Abstract
Objective
To test the hypothesis that beta-amyloid (Aβ) burden is associated with rates of brain atrophy.
Methods
Forty-five subjects who had been prospectively studied, died, and had an autopsy diagnosis of low, intermediate, or high probability of Alzheimer's disease that had two volumetric head MRI scans were identified. Compact, as well as total (compact + diffuse) Aβ burden was measured using a computerized image analyzer with software program to detect the proportion of grey matter occupied by Aβ. Visual ratings of Aβ burden were also performed. The boundary-shift integral (BSI) was used to calculate change over time in whole brain and ventricular volume. All BSI results were annualized by adjusting for scan interval. Demographics, cognitive measures, clinical diagnoses, apolipoprotein E genotype, neurofibrillary tangle pathology, and vascular lesion burden were determined.
Results
There was no correlation between compact or total Aβ burden, or visual Aβ ratings, and rates of brain loss or ventricular expansion in all subjects. However, significant correlations were observed between rates of brain loss and age, Braak stage, and change over time in cognitive measures. These features also correlated with rates of ventricular expansion. The rates of brain loss and ventricular expansion were greater in demented compared to non-demented subjects.
Interpretation
These findings suggest that rate of brain volume loss is not determined by the amount of insoluble Aβ in the grey matter.
Introduction
The deposition of beta-amyloid (Aβ) in senile plaques is a characteristic feature of Alzheimer's disease (AD),1 a disease which affects over 29.3 million people worldwide.2 It has been hypothesized that the presence of Aβ protein is associated with neuronal loss and brain atrophy, and hence the cognitive decline that occurs in subjects with AD.3 This hypothesis led to the development of a vaccine which aimed to reduce Aβ burden in the brain.4, 5
Rates of brain atrophy have been shown to correlate to cognitive decline in subjects with AD and have therefore been proposed as useful biomarkers of disease progression.6 In fact, rates of whole brain atrophy were one of the primary outcome measures in the recent Aβ vaccine trial.7 Two previous studies have found associations between the rates of atrophy and the presence of Aβ 8 or senile plaques9 in subjects with AD. However, neither of these studies performed a direct measure of Aβ burden. In addition, the contribution of age and additional pathological processes, such as the presence of neurofibrillary tangles (NFT), which is another characteristic feature of AD, and vascular disease, to rates of brain atrophy is unclear.
The primary aim of this study was to determine whether there is an association between Aβ burden and rates of brain atrophy in a prospectively studied cohort that came to autopsy. As a secondary aim we also set out to assess the contribution of NFT pathology, vascular pathology, apolipoprotein E (ApoE) genotype, and demographic features on rates of brain atrophy. These associations were assessed across the entire cohort, and then as a secondary analysis the associations were assessed separately for those subjects that fulfilled clinical criteria for dementia10 and those that did not.
Methods
Subjects
Subjects were identified from the neuropathology files of the Mayo Clinic, Rochester, MN. All subjects had been studied prospectively in our Alzheimer's Disease Research Center (ADRC) or Alzheimer's Disease Patient Registry (ADPR) during life and had an autopsy at death. The ADRC and ADPR are longitudinal studies of dementia and ageing in which subjects undergo yearly clinical evaluations and cognitive testing, including the Mini-Mental State Examination (MMSE)11 and the Clinical Dementia Rating scale sum of boxes (CDR-SOB).12 The diagnosis of dementia was made based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition10 and the diagnosis of AD was made based on National Institute on Neurologic and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders criteria.13 Written informed consent was obtained for participation in the studies, which were approved by the Mayo Institutional Review Board.
Subjects were included in the study if they had been given a pathological diagnosis of low, intermediate or high probability AD according to the National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's disease (NIA-Reagan).14 In addition, in order to be included in the study all subjects had to have had two volumetric head MRI scans with the second MRI performed within three years of death, and the interval from first to second MRI between six months and four years.
Subjects were excluded if there were any pathological features other than AD-type pathology. Therefore, any subjects with cortical infarcts, tumors, or another degenerative disorder such as Lewy body disease, hippocampal sclerosis, progressive supranuclear palsy, corticobasal degeneration, or frontotemporal lobar degeneration were excluded. In addition, the clinical histories of all cases were reviewed and subjects who had treatments or concurrent illnesses that could interfere with brain function or volume were excluded. All MRI were reviewed and scans were rejected for poor quality (such as motion or susceptibility artifacts) or the presence of other pathologies (such as large cortical infarcts, tumor or other structural lesion) that may influence the structural analysis.
