Abstract
Sulfolipid-I (SL-I) is an abundant metabolite found in the cell wall of Mycobacterium tuberculosis that is comprised of a trehalose 2-sulfate core modified with four fatty acyl substituents. The correlation of its abundance with the virulence of clinical isolates suggests a role for SL-I in pathogenesis, although its biological functions remain unknown. Here we describe the synthesis of a SL-I analogue bearing unnatural lipid substituents. A key feature of the synthesis was application of an intramolecular aglycon delivery reaction to join two differentially protected glucose monomers, one prepared with a novel α-selective glycosylation. The route developed for the model compound can be readily extended to the synthesis of native SL-I as well as additional analogues for use in the investigation of SL-I’s functions.
Introduction
While it was once considered a disease on the wane and largely a problem of the third world, the emergence of multidrug-resistant strains of tuberculosis (Tb) and the rise of HIV has led to a resurgence of Tb over the past several decades.1 Today, some 2 billion people, one-third of the world’s population, are thought to be infected with Mycobacterium tuberculosis (Mtb), the causative agent of Tb. Every year, nearly 9 million people contract Tb and 2 million die from complications of the disease, making it one of the leading causes of death from an infectious organism.2
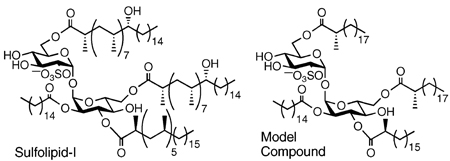
Mtb is most commonly found inside macrophages in the host’s lungs. The fact that it not only escapes destruction but thrives within host macrophages suggests that Mtb engages in some form of host immunomodulation. Several molecules from the loosely associated capsular layer outside the Mtb cell wall appear to affect the human immune system. One of the most interesting of these is Sulfolipid-I (SL-I) (Figure 1).
FIGURE 1.
Sulfolipid-I from Mycobacterium tuberculosis (average lipid lengths shown).
SL-I, which is preferentially expressed in virulent strains of Mtb, was isolated and characterized by Goren more than 30 years ago.3–6 The sulfoglycolipid is comprised of a trehalose 2-sulfate core modified with four acyl substituents. At the 2′ position is a straight-chain fatty acid, usually a palmitoyl or stearoyl group. The 3′ position bears a phthioceranoyl group, while the 6 and 6′ positions are modified with hydroxyphthioceranoyl substituents. These three multi-methylated long-chain fatty acids are found uniquely on the sulfatides of Mtb.7
While SL-I has been shown to elicit specific responses from immune cells, the mechanistic basis of these effects is unknown, and not all of them are well substantiated.8–12 By contrast, significant progress has been made in the last 5 years in understanding the biosynthesis of SL-I.13–21 One of its presumed precursors appears to have a hydroxyphthioceranoyl group at the 3′ position (rather than the 6′ position as shown in Figure 1), however, casting some doubt on the accuracy of Goren’s structure.19,20
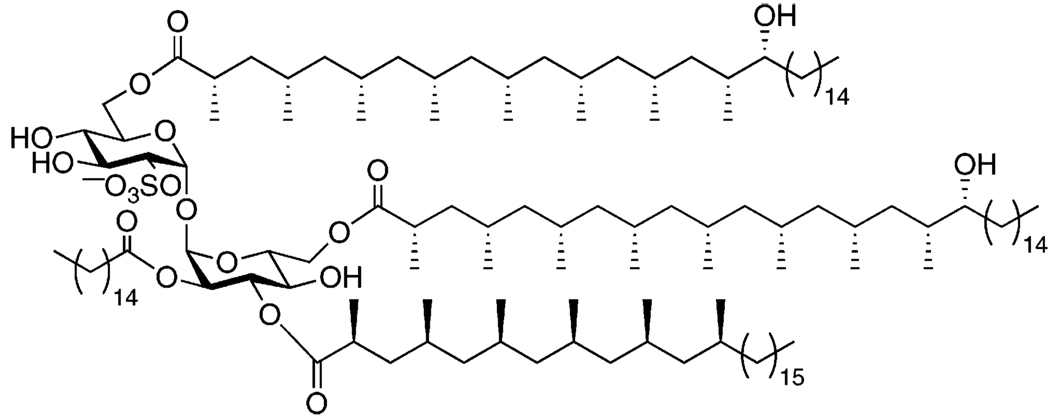
To investigate the biological activity of SL-I and to resolve questions concerning its precise structure, we set out to synthesize SL-I and analogs thereof. While mono- and diacylated trehalose derivatives have been synthesized,22,23 no derivatives approaching the complexity of the natural product have been described in the literature. Herein we report the first synthesis of a model compound related to SL-I (compound 1, Figure 2) that possesses all of the key modifications to the trehalose core: sulfation at the 2 position and distinct acyl substituents at the sites that are differentially modified in native SL-I. The route can be generalized to SL-I analogs bearing various unnatural acyl groups.
FIGURE 2.
Structure and retrosynthesis of model compound 1.
Results and Discussion
Synthetic Strategy
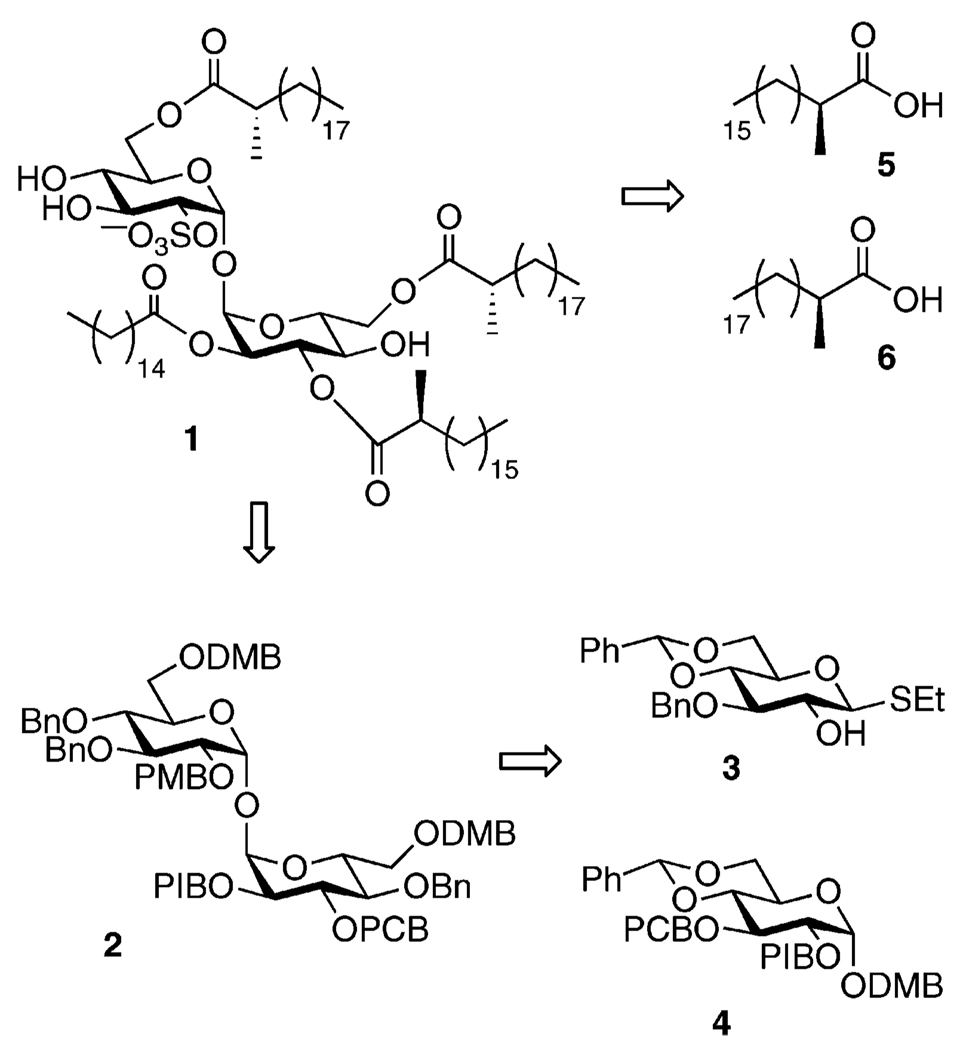
The central challenge in the synthesis of SL-I and its analogs is establishing a trehalose-based core structure that is properly differentially protected to enable installation of distinct acyl groups at the 2′, 3′, 6, and 6′ positions. In addition, the 2-sulfate group must be added toward the end of the synthesis, thereby minimizing its exposure to acidic or harsh basic conditions. We pursued compound 1 (Figure 2) to establish a viable route to SL-I analogues bearing the same substitution pattern as the natural product. The only differences between SL-I and 1 lie in the structures of the acyl substituents. We chose α-methylated acyl groups at the 3′, 6, and 6′ positions and a straight chain palmitoyl group at the 2′ position to reflect the relative reactivities of the lipids on SL-I.
Compound 2 (Figure 2) was envisioned as a key intermediate in the synthesis, and could later serve as a branching point to the synthesis of SL-I or its analogues. We conceived of compound 2 as deriving from convergent assembly of differentially protected glucose monomers 3 and 4, using the intramolecular aglycon delivery (IAD) technique developed in our lab.24 (Our variant was derived from the IAD glycosylation developed by Ito.25) The model lipids, 5 and 6, are both known compounds, as is 3.26–28
At least five mutually orthogonal protecting groups were required for this route. We chose to primarily employ benzyl ethers due to their stability and the range of available congeners. Among these were the p-methoxybenzyl (PMB), 3,4-dimethoxybenzyl (DMB), p-chlorobenzyl (PCB), and p-iodobenzyl (PIB) ethers. The two halobenzyl ethers were developed as protecting groups by Buchwald and Seeberger and can be selectively cleaved by Pd-catalyzed amination followed by acidic hydrolysis.29
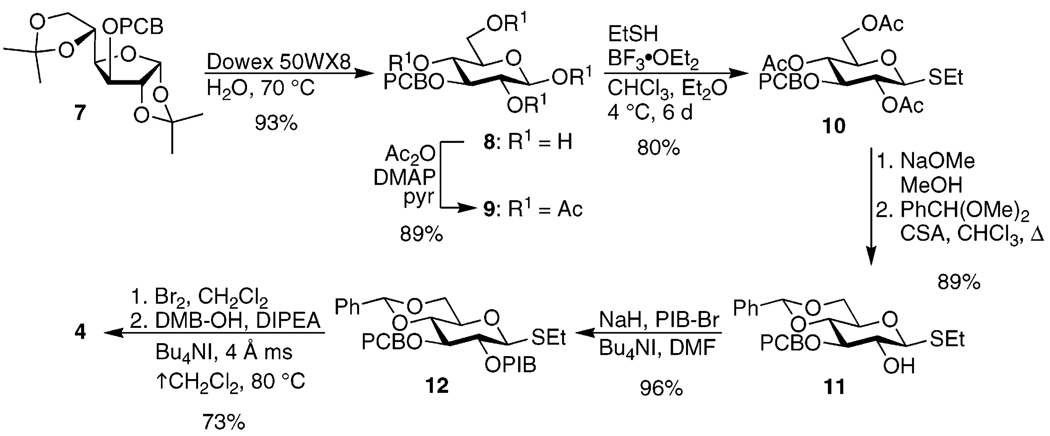
Preparation of Glucose Monomer 4
Synthesis of 4 began with 7,30 available in one step from commercially available diacetone glucose (Scheme 1). Cleavage of the isopropylidene groups with Dowex H+ resin in hot water afforded 8 in excellent yield.31 Conveniently, upon concentrating the crude reaction mixture, the β-anomer crystallized selectively. Acetylation proceeded with minimal epimerization at the anomeric center to produce 9. As a practical matter, the alkylation, de-isopropylidenation, and peracetylation steps were usually run sequentially with minimal purification between them. On a multigram scale, a yield of 89% over three steps could be obtained in this fashion. Several thioglycosylation protocols were screened to optimize yield and selectivity for the β thioglycoside and to minimize unproductive conversion to the unreactive α acetate.32 The best of these was slow and required a large excess of ethanethiol, but furnished 10 in good yield on a multigram scale.33 Deacetylation and formation of the benzylidene34 provided 11, which was alkylated with p-iodobenzyl bromide to afford 12 in excellent yield.
SCHEME 1.
Preparation of Glucose Monomer 4
Formation of the α,α-trehalose via the IAD reaction required exclusive use of the α-anomer of compound 4. Since the two anomers of 4 proved inseparable, it was particularly important to achieve high α-selectivity in the glycosylation of 12 with 3,4-dimethoxybenzyl alcohol. Two routes from 12 to 4 were initially explored. Gervay–Hague’s glycosyl iodide glycosylation technique,35 as was used in our initial report of the IAD methodology,24 provided excellent selectivity. However, application of this technique in the present context required conversion of thioglycoside 12 to the corresponding anomeric acetate, a process that we found resulted in only moderate yields. Alternatively, we envisioned that treatment of 12 with bromine would form the crude glycosyl bromide, which could then be subjected to Lemieux’s in situ halo-anomerization conditions.36 Preliminary investigation of this route, however, showed high α-selectivity but with only a moderate yield.
