Abstract
Xeroderma pigmentosum group C (XPC) protein initiates the DNA excision repair of helix-distorting base lesions. To understand how this versatile subunit searches for aberrant sites within the vast background of normal genomic DNA, the real-time redistribution of fluorescent fusion constructs was monitored after high-resolution DNA damage induction. Bidirectional truncation analyses disclosed a surprisingly short recognition hotspot, comprising ∼15% of human XPC, that includes two β-hairpin domains with a preference for non-hydrogen-bonded bases in double-stranded DNA. However, to detect damaged sites in living cells, these DNA-attractive domains depend on the partially DNA-repulsive action of an adjacent β-turn extension that promotes the mobility of XPC molecules searching for lesions. The key function of this dynamic interaction surface is shown by a site-directed charge inversion, which results in increased affinity for native DNA, retarded nuclear mobility and diminished repair efficiency. These studies reveal a two-stage discrimination process, whereby XPC protein first deploys a dynamic sensor interface to rapidly interrogate the double helix, thus forming a transient recognition intermediate before the final installation of a more static repair-initiating complex.
Keywords: DNA repair, genome stability, protein dynamics
Introduction
Nucleotide excision repair (NER) is a fundamental protective system that promotes genome stability by eliminating a wide range of DNA lesions (Gillet and Schärer, 2006). In addition to (6-4) photoproducts and cyclobutane pyrimidine dimers (CPDs) caused by ultraviolet (UV) light, the NER pathway removes DNA adducts generated by electrophilic chemicals as well as intrastrand DNA cross-links, DNA-protein cross-links and a subset of oxidative lesions (Huang et al, 1994; Kuraoka et al, 2000; Reardon and Sancar, 2006). The NER system operates through the cleavage of damaged strands on either side of injured sites, thus releasing defective bases as the component of oligomeric DNA fragments (Evans et al, 1997). Subsequently, the excised oligonucleotides are replaced by repair patch synthesis before DNA integrity is restored by ligation. Hereditary defects in this NER process cause devastating syndromes such as xeroderma pigmentosum (XP), a recessive disorder presenting with photosensitivity, a >1000-fold increased risk of skin cancer and, occasionally, internal tumours and neurological complications (Cleaver, 2005; Andressoo et al, 2006; Friedberg et al, 2006). XP patients are classified into seven repair-deficient complementation groups designated XP-A through XP-G (Cleaver et al, 1999; Lehmann, 2003).
In the NER pathway, the initial detection of DNA damage occurs by two alternative mechanisms. One subpathway, referred to as transcription-coupled repair, takes place when the transcription machinery is blocked by obstructing lesions in the transcribed strand (Hanawalt and Spivak, 2008). The second subpathway, known as global genome repair (GGR), is triggered by the binding of a versatile recognition complex, composed of XPC, Rad23B and centrin 2, to damaged DNA anywhere in the genome (Sugasawa et al, 1998; Nishi et al, 2005). XPC protein, which is the actual damage sensor of this initiator complex, displays a general preference for DNA substrates that contain helix-destabilizing lesions including (6-4) photoproducts (Batty et al, 2000; Sugasawa et al, 2001). In the particular case of CPDs, this recognition function depends on an auxiliary protein discovered by virtue of its characteristic UV-damaged DNA-binding (UV-DDB) activity (Nichols et al, 2000; Fitch et al, 2003). The affinity of this accessory factor for UV-irradiated substrates is conferred by a DNA-binding subunit (DDB2) mutated in XP-E cells (Scrima et al, 2008).
To achieve its outstanding substrate versatility, XPC protein interacts with an array of normal nucleic acid residues surrounding the lesion in a way that no direct contacts are made with the damaged bases themselves (Buterin et al, 2005; Trego and Turchi, 2006; Maillard et al, 2007). This exceptional binding strategy has been confirmed by structural analyses of Rad4 protein, a yeast orthologue that shares ∼40% similarity with the human XPC sequence. In co-crystals, Rad4 protein associates with DNA through a large transglutaminase-homology domain (TGD) flanked by the three β-hairpin domains BHD1, BHD2 and BHD3 (Supplementary Figure 1; Min and Pavletich, 2007). In view of the position of these structural elements relative to the accompanying model substrate, a recognition mechanism has been proposed in which BHD3 would ‘sample the DNA's conformational space to detect a lesion' (Min and Pavletich, 2007).
These earlier studies describing the features of an ultimately stable XPC/Rad4–DNA complex explain its ability to serve as a molecular platform for the recruitment of transcription factor IIH (TFIIH) or other downstream NER players (Yokoi et al, 2000; Uchida et al, 2002). However, one of the most challenging issues in the DNA repair field is the question of how a versatile sensor-like XPC/Rad4 examines the Watson–Crick double helix and faces the task of actually finding base lesions among a large excess of native DNA in a typical mammalian genome (Schärer, 2007; Sugasawa and Hanaoka, 2007). To address this long-standing question, we exploited fluorescence-based imaging techniques (Houtsmuller et al, 1999; Houtsmuller and Vermeulen, 2001; Politi et al, 2005) to visualize the mobility of XPC protein at work in the chromatin context of living cells. Our results point to a two-stage discrimination process, in which the rapid DNA quality check driven by a dynamic sensor of non-hydrogen-bonded bases precedes the final engagement of BHD3 with lesion sites.
Results
Instantaneous recognition of DNA lesions in human cells
Damage-induced changes of molecular dynamics in the nuclear compartment have been followed by C-terminal conjugation of the human XPC polypeptide with green-fluorescent protein (GFP). The time-dependent relocation of this fusion product was tested by transfection of repair-deficient XP-C fibroblasts that lack functional XPC because of a mutation leading to premature termination at codon 718 (Chavanne et al, 2000). Individual nuclei containing low levels of XPC-GFP (similar to the XPC expression in wild-type fibroblasts) were identified on the basis of their overall fluorescence (Supplementary Figure 2). To induce lesions, the nuclei were subjected to near-infrared irradiation using a pulsed multiphoton laser, thereby generating spatially confined and clearly detectable patterns of DNA damage with minimal collateral effects (Meldrum et al, 2003). The resulting laser tracks contained (6-4) photoproducts (Figure 1A) and CPDs (Figure 1B), representing the major UV lesions processed by the NER system. As expected, wild-type XPC-GFP was rapidly concentrated at nuclear sites containing such photolesions (Figure 1A and B). As earlier studies showed that the UV-induced accumulation of XPC is stimulated by DDB2 protein (Fitch et al, 2003; Moser et al, 2005), we applied the same procedure to XP-E cells, in which an R273H mutation generates a DDB2 product that is inactive in DNA binding and fails to be expressed to detectable levels (Nichols et al, 2000; Itoh et al, 2001). In this XP-E background, XPC-GFP is nevertheless effectively relocated to UV-irradiated tracks (Figure 1C), consistent with the known ability of XPC protein to detect (6-4) photoproducts in the absence of UV-DDB activity (Batty et al, 2000; Kusumoto et al, 2001).
Figure 1.
Instantaneous recognition of DNA damage by XPC protein in living cells. (A) High-resolution patterns of DNA damage and XPC-GFP accumulation. XP-C fibroblasts expressing low levels of XPC-GFP were laser treated to generate ∼5000 UV lesions along each linear irradiation track. The cells were fixed after 6 min and (6-4) photoproducts were detected by immunochemical staining using the red dye Alexa 546. B/W, black-and-white images illustrating the pattern of UV lesions (upper panel) and the accumulation of XPC-GFP (lower panel). Merged, superimposed images in which the relocation of XPC-GFP matches the pattern of DNA damage. Hoechst, DNA staining visualizing the nuclei. (B) Co-localization of XPC-GFP and CPDs. (C) Efficient relocation of XPC-GFP to UV irradiation tracks in XP-E cells devoid of UV-DDB activity. (D) Real-time kinetics of DNA damage recognition. A single 10-μm line of UV photoproducts was generated across each nucleus of XP-C cells. The accumulation of XPC-GFP at different time points is plotted as a percentage of the average fluorescence before irradiation (n=7). Error bars, standard errors of the mean.
