 2006). Octopamine mediates CREB activation as exogenous octopamine can activate cre∷gfp and starvation-mediated activation is not observed in tbh-1 mutants (Suo et al, 2006), which are defective in octopamine synthesis (Alkema et al, 2005). Although the tbh-1 gene is expressed in the RIC neurons and the gonadal sheath cells, the RIC neurons are most likely responsible for starvation-mediated CREB activation as the starvation response is also observed in larvae in which the gonadal sheath cells are not yet fully developed (data not shown). Furthermore, starvation-mediated CREB activation requires the unc-64 gene encoding syntaxin (Suo et al, 2006), which is not expressed in the gonadal sheath cells but is expressed in many neurons and is required for neurotransmitter release (Ogawa et al, 1998; Saifee et al, 1998). This further supports a non-gonadal function for octopamine.
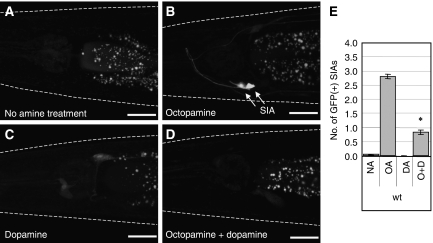
To detect a possible interaction between octopamine and dopamine signalling, we first examined whether exogenous dopamine could modify exogenous octopamine-induced CREB activation in the SIA neurons. We quantified CREB activation by determining the average number of the SIA neurons expressing GFP in each condition. As we demonstrated earlier (Suo et al, 2006), cre∷gfp animals cultured on plates containing bacteria as food source did not express GFP (Figure 1A and E), but did express GFP in the SIA neurons when incubated with 3 mg/ml octopamine (Figure 1B and E). This octopamine-induced expression was reduced by co-incubation of octopamine-treated animals with 1 mg/ml dopamine (Figure 1D and E), whereas dopamine treatment alone did not alter the lack of GFP expression observed in the SIA neurons in well-fed animals (Figure 1C and E). These results show that exogenous dopamine suppresses exogenous octopamine-mediated CREB activation in the SIA neurons.
Figure 1.
Exogenous octopamine and dopamine treatments of cre∷gfp animals. Animals carrying cre∷gfp were placed on agar plates containing (A) no amine, (B) 3 mg/ml octopamine, (C) 1 mg/ml dopamine, or (D) 3 mg/ml octopamine plus 1 mg/ml dopamine. Fluorescence images were taken after 6 h of incubation. GFP expression was observed in the SIA neurons in octopamine-treated animals and it was decreased by dopamine. White dotted lines outline the head of each animal. Scale bars, 20 μm. (E) The number of GFP-expressing SIA neurons per animal was determined after cre∷gfp animals were incubated on plates containing no amine (NA), octopamine (OA), dopamine (DA), or octopamine plus dopamine (O+D) for 6 h. Error bars indicate the standard errors of the means. At least 80 animals were tested. *P<0.001 (Tukey–Kramer multiple comparison test), compared with OA.
Endogenous dopamine suppresses endogenous octopamine-mediated CREB activation
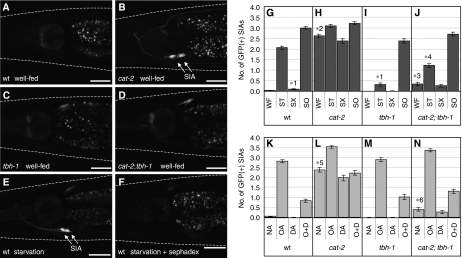
To investigate whether endogenous dopamine also suppresses CREB activation, we examined cat-2(e1112) mutant animals. The cat-2 gene encodes a tyrosine hydroxylase that is required for dopamine synthesis, hence cat-2 mutants have decreased but measurable levels of dopamine (Sulston et al, 1975; Sanyal et al, 2004). cat-2 mutant animals spontaneously induced CREB activation in the SIA neurons, even when they were cultured in the presence of food (Figure 2B and H). This result indicates that endogenous dopamine normally suppresses CREB activation in the presence of food.
Figure 2.
CRE-mediated GFP expression of dopamine- and octopamine-deficient mutants. Fluorescence images were taken of (A, E, F) wild-type (wt), (B) cat-2 mutant, (C) tbh-1 mutant, and (D) cat-2;tbh-1 double mutant animals carrying cre∷gfp under (A–D) well-fed, (E) starvation, or (F) starvation plus Sephadex conditions (see Materials and methods). White dotted lines outline the head of each animal. Scale bars, 20 μm. (G–J) The number of GFP-expressing SIA neurons per animal was determined after animals were incubated in well-fed (WF), starvation (ST), starvation+Sephadex (SX), or soaking (SO) conditions for 6 h. (K–N) The number of GFP-expressing SIA neurons per animal was determined after cre∷gfp animals were incubated on food containing plates also containing no amine (NA), octopamine (OA), dopamine (DA), or octopamine plus dopamine (O+D) for 6 h. (K) Data are also in Figure 1E and are shown here for comparison. Error bars indicate the standard errors of the means. At least 80 animals were tested. *1P<0.001 (Tukey–Kramer multiple comparison test), compared with ST of wt animals. *2P<0.001, compared with WF of wt animals. *3P<0.001, compared with WF of cat-2 mutants. *4P<0.001, compared with ST of tbh-1 mutants. *5P<0.001, compared with NA of wt animals. *6P<0.001, compared with NA of cat-2 mutants.
Octopamine-deficient tbh-1(ok1196) mutants exhibited no spontaneous GFP expression in the presence of food (Figure 2C and I). Well-fed cat-2;tbh-1 double mutants exhibited a greatly decreased level of spontaneous GFP expression (Figure 2D and J) compared with the cat-2 single mutant (Figure 2B and H). These results indicate that spontaneous CREB activation induced by the loss of dopamine in cat-2 mutants is dependent on the presence of endogenous octopamine. Taken together, these results suggest that endogenous dopamine suppresses endogenous octopamine signalling required for CREB activation in the SIA neurons, which parallels the above findings for exogenous octopamine and dopamine.