A total of 82 cases were identified that fulfilled the pathological criteria and had two volumetric MRI. Of these, 17 cases were excluded due to poor quality MRI or the presence of other structural lesions or tumors, and 20 were excluded because their last MRI was not within three years of death. A total of 45 subjects were identified that fulfilled our inclusion criteria. Thirty-two of these 45 subjects met criteria for dementia.10 The other thirteen subjects were recruited as normal controls and had not progressed to a clinical diagnosis of dementia.10
Apolipoprotein E genotyping
Apolipoprotein E testing was performed on all 45 subjects. Genomic DNA was extracted from fresh frozen brain using the QIAamp DNA mini kit (Qiagen, Bothel, Washington). Amplification was carried out using the single-day ApoE method used by Crook et al.15 with modifications as previously described.16
Neuropathologic procedure and diagnosis
Neuropathological examinations were performed according to the recommendations of the Consortium to Establish a Registry for Alzheimer's Disease.17 In all cases pathological assessment and diagnosis was conducted by one, or both, of two expert neuropathologists (DWD or JEP). After removal, the brain was divided into right and left hemibrains. One hemibrain was fixed in 10% buffered formaldehyde for 7 to 10 days, and then sectioned. Routinely sampled brain areas included: middle frontal gyrus (Brodmann area [BA] 9), inferior parietal lobule (BA 39), superior temporal gyrus (BA 22), calcarine cortex (BA 17), anterior cingulate gyrus (BA 24), hippocampus at the level of the lateral geniculate body, amygdala, transentorhinal and entorhinal cortices at the level of the mamillary bodies, nucleus basalis, cerebellum, dorsomedial thalamus with subthalamic nucleus, midbrain with substantia nigra, pons, and medulla. Samples were processed in paraffin and stained with hematoxylin and eosin, stained with modified Bielschowsky silver impregnation, and immunostained with antibodies to Aβ (clone 6F/3D, 1:10 dilution; Novocastra Vector Labs, Burlingame, CA), tau (clone AT8, 1:1,000 dilution; Endogen, Woburn, MA), alpha-synuclein (clone LB509, 1:200 dilution; Zymed, San Francisco, CA), neurofilament (clone 2F11, 1:75 dilution; DAKO, Carpinteria, CA), and ubiquitin (polyclonal, 1:100 dilution DAKO). A Braak NFT stage was assigned to each case based on the distribution of NFT’s with Bielchowsky silver stain.18 The diagnosis of AD was based on NIA-Reagan criteria.14
Amyloid analysis
In order to obtain a quantitative measure of Aβ burden an unbiased computer assisted image analyser was used to quantify the percentage area occupied by Aβ positive immunoreactive plaques. For each of the 45 subjects brain tissue slides were created from paraffin blocks of middle frontal gyrus (Brodmann area (BA 9)) and primary visual cortex (BA 17). All 90 slides were immunostained with an antibody against Aβ (Clone 6F/3D, 1/10 dilution: Novacastra Vector Labs, Burlingame, CA) using an automated immunohistochemical stainer with identical incubation times. Each immunostained frontal and occipital section was converted into a single high resolution digital image using an Aperio slide scanner (Aperio Technologies, Vista, CA). Two regions of interest were delineated on each image (Imagescope version 8, Aperio Technologies, Vista, CA) that represented a perpendicular strip of cortical grey matter (pial surface to grey-white junction) of about 1–2mm in width. An automated image analysis system was employed to visualise the Aβ within the selected regions applying a color deconvolution algorithm.19 Quantification of Aβ staining was performed to detect the proportion of grey matter occupied by Aβ immunoreactivity (expressed as the area of Aβ per the total area of the region-of-interest (Aβ burden)) for compact Aβ only using color translation and an automated thresholding algorithm (Aperio technology). A second automated threshold was designed to detect both compact and diffuse (total) Aβ deposits.
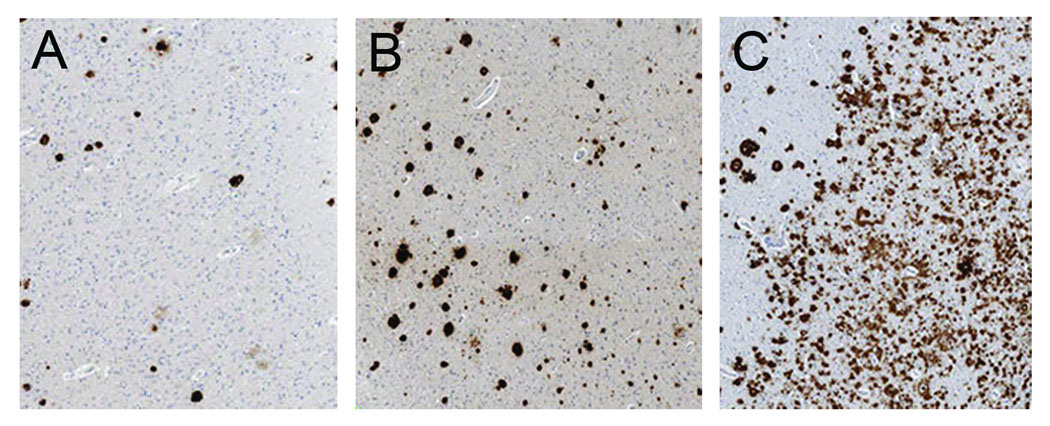
For each region-of-interest two raters (KAJ and ZA) performed an independent visual rating of the total Aβ burden on a four point scale (0=no Aβ, 1=scant Aβ deposition, 2=moderate Aβ, 3=severe Aβ burden) based on comparison to representative examples of Aβ burden (Figure 1). The concordance correlation coefficient for inter-rater reliability of visually rated Aβ burden was 0.96 for frontal Aβ and 0.92 for occipital Aβ. Since there was high concordance the results from the two raters were averaged for all statistical analysis.
Figure 1.
Examples of the different visual grading of Aβ burden including mild Aβ burden (A), moderate Aβ burden (B), and severe Aβ burden (C).
MRI analysis
All MRI studies were performed with a standardized imaging protocol that included a T1-weighted 3D coronal volumetric SPGR image with 124 contiguous partitions, and 1.6mm slice thickness (22×16.5cm field of view, minimum full TE, TR of 23 msec, and 25° flip angle) and a Fluid Attenuated Inversion Recovery (FLAIR) scan (TR=11,000 ms; TE=147ms; TI=2,250 ms; 3 mm interleaved images of the whole head).
Change in the whole brain and ventricle volumes were calculated using the SPGR scans and a software algorithm that has been developed in our lab and described in detail elsewhere.20 Repeat scans were registered, i.e. spatially matched, using 9 degrees of freedom (dof) rigid body registration, to the baseline scan for each subject. Change in brain and ventricle volume was calculated automatically from each registered scan pair using the boundary shift integral (BSI).20, 21 The BSI results were annualized and expressed as percentage change per year.
