Abstract
The bystander effect, whereby cells that are not traversed by ionizing radiation exhibit various responses when in proximity to irradiated cells, is well documented in the field of radiation biology, Here we demonstrate that considerable bystander responses are also observed after photodynamic stress using the membrane-localizing dye deuteroporphyrin (DP). Using cells of a WTK1 human lymphoblastoid cell line in suspension and a transwell insert system that precludes contact between targeted and bystander cells, we have shown that the bystander signaling is mediated by diffusing species. The extranuclear localization of the photosensitizer used suggests that primary DNA damage is not the trigger for initiating these bystander responses, which include elevated oxidative stress, DNA damage (micronucleus formation), mutagenesis and decreased clonogenic survival. In addition, oxidative stress in the bystander population was reduced by the presence of the membrane antioxidant vitamin E in the targeted cells, suggesting that lipid peroxidation may play a key role in mediating these bystander effects. The fluence responses for these bystander effects are non-linear, with larger effects seen at lower fluences and toxicity to the target cell population. Hence, when considering outcomes of photodynamic action in cells and tissue, bystander effects may be significant, especially at sublethal fluences.
INTRODUCTION
The long-accepted paradigm that damage induced by ionizing radiation and other sources of stress is restricted to those cells targeted directly by the insult has now come into question after the observation that ionizing radiation can elicit a variety of responses (1), such as altered gene expression (2), DNA damage (3, 4), mutation (5, 6), cell death (7) and malignant transformation (8) in bystander cells that were not directly targeted. The term bystander effects describes processes where naive (bystander) cells in proximity to cells that are directly subjected to certain stresses or stimuli exhibit responses that would not have occurred in the absence of the directly targeted cells. The bystander cells respond on receiving some type of “signal” from the targeted cells. Thus bystander effects constitute a relatively new paradigm that must be considered in evaluating the response of tissues to stress that arises from environmental sources (radiation, UV light, toxic chemicals, etc.) or therapeutic intervention [e.g., chemotherapy, radiotherapy, UV phototherapy and photodynamic therapy (PDT)].
While the existence of ionizing radiation-induced bystander effects is now clear, the mechanism(s) responsible for triggering these effects remain unclear. Different reports suggest that multiple signaling pathways could be involved in mediating these processes, including gap junction-mediated intercellular communication (9, 10), diffusion of soluble factors (9–11), including nitric oxide (12) and ROS (13, 14), and oxidative metabolism (15). Some studies suggest that direct cell-cell contact is required, but others have shown that this is not a prerequisite and that bystander effects can be elicited through application of conditioned medium taken from irradiated cell cultures, suggesting release from the targeted cells of signaling molecules such as different cytokines, ROS, calcium and nitric oxide (7, 16–19). It is also apparent that parallel pathways may be possible and that the magnitude and mechanisms of bystander responses could be dependent on the cell type and treatment.
Although ionizing radiation has been widely shown to induce bystander responses, it is not unique in generating these phenomena. A limited number of reports suggest that bystander responses are also produced by other stresses, including chemotherapeutic drugs, (20) ultraviolet light (21–24) and photodynamic treatment (22, 25–28). PDT has been investigated for treatment of a variety of pathological conditions and diseases such as some cancers (29, 30), age-related macular degeneration (31), rheumatoid arthritis (32), skin conditions (33, 34) and wound healing (35, 36). PDT uses a combination of visible light and a localized photosensitizer to produce acute oxidative stress from singlet oxygen generation to eradicate diseased tissue. However, the responses of surviving cells in the target tissue as well as normal cells in the periphery are important elements that contribute to outcome after PDT (or any other therapy), with particular regard to later carcinogenesis.
The present study was undertaken to evaluate the effects of targeted photodynamic stress on bystander cells and to determine whether medium-mediated factors play any role in extending the zone of response from the target cells alone. Previous work on photosensitized bystander effects adopted a statistical approach to the distribution of cellular response in an irradiated population (22, 26, 27). In this work we adopted a direct method that provides better delineation between targeted and bystander cells to study these phenomena in more detail; i.e., a transwell insert culture dish was used to co-culture bystander and targeted cells (37). Under these conditions the two populations are physically separated with no cell-cell contact but share the same medium, providing a useful approach to discern the involvement of soluble factors in mediating bystander effects and to provide information on the mechanisms involved. A more global understanding of the interaction and responses of directly targeted and bystander cells to oxidative insult would be valuable to understand the progression of diseases involving oxidative insult and in planning and interpretation of therapeutic modalities.
MATERIALS AND METHODS
Chemicals
Deuteroporphyrin (DP) was prepared from deuteroporphyrin IX dimethyl ester as described previously (38) and kept as a stock solution (3.5 mM) in dimethyl sulfoxide (DMSO). 5-(or 6)-Chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CMH2DCFDA) was purchased from Molecular Probes (Invitrogen, Eugene, OR). CM-H2DCFDA (50 μg) was dissolved in 50 μl of DMSO (Sigma, St. Louis, MO) to a stock concentration of 1.73 mM for further dilution in experiments. 2′-Deoxycytidine, hypoxanthine, aminopterin, thymidine and trifluorothymine deoxyriboside (TFT) were purchased from Sigma. Low-melting agarose, normal-melting agarose, EDTA, Trisma, Triton X-100, NaOH, α-tocopherol acetate (vitamin E) and 4′,6-diamidino-2-phenylindole (DAPI) were also purchased from Sigma.
Cell Culture
WTK1 cells, a human lymphoblastoid cell line, were grown at 37°C in a humidified atmosphere of 95% air/5% CO2 with RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen), 5% penicillin and streptomycin and 5% Hepes buffer (Sigma). The cells were subcultured every 2 days and were never allowed to exceed a density of 0.8 × 106 cells/ml. The WTK1 cell line contains a mutation (39–41) in the p53 gene (p53Ile237) that confers hypermutability (42, 43); this makes it ideal for mutagenesis studies in experimental situations where the use of relatively small cell numbers is desirable.