To further test the involvement of endogenous dopamine in the regulation of CREB activation in the SIA neurons, we used the Sephadex beads (SX). It was shown earlier that the Sephadex beads induce a dopamine-dependent behavioural change presumably by mimicking the tactile attribute of the bacterial food source without providing nutritional or chemosensory cues associated with bacteria (Sawin et al, 2000). As we showed earlier (Suo et al, 2006), cre∷gfp animals cultured in the absence of bacteria expressed GFP in the SIA neurons (Figure 2E and G). cre∷gfp animals cultured on agar growth plates covered with the Sephadex beads instead of bacteria showed a decrease in the frequency of GFP expression in the SIA neurons relative to animals cultured in the absence of bacteria (Figure 2F and G). These results strongly suggest that octopamine-mediated CREB activation in the absence of a bacterial food source is not initiated by the decrease in chemosensation of food or food intake but by the absence of a tactile perception of food by the dopaminergic neurons.
cat-2 and tbh-1 mutants were also tested with the Sephadex beads in the absence of food. cat-2 mutants, which manifest spontaneous CREB activation in the presence of food, showed a slight further increase in GFP expression in the absence of food (Figure 2H). This increase was eliminated by the addition of the Sephadex beads to the foodless plates. It is reported that cat-2 mutants are not completely dopamine deficient (Sanyal et al, 2004), suggesting that residual dopamine may be suppressing CREB activation in the presence of food or by the Sephadex beads in cat-2 mutants. tbh-1 mutants exhibited only a small increase in GFP expression in the absence of food (Figure 2I), as expected, as octopamine is required for CREB activation (Suo et al, 2006). cat-2;tbh-1 double mutants showed a significantly larger increase in GFP expression in the absence of food than tbh-1 single mutants. This increase was also suppressed in the presence of the Sephadex beads (Figure 2J). These results suggest the existence of some octopamine-independent regulation of CREB activation in the SIA neurons that is suppressed by dopamine, although it is also possible that they are attributable to residual octopamine, which may exist in tbh-1 mutants.
A possible explanation for the failure of cat-2;tbh-1 double mutants to express GFP would be because the SIA neurons were missing or grossly abnormal in this mutant strain. We showed earlier that soaking animals in water activates CRH-1-dependent gene expression in the SIA neurons independent of the octopamine signal (Suo et al, 2006) (Figure 2G and I). cat-2;tbh-1 double mutants also responded normally to soaking (Figure 2J), indicating that not only are the SIA neurons generated in this strain but they are also able to activate CREB. This result also shows that soaking activates CREB in the SIA neurons independently of dopamine.
We also examined the effects of exogenous octopamine and dopamine treatment on cat-2 and tbh-1 single mutants and the cat-2;tbh-1 double mutant (Figure 2K–N). These mutants increased GFP expression in response to octopamine and this increased expression was suppressed by adding dopamine as seen in the wild-type animals. Dopamine treatment did not decrease GFP expression of cat-2 mutants to the wild-type level, even though in principle the defect caused by the absence of endogenous dopamine should be rescued by the addition of exogenous dopamine. This is probably because cat-2 mutants already expressed GFP in the SIA neurons before the drug treatment and 6 h of incubation with dopamine was not sufficient for the preexisting GFP to be degraded and become undetectable. Taken together, these results indicate that loss of endogenous octopamine or dopamine does not have a major influence on the effects of exogenous octopamine and dopamine.
Responses of dopamine receptor mutants to exogenous dopamine
We next attempted to determine which dopamine receptors are responsible for the dopamine-mediated suppression of CREB activation in the SIA neurons. Four G protein-coupled dopamine receptors have been identified from C. elegans. DOP-1 is a D1-like receptor that couples to Gs when expressed in cultured mammalian cells (Suo et al, 2002; Sanyal et al, 2004). DOP-2 and DOP-3 are D2-like receptors that couple to Gi/o (Suo et al, 2003; Chase et al, 2004; Sugiura et al, 2005). DOP-4 is homologous to invertebrate-specific dopamine receptors identified in insects and is believed to be a Gs- or Gq-coupled receptor (Sugiura et al, 2005). Earlier analyses indicate that these four receptors are the only G protein-coupled receptors in C. elegans genome that show strong homology to known dopamine receptors (Wintle and Van Tol, 2001, data not shown). We showed earlier that octopamine works through the Gqα homologue EGL-30 in the SIA neurons to activate CREB and that this signal is inhibited by the Goα homologue GOA-1 (Suo et al, 2006). We, therefore, hypothesized that dopamine works through GOA-1 to suppress Gq-mediated CREB activation and deemed DOP-2 and DOP-3 to be likely candidates for the dopamine receptors that suppress octopamine signalling as these receptors are capable of coupling to Gi/o (Suo et al, 2003; Sugiura et al, 2005).
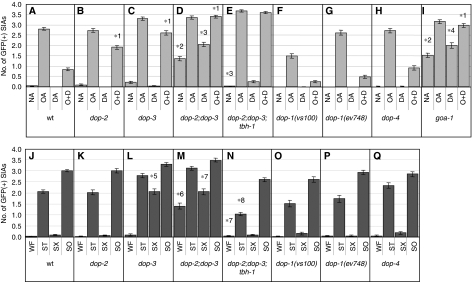
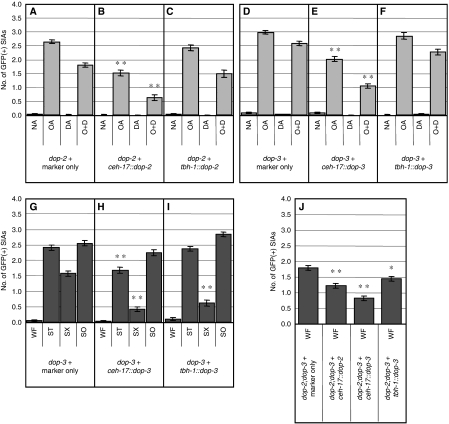
To test this hypothesis, dop-2(vs105) and dop-3(vs106) deletion mutants (Chase et al, 2004) were examined for their responses to exogenous dopamine treatment. We found that both dop-2 and dop-3 single mutants were partially defective in responding to exogenous dopamine in that CREB activation after incubation with octopamine plus dopamine was significantly higher in dop-2 and dop-3 single mutants than in wild-type animals (Figure 3A–C). Their defects were partial as, for each single mutant, GFP expression in animals treated with octopamine plus dopamine was lower than that of animals treated with octopamine alone (Figure 3B and C). These results raise the possibility that the two receptors act in parallel to mediate the dopamine response in the SIA neurons. This possibility was verified by finding that dop-2;dop-3 double mutants were completely defective in exogenous dopamine-mediated suppression of CREB activation and showed no reduction in octopamine-mediated GFP expression after addition of exogenous dopamine (Figure 3D). These results suggest that DOP-2 and DOP-3 receptors act in parallel and together account for all dopamine-induced suppression of CREB activation in the SIA neurons.
Figure 3.