To determine the kinetics of protein redistribution, DNA photoproducts were formed along a single 10-μm line crossing the nucleus of XP-C cells. Maximal accumulation of XPC protein was detected after treatment with a near-infrared radiation of 300–360 GW ·cm−2 (Supplementary Figure 3). Subsequently, DNA damage was induced with 314 GW cm−2 to generate ∼5000 UV lesions in each cell or, on the average, 1 UV lesion in ∼1.6 × 106 base pairs (see Materials and methods). Under these conditions, the local fluorescence in irradiated areas increased nearly instantaneously leading to a clearly distinguishable relocation of XPC fusion protein already 3 s after irradiation (Supplementary Movie 1). With progressive accumulation of wild-type XPC, a half-maximal increase in local fluorescence intensity was reached after ∼40 s (Figure 1D). A plateau level of fluorescence in the irradiation tracks, reflecting a steady-state situation with constant turnover, was detected after ∼300 s.
Concordance of relocation and DNA-binding activity
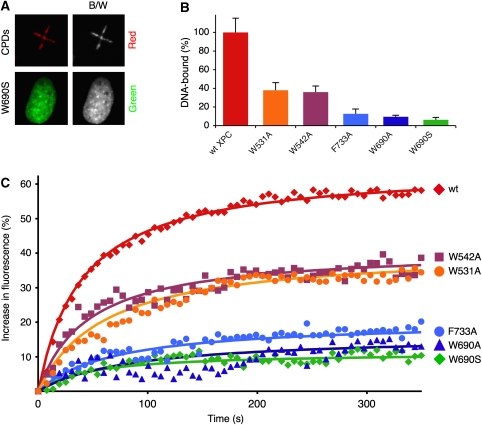
Besides the truncating XPC mutation, the XP-C fibroblasts used in this study (GM16093) are characterized by a comparably low level of DDB2 protein (Supplementary Figure 4). This reduced DDB2 expression suggested that the GM16093 fibroblasts may provide a cellular context in which, in contrast to an earlier report (Yasuda et al, 2007), the damage recognition defect of XPC mutants becomes evident without preceding DDB2 down-regulation. This view was confirmed by testing the nuclear dynamics of a repair-deficient W690S mutant with minimal DNA-binding affinity (Bunick et al, 2006; Maillard et al, 2007; Hoogstraten et al, 2008). In conjunction with the GFP fusion partner, this pathogenic mutant is expressed in similar amounts as the wild-type control and also localizes to the nuclei. However, in the XP-C fibroblasts of this study, the single W690S mutation causes >five-fold reduction in the relocation to UV-damaged areas (Figure 2A; Supplementary Movie 2). These findings were confirmed when another technique was used to inflict genotoxic stress, that is by UV-C irradiation (254 nm wavelength) through the pores of polycarbonate filters (Moné et al, 2004). In fact, compared with wild-type XPC, the W690S mutant exhibits only a marginal tendency to accumulate in UV-C radiation-induced foci (data not shown). Oligonucleotide-binding assays with XPC protein expressed in insect cells confirmed that this W690S mutation and the corresponding alanine substitution (W690A) abrogate the interaction with DNA (Figure 2B).
Figure 2.
Dependence on intrinsic DNA-binding activity. (A) Representative image (in colour and black-and-white) showing the low residual accumulation of the W690S mutant 6 min after irradiation. DNA lesions were counterstained by antibodies against CPDs. (B) DNA-binding activity determined by direct pull down. Wild-type (wt) XPC or mutants were expressed in Sf9 cells as fusion constructs with maltose-binding protein (MBP). Cell lysates containing similar amounts of XPC protein (Maillard et al, 2007) were incubated with a single-stranded 135-mer oligonucleotide. Subsequently, radiolabelled DNA molecules captured by XPC protein were separated from the free probes using anti-MBP antibodies linked to magnetic beads, and the radioactivity in each fraction was quantified in a scintillation counter. DNA binding is represented as the percentage of radioactivity immobilized by wt XPC protein after deduction of a background value determined with empty beads (n=3). Error bars, standard deviation. (C) Correlation between DNA binding and the kinetics of XPC accumulation in XP-C cells (n=7). See legend to Figure 1D for details.
The same analysis was extended to further repair-deficient XPC mutants targeting conserved aromatic residues (Maillard et al, 2007). A nearly complete loss of DNA binding is conferred by the F733A mutation, whereas the W531A and W542A substitutions are associated with more moderate defects (Figure 2B). When tested in GM16093 fibroblasts as GFP fusions, the damage-dependent redistribution of these different mutants correlates closely with the respective DNA-binding properties. In fact, the W690S, W690A and F733A derivatives display a poor ability to concentrate at damaged sites. In contrast, the residual DNA-binding activity of W531A and W542A leads to an intermediary level of accumulation in areas containing UV photoproducts (Figure 2C). From this tight correspondence between DNA binding and nuclear redistribution, we concluded that the rapid relocation of XPC protein to UV lesion sites reflects the intrinsic capacity of this sensor subunit to detect DNA damage through direct interactions with the nucleic acid substrate.
Role of the transglutaminase-like domain
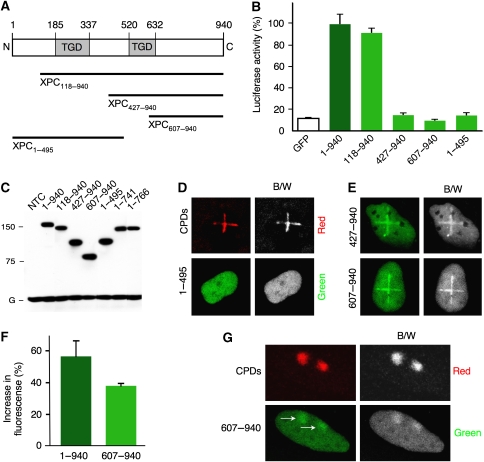
As the transglutaminase-like region maps to the N-terminal part of human XPC (Figure 3A), we generated N-terminal truncations (XPC118−940, XPC427−940 and XPC607−940) to test how the TGD sequences contribute to DNA damage recognition in living cells. The positions 118 and 607 were selected for these truncations to allow for comparisons with an earlier in vitro study monitoring the DNA-, Rad23B- and TFIIH-binding activity of XPC fragments (Uchida et al, 2002). Another truncate (XPC1−495) was included as a negative control that lacks the entire C-terminal half. The functionality of these constructs, conjugated to GFP at their C-terminus, was compared in a host-cell reactivation assay that has been developed to measure the cellular GGR activity (Carreau et al, 1995). Briefly, XP-C fibroblasts were transfected with a dual luciferase reporter system along with an expression vector coding for full-length or truncated XPC fusions. The reporter plasmid, which carries a Photinus luciferase gene, was damaged by exposure to UV-C light and supplemented with an undamaged vector that expresses the Renilla luciferase. GGR efficiency was assessed after 18-h incubations by determining Photinus luciferase activity in cell lysates, followed by normalization against the Renilla control.
Figure 3.
Mapping of the damage sensor domain to the C-terminal part of human XPC. (A) Scheme illustrating the position of the TGD sequences relative to the N-terminal XPC truncates. (B) GGR activity determined by host-cell reactivation assay (n=5; error bars, standard deviation). (C) Immunoblot analysis of XP-C cells transfected with expression vectors coding for the indicated fusions. The protein level was probed using anti-GFP antibodies. G, endogenous GAPDH control. (D) Representative image showing that an XPC fragment lacking the C-terminus (XPC1−495) fails to accumulate in laser-damaged areas. The XP-C fibroblasts were fixed 6 min after irradiation. B/W, black-and-white images showing that the tracks of DNA damage (upper panel) do not induce an accumulation of truncated XPC fusions (lower panels). (E) Representative images (in colour and black and white) showing that XPC427−940 and XPC607−940 accumulate in damaged areas of XP-C fibroblasts. The distribution of fluorescent fusion products was monitored 6 min after irradiation. (F) Local increase of fluorescence resulting from the damage-induced redistribution of full-length XPC or XPC607−940. A 10-μm line of UV photoproducts was generated across each nucleus and the resulting accumulation of fusion proteins (after a 6-min incubation) is plotted as a percentage of the average fluorescence before irradiation (n=7). Error bars, standard errors of the means. (G) Representative image illustrating that XPC607−940 accumulates in foci generated by UV-C irradiation (100 J m−2) through the pores of polycarbonate filters. The XP-C cells were fixed 15 min after treatment and CPDs were detected by immunochemical staining. The position of XPC607−940 foci is indicated by the arrows.
