Abstract
A pathway for cytochrome c maturation (Ccm) in bacteria, archaea and eukaryotes (mitochondria) requires the genes encoding eight membrane proteins (CcmABCDEFGH). The CcmABCDE proteins are proposed to traffic haem to the cytochrome c synthetase (CcmF/H) for covalent attachment to cytochrome c by unknown mechanisms. For the first time, we purify pathway complexes with trapped haem to elucidate the molecular mechanisms of haem binding, trafficking and redox control. We discovered an early step in trafficking that involves oxidation of haem (to Fe3+), yet the final attachment requires reduced haem (Fe2+). Surprisingly, CcmF is a cytochrome b with a haem never before realized, and in vitro, CcmF functions as a quinol:haem oxidoreductase. Thus, this ancient pathway has conserved and orchestrated mechanisms for trafficking, storing and reducing haem, which assure its use for cytochrome c synthesis even in limiting haem (iron) environments and reducing haem in oxidizing environments.
Keywords: cytochrome, haem, quinone, redox
Introduction
Haem proteins such as haemoglobins that carry gases and cytochromes that carry electrons are required by virtually all organisms. Nevertheless, specific mechanisms by which the haem cofactor is delivered to these apoproteins are poorly understood (Hamza, 2006). In a few cases, haem (Quigley et al, 2004; Shayeghi et al, 2005; Rajagopal et al, 2008) or porphyrin (Krishnamurthy et al, 2006) transporters have been identified, but none have been purified or trapped with haem. The last step of haem synthesis is the insertion of iron (Fe2+) into porphyrin by ferrochelatase, which occurs in the prokaryotic cytoplasm and the mitochondrial matrix, possibly at the inner membrane (Dailey, 2002; O'Brian and Thony-Meyer, 2002).
Cytochromes c are essential for aerobic and anaerobic respiration and photosynthesis, and in eukaryotes, they additionally function as a signal for apoptosis (Moore and Pettigrew, 1990; Ow et al, 2008). As all cytochromes c are present outside the inner membrane of prokaryotes and the mitochondria, haem must cross the inner membranes to these sites of assembly. In cytochrome c, the haem is covalently attached to the protein and the protein folds into its native conformation only after the attachment. Two thioether bonds form between the two vinyl groups of haem and two cysteines in cytochrome c at a CXXCH motif, and accessory proteins that comprise assembly pathways are needed for this attachment (see below). Various approaches have shown that for thioether bond formation, the iron of haem must be reduced (Fe2+) (Nicholson and Neupert, 1989; Barker et al, 1993), but the mechanism(s) by which haem is reduced in vivo is unknown.
One major pathway to attach the haem is called the system I cytochrome c maturation (ccm) pathway (Figure 1), present in the plant and protozoal mitochondria, Gram-negative and other bacterial phyla, and archaea (Kranz et al, 1998; Thony-Meyer, 2002; Ferguson et al, 2008; Hamel et al, 2009). There have been significant genetic studies to define the eight genes involved in system I (ccmABCDEFGH), but other than the periplasmic haem chaperone, CcmE (Schulz et al, 1998; Uchida et al, 2004), none of these integral membrane proteins proposed to be involved in haem trafficking have been purified for biochemical characterization. The assembly occurs at the surface of the bacterial or mitochondrial inner membrane, with the suggestion that haem is trafficked along the pathway to the final attachment step. This trafficking is believed to allow haem attachment at very low haem levels (thus iron levels), suggesting high affinities and potential reservoirs for haem in pathway proteins (Feissner et al, 2006b; Richard-Fogal et al, 2007). In addition, because the periplasmic space of prokaryotes and the mitochondrial intermembrane space of many eukaryotes (e.g., plants) can be quite oxidizing environments, the mechanisms for haem redox (Fe2+/Fe3+) control may have evolved. Briefly, it is proposed that the pathway begins with CcmC, which has six transmembrane domains (TMDs), binding haem in a periplasmic tryptophan-rich ‘WWD domain' (Beckman et al, 1992; Goldman et al, 1998). CcmC is required for the transfer of haem to CcmE by an unknown mechanism, forming a covalent adduct between the 2-vinyl of haem and His130 of CcmE (called holoCcmE; see Figure 1A, labelled 1* and 2*) (Schulz et al, 1998, 1999; Stevens et al, 2003). To date, CcmC has not been purified, although it has been shown to be a part of the CcmABCD ABC transporter complex, which is required to release the holoCcmE from CcmC (Figure 1A, complexes 3 and 4*) (Feissner et al, 2006a). The CcmF/H complex, proposed to accept the haem from holoCcmE and act as the cytochrome c synthetase (Figure 1A, complexes 5* and 6), has been studied using only immunological and topological approaches (Goldman et al, 1998; Ren et al, 2002; Sanders et al, 2008) or yeast two hybrid system (Rayapuram et al, 2008), and it has not been biochemically characterized.
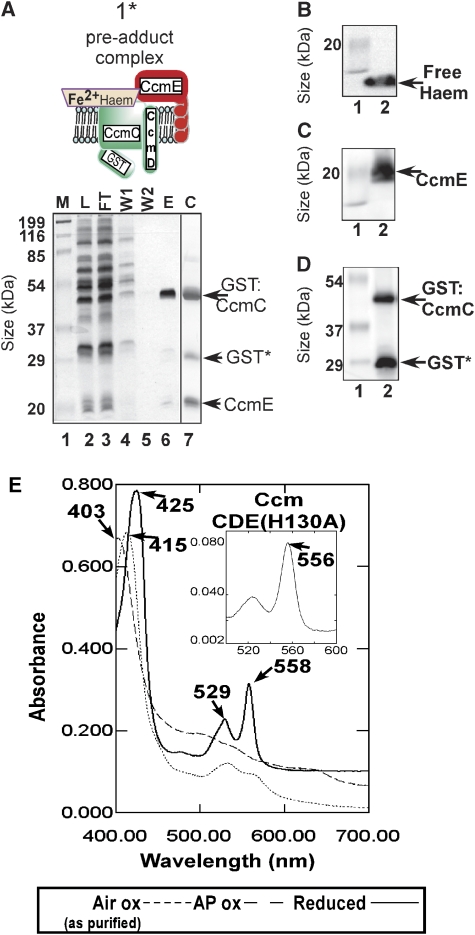
Figure 1.
A model of system I cytochrome c biogenesis pathway. All complexes with an asterisk (*) were purified and characterized in this study. (For simplicity, the Dsb and CcmG thiol redox components and the export of apocytochrome c by Sec are not shown.) (B) Coomassie and haem stain of purified GST:CcmCDE (A, 2*). Protein markers with indicated molecular masses are shown. (C, D) UV/visible absorption spectra of (C) purified holoCcmCDE post-adduct complex and (D) purified holoCcmE, under the conditions shown. Absorption maxima are indicated with arrows. AP, ammonium persulphate. Boxed insets are pyridine hemochromagen spectra.