Cell Irradiation and Co-culture
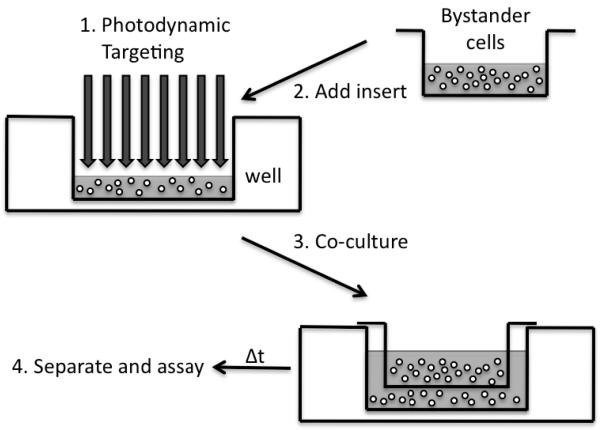
Cells were washed and resuspended at a density of 2 ×106 cells/ml in Hanks' balanced salt solution (HBSS, pH 7.4). Cells were then incubated with 0.5 μM. DP for 15 min at 37°C in a humidified atmosphere of 95% air/5% CO2. Then 1.5-ml samples of cell suspension were illuminated at 532 nm using a Nd/YAG laser (Spectra Physics GCR-150) operating at 20 mW (10 Hz rep rate, 2 mJ/pulse, ∼6-mm beam diameter). Samples were irradiated with total light fluences up to 3.6 J/cm2. Immediately after illumination, 375 μl of treated samples was placed in wells (with a growth area of 9.6 cm2) of six-well plates (Falcon) with complete RPMI 1640 medium (2.725 ml) at a density of 2.4 × 105 cells/ml. To study bystander effects, 3.9 × 105 cells in 3 ml of medium were placed in a transwell culture insert dish (Falcon, Franklin Lakes, NJ) with a growth area of 4.2 cm2. The insert dish bottom is a microporous membrane with 1-mm pores at a density of 1.6 × 106/cm2, allowing transfer of dissolved species in the medium without contact between targeted and bystander cells. The bottom surface of the insert is 0.9 mm from the bottom of the companion well. The experimental approach is summarized in Fig. 1. Plates with targeted (well) and bystander cells (insert) were cultured in the incubator at 37°C in a humidified atmosphere of 95% air/5% CO2.
FIG. 1.
Schematic diagram of experiments involving transwell/ insert co-culture system, with targeted cells in the well and bystander cells in a microporous insert added to the well immediately after photodynamic treatment.
Clonogenic Survival
Photodynamically targeted cells were plated in a 96-well plate at an average of 1 cell/well immediately after treatment over the range 0–3.6 J/cm2 and incubated for 14 days. To measure viability in bystander cells, the targeted cells were immediately placed in co-culture with bystander cells for 24 h at 37°C in a humidified atmosphere of 95% air/5% CO2. After 24 h the cells in the insert were counted and plated in a 96-well plate at ∼1 cell/well. Colonies from targeted and bystander populations were counted after 14 days, and cell survival was calculated by Poisson distribution, as described in earlier studies (44). Colonies consisted of at least 50 cells in a well but typically contained hundreds to thousands of cells.
Micronucleus Formation
Chromosome damage was assessed using the cytokinesis-block micronucleus technique (45) in the treated and bystander cells. Binucleated cells result from actively dividing cells that cannot advance, as cytokinesis is blocked by cytochalasin. Micronuclei are due to broken or detached chromosomes that separate from the mitotic spindle after mitosis. Photodynamically treated cells in companion wells were incubated for 24 h prior to addition of cytochalasin B (2 μg/ml) and then harvested after a further 36 h. For bystander studies, the inserts containing unirradiated cells were put into the companion wells immediately after treatment and cytochalasin B (2 μg/ml) was added. Cells were then harvested after 36 h co-culture with treated cells. The additional 24-h delay for the treated cells was necessary because photodynamic treatment induces temporary cell cycle arrest in the surviving cells. After harvest and centrifugation at 800 rpm, cells were treated with 5 ml hypotonic KCl (0.1 M) for 15 min. Cells in the pellet were fixed with two washes using 5 ml methanol:acetic acid (3:1, v/v) solution, then resuspended in 1 ml methanol:acetic acid solution. Samples were dropped onto slides, dried and stained with DAPI (10 μg/ml) for 5 min, then washed twice with distilled water. After drying, nuclei were observed under a fluorescence microscope (Nuance Multispectral Imaging System, CRI Inc., Woburn, MA). At least 500 binucleated cells were scored using standard criteria. Results are expressed as the percentage of micronucleated binucleated cells.