The full-length protein (XPC1−940) and an XPC118−940 derivative, isolated by functional complementation (Legerski and Peterson, 1992), were proficient in correcting the repair defect of XP-C cells (Figure 3B), thus showing that gene reactivation is determined by the ability of the GGR pathway to excise offending UV lesions. However, this repair activity could not be rescued by XPC427−940 and XPC607−940 (Figure 3B), implying that the N-terminal part of XPC protein is essential for the GGR reaction. All tested fragments were detected in transfected fibroblasts in similar amounts as the full-length control or the functional XPC118−940 derivative (Figure 3C), indicating that their repair deficiency does not result from reduced expression or enhanced degradation.
Next, all GGR-deficient truncates were tested for their damage recognition proficiency in XP-C fibroblasts. Neither XPC1−495 (Figure 3D) nor XPC1−718 (Supplementary Figure 4) were redistributed to sites of photoproduct formation in the irradiated nuclei of living cells, confirming that the C-terminal half of XPC protein is necessary for lesion recognition. However, unlike these C-terminal truncations, fragment XPC427−940 retains the ability to concentrate in laser-irradiated areas (Figure 3E). Even more surprising was the observation that the smaller fragment XPC607−940 readily accumulates at sites containing UV photolesions (Figure 3E). The quantification of defined 10-μm tracks showed that XPC607−940 is only ∼30% less efficient than full-length XPC in relocating to damaged sites (Figure 3F). Thus, a large N-terminal part of human XPC (65% of the full-length protein including its TGD regions) stimulates DNA damage recognition, but is not absolutely required for the sensing process itself. This conclusion is confirmed by the accumulation of XPC607−940 in UV-C foci generated by irradiation through the pores of polycarbonate filters (Figure 3G).
Differential contribution of -hairpin domains
According to the Rad4 crystal, three consecutive β-hairpin domains (BHD1, BHD2 and BHD3) mediate the interaction with damaged DNA (see Supplementary Figure 1). In the homologous XPC sequence, these structural elements range from residue 637 (start of BHD1) to residue 831 (end of BHD3). To examine how each of these domains contributes to DNA damage recognition in living cells, we generated the C-terminal truncations XPC1−741 (comprising BHD1 and BHD2) and XPC1−831, which includes all three BHDs (Figure 4A). Again, the truncation position 741 was chosen to allow for comparisons with an earlier in vitro study (Uchida et al, 2002). The constructs were conjugated to GFP at their C-terminus and tested for their ability to initiate the GGR reaction. In the case of XPC1−741, the repair function is reduced to a background level observed with empty GFP vector (Figure 4B). However, the reporter gene was reactivated to ∼40% of control in the presence of XPC1−831, indicating that despite its C-terminal truncation, this large fragment retains in part the ability to recruit NER factors to lesion sites. Although attempting to delineate the borders of a minimal sensor domain, we surprisingly found that essentially the same GGR activity was induced by XPC1−766, that is by adding only 25 amino acids to XPC1−741 (Figure 4B). A comparison with the Rad4 orthologue indicates that these 25 amino acids (residues 742–766) belong to an N-terminal extension of BHD3, which folds into a β-turn structure (see Figure 4A).
Figure 4.
BHD3 is not required for DNA damage detection. (A) Scheme illustrating the location of BHD and β-turn sequences relative to the C-terminal XPC truncates of this study. (B) GGR activity determined by host-cell reactivation assay in XP-C fibroblasts (n=5; error bars, standard deviation). (C) Representative images (taken 6 min after irradiation) comparing the accumulation of XPC1−766 and XPC1−741 at damaged sites. In the black-and-white representation, the linear irradiation tracks are surrounded by a dashed rectangle. (D) Representative image illustrating the accumulation of XPC1−766 along UV radiation tracks generated in XP-E fibroblasts devoid of UV-DDB activity. (E) The local increase in fluorescence, because of damage-induced redistributions of XPC truncates, was measured in XP-C and XP-E cells and plotted as the percentages of wt control as outlined in Figure 1D (n=5; error bars, standard errors of the mean). (F) XPC1−766 is also more efficient than XPC1−741 in accumulating in DNA damage foci generated by UV-C irradiation through the pores of polycarbonate filters (see Figure 3G for details). XPC1−766 (top) and XPC1−741 foci (bottom) are indicated by the arrows.
The UV-induced relocation of truncated XPC derivatives was tested in XP-C fibroblasts expressing similar low levels of each GFP construct (Supplementary Figure 5). Consistent with its distinctive functionality in the GGR assay, we observed that XPC1−766 accumulates more effectively than XPC1−741 to the 10-μm tracks of photolesions generated by laser irradiation (Figure 4C). An unequivocal pattern of XPC1−766 accumulation along the radiation tracks was also recorded in XP-E fibroblasts, that is in the absence of UV-DDB activity (Figure 4D). A quantitative comparison in both XP-C and XP-E cells highlights the increase in damage recognition when the truncation was introduced at residue 766 as compared with the truncation at position 741 (Figure 4E), thus showing that the damage-specific accumulation of XPC truncates as well as the effect of the β-turn structure takes place in the absence of DDB2 protein. A clear difference between XPC1−766 and XPC1−741 was reproduced when foci of fluorescence were monitored after UV-C irradiation through the pores of polycarbonate filters (Figure 4F). Taken together, this efficient redistribution of XPC1−766, irrespective of the cell type or technique used to inflict DNA damage, establishes for the first time that most of BHD3 is not required for the initial damage-sensing process.
The -turn structure enhances XPC dynamics
The GGR and relocation assays of Figure 4 revealed a striking difference between XPC1−741 and XPC1−766 because of the 25-amino-acid β-turn extension. To analyse the function of this β-turn structure, we compared the nuclear mobility of different truncates using fluorescence recovery after photobleaching (FRAP; Houtsmuller and Vermeulen, 2001). In cells that express similarly low levels of GFP fusion constructs, a nuclear area of 4 μm2 was bleached and, subsequently, protein movements were tested by recording the recovery of local fluorescence, which is dependent on the ability of the GFP fusions to move rapidly within the nuclear compartment.
The control experiment of Figure 5A shows how, in the absence of a fusion partner, the GFP moiety moves freely inside the cells. Instead, the nuclear mobility of full-length XPC-GFP is restrained by its larger size and propensity to undergo macromolecular interactions, as reported earlier (Hoogstraten et al, 2008). Surprisingly, in a direct comparison between XPC1−741, XPC1−766 and XPC1−831, a larger size correlated with increased nuclear mobility (Figure 5B). The FRAP curves obtained with these different truncates were used to calculate effective diffusion coefficients (Deff; Supplementary Table I). It was unexpected to find that, in undamaged cells, XPC1−766 (containing BHD1, BHD2 and the β-turn structure) and XPC1−831 (containing all three BHDs) move more rapidly inside the nucleus (Deff=0.44 and 0.49 μm2 s–1, respectively) than the shorter polypeptide XPC1−741 lacking the β-turn (Deff=0.34 μm2 s–1). We concluded that these C-terminal truncations disclose the existence of a dynamic interface, residing within the β-turn structure, which enhances the constitutive nuclear mobility of XPC protein in the absence of genotoxic stress.
Figure 5.
