Summary
Respiratory virus infections in hematopoietic cell transplant (HCT) recipients are a major cause of morbidity and mortality. While respiratory syncytial virus (RSV), human metapneumonvirus, parainfluenzaviruses, and influenza viruses are well known for their potential to cause fatal pneumonia, information has only recently emerged regarding the significance of the newly discovered viruses, such as human coronaviruses NL63 and HKU1, and human bocavirus. Lymphopenia seems to be the most important risk factor for progression to lower respiratory tract disease. Airflow obstruction is another complication of respiratory virus infections after HCT, and data to date indicate this complication may occur following parainfluenza virus and RSV infection. Infection control procedures are key for prevention. Unfortunately, there are no randomized treatment studies, which make the interpretation of the literature on interventions difficult. This article reviews the spectrum of pathogens, epidemiology, risk factors and clinical manifestations of infection, as well as recent advances in diagnostic and clinical management.
Keywords: hematopoietic cell transplantation, respiratory viruses, respiratory syncytial virus, parainfluenzaviruses, influenzaviruses
In immunocompetent persons, respiratory viruses cause a wide range of syndromes. These syndromes span from the mere common cold episodes to incidences of croup, bronchiolitis, and airflow obstruction in children. Moreover, respiratory viruses may lead to exacerbation of asthma or chronic obstructive pulmonary disease, and frank pneumonia in adults (Kim et al, 2007). While direct mortality is rarely associated with these viruses in immunocompetent people, the impact on quality of life and the economic losses are substantial. In contrast, the clinical spectrum of disease is much more severe in immunocompromised patients. Respiratory viruses can cause fatal pneumonia and trigger a late airflow obstruction syndrome, which is associated with significant morbidity and mortality (1993, 1996; Ljungman, 2001; Nichols et al, 2001a; Chemaly et al, 2006; Erard et al, 2006). This review will summarize the current status of respiratory virus diagnostics, disease associations, and management strategies.
Epidemiology
Most of the available information focuses on respiratory syncytial virus (RSV), parainfluenza viruses, and influenza viruses, probably due to the fact that these viruses are readily identified by traditional virological detection methods, such as viral cultures and immunofluorescence‐based methods. The more recently described human coronaviruses (HCoV), human metapneumovirus (HMPV), human bocavirus (HBoV), as well as human rhinoviruses (HRhV) require molecular detection methods for optimal detection; thus, information on their clinical importance has only begun to emerge.
The infection epidemiology or respiratory viruses in HCT recipients usually parallels that observed in the community, as these viruses circulate in immunocompetent individuals (including health care personnel and family members).
Importance of diagnostics
Non‐molecular methods available for testing include: standard viral cultures (results available in several days), shell vial centrifugation cultures using specific monoclonal antibodies (results after 1–3 d), direct fluorescent antibody (DFA) tests (2 h), and enzyme immunoassays (2 h). On tissue sections from lung biopsy or autopsy specimens, virus‐specific monoclonal antibody staining, viral cultures, or polymerase chain reaction (PCR) can be used.
For non‐molecular methods, and to a lesser extent, for molecular detection methods, specimen acquisition techniques and handling are important for maximal diagnostic yield. Nasal wash or swab specimens should be placed on ice or in the refrigerator immediately and transported to the laboratory without delay (Englund et al, 1996). For non‐molecular methods, specimen set‐up in the laboratory should occur within 2–4 h.
There has been a significant shift towards molecular detection techniques in recent years. Indeed, these techniques offer the potential for multiplex testing platforms (Lee et al, 2007; Mahony et al, 2007; Nolte et al, 2007). This is important because of the non‐specific clinical presentation of these infections. This “syndromic” nature of respiratory viral infections will ultimately require a multiplex testing platform for comprehensive detection of these pathogens. Several assays have been described in the literature. The Hexaplex™ assay detects seven respiratory viruses and has shown excellent performance characteristics in various clinical settings (Hindiyeh et al, 2001; Kehl et al, 2001). Another multiplex platform (MultiCode‐PLx system, EraGen Biosciences, Inc., Madison, WI) detected 17 respiratory viruses simultaneously and showed significantly increased diagnostic yield compared to DFA or culture methods. This was mainly caused by improved detection of influenza A virus and viruses not readily detected by standard virological methods, including HMPV, HcoV, and HRhV (Nolte et al, 2007). A 20‐respiratory virus microbead‐based assay also showed excellent sensitivity and specificity, as well as an increased yield for detection of viruses that are difficult to detect by culture or DFA (Mahony et al, 2007). Microarray and nanotechnologies are also being explored in order to develop large‐scale and efficient viral detection platforms (Liu et al, 2006; Chiu et al, 2007; Fournier‐Wirth & Coste, 2007).
Respiratory syncytial virus
Significance and risk factors
In patients with hematological malignancies, including HCT recipients, RSV causes upper respiratory infection (URI), which may progress to fatal pneumonia (Harrington et al, 1992). RSV lower respiratory tract infection has also been linked to late airflow obstruction, a debilitating condition of accelerated loss of lung function after HCT (Erard et al, 2006). During the respiratory virus season, the incidence may be as high as 10%, and both allogeneic and autologous transplant recipients may be infected (Nichols et al, 2001a; Small et al, 2002) (Table I, Fig 1). Acquisition of RSV may occur at any time after transplantation, but is most common when patients are in the outpatient setting.