The presence of cerebro vascular disease was assessed semi quantitatively on FLAIR scans by an experienced research technician (MMS) who was blinded to pathological and clinical diagnosis using a synthesis of published criteria.22–24 The number of lacunar infarcts was counted in each subject and white matter hyper-intensity (WMH) load was graded with a visual analog scale. Each incoming FLAIR study was registered to a common template and compared against a bank of example scans to assign it a WMH load in units of cm3. WMH burden in units of cm3 had been determined quantitatively for each example case using an algorithm developed in our lab.25 The technician assigned each new incoming scan a WMH burden on a continuous scale (i.e. visual analog scale) using an electronic slider bar. The intra-class correlation coefficient for inter-rater reliability of this visual analog WMH grading scale was 0.96. The concordance correlation coefficient between quantitative measures and visual WMH grading was 0.93.
Statistics
Mann-Whitney U tests were used to compare continuous variables between demented and non-demented subjects, and to compare rates of atrophy between genders, clinical diagnosis groups (demented versus non-demented), APOE genotypes (e4 carriers and noncarriers) and between those with and without lacunar infarcts. Chi-squared tests were used to compare nominal variables between demented and non-demented subjects. For cells with small numbers Fishers exact test was used. Spearman rank order correlations (rho) were used to assess the relationship, both unadjusted and adjusted (partial correlations) for the effects of age at the time of the second scan and duration from the second scan to death, between rates of atrophy and the primary measure Aβ burden, and rates of atrophy and the secondary measures (Braak NFT stage, white matter hyperintensity load, age at scan, and change over time in the MMSE and CDR-SOB). The Spearman rank order correlations were performed across the whole cohort, and then separately for the demented and non-demented subjects. Since there were only 13 non-demented subjects these correlations were not adjusted. All p values quoted are two-sided. Since each of the correlations evaluated in our study was of interest in its own right and not data-driven, we do not adjust for multiple comparisons26, 27. Statistical analyses were performed utilizing the R language and environment for statistical computing (R Foundation for Statistical Computing, version 2.4.1, Vienna, Austria) with statistical significance set at p < 0.05.
Results
Table 1 shows the demographic, clinical and pathological features of the study group. There were significant differences between the demented and non-demented subjects in all variables, except for gender, education, and the time from last scan to death.
Table 1.
Subject demographics
| All subjects (n=45) | Demented (n=32) | Non-demented (n=13) | ||
|---|---|---|---|---|
| No. females (%) | 28 (62%) | 17 (53%) | 11 (85%) | |
| Age at first scan (yrs)* | 80 [75–87] (48–98) | 79 [72–85] (48–97) | 85 [83–92] (73–98) | |
| Age at second scan (yrs)* | 82 [77–88] (49–101) | 80 [73–86] (49–98) | 88 [85–94] (74–101) | |
| Education (yrs) | 13 [12–16] (7–18) | 14 [12–16] (8–18) | 12 [9–13] (7–18) | |
| Time from last scan to death (yrs) | 2.0 [1.0–2.5] (0–3.0) | 2.0 [1.0–2.8] (0–3.0) | 2.0 [1.0–2.5] (1.0–3.0) | |