Since generation of the glycosyl bromide and glycosylation of the glycosyl iodide both seemed to be high yielding, we were intrigued by the possibility of in situ transformation of the glycosyl bromide to the glycosyl iodide by submitting it to Gervay–Hague’s glycosylation conditions.37 Glycosyl iodides were first synthesized from glycosyl bromides by halide exchange,38 but this kind of in situ generation for glycosylation purposes has received relatively little attention.39 After considerable experimentation, we found that the glycosyl bromide generated from 12 could be converted exclusively to the α-anomer of 4 in 75% yield by altering Gervay–Hague’s glycosylation conditions such that the reaction mixture was heated at 80 °C while allowing the solvent (CH2Cl2) to distill into another vessel so as to concentrate the mixture as the reaction progressed. The product could be readily purified from the resulting concentrated residue by chromatography.
Construction of the Trehalose Core and Final Protecting Groups
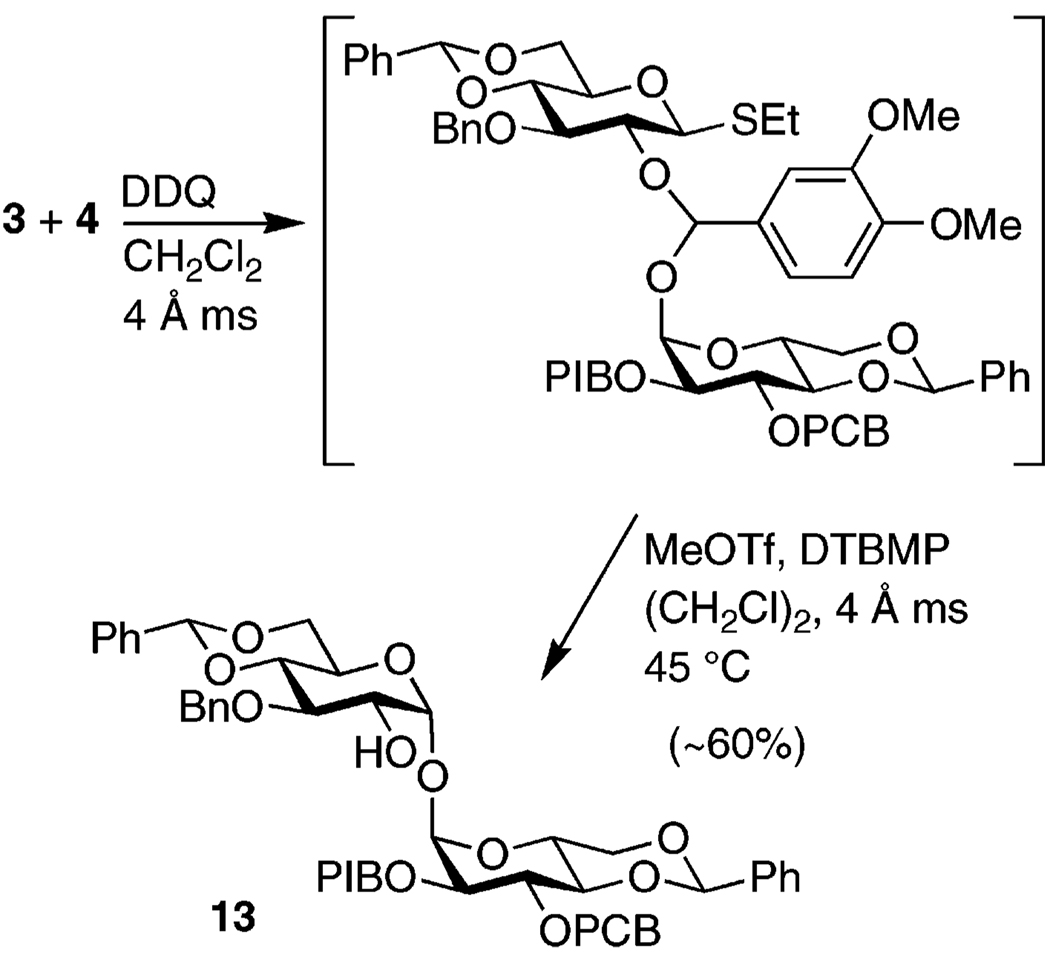
Glucose monomers 3 and 4 were then subjected to the IAD glycosylation methodology to afford 13 in good yield (Scheme 2). Purification of 13 has proven problematic, particularly its separation from the byproduct veratraldehyde and hydrolysis or elimination side products. Analytically pure material could be obtained by acetylating 13 or by very careful flash chromatography. Typically, therefore, partially purified material was carried on to the next step. The contaminants had no detrimental effect on the subsequent reaction and purification was much more facile afterward. Significantly, we did not observe either the α,β or β,β isomers among the byproducts of the IAD reaction.
SCHEME 2.
Construction of the Trehalose Core
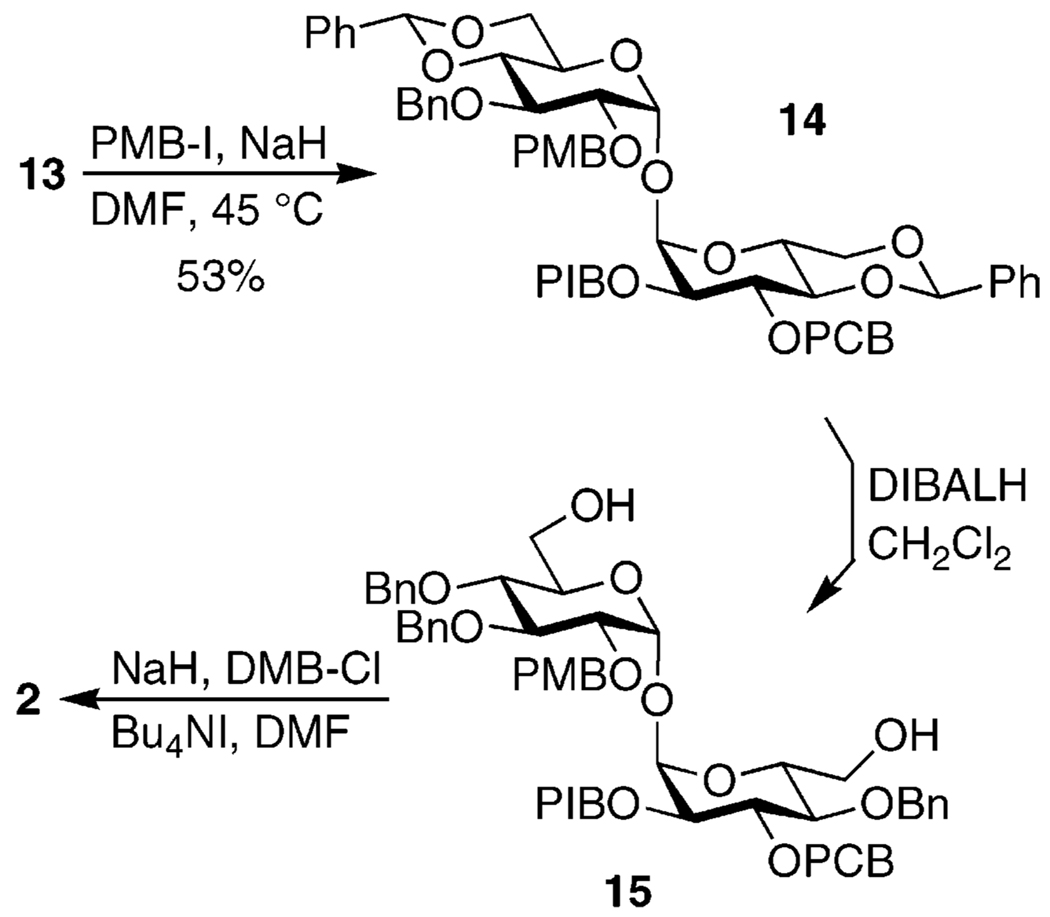
The remaining free hydroxyl group of 13 was alkylated by heating with p-methoxybenzyl iodide, affording 14 in 53% yield on a multigram scale (Scheme 3). Other reagents and conditions provided lower and frequently variable yields or simply failed to react.40 Further difficulties were encountered during reductive cleavage of the benzylidene groups. A number of reducing agents and conditions were investigated, but in all cases reduction of the aryl iodide (within the PIB group) was competitive.41 The de-iodinated product was inseparable from the desired 15 and the ratio of the two could only be estimated by the decrease in area of the PIB aromatic peaks in the 1H NMR spectrum. DIBALH reduction was found to be the best approach, affording a mixture of 15 and the de-iodinated byproduct in an apparent 3:1 ratio. (This would correspond to a 56% yield of 15.) Alkylation of this mixture was uncomplicated, providing 2 and the corresponding de-iodinated contaminant in essentially quantitative yield, though they remained inseparable.
SCHEME 3.
Synthesis of Intermediate 2
Completion of Model Compound 1
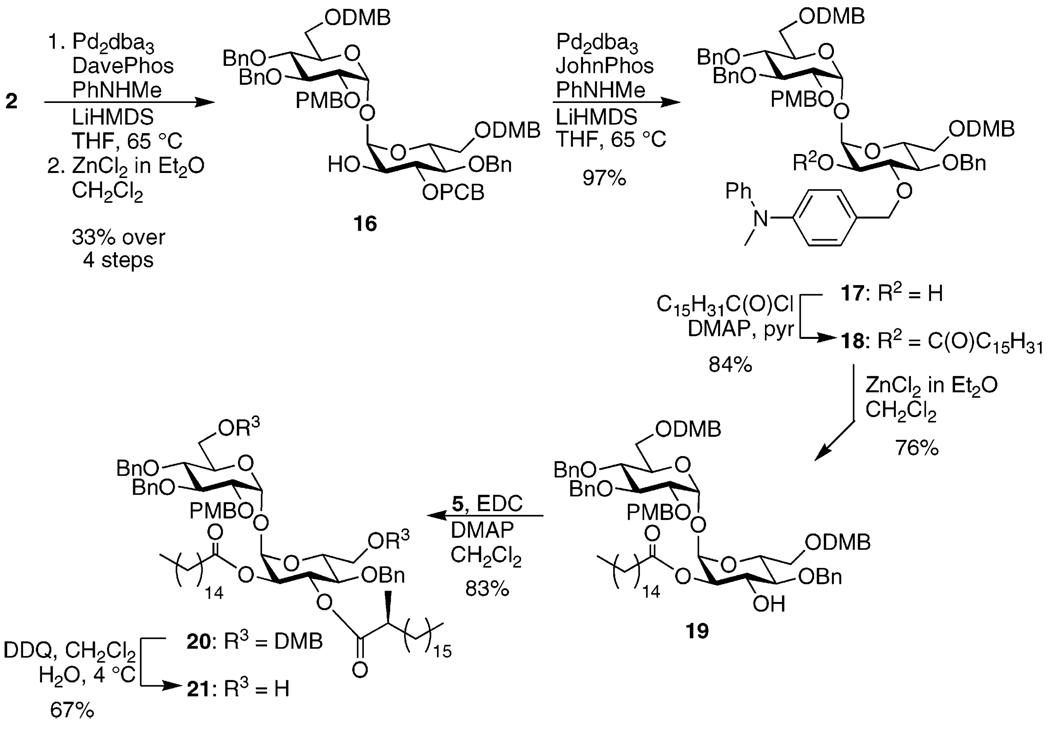
With key intermediate 2 in hand, the series of deprotections and functionalizations necessary to synthesize 1 were initiated. Due to their decreased sensitivity to adventitious water, Buchwald’s alcohol-tolerant conditions proved to be most convenient for the Pd-catalyzed amination of the PIB group (Scheme 4).42 The resulting aminated benzyl ether was then deprotected with zinc chloride. At this point the de-iodinated contaminant was finally separated; thus 16 was obtained in a 33% yield over four steps. While the obvious next step would be to palmitoylate the free hydroxyl of 16 and then remove the PCB group, the acylated compound proved unreactive to the published ester-tolerant amination conditions.29 As a result, 16 was aminated by using the alcohol-tolerant conditions to produce 17. Palmitoylation was followed by deprotection of the aminated benzyl ether to afford 19 in good yield. EDC-promoted esterification with model lipid 5 provided 20.26,43 The two DMB groups were then selectively cleaved to generate diol 21.44
SCHEME 4.
Initial Deprotections and Acylations of Intermediate 2
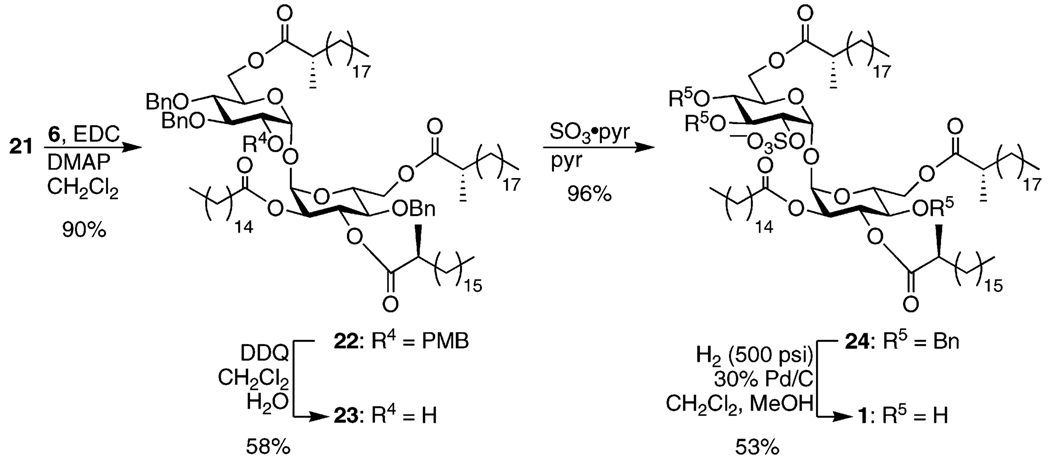
A second EDC-promoted esterification with model lipid 6 produced fully acylated 22 (Scheme 5).27,43 Oxidative deprotection of the PMB group was followed by sulfation of the resulting alcohol with SO3·pyridine to provide 24 in excellent yield. Finally, hydrogenolysis of the remaining benzyl ethers under high pressure produced the target compound 1.