Table I.
Respiratory virus infections after HCT: comparison of RSV, Parainfluenzavirus 3, and influenzaviruses.
| Virus | Incidence of infection (%) | Progression from URI to pneumonia (%) | Time from URI to pneumonia (median, d) | Proportion of pneumonia without URI (%) | Pulmonary copathogens in cases with pneumonia (%) | Overall mortality at 1 month after diagnosis of pneumonia (%) |
|---|---|---|---|---|---|---|
| RSV | 1·8–6* | 40 | 7 | 20–50 | 2·5–33 | 45 |
| Parainfluenza virus 3 | 4–7 | 18–44 | 7 | 31 | 53 | 35–37 |
| Influenzaviruses A and B | 1·3–2·6† | 18 | 11 | 18 | 50 | 25–28 |
*During the winter season, the incidence may be as high as 10%.
†Maybe significantly higher during outbreaks (Martino et al, 2003).
HCT, haematopoietic cell transplantation; RSV, respiratory syncytial virus; URI, upper respiratory infection.
Figure 1.
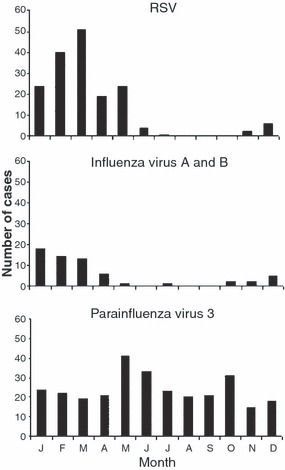
Monthly distribution of respiratory syncytial virus (RSV), parainfluenza virus 3, and influenza infections after HCT in the 1990’s at the Fred Hutchinson Cancer Research Center [from Boeckh (2004)]. With permission from Blackwell Publishing, Oxford, UK.
In one large cohort study, winter season, male gender, and use of bone marrow as a stem cell source were identified for the acquisition of RSV in HCT recipients (Nichols et al, 2001a). URI precedes pneumonia in 80–90% of patients, and approximately 30–40% of patients with RSV URI progress to pneumonia after a median of 7 d. However, in some patients with RSV pneumonia, URI is not present, is very mild, or occurs only concurrently with the onset of pneumonia (Nichols et al, 2001a). Strongest risk factors for progression to pneumonia are older age and lymphopenia (Nichols et al, 2001a). During the first 3 months after HCT, patients receiving non‐myeloablative conditioning regimens seem to be protected from progression to lower respiratory tract disease (Schiffer et al, 2006). Following reduced intensity conditioning, regimens may also have a somewhat lower risk of progression and fatal disease (Chakrabarti et al, 2002), however, larger studies are needed to better define the risk of progression. Most studies indicate that asymptomatic shedding of RSV is not a common phenomenon (Ljungman et al, 1989; Adams et al, 1999; Peck et al, 2007).
Treatment and prevention
Without treatment, RSV pneumonia is almost uniformly fatal in highly immunosuppressed HCT recipients (Harrington et al, 1992). However, recovery from RSV lower respiratory tract disease without specific treatment has been reported (Abdallah et al, 2003). These differences are probably due to less intense immunosuppressive regimens and whether lymphopenia is present at the time of diagnosis (Chemaly et al, 2006). Pulmonary copathogens are detected in up to one third of the patients with RSV pneumonia and require aggressive treatment. No adequately powered controlled trials exist for the treatment of RSV infection and pneumonia in the HCT setting. Available evidence comes from small uncontrolled cohort studies and one small randomized trial (Boeckh et al, 2007). The data suggest that treatment of early pneumonia (i.e. prior to mechanical ventilation) is associated with improved outcome. Intermittent short duration (2 g, over 2 h, three times per day) or continuous aerosolized ribavirin is considered the treatment of choice for RSV pneumonia. With this regimen, the 30‐d all‐cause mortality is approximately 40% (Table I). In a large multivariate analysis, RSV pneumonia (but not URI) was independently associated with mortality (Nichols et al, 2001a). Systemic ribavirin alone does not seem to be effective for the treatment of pneumonia (Lewinsohn et al, 1996; Sparrelid et al, 1997); better response rates were reported for treatment of RSV URI, however, the small sample size limits the strength of the data (Schleuning et al, 2004). There are no data on combined oral and aerosolized ribavirin.