CRE-mediated GFP expression of dopamine receptor and G protein mutants. (A–I) The number of GFP-expressing SIA neurons per animal was determined after animals were incubated on food containing plates also containing no amine (NA), octopamine (OA), dopamine (DA), or octopamine+dopamine (O+D) for 6 h. (J–Q) The number of GFP-expressing SIA neurons per animal was determined after animals were incubated in well-fed (WF), starvation (ST), starvation+Sephadex (SX), or soaking (SO) conditions for 6 h. The data in panels A and J are also shown in Figures 1E and 2K, respectively, and are shown here for comparison. Error bars indicate the standard errors of the means. At least 80 animals were tested. *1P<0.001 (Tukey–Kramer multiple comparison test), compared with O+D of wt animals. *2P<0.001, compared with NA of wt animals. *3P<0.001, compared with WF of dop-2;dop-3 double mutants. *4P<0.01, compared with WF of goa-1 mutants. *5P<0.001, compared with SX of wt animals. *6P<0.001, compared with WF of wt animals. *7P<0.01, compared with WF of dop-2;dop-3 double mutants. *8P<0.001, compared with WF of dop-2;dop-3;tbh-1 triple mutants.
dop-2;dop-3 double mutants also showed spontaneous GFP expression when no amine was administered even in the presence of food (Figure 3D), paralleling what was observed for cat-2 mutants (Figure 2L). This result suggests that endogenous dopamine also works through DOP-2 and DOP-3. However, spontaneous GFP expression in the dop-2;dop-3 double mutants was not as strong as in the cat-2 mutants. This suggests that either another dopamine receptor is involved in the dopamine-mediated suppression of CREB or that the dop-2 and dop-3 alleles are not nulls. Furthermore, spontaneous CREB activation was dependent on octopamine as the dop-2;dop-3;tbh-1 triple mutants did not show spontaneous GFP expression in the SIA neurons (Figure 3E).
We observed a slight increase in the frequency of GFP expression by dopamine compared with the no amine control in the dop-2;dop-3 double mutants (Figure 3D). This result indicates that dopamine may positively regulate CREB in the SIA neurons in the absence of suppression of CREB activation by the activities of DOP-2 and DOP-3.
We also tested deletion mutants of the other known dopamine receptors, dop-1(vs100), dop-1(ev748), and dop-4(ok1321). dop-1(vs100) showed a smaller response to exogenous octopamine alone (Figure 3F). However, another allele of dop-1, ev748, exhibited a normal response to octopamine (Figure 3G). Therefore, it is unclear whether the smaller response to octopamine is attributable to a dop-1 defect. Nonetheless, dop-1(vs100), dop-1(ev748), and dop-4(ok1321) did not show defects in responding to exogenous dopamine (Figure 3F–H), which is consistent with the notion that DOP-2 and DOP-3 receptors account for all exogenous dopamine-induced suppression of CREB activation in the SIA neurons.
It has been found that D1-like and D2-like dopamine receptors have opposite effects on intracellular signalling in mammals (Vallone et al, 2000). In C. elegans, D1-like receptor DOP-1 and D2-like receptor DOP-3 are shown to have opposing function in regulating locomotory activity (Chase et al, 2004). We separately tested dop-1 and dop-4 in the dop-2;dop-3 double mutant background to examine whether dop-1 and dop-4 have functions that oppose dop-2 and dop-3 function in the regulation of CREB activation (Supplementary Figure S1). dop-1;dop-2;dop-3 triple mutants responded to octopamine and dopamine in a similar manner as dop-2;dop-3 double mutants (Supplementary Figure S1B and C). On the other hand, loss of dop-4 mildly suppressed spontaneous CREB activation induced by loss of dop-2 and dop-3 (Supplementary Figure S1D). Furthermore, the small dopamine-induced increase in CREB activation, which was observed in dop-2;dop-3 double mutants, was not observed in dop-2;dop-3;dop-4 triple mutants. These results suggest that DOP-4, but not DOP-1, may positively regulate CREB activation in the SIA neurons thereby opposing the suppressive effects of DOP-2 and DOP-3 activity.
We also examined goa-1(sa734) mutants to determine whether, as expected, dopamine works through goa-1 to suppress the octopamine-mediated signal. goa-1 mutant animals behaved very similar to dop-2;dop-3 double mutant animals in response to exogenous octopamine and dopamine. goa-1 mutants exhibited spontaneous GFP expression as reported earlier (Suo et al, 2006), and this expression was slightly increased by exogenous dopamine. goa-1 mutants showed little, if any, change in octopamine-mediated GFP expression in response to exogenous dopamine (Figure 3I). These results indicate that GOA-1 works downstream of dopamine and suggest that DOP-2 and DOP-3 receptors work largely through GOA-1 in the SIA neurons to suppress CREB activation.
Responses of dopamine receptor mutants to Sephadex beads
We next examined Sephadex bead treatment of dopamine receptor mutants. dop-2 mutants responded normally to the presence or absence of food and to Sephadex bead treatment (Figure 3K). By contrast, the addition of the Sephadex beads in the absence of food did not entirely suppress CREB activation in dop-3 mutants, showing that dop-3 mutants are at least partially defective in responding to the Sephadex beads (Figure 3L). The failure of the Sephadex beads to suppress CREB activation was also observed in dop-2;dop-3 double mutants (Figure 3M). dop-1 and dop-4 mutants, on the other hand, did not show any defect (Figure 3O–Q). These results suggest that DOP-3, but not DOP-1, DOP-2, or DOP-4, is partially required for Sephadex-induced suppression of CREB activation in the SIA neurons.
We also found that mutations in dop-2 and dop-3 partially restore CREB activation in the absence of food in the tbh-1 mutant background and that this activation was suppressed by Sephadex beads treatment (Figure 3N). This partial restoration of CREB activation and its suppression by the Sephadex beads was similarly observed in cat-2;tbh-1 double mutants (Figure 2J). Suppression of CREB activation by the Sephadex beads in the absence of DOP-2 and DOP-3 receptors suggests that there is another dopamine receptor involved in this suppression (with the caveat that dop-2 and dop-3 alleles may not be nulls) or that the Sephadex beads can also work through a dopamine-independent mechanism to suppress CREB activation.
Cell-specific expression and activity of D2-like dopamine receptors
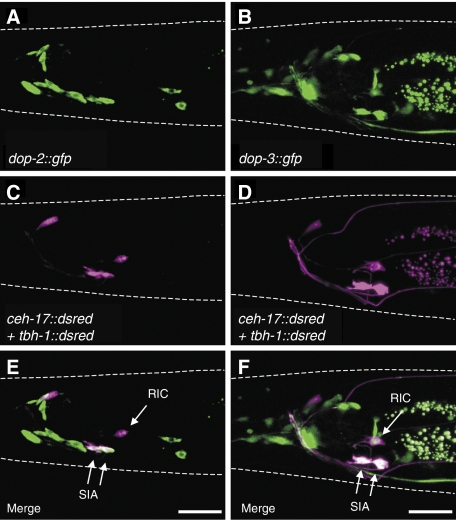
The most suitable locations for the dopamine receptors to regulate octopamine-mediated CREB activation would be in the SIA neurons and/or the RIC neurons. We, thus, examined whether dop-2 and dop-3 are expressed in these neurons. ceh-17∷dsred (Pujol et al, 2000; Suo et al, 2006) and tbh-1∷dsred (Alkema et al, 2005; Suo et al, 2006) expressing animals were used to mark the SIA neurons and RIC neurons, respectively. Promoter–reporter fusions, dop-2∷gfp and dop-3∷gfp, were introduced to the DsRed reporter strains to look for co-localization. Fluorescence microscopy showed that dop-2 is expressed in the SIA neurons but not in the RIC neurons, whereas dop-3 is expressed in both the SIA neurons and RIC neurons (Figure 4), indicating that these dopamine receptors are made by cells that we expect a priori to regulate octopamine signalling.