SCHEME 5.
Completion of Compound 1
Conclusion
We achieved the first synthesis of a model compound (1) closely related to the Mtb metabolite SL-I. A novel α-selective glycosylation reaction was developed and the utility of the trehalose-forming IAD glycosylation technique was further demonstrated. The appropriate choice of a large set of orthogonal protecting groups was crucial to this synthesis, rendering it flexible and readily extensible. Key intermediate 2 will provide a useful point of divergence for the synthesis of a range of SL-I analogues. Notably, Minnaard and co-workers recently reported a synthetic route to phthioceranic acids.45 Merger of their methods with the route reported here should provide direct access to native SL-I. These compounds should facilitate the study of SL-I’s biological functions as well as resolving questions about its precise structure.
Experimental Section
3-O-(p-Chlorobenzyl)-β-d-glucopyranose (8)
To 11.09 g (28.82 mmol) of 7 were added 60 mL of H2O, followed by 20 g of Dowex 50WX8–400 resin (H+ form). The reaction mixture was heated to 70 °C and left stirring overnight. The resin was filtered off and the filtrate concentrated, yielding 8.17 g (93%) of 8. 1H NMR (DMSO-d 6, 300 MHz): δ 3.03–3.30 (m, 4 H), 3.45 (dd, 1 H, J = 5.7, 11.7 Hz), 3.68 (d, 1 H, J = 11.7 Hz), 4.33 (d, 1 H, J = 7.5 Hz), 4.76 (d, 1 H, J = 12.3 Hz), 4.80 (d, 1 H, J = 12.3 Hz), 7.33–7.48 (m, 4 H). 13C NMR (DMSO-d 6, 100 MHz): δ 61.04, 69.81, 72.78, 74.74, 76.69, 85.40, 96.89, 127.92, 129.21, 131.50, 138.75. Anal. Calcd for C13H17O6Cl: C, 51.24; H, 5.62. Found: C, 50.94; H, 5.81.
1,2,4,6-Tetra-O-acetyl-3-O-(p-chlorobenzyl)-β-d-glucopyra-nose (9)
To a solution of 40.66 g (134.1 mmol) of 8 in 800 mL of dry pyridine were added 1.8 g (15 mmol, 0.11 equiv) of DMAP, followed by 300 mL (3.2 mol, 24 equiv) of Ac2O. After stirring overnight, the reaction appeared complete by TLC (Rf 0.5, 65:35 hexanes/EtOAc). The reaction mixture was concentrated under reduced pressure to yield a thin syrup. This was diluted with EtOAc and washed with ice-cold H2O, satd. CuSO4, H2O, satd. NaHCO3, and satd. NaCl and dried over Na2SO4. The reaction mixture was dissolved in minimal 65:35 hexanes/EtOAc and subjected to flash chromatography by using the same solvent system, affording 56.30 g (89%) of 9 as a mixture of anomers (1:7 α/β). 1H NMR (300 MHz, β anomer): δ 2.00 (s, 6 H), 2.09 (s, 3 H), 2.11 (s, 3 H), 3.69–3.80 (m, 2 H), 4.09 (dd, 1 H, J = 2.1, 12.3 Hz), 4.24 (dd, 1 H, J = 4.8, 12.3 Hz), 4.59 (s, 2 H), 5.16 (t, 1 H, J = 8.4 Hz), 5.17 (t, 1 H, J = 8.7 Hz), 5.65 (d, 1 H, J = 8.4 Hz), 7.12–7.38 (m, 4 H). 13C NMR (100 MHz, β anomer): δ 20.65, 20.78, 61.64, 68.79, 71.28, 72.86, 73.06, 80.11, 91.84, 128.50, 128.55, 133.62, 135.99, 168.97, 169.11, 169.17, 170.61. Anal. Calcd for C21H25O10Cl: C, 53.34; H, 5.33. Found: C, 53.22; H, 5.41.
Ethyl 2,4,6-Tri-O-acetyl-3-O-(p-chlorobenzyl)-1-thio-β-d-glu-copyranoside (10)
To a solution of 48.83 g (103.3 mmol) of 9 in 200 mL of 3:2 CHCl3/Et2O (previously dried over 3 Å ms) at 0 °C were added 40 mL (0.5 mol, 5 equiv) of ethanethiol followed by 16 mL (130 mmol, 1.2 equiv) of BF3·OEt2. The flask was sealed with a Teflon-sleeved glass stopper and left stirring at 4 °C. After 6 d, the reaction mixture was diluted with CH2Cl2, washed with ice-cold H2O, satd. NaHCO3, H2O, and satd. NaCl, and dried over Na2SO4. The reaction mixture was dissolved in toluene and subjected to flash chromatography with use of 95:5, 92.5:7.5, and 90:10 toluene/EtOAc, yielding 39.18 g (80%) of 10 (Rf = 0.6, 80: 20 toluene/EtOAc). 1H NMR (300 MHz): δ 1.26 (t, 3 H, J = 7.5 Hz), 1.99 (s, 3 H), 2.03 (s, 3 H), 2.07 (s, 3 H), 2.62–2.80 (m, 2 H), 3.62 (ddd, 1 H, J = 2.4, 5.1, 9.9 Hz), 3.70 (t, 1 H, J = 9.3 Hz), 4.11 (dd, 1 H, J = 2.4, 12.3 Hz), 4.20 (dd, 1 H, J = 5.1, 12.3 Hz), 4.41 (d, 1 H, J = 9.9 Hz), 4.56 (d, 1 H, J = 12.0 Hz), 4.61 (d, 1 H, J = 12.0 Hz), 5.08 (t, 1 H, J = 9.6 Hz), 5.11 (t, 1 H, J = 9.6 Hz), 7.17 (d, 2 H, J = 8.4 Hz), 7.30 (d, 2 H, J = 8.4 Hz). 13C NMR (100 MHz): δ 14.35, 20.33, 20.50, 20.61, 23.82, 62.05, 69.19, 72.96, 75.59, 81.33, 83.31, 128.11, 128.50, 133.00, 136.04, 168.89, 168.97, 170.17. Anal. Calcd for C21H27O8ClS: C, 53.11; H, 5.73; S, 6.75. Found: C, 52.99; H, 5.83, S, 6.80.
Ethyl 4,6-O-Benzylidene-3-O-(p-chlorobenzyl)-1-thio-β-d-glu-copyranoside (11)
To a solution of 16.92 g (35.60 mmol) of 10 in 375 mL of MeOH was added 2.03 g (37.5 mmol) of NaOMe to give a 0.1 M solution. After 5 h the reaction appeared complete by TLC (Rf 0.4, 65:35 EtOAc/hexanes). The reaction was quenched with 7 g of Dowex 50WX8–400 resin then filtered, and the solvent was removed under reduced pressure. The resulting residue was dissolved in 180 mL of CHCl3 and 0.42 g (1.8 mmol, 0.05 equiv) of CSA was added followed by 6.8 mL (45 mmol, 1.25 equiv) of benzaldehyde dimethyl acetal. Azeotropic removal of MeOH generated during the benzylidenation was accomplished by distilling the reaction mixture into a Dean–Stark trap. This was emptied when full (30 mL) and fresh CHCl3 was added to the pot. After 4 iterations, the reaction appeared complete (product Rf 0.6, 85:15 toluene/Et2O). The reaction was quenched with 2 g of solid Na2CO3 and reheated to reflux for 30 min. The hot suspension was filtered and the filtrate concentrated, redissolved in toluene, and subjected to flash chromatography with 98:2, 95:5, and 80:20 toluene/Et2O to yield 13.80 g (89%) of 11. 1H NMR (300 MHz): δ 1.32 (t, 3 H, J = 7.5 Hz), 2.50 (d, 1 H, J = 2.1 Hz), 2.75 (dq, 2 H, J = 1.5, 7.5 Hz), 3.45–3.74 (m, 4 H), 3.78 (t, 1 H, J = 10.2 Hz), 4.36 (dd, 1 H, J = 4.8, 10.5 Hz), 4.46 (d, 1 H, J = 9.3 Hz), 4.80 (d, 1 H, J = 12.0 Hz), 4.91 (d, 1 H, J = 12.0 Hz), 5.56 (s, 1 H), 7.23–7.51 (m, 9 H). 13C NMR (100 MHz): δ 15.27, 24.64, 68.61, 73.08, 73.82, 81.05, 81.54, 86.75, 101.33, 126.00, 128.28, 128.52, 129.09, 129.32, 133.47, 136.83, 137.12. Anal. Calcd for C22H25O5ClS: C, 60.47; H, 5.77; S, 7.34. Found: C, 60.49; H, 5.73, S, 7.37.
Ethyl 4,6-O-Benzylidene-3-O-(p-chlorobenzyl)-2-O-(p-iodo-benzyl)-1-thio-β-d-glucopyranoside (12)
To a solution of 13.51 g (30.90 mmol) of 11 and 0.12 g (0.31 mmol, 0.01 equiv) of Bu4NI in 300 mL of dry DMF was added 1.5 g (38 mmol, 1.2 equiv) of NaH (60% suspension in mineral oil), followed after several minutes by 11.02 g (37.11 mmol, 1.2 equiv) of p-iodobenzyl bromide. After stirring overnight, the reaction was complete by TLC analysis (Rf 0.75, 95:5 toluene/Et2O). The reaction was terminated by addition of H2O and the solvent removed under reduced pressure. The resulting residue was dissolved in EtOAc and washed with H2O and satd. NaCl and dried over Na2SO4. The reaction mixture in toluene was subjected to flash chromatography with use of 100:0 and 99:1 toluene/Et2O to afford 19.45 g (96%) of 12. 1H NMR (300 MHz): δ 1.33 (t, 3 H, J = 7.5 Hz), 2.71–2.84 (m, 2 H), 3.38–3.50 (m, 2 H), 3.65–3.76 (m, 2 H), 3.78 (t, 1 H, J = 10.2 Hz), 4.37 (dd, 1 H, J = 5.1, 10.5 Hz), 4.54 (d, 1 H, J = 9.9 Hz), 4.69 (d, 1 H, J = 10.5 Hz), 4.71 (d, 1 H, J = 11.7 Hz), 4.83 (d, 1 H, J = 10.5 Hz), 4.88 (d, 1 H, J = 11.7 Hz), 5.57 (s, 1 H), 7.09 (d, 2 H, 8.1 Hz), 7.22–7.49 (m, 9 H), 7.67 (d, 2 H, 8.1 Hz). 13C NMR (100 MHz): δ 15.11, 25.21, 68.65, 70.18, 74.23, 75.12, 81.30, 81.48, 82.65, 85.74, 101.19, 125.96, 128.28, 128.49, 129.05, 129.28, 129.93, 133.46, 137.14, 137.43, 137.55. Anal. Calcd for C29H30O5ClIS: C, 53.34; H, 4.63; S, 4.91. Found: C, 53.52; H, 4.69; S, 4.89.