| Scan interval (yrs)* | 1.5 [1.1–2.4] (0.6–3.9) | 1.4 [1.0–1.8] (0.6–3.0) | 2.0 [1.7–2.6] (1.0–3.9) | |
| Baseline MMSE (/30)** | 25 [20–28] (8 – 30) | 24 [18–27] (8–30) | 29 [27–30] (23–30) | |
| Baseline CDR-SOB (/18)** | 3.0 [0.5–6.5] (0 – 17) | 4.8 [2.6–7.8] (0–17) | 0 [0–1.3] (0–3) | |
| ApoE 4 allele carrier No. (%)* | 26 (58%) | 15 (71%) | 1 (11%) | |
| Braak NFT stage No. (%)** | ||||
| 2 | 1 (2%) | 0 (0%) | 1 (8%) | |
| 3 | 9 (20%) | 3 (9%) | 6 (46%) | |
| 4 | 6 (13%) | 3 (9%) | 3 (23%) | |
| 5 | 14 (31%) | 11 (34%) | 3 (23%) | |
| 6 | 15 (33%) | 15 (100%) | 0 (0%) | |
| NIA-Reagan No. (%)** | ||||
| Low | 7 (15%) | 2 (6%) | 5 (38%) | |
| Intermediate | 8 (18%) | 3 (9%) | 5 (38%) | |
| High | 30 (67%) | 27 (84%) | 3 (23%) | |
Results are shown as median [inter-quartile range] (range)
MMSE = Mini-Mental State Examination; CDR-SOB = Clinical Dementia Rating sum of boxes; NIA = National Institute of Aging; ApoE = Apolipoprotein E
Significant difference between demented and non-demented
p<0.01
p<0.001
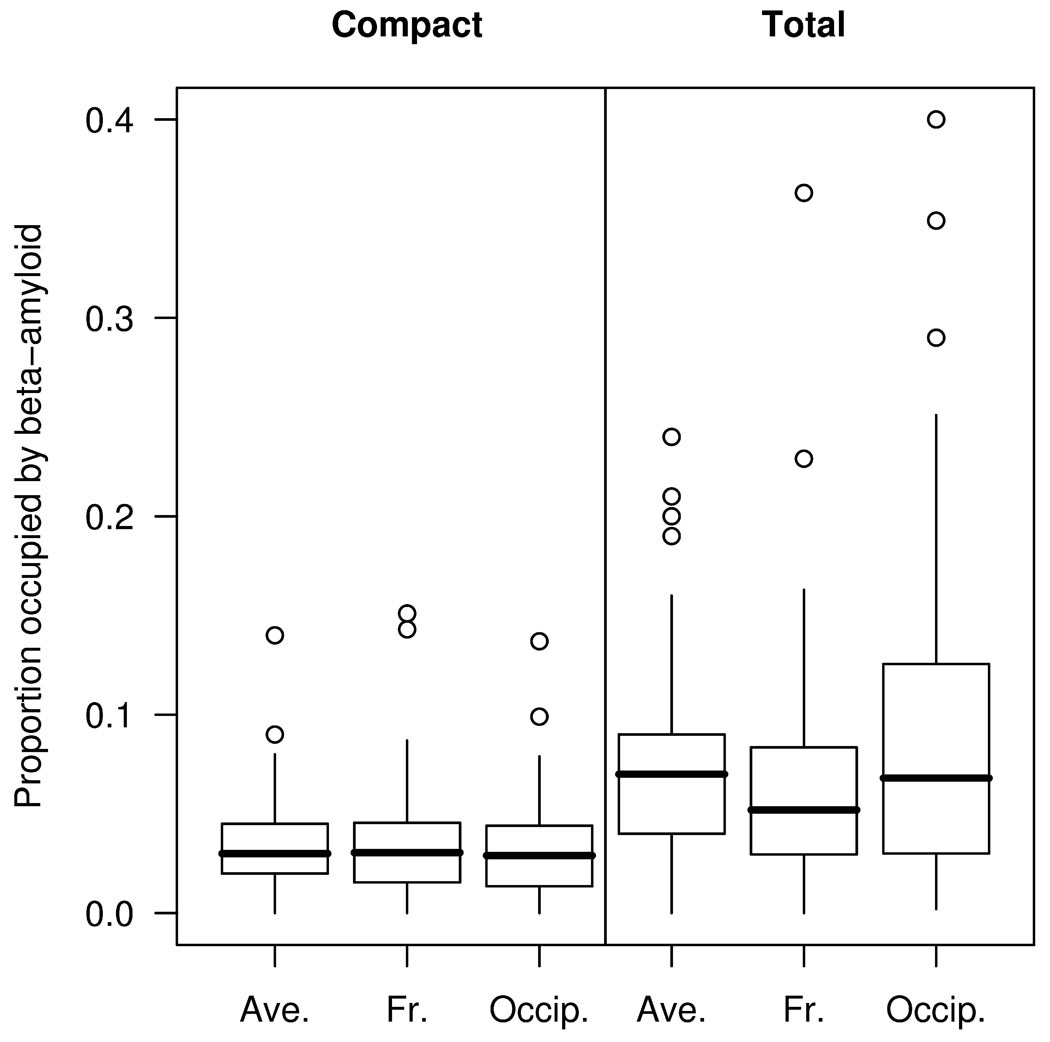
The median proportion (averaged across frontal and occipital regions) of grey matter occupied by compact Aβ was 0.031 (range 0.001–0.14), while the median proportion occupied by total Aβ was 0.067 (range 0.004–0.24) (Figure 2). The MMSE score declined between the first and second MRI by a median 3 points (range −3 – 17), and the CDR-SOB increased by 2 points (range −6 – 15). No correlations were observed between any of the measures of Aβ burden and change over time in MMSE or CDR-SOB, in the group as a whole, or in the demented or non-demented subjects.
Figure 2.
Box plots of proportion area occupied by Aβ. The horizontal lines of the boxes represent the 25th, 50th (median), and 75th percentiles of the distributions. The vertical lines extending from the boxes stop at the most extreme data point within 1.5 inter-quartile ranges of the box. Points beyond this are individually identified. Ave = average across frontal (Fr) and occipital (Occip) regions.
The median whole brain BSI was −0.92 %/year (range −3.8, 0.4) and ventricular BSI was 5.5 %/year (range −0.8, 20.3) across the whole group. There was no difference in the median whole brain (−0.8 (−3.8, 0.4) female, −1.1 (−2.4, 0.4) male, p=0.30) or ventricular BSI (5.1 (1.4, 20.3) female, 6.3 (−0.8, 13.3) male, p=0.30) across genders. There was also no difference in the median whole brain (−1.1 (−3.3, 0.4) carriers, −0.7 (−3.8, 0.1) non-carriers, p=0.72) or ventricular BSI (5.7 (−0.8, 17.0) carriers, 5.1 (1.4, 20.3) non-carriers, p=1.00) between those with and without an APOE e4 allele. However the rate of whole brain loss was greater in those with a clinical diagnosis of dementia compared to those that were non-demented (−1.4 (−3.8, 0.4) demented, −0.2 (−1.5, 0.4) non-demented, p=0.0006). This was also true for ventricular expansion (6.5 (−0.8, 20.3) demented, 4.2 (1.4, 6.9) non-demented, p=0.004). Eight of the 45 subjects had lacunar infarcts on the MRI but there was no difference in the whole brain (−0.3 (−2.5, 0.0) infarcts, −1.0 (−3.8, 0.4) no infarcts, p=0.29) or ventricular BSI (5.8 (1.4, 10.7) infarcts, 5.5 (−0.8, 20.3) no infarcts, p=0.68) between those with and without infarcts. The median WMH burden across all subjects was 23cm3 (range 4–103).