The role of concomitant intravenous immunoglobulin, or RSV‐specific immunoglobulin, or palivizumab, an RSV‐specific monoclonal antibody directed at the F protein of RSV, for the treatment of RSV pneumonia is poorly defined. Uncontrolled data suggest that high‐titer antibody preparations or palivizumab may be required if such adjunctive therapy is given (DeVincenzo et al, 2000; Boeckh et al, 2001; Small et al, 2002). There are several factors that may account for differences in outcome in the available cohort studies. One important factor appears to be the timing of initiation of therapy. Several studies suggest that, when treatment is started after respiratory failure has occurred, it is almost uniformly unsuccessful (Whimbey et al, 1995). Other factors that may explain the differences in outcome between studies include: the presence of lymphopenia at the time of diagnosis, the presence of co‐pathogens (e.g. invasive moulds), and the use of immunosuppressive agents (Ljungman, 2001; Boeckh et al, 2004; Chemaly et al, 2006). Available studies are too small to control for these parameter, making the interpretation of results of pharmacological interventions difficult. Most centers now agree that aerosolized ribavirin should be given to HCT patients with RSV lower respiratory tract disease. There is no consensus on the role of antibody preparations, however, the author uses palivizumab for radiographically and virologically proven RSV pneumonia.
Due to the high mortality of RSV pneumonia, much interest has focused on prevention. A recent small controlled study of preemptive, antiviral therapy of aerosolized ribavirin, given at the time of RSV URI, showed a decline in viral load, but no significant difference in RSV pneumonia; the study was prematurely stopped due to slow accrual(Boeckh et al, 2007). A larger retrospective study suggests that this strategy may be effective (Chemaly et al, 2006). Whether ribavirin is indicated for RSV URI remains controversial; some centers (including the author’s) administer it in the presence of lymphopenia (lymphocyte count <0·3 × 109/l) when the risk of progression is particularly high (Boeckh et al, 2004; Chemaly et al, 2006). Lymphopenia is a highly significant risk factor for progression to lower tract disease during the first 3 months after HCT (Ljungman et al, 2001; Chemaly et al, 2006). There is no data on the risk of progression later after HCT; anecdotal experience suggests that other factors, such as poor lung function, may be more important in patients with prolonged immunosuppression; further studies are needed to define the risk factors in this setting. Whether palivizumab given preemptively to patients with RSV URI to prevent progression to lower respiratory tract disease is effective has not been definitively determined (Chavez‐Bueno et al, 2007; Khanna et al, 2008).
Prophylactic measures recommended throughout the respiratory virus season include: isolation of infected patients, hand washing before and after every patient contact, educational efforts targeted at health care personnel and family members, and avoiding patient contact of health care personnel and family members with uncontrolled secretions (Boeckh et al, 2004). No studies exist on pharmacological prophylaxis for RSV acquisition.
For pretransplant infections with RSV, parainfluenzavirus or influenzavirus, most transplant centers postpone the transplant procedure until resolution of symptoms and cessation of viral shedding, especially when a myeloablative allogeneic HCT is planned. This approach was supported by a recent study of pretransplant RSV infection, which showed rapid progression to RSV pneumonia in patients in whom the transplant procedure was not postponed (Peck et al, 2004). However, there is evidence that low‐risk autologous transplant patients may be transplanted without adverse outcome (Aslan et al, 1999). Additionally, the outcome of respiratory virus infections appears to be less severe with non‐myeloablative or reduced‐toxicity conditioning regimens (Schiffer et al, 2006).
Human metapneumovirus
Significance and risk factors
Human metapneumovirus is a newly discovered negative‐sense non‐segmented RNA paramyxovirus (van den Hoogen et al, 2001). Structurally, the virus is closely related to RSV. By the age of 5 years, virtually all children are seropositive. The virus can cause upper and lower tract infection during the winter season (Boivin et al, 2002). Serious lower respiratory tract disease associated with HMPV has been reported in immunocompromised patients, but no risk factors for acquisition have been described (Table II). HMPV infection occurs in up to 5% of HCT recipients (Fig 2) (Peck et al, 2007). Whether asymptomatic shedding occurs is controversial (Debiaggi et al, 2007; Peck et al, 2007). The progression rate to lower respiratory tract disease has not been determined. Among HCT recipients undergoing bronchoalveolar lavage (BAL) for clinical and radiographic pneumonia, 3–4% had HMPV detected (Englund et al, 2006). Most of these patients were previously classified as having idiopathic pneumonia syndrome (Englund et al, 2006). HMPV disease in HCT recipients immediately post‐transplant presented with upper respiratory symptoms, including: fever, nasal congestion, and cough. Once pneumonia developed, rapidly progressive pulmonary infiltrates, frequently accompanied by hypotension, septic shock, or both, were observed (Englund et al, 2006). Radiographic findings ranged from diffuse bilateral alveolar and interstitial infiltrates to emphysema without infiltrate. A high viral load was observed in the bronchoalveolar lavage fluid. Histological assessment showed that diffuse alveolar damage with hyaline membrane formation, foci of bronchiolitis obliterans and organizing pneumonia, and diffuse alveolar hemorrhage are common (Englund et al, 2006).
Table II.