3,4-Dimethoxybenzyl 4,6-O-Benzylidene-3-O-(p-chlorobenzyl)-2-O-(p-iodobenzyl)-α-d-glucopyranoside (4)
After azeotropically drying 15.88 g (24.30 mmol) of 12 with toluene (3×), the residue was dissolved in 250 mL of dry CH2Cl2 and treated with 4.36 mL (85.1 mmol, 3.5 equiv) of Br2. The solution was stirred for 45 min, then concentrated and azeotroped 2× from toluene. The residue of crude glycosyl bromide was dissolved in 40 mL of dry CH2Cl2 and added to a mixture of 7.1 mL (49 mmol, 2 equiv) of 3,4-dimethoxybenzyl alcohol, 18.1 g (48.6 mmol, 2 equiv) of Bu4NI, 8.5 mL (49 mmol, 2 equiv) of DIPEA, and 9 g of 4 Å ms in 50 mL of dry CH2Cl2. The flask was fitted with a distillation head and heated in an 80 °C oil bath for 3 h, after which the bulk of the solvent had distilled off. After cooling to rt, the solidified reaction mixture was resuspended in 125 mL of dry CH2Cl2 and filtered through Celite. The reaction mixture in toluene was subjected to flash chromatography with use of 100:0, 98:2, and 95:5 toluene/Et2O to yield 13.49 g (73%) of 4 (Rf 0.6, 85:15 toluene/Et2O). 1H NMR (400 MHz): δ 3.49 (dd, 1 H, J = 3.6, 9.2 Hz), 3.59 (t, 1 H, J = 9.6 Hz), 3.72 (t, 1 H, J = 10.4 Hz), 3.82 (s, 3 H), 3.91 (s, 3 H), 3.92 (td, 1 H, J = 4.8, 10.0 Hz), 4.05 (t, 1 H, J = 9.2 Hz), 4.27 (dd, 1 H, J = 4.8, 10.0 Hz), 4.46 (d, 1 H, J = 12.0 Hz), 4.51 (d, 1 H, J = 12.0 Hz), 4.60 (d, 1 H, J = 12.4 Hz), 4.68 (d, 1 H, J = 12.0 Hz), 4.76 (d, 1 H, J = 11.6 Hz), 4.81 (d, 1 H, J = 3.6 Hz), 4.85 (d, 1 H, J = 11.6 Hz), 5.55 (s, 1 H), 6.82 (d, 1 H, J = 8.0 Hz), 6.92 (d, 2 H, J = 8.8 Hz), 6.94 (d, 2 H, J = 8.0 Hz), 7.19–7.50 (m, 9 H), 7.57 (d, 2 H, J = 8.0 Hz). 13C NMR (100 MHz): δ 55.87, 55.95, 62.59, 68.85, 69.02, 72.52, 74.34, 78.57, 79.20, 82.14, 93.23, 95.38, 101.34, 110.76, 111.79, 121.43, 125.99, 128.26, 128.41, 128.96, 129.02, 129.21, 129.49, 133.28, 137.18, 137.26, 137.42, 137.68, 149.01, 149.07. Anal. Calcd for C36H36O8ClI: C, 56.97; H, 4.78. Found: C, 56.79; H, 4.74.
3-O-Benzyl-4,6;4′,6′-di-O-benzylidene-3′-O-(p-chlorobenzyl)-2′-O-(p-iodobenzyl)-α,α-d-trehalose (13)
A mixture of 10.00 g (13.17 mmol) of 4 and 4.25 g (10.5 mmol, 0.8 equiv) of 3 28 was azeotropically dried with toluene 3×. To the residue were added 6 g of 4 Å ms and 150 mL of dry CH2Cl2. The mixture was cooled to 0 °C and 3.60 g (15.80 mmol, 1.2 equiv) of DDQ (freshly recrystallized from benzene) was added. After 10 min, the reaction was allowed to warm to rt and left stirring for 4 h. TLC analysis then showed none of the purple spots characteristic of the 3,4-dimethoxybenzyl group, which develop following treatment of the plate with ethanolic H2SO4 and charring. The reaction was therefore terminated with 165 mL of freshly prepared ascorbate buffer (3.5 g of ascorbic acid, 6.3 g of citric acid, and 4.6 g of NaOH in 500 mL of H2O), diluted with EtOAc, and stirred vigorously for 10 min, during which time the muddy brown solution turned lemon yellow. The reaction mixture was filtered through Celite and the organic layer washed with satd. NaHCO3 and satd. NaCl (2×) and dried over Na2SO4. The pale yellow EtOAc solution was concentrated under reduced pressure and the residue dried azeotropically with toluene (3×), after which 8.20 g (39.5 mmol, 3 equiv) of DTBMP, 8 g of 4 Å ms, and 300 mL of dry (CH2Cl)2 were added. The mixture was cooled to 0 °C and 3.9 mL (34 mmol, 2.6 equiv) of MeOTf were added. The reaction mixture was stirred for 15 min at 0 °C and heated to 45 °C overnight. The reaction mixture, now salmon-colored, was cooled to 0 °C and quenched with 4 mL of Et3N, turning blue-green. After being diluted with EtOAc and satd. NaHCO3, the reaction mixture was stirred for 15 min at rt and filtered through Celite. The grey-green organic layer was washed with H2O and satd. NaCl and dried over Na2SO4, turning pale yellow again upon sitting. The reaction mixture in toluene was subjected to flash chromatography with use of 100:0, 98:2, 95:5, 92.5:7.5, 90:10, and 87.5:12.5 toluene/Et2O to yield 6.21 g (est. 60%) of semipure 13 (Rf 0.3, 85:15 toluene/Et2O).
In most cases, this semipure material was carried on to the next step. However, isolation of pure 13 for characterization could be achieved by using a much slower toluene/Et2O gradient (100:0, 99:1, 98:2, 97:3, and 95:5 toluene/Et2O) on a longer column of silica gel. 1H NMR (400 MHz): δ 3.59 (dd, 1 H, J = 3.6, 9.2 Hz), 3.63 (t, 1 H, J = 9.6 Hz), 3.69 (t, 1 H, J = 9.2 Hz), 3.73 (t, 1 H, J = 10.4 Hz), 3.67–3.85 (m, 2 H), 3.93–3.98 (m, 1 H), 3.94 (t, 1 H, J = 9.2 Hz), 4.03 (t, 1 H, J = 9.6 Hz), 4.12 (dt, 1 H, J = 4.8, 10.0 Hz), 4.15–4.24 (m, 1 H), 4.29 (dd, 1 H, J = 5.2, 10.4 Hz), 4.65 (d, 1 H, J = 11.6 Hz), 4.72 (d, 1 H, J = 10.8 Hz), 4.74 (d, 1 H, J = 11.6 Hz), 4.76 (d, 1 H, J = 11.6 Hz), 4.91 (d, 1 H, J = 11.6 Hz), 5.02 (d, 1 H, J = 10.8 Hz), 5.17 (d, 1 H, J = 3.6 Hz), 5.18 (d, 1 H, J = 3.6 Hz), 5.56 (s, 1 H), 5.58 (s, 1 H), 7.07 (d, 2 H, J = 8.0 Hz), 7.23–7.55 (m, 19 H), 7.62 (d, 2 H, J = 8.0 Hz). 13C NMR (125 MHz): δ 62.95, 63.32, 68.88, 68.91, 71.63, 73.14, 74.41, 75.05, 78.63, 78.71, 78.85, 82.12, 82.22, 93.43, 94.25, 95.37, 101.16, 101.25, 125.97, 126.01, 127.90, 128.01, 128.27, 128.37, 128.48, 128.50, 129.00, 129.02, 129.17, 129.50, 133.38, 137.13, 137.26, 137.28, 137.42, 137.66, 138.32. HRMS (FAB) m/z 949.1868 (MH+ C47H47O11ClI requires 949.1852).
Alternatively, the semipure material could be acetylated to obtain an analytically pure compound: To a solution of 0.200 g (0.211 mmol) of semipure 13 and a few crystals of DMAP in 2 mL of dry pyridine were added 0.10 mL (1.06 mmol, 5 equiv) of Ac2O. After stirring overnight, the reaction mixture was poured over ice and extracted with EtOAc. The organic layer was washed with satd. CuSO4, H2O, satd. NaHCO3, and satd. NaCl and dried over Na2SO4. The reaction mixture in toluene was subjected to flash chromatography with use of 50:50, 60:40, and 70:30 Et2O/hexanes. The cleanest fractions (as judged by TLC) were dried, affording 0.100 g (48%) of acetylated 13 (Rf 0.6, 70:30 Et2O/hexanes). 1H NMR (400 MHz): δ 2.11 (s, 3 H), 3.57 (dd, 1 H, J = 3.6, 9.6 Hz), 3.63 (t, 1 H, J = 9.6 Hz), 3.68–3.79 (m, 3 H), 3.91–3.97 (m, 1 H), 4.01 (t, 1 H, J = 9.6 Hz), 4.05–4.19 (m, 3 H), 4.24 (td, 1 H, J = 4.8, 10.0 Hz), 4.64 (d, 1 H, J = 12.0 Hz), 4.71 (d, 1 H, J = 12.0 Hz), 4.72 (d, 1 H, J = 12.0 Hz), 4.76 (d, 1 H, J = 11.6 Hz), 4.89 (d, 1 H, J = 12.0 Hz), 4.93 (d, 1 H, J = 11.6 Hz), 4.95 (dd, 1 H, J = 4.0, 9.2 Hz), 5.13 (d, 1 H, J = 3.6 Hz), 5.31 (d, 1 H, J = 3.6 Hz), 5.55 (s, 1 H), 5.59 (s, 1 H), 7.06 (d, 2 H, J = 8.0 Hz), 7.21–7.56 (m, 19 H), 7.62 (d, 2 H, J = 8.0 Hz). 13C NMR (100 MHz): δ 20.71, 63.03, 63.13, 68.70, 68.80, 72.53, 73.14, 74.41, 76.11, 78.60, 78.72, 81.88, 93.44, 94.40, 101.17, 101.39, 125.93, 125.99, 127.46, 127.59, 128.27, 128.44, 128.99, 129.09 129.42, 133.35, 137.03, 137.16, 137.27, 137.64, 138.45, 170.17. Anal. Calcd for C49H48O10ClI: C, 59.37; H, 4.88. Found: C, 59.77; H, 5.04.
3-O-Benzyl-4,6;4′,6′-di-O-benzylidene-3′-O-(p-chlorobenzyl)-2′-O-(p-iodobenzyl)-2-O-(p-methoxybenzyl)-α,α-d-trehalose (14)
To a solution of 12.48 g (47.60 mmol, 6.5 equiv) of PPh3 in 120 mL of dry CH2Cl2 were added sequentially 3.24 g (47.6 mmol, 6.5 equiv) of imidazole and 12.06 g (47.53 mmol, 6.5 equiv) of I2.46 As the I2 dissolved, the solution became dark orange and developed a white precipitate. When dissolution was complete, 4.60 mL (36.9 mmol, 5.1 equiv) of p-methoxybenzyl alcohol was added and the suspension was stirred for 30 min, at which time the reaction appeared complete (product Rf 0.8, 90:10 hexanes/Et2O). The reaction mixture was filtered, diluted with Et2O, washed with satd. Na2S2O3, H2O, and satd. NaI, and dried over Na2SO4. The solvent was removed under reduced pressure and the residue was dissolved in a small amount of CH3CN. This was extracted 5× with hexanes, at which point virtually all the p-methoxybenzyl iodide had been removed, as shown by TLC. (This set of dissolution and extractions can be omitted, but the crude p-methoxybenzyl iodide contains much more water and large amounts of Ph3PO.) The combined hexanes layers were concentrated under reduced pressure and the residue was dried under vacuum for several hours in a foil-wrapped flask. To a solution of 6.917 g (7.288 mmol) of 13 in 100 mL of dry DMF was added 1.8 g (45 mmol, 6.2 equiv) of NaH (60% suspension in mineral oil). After stirring for several minutes, a solution of the freshly prepared p-methoxybenzyl iodide in 20 mL of dry DMF was added. The reaction mixture was heated to 45 °C and stirred overnight. The reaction was terminated by addition of H2O and the solvent removed under reduced pressure. The resulting residue was dissolved in Et2O and washed with H2O and satd. NaCl and dried over Na2SO4. Flash chromatography of the reaction mixture in toluene with use of 100:0, 99:1, 98:2, and 97:3 toluene/Et2O yielded 4.15 g (53%) of 14 (Rf 0.7, 85:15 toluene/Et2O). 1H NMR (300 MHz): δ 3.57 (dd, 1 H, J = 3.6, 11.1 Hz), 3.58–3.82 (m, 4 H), 3.61 (t, 1 H, J = 9.0 Hz), 3.71 (s, 3 H), 4.03–4.31 (m, 6 H), 4.63 (d, 1 H, J = 12.0 Hz), 4.65 (d, 1 H, J = 11.4 Hz), 4.71 (d, 1 H, J = 11.7 Hz), 4.74 (d, 1 H, J = 11.4 Hz), 4.75 (d, 1 H, J = 11.1 Hz), 4.84 (d, 1 H, J = 12.3 Hz), 4.88 (d, 1 H, J = 11.7 Hz), 4.95 (d, 1 H, J = 11.1 Hz), 5.09 (d, 1 H, J = 3.6 Hz), 5.14 (d, 1 H, J = 3.6 Hz), 5.54 (s, 1 H), 5.55 (s, 1 H), 6.81 (d, 2 H, J = 8.7 Hz), 7.05 (d, 2 H, J = 8.1 Hz), 7.08–7.52 (m, 21 H), 7.60 (d, 2 H, J = 8.1 Hz). 13C NMR (100 MHz): δ 55.17, 62.86, 62.94, 68.91, 68.95, 73.08, 73.54, 74.44, 75.27, 78.33, 78.60, 78.72, 78.88, 82.23, 93.31, 94.33, 94.66, 101.11, 101.29, 126.01, 126.08, 127.56, 127.88, 128.20, 128.31, 128.43, 128.92, 129.02, 129.17, 129.42, 130.00, 133.29, 137.19, 137.37, 137.42, 137.49, 137.60, 138.77, 159.26. HRMS (FAB) m/z 1075.2504 (MLi+ C55H54LiO12ClI requires 1075.2508).