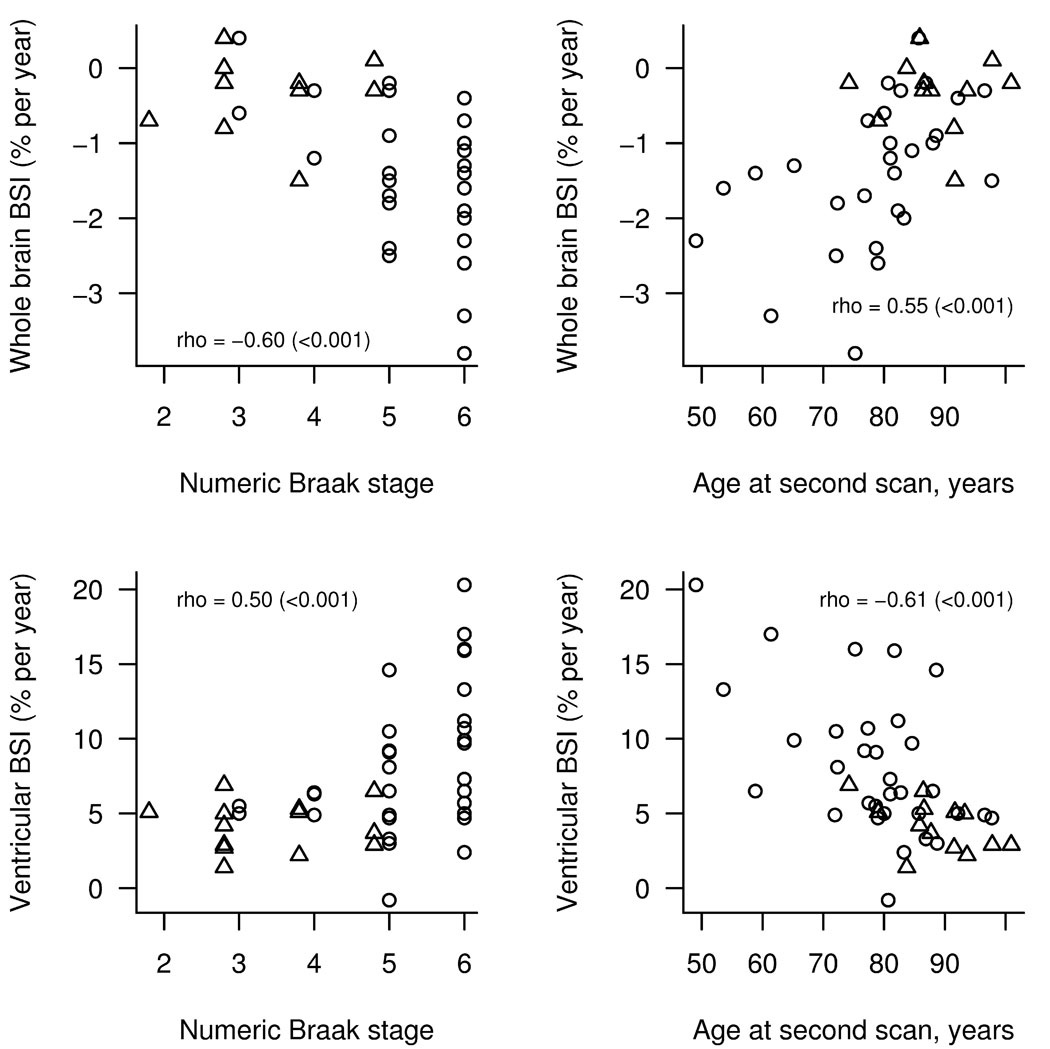
The Spearman rank correlations between rates of atrophy (whole brain BSI and ventricular BSI) and the other variables unadjusted and adjusted for age at the time of second scan and time from second scan to death across the whole cohort are shown in Table 2. The Spearman rank correlations are shown separately for the demented and non-demented subjects in Table 3. No significant correlations were observed between whole brain or ventricular BSI and any of the measures of Aβ burden (Supp Figure 1 and Supp Figure 2), except for a borderline significance between the visual grading of frontal Aβ and the whole brain BSI unadjusted for age or time from scan to death in the whole group. Correlations were observed between both whole brain BSI and ventricular BSI and the age at scan (Figure 3), Braak NFT stage (Figure 3), and change over time in MMSE and CDR-SOB in both the unadjusted and adjusted data for the whole group and in the unadjusted data for the demented subjects. The white matter hyperintensity load also did not correlate to whole brain or ventricular BSI.
Table 2.
Spearman rank correlations (p values) unadjusted and adjusted for age at time of second scan and time from second scan to death in all subjects
| Variable | Whole brain BSI | Ventricular BSI | ||
|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |
| Age at follow-up scan | 0.55 (<0.001) | n/a | −0.61 (<0.001) | n/a |
| Time from second scan to death | −0.26 (0.11) | n/a | 0.11 (0.49) | n/a |
| Compact Aβ via digital image analysis | ||||
| Average | −0.16 (0.33) | −0.07 (0.69) | 0.06 (0.68) | −0.12 (0.5) |
| Frontal | −0.02 (0.9) | 0.03 (0.88) | −0.01 (0.94) | −0.17 (0.33) |
| Occipital | −0.16 (0.35) | −0.07 (0.68) | 0.06 (0.71) | −0.06 (0.75) |
| Total Aβ via digital image analysis | ||||
| Average | −0.03 (0.88) | 0.05 (0.8) | 0.11 (0.47) | 0.02 (0.91) |
| Frontal | −0.04 (0.8) | 0.03 (0.86) | 0.08 (0.6) | −0.08 (0.67) |
| Occipital | −0.03 (0.86) | 0.01 (0.94) | 0.07 (0.64) | 0.03 (0.88) |
| Aβ via visual grading | ||||
| Frontal | −0.32 (0.048) | −0.27 (0.12) | 0.14 (0.37) | 0.03 (0.85) |
| Occipital | −0.23 (0.17) | −0.14 (0.42) | 0.22 (0.16) | 0.16 (0.38) |
| Braak stage | −0.60 (<0.001) | −0.54 (<0.001) | 0.50 (<0.001) | 0.39 (0.022) |
| White matter hyperintensity load | 0.24 (0.13) | 0.06 (0.73) | −0.01 (0.93) | 0.25 (0.15) |
| Annualized change in MMSE | 0.47 (0.0026) | 0.34 (0.048) | −0.59 (<0.001) | −0.60 (<0.001) |
| Annualized change in CDR-SOB | −0.60 (<0.001) | −0.47 (0.0049) | 0.58 (<0.001) | 0.47 (0.005) |
Adjusted correlations are partial correlations controlling for the effect of age at the time of the second scan and the time from the second scan until death.