Seasonality, symptoms, and diagnosis of respiratory viruses in immunocompetent subjects and HCT recipients [adapted from Nichols et al (2008), with permission from American Society for Microbiology].
| Virus | Seasonality | Disease manifestations in immunocompetent persons | Disease in HCT recipients | Diagnosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| URI | Otitis media | Croup | Bronchiolitis | Pneumonia | AFO and/or wheezing | URI | Pneumonia | AFO | Culture | EIA | FA rapid cultures | PCR | ||
| RSV | ++ | +++ | ++ | + | +++ | +++ | +++ | +++ | +++ | ++ | + | + | ++ | +++ |
| HMPV | ++ | +++ | ++ | + | ++ | +++ | ++ | +++ | +++ | ++ | Research | NA | Research | +++ |
| PIV 1 | + | +++ | + | +++ | + | ++ | ++ | +++ | +++ | +++ | + | NA | + | +++ |
| PIV 2 | + | +++ | + | ++ | + | ++ | ++ | +++ | +++ | +++ | + | NA | + | +++ |
| PIV 3 | − | +++ | + | ++ | ++ | ++ | +++ | +++ | +++ | +++ | + | NA | + | +++ |
| PIV 4 | − | +++ | + | + | + | + | + | +++ | +++ | ND | + | NA | ++ | +++ |
| Influenza A, B | ++ | +++ | + | + | + | ++ | + | +++ | +++ | + | + | + | ++ | +++ |
| Adenoviruses | + | +++ | + | + | ++ | + | − | + | +++ | − | + | ++ | ++ | +++ |
| Rhinoviruses | + | +++ | + | + | + | + | +++ | +++ | + | ++ | + | NA | NA | ++ |
| Coronaviruses | + | +++ | + | ++ | + | + | ++ | ND | ND | ND | Research | NA | NA | +++ |
| Bocavirus | + | +++ | ND | + | + | + | + | ND | ND | ND | NA | NA | NA | +++ |
HCT, haematopoietic cell transplantation; RSV, respiratory syncytial virus; HMPV, human metapneumovirus; PIV, parainfluenza virus; URI, upper respiratory infection; AFO, airflow obstruction; EIA, enzyme immunoassay; FA, fluorescent antibody; PCR, polymerase chain reaction; ND, no data; NA, not available.
Figure 2.
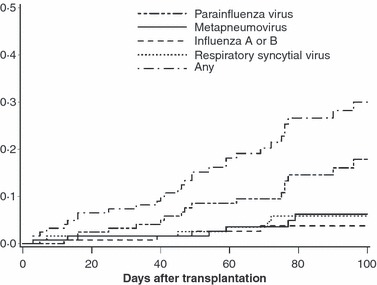
Time to first respiratory virus infection after haematopoietic cell transplantation. This research was originally published in Blood. Peck et al (2007). © the American Society of Hematology.
Treatment and prevention
To date, there is no established treatment for HMPV disease, which may be fatal in HCT recipients (Englund et al, 2006). Although IVIG has been shown to effectively neutralize HMPV in vitro, its effect in vivo is unknown (Wyde et al, 2003). The role of steroids is also not defined. Ribavirin has been shown to have in vitro antiviral activity against HMPV (Wyde et al, 2003), however, there are no reports on treatment with ribavirin. Infection control measures similar to those outlined for RSV are the mainstay of prevention.
Parainfluenza viruses
Significance and risk factors
Parainfluenza viruses are classified into four serotypes. Of the four types of parainfluenza viruses, parainfluenzavirus 3 is the most common (approximately 90%), followed by serotypes 1 and 2. The incubation time is 1–4 d. Parainfluenzavirus infections remain detectable throughout the summer season (Fig 1). Even with standard virological methods, the incidence of parainfluenzaviruses is higher than that of RSV in different cancer centers (Nichols et al, 2001a). PCR detection significantly increases the diagnostic yield (Peck et al, 2007).
Other than HCT from an unrelated donor, no risk factor for acquisition of the parainfluenzavirus has been found (Nichols et al, 2001a). In T cell‐depleted patients, CD4 lymphopenia has been reported to increase the risk of all respiratory virus infections, including parainfluenzavirus infections (Chakrabarti et al, 2002).
Similar to RSV and HMPV, URI is the predominant clinical presentation (Table II). Progression to pneumonia seems to be less common than with RSV (Nichols et al, 2001a). Late airflow obstruction has been linked, not only to parainfluenzavirus pneumonia, but also to URI (Erard et al, 2006). The most important risk factors for the progression from URI to pneumonia is use of systemic corticosteroids and lymphopenia.( Nichols et al, 2001b; Chakrabarti et al, 2002). During the first 3 months after HCT, recipients of non‐myeloablative conditioning regimens appear to have a small risk of progression to lower respiratory tract disease (Schiffer et al, 2006). Reduced intensity conditioning regimens may also have a somewhat lower risk of progression and fatal disease (Chakrabarti et al, 2002), however, larger studies are needed to better define the risk of progression. Although the overall progression rate to pneumonia is only 18%, in allograft recipients the risk is 40% if 1 mg/kg of prednisone is given (for treatment of graft‐versus‐host disease, GvHD), and 65% with 2 mg/kg (Nichols et al, 2001b). Parainfluenzavirus 3 pneumonia may also occur after autologous transplantation, however, mainly in the setting of CD34 selection or use of high‐dose steroids (Nichols et al, 2001b). Parainfluenzavirus 3 pneumonia is associated with serious pulmonary co‐pathogens, such as Aspergillus fumigatus, in approximately half of the cases. Factors associated with poor outcome after pneumonia include presence of co‐pathogens and mechanical ventilation (Nichols et al, 2001b). Thus, aggressive diagnostic intervention (i.e. BAL) and therapy are indicated in patients with suspected parainfluenzavirus pneumonia. In a large retrospective analysis both URI and pneumonia, due to parainfluenzavirus 3, were associated with overall mortality in multivariable models (Nichols et al, 2001b).