Determination of Mutagenicity at the tk Locus
Cells at a density of 105 cells/ml were treated for 48 h with CHAT (C: 2′-deoxycytidine 10−5 M, H: hypoxanthine 2 × 10−4 M, A: aminopterin 10−7 M, T: thymidine 1.75 × 10−5 M) to reduce the background mutant fraction prior to photodynamic stress. After CHAT treatment, cells were centrifuged and resuspended in fresh medium containing THC (CHAT minus aminopterin). After a further 24 h, the cells were subcultured with fresh medium and subjected to photodynamic stress, as described above. Immediately after treatment, the cells (105 cells/ml) were transferred to nonselective RPMI 1640 medium and cultured for 3 days to allow phenotypic expression of newly induced mutants. For the bystander experiment, naive cells were co-cultured in the well/insert system with directly irradiated cells for 24 h and then removed and cultured separately in RPMI 1640 medium for a further 2 days. Cells from each culture were then plated in 96-well plates (2 plates/group) at 1 cell/well in the absence of TFT (trifluorothymine deoxyriboside). Plating efficiency (PE) was evaluated 2 weeks later by counting the number of wells with colonies in each plate; colonies generally contained ≥10,000 cells. In another set, the mutant fraction was determined by plating 2000 cells in each well in the presence of TFT (2.0 μg/ml) and incubating for 11 days prior to scoring colonies. Plates were rechallenged with fresh TFT medium 11 days after initial seeding and incubated for an additional 10 days to observe the appearance of any late-appearing mutants. Mutant colonies also consisted of ≥10,000 cells and therefore were easily distinguished from the 2000 wild-type cells that had been killed by TFT. The mutant fraction and PE were calculated using the Poisson distribution as described (44).
Microplate Spectrofluorometric Analysis of Oxidative Stress
Oxidative stress was assayed using the probe CM-H2DCFDA. This compound is converted to the fluorescent chloromethyl-dichlorofluorescein (CMDCF) structure and oxidation and cleavage of the acetate groups by intracellular esterases. Cells were co-incubated with 0.5 μM DP and 1.5 μM. CM-H2DCFDA for 20 min before treatment. After illumination the cells were washed once with HBSS and resuspended in 1 ml HBSS for immediate microplate spectrofluorometric analysis, as described below. Bystander cells were placed in co-culture with directly targeted cells in a transwell insert system immediately after treatment for 6 h to allow the appearance of delayed ROS generation. After co-incubation, 1.5 μM CMH2DCFDA was added to the bystander cells for 20 min followed by removal of bystander cells from the insert, washing and resuspension in 1 ml HBSS. Then 200 μl of this cell suspension was added to a well of a 96-well plate (3 wells per sample). The oxidized probe (CMDCF) fluorescence was measured immediately at 37°C using a microplate spectrofluorometer (Spectramax, Molecular Devices, CA) with excitation at 488 nm and an emission maximum of 535 nm. Samples were scanned only once because of potential perturbation by analyzing light.
Statistical significance was determined in all experiments using the Student's t test, and a P value of <0.05 was considered significant.
RESULTS
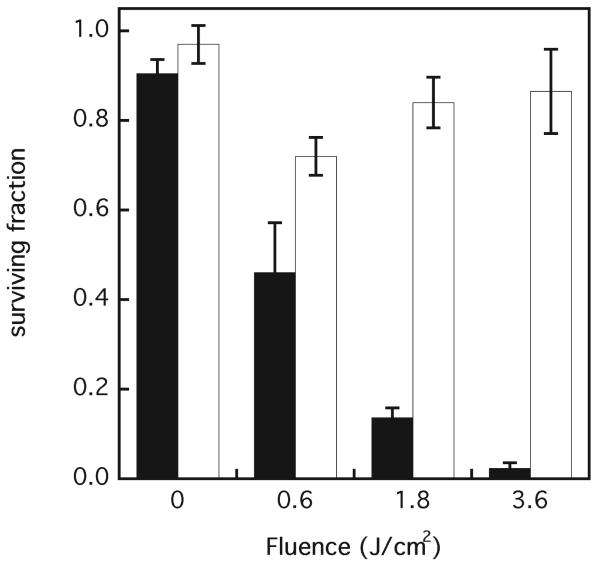
The viability of photodynamically targeted and bystander WTK1 cells was studied by clonogenicity. Directly targeted cells exhibit a clear fluence-dependent decrease of clonogenic survival, as shown in Fig. 2. However, the survival data for bystander cells that were immediately co-cultured with treated cells for 24 h was not fluence-dependent and exhibited the greatest loss of clonogenicity at the lowest fluence of 0.6 J/cm2 of 532 nm light to the targeted cells. This difference was statistically significant (P < 0.05) compared to the higher doses, but no significant difference was observed in clonogenic survival between bystander cells at 1.8 and 3.6 J/cm2. The slight decrease in cell viability in the targeted population at 0 J/cm2 is probably due to long-term effects of the photodynamic agent (DP) in the clonogenic assay because the bystander population control does not contain DP and the cell viability is slightly higher.
FIG. 2.
Clonogenic survival of directly targeted (black bars) and bystander (white bars) WTK1 cells. Results are the means of three independent experiments ± SD. Clonogenic surviving fraction is referenced to a survival of 1.0 for cells with no DP or light treatment.
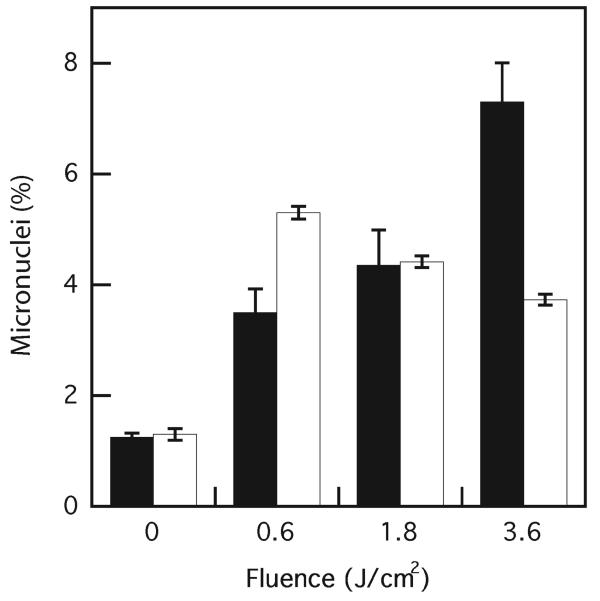
We have demonstrated previously that photodynamic treatment with DP gives rise to DNA damage in WTK1 cells via secondary reaction processes (46). In the present study we extended our observations to possible effects on bystander cells, because extensive DNA alterations have been demonstrated previously in similar experiments using ionizing radiation. Figure 3 shows the dependence of micronucleus formation in targeted and bystander cells on fluence delivered to the target cell population. Targeted cells show a fluence-dependent increase of micronucleus formation, as expected. However, the micronucleus formation in the bystander cells again shows the highest levels at the lowest photodynamic dose delivered to the target cells and decreases slightly at the higher doses, with all values decreasing by a statistically significant amount (P < 0.05). Although the difference in micronucleus formation between targeted and bystander cells is not significantly different at a fluence of 1.8 J/cm2, unlike 0.6 and 3.6 J/cm2, the response profiles are clearly different for each population.