Figure 4.
Expression patterns of dop-2 and dop-3. Fluorescence images for (A, B) GFP expression and (C, D) DsRed expression were obtained from animals carrying ceh-17∷dsred and tbh-1∷dsred in addition to (A, C) dop-2∷gfp or (B, D) dop-3∷gfp. (E, F) Merged images of (A+C) and (B+D), respectively. dop-2∷gfp induced GFP expression in the SIA neurons but not in the RIC neurons. dop-3∷gfp induced GFP expression in the SIA neurons and the RIC neurons. Both dop-2∷gfp and dop-3∷gfp also each induced GFP expression in some other cells as reported earlier (Suo et al, 2003; Tsalik et al, 2003; Chase et al, 2004). White dotted lines outline the head of each animal. Scale bars, 20 μm.
As dop-2 is expressed in the SIA neurons, it is likely that it works in the SIA neurons for dopamine-mediated suppression of CREB activation. In this case, expression of DOP-2 in the SIA neurons of the dop-2 mutant should restore the ability of exogenous dopamine to suppress CREB activation. cDNAs for the two splice variants of dop-2, dop-2S and dop-2L, were fused with the ceh-17 promoter (ceh-17∷dop-2S and ceh-17∷dop-2L, respectively) that drives gene expression only in the SIA and ALA neurons (Pujol et al, 2000). ceh-17∷ dop-2S, ceh-17∷dop-2L, and a co-transformation marker were co-injected into the dop-2 mutants. dop-2 mutants expressing dop-2S and dop-2L in the SIA neurons (Figure 5B) showed decreased GFP expression in the SIA neurons after octopamine plus dopamine treatment compared with the control strain, which was injected only with the co-transformation marker (Figure 5A). Their response to octopamine treatment by itself was also reduced compared with the control animals, which may be because multiple copies of transgenes are usually introduced by microinjection-mediated transgenesis, possibly leading to excessive dopamine receptor signalling. We also expressed dop-2S and dop-2L in the RIC neurons using fusions of the tbh-1 promoter (tbh-1∷dop-2S and tbh-1∷dop-2L, respectively) that drives gene expression in the RIC neurons (Alkema et al, 2005). However, expression of dop-2S and dop-2L in the RIC neurons did not significantly rescue the response of dop-2 mutants to exogenous dopamine (Figure 5C). Collectively, these results suggest that dop-2 works in the SIA neurons to suppress CREB activation by exogenous dopamine, which is in agreement with the dop-2 expression pattern.
Figure 5.
Cell-specific rescue of the dop-2 and dop-3 mutant CREB activation phenotype. dop-2 mutant animals carrying cre∷gfp were injected with (A) the co-transformation marker transgene lin-44∷gfp only, (B) ceh-17∷dop-2S+L plus the marker, or (C) tbh-1∷dop-2S+L plus the marker. dop-3 mutants carrying cre∷gfp were injected with (D) the co-transformation marker only, (E) ceh-17∷dop-3fl plus the marker, or (F) tbh-1∷dop-3fl plus the marker. The number of GFP-expressing SIA neurons per animal was determined after the transformed animals were incubated on plates containing no amine (NA), octopamine (OA), dopamine (DA), or octopamine plus dopamine (O+D) for 6 h. (G–I) The number of GFP-expressing SIA neurons per animal was determined after animals were incubated in well-fed (WF), starvation (ST), starvation+Sephadex (SX), or soaking (SO) conditions for 6 h for the transformed dop-3 mutants. (J) dop-2;dop-3 double mutants carrying cre∷gfp were injected with the co-transformation marker only, ceh-17∷dop-2S+L and the marker, ceh-17∷dop-3fl and the marker, or tbh-1∷dop-3fl and the marker. The number of GFP-expressing SIA neurons per animal was determined after animals were incubated in well-fed condition for 6 h. Error bars indicate the standard errors of the means. At least 80 animals were tested. *P<0.01, **P<0.001 (Tukey–Kramer multiple comparison test), compared with the corresponding marker-only control strains.
Similar experiments for dop-3 were also carried out (Figure 5D–F). Fusions of the ceh-17 and tbh-1 promoter with the cDNA for dop-3fl, the functional splice variant of dop-3 (Sugiura et al, 2005) (ceh-17∷dop-3fl and tbh-1∷dop-3fl, respectively) were introduced into dop-3 mutants. Expression of dop-3fl in the SIA neurons (Figure 5E) decreased the number of the GFP-expressing SIA neurons after octopamine plus dopamine and octopamine-alone treatment, compared with the control dop-3 mutants (Figure 5D). In contrast, expression of dop-3fl in the RIC neurons had almost no effect (Figure 5F). These results suggest that dop-3 works in the SIA neurons to suppress CREB activation by exogenously applied dopamine.
dop-3 mutants also fail to suppress CREB activation in response to the Sephadex beads. Therefore, dop-3 mutants carrying ceh-17∷dop-3fl or tbh-1∷dop-3fl were also tested for their response to the Sephadex beads (Figure 5G–I). Expression of dop-3fl in the SIA neurons (Figure 5H) or the RIC neurons (Figure 5I) in the dop-3 mutants decreased the numbers of GFP-expressing SIA neurons after Sephadex bead treatment. On the other hand, the animals responded to soaking normally, suggesting that overexpression of dop-3fl did not cause a developmental defect that indirectly abrogates CREB-dependent transcription or its detection in the SIA neurons. These results suggest that dop-3 can work in either the SIA neurons or the RIC neurons to mediate Sephadex-induced suppression of CREB activation in the SIA neurons.
ceh-17∷dop-2S+L, ceh-17∷dop-3fl, and tbh-1∷dop-3fl that rescued dop-2 or dop-3 mutants were introduced into dop-2;dop-3 double mutants to examine whether they suppress spontaneous CREB activation in the presence of food (Figure 5J). Expression of dop-2S+L in the SIA neurons, dop-3fl in the SIA neurons, or dop-3fl in the RIC neurons resulted in an incomplete but significant reduction in GFP expression in the dop-2;dop-3 double mutants compared with control animals. These results suggest that dop-2 in the SIA neurons and dop-3 in the SIA neurons and the RIC neurons function to respond to endogenous dopamine.