Identification of a dynamic core and two-stage damage recognition. (A) Principle of FRAP analysis. An area of 4 μm2 in the nuclei of XP-C fibroblasts expressing a particular GFP construct is bleached with a 488-nm wavelength laser. The kinetics and extent of fluorescence recovery (shown for GFP and XPC-GFP) depends on diffusion rate, molecular interactions as well as the fraction of immobile molecules. (B) Recovery plots of XPC truncates normalized to prebleach intensity (n=12). Error bars, standard errors of the mean. The difference between XPC1−766 and XPC1−831 is not significant. (C) The nuclear mobility of XPC1−741 remains unaffected by UV-C irradiation at a dose of 10 J m−2 (n=12). (D) The initial diffusion of XPC1−766 is reduced by UV light (10 J m−2, n=12), reflecting transient molecular interactions during stage 1 of the damage recognition process. (E) A fraction of XPC1−831 is stably immobilized after UV irradiation (10 J cm−2, n=12), reflecting stage 2 of the damage recognition process.
Subsequently, the FRAP approach was used to assess the corresponding responses to UV-C irradiation. In accord with its poor accumulation along DNA damage tracks (Figure 4C), the mobility of XPC1−741 is only marginally affected by the induction of photolesions (Figure 5C). In contrast, the diffusion rates of XPC1−766 (Figure 5D) and XPC1−831 (Figure 5E), which accumulate in UV lesion tracks, are significantly reduced (the respective Deff values are listed in Supplementary Table I). In the case of XPC1−831, the induction of DNA damage had a two-fold effect. First, UV lesions decreased the initial rate of protein diffusion exactly as observed with XPC1−766. Second, similar to the response of full-length XPC (Hoogstraten et al, 2008), the overall fluorescence recovery is less complete on UV irradiation (Figure 5E), indicating that a fraction of XPC1−831 is immobilized in a damage-specific manner. In summary, these protein mobility studies show that BHD3 induces the formation of a stable nucleoprotein complex once the lesion has been detected.
Antagonistic composition of the dynamic sensor domain
The truncation studies of Figures 4 and 5 suggested that residues 607–766 may be sufficient to find lesion sites in the genome. This hypothesis was confirmed by expressing short protein fragments in XP-C fibroblasts (Figure 6A). In the case of XPC607−766 (consisting of BHD1/BHD2 and the β-turn structure), a clear pattern of damage-induced accumulation was detected immediately after laser irradiation (Figure 6B). In contrast, XPC607−741 (lacking the β-turn) failed to accumulate in the tracks of UV lesions. XPC607−741 was unable to relocate to damaged areas regardless of whether the GFP moiety was placed at the C- (Figure 6C) or at the N-terminus (data not shown). These results support the conclusion that XPC607−766 displays a minimal sensor surface with damage recognition activity in living human cells.
Figure 6.
Antagonistic composition of the minimal damage sensor. (A) Immunoblot analysis of XP-C fibroblasts after transfection with vectors coding for the indicated XPC-GFP sequences. The expression was probed using anti-GFP antibodies. NTC, non-transfected cells; GFP, cells transfected with the GFP sequence alone; G, GAPDH control. (B) Representative image illustrating that fragment XPC607−766 readily accumulates in damaged areas containing DNA photolesions. The distribution of fluorescent fusion products was monitored 1 min after laser irradiation. B/W, black-and-white image. (C) XPC607−741 is unable to recognize UV lesions in living cells. Fibroblasts were subjected to fixation 1 min after irradiation and (6-4) photoproducts were detected by immunochemical staining. B/W, black-and-white images showing that UV lesions (upper panel) did not lead to accumulation of the fusion protein (lower panel). (D) Gel electrophoretic analysis of purified XPC fragments expressed as glutathione-S-transferase (GST) fusions in E. coli or with a histidine (His) tag in Sf9 cells. (E) DNA binding of XPC607−741 determined by oligonucleotide capture. The indicated concentrations of XPC-GST fragments were incubated with radiolabelled 135-mer oligonucleotides (3-mismatch heteroduplexes, homoduplexes and single strands). Thereafter, DNA molecules immobilized by XPC fragments were separated from the free oligonucleotides using glutathione-Sepharose beads, followed by the quantification of radioactivity associated with the beads. DNA binding is represented as the percentage of total input radioactivity captured by XPC fragments after deduction of a background value determined with empty beads (n=6; error bars, standard deviation). (F) DNA-binding profile of the minimal damage sensor (XPC607−766) determined as described in the legend to Figure 6E. (G) Contribution of BHD3. The DNA-binding profile of XPC607−831 was determined as outlined in the legend to Figure 6E, except that pull downs were performed with Ni-NTA agarose beads.
The fragments XPC607−741, XPC607−766 and XPC607−831 have been isolated to assess their DNA-binding properties using 135-mer DNA substrates. All three fragments were expressed and purified as soluble polypeptides without any signs of aggregation or precipitation that would be indicative of defective protein folding (Figure 6D). We compared their binding with three different DNA conformations: homoduplexes, heteroduplexes with three contiguous base mismatches or single-stranded oligonucleotides of the same length. Although XPC607−741 (containing BHD1 and BHD2) is unable to find DNA lesions in living cells, this fragment displays a preference for unpaired bases embedded in double-stranded DNA. In fact, XPC607−741 binds with higher affinity to heteroduplex DNA relative to homoduplexes or single-stranded oligonucleotides (Figure 6E).
A similar preference for hetero- over homoduplexes is retained by XPC607−766, which includes both BHD1/BHD2 and the β-turn structure (Figure 6F), thus supporting the notion that this minimal sensor is active in living cells by searching for destabilized base pairs. A side-by-side comparison of dose-dependent DNA-binding activities with XPC607−741 and XPC607−766 showed that the β-turn structure leads to a substantial reduction in nucleic acid binding (Figure 6F). In particular, we found that the association constant representing the interaction with homoduplex DNA decreases nearly 10-fold from 2.7 × 109 M−1 for XPC607−741 to 2.8 × 108 M−1 for XPC607−766. This drop in binding to the native double helix implies that the enhanced nuclear mobility conferred by amino acids 742–766 (Figure 5B) results from an antagonistic DNA-repulsive effect.
Finally, to test the contribution of BHD3, the same 135-mer substrates were used to monitor the DNA-binding properties of a longer fragment (XPC607−831) comprising all three BHDs. Figure 6G shows that this larger fragment has the characteristics of a single-stranded DNA-binding protein, indicating that BHD3 itself confers a pronounced selectivity for single-stranded conformations. The characteristic DNA-binding profile of this larger fragment XPC607−831 corresponds roughly to that detected when identical reactions were carried out with full-length XPC protein (Supplementary Figure 6).
Design of an XPC mutant with retarded nuclear mobility
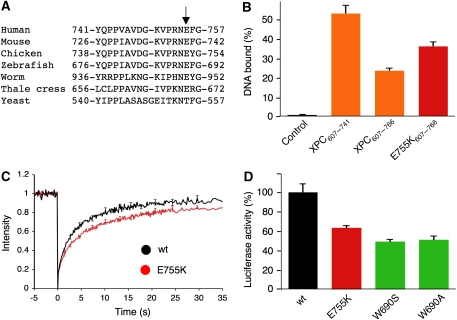
We postulated that part of the DNA-repulsive action mediated by the β-turn structure (Figure 6F) arises from negatively charged side chains that clash with the phosphates of the nucleic acid backbone. This hypothesis predicts that it should be possible to mitigate this DNA-repellent effect by replacing negatively charged amino acids with positively charged analogues. We identified a glutamate moiety at position 755 of the human β-turn motif that is conserved among higher eukaryotes (Figure 7A) and inverted the charge of this particular side chain by substitution with lysine.
Figure 7.