To track haem, as it is delivered to apocytochrome c, we purify and characterize complexes in the system I pathway (Figure 1A, all complexes noted with * purified here). The aims were to establish which proteins provide axial ligands to the haem as it is trafficked, as well as the oxidation state of the haem iron throughout. By eliminating ccmA and ccmB, we trapped stable intermediates of the haem delivery to CcmE (Figure 1A, 1* and 2*). A purified holoCcmCDE complex (Figure 1A, 2*) contains the CcmE His130 adduct with oxidized haem (Fe3+). Haem in this holoCcmCDE complex is bound using periplasmic axial ligands His60 and His184 of CcmC, establishing that CcmC binds haem and delineating the ‘external haem-binding domain' of CcmC, clearly the active site for holoCcmE adduct formation. We purified a CcmCDE(H130A) ‘pre-adduct complex' that has reduced haem (Fe2+) (Figure 1A, 1*), providing evidence that the adduct formation in holoCcmE involves oxidation of the haem iron to the Fe3+ state (Figure 1A, 2*). Haem, CcmC and apoCcmE are mutually required to form the very stable intermediate complexes, which elucidates why an ATP-dependent CcmABCD-mediated release is necessary. Upon release, the histidine axial ligands from CcmC are switched to Tyr134 of CcmE (Uchida et al, 2004), whereas the purified holoCcmE remains oxidized (Fe3+) (Figure 1A, 4*). We purified the final complex for system I, the CcmF/H complex (Figure 1A, 5*), thus discovering that CcmF contains haem in an equimolar stoichiometry, which has never before been identified. CcmF, shown to have 13 TMDs, is therefore a cytochrome b, and we show that it acts in vitro as a quinol:haem oxidoreductase. A completely conserved histidine in TMD5 of CcmF is an axial ligand to this haem, suggesting a conserved quinol-mediated mechanism to reduce the haem for covalent attachment. Thus, CcmF is a multifunctional protein, acting as a haem reductase and a cytochrome c synthetase.
Results
Haem in the wild-type CcmCDE intermediate complex (post-adduct intermediate complex)
To date, a major obstacle in characterizing complexes in the system I pathway has been obtaining sufficient quantities of pure integral membrane proteins. We optimized expression to purify the intermediate CcmCDE complexes using the pGEX-based plasmid (pSysI-ΔccmAB) that contains a glutathione S-transferase (GST) fusion to ccmC and the remainder of the system I operon (ccmDEFGH). To test for functionality of GST-tagged CcmC, we used a system that relies on the correct assembly of recombinant holocytochrome c4 (from Bordetella pertussis), as described earlier (Feissner et al, 2006b). GST:CcmC is functional; upon ccmAB expression, holoCcmE is released and cytochrome c4 is synthesized (Supplementary Figure 1). Yields of purified GST:CcmC are ∼0.2 mg of >95% pure CcmCDE per liter of culture, and Coomassie stains show three polypeptides upon SDS–PAGE (Figure 1B). These include GST:CcmC (49 kDa), proteolyzed GST* (29 kDa) and CcmE (20 kDa). Co-purified CcmE is in the holo(haem-adduct) form, as detected using a chemiluminescent stain for haem (Figure 1B). Although CcmD (8 kDa) is not detectable using Coomassie staining, the 8-kDa CcmD polypeptide has been detected by silver staining and FLAG tagging (Richard-Fogal et al, 2008) (data not shown).
The UV/visible absorption spectrum of the purified GST:CcmCDE (Figure 1C) post-adduct complex shows that the haem is oxidized (Figure 1C) with a Soret maximum at 411 nm. (Note that all spectra are ±1 nm in resolution.) Upon reduction using sodium dithionite, a shift of 10 nm in the Soret (to 421 nm) and a β-maximum at 526 nm are observed (Figure 1C). Surprisingly, an unusual split α-peak is observed with maxima at 553 and 559 nm (Figure 1C, Supplementary Figure 2 for magnification). The split α is strikingly different than that of a His-tagged, ‘released' holoCcmE (Schulz et al, 1998; Enggist et al, 2003; Uchida et al, 2004), suggesting a distinct haem environment in the CcmCDE complex. We purified holoCcmE:6xHis (>90% pure, data not shown), confirming that it shows a single α-maxima at 556 nm (Figure 1D) when reduced. The unique split α in the holoCcmCDE complex could be due to an asymmetrical electronic distribution around the haem in a homogenous environment or due to two populations of haem, although all detectable haem using haem stain clearly is covalent to CcmE. Low-temperature (77 K) spectra on CcmCDE yielded the same split α, indicating that it did not induce one potential population of haem over the other (data not shown). We carried out pyridine extraction (Berry and Trumpower, 1987) and obtained spectra on the CcmCDE complex and released holoCcmE (Figure 1C and D, insets). These spectra yielded the same single α-maxima at 552 nm, indicating that the haem in the post-adduct complex is not a mixture of b-haem and adduct (to CcmE). It can be noted that 552 nm is a wavelength indicative of a single covalent bond, whereas the pyridine hemochromes of the horse heart cytochrome c (with two covalent bonds) has an absorption maxima at 550 nm and the horse myoglobin, a typical b-type haem, has an absorption maxima at 555 nm (Supplementary Figure 3). We suggest that the split α is the result of an asymmetrical electronic state provided by the unique environment.
To determine the oxidation state of the haem in both the CcmCDE complex and holoCcmE, we analyzed the air-oxidized (as purified) spectra. HoloCcmE is purified in the oxidized (Fe3+) form (Figure 1D). The addition of ammonium persulphate did not change the spectrum (Figure 1D), whereas sodium dithionite reduction yielded the characteristic α- and β-maxima and the shift in Soret (Figure 1D). We conclude that at least 95% of the released holoCcmE and CcmCDE intermediate complex (post-adduct; Figure 1C) is purified in the oxidized form. Trapping an intermediate in the ‘pre-adduct' form, with reduced (Fe2+) haem is described below.
CcmC provides the fifth and sixth axial ligands (His60 and His184) to the haem in the CcmCDE complex
Candidate ligands to haem in holoCcmCDE include the known ligand in CcmE, Tyr134, a conserved tyrosine in CcmD (Tyr17) and the two histidines flanking the WWD domain in CcmC (His60 and His184; Figure 2A, starred residues). To determine the axial ligands, we used a strain of E. coli Δccm (RK103) harbouring a pGEX-derived plasmid that contains only ccmCDE with a GST fusion to the N-terminus of ccmC. The purified WT–CcmCDE complex was >95% pure, contained only covalently bound haem (Supplementary Figure 4), was spectrally indistinguishable from the CcmCDE complex purified from overexpression using pSysI-ΔccmAB (data not shown), and consistently yielded three times more complex than with pSysI-ΔccmAB. Plasmid pccmCDE derivatives were used for overexpression of all CcmCDE with putative axial ligands mutated; these CcmCDE mutant complexes were >95% pure and contained covalently bound holoCcmE (Supplementary Figure 4). We initially changed Tyr134 of CcmE (pccmCDE(Y134A); Figure 2A). Ferguson's group showed that when Tyr134 of soluble holoCcmE* (i.e., no TMD) was mutated to phenylalanine, the reduced protein exhibited a 9-nm red shift in the Soret with no detectable α or β absorption, suggesting that Tyr134 is an axial ligand (Uchida et al, 2004). The CcmCDE(Y134A) complex showed the same air-oxidized spectrum as the WT–CcmCDE complex (Figure 2B). Ammonium persulphate treatment did not further oxidize the haem (Figure 2B), indicating that it is also purified in the oxidized state. The reduced sodium dithionite (Figure 2B) and pyridine hemochrome spectrum (Figure 2B, inset) are nearly identical to the WT–CcmCDE complex, including the distinct split α, indicating that Tyr134 is not an axial ligand to haem iron in the CcmCDE complex.