Effect of serotonin on CREB activation
It is known that exogenous serotonin treatment in the absence of food induces behavioural responses like those observed in well-fed C. elegans (Horvitz et al, 1982). Furthermore, exogenous serotonin and octopamine elicit opposite behavioural responses in C. elegans. We, therefore, investigated whether serotonin has any effect on octopamine-mediated CREB activation as observed for dopamine.
We first tested the effect of exogenous serotonin on exogenous octopamine-mediated CREB activation. Octopamine-mediated GFP expression in the SIA neurons was slightly suppressed by 1.0 mg/ml serotonin (Supplementary Figure S2A). However, animals incubated with serotonin plus octopamine were pale and had a disrupted morphology. Therefore, the apparent suppression of octopamine-mediated CREB activation by serotonin could be attributable to a general unhealthiness of the animals. At lower concentrations of serotonin, in which animals appeared healthy, octopamine-mediated GFP expression was not suppressed. Furthermore, mutants of ser-4, which encodes the only Gi/o-coupled serotonin receptor identified in C. elegans (Olde and McCombie, 1997), also exhibited a slight reduction in octopamine-mediated CREB activation at the higher concentration of serotonin (Supplementary Figure S2B). In addition, ser-4 mutants also responded normally to food and starvation (Supplementary Figure S2E). tph-1 mutants, which are defective in serotonin synthesis (Sze et al, 2000), showed only slight spontaneous CREB activation in the presence of food and did not enhance the starvation response (Supplementary Figure S2D). Taken together, the above results suggest that serotonin does not have a major function in food-mediated regulation of CREB activation in the SIA neurons.
Effect of DAF-7 signalling on CREB activation
The DAF-7 TGFβ ligand is expressed only in the ASI sensory neurons in the presence of food and serves as a gauge of environmental conditions in C. elegans (Ren et al, 1996; Schackwitz et al, 1996). DAF-7 expression is reduced in the absence of food. DAF-7 signals through the TGFβ receptor DAF-1 (Georgi et al, 1990), which inactivates the co-SMAD DAF-3 (Patterson et al, 1997). Accordingly, daf-7 and daf-1 mutants exhibit features of starved animals (constitutive dauer formation, fat accumulation, egg laying defect, and decreased pharyngeal pumping) in the presence of food and daf-3 mutations suppress these features. Greer et al showed that DAF-7-mediated signalling in the tyraminergic RIM neurons is sufficient for the regulation of all the behavioural and metabolic responses. They also found that the octopaminergic RIC neurons have a function in this regulation although their effect was partial. Furthermore, it was shown that tyramine and octopamine work downstream of DAF-7 signalling to regulate pumping rate. These results suggest that, in the absence of food, activation of DAF-3 by loss of DAF-7 signalling leads to increased octopamine signalling from the RIC neurons. We, therefore, examined whether DAF-7 signalling has a function in CREB activation in the SIA neurons.
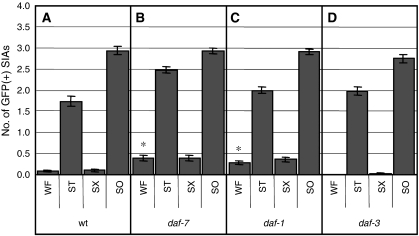
daf-7 and daf-1 mutants showed only slight spontaneous CREB activation in the presence of food (Figure 6B and C). Furthermore, daf-3 mutants exhibited a normal response to the absence of food (Figure 6D). These results suggest that the DAF-7 signalling pathway has a minor function in the regulation of CREB in the SIA neurons and that, even in the absence of DAF-7 signalling, dopamine signalling can regulate octopamine signalling in response to food.
Figure 6.
CRE-mediated GFP expression of DAF-7 signalling mutants. (A–D) The number of GFP-expressing SIA neurons per animal was determined after animals were incubated in well-fed (WF), starvation (ST), starvation+Sephadex (SX), or soaking (SO) conditions for 6 h. Error bars indicate the standard errors of the means. At least 80 animals were tested. *P<0.001 (Tukey–Kramer multiple comparison test), compared with WF of wt animals.
Discussion
We showed earlier that octopamine made by the RIC neurons in the head works directly on the SIA neurons of C. elegans to activate CRE-mediated gene expression through the octopamine receptor SER-3 and Gqα EGL-30 (Suo et al, 2006). In this study, we show for the first time that dopamine signalling in C. elegans suppresses octopamine signalling and that the cessation of dopamine signalling in the absence of food is an important mechanism for up-regulating octopamine signalling.
The dopamine signal antagonizes the octopamine signal in the SIA neurons
We found that exogenously applied dopamine suppresses exogenous octopamine-mediated CREB activation in the SIA neurons. dop-2 and dop-3 mutants are defective in response to exogenous dopamine, indicating that this activity is mediated by Gi/o-coupled D2-like dopamine receptors DOP-2 and DOP-3. Cell-specific rescue experiments showed that both dop-2 and dop-3 work in the SIA neurons, showing that dopamine works directly on these neurons. Chase et al showed earlier that exogenous dopamine works on dop-3 in the ventral nerve cord cholinergic neurons and promotes paralysis of C. elegans through goa-1 (Chase et al, 2004). Consistent with this report, we also found that goa-1 mutants are insensitive to the CREB-suppressive effect of exogenous dopamine, suggesting that exogenous dopamine uses the Gi/o class of G proteins to suppress octopamine-mediated Gq signalling in the SIA neurons.
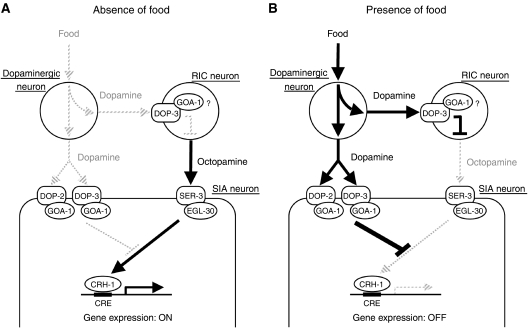
It is likely that activation of CREB in the SIA neurons is determined by the relative strength of the dopamine and octopamine signals. The animals in the presence of food show no CREB activation in the SIA neurons because endogenous dopamine is being released in these conditions. The animals treated with exogenous octopamine activate CREB in the SIA neurons even when the octopamine treatment plates contained food, which should induce endogenous dopamine release. This suggests that exogenously applied octopamine can overcome the suppression by endogenous dopamine. Furthermore, exogenous octopamine-mediated CREB activation is suppressed by addition of exogenous dopamine, which presumably is in higher concentration than that of endogenous dopamine. Thus, the SIA neurons, in which both the dopamine and octopamine receptors act, appear to compare the relative strength of dopamine and octopamine signals to determine whether CREB will be activated (Figure 7).