FIG. 3.
Photodynamic DNA damage in WTK1 cells assessed by percentage of WTK1 cells containing micronuclei after cyctochalasin B treatment of photodynamically targeted cells (black bars) and bystander cells (white bars). Control cells contained DP but received no light. Results are the means of three independent experiments ± SD.
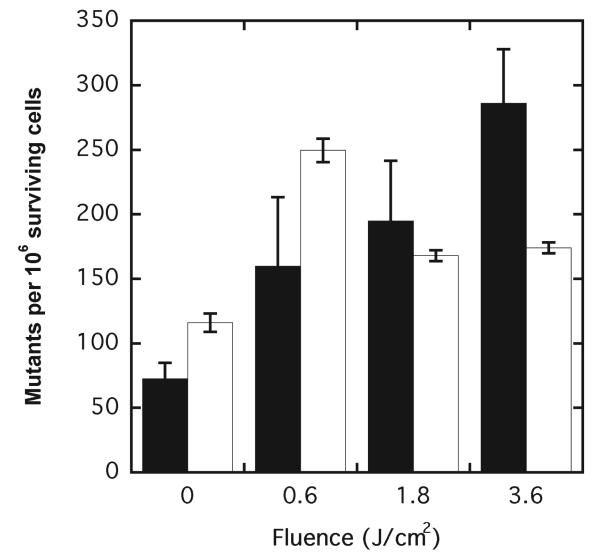
An obvious consequence of DNA damage is potential mutagenicity if the lesion is unrepaired or misrepaired. Thus a mutagenesis assay was performed on directly treated and bystander WTK1 cells at the thymidine kinase (tk) locus, which reflects mainly deletions, translocations and recombinations. Cells mutated at the tk locus are able to proliferate in the presence of the pyrimidine analogue TFT, whereas normal WTK1 cells cannot. Figure 4 shows the mutation frequency as a function of applied fluence. In directly targeted cells, the induction of tk-locus mutants increased with treatment fluence, rising to a fourfold increase over background with 3.6 J/cm2. Bystander cells also displayed increased mutant frequency but not in a fluence-dependent manner, with the highest mutation frequency seen at the lowest photodynamic fluence of 0.6 J/cm2 to the target cells. A further increase in the dose to the treated cells produced a statistically significant decrease in mutation frequency (P < 0.05) in the bystander cells. However, no significant difference in mutation frequency was observed between the two highest doses used. These data indicate that photodynamic stress can cause mutation in the DNA of bystander cells in addition to that in directly targeted cells.
FIG. 4.
Photodynamic induction of mutation at the tk− locus in directly targeted cells (black bars) and bystander (white bars) WTK1 cells. Control cells contained DP but received no light. Results are the means of three independent experiments ± SD.
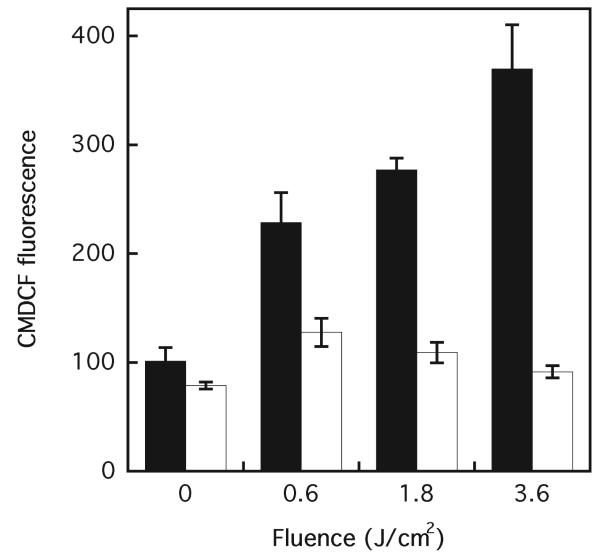
An increased level of ROS in mammalian cells after photodynamic stress has been well documented. Oxidative stress has also been suggested to play a critical role in mediating the bystander response (9, 20, 47). DP is a type II photosensitizer, exclusively generating singlet oxygen under the conditions used here (38). Singlet oxygen itself is very short-lived and cannot be responsible for ROS levels that persist long after the photosensitizing light is removed. However, some products of the reaction of 1O2 with biomolecules are also ROS, or precursors of ROS, that are longer-lived and can be produced over extended periods (46). Figure 5 demonstrates a significant fluence-dependent increase of oxidized CMDCF fluorescence, a marker for intracellular oxidative stress, when targeted WTK1 cells were photosensitized with DP in the presence of CMH2DCFDA and measured immediately after irradiation. To allow evolution of delayed oxidative stress, the ROS levels were measured in bystander cells 6 h after co-culture with DP-photosensitized cells. Delayed ROS generation in bystander cells was not dependent on fluence. Although a trend of decreased CMDCF fluorescence with increased fluence was observed, there was no significant difference between the 0.6 and 1.8 J/cm2 fluences, although both were significantly higher than the 3.6 J/cm2 fluence (P < 0.05).