3,4,4′-Tri-O-benzyl-3′-O-(p-chlorobenzyl)-2′-O-(p-iodobenzyl)-2-O-(p-methoxybenzyl)-α,α-d-trehalose (15)
To a solution of 1.56 g (1.46 mmol) of 14 in 60 mL of dry CH2Cl2 at 0 °C was added 30 mL (30 mmol, 20 equiv) of 1 M DIBALH in toluene. The reaction mixture was allowed to warm to rt and left stirring overnight. Excess hydride was quenched, slowly at first, with MeOH, after which 100 mL of half-satd. Rochelle’s salt was added and the mixture was stirred vigorously for several hours to help break up the resulting solid gel. The reaction mixture was diluted with CH2Cl2 and satd. NH4Cl and the aqueous layer extracted 2× with CH2Cl2. The combined organic layers were washed with satd. NaCl and dried over Na2SO4. Flash chromatography of the reaction mixture in toluene with use of 95:5 and 90:10 toluene/acetone afforded 1.125 g of a mixture of 15 and the de-iodinated side product in an apparent 3:1 ratio, as judged by the decrease in area of the PIB aromatic peaks in the 1H NMR spectrum (Rf 0.3, 80:20 toluene/acetone). This would correspond to 0.869 g (56%) of 15 and 0.256 g (19%) of the side product. 1H NMR (300 MHz): δ 3.44–3.70 (m, 8 H), 3.76 (s, 3 H), 3.95–4.13 (m, 2 H), 4.01 (t, 1 H, J = 9.3 Hz), 4.03 (t, 1 H, J = 9.3 Hz), 4.55 (d, 1 H, J = 12.0 Hz), 4.59 (d, 1 H, J = 12.9 Hz), 4.61 (d, 1 H, J = 12.9 Hz), 4.63 (d, 2 H, J = 11.4 Hz), 4.65 (d, 1 H, J = 11.4 Hz), 4.82 (d, 2 H, J = 11.1 Hz), 4.88 (d, 3 H, J = 11.1 Hz), 4.99 (d, 1 H, J = 11.1 Hz), 5.09 (d, 1 H, J = 3.3 Hz), 5.13 (d, 1 H, J = 3.3 Hz), 6.80 (d, 2 H, J = 8.7 Hz), 7.00 (d, 2 H, J = 8.1 Hz), 7.18–7.50 (m, 21 H), 7.57 (d, 2 H, J = 8.4 Hz). 13C NMR (100 MHz): δ 55.22, 61.48, 61.53, 61.60, 71.27, 72.15, 72.69, 72.87, 74.62, 75.02, 75.16, 75.52, 79.00, 79.47, 81.38, 81.53, 93.44, 93.82, 113.76, 127.45, 127.57, 127.82, 127.94, 128.03, 128.10, 128.18, 128.38, 128.46, 128.50, 128.59, 128.94, 129.07, 129.17, 129.25, 130.00, 133.27, 137.13, 137.44, 137.85, 138.70, 159.20. HRMS (FAB) m/z 1079.2827 (MLi+ C55H58LiO12ClI requires 1079.2822).
3,4,4′-Tri-O-benzyl-3′-O-(p-chlorobenzyl)-6,6′-di-O-(3,4-dimethoxybenzyl)-2′-O-(p-iodobenzyl)-2-O-(p-methoxybenzyl)-α,α-d-trehalose (2)
To 0.99 g (8.3 mmol, 10 equiv) of benzotriazole was added 0.60 mL (8.3 mmol, 10 equiv) of SOCl2.47 The resulting viscous yellow solution was diluted with 5 mL of dry CH2Cl2 and added over 5 min to a solution of 1.00 mL (6.88 mmol, 8 equiv) of 3,4-dimethoxybenzyl alcohol in 100 mL of dry CH2Cl2. After an additional 10 min, the reaction appeared complete (product Rf 0.4, 80:20 hexanes/Et2O). The reaction was quenched with 0.100 mL (2.47 mmol, 3 equiv) of MeOH, stirred for several min, then filtered and the filtrate was concentrated to afford 1.250 g (97%) of 3,4-dimethoxybenzyl chloride. To a solution of 0.886 g (0.850 mmol, assuming a 3:1 ratio) of the mixture of 15 and the de-iodinated contaminant and 15 mg (0.041 mmol, 0.05 equiv) of Bu4NI in 10 mL of dry DMF was added 0.37 g (9.3 mmol, 11 equiv) of NaH (60% suspension in mineral oil). After stirring for several minutes, a solution of the freshly prepared 3,4-dimethoxybenzyl chloride in 10 mL of dry DMF was added, causing the reaction mixture to turn briefly bright purple before fading back to amber. After stirring overnight, the reaction was terminated by addition of H2O and the solvent was removed under reduced pressure. The resulting residue was dissolved in Et2O and washed with H2O and satd. NaCl and dried over Na2SO4. Flash chromatography of the reaction mixture in toluene with use of 95:5, 85: 15, and 80:20 toluene/EtOAc produced 1.113 g (98%, assuming the same ratio) of an inseparable mixture of 2 and the corresponding de-iodinated contaminant (Rf 0.5, 70:30 toluene/EtOAc). 1HNMR (500 MHz): δ 3.23–4.00 (m, 6 H), 3.47 (dd, 1 H, J = 3.5, 10.5 Hz), 3.63 (t, 1 H, J = 9.5 Hz), 3.75 (s, 3 H), 3.77 (s, 6 H), 3.81 (s, 3 H), 3.82 (s, 3 H), 3.95 (t, 1 H, J = 9.0 Hz), 3.96 (t, 1 H, J = 8.5 Hz), 4.09 (br d, 1 H, J = 10.0 Hz), 4.15 (br d, 1 H, J = 10.0 Hz), 4.32 (d, 1 H, J = 12.0 Hz), 4.34 (d, 1 H, J = 11.5 Hz), 4.40 (d, 1 H, J = 11.0 Hz), 4.42 (d, 1 H, J = 11.5 Hz), 4.45 (d, 1 H, J = 12.0 Hz), 4.47 (d, 1 H, J = 11.5 Hz), 4.53 (d, 1 H, J = 11.5 Hz), 4.54 (d, 1 H, J = 12.0 Hz), 4.56 (d, 1 H, J = 11.5 Hz), 4.60 (s, 2 H), 4.71 (d, 1 H, J = 11.0 Hz), 4.80 (d, 1 H, J = 11.0 Hz), 4.83 (d, 1 H, J = 11.0 Hz), 4.84 (d, 1 H, J = 11.0 Hz), 4.96 (d, 1 H, J = 10.5 Hz), 5.17 (d, 1 H, J = 3.5 Hz), 5.21 (d, 1 H, J = 3.5 Hz), 6.72–6.85 (m, 8 H), 6.95 (d, 2 H, J = 8.0 Hz), 7.07–7.38 (m, 21 H), 7.52 (d, 2 H, J = 8.0 Hz). 13C NMR (125 MHz): δ 55.39, 55.95, 55.97, 56.02, 67.38, 67.96, 70.78, 72.05, 72.53, 73.65, 74.79, 75.15, 75.30, 75.69, 77.85, 79.01, 79.59, 81.77, 81.89, 93.12, 94.18, 94.55, 110.96, 111.55, 111.63, 113.87, 120.97, 127.56, 127.69, 127.85, 128.99, 128.01, 128.09, 128.52, 128.54, 128.56, 128.61, 128.65, 129.10, 129.23, 129.25, 129.37, 130.36, 130.41, 133.37, 137.46, 137.53, 137.92, 138.31, 139.02, 148.85, 149.09, 159.30. HRMS (FAB) m/z 1379.4164 (MLi+ C73H78LiO16ClI requires 1379.4183).
3,4,4′-Tri-O-benzyl-3′-O-(p-chlorobenzyl)-6,6′-di-O-(3,4-dimethoxybenzyl)-2-O-(p-methoxybenzyl)-α,α-d-trehalose (16)
After azeotropically drying 0.893 g (0.665 mmol, assuming a 3:1 ratio) of the mixture of 2 and the corresponding de-iodinated contaminant with toluene (3×) and drying under vacuum for 1 h, 30 mg (0.03 mmol, 0.05 equiv) of Pd2dba3 and 30 mg (0.08 mmol, 0.12 equiv) of 2-(dicyclohexylphosphino)-2′-(N,N-dimethylamino)-biphenyl were added to the residue. The mixture was dissolved in 4.5 mL of dry THF and 55 µL (0.68 mmol, 1.02 equiv) of N-methylaniline was added, followed by 1.2 mL (1.2 mmol, 1.3 equiv) of a 1 M solution of lithium bis(trimethylsilyl)amide in THF. The rubber septum was replaced with a Teflon-sleeved glass stopper (Teflon sleeves were already in use in all other glass joints) and the reaction mixture was heated to 65 °C. After stirring overnight, the reaction was terminated with satd. NH4Cl and stirred for several minutes before being neutralized with satd. NaHCO3. The reaction mixture was diluted with Et2O and filtered through Celite. The filtrate was washed with satd. NaCl and dried over Na2SO4. Flash chromatography of the reaction mixture in toluene with use of 60: 40 and 50:50 hexanes/EtOAc yielded 0.757 g of mixed aminated 2 and the unreacted de-iodinated contaminant (Rf 0.6, 60:40 EtOAc/hexanes). The mixture was dissolved in 25 mL of dry CH2Cl2 and 1.2 mL (1.2 mmol, 2.1 equiv) of 1 M ZnCl2 in Et2O was added. After stirring 1 h, the reaction mixture had turned a milky blue-green. It was diluted with CH2Cl2, washed with H2O, satd. NaHCO3, and satd. NaCl, and dried over Na2SO4. Flash chromatography of the reaction mixture in toluene with use of 60:40, 50:50, and 40: 60 hexanes/EtOAc afforded 0.337 g (33% over 4 steps) of 16 (Rf 0.4, 60:40 EtOAc/hexanes). 1H NMR (500 MHz): δ 2.06 (d, 1 H, J = 9.0 Hz), 3.49 (br d, 1 H, J = 9.5 Hz), 3.57–3.86 (m, 8 H), 3.74 (s, 3 H), 3.77 (s, 3 H), 3.80 (s, 3 H), 3.81 (s, 6 H), 3.93 (t, 1 H, J = 9.5 Hz), 4.00 (br d, 1 H, J = 9.5 Hz), 4.14 (br d, 1 H, J = 10.0 Hz), 4.39 (d, 1 H, J = 12.0 Hz), 4.40 (d, 1 H, J = 11.5 Hz), 4.47 (d, 2 H, J = 10.5 Hz), 4.56 (d, 2 H, J = 11.5 Hz), 4.61 (s, 2 H), 4.72 (d, 1 H, J = 10.5 Hz), 4.77–4.87 (m, 1 H), 4.81 (d, 1 H, J = 11.5 Hz), 4.83 (d, 1 H, J = 10.5 Hz), 4.84 (d, 1 H, J = 11.5 Hz), 4.96 (d, 1 H, J = 11.0 Hz), 5.17 (d, 1 H, J = 3.5 Hz), 5.23 (d, 1 H, J = 3.5 Hz), 6.74–6.92 (m, 8 H), 7.10–7.38 (m, 21 H). 13C NMR (125 MHz): δ 55.27, 55.84, 55.87, 55.90, 67.95, 67.98, 70.88, 71.03, 72.59, 73.42, 73.57, 74.52, 74.87, 75.29, 75.57, 77.68, 78.93, 81.83, 82.90, 93.73, 95.46, 110.91, 111.50, 111.52, 113.82, 120.80, 120.83, 127.64, 127.71, 127.87, 127.88, 127.91, 128.03, 128.45, 128.46, 128.48, 128.62, 129.12, 129.30, 130.16, 130.29, 130.36, 133.38, 137.32, 138.10, 138.20, 138.83, 148.76, 149.02, 159.27. HRMS (FAB) m/z 1163.4767 (MLi+ C66H73LiO16Cl requires 1163.4747).
3,4,4′-Tri-O-benzyl-3′-O-(p-(N-methyl-N-phenylamino)benzyl)-6,6′-di-O-(3,4-dimethoxybenzyl)-2-O-(p-methoxybenzyl)-α,α-d-trehalose (17)
Subjecting 1.192 g (1.030 mmol) of 16 to the amination conditions used for 16 but with 0.08 equiv of 2-(di-tert-butylphosphino)biphenyl in place of the 2-(dicyclohexylphosphino)-2′-(N,N-dimethylamino)biphenyl and with use of 1.3 equiv of N-methylaniline and 2.5 equiv of lithium bis(trimethylsilyl)amide solution produced, after flash chromatography of the reaction mixture in toluene with 60:40, 50:50, and 40:60 hexanes/EtOAc, 1.223 g (97%) of 17 (Rf 0.4, 60:40 EtOAc/hexanes). 1H NMR (400 MHz): δ 1.95 (d, 1 H, J = 6.6 Hz), 3.23 (s, 3 H), 3.50 (d, 1 H, J = 9.2 Hz), 3.56–3.88 (m, 5 H), 3.60 (t, 1 H, J = 9.6 Hz), 3.61 (t, 1 H, J = 9.6 Hz), 3.67 (dd, 1 H, J = 3.2, 9.6 Hz), 3.74 (s, 3 H), 3.78 (s, 3 H), 3.80 (s, 3 H), 3.82 (s, 6 H), 3.95 (t, 1 H, J = 9.2 Hz), 4.02 (d, 1 H, J = 10.0 Hz), 4.15 (d, 1 H, J = 12.0 Hz), 4.38 (d, 1 H, J = 11.6 Hz), 4.39 (d, 1 H, J = 12.0 Hz), 4.47 (d, 1 H, J = 10.8 Hz), 4.49 (d, 1 H, J = 10.4 Hz), 4.55 (d, 1 H, J = 12.0 Hz), 4.56 (d, 1 H, J = 12.0 Hz), 4.61 (s, 2 H), 4.77 (d, 1 H, J = 11.2 Hz), 4.80 (d, 1 H, J = 10.8 Hz), 4.82 (d, 1 H, J = 10.8 Hz), 4.83 (d, 1 H, J = 11.2 Hz), 4.97 (d, 1 H, J = 11.2 Hz), 5.18 (d, 1 H, J = 3.6 Hz), 5.23 (d, 1 H, J = 3.6 Hz), 6.76 (d, 4 H, J = 8.8 Hz), 6.81–6.87 (m, 4 H), 6.92–7.02 (m, 5 H), 7.13–7.48 (m, 21 H). 13C NMR (100 MHz): δ 40.32, 55.34, 55.91, 55.95, 55.97, 68.05, 68.10, 70.88, 71.19, 72.55, 72.58, 73.48, 73.61, 74.95, 75.35, 75.45, 75.64, 77.79, 77.88, 79.02, 81.93, 82.72, 93.78, 95.48, 110.99, 111.54, 111.60, 113.88, 120.21, 120.83, 120.87, 121.61, 127.67, 127.87, 127.96, 128.09, 128.52, 129.32, 129.36, 129.38, 130.29, 130.45, 130.49, 131.21, 138.26, 138.40, 138.95, 148.81, 149.01, 149.09, 159.32. HRMS (FAB) m/z 1234.5747 (MLi+ C73H81LiNO16 requires 1234.5715).