Figure 7.
Model for dopamine regulation of the octopamine signalling in the presence and absence of food. (A) In the absence of food or food-like mechanical stimulation, dopamine is not released, and octopamine, which is released from the octopaminergic RIC neurons, is able to activate the octopamine receptor SER-3 in the SIA neurons. SER-3 works through EGL-30 (Gqα) to activates CRH-1 (CREB). (B) In the presence of food, dopamine is released and suppresses CREB activation through the D2-like dopamine receptor DOP-3 in the RIC neurons probably by decreasing octopamine release. Simultaneously, dopamine inhibits Gq signalling in the SIA neurons by activating GOA-1 (Goα) through D2-like dopamine receptors DOP-2 and DOP-3, which also results in suppression of CREB activation in the SIA neurons.
Dopamine regulation of octopaminergic neurons
We also found that endogenous dopamine suppresses endogenous octopamine signalling. This is suggested by the observation that dopamine-deficient cat-2 mutants exhibit spontaneous CREB activation in the presence of food in a manner that depends on endogenous octopamine. Furthermore, Sephadex bead treatment, which is shown to mediate a food-related behaviour in a dopamine-dependent manner (Sawin et al, 2000), suppressed activation of octopamine-dependent CREB activation in the SIA neurons of starved animals. These results show that the perception of food through dopamine signalling can regulate octopamine signalling independently of food intake or chemosensation.
Endogenous dopamine also works through dop-2 and dop-3, as revealed by finding that dop-2;dop-3 double mutants show spontaneous CREB activation. In addition, dop-3 mutants exhibit a partial defect in the Sephadex response, whereas dop-2 mutants were normal in this response. It is somewhat surprising that CREB activation in the SIA neurons of dop-3 mutants can be suppressed by food but not by the Sephadex beads. A possible explanation for this is that food activates the dopaminergic neurons more strongly than the Sephadex beads do, possibly through a stronger mechanical stimulation or an additional non-mechanical stimulus, and dop-3 is required to respond to the weaker dopamine signal. Regardless of the mechanism, a defective Sephadex response in dop-3 mutants indicates that the Sephadex beads work through dopamine signalling to suppress octopamine signalling.
We found that expression of dop-3 in the RIC neurons rescues the Sephadex response of dop-3 mutants and suppresses spontaneous CREB activation of dop-2;dop-3 double mutants, but does not rescue exogenous dopamine-mediated suppression of exogenous octopamine signalling. These results indicate that dop-3 in the RIC neurons can suppress endogenous octopamine signalling but not exogenous octopamine-mediated signalling. These results are consistent with a model whereby DOP-3 transduces a dopamine signal to the RIC neurons that suppresses their ability to release octopamine. This model is also consistent with the ability of DOP-3 to couple to an inhibitory Gi/o (Sugiura et al, 2005) as Goα homologue GOA-1, which is expressed in all the neurons, is shown to inhibit neurotransmitter release (Miller et al, 1999; Nurrish et al, 1999). Thus, it appears that dopamine can downregulate octopamine signalling in two ways, one is by affecting its release from the RIC neurons and the other is by negatively regulating the ability of octopamine to activate CREB in the SIA neurons.
In a recent report, Greer et al showed that loss of DAF-7 release from the ASI neurons in starved animals activates octopamine signalling by disinhibiting DAF-3 in the RIC neurons (Greer et al, 2008). This finding suggests that the RIC neurons could also be inactivated by food through DAF-7 signalling. However, considering that well-fed cat-2 animals exhibit CREB activation, a block in dopamine signalling is sufficient to allow octopamine signalling without invoking additional activation of octopamine signalling by loss of DAF-7 function. Furthermore, daf-3 mutants exhibited CREB activation in the absence of food, indicating that activation of DAF-3 in the absence of food is not necessary for octopamine signalling. Our results show that the DAF-7 signalling has a minor function in the regulation of octopamine signalling and that most of the negative regulation of octopamine signalling that occurs in response to food is caused by dopamine signalling.
A two-transmitter, three-neuron-type network regulates the response to food
To activate a signal in response to the absence of food in the environment, organisms need to shut off an inhibitory process that is active in the presence of food. This study shows that C. elegans uses two bioamines and a three-neuron-type circuit to activate a signal in the absence of food (Figure 7). In the presence of food, dopamine is released by the dopaminergic neurons. The released dopamine activates DOP-3 in the RIC neurons, possibly to decrease octopamine release. Simultaneously, dopamine also inhibits Gq signalling in the SIA neurons by activating Gi/o signalling through DOP-2 and DOP-3. In the absence of food, dopamine is not released, which inactivates DOP-3 in the RIC neurons, potentially increasing octopamine release. The released octopamine activates the Gq-coupled octopamine receptor SER-3 in the SIA neurons. The Gq signal can activate CREB, as negative regulation by dopamine through Gi/o is not present when dopamine is not released.
It is interesting to note that dopamine controls octopamine signalling at two sites, even though, in principle, regulation in either one of the two sites should be sufficient for the suppression. The nervous system may have evolved to use this circuit because controlling function at two sites would allow for a more precise and reliable control of the octopamine signalling.
We speculate that this type of three-neuron-type circuit may be a common mechanism for inhibition of one neurotransmitter signal by another neurotransmitter and may be used in many different circumstances. For example, there are striking analogies between this three-neuron-type circuit in C. elegans and a three-neuron-type circuit possibly involved in food response in the mammalian brain. First, food stimuli increase dopamine and decrease noradrenaline (the vertebrate equivalent of octopamine) release in the mammalian brain (Hajnal and Norgren, 2004; Hajnal et al, 2004). Second, noradrenergic neurons in the locus coeruleus receive projections from dopaminergic neurons in the ventral tegmental area (Beckstead et al, 1979) and their firing rate is negatively regulated by dopamine (Guiard et al, 2008). Third, both the noradrenergic neurons and the dopaminergic neurons innervate basal forebrain cholinergic neurons (Jones and Cuello, 1989). Noradrenaline positively regulates these cholinergic neurons primarily through the α1 adrenergic receptor (Fort et al, 1995), which is highly homologous to SER-3. Meanwhile, dopamine positively regulates the cholinergic neurons through the D1 receptor (Day and Fibiger, 1992; Acquas et al, 1994) and negatively regulates them through the D3 receptor (Millan et al, 2007), which is a homologue of DOP-2 and DOP-3. Thus, we postulate that the neuronal and molecular circuitry for food sensing we have discovered in C. elegans is conserved in vertebrates and therefore, further study of this circuitry in C. elegans should help to elucidate the mechanisms of food sensation in humans.