FIG. 5.
Photosensitized generation of ROS in directly irradiated and bystander WTK1 cells. With photodynamically targeted cells (black bars) ROS measurement was performed immediately after treatment, and with bystander cells (white bars) after 6 h of co-culture with irradiated cells. Control cells contained DP but received no light. Results are the means of three independent experiments ± SD.
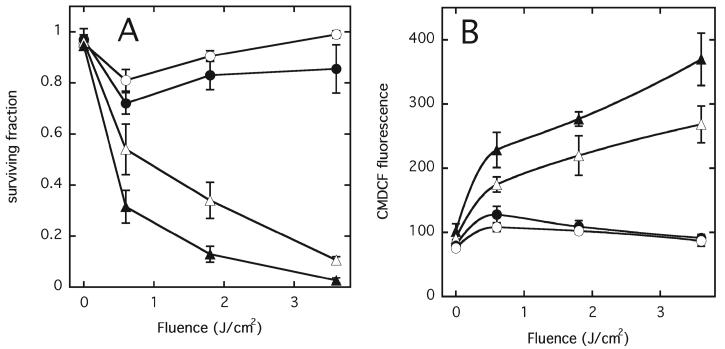
To determine the possible importance of lipid peroxidation and membrane damage in bystander signaling, we employed α-tocopherol acetate (vitamin E, 30 μM) as a membrane antioxidant. Vitamin E can act as a ROS scavenger on two levels (1) by quenching singlet oxygen and (2) by acting as a radical chain-breaking agent. Cells that were scheduled for photodynamic treatment were preincubated with vitamin E for 1 h, washed and then used for experiments, whereas bystander cells were not pretreated with vitamin E. Control cells incubated with vitamin E alone did not show toxicity under these conditions. Figure 6A shows the protective effect of vitamin E on the targeted and bystander cells in terms of clonogenic survival. Statistically significant increases in clonogenic survival are seen in both populations, with the exception of the 1.8 J/cm2 fluence in the bystander population, when the targeted cells were pretreated with vitamin E. Similarly, levels of oxidative stress in both targeted and bystander cells were reduced after the same treatment, as shown in Fig. 6B. Reduction in the case of targeted cells was statistically significant at all fluences used (P < 0.05). Although the trend was for a decreased fluorescence in the bystander population, this did not reach the level of statistical significance at any of the fluences used. Treatment of unirradiated control cells with vitamin E did not induce any changes in cell viability.
FIG. 6.
Effects of 1 h preincubation of target cells with 30 μM vitamin E on (panel A) clonogenic survival and (panel B) ROS levels measured by oxidized CMDCF fluorescence, on (▲) target cells, no vitamin E; (△) target cells + vitamin E; (●) bystander cells, no vitamin E; (○) bystander cells + vitamin E. Control cells contained DP but received no light. Results are the means of three independent experiments ± SD.
DISCUSSION
Previous work by Dahle and colleagues on photodynamic treatment of cells in culture has implied bystander effects on the basis of the probability distribution of cells responding under conditions where all cells were treated (27). The observation of clustered effects distinct from a random distribution implied some form of co-operativity between cells attached on cell culture dishes (22, 26). Further experiments from that group showed that cell-cell contact was not required and that gap junctions were not exclusively used in the communication process (28). In this study we have confirmed that bystander responses do occur after photodynamic oxidative stress in cell culture and have shown, by using WTK1 cells in suspension rather than attached cell lines, that extracellular diffusing species can mediate bystander effects. Under these conditions, gap junction communication is not possible because physical separation of treated and bystander cells is guaranteed by the nature of the transwell insert system (37). Our results show that bystander effects are indeed generated under photodynamic stress in WTK1 cells and that these can be considerable, approaching the levels of response generated in the targeted cells, especially at low light fluences. Increased oxidative stress, DNA damage and mutagenesis are accompanied by a decrease in clonogenic survival in bystander cells.
While the targeted cell responses are dependent on fluence, we have also shown here that the magnitudes of these bystander effects typically do not increase with fluence in the same manner. Rather, larger effects are seen at lower fluences to the target cells where survival of this population is higher. Figures 2–5 all show the highest bystander responses in toxicity, DNA damage, mutation fraction and elevated oxidative stress at the lowest fluence used (0.6 J/cm2), where around 50% of the targeted cells survive the insult. At higher levels of lethality, e.g., the 3.6 J/cm2 fluence used here that reduced clonogenicity to <5%, the bystander effect tends to diminish. Similar behavior has been observed in ionizing radiation experiments looking at a variety of end points (48, 49). These observations infer that cell stress but not cell death is important in the generation of the bystander response and that active signaling is taking place, rather than simple release of intracellular contents from dying cells.
In this study we have confirmed our previous observation of DNA damage in WTK1 cells that are treated with an amphiphilic photosensitizer that localizes in cellular membranes and does not enter the nucleus (Fig. 3). However, taking this a step further, we have now shown that the DNA damage leads to increased mutagenesis in surviving WTK1 cells (Fig. 4). DNA damage and mutagenesis have not been the focus of much attention in PDT because the photosensitizers used typically do not enter the nucleus and the short lifetime of singlet oxygen in cells is not favorable for extranuclear formation, diffusion and subsequent reaction with DNA (50). A more likely scenario is membrane damage generated by the primary singlet oxygen and the generation of secondary mediators that can diffuse both within and between cells to initiate oxidative damage. This is supported by the action of the membrane antioxidant, vitamin E, which reduced toxicity and oxidative stress in both targeted and bystander WTK1 cells. Certainly the bystander signaling process cannot be induced by singlet oxygen because the co-incubation is initiated after photodynamic treatment of the target cells. However, both DNA damage (51–53) and mutagenesis (54, 55) have been reported in a variety of cell lines after photodynamic targeting, and our results with directly targeted WTK1 cells confirm that extranuclear-localizing photosensitizers can cause DNA damage by secondary processes and that these lesions can be mutagenic.