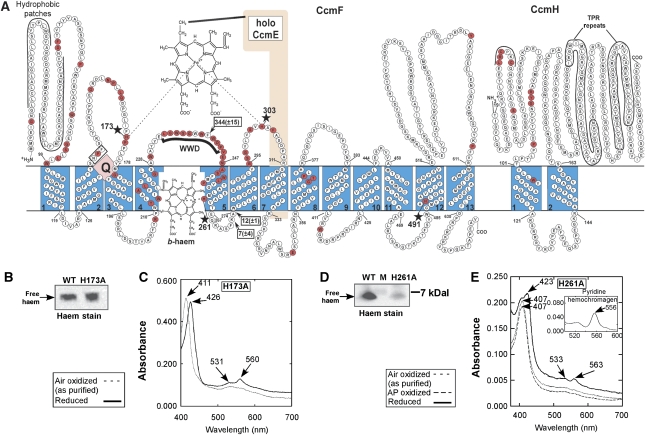
Figure 2.
E. coli CcmC, CcmD and CcmE membrane topologies; conserved amino acid residues; and spectra of mutant CcmCDE complexes. (A) On the basis of previously determined topologies (Goldman et al, 1998). Conserved residues (in red) were determined by protein alignments from representative organisms (Supplementary Table 1). The WWD domain of CcmC and haem with its iron axial ligands are shown. All potential axial ligands to haem iron in the CcmCDE complex are starred (in red). The site of covalent adduct formation with CcmE (H130) is green starred. (B–E) Absorption and pyridine hemochrome (insets) spectra of purified CcmCDE post-adduct complexes (Figure 1A, 2*) with mutations in the indicated residues; AP, ammonium persulphate.
We have shown earlier that the small polypeptide, CcmD, is a part of the CcmABC holoCcmE release complex, but CcmD is not required for the covalent adduct formation of haem from CcmC to CcmE, nor for the interaction of CcmE with the CcmABC complex (Feissner et al, 2006a; Richard-Fogal et al, 2008). However, CcmD is essential for the release of holoCcmE (Richard-Fogal et al, 2008). CcmD contains a conserved Tyr17 in its periplasmic N terminus (Figure 2A). Spectra of purified, reduced, and oxidized CcmCD(Y17A)E and of the pyridine hemochrome (Figure 2C) were nearly identical to the WT–CcmCDE complex. We conclude that Tyr17 of CcmD is not an axial ligand and CcmD Tyr17 is not necessary for the covalent attachment to CcmE.
We next mutated CcmC His60 and His184 (Figure 2A) to alanines. Purified CcmC(H60A)DE and CcmC(H184A)DE each possess ∼50% less haem when compared with WT–CcmCDE (Supplementary Figure 4). Spectral analyses of CcmC(H60A)DE and CcmC(H184A)DE (Figure 2D and E) complexes show significantly different absorptions than those of WT–CcmCDE complex. For the sodium dithionite reduced CcmC(H60A)DE complex, broad-β (536 nm) and α (558 nm) absorptions are present, but no split α or red shift (from oxidized) in the Soret peak are observed (Figure 2D). In addition, there is a difference of 8 nm in the oxidized Soret absorption peaks (419 versus 411 nm) compared with the WT–CcmCDE complex (Figure 2D), and ammonium persulphate oxidation did not change the air-oxidized spectrum (Figure 2D). The CcmC(H184A)DE complex showed similar spectral differences to CcmC(H60A)DE and, in addition, showed absorption at 654 nm (Figure 2E). The pyridine extractions of both CcmC(H60A)DE and CcmC(H184A)DE show α-peaks at 553 (Figure 2D and E, inset), indicating that the haem in each has one covalent bond (i.e., to CcmE), confirming the haem stain results. We conclude that His60 and His184 act as the fifth and sixth axial ligands to the haem iron in the CcmCDE post-adduct intermediate complex (see Discussion).
Trapping of a ‘pre-adduct' CcmCDE intermediate complex
His130 of CcmE has been shown to form the covalent bond with the β-carbon of the 2-vinyl group of haem (Schulz et al, 1998; Lee et al, 2005). Although we have defined the ‘active site' of the CcmC protein (i.e., His60 and His184) for the holoCcmE haem adduct formation, we aimed to analyse this haem-binding site without the covalent product (holoCcmE) and to test whether the covalent bond contributes to the split α-absorption, as well as to determine the haem oxidation state. However, we have been unable to detect haem in purified CcmC when it is overexpressed without CcmE, suggesting that CcmE is required for binding of haem to CcmC (CL Richard-Fogal and RG Kranz, unpublished). To capture a ‘pre-adduct' CcmCDE intermediate (i.e., before covalent haem attachment), we mutated CcmE His130, thus preventing covalent bond formation (see 1* in Figure 1A). The purified CcmCDE(H130A) pre-adduct complex is >95% pure by Coomassie stain of SDS–PAGE (Figure 3A, lane 7). No covalent haem (to CcmE) was detected in the purified GST:CcmCDE(H130A) complex, but upon SDS–PAGE analysis free haem was apparent at the dye front (Figure 3B, lane 2). CcmE was detected by western blot at 20 kDa (Figure 3C, lane 2) and both GST:CcmC and GST* were detected by GST western blot (Figure 3D, lane 2). Interestingly, spectra of the purified pre-adduct complex showed significant levels of reduced (Fe2+) haem, with β- and α-maxima at 529 and 558 nm, respectively (Figure 3E). Upon ammonium persulphate oxidation, a 12-nm blue shift in the Soret (415 to 403 nm) and a flattening of α- and β-peaks were observed (Figure 3E). Sodium dithionite completely reduced this complex with an α-peak of 558, typical of b-type haem and a Soret shift to 425 nm (Figure 3E). Comparison of the chemically reduced, air-oxidized and ammonium persulphate-oxidized spectra suggests that ∼50% of the CcmCDE(H130A) complex is purified in the reduced form, which explains why the Soret peak (415 nm) is intermediate to the fully oxidized (403 nm) and fully reduced (425 nm) forms. Pyridine extracts of this complex also confirm a b-type haem (Figure 3E, inset). The spectral results indicate that covalent attachment of haem to CcmE contributes to the split α observed with purified WT–GST:CcmCDE post-adduct complex. In addition, these results indicate that other CcmE interactions (besides His130) are necessary for the stable formation of the CcmCDE haem complex.
Figure 3.
Purification and spectral analysis of purified CcmCDE(H130A). (A) Coomassie stain of the GST purification of the CcmCDE(H130A) pre-adduct complex showing the load (lane 2), flow through (lane 3), wash 1 (lane 4), wash 2 (lane 5), elution (lane 6) and concentrated elution (lane 7). Protein markers in kDa are shown. (B–D) Haem stain showing free haem, anti-CcmE and anti-GST western blot analysis, respectively. (E) UV/Vis spectra and pyridine spectra (inset) of the complex under indicated conditions; AP, ammonium persulphate.