Treatment and prevention
Mortality of pneumonia is approximately 35% in recipients of allografts following myeloablative conditioning (Nichols et al, 2001b). Outcome appears to be somewhat better in patients who received reduced toxicity conditioning regimens (Chakrabarti et al, 2002). In a retrospective analysis, neither aerosolized ribavirin nor intravenous immunoglobulin led to improved outcome of pneumonia or a reduction in viral shedding following pneumonia (Nichols et al, 2001b). Systemic ribavirin has only been reported in case reports (Chakrabarti et al, 2000a). Randomized treatment studies have not been performed. No definitive assessment can be made as to whether earlier antiviral treatment (i.e. preemptive treatment for URI) is effective in the prevention of pneumonia or late airflow obstruction (Chakrabarti et al, 2000a). The association of high‐dose steroid treatment with progression to disease might suggest that reduction on immunosuppression may be useful (Nichols et al, 2001b), however, such an approach has not been tested. The role of immunoglobulin in the treatment of parainfluenzavirus infections is also poorly defined. One retrospective analysis did not find a benefit of pooled immunoglobulin given for parainfluenzavirus pneumonia (Nichols et al, 2001b). However, antibody content in pooled preparations is highly variable. The highest titers can be obtained from RSV‐specific immunoglobulin that also contains high titer of antibodies directed against parainfluenzavirus (Cortez et al, 2002).
Infection control is the mainstay of prevention strategies. Unfortunately, current infection control practices seem to be far from perfect in keeping parainfluenza virus out of HCT units, as indicated by the high incidence figures and repeated outbreaks (Zambon et al, 1998; Cortez et al, 2001; Nichols et al, 2004b). Possible explanations for the difficulty to prevent parainfluenzavirus from entering HCT units include: lack of a vaccine for health care workers and close contacts, very mild or lacking symptoms in immunocompetent individuals in combination with prolonged shedding, prolonged asymptomatic shedding in infected patients, and persistence of the virus on environmental surfaces (Peck et al, 2007).
Influenzaviruses
Significance and risk factors
Influenza is classified into three major types, of which type A is most common, followed by type B (Ljungman et al, 1993; Whimbey et al, 1996; Bowden, 1997). Influenza type C is very uncommon, even in the immunocompetent population; there are no reports of influenza C in HCT recipients. Influenza virus infections seem to be less common than RSV and parainfluenza virus infections (Table I, Fig 1).
Acquisition of influenza is increased in patients with high‐risk underlying disease (Nichols et al, 2004a). Progression to severe pneumonia can occur similar to RSV and parainfluenzavirus (Bowden, 1997), however, progression rates appear to be lower and risk factors for progression differ (Table II). In contrast to parainfluenzavirus infection, where corticosteroids are an important risk factor for the development of pneumonia, influenza virus lower tract disease appears to be less common in patients treated with corticosteroids (Nichols et al, 2004a; Machado et al, 2005). Also, patients receiving non‐myeloablative conditioning regimens appear to be less likely to develop pneumonia (Schiffer et al, 2006). Interestingly, the clinical presentation in HCT often lacks myalgia and high fever, which are commonly seen in immunocompetent individuals (Fig 3).
Figure 3.
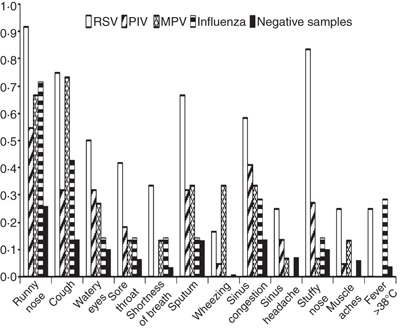
Symptoms associated with respiratory virus infection after haematopoietic cell transplantation. This research was originally published in Blood. Peck et al (2007). © the American Society of Hematology. RSV, respiratory syncytial virus; PIV, parainfluenza virus; MPV, metapneumovirus.
Treatment and prevention
Effective prevention is available for influenza, which may explain the lower incidence. Health care personnel, family members, and visitors are advised to get vaccinated against influenza early in the season. Antiviral therapy is available for influenza virus infection; however, available agents have not been studied systematically in HCT recipients. Due to the recent emergence of resistance against the M2‐inhibitors, amantidine and rimantidine, these agents should not be used to treat or prevent influenza A (Bright et al, 2006). Neuraminidase inhibitors (i.e. zanamavir, oseltamivir) are now available (Hayden et al, 1997; Nicholson et al, 2000) and are active against influenza A and B. Uncontrolled studies suggest that preemptive therapy with neuraminidase inhibitors (i.e. oseltamivir, zanamivir) is effective in preventing progression to lower tract disease (Johny et al, 2002; Nichols et al, 2004a; Chemaly et al, 2006). Widespread chemoprophylaxis for susceptible immunosuppressed patients in outbreak situations has been recommended [Centers for Disease Control; Prevention; Infectious Diseases Society of America; American Society of Blood; Marrow Transplantation (CDC/IDSA/ASBMT), 2000], and a case‐control study of oseltamivir suggests that this approach is safe in HCT recipients (Vu et al, 2007).