MMSE = Mini-Mental State Examination; CDR-SOB = Clinical Dementia Rating sum of boxes; Aβ = Beta-amyloid
Table 3.
Spearman rank correlations (p values) unadjusted and adjusted for age at time of second scan and time from second scan to death in demented and non-demented subjects
| Variable | Demented patients (n=32) | Non-demented patients (n=13)† | ||||
|---|---|---|---|---|---|---|
| Whole brain BSI | Ventricular BSI | Whole brain BSI | Ventricular BSI | |||
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | Unadjusted | Unadjusted | |
| Age at follow-up scan | 0.57 (0.0015) | n/a | −0.50 (0.0035) | n/a | −0.11 (0.74) | −0.45 (0.12) |
| Time from second scan to death | −0.31 (0.12) | n/a | 0.04 (0.83) | n/a | −0.17 (0.6) | −0.03 (0.91) |
| Compact Aβ via digital image analysis | ||||||
| Average | −0.02 (0.92) | 0.01 (0.96) | −0.11 (0.56) | −0.31 (0.15) | −0.17 (0.63) | 0.21 (0.5) |
| Frontal | 0.08 (0.68) | 0.04 (0.87) | −0.11 (0.55) | −0.28 (0.20) | −0.10 (0.77) | 0.07 (0.83) |
| Occipital | −0.11 (0.59) | −0.00 (0.98) | −0.13 (0.49) | −0.28 (0.19) | −0.31 (0.36) | 0.35 (0.26) |
| Total Aβ via digital image analysis | ||||||
| Average | 0.19 (0.32) | 0.19 (0.38) | −0.14 (0.44) | −0.23 (0.3) | −0.14 (0.68) | 0.31 (0.33) |
| Frontal | 0.04 (0.84) | −0.01 (0.96) | −0.07 (0.72) | −0.23 (0.3) | −0.05 (0.9) | 0.29 (0.36) |
| Occipital | 0.08 (0.69) | 0.09 (0.67) | −0.15 (0.42) | −0.19 (0.38) | −0.22 (0.51) | 0.39 (0.21) |
| Aβ via visual grading | ||||||
| Frontal | −0.28 (0.15) | −0.20 (0.36) | 0.09 (0.63) | −0.09 (0.69) | −0.04 (0.9) | −0.00 (1) |
| Occipital | −0.28 (0.15) | −0.18 (0.41) | 0.06 (0.74) | −0.04 (0.87) | −0.15 (0.66) | 0.42 (0.18) |
| Braak stage | −0.42 (0.026) | −0.37 (0.084) | 0.43 (0.015) | 0.34 (0.11) | −0.01 (0.98) | 0.05 (0.86) |
| White matter hyperintensity load | 0.29 (0.13) | 0.22 (0.31) | 0.04 (0.83) | 0.21 (0.34) | −0.14 (0.66) | −0.08 (0.8) |
| Annualized change in MMSE | 0.40 (0.041) | 0.23 (0.29) | −0.67 (<0.001) | −0.72 (<0.001) | −0.12 (0.7) | 0.14 (0.65) |
| Annualized change in CDR-SOB | −0.45 (0.017) | −0.28 (0.20) | 0.47 (0.0076) | 0.37 (0.085) | −0.55 (0.062) | 0.43 (0.15) |
Adjusted correlations are partial correlations controlling for the effect of age at the time of the second scan and the time from the second scan until death.
Adjusted correlations were not assessed for the non-demented subjects due to the small number of subjects.
MMSE = Mini-Mental State Examination; CDR-SOB = Clinical Dementia Rating sum of boxes; Aβ = Beta-amyloid
Figure 3.
Relationship between BSI volumes and numeric Braak stage or age. The open circles indicate demented subjects while the triangles indicate non-demented subjects. The partial correlation for the whole group from table 2 has been added for reference.
Discussion
The results of this study suggest that Aβ burden is not associated with rates of brain atrophy in subjects with varying degree of Alzheimer's type pathology.
Beta-amyloid deposition has been shown to correlate with rates of ventricular expansion but not whole brain atrophy in one clinicopathological study,9 and rates of whole brain atrophy was shown to correlate with Aβ deposits as measured by 11C-PIB positron emission tomography.8 Unfortunately in neither of these studies was there direct measurement of Aβ burden. In the clinicopathologic study Aβ burden was indirectly measured by counting senile plaques, a technique which has been shown to have average inter-rater reliability28. Furthermore, Aβ was detected using congo red and silver stains which are less sensitive to detecting diffuse Aβ.29 The results of the PIB study are difficult to interpret since PIB may bind preferentially to fibrillar Aβ which may constitute a relatively small proportion of Aβ deposits in many brains, especially those with no or only mild cognitive impairment.30, 31 Moreover, fibrillar Aβ detected by PIB may be located in amyloid laden vessels.32
In our study we directly measured Aβ burden in the brain parenchyma and with a pan Aβ antibody that recognises both diffuse and compact Aβ deposits. Furthermore, we used a novel technique that significantly improves Aβ sampling and is more representative of the true Aβ burden by scanning the entire slide and not just random fields using the Aperio slide scanner (Aperio Technologies, Vista, CA). The regions of interest were relatively large in comparison to some analytic techniques, included Aβ deposits at all cortical layers, and were selected to be representative of Aβ in the areas of interest, namely multimodal association cortex (BA9) and a primary sensory cortex (BA17). The former is affected early in the disease process, while the latter is affected later.33 Thus, the Aβ measures reflected anatomic and lesion type heterogeneities. We did not find any significant correlation between Aβ burden and rates of whole brain atrophy or ventricular expansion in the whole group, or in the demented or non-demented subjects. The lack of any convincing evidence that Aβ burden is associated with rates of brain volume loss has significant implications for future treatment studies that intend to investigate the removal of Aβ. Indeed, preliminary evidence suggests that brains of subjects treated with Aβ vaccines, which presumably cleared Aβ from their brain, may actually decrease over time.7 As previously suggested, the presence of soluble Aβ conformers, such as dimmers and oligomers, may play a more important role in neuronal loss and cognitive dysfunction than insoluble Aβ deposits34.