Purification of the E. coli CcmF/H cytochrome c synthetase complex: discovery that it contains b-haem (i.e., is a cytochrome b)
All post-adduct holoCcmE intermediates and released holoCcmE possess haem in the oxidized state (Fe3+). The next step in the pathway is proposed as the final step involving the CcmF/H complex; hence, we purified and characterized it. We added a hexahistidine tag to the C-terminus of CcmF. CcmF:6xHis was shown to be functional using synthesis of cytochrome c4 as the reporter (Supplementary Figure 5). Using antibodies to His6 and to CcmH, it was shown that CcmH co-purifies (37 kDa) on cobalt columns with a His6 reactive polypeptide (54 kDa) (Supplementary Figure 6). This is consistent with the recently published results of the Rhodobacter capsulatus CcmF/H, in which a complex was shown using the same immunological approach (Sanders et al, 2008). Purification of CcmF:6xHis was optimized to yield ∼0.5 mg of the CcmF/H complex per liter of culture. Upon SDS–PAGE, staining with Coomassie (Figure 4A) indicates at least 95% purity, with CcmF (Figure 4B) and CcmH (Figure 4C) as the major polypeptides. There is always more CcmF than CcmH, yet the stoichiometry is varied. In addition, the association seems to be salt dependent, suggesting that CcmH interaction with CcmF is based on weak ionic interactions.
Figure 4.
Purification, haem stain and UV/Vis absorption spectra of the CcmF/H complex. (A) Coomassie stain of CcmF:6xHis purification. The fractions are given at the top with crude sonicate (Cr; lane 1), L (load; 1% dodecyl maltoside solubilized membranes; lane 2), FT (flow through; lane 3), wash (lane 4), E (elution; lane 5), and M (molecular size standards); compare elution fraction with the load fraction. (B) His6 western and (C) CcmH western. (D) Haem stain of fractions of CcmF:6xHis purification with holoCcmE and free haem indicated. (E) UV/Vis spectra and pyrimidine hemochrome (inset) spectra of the purified CcmF/H complex, as indicated; AP, ammonium persulphate. (F–H) Coomassie stain of the purification of CcmF:6xHis from pSysI-ΔccmEGH, (G) haem stain and His6 western and (H) UV/Vis absorption spectra and pyridine hemochrome (inset) of purified CcmF:6xHis.
We noticed in all purified CcmF/H preparations a reddish colour, reminiscent of haem. Given the published interaction of holoCcmE with CcmF (Ren et al, 2002), we used antisera to CcmE to determine whether this was the source of haem. No CcmE was immunologically detected in the purified preparations (data not shown). Moreover, a haem stain of the preparations upon SDS–PAGE did not show holoCcmE in pure fractions (Figure 4D, lanes 7–9), whereas it is easily detectable in the crude extracts (Figure 4D, lanes 1–3). However, there is haem in the pure CcmF/H fractions that upon SDS–PAGE migrates with the dye front, suggestive of free haem (Figure 4D, lanes 7–9).
To characterize further the properties of the haem in the CcmF/H complex, room temperature and low-temperature absorption spectra were recorded, both yielding similar profiles. The room temperature spectra (Figure 4E) of the CcmF/H complex show a Soret maxima at 411 nm, even upon oxidation with ammonium persulphate. Upon reduction with sodium dithionite, the Soret maximum shifts from 411 to 427 nm and an α-absorption at 560 nm and β at 531 nm appear. These are characteristics of b-haem in a cytochrome b, as also indicated by a reduced minus oxidized spectra (Figure 4E). A pyridine hemochrome analysis indicates the characteristic absorptions of b-type haem (Figure 4E, inset). To verify that this haem is associated with the CcmF/H complex, gel filtration in 0.1% dodecyl maltoside on a Sephacryl S-200 column was carried out (Supplementary Figure 7). Fractions were stained for protein (Supplementary Figure 7A) and probed with His6 antibodies (Supplementary Figure 7B), CcmH antibodies (Supplementary Figure 7C), and for haem (Supplementary Figure 7D), showing that CcmF, CcmH and haem all co-purify as a complex.
The b-haem is present in CcmF and it does not arise from holoCcmE
To establish unambiguously that the b-haem is in CcmF (not CcmH) and show that it did not come from CcmE (i.e., inserted from holoCcmE prior to ligation), we purified the CcmF:6xHis-tagged protein expressed from three different plasmids. The first was deleted of ccmH, in which the same levels of haem and spectral properties were observed in purified CcmF devoid of CcmH (data not shown). The second construct was deleted of ccmE and ccmH. Purification of the CcmF:6xHis protein yielded a single polypeptide fragment detected using Coomassie (Figure 4F) and His6 western analysis (Figure 4G), and free haem is detected (Figure 4G). The spectra were identical to the CcmF/H complex (Figure 4H). These results prove that the b-haem is associated with CcmF and that it is not derived from holoCcmE. This has been confirmed by overexpressing CcmF/H from an arabinose-inducible promoter in pBAD, and this preparation possessed b-haem with identical spectral properties (Supplementary Figure 8).
Stoichiometry of the b-haem of CcmF
To determine the stoichiometry of haem:CcmF/H complex, we carried out two types of haem quantitative analyses (Supplementary Table 2). On account of the variable CcmF/H ratios, all calculations are based on the CcmF polypeptide levels in preparations that are at least 90% pure. Initially, we used the pyridine extractions. Although the pyridine hemochrome method yielded ∼0.7 moles of haem per mole CcmF, precipitation of the preparation was problematic for haem quantification, and 0.7 is an underestimate of the levels of haem. As a complementary approach for quantifying haem, we used a method that depends on a haem standard curve and the alkali/Triton X-100/methanol extraction (Pandey et al, 1999). This method does not precipitate the CcmF/H preparation, and was performed on four different preparations (Supplementary Table 2). This method yielded 1.2±0.2 moles of haem per mole of CcmF. We conclude that the CcmF/H complex contains a stable, reducible b-haem in a stoichiometry of 1:1 (haem:CcmF), and thus it can be considered a b-type cytochrome.