Materials and methods
Strains and fusion gene constructs
The strains and the fusion gene constructs used in this study are described in the Supplementary Materials and methods.
Analyses of CRE-mediated gene expression
For amine treatment, the assay plates contained 1.7% AgarNoble (BD Diagnostics) with or without 3 mg/ml octopamine-hydrochloride (Fluka), 1 mg/ml dopamine-hydrochloride (Sigma-Aldrich), and various concentration of serotonin creatinine sulphate (Sigma-Aldrich). An overnight culture of Escherichia coli OP50 (Brenner, 1974) in L broth was spun down and resuspended in 1/20 volume of water. A measure of 50 μl of the concentrated OP50 was spread on the assay plates and was left without the lids until the surface of the plates became dry.
Eggs from strains carrying cre∷gfp were isolated by alkaline hypochlorite treatment and grown for 2 days at 20°C on nematode growth media (NGM) plates seeded with OP50. Animals were collected in water and transferred to fresh NGM plates seeded with OP50 and incubated for one more day. For daf-7 and daf-1 mutants, animals were grown at 16°C until they became L4 larva and grown one more day at 20°C. Animals were again collected in water and transferred onto the assay plates in a drop of water. The water remaining on the plates was removed with a Kimwipe (Kimberly-Clark). After 6 h of incubation at 20°C, animals were collected in M9 buffer (Brenner, 1974) containing 50 mM NaN3 and mounted on glass slides.
Assays for well-fed, starvation, and soaking conditions were performed as described (Suo et al, 2006). In brief, synchronized animals were placed on NGM plates with bacteria for the well-fed condition, and NGM plates without bacteria for the starvation condition. Approximately 5 ml of water was poured onto 60 mm seeded NGM plates in the soaking condition. For the Sephadex beads treatment, 1 ml of 30 mg/ml Sephadex G50 DNA Grade Fine (Pharmacia) suspended in water was spread on NGM plates without bacteria. With this amount, the entire surface of the 60 mm diameter Petri dish was covered by the Sephadex beads. The plates were dried for at least 3 h without their lids. After 6 h of incubation on the Sephadex beads plates, animals were transferred into 35% sucrose solution to separate them from the Sephadex beads. Animals floating on the surface of the sucrose solution were collected in M9 buffer containing NaN3 and mounted on glass slides. We confirmed that the sucrose floating procedure alone does not increase or decrease the CRE-mediated GFP expression (data not shown).
The animals mounted on slides were examined under a fluorescence microscope (DMRA2, Leica). In the earlier study (Suo et al, 2006), we counted the frequency of the animals showing detectable GFP expression in the ventral ganglia. In this study, we counted the numbers of neurons expressing GFP for each animal to obtain more information from each animal. This new counting method allowed for more reliable measurements with strong correlation to the original counting method. The counting was done by the experimenter blind to the genotype and the condition of the animals. Statistical significance was evaluated by analysis of variance followed by Tukey–Kramer multiple comparison test using GraphPad Prism (GraphPad Software). The images presented in figures were obtained with a confocal laser microscope (LSM510, Zeiss).
Analyses of expression pattern
dop-2∷gfp (Suo et al, 2003) and dop-3∷gfp were injected into wild-type animals together with ceh-17∷dsred, tbh-1∷dsred, and a transformation marker pRF4, which contains the dominant roller mutation rol-6(su1006) (Kramer et al, 1990) as described earlier (Mello et al, 1991). Images from Rol animals were obtained with the confocal laser microscope.
Cell-specific rescue experiments
The fusion genes were injected into dop-2;tzIs3, dop-3;tzIs3, or dop-2;dop-3;tzIs3 together with an injection marker lin-44∷gfp (Murakami et al, 2001) and pBluescript (Stratagene). For the control strains, only the injection marker and pBluescript were injected. The final concentration of the dop-2/3 expression plasmids and lin-44∷gfp were 10 ng/μl, and the concentration of pBluescript was adjusted so that the total DNA concentration was 100 ng/μl. Animals carrying lin-44∷gfp, reflected by expression of GFP in the tail hypodermis, were analysed in the rescue experiments. The GFP expression from lin-44∷gfp was often limited to the tail region and rarely interfered with the GFP expression in the SIA neurons induced from cre∷gfp.
Supplementary Material
Supplementary Materials and Methods
Review Process Article
Acknowledgments
We thank Y Ohshima for lin-44∷gfp. We also thank SH Ordog for excellent technical assistance and SP Cordes, F Liu, H Suzuki, LT MacNeil, and JT Plummer for comments on the manuscript. dop-4 (ok1321) was isolated by the C. elegans Gene Knockout Project at Oklahoma Medical Research Foundation. Some strains were obtained through the Caenorhabditis Genetic Center. This work was supported in part by the Canadian Institutes of Health Research grant MOP-77722 and MOP-82909 to JGC. JGC and HHMVT are holders of Canadian Research Chairs. SS is a recipient of a Parkinson Society Canada Basic Research Fellowship. This paper is dedicated to the memory of Dr Hubert HM Van Tol.