Human rhinoviruses and enteroviruses
Significance and risk factors
Human rhinovirus is classified as a picornavirus and is characterized by small, naked, single‐stranded RNA. There are approximately 100 serotypes, which usually cause mild upper respiratory tract symptoms in immunocompetent hosts. Using culture techniques, rhinovirus is infrequently reported as a cause of respiratory infection in HCT recipients. However, several reports found the organism in patients with pneumonia. Ghosh et al (1999) reported lower tract disease in seven of 22 patients with rhinovirus infection while Bowden (1997) documented lower tract disease in only one of 29 infected patients. Rhinovirus appears to occasionally cause lower respiratory tract disease (Gutman et al, 2007), although a clear causative role is sometimes difficult to ascertain because of the presence of co‐pathogens (Ghosh et al, 1999; Ison et al, 2003). Surveillance studies using reverse transcription (RT)‐PCR are ongoing in order to more precisely define the role of HRhV in HCT recipients. Enteroviruses are also picornaviruses. They are transmitted by the fecal‐oral and respiratory route. Respiratory infections can occur, but limited evidence exists as to their significance as respiratory pathogens.
Treatment and prevention
There are presently no drugs or vaccines available for the treatment or prevention of HRhV infections. Pleconaril, a capsid‐binding compound, was the first anti‐rhinovirus antiviral to be submitted to the US food and Drug Administration for regulatory approval, but was not approved for licensure for the treatment of colds in adults, primarily based on drug interactions and modest treatment effects. Other antiviral agents, including protease inhibitors, are active in vitro but whether these compounds will be developed is uncertain (Patick, 2006). Zinc salts and preparations of Echinacea are also weakly active in vitro. Meta‐analyses have shown no benefit for zinc salts (Jackson et al, 1997) and have displayed a moderate effect for Echinacea in immunocompetent subjects, although results are not consistent (Turner et al, 2005) (Linde, et al 2006). No studies have been performed in immunosuppressed patients with these compounds.
Human coronaviruses
Significance and risk factors
Human coronaviruses are RNA viruses that can cause URI, croup, wheezing, and pneumonia in immunocompetent individuals. Since 2004, several new strains have been described, including NL63 and HKU1, in addition to the previously described OC43 and 229E strains. In a recent study, 5·7% of 823 patients admitted to the hospital had HCoV detected; HCoV infection occurred in 8·8% of immunocompromised patients, compared with 4·5% of immunocompetent patients. Fourteen of the 47 HCoV‐infected patients (30%), including three HCT recipients, were also infected with significant co‐pathogens (Gerna et al, 2006). The four HCoVs have also been detected in solid organ transplant recipients, patients with underlying malignancies, and HIV‐infected individuals (Kumar et al, 2005). HCoV infections have also been associated with long‐term decline in FEV1 (forced expiratory volume in 1 s) in lung transplant recipients (Kumar et al, 2005). Surveillance studies are ongoing to define the incidence and disease associations of the HCoVs in HCT recipients.
Treatment and prevention
There is no specific treatment for HCoV. Infection control measures are recommended, similar to those for other respiratory viruses.
Human bocavirus
Significance and risk factors
Human bocavirus is a newly identified human parvovirus, originally identified by random PCR amplification/cloning technique from hospitalized children in Sweden (Allander et al, 2005). HBoV has been detected worldwide (Chung et al, 2006; Foulongne et al, 2006; Manning et al, 2006), and appears to be common in young children. Symptoms associated with HBoV infections include rhinorrhea, cough, fever, wheezing, hypoxia, and diarrhea (Arnold et al, 2006). To date, there is only limited information on the clinical significance of HBoV infection in immunocompromised patients (Schenk et al, 2007). Surveillance studies are ongoing to better define the incidence and disease associations of HBoV in HCT recipients.
Treatment and prevention
There is no specific treatment for HBoV. The infection control measures that are recommended are similar to those for other respiratory viruses.
Adenoviruses
Significance and risk factors
Adenoviruses are categorized in subgroups A to F, and there a presently 51 human serotypes known. Serotypes from all five subgroups can cause disease in both immunocompetent and immunocompromised subjects, although the disease spectrum associated with strains may differ (Horwitz, 2001; Bruno et al, 2003). After primary infection, adenovirus establishes latency in adenoidal tissues (Horwitz, 2001) with lifelong persistence of specific antibodies.
Transmission of adenovirus occurs by either respiratory droplet or the oral‐fecal route. Adenovirus enters the mucosa and infects epithelial cells, resulting in inflammation and necrosis. Subsequent viremia may lead to disseminated disease. After HCT, reactivation from latency is probably more problematic than exogenous infection (Bruno et al, 2003), however, transmission via the respiratory route can occur.