Topology of CcmF and H and prospective analysis of the potential axial ligands to the b-haem and the haem from holoCcmE
The discovery of a b-haem in the CcmF/H complex raises the question of what axial ligands in CcmF bind the b-haem iron, and importantly what is its function. To address these questions, we first reinvestigated the topological profiles of CcmF and CcmH (Figure 5A). The conserved residues in CcmF and CcmH are displayed in red, including potential axial ligands to haem (Figure 5A, stars). The topology of two CcmF proteins have been previously analyzed using phoA and lacZ fusions, one using the R. capsulatus ccmF (ccl1) gene (Goldman et al, 1998) and the other using the Rhodobacter sphaeroides gene (Rios-Velazquez et al, 2003). In each case, 11 TMDs were experimentally suggested, although current membrane topology programmes predict 15 TMDs (Lee et al, 2007). For the E. coli CcmF diagrammed in Figure 5A, the first two computer-predicted TMDs are labelled as ‘hydrophobic patches' because experimentally, a phoA fusion between these two patches yielded a high alkaline phosphatase activity, suggestive of a periplasmic location (Goldman et al, 1998; Rios-Velazquez et al, 2003). Another computer-predicted TMD5 (in Figure 5A) was not previously tested with a phoA or lacZ fusion in the loop between TMD5 and TMD6; hence, TMD5, the loop, and TMD6 were placed in the periplasm in the previous 11 TMD diagrams. We constructed and analyzed phoA fusions at two different residues (Lys273 and Ala274) in the third cytoplasmic loop between TMD5 and TMD6. These were compared with a proven periplasmic loop comprising the WWD domain, in which a phoA fusion after Phe241 was constructed. Results indicate that the Phe241 phoA fusion was at least 20 times higher in alkaline phosphatase activity than the Lys273 or Ala274 fusions (data displayed on Figure 5A at these residues). We conclude that TMD5 and TMD6 are present and the intervening loop is cytoplasmic. Importantly, this brings a completely conserved histidine (His261) into TMD5, and it leaves another histidine (His303) in a periplasmic loop that is adjacent to the WWD domain. There are four completely conserved histidines in CcmF that may be important as haem ligands to holoCcmE and/or the b-haem (see below).
Figure 5.
E. coli CcmF and CcmH membrane topologies along with purified CcmF(H173A) and CcmF(H261A) complexes. (A) The conserved residues (red) were determined by protein alignments of representative proteobacterial organisms; in addition, the four His residues from CcmF and the WWD domain are conserved in Oryza sativa, Triticum aestivum, Arabidopsis thaliana and Tetrahymena thermophila (Supplementary Table 1). The position of holoCcmE haem molecule is shown at the conserved WWD domain with the iron axial ligands provided by His173 and His 303. Potential iron axial ligands are starred. The position of the b-haem and iron axial ligand His 261 are shown along with the potential quinone binding site (Q) boxed NPF. Hydrophobic patches (underlined), TPR repeats (circled), locations of phoA fusions (arrows), and alkaline phosphatase activities (boxed) are shown. (B) Haem stain and (C) UV/vis spectra of CcmF(H173A), as indicated. (D) Haem stain and (E) UV/visible spectra of CcmF (H261A), as indicated; AP, ammonium persulphate.
An axial ligand for the b-haem is provided by His261 in TMD5 of CcmF
It is commonly accepted but not proven that holoCcmE docks onto CcmF, whereby the haem is bound in the WWD domain (see Figure 5A). Two conserved histidines (His173 and His303) in the two periplasmic domains flanking the WWD domain could provide the axial ligands to this haem, thus positioning it in the active site that will accept the CXXCH motif of apocytochrome c (from CcmH). We focus here on His173, the putative axial ligand for haem from holoCcmE, and His261 in TMD5, with unknown function but potentially an axial ligand to the b-haem in CcmF. We mutated each to an alanine and purified these CcmF/H complexes. Each of the CcmF mutants was not functional for cytochrome c synthesis (data not shown), and the CcmF proteins from each were undegraded and purified with similar yields (not shown). Purified CcmF/H complex with the first mutated histidine (H173A) yielded similar haem stoichiometries (Figure 5B, Supplementary Table 2) and spectra to wild-type CcmF (Figure 5C); therefore, His173 is not (or is unlikely to be) a ligand to the b-haem. However, CcmF(H261A)H possessed at least four times less haem than wild-type by haem stain (Figure 5D) and haem quantitative analyses (Supplementary Table 2). CcmH still co-purifies with CcmF(H261A)H, indicating that the proper protein–protein interactions are maintained, thus suggesting that CcmF(H261A)H is properly folded. Moreover, the oxidized and reduced spectra of CcmF(H261A)H show major differences to wild-type (Figure 5E), including a red shift of 3 nm in the reduced α-maxima (560–563 nm). In the oxidized Soret region, CcmF(H261A) shows an absorption at 407 nm rather than at 411 nm. The reduced form shows shoulders at 407 and 423 nm in the Soret; we cannot fully reduce this haem before the protein precipitates with sodium dithionite, thus the ratio of these two maxima vary from spectra to spectra. On account of the lower levels of b-haem and absorption differences, we conclude that His261 in TMD5 of CcmF is an axial ligand to the b-haem.
The b-haem of CcmF is reduced by quinols, thus it functions in vitro as a quinol:haem oxidoreductase
An obvious suggestion for the role of b-haem of CcmF is to reduce the iron (Fe3+ to Fe2+) of holoCcmE before covalent bonding to the CXXCH of apocytochromes c. The proximity of this b-haem (see His261 in TMD5 in Figure 5A) to the WWD domain is consistent with this idea. What provides the in vivo reductant for this b-haem? As quinones are central to nearly all electron transport chains, they are membrane bound and poised at intermediate redox potentials, we tested whether purified CcmF is reduced by various quinones. The CcmF cytochrome b is reduced by ubiquinol 1 (Figure 6). Upon the addition of ubiquinol 1 (CoQ1) and DTT, spectra show a gradual Soret shift from 411 to 425 nm and the appearance of the typical α-absorption at 560 and β at 531 nm, all indicating reduction by CoQ1. In this assay, DTT is used to maintain the quinols in the reduced state, but DTT alone is unable to reduce the b-haem (Figure 6). Dimethylquinone was also able to reduce CcmF, although it was more rapidly re-oxidized under the ambient air conditions used (data not shown). We note that CcmF contains a region (-NPF) that is similar to the known quinone binding site of RegB (Swem et al, 2006) and that the location of this region is in close proximity to the b-haem of CcmF (labeled ‘Q' in Figure 5A).
Figure 6.
Coenzyme Q-1 reduction of the purified CcmF/H complex. Time points for spectra after indicated additions are shown. The positions of the α, β and Soret absorption maxima are shown (arrows) with the vertical dashed line showing the beginning position of complete oxidation.
Discussion
The system I cytochrome c biogenesis proteins CcmC and CcmF (and the system II CcsA protein) are the three members that form a superfamily of proteins recently called the ‘haem handling proteins' (HHP; Lee et al, 2007). This superfamily is characterized by the highly conserved tryptophan-rich WWD domain (Beckman et al, 1992), which is flanked by conserved histidine residues. These proteins are proposed to function in haem delivery, but a system I HHP has never been purified or shown to contain haem. Here, we show by purification and characterization of the CcmC protein, its interaction with haem, thus validating the HHP designation for this superfamily of proteins.