3,4,4′-Tri-O-benzyl-3′-O-(p-(N-methyl-N-phenylamino)benzyl)-6,6′-di-O-(3,4-dimethoxybenzyl)-2-O-(p-methoxybenzyl)-2′-O-palmitoyl-α,α-d-trehalose (18)
To a solution of 0.257 g (0.209 mmol) of 17 and a few crystals of DMAP in 5 mL of dry pyridine was added 0.10 mL (0.33 mmol, 1.6 equiv) of palmitoyl chloride. After stirring overnight, the reaction appeared complete (product Rf 0.4, 60:40 hexanes/EtOAc). The reaction mixture was poured over crushed ice and the aqueous layer extracted 3× with Et2O. The combined organic layers were washed with H2O, satd. NaHCO3, and satd. NaCl and dried over Na2SO4. Flash chromatography of the reaction mixture in toluene with use of 90:10, 80: 20, 73:30, and 65:35 hexanes/EtOAc yielded 0.259 g (84%) of 18. 1H NMR (500 MHz): δ 0.88 (t, 3 H, J = 7.0 Hz), 1.12–1.43 (m, 24 H), 1.47–1.63 (m, 2 H), 2.21–2.28 (m, 2 H), 3.27 (s, 3H), 3.46 (d, 1 H, J = 9.0 Hz), 3.54 (d, 1 H, J = 9.5 Hz), 3.56 (dd, 1 H, J = 3.5, 10.0 Hz), 3.59 (dd, 1 H, J = 3.5, 11.0 Hz), 3.65 (t, 1 H, J = 9.5 Hz), 3.66–3.90 (m, 2 H), 3.71 (td, 1 H, J = 3.0, 7.0 Hz), 3.75 (s, 3 H), 3.79 (s, 3 H), 3.80 (s, 3 H), 3.82 (s, 6 H), 3.96 (t, 1 H, J = 9.5 Hz), 4.04 (t, 1 H, J = 9.5 Hz), 4.19 (d, 1 H, J = 10.0 Hz), 4.36 (d, 2 H, J = 12.0 Hz), 4.47 (d, 1 H, J = 11.0 Hz), 4.48 (d, 1 H, J = 10.5 Hz), 4.54 (d, 1 H, J = 12.0 Hz), 4.55 (d, 1 H, J = 11.5 Hz), 4.60 (s, 2 H), 4.69 (d, 1 H, J = 10.5 Hz), 4.74 (d, 1 H, J = 10.5 Hz), 4.80 (d, 1 H, J = 12.0 Hz), 4.81 (d, 1 H, J = 11.0 Hz), 4.82 (d, 1 H, J = 11.0 Hz), 4.96 (dd, 1 H, J = 4.0, 9.5 Hz), 4.97 (d, 1 H, J = 11.0 Hz), 5.17 (d, 1 H, J = 3.5 Hz), 5.29 (d, 1 H, J = 4.0 Hz), 6.74–6.78 (m, 3 H), 6.82–6.97 (m, 4 H), 6.92–6.97 (m, 2 H), 6.98–7.02 (m, 2 H), 7.10–7.36 (m, 23 H). 13C NMR (125 MHz): δ 14.13, 22.69, 24.80, 29.30, 29.37, 29.56, 29.68, 29.72, 31.92, 34.20, 40.22, 55.21, 55.79, 55.82, 67.84, 67.93, 70.89, 71.11, 72.51, 73.04, 73.46, 73.58, 75.03, 75.15, 75.29, 75.52, 77.61, 77.79, 78.77, 79.79, 81.70, 93.16, 93.73, 110.83, 111.33, 111.37, 113.75, 120.00, 120.60, 120.63, 120.67, 121.44, 127.53, 127.74, 127.79, 128.36, 128.84, 129.18, 129.20, 130.12, 130.27, 130.37, 131.07, 138.22, 138.25, 138.81, 148.56, 148.64, 148.70, 148.89, 148.96, 148.98, 159.18, 172.86. HRMS (FAB) m/z 1472.8036 (MLi+ C89H111LiNO17 requires 1472.8012).
3,4,4′-Tri-O-benzyl-6,6′-di-O-(3,4-dimethoxybenzyl)-2-O-(p-methoxybenzyl)-2′-O-palmitoyl-α,α-d-trehalose (19)
Subjecting 0.250 g (0.170 mmol) of 18 to the ZnCl2 cleavage conditions used for 16 afforded, after flash chromatography of the reaction mixture in toluene with use of 80:20 and 70:30 hexanes/EtOAc, 0.166 g (76%) of 19 (Rf 0.8, 50:50 hexanes/EtOAc). 1H NMR (400 MHz): δ 0.88 (t, 3 H, J = 6.8 Hz), 1.10–1.48 (m, 24 H), 1.52–1.65 (m, 2 H), 2.16–2.40 (m, 2 H), 3.43–3.98 (m, 5 H), 3.47 (d, 1 H, J = 10.0 Hz), 3.53 (d, 1 H, J = 9.6 Hz), 3.55 (td, 1 H, J = 3.2, 10.0 Hz), 3.63 (t, 1 H, J = 9.6 Hz), 3.74 (s, 3 H), 3.80 (s, 6 H), 3.82 (s, 3 H), 3.84 (s, 3 H), 3.93 (t, 1 H, J = 9.6 Hz), 4.16 (t, 2 H, J = 9.6 Hz), 4.35 (d, 1 H, J = 12.4 Hz), 4.39 (d, 1 H, J = 12.8 Hz), 4.46 (d, 1 H, J = 10.8 Hz), 4.53 (d, 1 H, J = 12.0 Hz), 4.54 (d, 1 H, J = 10.8 Hz), 4.57 (d, 1 H, J = 10.8 Hz), 4.58 (s, 2 H), 4.73 (d, 1 H, J = 11.2 Hz), 4.78 (d, 1 H, J = 10.8 Hz), 4.80 (d, 1 H, J = 10.0 Hz), 4.83 (dd, 1 H, J = 3.6, 8.8 Hz), 4.92 (d, 1 H, J = 11.2 Hz), 5.15 (d, 1 H, J = 3.2 Hz), 5.27 (d, 1 H, J = 3.6 Hz), 6.73–6.88 (m, 8 H), 7.10–7.41 (m, 17 H). 13C NMR (100 MHz): δ 14.12, 22.69, 24.78, 29.17, 29.32, 29.36, 29.53, 29.66, 29.71, 31.92, 34.08, 55.22, 55.81, 55.84, 67.86, 67.98, 70.54, 71.16, 71.77, 72.54, 72.86, 73.49, 73.53, 74.70, 74.94, 75.57, 78.07, 78.80, 81.77, 92.97, 93.72, 110.89, 111.36, 111.39, 113.75, 120.57, 120.67, 127.57, 127.62, 127.84, 127.91, 128.30, 128.35, 128.51, 129.12, 130.15, 130.31, 138.24, 138.35, 138.75, 148.71, 149.00, 159.18, 173.44. HRMS (FAB) m/z 1277.6964 (MLi+C75H98LiO17 requires 1277.6964).
3,4,4′-Tri-O-benzyl-6,6′-di-O-(3,4-dimethoxybenzyl)-2-O-(p-methoxybenzyl)-3′-O-((S)-2-methylstearoyl)-2′-O-palmitoyl-α,α-d-trehalose (20)
To a solution of 0.616 g (0.484 mmol) of 19 and 0.248 g (0.829 mmol, 1.7 equiv) of 5 26,43 in 15 mL of dry CH2Cl2 at 0 °C was added a solution of 0.650 g (3.39 mmol, 7 equiv) of EDC and 0.240 g (1.97 mmol, 4 equiv) of DMAP in 10 mL of dry CH2Cl2. The resulting solution was allowed to warm to rt and left stirring overnight. The reaction mixture was diluted with satd. NH4Cl and extracted 5× with Et2O. The combined organic layers were dried over Na2SO4. Flash chromatography of the reaction mixture in toluene with use of 75:25 hexanes/EtOAc provided 0.627 g (83%) of 20 (Rf 0.6, 60:40 hexanes/EtOAc). 1H NMR (500 MHz): δ 0.88 (t, 6 H, J = 7.0 Hz), 1.07 (d, 3 H, J = 7.0 Hz), 1.13–1.38 (m, 52 H), 1.43–1.66 (m, 4 H), 2.16–2.24 (m, 2 H), 2.32 (q, 1 H, J = 7.0 Hz), 3.40 (d, 1 H, J = 10.0 Hz), 3.47–3.56 (m, 1 H), 3.50 (d, 1 H, J = 8.5 Hz), 3.52 (td, 1 H, J = 3.5, 10.0 Hz), 3.62 (t, 1 H, J = 10.0 Hz), 3.65 (dd, 1 H, J = 3.5, 10.5 Hz), 3.70 (s, 3 H), 3.75–3.87 (m, 1 H), 3.78 (t, 1 H, J = 10.0 Hz), 3.81 (s, 3 H), 3.82 (s, 3 H), 3.83 (s, 3 H), 3.86 (s, 3 H), 4.02 (t, 1 H, J = 9.5 Hz), 4.23 (d, 1 H, J = 10.0 Hz), 4.34 (d, 1 H, J = 11.5 Hz), 4.35 (d, 1 H, J = 12.0 Hz), 4.41 (d, 1 H, J = 11.0 Hz), 4.47 (d, 1 H, J = 11.0 Hz), 4.50 (d, 1 H, J = 11.5 Hz), 4.51 (d, 1 H, J = 10.5 Hz), 4.54 (d, 1 H, J = 11.5 Hz), 4.62 (d, 1 H, J = 11.5 Hz), 4.82 (d, 1 H, J = 11.0 Hz), 4.84 (d, 1 H, J = 11.0 Hz), 4.97 (dd, 1 H, J = 3.5, 10.0 Hz), 4.97 (d, 1 H, J = 11.0 Hz), 5.13 (d, 1 H, J = 3.5 Hz), 5.28 (d, 1 H, J = 3.5 Hz), 5.65 (t, 1 H, J = 10.0 Hz), 6.68–6.81 (m, 8 H), 7.08–7.16 (m, 5 H), 7.22–7.34 (m, 12 H). 13C NMR (125 MHz): δ 14.14, 16.68, 22.70, 24.60, 27.25, 29.26, 29.39, 29.55, 29.60, 29.69, 29.71, 29.74, 29.76, 31.94, 33.70, 33.93, 39.53, 55.16, 55.78, 55.81, 55.83, 67.92, 70.22, 70.79, 71.22, 71.38, 72.71, 73.48, 73.51, 74.00, 74.96, 75.66, 75.93, 77.52, 78.65, 81.65, 92.79, 93.91, 110.73, 110.78, 111.23, 111.34, 113.69, 120.51, 120.71, 127.52, 127.61, 127.67, 127.93, 128.27, 128.29, 128.36, 129.03, 130.18, 130.25, 137.80, 138.28, 138.77, 148.63, 148.68, 148.95, 159.08, 172.89, 175.62. HRMS (FAB) m/z 1557.9704 (MLi+ C94H134LiO18 requires 1557.9730).