Adenovirus infections are common after HCT and some recent reports suggest that they may be increasing, possibly related to transplantation practices (e.g. T cell‐depletion) (Flomenberg et al, 1994; Bruno et al, 2003). However, the incidence of adenovirus disease is dependent on the degree of immunosuppression and seems to be increasing only in recipients of T cell‐depleted grafts (Flomenberg et al, 1994). Clinical manifestations include pneumonia, hepatitis, gastrointestinal disease, nephritis, cystitis, and eye infections (Cox et al, 1994; Flomenberg et al, 1994; 2003, 2004).
Risk factors for adenovirus asymptomatic infection after HCT include: GvHD, unrelated donor status, use of total body irradiation, T cell depletion, and younger age (Flomenberg et al, 1994; Baldwin et al, 2000; Bruno et al, 2003). The degree of T cell depletion and post‐transplant immunosuppression directed at T cell function appears to be particularly important (Lion et al, 2003). Almost all studies have reported a higher incidence in children (Wasserman et al, 1988; Bruno et al, 2003).
Adenovirus end‐organ disease is most commonly seen following conditioning regimens that include in vivo or ex vivo T cell depletion (Flomenberg et al, 1994). Risk factors for adenovirus disease include: younger age (Howard et al, 1999), viremia and shedding from more than two sites (Howard et al, 1999; Baldwin et al, 2000; Schilham et al, 2002), total body irradiation (Hale et al, 1999), GvHD (Flomenberg et al, 1994), and transplantation from an unrelated donor (Baldwin et al, 2000). Plasma viremia is an important risk factor for disease (Erard et al, 2007). Adenovirus disease is associated with a fatality rate of 30–50% (Kim et al, 2007). Response to treatment seems to be particularly poor in patients with pneumonia or disseminated disease (Ljungman et al, 2003). A recent multivariate analysis in non‐T cell‐depleted HCT recipients indicated that adenovirus infection was independently associated with mortality (Bruno et al, 2003).
Prevention and treatment
There are no controlled treatment studies for adenovirus infection in immunocompromised patients. Intravenous ribavirin has been used, but results are conflicting (Liles et al, 1993; Chakrabarti et al, 1999; Bordigoni et al, 2001; Gavin & Katz, 2002). Recently, cidofovir has been shown to have in vitro activity, and several uncontrolled case series showed promising results (Legrand et al, 2001; Ljungman et al, 2003; Yusuf et al, 2006; Neofytos et al, 2007; Sivaprakasam et al, 2007). Ganciclovir has in vitro activity against adenovirus and has a moderate effect in the prevention of adenovirus infection in non‐T cell‐depleted patients (Bruno et al, 2003). However, ganciclovir is not recommended for the treatment of adenovirus disease. The antiretroviral drugs, zalzitabine (DDC), alovudine, and stavudine, have activity in vitro (Naesens et al, 2005; Uchio et al, 2007). Differences in in vitro susceptibility and treatment outcome results between studies may be due to strain and serotype differences, which often were not reported in these studies. Specific and non‐specific T cell therapy have been reported in small series (Chakrabarti et al, 2000b; Regn et al, 2001; Feuchtinger et al, 2006; Leen et al, 2006).
In high‐risk settings, such as haploidentical transplantation or cord blood transplantation, weekly PCR surveillance for adenovirus viremia and preemptive treatment with cidofovir is now used at several centers (Lion et al, 2003; Yusuf et al, 2006). However, this strategy does not completely eliminate adenovirus disease in highest‐risk patients (Yusuf et al, 2006) even if cidofovir is initiated at any viral load. In T cell‐replete transplant recipients, plasma DNAemia of greater than 1000 copies/mL correlated well with the presence of adenovirus disease (Erard et al, 2007). At what level preemptive antiviral therapy should be initiated depends both on the PCR assay performance characteristics and the underlying immunosuppression. The latter determines the in vivo viral dynamics. At this time, no definitive threshold has been established, however, repeated testing after 2–3 d of a low viral load test can identify patients with a rapid increase in viral load. The use of adenovirus‐specific T cells for prevention and treatment of adenovirus disease are an area of active research (Feuchtinger et al, 2006). Randomized trials are needed to evaluate prevention strategies.
Isolation practices
Recommendations have been summarized by the CDC/IDSA/ASBMT (2000); a revision of this document is expected to be published early in 2009. Hand washing is the single most effective way of preventing spread of respiratory viruses, and should be performed after each patient contact by all health care personnel and visitors. Respiratory isolation is used for HCT recipients with documented respiratory virus infections, as well as upper respiratory symptoms during the diagnostic work‐up period (masks, gowns, gloves, and eye protection). One area of uncertainty is when to lift isolation in patients who have become asymptomatic, but have persistent low‐level shedding which is only detected by molecular methods. To what extent these patients are contagious is presently unknown. For the viruses that are known to cause serious disease (i.e. RSV, parainfluenzaviruses, influenzaviruses, HMPV), it appears prudent to isolate patients that are positive by PCR, even if they are asymptomatic. Whether this is necessary for viruses with an extremely uncommon or unclear disease association (e.g. HRhV, HCoV), is presently an area of active research.