The purified WT–CcmCDE complex possesses haem that shows an exceptional split α-absorption upon reduction (maxima at 553 and 559 nm; spectra summarized in Supplementary Table 3). The split α-absorption peak is due to a unique haem environment, contributions from which are shown to include CcmC His60 and His184 as iron axial ligands and the CcmE His130 as a covalent adduct. The CcmC(H60A)DE and CcmC(H184A)DE complexes show significantly different absorption spectra than the WT–CcmCDE. Similar differences in absorption spectra (i.e., red shift in Soret and α-peak maxima) have been observed with axial ligand mutants of cytochrome b5 (Wang et al, 2003), cytochrome b562 (Oyedotun et al, 2004) and others (Moore and Pettigrew, 1990; Ito-Maki et al, 1995). Our data indicate that this haem is present in an ‘external haem-binding domain' in CcmC consisting of the tryptophan-rich ‘WWD domain' (proposed) and the proven flanking histidine residues (His60 and His184; see Figure 2A). Released ferric holoCcmE has iron axial coordination to Tyr134 and an unidentified ligand (Uchida et al, 2004; Garcia-Rubio et al, 2007). Therefore, the release of holoCcmE from CcmC by the ABC transporter, CcmABCD complex, must require the axial ligands to be switched from those in CcmC to those in CcmE. It can be noted that both CcmC(H60A)DE and CcmC(H184A)DE complexes still contain the CcmE adduct (holoCcmE), although at approximately 50% lower levels. These CcmC mutants are partially functional (Ren and Thony-Meyer, 2001); hence, we conclude that the holoCcmE can still be released from these intermediates, and once released are functional. All intermediate CcmCDE/haem complexes are remarkably stable, with no detectable spectral changes over months at 4°C. Such stability reinforces the importance of the release step.
Haem binding to the CcmC ‘external haem-binding domain' is only detected when CcmE is present and always with complete adduct formation (when His130 is present), consistent with a rapid formation of holoCcmE. Determinants on apoCcmE were required to form the stable CcmCDE:haem intermediate complex that did not involve CcmE His130 because a stable pre-adduct intermediate (CcmCDE(H130A)) was purified and characterized. We suggest that other residues in the soluble domain of CcmE, possibly hydrophobic residues as have been proposed (Enggist et al, 2003; Harvat et al, 2009), interact with the haem that is liganded by CcmC. As CcmD is not essential to form holoCcmE but is for the release (Richard-Fogal et al, 2008), we conclude that a stable CcmC:haem:CcmE ternary complex forms with residues in CcmC (e.g., His60 and His184) and CcmE contributing to complex formation. Recently, we purified another member of the HHP superfamily, CcsBA, which represents the system II cytochrome c synthetase. We have shown that CcsBA binds haem in its WWD domain (‘external haem-binding domain') through analogous, periplasmic histidine axial ligands (Frawley and Kranz, 2009). However, CcsBA binds haem independent of other accessory proteins. Clearly, the CcmC and CcsA proteins show similarities in topology, the WWD domain and the now proven functions of external axial histidine ligands. The co-evolution of CcmE with CcmC to form haem-binding determinants is unexpected, but certainly reflects the distinct mechanisms operating in the system I pathway.
In the case of CcsBA, which is the system II cytochrome c synthetase (Beckett et al, 2000; Feissner et al, 2006b), haem vinyl groups are directly attached to the CXXCH of apocytochrome c. For the CcmCDE complex, the acceptor is the CcmE His130 residue. As would be predicted, all the haem in purified CcsBA is in the reduced (Fe2+) state, mutations in the flanking histidine ligands are completely non-functional and these mutant CcsBA proteins have oxidized haem (Frawley and Kranz, 2009). Much of the haem in the CcmCDE pre-adduct complex is reduced, but all haem present in the CcmCDE post-adduct complexes and holoCcmE is oxidized (Fe3+). Allen et al (2005) and Lee et al (2005) have discussed the anti-Markovnikov chemistry underlying His130 adduct formation but the oxidation state of the haem has received little attention. Both reports suggest the reaction would be consistent with a free radical mechanism (of the His130 imidazole group). The alternative is a Michael addition (Lewis base reaction) that would prefer Fe3+ haem (J Taylor, personal communication). In vitro studies with CcmE* by the Ferguson group indicate that reduced haem is necessary for adduct formation, leading them to favour the imidazole radical mechanism (Daltrop et al, 2002). In either mechanism, an unidentified oxidant would be needed to catalyze formation of the imidazole radical or the Fe3+ haem intermediates, noting that addition of imidazole to the haem is redox neutral (J. Taylor, personal communication). Although our results with pre-adduct and post-adduct complexes favour a free radical mechanism with reduced haem (Fe2+) as a substrate, it is clear that Fe3+ haem is the major intermediate in the trafficking pathway.
Purification of the CcmF/H complex in milligram quantities led to the discovery of a b-type haem. This haem is shown not to arise from holoCcmE, which is the haem moiety that is covalently attached to the CXXCH of apocytochromes c at the CcmF/H active site (Ahuja and Thony-Meyer, 2003; Feissner et al, 2006b). Rather, the b-haem is a stable component of the CcmF protein in a 1:1 (molar) stoichiometry. Topological and mutational studies here and previously indicate that CcmF has 13 TMDs with four completely conserved and essential histidines (Figure 5A). Two conserved histidines (His173 and His303) are in the external (p-side), periplasmic domain, flanking the highly conserved WWD domain, which is proposed to bind haem from holoCcmE. Consistent with this, the purified CcmF(H173A)H complex had wild-type levels and spectroscopic properties of b-haem, yet is non-functional. We show evidence that His261 in TMD5 acts as an axial ligand to the b-type haem, as fourfold less haem is detected in the purified CcmF(H261A)H complex, and the spectra are significantly altered compared with wild-type and the CcmF(H173A)H complex.
The b-haem in the CcmF/H complex (and in CcmF without CcmH) is reduced by ubiquinol 1 and by dimethylquinone. We note that a periplasmic loop at the TMD/p-side interface has a motif (sequence -NPF-) similar to a known quinone-binding region (in RegB; Swem et al, 2006) and this motif is near the His261-liganded b-haem (see ‘Q' in Figure 5A). Thus, we tentatively assign this motif as the quinol oxidation site in CcmF. The data indicate that CcmF functions in vitro as a quinol:haem oxidoreductase, accepting electrons from quinol to reduce holoCcmE through the b-haem. The proximity of b-haem to the holoCcmE haem (in the WWD domain) is suggested by the location of His261 in TMD5 (see Figure 5A).
We show that the intricate system I cytochrome c biogenesis pathway has evolved complex but elegant mechanisms to deliver, oxidize, release, store and penultimately reduce haem before it is covalently attached to the apocytochrome c polypeptide (Figure 1A). Thus, the final CcmF/H complex is even more complicated than originally envisioned, functioning as a cytochrome c synthetase and as a quinol:haem oxidoreductase. The conservation of His261 in CcmF and other residues noted here among all plants and the primitive mitochondria, Gram-negative bacteria and archaea, suggest that these mechanisms in system I cytochrome c biogenesis are ancient and highly conserved.
Materials and methods
Bacterial strains and growth conditions
The E. coli strains used in this study are listed in Supplementary Table 4 and were grown aerobically, with shaking at 300 r.p.m. in Luria–Bertani broth (LB; Difco) at 37 °C. Concentrations of antibiotic (Sigma-Aldrich) and other compounds were carbenicillin 50 μg ml−1 and chloramphenicol 20 μg ml−1, isopropyl-β-D-thiogalactopyranoside (Gold Biotechnology) 1 mM and arabinose (Sigma-Aldrich) 0.2% (wt/vol).
Construction of plasmids
All plasmid constructions were by previously described (Richard-Fogal et al, 2008) or are described in detail in Supplementary data. Oligonucleotides and plasmid constructs are given in Supplementary Table 4.