3,4,4′-Tri-O-benzyl-2-O-(p-methoxybenzyl)-3′-O-((S)-2-meth-ylstearoyl)-2′-O-palmitoyl-α,α-d-trehalose (21)
To a solution of 0.093 g (0.060 mmol) of 20 in 2 mL of CH2Cl2 was added 0.1 mL of H2O. The reaction mixture was cooled to 0 °C and 26 mg (0.12 mmol, 2 equiv) of DDQ was added. After 2 h at 4 °C, the reaction mixture was quenched with satd. NaHCO3 and diluted with CH2Cl2. The organic layer was washed with satd. NaHCO3 and satd. NaCl and dried over Na2SO4. Flash chromatography of the reaction mixture in toluene with use of 80:20, 70:30, and 60:40 hexanes/EtOAc yielded 0.050 g (67%) of 21 (Rf = 0.2, 60:40 hexanes/EtOAc). 1H NMR (500 MHz): δ 0.88 (t, 6 H, J = 7.0 Hz), 1.10 (d, 3 H, J = 7.0 Hz), 1.13–1.38 (m, 52 H), 1.45–1.68 (m, 4 H), 2.26 (t, 2 H, J = 8.0 Hz), 2.36 (q, 1 H, J = 7.0 Hz), 3.50 (td, 1 H, J = 3.5, 10.0 Hz), 3.50 (d, 1 H, J = 7.5 Hz), 3.54–3.76 (m, 5 H), 3.72 (s, 3 H), 3.73 (t, 1 H, J = 10.0 Hz), 4.06 (t, 1 H, J = 9.5 Hz), 4.14 (d, 1 H, J = 10.0 Hz), 4.56 (d, 1 H, J = 11.5 Hz), 4.59 (s, 2 H), 4.64 (d, 1 H, J = 11.0 Hz), 4.66 (d, 1 H, J = 11.5 Hz), 4.87 (dd, 1 H, J = 3.5, 10.0 Hz), 4.88 (d, 1 H, J = 11.0 Hz), 4.89 (d, 1 H, J = 11.0 Hz), 5.00 (d, 1 H, J = 11.0 Hz), 5.04 (d, 1 H, J = 3.5 Hz), 5.24 (d, 1 H, J = 3.5 Hz), 5.70 (t, 1 H, J = 10.0 Hz), 6.77 (d, 2 H, J = 8.5 Hz), 7.18 (d, 2 H, J = 8.5 Hz), 7.23–7.36 (m, 15 H). 13C NMR (125 MHz): δ 14.14, 16.71, 22.70, 24.58, 27.28, 29.23, 29.36, 29.39, 29.56, 29.68, 29.71, 29.74, 31.94, 33.73, 33.93, 39.57, 55.20, 61.16, 61.72, 70.90, 70.92, 71.09, 71.78, 72.97, 74.18, 75.05, 75.57, 75.68, 78.79, 81.55, 92.61, 93.80, 113.78, 127.56, 127.78, 127.85, 127.87, 127.93, 128.02, 128.41, 128.43, 128.45, 129.12, 130.01, 137.67, 138.04, 138.67, 159.19, 173.24, 175.65. HRMS (FAB) m/z 1257.8329 (MLi+ C76H114LiO14 requires 1257.8369).
3,4,4′-Tri-O-benzyl-2-O-(p-methoxybenzyl)-6,6′-di-O-((S)-2-methylarachidoyl)-3′-O-((S)-2-methylstearoyl)-2′-O-palmitoyl-α,α-d-trehalose (22)
To a solution of 0.269 g (0.215 mmol) of 21 and 0.285 g (0.872 mmol, 4 equiv) of 6 27,43 in 10 mL of dry CH2Cl2 at 0 °C was added a solution of 0.581 g (3.03 mmol, 14 equiv) of EDC and 0.212 g (1.74 mmol, 8 equiv) of DMAP in 10 mL of dry CH2Cl2. The resulting solution was allowed to warm to rt and left stirring overnight. The reaction mixture was diluted with satd. NH4Cl and extracted 5× with Et2O. The combined organic layers were dried over Na2SO4. Flash chromatography of the reaction mixture in toluene with use of 100:0, 98:2, 95:5, and 92.5: 7.5 hexanes/EtOAc produced 0.360 g (90%) of 22 (Rf 0.7, 85:15 hexanes/EtOAc). 1H NMR (400 MHz): δ 0.88 (t, 12 H, J = 6.8 Hz), 1.10 (d, 3 H, J = 6.0 Hz), 1.11 (d, 3 H, J = 6.4 Hz), 1.15 (d, 3 H, J = 6.8 Hz), 1.05–1.47 (m, 116 H), 1.48–1.73 (m, 8 H), 2.24 (t, 1 H, J = 8.0 Hz), 2.25 (t, 1 H, J = 8.0 Hz), 2.36 (q, 1 H, J = 6.8 Hz), 2.43 (q, 1 H, J = 6.8 Hz), 2.47 (q, 1 H, J = 6.8 Hz), 3.48 (t, 1 H, J = 9.6 Hz), 3.54 (dd, 1 H, J = 3.6, 10.0 Hz), 3.67 (t, 1 H, J = 10.0 Hz), 3.71 (s, 3 H), 3.90 (dd, 1 H, J = 3.2, 8.0 Hz), 4.07 (t, 1 H, J = 9.2 Hz), 4.08 (d, 1 H, J = 10.8 Hz), 4.16 (t, 1 H, J = 5.6 Hz), 4.17 (t, 1 H, J = 5.6 Hz), 4.26 (dd, 1 H, J = 4.4, 10.4 Hz), 4.29 (d, 1 H, J = 10.0 Hz), 4.49 (d, 1 H, J = 10.8 Hz), 4.55 (d, 2 H, J = 10.8 Hz), 4.57 (d, 1 H, J = 11.2 Hz), 4.66 (d, 1 H, J = 11.2 Hz), 4.86 (d, 1 H, J = 10.8 Hz), 4.89 (d, 1 H, J = 11.2 Hz), 4.97 (dd, 1 H, J = 3.6, 10.0 Hz), 5.00 (d, 1 H, J = 10.8 Hz), 5.10 (d, 1 H, J = 3.6 Hz), 5.18 (d, 1 H, J = 3.6 Hz), 5.70 (t, 1 H, J = 10.0 Hz), 6.77 (d, 2 H, J = 8.4 Hz), 7.15–7.40 (m, 17 H). 13C NMR (100 MHz): δ 14.13, 16.61, 16.97, 17.07, 22.71, 24.68, 27.26, 27.28, 27.31, 29.26, 29.38, 29.56, 29.69, 29.74, 31.94, 33.69, 33.83, 34.01, 39.43, 39.51, 39.56, 55.19, 62.05, 62.45, 68.71, 69.60, 70.47, 71.32, 73.10, 74.24, 75.20, 75.79, 76.26, 77.86, 78.99, 81.53, 92.19, 93.09, 113.90, 127.64, 127.82, 127.91, 128.01, 128.43, 129.11, 129.84, 137.37, 137.86, 138.56, 159.30, 172.75, 175.57, 176.43, 176.55. LRMS (MALDI) m/z 1890 (MNa+).
3,4,4′-Tri-O-benzyl-6,6′-di-O-((S)-2-methylarachidoyl)-3′-O-((S)-2-methylstearoyl)-2′-O-palmitoyl-α,α-d-trehalose (23)
Subjecting 0.317 g (0.170 mmol) of 22 to the reaction conditions used for 21 but with use of 3 equiv of DDQ and running the reaction at rt afforded, after flash chromatography of the reaction mixture in toluene with 95:5, 92.5:7.5, and 90:10 hexanes/EtOAc, 0.172 g (58%) of 23 (Rf 0.5, 85:15 hexanes/EtOAc) as well as 0.081 g (26%) of recovered starting material. 1HNMR (500 MHz): δ 0.88 (t, 12 H, J = 7.0 Hz), 1.12 (d, 3 H, J = 7.0 Hz), 1.13 (d, 3 H, J = 7.0 Hz), 1.16 (d, 3 H, J = 7.0 Hz), 1.07–1.47 (m, 116 H), 1.49–1.73 (m, 8 H), 1.90 (br s, 1 H), 2.25 (t, 1 H, J = 8.0 Hz), 2.26 (t, 1 H, J = 8.0 Hz), 2.38 (q, 1 H, J = 7.0 Hz), 2.45 (q, 1 H, J = 7.0 Hz), 2.49 (q, 1 H, J = 7.0 Hz), 3.49 (t, 1 H, J = 9.5 Hz), 3.65 (dd, 1 H, J = 4.0, 9.0 Hz), 3.66 (t, 1 H, J = 9.5 Hz), 3.84–3.91 (m, 1 H), 3.90 (t, 1 H, J = 9.5 Hz), 4.15–4.20 (m, 1 H), 4.20 (t, 1 H, J = 12.0 Hz), 4.21 (t, 1 H, J = 12.0 Hz), 4.26 (dd, 1 H, J = 5.0, 12.0 Hz), 4.34 (d, 1 H, J = 10.0 Hz), 4.51 (d, 1 H, J = 11.0 Hz), 4.58 (d, 1 H, J = 11.0 Hz), 4.61 (d, 1 H, J = 11.0 Hz), 4.85 (d, 1 H, J = 11.5 Hz), 4.88 (d, 1 H, J = 11.5 Hz), 4.92 (d, 1 H, J = 11.5 Hz), 4.97 (dd, 1 H, J = 3.5, 10.0 Hz), 5.08 (d, 1 H, J = 3.5 Hz), 5.22 (d, 1 H, J = 3.5 Hz), 5.64 (t, 1 H, J = 10.0 Hz), 7.20–7.41 (m, 15 H). 13C NMR (125 MHz): δ 14.14, 16.65, 16.98, 17.09, 22.71, 24.71, 27.26, 27.29, 27.32, 29.26, 29.38, 29.51, 29.56, 29.60, 29.63, 29.68, 29.74, 31.94, 33.70, 33.79, 33.82, 34.01, 39.44, 39.51, 39.59, 68.95, 69.96, 70.40, 71.41, 71.87, 74.62, 75.17, 75.69, 76.27, 77.85, 81.98, 91.84, 94.19, 127.79, 127.88, 127.96, 128.07, 128.19, 128.50, 128.77, 137.18, 137.63, 138.15, 172.71, 175.71, 176.50, 176.53. LRMS (MALDI) m/z 1770 (MNa+).
3,4,4′-Tri-O-benzyl-6,6′-di-O-((S)-2-methylarachidoyl)-3′-O-((S)-2-methylstearoyl)-2′-O-palmitoyl-α,α-d-trehalose-2–O-sulfate, Sodium Salt (24)
To a solution of 0.160 g (91.5 µmol) of 23 in 8 mL of dry pyridine was added 0.150 g (0.940 mmol, 10 equiv) of SO3·pyridine. After 10 h, the reaction was quenched with MeOH and concentrated under reduced pressure. The residue was redissolved in minimal CH2Cl2 and subjected to flash chromatography with use of 85:15, 75:25, and 70:30 hexanes/acetone. The product-containing fractions were pooled and concentrated and the residue dissolved in 9:1 CH2Cl2/MeOH and passed through a Dowex 50WX8–400 column (Na+ form) with use of the same solvent system to provide 0.162 g (96%) of 24 (Rf 0.7, 60:40 hexanes/acetone). 1H NMR (500 MHz, 70:30 CDCl3/CD3OD): δ 0.88 (t, 12 H, J = 7.0 Hz), 1.09 (d, 3 H, J = 7.0 Hz), 1.12 (d, 3 H, J = 7.0 Hz), 1.19 (d, 3 H, J = 7.0 Hz), 1.08–1.74 (m, 124 H), 2.28 (t, 2 H, J = 8.0 Hz), 2.36 (q, 1 H, J = 7.0 Hz), 2.44 (q, 1 H, J = 7.0 Hz), 2.56 (q, 1 H, J = 7.0 Hz), 3.52 (t, 1 H, J = 9.5 Hz, H-4), 3.79 (t, 1 H, J = 10.0 Hz, H-4′), 3.92 (ddd, 1 H, J = 2.0, 5.0, 9.5 Hz, H-5), 4.10 (t, 1 H, J = 9.5 Hz, H-3), 4.18 (d, 1 H, J = 10.5 Hz, H-6), 4.25 (dd, 1 H, J = 5.0, 12.0 Hz, H-6), 4.37 (d, 1 H, J = 11.5 Hz, H-6′), 4.46 (dd, 1 H, J = 4.0, 9.5 Hz, H-2), 4.52 (d, 1 H, J = 10.5 Hz, H-5′), 4.53 (d, 1 H, J = 11.0 Hz), 4.59 (d, 2 H, J = 11.0 Hz), 4.63 (d, 1 H, J = 11.0 Hz, H-6′), 4.74 (d, 1 H, J = 10.5 Hz), 4.88 (d, 1 H, J = 11.0 Hz), 4.99 (dd, 1 H, J = 3.5, 10.0 Hz, H-2′), 5.16 (d, 1 H, J = 10.5 Hz), 5.24 (d, 1 H, J = 3.5 Hz, H-1′), 5.53 (d, 1 H, J = 4.0 Hz, H-1), 5.68 (t, 1 H, J = 10.0 Hz, H-3′), 7.20–7.35 (m, 12 H), 7.44–7.53 (m, 3 H). 13C NMR (125 MHz, 70:30 CDCl3/CD3OD): δ 14.23, 16.82, 17.06, 17.32, 22.98, 24.98, 27.54, 27.60, 27.69, 29.17, 29.57, 29.68, 29.70, 29.82, 29.85, 29.86, 29.88, 29.93, 29.97, 30.02, 30.08, 31.95, 32.24, 34.08, 34.10, 34.23, 34.31, 39.78, 39.91, 40.00, 62.53, 63.09, 69.09, 69.64, 71.09, 71.95, 74.69, 75.50, 75.88, 76.30, 80.18, 92.43, 93.55, 127.90, 127.92, 128.11, 128.12, 128.15, 128.54, 128.77, 128.63, 128.67, 129.01, 137.77, 138.19, 138.74, 173.26, 176.46, 177.48, 177.82. HRMS (ESI) m/z 1872.3111 (MNa+ C110H185Na2O18S requires 1872.3071).