Preincubation of targeted cells with vitamin E protects WTK1 cells from damage and reduces phototoxicity, oxidative stress and DNA damage. However, more interestingly, when vitamin E is administered only to the target cells, a significant reduction in toxicity, ROS levels and DNA damage was also seen in the bystander cells. This key observation suggests that membrane damage in the target cells is a key initiator for the bystander signal and that lipid peroxidation is initiated early in the bystander response pathway. This is supported by earlier experiments of Dahle et al. where the bystander effect was seen to be greater after treatments that produced membrane damage (22). This action could be in the form of diffusing ROS that act as oxidizing agents in the bystander cells. Hydrogen peroxide and products of lipid hydroperoxides would have sufficient lifetime to diffuse to bystander cells to initiate oxidation processes. An interesting observation is the delayed increase (peaking at ∼6 h) in oxidative stress that occurs in bystander WTK1 cells. Clearly, signaling occurs that produces elevated ROS levels in the bystander cells that subsequently lead to DNA damage, mutagenesis and cell death at later times.
The observation that the bystander responses observed here were highest at low toxicity to the targeted cells implies that the stressed cells are actively involved in secondary generation of ROS and signaling. We have also observed increased cytosolic calcium levels in the targeted cells (not shown) and are currently investigating the potential role of enzymes involved in arachidonic acid metabolism, including phospholipase A2 (PLA2) and cyclooxygenase 2 (COX2), the latter having been implicated in the ionizing radiation-induced bystander effect (56). Other potential sources of secondary ROS under investigation include the membrane-localized NADPH oxidases that are an attractive answer to the problem of generation of long-lived and extracellular ROS that can diffuse to bystander cells to initiate remote oxidative damage (57, 58).
In summary, we have demonstrated that photodynamic treatment of WTK1 human lymphoblastoid cells in suspension under conditions where targeted and bystander cells cannot be in physical contact can lead to considerable bystander effects that can be important if extended to progression of disease that involved oxidative stress or in response to acute therapy. WTK1 bystander cells can experience increased oxidative stress, leading to DNA damage, mutagenesis and cell death, thus expanding the range of cells affected from those that are directly targeted. While presently limited to the WTK1 line, these findings should be explored using other cell lines and photodynamic agents to determine whether the effects are general or specific in nature.
ACKNOWLEDGMENTS
The authors are grateful for the advice and assistance of Chelvi Rajadurai, Noemí Rubio, Larry Booth, Gladys Ouédraogo and Irene Kochevar. This work was supported by funding from NIH P01 CA095227 and NIH T32 CA009078 (AC).
REFERENCES
- 1.Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 2.Iyer R, Lehnert BE, Svensson R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000;60:1290–1298. [PubMed] [Google Scholar]
- 3.Little JB, Lauriston S. Taylor lecture: Nontargeted effects of radiation: implications for low-dose exposures. Health Phys. 2006;91:416–426. doi: 10.1097/01.HP.0000232847.23192.3e. [DOI] [PubMed] [Google Scholar]
- 4.Koturbash I, Rugo RE, Hendricks CA, Loree J, Thibault B, Kutanzi K, Pogribny I, Yanch JC, Engelward BP, Kovalchuk O. Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene. 2006;25:4267–4275. doi: 10.1038/sj.onc.1209467. [DOI] [PubMed] [Google Scholar]
- 5.Nagasawa H, Peng Y, Wilson PF, Lio YC, Chen DJ, Bedford JS, Little JB. Role of homologous recombination in the alpha-particle-induced bystander effect for sister chromatid exchanges and chromosomal aberrations. Radiat. Res. 2005;164:141–147. doi: 10.1667/rr3420. [DOI] [PubMed] [Google Scholar]
- 6.Nagasawa H, Huo L, Little JB. Increased bystander mutagenic effect in DNA double-strand break repair-deficient mammalian cells. Int. J. Radiat. Biol. 2003;79:35–41. [PubMed] [Google Scholar]
- 7.Lyng FM, Seymour CB, Mothersill C. Early events in the apoptotic cascade initiated in cells treated with medium from the progeny of irradiated cells. Radiat. Prot. Dosimetry. 2002;99:169–172. doi: 10.1093/oxfordjournals.rpd.a006753. [DOI] [PubMed] [Google Scholar]
- 8.Mothersill C, Seymour C. Radiation-induced bystander effects, carcinogenesis and models. Oncogene. 2003;22:7028–7033. doi: 10.1038/sj.onc.1206882. [DOI] [PubMed] [Google Scholar]
- 9.Azzam EI, de Toledo SM, Little JB. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 2003;22:7050–7057. doi: 10.1038/sj.onc.1206961. [DOI] [PubMed] [Google Scholar]
- 10.Ballarini F, Biaggi M, Ottolenghi A, Sapora O. Cellular communication and bystander effects: a critical review for modelling low-dose radiation action. Mutat. Res. 2002;501:1–12. doi: 10.1016/s0027-5107(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 11.Little MP, Wakeford R. The bystander effect in experimental systems and compatibility with radon-induced lung cancer in humans. J. Radiol. Prot. 2002;22:A27–A31. doi: 10.1088/0952-4746/22/3a/305. [DOI] [PubMed] [Google Scholar]