Membrane preparation and protein purification
E. coli cultures were grown in LB with the appropriate antibiotics at 37 °C to an OD600 of ∼1.8 and induced with 1 mM IPTG (or 0.2% arabinose for pBADccmE:6xHis and pBADccmF:6xHisGH) for 14–16 h. Cells were harvested at 9000 g for 10 min, resuspended in either 1 × GST buffer (4.3 mM Na3HPO4, 1.47 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl (pH 7.3)) or a modified 1 × Talon (Clontech) binding buffer (20 mM Tris-HCl (pH 7.9), 100 mM NaCl), treated with 1 mg−1 egg white lysozyme (Sigma-Aldrich) for 20 min and sonicated at 50% duty, 80% output on a Branson 250 sonicator. Membrane proteins were isolated at 207 000 g for 75 min, resuspended in either 1 × GST buffer or modified 1 × Talon binding buffer and solubilized in 1% dodecyl maltoside as described previously (Feissner et al, 2006a). Solubilized membranes were purified over GST Bind resin (Novagen-EMD Biosciences) or Talon resin (Clontech) as per the manufacturers' recommendations, with the exception of the modified 1 × Talon binding buffer.
Haem quantification and protein purity determination
The haem concentration in the different protein preparations was determined by at least one of the following methods: (1) pyridine extraction as described by Berry and Trumpower (1987), (2) triton–methanol extraction as described by Pandey et al (1999) and (3) chemiluminescent haem stain as described by Richard-Fogal et al (2007). Protein purity was assessed by scanning dried Coomassie-stained SDS–PAGE and determining the relative concentration of polypeptides with the ImageJ software package (Rasband, 1997-2008).
Other methods
Haem stains and western blots were performed on un-boiled samples suspended in 6 × SDS–PAGE dye without DTT as described previously (Feissner et al, 2006a). Antibodies were used at the following dilutions: anti-CcmE (1:5000; Feissner et al, 2006b) anti-GST (1:5000; Sigma-Aldrich) and anti-His (1:5000; Santa Cruz Biotechnology). Protein A peroxidase (Sigma-Aldrich) was used as the secondary label. Chemiluminescence was developed using the SuperSignal Femto kit (Pierce) and detected in an LAS-1000plus detection system (Fujifilm). Protein concentrations were determined using the BCA assay (Pierce) with bovine serum albumin as a standard. Absorption spectra were obtained as described previously (Feissner et al, 2006b) on a UV2101PC scanning spectrophotometer (Shimadzu). Coenzyme Q1 (Sigma-Aldrich) and dimethylquinone (Sigma) reductions were carried out as previously described by Swem et al (2006) with the following exception for the CoQ1 reduction, all manipulations with purified protein complex were carried out under air or nitrogen.
Supplementary Material
Supplementary Materials and methods
Review Process File
Acknowledgments
We thank Robert Blankenship (Washington University, St Louis) for use of equipment; Aaron Collins for technical assistance on low-temperature spectra; Alison Goldberger for optimizing the purification of His-tagged CcmE, Andrew Mutka for CcmC:6xHis purifications; Joe Argus and Silvano Ciani for optimizing the purification of His-tagged CcmF; and Dewey Holten, John Taylor; and Robert Blankenship (Washington University, St Louis) for helpful discussions. The author contributions were as follows: Cindy Richard-Fogal worked on CcmCDE, CcmE and CcmF; Elaine Frawley worked on CcmE; Eric Bonner worked on CcmF; Huifen Zhu worked on CcmF; Brian San Francisco worked on CcmF; and Robert Kranz supervised experimental design, data analysis and paper preparation. This study was funded by National Institutes of Health Grant GM47909 to RGK.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahuja U, Thony-Meyer L (2003) Dynamic features of a heme delivery system for cytochrome C maturation. J Biol Chem 278: 52061–52070 [DOI] [PubMed] [Google Scholar]
- Allen JW, Barker PD, Daltrop O, Stevens JM, Tomlinson EJ, Sinha N, Sambongi Y, Ferguson SJ (2005) Why isn't ‘standard' heme good enough for c-type and d1-type cytochromes?. Dalton Trans 21: 3410–3418 [DOI] [PubMed] [Google Scholar]
- Barker PD, Ferrer JC, Mylrajan M, Loehr TM, Feng R, Konishi Y, Funk WD, MacGillivray RT, Mauk AG (1993) Transmutation of a heme protein. Proc Natl Acad Sci USA 90: 6542–6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett CS, Loughman JA, Karberg KA, Donato GM, Goldman WE, Kranz RG (2000) Four genes are required for the system II cytochrome c biogenesis pathway in Bordetella pertussis, a unique bacterial model. Mol Microbiol 38: 465–481 [DOI] [PubMed] [Google Scholar]
- Beckman DL, Trawick DR, Kranz RG (1992) Bacterial cytochromes c biogenesis. Genes Dev 6: 268–283 [DOI] [PubMed] [Google Scholar]
- Berry EA, Trumpower BL (1987) Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem 161: 1–15 [DOI] [PubMed] [Google Scholar]
- Dailey HA (2002) Terminal steps of haem biosynthesis. Biochem Soc Trans 30: 590–595 [DOI] [PubMed] [Google Scholar]
- Daltrop O, Stevens JM, Higham CW, Ferguson SJ (2002) The CcmE protein of the c-type cytochrome biogenesis system: unusual in vitro heme incorporation into apo-CcmE and transfer from holo-CcmE to apocytochrome. Proc Natl Acad Sci USA 99: 9703–9708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enggist E, Schneider MJ, Schulz H, Thony-Meyer L (2003) Biochemical and mutational characterization of the heme chaperone CcmE reveals a heme binding site. J Bacteriol 185: 175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feissner RE, Richard-Fogal CL, Frawley ER, Kranz RG (2006a) ABC transporter-mediated release of a haem chaperone allows cytochrome c biogenesis. Mol Microbiol 61: 219–231 [DOI] [PubMed] [Google Scholar]
- Feissner RE, Richard-Fogal CL, Frawley ER, Loughman JA, Earley KW, Kranz RG (2006b) Recombinant cytochromes c biogenesis systems I and II and analysis of haem delivery pathways in Escherichia coli. Mol Microbiol 60: 563–577 [DOI] [PubMed] [Google Scholar]
- Ferguson SJ, Stevens JM, Allen JW, Robertson IB (2008) Cytochrome c assembly: a tale of ever increasing variation and mystery? Biochim Biophys Acta 1777: 980–984 [DOI] [PubMed] [Google Scholar]
- Frawley ER, Kranz RG (2009) CcsBA is a cytochrome c synthetase that also functions in heme transport. Proc Natl Acad Sci USA 106: 10201–10206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rubio I, Braun M, Gromov I, Thony-Meyer L, Schweiger A (2007) Axial coordination of heme in ferric CcmE chaperone characterized by EPR spectroscopy. Biophys J 92: 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman BS, Beck DL, Monika EM, Kranz RG (1998) Transmembrane heme delivery systems. Proc Natl Acad Sci USA 95: 5003–5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel P, Corvest V, Giege P, Bonnard G (2009) Biochemical requirements for the maturation of mitochondrial c-type cytochromes. Biochim Biophys Acta 1793: 125–138 [DOI] [PubMed] [Google Scholar]