Analysis of the dynamic interface by site-directed mutagenesis. (A) Identification of a conserved glutamate (arrow) in the β-turn motif of higher eukaryotes. This residue is not conserved in the Rad4 sequence, suggesting that the yeast orthologue may have different dynamic properties. (B) A single E755K mutation reduces the DNA-repellent effect of the β-turn structure. The association of XPC607−741, XPC607−766 and E755K607−766 with homoduplex DNA was compared at a polypeptide concentration of 150 nM, as outlined in the legend to Figure 6E. DNA binding is represented as the percentage of total input radioactivity captured by XPC fragments (n=6; error bars, standard deviation). A control reaction was carried out with empty beads. (C) FRAP analysis showing that, in undamaged cells, the nuclear mobility of the full-length E755K mutant is retarded relative to the wt control (n=12; error bars, standard error of the mean). (D) Host-cell reactivation assay showing that the E755K mutation confers a significant GGR defect. All results were corrected for the background activity in XP-C cells transfected with the GFP vector (n=5; error bars, standard deviation).
The consequence of this engineered charge inversion was first tested by comparing the interaction with native double-stranded DNA in biochemical assays. For that purpose, the lysine substitution was introduced into XPC607−766, thus generating a mutated fragment of 160 amino acids (E755K607−766) that, similar to its wild-type counterpart (XPC607−766), is amenable to expression and purification as a soluble polypeptide. DNA homoduplexes of 135 base pairs were used to determine the DNA-binding capacity of this mutated fragment in relation to the wild-type control. As illustrated in the comparison of Figure 7B, the E755K mutation was able to partially reverse the drop in DNA binding resulting from the presence of the β-turn structure in XPC607−766. Binding saturation studies with homoduplex DNA indicates that the association constant increased from 2.8 × 108 M−1 for XPC607−766 containing the wild-type sequence (determined in the earlier section) to 7.4 × 108 M−1 for the E755K607−766 derivative, which carries the single charge inversion.
These findings led us to generate a mutant GFP fusion construct to confirm that the effect of the β-turn structure in enhancing the XPC dynamics, observed with truncated derivatives (Figure 5B), is retained in the full-length protein context. Unlike other repair-defective XPC mutants (W531A, W542A, W690A, W690S and F733A), all of which display a higher nuclear mobility than the wild-type control (Hoogstraten et al, 2008 and data not shown), the novel E755K mutant is characterized by a strikingly reduced nuclear mobility (Figure 7C) accompanied by a significant GGR defect (Figure 7D). Collectively, these effects induced by a single site-directed mutation confirm that the dynamic properties of its minimal sensor surface, conferred by the β-turn structure, are critical for the ability of human XPC protein to act as a sensor of DNA damage.
Discussion
We elucidated the mechanism by which XPC protein scrutinizes DNA quality in living cells. The most outstanding finding is the identification of a two-stage discrimination process triggered by a dynamic sensor interface that detects DNA damage without the involvement of a prominent DNA-binding domain (BHD3), which was thought to represent the primary lesion recognition module on the basis of the Rad4 crystal structure (Min and Pavletich, 2007). The newly identified sensor interface serves to rapidly screen the double helix for the presence of unpaired bases, thus localizing damaged target sites that are amenable to the subsequent installation of an ultimate repair-initiating complex.
Dynamic molecular dialogue with the DNA double helix
According to the aforementioned Rad4 structure, the TGD region cooperates with BHD1 to associate with a portion of double-stranded DNA flanking the lesion (see Supplementary Figure 1). However, we observed that a large N-terminal segment (65% of the human sequence including most TGD sequences) has a stimulatory role, but is not directly required for the relocation of XPC protein to focus on DNA lesions (Figure 3). In the absence of this TGD segment, a strong interaction with the normal duplex is nevertheless mediated by the earlier described (Uchida et al, 2002) minimal DNA-binding fragment XPC607−741, which consists of BHD1 and BHD2 (Figure 6E). Instead, a longer fragment covering all three BHDs displays a comparably low affinity for the normal duplex (Figure 6G), indicating that the double-stranded DNA-binding activity of BHD1/BHD2 is opposed by the neighbouring BHD3 sequence. The further dissection of this critical XPC region revealed that a short β-turn extension of BHD3 is sufficient to mediate in part such an antagonistic effect (Figure 6F).
Several observations in living cells support the notion that the addition of this β-turn extension conveys a true gain of function rather than causing the destabilization of adjacent structural elements in the respective XPC constructs. First, XPC1−766 and XPC1−831 display a residual GGR function that is missing in the case of XPC1−741, which lacks the β-turn structure (Figure 4B). The fact that XPC1−766 and XPC1−831 exert a similarly low complementing activity is likely because of the absence of at least some components of the TFIIH-recruiting domain in their C-terminal region (Uchida et al, 2002). Second, a side-by-side comparison of the same C-terminal truncates shows that the enhanced nuclear mobility conferred by the β-turn structure (Figure 5B) correlates with a more efficient relocation to UV lesions (Figure 4E). Third, the nuclear mobility of XPC1−766, but not XPC1−741, is retarded by UV damage (Figure 5C and D), confirming that the former detects DNA lesions more effectively. Fourth, in living cells, the damage-induced accumulation of an earlier defined minimal DNA-binding fragment (XPC607−741) is strictly dependent on the presence of the β-turn structure (Figure 6B). Finally, the critical role of this dynamic β-turn subdomain is supported by a site-directed E755K substitution that reverts in part its DNA-repellent action. The increased affinity of this novel mutant for the native double helix results in decreased nuclear mobility and markedly reduced repair activity (Figure 7). According to the Rad4 structure, the critical position 755 maps to an amino-acid sequence that is in close contact with the DNA substrate (Min and Pavletich, 2007). Thus, our findings indicate that the β-turn structure displays both DNA-attractive and DNA-repulsive forces that dictate the dynamic interplay with duplex DNA such that, in the full genome context, this subdomain facilitates damage recognition by providing sufficient mobility to the XPC molecules searching for lesions.
Identification of a transient recognition intermediate
On binding to damaged substrates, XPC protein induces local DNA melting and kinking (Evans et al, 1997; Janicijevic et al, 2003; Mocquet et al, 2007). A structural basis for these rearrangements is again provided by the Rad4 crystal, in which the β-hairpin of BHD3 is inserted through the DNA duplex, causing two base pairs to entirely flip out of the double helix (see Supplementary Figure 1). In view of these features of the Rad4–DNA complex, it was unexpected to find that most of BHD3 including the protruding β-hairpin is actually not necessary to sense DNA damage in living cells. In fact, an XPC fragment that contains the β-turn structure, but is devoid of the remaining BHD3 sequence because of a truncation at position 766 (XPC1−766), accumulates in UV foci with remarkable efficiency (∼60% of the full-length control; Figure 4E), but without forming stable nucleoprotein complexes (Figure 5D). Similar to the W690S mutant, this truncated XPC1−766 derivative is even able to induce GGR activity (Figure 4B), although to moderate levels that are not sufficient to complement the repair defect of XP-C cells. A damage-specific accumulation of XPC1−766 was also detected in DDB2-deficient XP-E fibroblasts (Figure 4D and E) and V79 hamster cells (data not shown), thus excluding that the BHD3-independent relocation occurs in an indirect manner by association with UV-DDB. Finally, the conclusion that XPC protein forms a transient damage recognition intermediate without the involvement of BHD3 is supported by the finding that a small fragment (XPC607−766) consisting only of BHD1/BHD2 and the β-turn structure (together ∼15% of the human XPC sequence) still functions as a cellular DNA damage sensor (Figure 6B). This minimal sensor surface displays a binding preference for duplexes containing non-hydrogen-bonded bases, a generic feature of damaged DNA, and hence functions as a molecular caliper of thermodynamic base-pair stability.
A two-stage quality-control inspection
Although the BHD3 segment (residues 767–831) and its β-hairpin are not required to attract XPC protein to lesion sites, this additional domain favours the subsequent formation of stable nucleoprotein complexes, resulting in an immobile fraction of XPC protein in response to DNA damage (Figure 5E). The biochemical analysis of purified fragments shows that, unlike the BHD1/BHD2/β-turn minimal sensor, which displays a preference for duplexes with unpaired bases, BHD3 confers an exquisite selectivity for single-stranded DNA conformations (Figure 6G). In conjunction with the earlier mentioned Rad4 structure, these findings indicate that BHD3 does not participate in the early and transient recognition intermediate, but, instead, facilitates the subsequent stabilization of a repair-initiating complex using its single-stranded DNA-binding activity to encircle the undamaged strand across lesion sites.