Given that some cognitively normal people have substantial Aβ burden,30, 31, 35, 36 the results of our study are not too surprising. While some studies have found an association between Aβ burden and cognitive impairment in AD,37, 38 others have not.39, 40 In fact, some evidence suggests that because Aβ deposition may be so early in the disease process, particularly in areas of the brain where neurofibrillary degeneration and neuronal loss are late events, that the effects of Aβ on clinical measures such as cognitive tests and presumably also including MRI volume measurements are mediated indirectly by markers of neuronal pathology, such as NFT.41
As a secondary analysis we also correlated Braak NFT stage to rates of whole brain atrophy and ventricular expansion. The Braak NFT stage is a semiquantitative measure of neurofibrillary degeneration that represents a topographic distribution of NFT across multiple brain regions that correlates well with neurofibrillary tangle density42 and hence an indirect measure of the microtubule association protein tau density since tau is the major protein present in neurofibrillary tangles.43 Unlike Aβ we found a significant correlation between Braak NFT stage and whole brain volume loss and between Braak NFT stage and ventricular expansion in the whole group. The unadjusted correlations were also significant in the demented group. The findings of this study mirror those of another study that correlated neurofibrillary tangle burden with brain atrophy.9 Other studies have also found significant correlations between neurofibrillary tangle burden or Braak NFT stage and measurements of regional hippocampal volumes or medial temporal lobe width in subjects with AD.44–46 One other previous study has also found a stronger association between the degree of brain loss and neurofibrillary tangle burden than between the degree of brain loss and Aβ burden.44
An association was also observed between rates of brain loss and age at the time of scan in the whole group, with the rate of both brain volume loss and ventricular expansion decreasing as the average age increases. Although the non-demented subjects in the cohort were older than the demented subjects this does not explain the correlation since it remained significant in the demented subjects alone. This may be somewhat counter-intuitive since it has been shown that the rate of brain atrophy increases with age in normal subjects.47 Since the majority of subjects in our cohort had AD this correlation actually reflects the fact that younger subjects with AD have higher rates of atrophy than older subjects with AD. This trend has not previously been demonstrated in a cohort of pathologically confirmed subjects although it has been alluded to in previous cross-sectional studies that have shown more widespread patterns of grey matter loss in subjects with young onset AD compared to old onset AD.48
No associations were identified between rates of whole brain loss or ventricular expansion and the degree of vascular pathology, as assessed by the white matter hyperintensity burden or the presence of lacunar infarcts. Similarly there was no difference in the rate of brain loss or ventricular expansion between those subjects with and without an ApoE 4 allele. Previous studies have reported conflicting results, with some reporting an association between the presence of the ApoE 4 allele and rates of atrophy,49 while others have failed to find a significant association47. However, the rates of atrophy did map well onto the clinical features of the cohort. The rates of atrophy were larger in the subjects with dementia than the non-demented subjects as would be expected and has been previously reported.6, 50 In addition, significant correlations were identified between change in MMSE and CDR-SOB scores, and whole brain loss and ventricular expansion supporting the notion that rates of brain atrophy are good markers of clinical disease progression.6
The strengths of this study are the techniques that were utilized to measure Aβ burden; the detection of Aβ with anti-Aβ immunohistochemistry, and the ability to measure both compact and total Aβ burden. In addition, we had a relatively large cohort, and the rates of atrophy were calculated on average just 2 years prior to death in these subjects. However, the power of the Spearman Rank correlations performed within the demented and non-demented subjects separately could have been limited by the smaller number of subjects in each group, particularly in the non-demented group which only contained 13 subjects. This may explain why the adjusted correlations between rates of atrophy and Braak stage, age, MMSE and CDR-SOB, did not quite reach significance in the demented subjects, and why these correlations were not significant in the non-demented group. Another possible limitation is that the BSI assumes that volume change occurs at a linear rate over time, whereas it is possible that change over time is nonlinear. However, this nonlinearity is not likely to be pronounced over short inter-scan intervals of only a few years, and therefore the change in volume is likely to be well approximated by a linear path.
The results of this study demonstrate that Aβ burden plays a limited and perhaps indirect role in rates of brain loss in subjects with varying degrees of Alzheimer's type pathology. Conversely, rates of brain loss do appear to be better and more directly related to age, clinical measures of cognitive dysfunction, and neurofibrillary degeneration.
Supplementary Material
Acknowledgements
This study was supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078, by grants P50 AG16574, U01 AG06786, and R01 AG11378 from the National Institute on Aging, Bethesda MD, NIRG-03-4842 from the Alzheimer's Association, and the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimers' Disease Research Program of the Mayo Foundation, U.S.A. DSK has been a consultant to GE HealthCare, GlaxoSmithKline and Myriad Pharmaceuticals, has served on a Data Safety Monitoring Board for Neurochem Pharmaceuticals, and is an investigator in a clinical trial sponsored by Elan Pharmaceuticals. BFB is an investigator in a clinical trial sponsored by Myriad Pharmaceuticals. RCP has been a consultant to GE Healthcare and has served on a data safety monitoring board in a clinical trial sponsored by Elan Pharmaceuticals.