- 12.Shao C, Furusawa Y, Aoki M, Matsumoto H, Ando K. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int. J. Radiat. Biol. 2002;78:837–844. doi: 10.1080/09553000210149786. [DOI] [PubMed] [Google Scholar]
- 13.Little JB, Azzam EI, de Toledo SM, Nagasawa H. Bystander effects: intercellular transmission of radiation damage signals. Radiat. Prot. Dosimetry. 2002;99:159–162. doi: 10.1093/oxfordjournals.rpd.a006751. [DOI] [PubMed] [Google Scholar]
- 14.Rubio N, Fleury SP, Redmond RW. Spatial and temporal dynamics of in vitro photodynamic cell killing: Extracellular hydrogen peroxide mediates neighbouring cell death. Photochem. Photobiol. Sci. 2009 doi: 10.1039/b815343d. DOI: 10.1039/B815343D. [DOI] [PubMed] [Google Scholar]
- 15.Shao C, Furusawa Y, Kobayashi Y, Funayama T, Wada S. Bystander effect induced by counted high-LET particles in confluent human fibroblasts: a mechanistic study. FASEB J. 2003;17:1422–1427. doi: 10.1096/fj.02-1115com. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Zhou H, Geard CR, Hei TK. Effect of medium on chromatin damage in bystander mammalian cells. Radiat. Res. 2004;162:264–269. doi: 10.1667/rr3226. [DOI] [PubMed] [Google Scholar]
- 17.Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD. A proliferation-dependent bystander effect in primary porcine and human urothelial explants in response to targeted irradiation. Br. J. Cancer. 2003;88:767–774. doi: 10.1038/sj.bjc.6600804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiat. Res. 2006;165:400–409. doi: 10.1667/rr3527.1. [DOI] [PubMed] [Google Scholar]
- 19.Demidem A, Morvan D, Madelmont JC. Bystander effects are induced by CENU treatment and associated with altered protein secretory activity of treated tumor cells: a relay for chemotherapy? Int. J. Cancer. 2006;119:992–1004. doi: 10.1002/ijc.21761. [DOI] [PubMed] [Google Scholar]
- 20.Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee G, Gupta N, Kapoor A, Raman G. UV induced bystander signaling leading to apoptosis. Cancer Lett. 2005;223:275–284. doi: 10.1016/j.canlet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 22.Dahle J, Angell-Petersen E, Steen HB, Moan J. Bystander effects in cell death induced by photodynamic treatment UVA radiation and inhibitors of ATP synthesis. Photochem. Photobiol. 2001;73:378–387. doi: 10.1562/0031-8655(2001)073<0378:beicdi>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Dahle J, Kaalhus O, Stokke T, Kvam E. Bystander effects may modulate ultraviolet A and B radiation-induced delayed mutagenesis. Radiat. Res. 2005;163:289–295. doi: 10.1667/rr3305. [DOI] [PubMed] [Google Scholar]
- 24.Dahle J, Kvam E. Induction of delayed mutations and chromosomal instability in fibroblasts after UVA-, UVB-, and X-radiation. Cancer Res. 2003;63:1464–1469. [PubMed] [Google Scholar]
- 25.Dabrowska A, Gos M, Janik P. “Bystander effect” induced by photodynamically or heat-injured ovarian carcinoma cells (OVP10) in vitro. Med. Sci. Monit. 2005;11:BR316–BR324. [PubMed] [Google Scholar]
- 26.Dahle J, Bagdonas S, Kaalhus O, Olsen G, Steen HB, Moan J. The bystander effect in photodynamic inactivation of cells. Biochim. Biophys. Acta. 2000;1475:273–280. doi: 10.1016/s0304-4165(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 27.Dahle J, Kaalhus O, Moan J, Steen HB. Cooperative effects of photodynamic treatment of cells in microcolonies. Proc. Natl. Acad. Sci. USA. 1997;94:1773–1778. doi: 10.1073/pnas.94.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahle J, Mikalsen SO, Rivedal E, Steen HB. Gap junctional intercellular communication is not a major mediator in the bystander effect in photodynamic treatment of MDCK II cells. Radiat. Res. 2000;154:331–341. doi: 10.1667/0033-7587(2000)154[0331:gjicin]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 30.Triesscheijn M, Baas P, Schellens JH, Stewart FA. Photodynamic therapy in oncology. Oncologist. 2006;11:1034–1044. doi: 10.1634/theoncologist.11-9-1034. [DOI] [PubMed] [Google Scholar]
- 31.van den Bergh H. Photodynamic therapy of age-related macular degeneration: History and principles. Semin. Ophthalmol. 2001;16:181–200. doi: 10.1076/soph.16.4.181.10299. [DOI] [PubMed] [Google Scholar]
- 32.Trauner KB, Hasan T. Photodynamic treatment of rheumatoid and inflammatory arthritis. Photochem. Photobiol. 1996;64:740–750. doi: 10.1111/j.1751-1097.1996.tb01829.x. [DOI] [PubMed] [Google Scholar]
- 33.Ibbotson SH. Topical 5-aminolaevulinic acid photodynamic therapy for the treatment of skin conditions other than non-melanoma skin cancer. Br. J. Dermatol. 2002;146:178–188. doi: 10.1046/j.0007-0963.2001.04689.x. [DOI] [PubMed] [Google Scholar]
- 34.Taub AF. Photodynamic therapy: other uses. Dermatol. Clin. 2007;25:101–109. doi: 10.1016/j.det.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Lambrechts SA, Demidova TN, Aalders MC, Hasan T, Hamblin MR. Photodynamic therapy for Staphylococcus aureus infected burn wounds in mice. Photochem. Photobiol. Sci. 2005;4:503–509. doi: 10.1039/b502125a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva JC, Lacava ZG, Kuckelhaus S, Silva LP, Neto LF, Sauro EE, Tedesco AC. Evaluation of the use of low level laser and photosensitizer drugs in healing. Lasers Surg. Med. 2004;34:451–457. doi: 10.1002/lsm.20062. [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Asaad N, Held KD. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene. 2005;24:2096–2103. doi: 10.1038/sj.onc.1208439. [DOI] [PubMed] [Google Scholar]