To conclude, this is the first report providing evidence for a two-stage discrimination mechanism by which XPC protein carries out its versatile recognition function (Figure 8). This two-stage process obviates the difficulty of probing every genomic base pair for its susceptibility to undergo a BHD3-mediated β-hairpin insertion. Instead, the energetically less demanding search conducted by the dynamic BHD1/BHD2/β-turn interface is likely to precede more extensive BHD3-dependent structural adjustments. This initial search leads to the detection of non-hydrogen-bonded residues that are more prone than native base pairs to be flipped out of the double helix and, hence, become an interaction partner for the single-stranded DNA-binding activity of BHD3. A critical step of this two-stage quality-control process is the transition from an initially labile sensor intermediate to the more stable ultimate recognition complex. Two constitutive interaction partners of XPC protein, Rad23B and centrin 2, are thought to exert an accessory function not only by inhibiting XPC degradation, but also by stimulating its DNA-binding activity (Ng et al, 2003; Xie et al, 2004; Nishi et al, 2005). Such an auxiliary role is supported for Rad23B by the observation that XPC607−940, a fragment that fails to associate with Rad23B (Uchida et al, 2002), has a reduced DNA damage recognition capacity in living cells (Figure 3F). In addition, the two-step discrimination process identified in this study raises the possibility that Rad23B, centrin 2 or other binding partners may facilitate the installation of an ultimate XPC–DNA complex by lowering the energetic cost of critical nucleoprotein rearrangements required for the final β-hairpin insertion.
Figure 8.
Two-stage detection of DNA lesions by XPC protein. Model depicting the switch from a dynamic damage sensor intermediate to the ultimate recognition complex. (A) This study identifies a minimal sensor interface that rapidly scrutinizes base-pair integrity. This initial search, carried out by BHD1/BHD2 in conjunction with the β-turn structure, results in the formation of a labile nucleoprotein intermediate. (B) The single-stranded DNA-binding activity of BHD3 promotes the subsequent transition to a stable recognition complex by capturing extruded nucleotides in the undamaged strand.
Materials and methods
XPC constructs
The human XPC complementary DNA was cloned into pEGFP-N3 (Clontech) using the restrictions enzymes KpnI and XmaI. The same enzymes were used to generate the truncated XPC fragments. Primers for the insertion of restrictions sites and site-directed mutagenesis (QuickChange, Stratagene) are listed in the Supplementary Table II. All clones were sequenced (Microsynth) to exclude accidental mutations.
Cell culture
Simian virus 40-transformed human XP-C fibroblasts (GM16093) and untransformed XP-E fibroblasts (GM02415), derived from patients XP14BR and XP2RO, respectively, were purchased from the Coriell Institute for Medical Research (Camden, New Jersey, USA). The XP-C cells carry a homozygous C → T transition at position 2152 of the XPC sequence (Chavanne et al, 2000). The GM02415 cells carry a G → A transition in the DDB2 sequence generating an inactive R273H mutant that is not expressed to detectable levels (Nichols et al, 2000; Itoh et al, 2001). These fibroblasts, as well as V79 hamster cells deficient in UV-DDB activity (Tang et al, 2000) were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco), supplemented with 10% fetal calf serum (FCS), penicillin G (100 units ml–1) and streptomycin (100 μg ml–1). The cells were maintained at 37°C in a humidified incubator containing 5% CO2.
Transfections
One day before transfection, 600 000 cells were seeded into 6-well plates containing glass cover slips. At a confluence of 90–95%, the cells were transfected with 1 μg XPC-pEGFP-N3 (or truncated constructs) using 4 μl FuGENE HD reagent (Roche) and incubated for another 18 h. Expression of XPC polypeptides was monitored by western blotting (Maillard et al, 2007).
High-resolution DNA damage induction
The growth medium was replaced by phenol red-free DMEM (Gibco) supplemented with 10% FCS and 25 mM HEPES (pH 7.2). Single cells were irradiated with a femtosecond fibre laser (Träutlein et al, 2008) coupled to a confocal microscope (LSM Pascal, Zeiss) that generates pulses of 775 nm (duration 230 fs, repetition rate 107 MHz). The peak power density at the focal plane was 350 GW cm−2 and the pixel dwell time was 44.2 ms. Nuclei were irradiated along a single track or two intersecting lines. The area of each irradiation track was <10 μm2 and its volume <20 μm3.
By multiphoton excitation, three photons of low energy (775 nm wavelength) cause DNA lesions normally produced by the absorption of a single photon of higher energy (equivalent to 258 nm wavelength). Irradiation in the near-infrared range induces CPDs, (6-4) photoproducts and oxidative lesions (Lan et al, 2004; Dinant et al, 2007). In an earlier report (Meldrum et al, 2003), it has been calculated that three-photon irradiation with a peak power density of 350 GW cm−2 generates ∼7000 UV lesions in each treated cell. Taking into account our slightly modified parameters, we calculated that in this study, the same power density produced ∼5000 UV lesions along each linear 10-μm track.
Image analysis
Fluorescence measurements were carried out through a × 40 oil immersion objective lens with a numerical aperture of 1.4 (EC-Plan-Neo-Fluar, Zeiss) using an Ar+ source (488 nm). The selected parameters, including laser power and magnification factor, were kept constant throughout all experiments. To monitor the distribution of fluorescent fusions, at least 60 images were taken for up to 10 min after irradiation and analysed using the ImageJ software (http://rsb.info.nih.gov/ij). An initial-control image was taken immediately before damage induction. Signals were corrected for bleaching (http://www.embl-heidelberg.de/eamnet/html/body_bleach_correction.html) and cell movements (http://bigwww.epfl.ch/thevenaz/stackreg). For every time point, the average fluorescence intensities were measured in the area of accumulation and, as a background reference, in a neighbouring area of identical size. Finally, the background-corrected values were normalized to the mean intensity of the same nuclear region before irradiation.
Induction of UV foci
After removal of the culture medium, the cells were rinsed with phosphate-buffered saline (PBS), covered by a polycarbonate filter (Millipore) with 5-μm pores and irradiated using a UV-C source (254 nm, 100 J m−2). Subsequently, the filter was removed and the cells were returned to complete DMEM for 15 min at 37°C before paraformaldehyde fixation.
Immunocytochemistry
All wash steps and incubations were performed in PBS. At the indicated times after irradiation, cells were washed and fixed for 15 min at room temperature using 4% (v/v) paraformaldehyde. The cells were then permeabilized twice with 0.1% (v/v) TWEEN 20 for 10 min and DNA was denatured with 0.07 M NaOH for 8 min. Subsequently, the samples were washed five times with 0.1% TWEEN 20 and incubated (30 min at 37°C) with 20% FCS to inhibit unspecific binding. The samples were incubated (1 h at 37°C in 5% FCS) with primary antibodies (MBL International Corporation) directed against CPDs (TDM-2, dilution 1:3000) or (6-4) photoproducts (64M-2, dilution 1:1000). Next, the samples were washed with 0.1% TWEEN 20, blocked twice for 10 min with 20% FCS and treated with Alexa Fluor 546 dye-conjugated secondary antibodies (Invitrogen, dilution 1:400) for 30 min at 37°C. After washing with 0.1% TWEEN 20, the nuclei were stained for 10 min with Hoechst dye 33258 (200 ng ml–1). Finally, the samples were washed three times and analysed using an oil immersion objective.
GGR assay
Triplicate samples of XP-C fibroblasts, at a confluence of 90–95%, were transfected in a 6-well plate. The total amount of plasmid DNA (1 μg) included 0.45 μg pGL3 (UV irradiated at 1000 J m−2, coding for Photinus luciferase), 0.05 μg phRL-TK (unirradiated, coding for Renilla luciferase) and 0.5 μg of XPC-pEGFP expression vector. After 4 h, the transfection mixture was replaced by complete culture medium. After another 18 h, the cells were disrupted in 500 μl Passive Lysis Buffer (Promega). The lysates were cleared by centrifugation and the ratio of Photinus and Renilla luciferase activity was determined in a Dynex microtiter luminometer using the Dual-Luciferase assay system (Promega).