Footnotes
Conflict of interest statement: There is no conflict of interest
References
- 1.Masters CL, Simms G, Weinman NA, et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wimo A, Winblad B, Jonsson L. An estimate of the total worldwide societal costs of dementia in 2005. Alzheimer's and Dementia. 2007;3:81–91. doi: 10.1016/j.jalz.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 4.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 5.Gilman S, Koller M, Black RS, et al. Clinical effects of A{beta} immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005 doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox NC, Black RS, Gilman S, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 8.Archer HA, Edison P, Brooks DJ, et al. Amyloid load and cerebral atrophy in Alzheimer's disease: an 11C-PIB positron emission tomography study. Ann Neurol. 2006;60:145–147. doi: 10.1002/ana.20889. [DOI] [PubMed] [Google Scholar]
- 9.Silbert LC, Quinn JF, Moore MM, et al. Changes in premorbid brain volume predict Alzheimer's disease pathology. Neurology. 2003;61:487–492. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- 10.American Pyschiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th Ed) (DSM IV) Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer' disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 15.Crook R, Hardy J, Duff K. Single-day apolipoprotein E genotyping. J Neurosci Methods. 1994;53:125–127. doi: 10.1016/0165-0270(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 16.Josephs KA, Tsuboi Y, Cookson N, et al. Apolipoprotein E epsilon 4 is a determinant for Alzheimer-type pathologic features in tauopathies, synucleinopathies, and frontotemporal degeneration. Arch Neurol. 2004;61:1579–1584. doi: 10.1001/archneur.61.10.1579. [DOI] [PubMed] [Google Scholar]
- 17.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 18.Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- 19.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291–299. [PubMed] [Google Scholar]
- 20.Gunter JL, Shiung MM, Manduca A, et al. Methodological considerations for measuring rates of brain atrophy. J Magn Reson Imaging. 2003;18:16–24. doi: 10.1002/jmri.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imaging. 1997;16:623–629. doi: 10.1109/42.640753. [DOI] [PubMed] [Google Scholar]
- 22.Bryan RN, Manolio TA, Schertz LD, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: the cardiovascular health study. AJNR Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 23.Scheltens P, Barkhof F, Leys D, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- 24.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 25.Jack CR, Jr, O'Brien PC, Rettman DW, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging. 2001;14:668–676. doi: 10.1002/jmri.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien PC. The appropriateness of analysis of variance and multiple-comparison procedures. Biometrics. 1983;39:787–794. [PubMed] [Google Scholar]
- 27.Perneger TV. What's wrong with Bonferroni adjustments. Bmj. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirra SS, Gearing M, McKeel DW, Jr, et al. Interlaboratory comparison of neuropathology assessments in Alzheimer's disease: a study of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) J Neuropathol Exp Neurol. 1994;53:303–315. doi: 10.1097/00005072-199405000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi H, Hirai S, Morimatsu M, et al. Diffuse type of senile plaques in the brains of Alzheimer-type dementia. Acta Neuropathol (Berl) 1988;77:113–119. doi: 10.1007/BF00687420. [DOI] [PubMed] [Google Scholar]
- 30.Dickson DW, Crystal HA, Mattiace LA, et al. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 31.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 32.Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol. 2007;64:431–434. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 33.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 34.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 35.Davis DG, Schmitt FA, Wekstein DR, et al. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Lue LF, Kuo YM, Roher AE, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cummings BJ, Pike CJ, Shankle R, et al. Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer's disease. Neurobiol Aging. 1996;17:921–933. doi: 10.1016/s0197-4580(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 38.Bussiere T, Friend PD, Sadeghi N, et al. Stereologic assessment of the total cortical volume occupied by amyloid deposits and its relationship with cognitive status in aging and Alzheimer's disease. Neuroscience. 2002;112:75–91. doi: 10.1016/s0306-4522(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 39.Giannakopoulos P, Herrmann FR, Bussiere T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 40.Arriagada PV, Growdon JH, Hedley-Whyte ET, et al. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 41.Bennett DA, Schneider JA, Wilson RS, et al. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 42.Gertz HJ, Xuereb J, Huppert F, et al. Examination of the validity of the hierarchical model of neuropathological staging in normal aging and Alzheimer's disease. Acta Neuropathol (Berl) 1998;95:154–158. doi: 10.1007/s004010050780. [DOI] [PubMed] [Google Scholar]
- 43.Brion JP, Flament-Durand J, Dustin P. Alzheimer's disease and tau proteins. Lancet. 1986;2:1098. doi: 10.1016/s0140-6736(86)90495-2. [DOI] [PubMed] [Google Scholar]
- 44.Nagy Z, Jobst KA, Esiri MM, et al. Hippocampal pathology reflects memory deficit and brain imaging measurements in Alzheimer's disease: clinicopathologic correlations using three sets of pathologic diagnostic criteria. Dementia. 1996;7:76–81. doi: 10.1159/000106857. [DOI] [PubMed] [Google Scholar]
- 45.Jack CR, Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gosche KM, Mortimer JA, Smith CD, et al. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology. 2002;58:1476–1482. doi: 10.1212/wnl.58.10.1476. [DOI] [PubMed] [Google Scholar]
- 47.Enzinger C, Fazekas F, Matthews PM, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64:1704–1711. doi: 10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed] [Google Scholar]
- 48.Ishii K, Kawachi T, Sasaki H, et al. Voxel-based morphometric comparison between early- and late-onset mild Alzheimer's disease and assessment of diagnostic performance of z score images. AJNR Am J Neuroradiol. 2005;26:333–340. [PMC free article] [PubMed] [Google Scholar]
- 49.Wahlund LO, Julin P, Lannfelt L, et al. Inheritance of the ApoE epsilon4 allele increases the rate of brain atrophy in dementia patients. Dement Geriatr Cogn Disord. 1999;10:262–268. doi: 10.1159/000017130. [DOI] [PubMed] [Google Scholar]
- 50.Whitwell JL, Jack CR, Jr, Parisi JE, et al. Rates of cerebral atrophy differ in different degenerative pathologies. Brain. 2007;130:1148–1158. doi: 10.1093/brain/awm021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.