References
- Acquas E, Day JC, Fibiger HC (1994) The potent and selective dopamine D1 receptor agonist A-77636 increases cortical and hippocampal acetylcholine release in the rat. Eur J Pharmacol 260: 85–87 [DOI] [PubMed] [Google Scholar]
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR (2005) Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46: 247–260 [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJ (1979) Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res 175: 191–217 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DL, Pepper JS, Koelle MR (2004) Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci 7: 1096–1103 [DOI] [PubMed] [Google Scholar]
- Day J, Fibiger HC (1992) Dopaminergic regulation of cortical acetylcholine release. Synapse 12: 281–286 [DOI] [PubMed] [Google Scholar]
- Finn PF, Dice JF (2006) Proteolytic and lipolytic responses to starvation. Nutrition 22: 830–844 [DOI] [PubMed] [Google Scholar]
- Fort P, Khateb A, Pegna A, Mühlethaler M, Jones BE (1995) Noradrenergic modulation of cholinergic nucleus basalis neurons demonstrated by in vitro pharmacological and immunohistochemical evidence in the guinea-pig brain. Eur J Neurosci 7: 1502–1511 [DOI] [PubMed] [Google Scholar]
- Georgi LL, Albert PS, Riddle DL (1990) daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell 61: 635–645 [DOI] [PubMed] [Google Scholar]
- Giles AC, Rose JK, Rankin CH (2006) Investigations of learning and memory in Caenorhabditis elegans. Int Rev Neurobiol 69: 37–71 [DOI] [PubMed] [Google Scholar]
- Greer ER, Pérez CL, Van Gilst MR, Lee BH, Ashrafi K (2008) Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metab 8: 118–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Merali Z, Blier P (2008) Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol 11: 625–639 [DOI] [PubMed] [Google Scholar]
- Hajnal A, Norgren R (2004) Sucrose sham feeding decreases accumbens norepinephrine in the rat. Physiol Behav 82: 43–47 [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R (2004) Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 286: 31–37 [DOI] [PubMed] [Google Scholar]
- Hills T, Brockie PJ, Maricq AV (2004) Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci 24: 1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD (1982) Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216: 1012–1014 [DOI] [PubMed] [Google Scholar]
- Jones BE, Cuello AC (1989) Afferents to the basal forebrain cholinergic cell area from pontomesencephalic—catecholamine, serotonin, and acetylcholine—neurons. Neuroscience 31: 37–61 [DOI] [PubMed] [Google Scholar]
- Kaeberlein TL, Smith ED, Tsuchiya M, Welton KL, Thomas JH, Fields S, Kennedy BK, Kaeberlein M (2006) Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell 5: 487–494 [DOI] [PubMed] [Google Scholar]
- Kahn BB, Flier JS (2000) Obesity and insulin resistance. J Clin Invest 106: 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Corcoran EE, Eto K, Gengyo-Ando K, Muramatsu M, Kobayashi R, Freedman JH, Mitani S, Hagiwara M, Means AR, Tokumitsu H (2002) A CaMK cascade activates CRE-mediated transcription in neurons of Caenorhabditis elegans. EMBO Rep 3: 962–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR (2007) Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron 55: 662–676 [DOI] [PubMed] [Google Scholar]
- Kramer JM, French RP, Park EC, Johnson JJ (1990) The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol Cell Biol 10: 2081–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GD, Wilson MA, Zhu M, Wolkow CA, de Cabo R, Ingram DK, Zou S (2006) Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell 5: 515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Dillin A (2008) Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem 77: 727–754 [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10: 3959–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Di Cara B, Dekeyne A, Panayi F, De Groote L, Sicard D, Cistarelli L, Billiras R, Gobert A (2007) Selective blockade of dopamine D(3) versus D(2) receptors enhances frontocortical cholinergic transmission and social memory in rats: a parallel neurochemical and behavioural analysis. J Neurochem 100: 1047–1061 [DOI] [PubMed] [Google Scholar]
- Miller KG, Emerson MD, Rand JB (1999) Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron 24: 323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Koga M, Ohshima Y (2001) DAF-7/TGF-beta expression required for the normal larval development in C. elegans is controlled by a presumed guanylyl cyclase DAF-11. Mech Dev 109: 27–35 [DOI] [PubMed] [Google Scholar]
- Nurrish S, Ségalat L, Kaplan JM (1999) Serotonin inhibition of synaptic transmission: Galpha(0) decreases the abundance of UNC-13 at release sites. Neuron 24: 231–242 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Harada S, Sassa T, Yamamoto H, Hosono R (1998) Functional properties of the unc-64 gene encoding a Caenorhabditis elegans syntaxin. J Biol Chem 273: 2192–2198 [DOI] [PubMed] [Google Scholar]
- Olde B, McCombie WR (1997) Molecular cloning and functional expression of a serotonin receptor from Caenorhabditis elegans. J Mol Neurosci 8: 53–62 [DOI] [PubMed] [Google Scholar]
- Patterson GI, Koweek A, Wong A, Liu Y, Ruvkun G (1997) The DAF-3 Smad protein antagonizes TGF-beta-related receptor signaling in the Caenorhabditis elegans dauer pathway. Genes Dev 11: 2679–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol N, Torregrossa P, Ewbank JJ, Brunet JF (2000) The homeodomain protein CePHOX2/CEH-17 controls antero-posterior axonal growth in C. elegans. Development 127: 3361–3371 [DOI] [PubMed] [Google Scholar]
- Ramos EJB, Meguid MM, Campos ACL, Coelho JCU (2005) Neuropeptide Y, alpha-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition 21: 269–279 [DOI] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL (1996) Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science 274: 1389–1391 [DOI] [PubMed] [Google Scholar]
- Robertson MD (2006) Food perception and postprandial lipid metabolism. Physiol Behav 89: 4–9 [DOI] [PubMed] [Google Scholar]
- Roeder T (1999) Octopamine in invertebrates. Prog Neurobiol 59: 533–561 [DOI] [PubMed] [Google Scholar]
- Saifee O, Wei L, Nonet ML (1998) The Caenorhabditis elegans unc-64 locus encodes a syntaxin that interacts genetically with synaptobrevin. Mol Biol Cell 9: 1235–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hébert TE, van der Kooy D, Schafer WR, Culotti JG, Van Tol HHM (2004) Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J 23: 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631 [DOI] [PubMed] [Google Scholar]
- Schackwitz WS, Inoue T, Thomas JH (1996) Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron 17: 719–728 [DOI] [PubMed] [Google Scholar]
- Sugiura M, Fuke S, Suo S, Sasagawa N, Van Tol HHM, Ishiura S (2005) Characterization of a novel D2-like dopamine receptor with a truncated splice variant and a D1-like dopamine receptor unique to invertebrates from Caenorhabditis elegans. J Neurochem 94: 1146–1157 [DOI] [PubMed] [Google Scholar]
- Sulston J, Dew M, Brenner S (1975) Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol 163: 215–226 [DOI] [PubMed] [Google Scholar]
- Suo S, Kimura Y, Van Tol HHM (2006) Starvation induces cAMP response element-binding protein-dependent gene expression through octopamine-Gq signaling in Caenorhabditis elegans. J Neurosci 26: 10082–10090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo S, Sasagawa N, Ishiura S (2002) Identification of a dopamine receptor from Caenorhabditis elegans. Neurosci Lett 319: 13–16 [DOI] [PubMed] [Google Scholar]
- Suo S, Sasagawa N, Ishiura S (2003) Cloning and characterization of a Caenorhabditis elegans D2-like dopamine receptor. J Neurochem 86: 869–878 [DOI] [PubMed] [Google Scholar]
- Sze JY, Victor M, Loer C, Shi Y, Ruvkun G (2000) Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403: 560–564 [DOI] [PubMed] [Google Scholar]
- Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, Hobert O (2003) LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol 263: 81–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallone D, Picetti R, Borrelli E (2000) Structure and function of dopamine receptors. Neurosci Biobehav Rev 24: 125–132 [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S (1986) The structure of the nervous system of the nematode C. elegans. Philos Trans R Soc Lond B Biol Sci 314: 1–340 [DOI] [PubMed] [Google Scholar]
- Wintle RF, Van Tol HHM (2001) Dopamine signaling in Caenorhabditis elegans-potential for parkinsonism research. Parkinsonism Relat Disord 7: 177–183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods
Review Process Article