FRAP analysis
Protein mobility was analysed at high time resolution using a Leica TCS SP5 confocal microscope equipped with an Ar+ laser (488 nm, not inducing DNA lesions) and a × 60 oil immersion lens (numerical aperture of 1.4). The assays were performed in a controlled environment at 37°C and CO2 supply of 5%. A region of interest (ROI) covering 4 μm2 was photobleached for 2.3 s at 100% laser intensity. Fluorescence recovery within the ROI was monitored 200 times using 115-ms intervals followed by 30 frames at 250-ms and 10 frames at 500 ms. Simultaneously, a reference ROI of the same size was measured for each time point to correct for overall bleaching. All data were normalized to the prebleach intensity and the effective diffusion model (Sprague et al, 2004) was used to estimate diffusion coefficients (see Supplementary Table I).
DNA-binding assays
Full-length MBP-XPC fusions were expressed in Sf9 cells (Maillard et al, 2007). Insect cell lysates (5–20 μl) were incubated with 32P-labelled 135-mer oligonucleotides (4 nM) in 200 μl buffer A (25 mM Tris–HCl, pH 7.5, 0.3 M NaCl, 10% glycerol, 0.01% Triton X-100, 0.25 mM phenylmethane sulfonyl fluoride and 1 mM EDTA). After 1 h at 4°C, the reaction mixtures were supplemented with monoclonal antibodies against MBP linked to paramagnetic beads (0.2 mg, New England BioLabs). After another 2 h at 4°C, the beads were washed four times with 200 μl buffer A and the oligonucleotides associated with paramagnetic beads were quantified by liquid scintillation counting. All values were corrected for the background radioactivity resulting from unspecific binding to empty beads. The amount of immobilized XPC protein was controlled by denaturing gel electrophoresis.
GST-XPC607−741, GST-XPC607−766 and GST-K755E607−766 (expressed in Escherichia coli) as well as His-XPC607−831 (expressed in Sf9 cells) were purified as described (Uchida et al, 2002). The indicated concentrations of XPC fragments were incubated with radiolabelled 135-mer oligonucleotides (4 nM) in 200 μl buffer B (25 mM Tris–HCl, pH 7.5, 0.15 M NaCl, 10% glycerol, 0.01% Triton X-100, 0.25 mM phenylmethane sulfonyl fluoride and 1 mM EDTA). After 1 h at 4°C, the reaction mixtures were supplemented with glutathione-Sepharose (10 μl, Amersham) or Ni-NTA agarose beads (10 μl, Qiagen). After another 1 h at 4°C, the beads were washed twice with 200 μl buffer B and the immobilized oligonucleotides were quantified by liquid scintillation counting. All values were corrected for the background radioactivity resulting from unspecific binding to empty beads. To estimate binding constants, the data from saturation experiments (50–250 nM protein) were subjected to Scatchard analysis by plotting the ratio of bound and free XPC fragments as a function of the fraction of bound protein (Husain and Sancar, 1987). The double-stranded homoduplex or heteroduplex probes were obtained by hybridization of complementary 135-mers in 50 mM Tris–HCl (pH 7.4), 10 mM MgCl2 and 1 mM dithiothreitol. Equal amounts of each oligonucleotide were heated at 95°C for 10 min followed by slow cooling (3 h at 25°C).
Supplementary Material
Supplementary Information
Supplementary Movie 1
Supplementary Movie 2
Review Process File
Acknowledgments
We thank D Hermann, A Lenisa, M Träxler and M Vitanescu for excellent technical assistance and W Vermeulen for introduction into the FRAP technique. We also thank CT Craescu, A Scrima and N Thomä for critical reading and discussion of the manuscript. This work was supported by the Swiss National Science Foundation (grant 3100A0-113694), Oncosuisse (grant KLS-01827-02-2006), the German Research Foundation (grant DFG MA/2385/2-3) and the Ministry of Science, Research and the Arts of Baden-Württemberg.
References
- Andressoo JO, Hoeijmakers JH, Mitchell JR (2006) Nucleotide excision repair disorders and the balance between cancer and aging. Cell Cycle 5: 2886–2888 [DOI] [PubMed] [Google Scholar]
- Batty D, Rapic'-Otrin V, Levine AS, Wood RD (2000) Stable binding of human XPC complex to irradiated DNA confers strong discrimination for damaged sites. J Mol Biol 300: 275–290 [DOI] [PubMed] [Google Scholar]
- Bunick CG, Miller MR, Fuller BE, Fanning E, Chazin WJ (2006) Biochemical and structural domain analysis of xeroderma pigmentosum complementation group C protein. Biochemistry 45: 14965–14979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buterin T, Meyer C, Giese B, Naegeli H (2005) DNA quality control by conformational readout on the undamaged strand of the double helix. Chem Biol 12: 913–922 [DOI] [PubMed] [Google Scholar]
- Carreau M, Eveno E, Quilliet X, Chevalier-Lagent O, Benoit A, Tanganelli B, Stefanini M, Vermeulen W, Hoeijmakers JH, Sarasin A, Mezzina M (1995) Development of a new easy complementation assay for DNA repair deficient human syndromes using cloned repair genes. Carcinogenesis 16: 1003–1009 [DOI] [PubMed] [Google Scholar]
- Chavanne F, Broughton BC, Pietra D, Nardo T, Browitt A, Lehmann AR, Stefanini M (2000) Mutations in the XPC gene in families with xeroderma pigmentosum and consequenced at the cell, protein and transcription level. Cancer Res 60: 1974–1982 [PubMed] [Google Scholar]
- Cleaver JE (2005) Cancer in xeroderma pigmentosum and related disorders of DNA repair. Nat Rev Cancer 5: 564–573 [DOI] [PubMed] [Google Scholar]
- Cleaver JE, Thompson LH, Richardson AS, States JC (1999) A summary of mutations in the UV-sensitive disorders: xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy. Hum Mutat 14: 9–22 [DOI] [PubMed] [Google Scholar]
- Dinant C, de Jager M, Essers J, van Cappellen WA, Kanaar R, Houtsmuller AB, Vermeulen W (2007) Activation of multiple DNA repair pathways by subnuclear damage induction methods. J Cell Sci 120: 2731–2740 [DOI] [PubMed] [Google Scholar]
- Evans E, Moggs JG, Hwang JR, Egly JM, Wood RD (1997) Mechanism of open complex and dual incision formation by human nucleotide excision repair factors. EMBO J 16: 6559–6573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch ME, Nakajima S, Yasui A, Ford JM (2003) In vivo recruitment of XPC to UV-induced cyclobutane pyrimidine dimers by the DDB2 gene product. J Biol Chem 278: 46906–46910 [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T (2006) DNA Repair and Mutagenesis. Washington DC: ASM Press [Google Scholar]
- Gillet LC, Schärer OD (2006) Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem Rev 106: 253–276 [DOI] [PubMed] [Google Scholar]
- Hanawalt PC, Spivak G (2008) Transcription-coupled DNA repair: two decades of progress and surprises. Nat Rev Mol Cell Biol 9: 958–970 [DOI] [PubMed] [Google Scholar]
- Hoogstraten D, Bergink S, Verbiest VH, Luijsterburg MS, Geverts B, Raams A, Dinant C, Hoeijmakers JH, Vermeulen W, Houtsmuller AB (2008) Versatile DNA damage detection by the global genome nucleotide excision repair protein XPC. J Cell Sci 121: 2850–2859 [DOI] [PubMed] [Google Scholar]
- Houtsmuller AB, Rademakers S, Nigg AL, Hoogstraten D, Hoeijmakers JH, Vermeulen W (1999) Action of DNA repair endonuclease ERCC1/XPF in living cells. Science 284: 958–961 [DOI] [PubMed] [Google Scholar]
- Houtsmuller AB, Vermeulen W (2001) Mocromolecular dynamics in living cell nuclei revealed by fluorescence redistribution after photobleaching. Histochem Cell Biol 115: 13–21 [DOI] [PubMed] [Google Scholar]