6,6′-Di-O-((S)-2-methylarachidoyl)-3′-O-((S)-2-methylstearoyl)-2′-O-palmitoyl-α,α-d-trehalose-2-O-sulfate, Sodium Salt (1)
To a solution of 0.020 g (1.1 µmol) of 24 in 1 mL of CH2Cl2 and 1 mL of MeOH was added 0.010 g of 30% Pd/C. The reaction mixture was placed under 500 psi of H2 in a Parr bomb and stirred vigorously overnight. After filtering through Celite, the reaction mixture was concentrated under reduced pressure and the residue was redissolved in minimal CH2Cl2 and subjected to flash chromatography with use of 70:30, 65:35, 60:40, 55:45, 50:50, and 40: 60 hexanes/acetone. The product-containing fractions were pooled and dried and the residue was dissolved in 9:1 CH2Cl2/MeOH and passed through a Dowex 50WX8-400 column (Na+ form) with the same solvent system to yield 0.009 g (53%) of 1 (Rf 0.6, 50:50 hexanes/acetone). 1H NMR (500 MHz, 70:30 CDCl3/CD3OD): δ 0.88 (t, 12 H, J = 7.0 Hz), 1.14 (d, 3 H, J = 7.0 Hz), 1.16 (d, 6 H, J = 7.0 Hz), 1.09–1.77 (m, 124 H), 2.30 (t, 1 H, J = 8.0 Hz), 2.31 (t, 1 H, J = 8.0 Hz), 2.46 (q, 1 H, J = 7.0 Hz), 2.47 (q, 1 H, J = 7.0 Hz), 2.53 (q, 1 H, J = 7.0 Hz), 3.39 (t, 1 H, J = 9.5 Hz, H-4), 3.60 (t, 1 H, J = 10.0 Hz, H-4′), 3.84 (ddd, 1 H, J = 3.5, 6.0, 13.0 Hz, H-5), 3.94 (t, 1 H, J = 9.5 Hz, H-3), 4.25 (dd, 1 H, J = 4.0, 9.5 Hz, H-2), 4.22–4.34 (m, 3 H, H-6, H-5′), 4.39 (t, 1 H, J = 12.0 Hz, H-6′), 4.43 (dt, 1 H, J = 2.5, 12.5 Hz, H-6′), 4.94 (dd, 1 H, J = 3.5, 10.5 Hz, H-2′), 5.24 (d, 1 H, J = 3.5 Hz, H-1′), 5.43 (t, 1 H, J = 9.5 Hz, H-3′), 5.45 (d, 1 H, J = 3.5 Hz, H-1). 13C NMR (125 MHz, 70:30 CDCl3/CD3OD): δ 14.21, 17.03, 17.08, 17.13, 22.96, 25.07, 27.50, 27.54, 27.63, 29.19, 29.60, 29.66, 29.73, 29.85, 29.88, 29.92, 29.97, 30.03, 31.92, 32.24, 34.03, 34.08, 34.10, 34.19, 34.83, 39.90, 40.06, 62.46 (C-6′), 63.85 (C-6), 68.96 (C-4′), 70.36 (C-5′), 70.46 (C-5), 70.84 (C-2′), 71.05 (C-4), 71.97 (C-3), 72.49 (C-3′), 76.76 (C-2), 91.79 (C-1′), 92.32 (C-1), 173.14, 177.82, 178.22. HRMS (ESI) m/z 1556.1802 ([M – Na]− C89H167O18S requires 1556.1878).
Supplementary Material
Acknowledgment
This research was sponsored by grants to C.R.B. from the National Institutes of Health (AI51622) and Elan Pharmaceuticals.
Footnotes
Supporting Information Available: Note on the glycosylation of 12, table of reduction conditions tested for 14, general experimental information, and copies of NMR spectra for compounds 1, 2, 4, and 8–24. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1. TB Elimination: Now Is the Time!; National Center for HIV, STD, and TB Prevention: Division of Tuberculosis Elimination, http://www.cd-c.gov/tb/pubs/nowisthetime/pdfs/nowisthetime.pdf.
- 2. WHO Fact Sheets: Tuberculosis, http://www.who.int/mediacentre/factsheets/fs104/en/index.html.
- 3.Goren MB. Biochim. Biophys. Acta. 1970;210:116–126. doi: 10.1016/0005-2760(70)90067-6. [DOI] [PubMed] [Google Scholar]
- 4.Goren MB. Biochim. Biophys. Acta. 1970;210:127–138. doi: 10.1016/0005-2760(70)90068-8. [DOI] [PubMed] [Google Scholar]
- 5.Goren MB, Brokl O, Das BC, Lederer E. Biochemistry. 1971;10:72–81. doi: 10.1021/bi00777a012. [DOI] [PubMed] [Google Scholar]
- 6.Goren MB, Brokl O, Roller P, Fales HM, Das BC. Biochemistry. 1976;15:2728–2735. doi: 10.1021/bi00658a003. [DOI] [PubMed] [Google Scholar]
- 7.The acids are dextrorotary, and as such are assumed to be in the l configuration by analogy with similar compounds. The hydroxyl group of the hydroxyphthioceranic acids is assumed to be in the d configuration based on the positive shift in optical rotation between the phthioceranic and hydroxyphthioceranic acids and judging from the neighboring functionalities. See: Goren MB. Handbook Lip. Res. 1990;6:363–461..
- 8.Pabst MJ, Gross JM, Brozna JP, Goren MB. J. Immunol. 1988;140:634–640. [PubMed] [Google Scholar]
- 9.Brozna JP, Horan M, Rademacher JM, Pabst KM, Pabst MJ. Infect. Immun. 1991;59:2542–2548. doi: 10.1128/iai.59.8.2542-2548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goren MB, D'Arcy Hart P, Young MR, Armstrong JA. Proc. Natl. Acad. Sci. U.S.A. 1976;73:2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Goren MB, Holzer TJ, Andersen BA. Infect. Immun. 1988;56:2876–2883. doi: 10.1128/iai.56.11.2876-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, English D, Andersen BA. J. Immunol. 1988;146:2730–2736. [PubMed] [Google Scholar]
- 13.Sirakova TD, Thirumala AK, Dubey VS, Sprecher H, Kolattukudy PE. J. Biol. Chem. 2001;276:16833–16839. doi: 10.1074/jbc.M011468200. [DOI] [PubMed] [Google Scholar]
- 14.Domenech P, Pym AS, Cellier M, Barry CE, III, Cole ST. FEMS Microbiol. Lett. 2002;207:81–86. doi: 10.1111/j.1574-6968.2002.tb11032.x. [DOI] [PubMed] [Google Scholar]
- 15.Mougous JD, Leavell MD, Senaratne RH, Leigh CD, Williams SJ, Riley LW, Leary JA, Bertozzi CR. Proc. Natl. Acad. Sci. U.S.A. 2002;99:17037–17042. doi: 10.1073/pnas.252514899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Converse SE, Mougous JD, Leavell MD, Leary JA, Bertozzi CR, Cox JS. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6121–6126. doi: 10.1073/pnas.1030024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rousseau C, Turner OC, Rush E, Bordat Y, Sirakova TD, Kolattukudy PE, Ritter S, Orme IM, Gicquel B, Jackson M. Infect. Immun. 2003;71:4684–4690. doi: 10.1128/IAI.71.8.4684-4690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mougous JD, Petzold CJ, Senaratne RH, Lee DH, Akey DL, Lin FL, Munchel SE, Pratt MR, Riley LW, Leary JA, Berger JM, Bertozzi CR. Nature Struct. Mol. Biol. 2004;11:721–729. doi: 10.1038/nsmb802. [DOI] [PubMed] [Google Scholar]
- 19.Domenech P, Reed MB, Dowd CS, Manca C, Kaplan C, Barry CE., III J. Biol. Chem. 2004;279:21257–21265. doi: 10.1074/jbc.M400324200. [DOI] [PubMed] [Google Scholar]
- 20.Gilleron M, Stenger S, Mazorra Z, Wittke F, Mariotti S, Böhmer G, Prandi J, Mori L, Puzo G, De Libero G. J. Exp. Med. 2004;199:649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar P, Schelle MW, Jain M, Lin FL, Petzold CJ, Leavell MD, Leary JA, Cox JS, Bertozzi CR. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11221–11226. doi: 10.1073/pnas.0611649104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baer HH, Wu X. Carbohydr. Res. 1993;238:215–230. doi: 10.1016/0008-6215(93)87014-j. [DOI] [PubMed] [Google Scholar]
- 23.Chapleur Y, Castro B. J. Chem. Soc., Perkin Trans. 1. 1980:1940–1943. [Google Scholar]
- 24.Pratt MR, Leigh CD, Bertozzi CR. Org. Lett. 2003;5:3185–3188. doi: 10.1021/ol034836t. [DOI] [PubMed] [Google Scholar]
- 25.(a) Dan A, Lergenmüller M, Amano M, Nakahara Y, Ogawa T, Ito Y. Chem. Eur. J. 1998;4:2182–2190. [Google Scholar]; (b) Seifert J, Lergenmüller M, Ito Y. Angew. Chem., Int. Ed. 2000;39:531–534. doi: 10.1002/(sici)1521-3773(20000204)39:3<531::aid-anie531>3.3.co;2-6. [DOI] [PubMed] [Google Scholar]; (c) Ito Y, Ando H, Wada M, Kawai T, Ohnish Y, Nakahara Y. Tetrahedron. 2001;57:4123–4132. [Google Scholar]
- 26.Ade E, Helmchen G, Heiligenmann G. Tetrahedron Lett. 1980;21:1137–1140. [Google Scholar]
- 27.Besra GS, Minnikin DE, Wheeler PR, Ratledge C. Chem. Phys. Lipids. 1993;66:23–34. doi: 10.1016/0009-3084(93)90027-z. [DOI] [PubMed] [Google Scholar]
- 28.Garegg PJ, Helland A-C. J. Carbohydr. Chem. 1993;12:105–117. [Google Scholar]
- 29.Plante OJ, Buchwald SL, Seeberger PH. J. Am. Chem. Soc. 2000;122:7148–7149. [Google Scholar]
- 30.Qureshi S, Shaw G, Burgess GE. J. Chem. Soc, Perkin Trans. I. 1985;7:1557–1563. [Google Scholar]
- 31.Lichtenthaler FW, Klares U, Szurmai Z, Werner B. Carbohydr. Res. 1998;305:293–303. doi: 10.1016/s0008-6215(97)00249-8. [DOI] [PubMed] [Google Scholar]
- 32.Because the a-acetate is unreactive under common thioglycosylation conditions, the preferential crystallization of the β anomer of 8 was particularly fortuitous.
- 33.Das SK, Roy N. Carbohydr. Res. 1996;296:275–277. [Google Scholar]
- 34.Ferro V, Mocerino M, Stick RV, Tilbrook DMG. Aust. J. Chem. 1988;41:813–815. [Google Scholar]
- 35.Hadd MJ, Gervay J. Carbohydr. Res. 1999;320:61–69. [Google Scholar]
- 36.Polt R, Szabó L, Treiberg J, Li Y, Hruby VJ. J. Am. Chem. Soc. 1992;774:10249–10258. [Google Scholar]
- 37.In fact, Gervay–Hague demonstrates this sort of transformation,35 but only for fucosyl bromide. The article states that study of less reactive donors was underway.
- 38.Helferich B, Gootz R. Ber. 1929;62:2788–2792. [Google Scholar]
- 39.(a) Jennings HJ. Can. J. Chem. 1971;49:1355–1359. [Google Scholar]; (b) Kronzer FJ, Schuerch C. Carbohydr. Res. 1974;34:71–78. [Google Scholar]; (c) Nukada T, Lucas H, Konradsson P, van Boeckel CAA. Synlett. 1991:365–367. [Google Scholar]
- 40.As stated previously, this does not appear to be a result of the use of impure starting material; essentially the same results were obtained from analytically pure 13.
- 41.A table of the reduction conditions tested may be found in the Supporting Information.
- 42.Harris MC, Huang X, Buchwald SL. Org. Lett. 2002;4:2885–2888. doi: 10.1021/ol0262688. [DOI] [PubMed] [Google Scholar]
- 43.Acids 5 and 6 were obtained in 95% and 84% ee, respectively, as determined by GC comparison of their (ft)-α-methylbenzyl amides with those of the racemates. No alternate diastereomers were evident in the NMR spectra of 22; these were presumably removed during purification.
- 44.Horita K, Yoshioka T, Tanaka T, Oikawa Y, Yonemitsu O. Tetrahedron. 1986;42:3021–3028. [Google Scholar]
- 45.ter Horst B, Feringa BL, Minnaard AJ. Org. Lett. 2007;9:3013–3015. doi: 10.1021/ol071078o. [DOI] [PubMed] [Google Scholar]
- 46.The synthesis of p-methoxybenzyl iodide was adapted from the following: Lange GL, Gottardo C. Synth. Commun. 1990;20:1473–1479..
- 47.The synthesis of 3,4-dimethoxybenzyl chloride was adapted from the following: Chaudhari SS, Akamanchi KG. Synlett. 1999;11:1763–1765..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.