- Hamza I (2006) Intracellular trafficking of porphyrins. ACS Chem Biol 1: 627–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvat EM, Redfield C, Stevens JM, Ferguson SJ (2009) Probing the heme-binding site of the cytochrome c maturation protein CcmE. Biochemistry 48: 1820–1828 [DOI] [PubMed] [Google Scholar]
- Ito-Maki M, Ishikawa K, Matera KM, Sato M, Ikeda-Saito M, Yoshida T (1995) Demonstration that histidine 25, but not 132, is the axial heme ligand in rat heme oxygenase-1. Arch Biochem Biophys 317: 253–258 [DOI] [PubMed] [Google Scholar]
- Kranz R, Lill R, Goldman B, Bonnard G, Merchant S (1998) Molecular mechanisms of cytochrome c biogenesis: three distinct systems. Mol Microbiol 29: 383–396 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG, Schuetz JD (2006) Identification of a mammalian mitochondrial porphyrin transporter. Nature 443: 586–589 [DOI] [PubMed] [Google Scholar]
- Lee D, Pervushin K, Bischof D, Braun M, Thony-Meyer L (2005) Unusual heme–histidine bond in the active site of a chaperone. J Am Chem Soc 127: 3716–3717 [DOI] [PubMed] [Google Scholar]
- Lee JH, Harvat EM, Stevens JM, Ferguson SJ, Saier MH Jr (2007) Evolutionary origins of members of a superfamily of integral membrane cytochrome c biogenesis proteins. Biochim Biophys Acta 1768: 2164–2181 [DOI] [PubMed] [Google Scholar]
- Moore GR, Pettigrew GW (1990) Cytochromes c: Evolutionary, Structural, and Physicochemical Aspects. Springer-Verlag, Berlin: New York [Google Scholar]
- Nicholson DW, Neupert W (1989) Import of cytochrome c into mitochondria: reduction of heme, mediated by NADH and flavin nucleotides, is obligatory for its covalent linkage to apocytochrome c. Proc Natl Acad Sci USA 86: 4340–4344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian MR, Thony-Meyer L (2002) Biochemistry, regulation and genomics of haem biosynthesis in prokaryotes. Adv Microb Physiol 46: 257–318 [DOI] [PubMed] [Google Scholar]
- Ow YP, Green DR, Hao Z, Mak TW (2008) Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol 9: 532–542 [DOI] [PubMed] [Google Scholar]
- Oyedotun KS, Yau PF, Lemire BD (2004) Identification of the heme axial ligands in the cytochrome b562 of the Saccharomyces cerevisiae succinate dehydrogenase. J Biol Chem 279: 9432–9439 [DOI] [PubMed] [Google Scholar]
- Pandey AV, Joshi SK, Tekwani BL, Chauhan VS (1999) A colorimetric assay for heme in biological samples using 96-well plates. Anal Biochem 268: 159–161 [DOI] [PubMed] [Google Scholar]
- Quigley JG, Yang Z, Worthington MT, Phillips JD, Sabo KM, Sabath DE, Berg CL, Sassa S, Wood BL, Abkowitz JL (2004) Identification of a human heme exporter that is essential for erythropoiesis. Cell 118: 757–766 [DOI] [PubMed] [Google Scholar]
- Rajagopal A, Rao AU, Amigo J, Tian M, Upadhyay SK, Hall C, Uhm S, Mathew MK, Fleming MD, Paw BH, Krause M, Hamza I (2008) Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature 453: 1127–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband W (1997-2008) ImageJ. National Institutes of Health: Bethesda, MD [Google Scholar]
- Rayapuram N, Hagenmuller J, Grienenberger JM, Bonnard G, Giege P (2008) The three mitochondrial encoded CcmF proteins form a complex that interacts with CCMH and c-type apocytochromes in Arabidopsis. J Biol Chem 283: 25200–25208 [DOI] [PubMed] [Google Scholar]
- Ren Q, Ahuja U, Thony-Meyer L (2002) A bacterial cytochrome c heme lyase. CcmF forms a complex with the heme chaperone CcmE and CcmH but not with apocytochrome c. J Biol Chem 277: 7657–7663 [DOI] [PubMed] [Google Scholar]
- Ren Q, Thony-Meyer L (2001) Physical interaction of CcmC with heme and the heme chaperone CcmE during cytochrome c maturation. J Biol Chem 276: 32591–32596 [DOI] [PubMed] [Google Scholar]
- Richard-Fogal CL, Frawley ER, Feissner RE, Kranz RG (2007) Heme concentration dependence and metalloporphyrin inhibition of the system I and II cytochrome c assembly pathways. J Bacteriol 189: 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard-Fogal CL, Frawley ER, Kranz RG (2008) Topology and function of CcmD in cytochrome c maturation. J Bacteriol 190: 3489–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Velazquez C, Coller R, Donohue TJ (2003) Features of Rhodobacter sphaeroides CcmFH. J Bacteriol 185: 422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C, Turkarslan S, Lee DW, Onder O, Kranz RG, Daldal F (2008) The cytochrome c maturation components CcmF, CcmH, and CcmI form a membrane-integral multisubunit heme ligation complex. J Biol Chem 283: 29715–29722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H, Fabianek RA, Pellicioli EC, Hennecke H, Thony-Meyer L (1999) Heme transfer to the heme chaperone CcmE during cytochrome c maturation requires the CcmC protein, which may function independently of the ABC-transporter CcmAB. Proc Natl Acad Sci USA 96: 6462–6467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H, Hennecke H, Thony-Meyer L (1998) Prototype of a heme chaperone essential for cytochrome c maturation. Science 281: 1197–1200 [DOI] [PubMed] [Google Scholar]
- Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT (2005) Identification of an intestinal heme transporter. Cell 122: 789–801 [DOI] [PubMed] [Google Scholar]
- Stevens JM, Daltrop O, Higham CW, Ferguson SJ (2003) Interaction of heme with variants of the heme chaperone CcmE carrying active site mutations and a cleavable N-terminal His tag. J Biol Chem 278: 20500–20506 [DOI] [PubMed] [Google Scholar]
- Swem LR, Gong X, Yu CA, Bauer CE (2006) Identification of a ubiquinone-binding site that affects autophosphorylation of the sensor kinase RegB. J Biol Chem 281: 6768–6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thony-Meyer L (2002) Cytochrome c maturation: a complex pathway for a simple task? Biochem Soc Trans 30: 633–638 [DOI] [PubMed] [Google Scholar]
- Uchida T, Stevens JM, Daltrop O, Harvat EM, Hong L, Ferguson SJ, Kitagawa T (2004) The interaction of covalently bound heme with the cytochrome c maturation protein CcmE. J Biol Chem 279: 51981–51988 [DOI] [PubMed] [Google Scholar]
- Wang WH, Lu JX, Yao P, Xie Y, Huang ZX (2003) The distinct heme coordination environments and heme-binding stabilities of His39Ser and His39Cys mutants of cytochrome b5. Protein Eng 16: 1047–1054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and methods
Review Process File