- 38.Aveline BM, Sattler RM, Redmond RW. Environmental effects on cellular photosensitization: correlation of phototoxicity mechanism with transient absorption spectroscopy measurements. Photochem. Photobiol. 1998;68:51–62. [PubMed] [Google Scholar]
- 39.Carrier F, Smith ML, Bae I, Kilpatrick KE, Lansing TJ, Chen CY, Engelstein M, Friend SH, Henner WD, Gilmer TM. Characterization of human Gadd45, a p53-regulated protein. J. Biol. Chem. 1994;269:32672–32677. [PubMed] [Google Scholar]
- 40.Xia F, Liber HL. Electroporation of human lymphoblastoid cells. Methods Mol. Biol. 1995;48:151–160. doi: 10.1385/0-89603-304-X:151. [DOI] [PubMed] [Google Scholar]
- 41.Zhen W, Denault CM, Loviscek K, Walter S, Geng L, Vaughan AT. The relative radiosensitivity of TK6 and WI-L2-NS lymphoblastoid cells derived from a common source is primarily determined by their p53 mutational status. Mutat. Res. 1995;346:85–92. doi: 10.1016/0165-7992(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 42.Amundson SA, Xia F, Wolfson K, Liber HL. Different cytotoxic and mutagenic responses induced by X-rays in two human lymphoblastoid cell lines derived from a single donor. Mutat. Res. 1993;286:233–241. doi: 10.1016/0027-5107(93)90188-l. [DOI] [PubMed] [Google Scholar]
- 43.Xia F, Amundson SA, Nickoloff JA, Liber HL. Different capacities for recombination in closely related human lymphoblastoid cell lines with different mutational responses to X-irradiation. Mol. Cell. Biol. 1994;14:5850–5857. doi: 10.1128/mcb.14.9.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furth EE, Thilly WG, Penman BW, Liber HL, Rand WM. Quantitative assay for mutation in diploid human lymphoblasts using microtiter plates. Anal. Biochem. 1981;110:1–8. doi: 10.1016/0003-2697(81)90103-2. [DOI] [PubMed] [Google Scholar]
- 45.Fenech M. Cytokinesis-block micronucleus assay evolves into a “cytome” assay of chromosomal instability, mitotic dysfunction and cell death. Mutat. Res. 2006;600:58–66. doi: 10.1016/j.mrfmmm.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 46.Ouedraogo GD, Redmond RW. Secondary reactive oxygen species extend the range of photosensitization effects in cells: DNA damage produced via initial membrane photosensitization. Photochem. Photobiol. 2003;77:192–203. doi: 10.1562/0031-8655(2003)077<0192:sroset>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 47.Azzam EI, De Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 48.Prise KM, Belyakov OV, Folkard M, Michael BD. Studies of bystander effects in human fibroblasts using a charged particle microbeam. Int. J. Radiat. Biol. 1998;74:793–798. doi: 10.1080/095530098141087. [DOI] [PubMed] [Google Scholar]
- 49.Zhou H, Suzuki M, Randers-Pehrson G, Vannais D, Chen G, Trosko JE, Waldren CA, Hei TK. Radiation risk to low fluences of alpha particles may be greater than we thought. Proc. Natl. Acad. Sci. USA. 2001;98:14410–14415. doi: 10.1073/pnas.251524798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Redmond RW, Kochevar IE. Spatially resolved cellular responses to singlet oxygen. Photochem. Photobiol. 2006;82:1178–1186. doi: 10.1562/2006-04-14-IR-874. [DOI] [PubMed] [Google Scholar]
- 51.McNair FI, Marples B, West CM, Moore JV. A comet assay of DNA damage and repair in K562 cells after photodynamic therapy using haematoporphyrin derivative, methylene blue and meso-tetrahydroxyphenylchlorin. Br. J. Cancer. 1997;75:1721–1729. doi: 10.1038/bjc.1997.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oleinick NL, Evans HH. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat. Res. 1998;150(Suppl):S146–S156. [PubMed] [Google Scholar]
- 53.Rousset N, Keminon E, Eleouet S, Le Neel T, Auget JL, Vonarx V, Carre J, Lajat Y, Patrice T. Use of alkaline comet assay to assess DNA repair after m-THPC-PDT. J. Photochem. Photobiol. B. 2000;56:118–131. doi: 10.1016/s1011-1344(00)00053-1. [DOI] [PubMed] [Google Scholar]
- 54.Deahl JT, Oleinick NL, Evans HH. Large mutagenic lesions are induced by photodynamic therapy in murine L5178Y lymphoblasts. Photochem. Photobiol. 1993;58:259–264. doi: 10.1111/j.1751-1097.1993.tb09558.x. [DOI] [PubMed] [Google Scholar]
- 55.Evans HH, Horng MF, Ricanati M, Deahl JT, Oleinick NL. Mutagenicity of photodynamic therapy as compared to UVC and ionizing radiation in human and murine lymphoblast cell lines. Photochem. Photobiol. 1997;66:690–696. doi: 10.1111/j.1751-1097.1997.tb03208.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, Hei TK. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc. Natl. Acad. Sci. USA. 2005;102:14641–14646. doi: 10.1073/pnas.0505473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehnert BE, Goodwin EH, Deshpande A. Extracellular factor(s) following exposure to alpha particles can cause sister chromatid exchanges in normal human cells. Cancer Res. 1997;57:2164–2171. [PubMed] [Google Scholar]
- 58.Narayanan PK, Goodwin EH, E. Lehnert B. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997;57:3963–3971. [PubMed] [Google Scholar]