- Huang JC, Hsu DS, Kazantsev A, Sancar A (1994) Substrate spectrum of human excinuclease: repair of abasic sites, methylated bases, mismatches and bulky adducts. Proc Natl Acad Sci USA 91: 12213–12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I, Sancar A (1987) Binding of E. coli DNA photolyase to a defined substrate containing a single T<>T dimer. Nucleic Acids Res 15: 1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Nichols A, Linn S (2001) Abnormal regulation of DDB2 gene expression in xeroderma pigmentosum group E strains. Oncogene 20: 7041–7050 [DOI] [PubMed] [Google Scholar]
- Janicijevic A, Sugasawa K, Shimizu Y, Hanaoka F, Wijgers N, Djurica M, Hoeijmakers JH, Wyman C (2003) DNA bending by the human damage recognition complex XPC-HR23B. DNA Rep 2: 325–336 [DOI] [PubMed] [Google Scholar]
- Kuraoka I, Bender C, Romieu A, Cadet J, Wood RD, Lindahl T (2000) Removal of oxygen free-radical-induced 5′,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc Natl Acad Sci USA 97: 3832–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumoto R, Masutani C, Sugasawa K, Iwai S, Araki M, Uchida A, Mizukoshi T, Hanaoka F (2001) Diversity of the damage recognition step in the global genomic nucleotide excision repair in vitro. Mutat Res 485: 219–227 [DOI] [PubMed] [Google Scholar]
- Lan L, Nakajima S, Oohata Y, Takao M, Okano S, Masutani M, Wilson SH, Yasui A (2004) In situ analysis of repair processes for oxidative DNA damage in mammalian cells. Proc Natl Acad Sci USA 101: 13738–13743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R, Peterson C (1992) Expression cloning of a human DNA repair gene involved in xeroderma pigmentosum group C. Nature 359: 70–73 [DOI] [PubMed] [Google Scholar]
- Lehmann AR (2003) DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie 85: 1101–1111 [DOI] [PubMed] [Google Scholar]
- Maillard O, Solyom S, Naegeli H (2007) An aromatic sensor with aversion to damaged strands confers versatility to DNA repair. PLoS Biol 5: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum RA, Botchway SW, Wharton CW, Hirst GJ (2003) Nanoscale spatial induction of ultraviolet photoproducts in cellular DNA by three-photon near-infrared absorption. EMBO Rep 4: 1144–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J-H, Pavletich NP (2007) Recognition of DNA damage by the Rad4 nucleotide excision repair protein. Nature 449: 570–575 [DOI] [PubMed] [Google Scholar]
- Mocquet V, Kropachev K, Kolbanovskiy M, Kolbanovskiy A, Tapias A, Cay Y, Broyde S, Geacintov NE, Egly JM (2007) The human DNA repair factor XPC-HR23B distinguishes stereoisomeric benzo[a]pyrenyl-DNA lesions. EMBO J 26: 2923–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moné JJ, Bernas T, Dinant C, Goedvree FA, Manders EM, Volker M, Houtsmuller AB, Hoeijmakers JH, Vermeulen W, van Driel R (2004) In vivo dynamics of chromatin-associated complex formation in mammalian nucleotide excision repair. Proc Natl Acad Sci USA 101: 15933–15937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J, Volker M, Kool H, Alekseev S, Vrieling H, Yasui A, van Zeeland AA, Mullenders LH (2005) The UV-damaged DNA binding protein mediates efficient targeting of the nucleotide excision repair complex to UV-induced photo lesions. DNA Rep 4: 571–582 [DOI] [PubMed] [Google Scholar]
- Ng JM, Vermeulen W, van der Horst GT, Bergink S, Sugasawa K, Vrieling H, Hoeijmakers JH (2003) A novel regulation mechanism of DNA repair by damage-induced and RAD23-dependent stabilization of xeroderma pigmentosum group C protein. Genes Dev 17: 1630–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols AF, Itoh T, Graham JA, Liu W, Yamaizumi M, Linn S (2000) Human damage-specific DNA-binding protein p48. J Biol Chem 275: 21422–21428 [DOI] [PubMed] [Google Scholar]
- Nishi R, Okuda Y, Watanabe E, Mori T, Iwai S, Masutani C, Sugasawa K, Hanaoka F (2005) Centrin 2 stimulates nucleotide excision repair by interacting with xeroderma pigmentosum group C protein. Mol Cell Biol 25: 5664–5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi A, Moné MJ, Houtsmuller AB, Hoogstraaten D, Vermeulen W, Heinrich R, van Driel R (2005) Mathematical modeling of nucleotide excision repair reveals efficiency of sequential assembly strategies. Mol Cell 19: 679–690 [DOI] [PubMed] [Google Scholar]
- Reardon JT, Sancar A (2006) Repair of DNA-polypeptide crosslinks by human excision nuclease. Proc Natl Acad Sci USA 103: 4056–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schärer OD (2007) Achieving broad substrate specificity in damage recognition by binding accessible nondamaged DNA. Mol Cell 28: 184–185 [DOI] [PubMed] [Google Scholar]
- Scrima A, Konickova R, Czyzewski BK, Kawasaki Y, Jeffrey PD, Groisman R, Nakatani Y, Iwai S, Pavletich NP, Thomä NH (2008) Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell 135: 1213–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague BL, Pego RL, Stavreva DA, McNally JG (2004) Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys J 86: 3473–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa K, Hanaoka F (2007) Sensing of DNA damage by XPC/Rad4: one protein for many lesions. Nat Struct Mol Biol 14: 887–888 [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Ng JM, Masutani C, Iwai S, van der Spek PJ, Eker AP, Hanaoka F, Bootsma D, Hoeijmakers JH (1998) Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol Cell 2: 223–232 [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Okamoto T, Shimizu Y, Masutani C, Iwai S, Hanaoka F (2001) A multistep damage recognition mechanism for global genomic nucleotide excision repair. Genes Dev 15: 507–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JY, Hwang BJ, Ford JM, Hanawalt PC, Chu G (2000) Xeroderma pigmentosum p48 gene enhances global gemomic repair and suppresses UV-induced mutagenesis. Mol Cell 5: 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träutlein D, Adler F, Moutzouris K, Jeromin A, Leitenstorfer A, Ferrando-May E (2008) Highly versatile confocal microscopy system based on a tunable femtosecond Er:fiber source. J Biophoton 1: 53–61 [DOI] [PubMed] [Google Scholar]
- Trego KS, Turchi JJ (2006) Pre-steady-state binding of damaged DNA by XPC-hHR23B reveals a kinetic mechanism for damage discrimination. Biochemistry 45: 1961–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida A, Sugasawa K, Masutani C, Dohmae N, Araki M, Yokoi M, Ohkuma Y, Hanaoka F (2002) The C-terminal domain of the XPC protein plays a crucial role in nucleotide excision repair through interactions with transcription factor IIH. DNA Rep 1: 449–461 [DOI] [PubMed] [Google Scholar]
- Xie Z, Liu S, Zhang Y, Wang Z (2004) Roles of Rad23 protein in yeast nucleotide excision repair. Nucl Acids Res 15: 5981–5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda G, Nishi R, Watanabe E, Mori T, Iwai S, Orioli D, Stefanini M, Hanaoka F, Sugasawa K (2007) In vivo destabilization and functional defects of the xeroderma pigmentosum C protein caused by a pathogenic missense mutation. Mol Cell Biol 27: 6606–6614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Masutani C, Maekawa T, Sugasawa K, Ohkuma Y, Hanaoka F (2000) The xeroderma pigmentosum group C protein complex XPC-HR23B plays an important role in the recruitment of transcription factor IIH to damaged DNA. J Biol Chem 275: 9870–9875 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Movie 1
Supplementary Movie 2
Review Process File