The ubiquitous use of masks among health care workers, family members, and asymptomatic patients remains controversial. One non‐randomized study suggests a benefit of masks (Raad et al, 1997). However, questions remain on the type of mask, frequency of changing the mask, and who should wear masks. Not all transplant centers have a universal mask policy. Recent CDC guidelines recommend using masks during patient transport [CDC/IDSA/ASBMT (2000)]. Another approach that is likely to reduce transmission is to restrict health care workers and family members from patient contact if they have URI and systemic symptoms, such as rhinorhea, watery eyes, sneezing, fever, and myalgia (Boeckh et al, 2004). Such a strategy will only be effective for infections that present with significant drainage and symptoms, such as RSV, rhinovirus, HMPV, and influenza infections. Parainfluenzavirus infections, which may present with only mild symptoms, may be missed by this approach (Nichols et al, 2004a; Peck et al, 2007). Most centers restrict small children from direct patient contact during the respiratory virus season, due to their predisposition to URIs and prolonged high‐titer shedding [CDC/IDSA/ASBMT (2000)].
Future perspective
Respiratory viruses are now recognized as causes of pneumonia and late airflow obstruction after HCT. Although major progress has been made in understanding disease associations and in the development of diagnostic tests, major gaps remain. One such gap is the availability of a sensitive, specific, and inexpensive multiplex diagnostic platform that detects all relevant respiratory viruses, and optimally, important bacterial pathogens, as well. Fortunately, the development of such testing methods is advancing. The establishment of these methods will enable the study of disease associations, and will also be essential for the development of more therapeutic options, not only in the immunosuppressed, but also in immunocompetent subjects. One key reason for the slow progress in drug development is the absence of widespread office‐based testing (Nichols et al, 2008). A systematic and comprehensive evaluation of the disease burden of these infections is also needed. Historically, only pneumonia and death were reported, however, data on airflow obstruction, health care utilization including duration of hospitalization, intensive care, and mechanical ventilation are important as well.
Another area of need is that of disease pathogenesis. Although several studies point towards lymphopenia as an important risk factor for severe manifestations of disease, the exact host defence mechanisms that lead to the devastating consequences of respiratory virus infections in HCT recipients are virtually unknown. Studies of the importance of virus load, T cell and humoral immunity, as well as cytokine and chemokine expression, and genetics, are needed. To date, most studies focus on the early period after transplantation. More information is needed on the risk of progression and outcome, late after HCT (i.e. after the first 3 months), when lymphopenia is rare. There may be a birectional relationship between respiratory viruses and lung function late after transplantion; viral infections may lead to prolonged airflow obstruction, and preexisting airflow obstruction may predispose individuals to progression of lower tract disease and poor outcome.
There is also very limited knowledge of what determines the risk of virus acquisition. Studies are ongoing to determine whether there are genetic predispositions. Furthermore, major progress is needed in regards to treatment options for respiratory viruses.
With the exception of treatment for influenza virus, there are no potent and easy to administer drug treatments. The development of novel treatment and prevention options for RSV has the highest priority. Existing options (ribavirin, palivizumab, polyclonal antibody preparations) are of only moderate efficacy, complicated to give, and/or prohibitively expensive‐‐especially when given to adult patients. Several lead compounds are currently being evaluated (Nichols et al, 2008), and a recently established volunteer infection model could speed up drug and vaccine development (Devincenzo et al, 2007). In the past, it has been difficult to conduct randomized clinical trials for RSV in HCT recipients (Boeckh et al, 2007). However, the failure to complete these trials was directly linked to the level of complexity required to give aerosolized ribavirin (Boeckh et al, 2007). With novel antiviral compounds that are easy to administer, do not require hospitalization, and lack serious side effects it should be highly feasible to conduct clinical trials in the HCT setting.
Another unmet medical need is a treatment option for parainfluenzavirus, the most prevalent of the respiratory viruses with known disease associations (Nichols et al, 2008). Also, well designed prospective observational studies are needed to define the disease burden of recently discovered respiratory viruses, such as novel coronaviruses, human bocavirus, and polyomaviruses. Finally, even today we see symptomatic patients in whom we are unable to make a virological diagnosis despite multiplex PCR testing. Thus, we will probably see additional, yet to be discovered, respiratory viruses in the future.
Acknowledgements
Michael Boeckh was supported in part by grants awarded by the National Institute of Health (CA 18029, HL 081585).
References
- Abdallah, A. , Rowland, K.E. , Schepetiuk, S.K. , To, L.B. & Bardy, P. (2003) An outbreak of respiratory syncytial virus infection in a bone marrow transplant unit: effect on engraftment and outcome of pneumonia without specific antiviral treatment. Bone Marrow Transplantation, 32, 195–203. [DOI] [PubMed] [Google Scholar]
- Adams, R. , Christenson, J. , Petersen, F. & Beatty, P. (1999) Pre‐emptive use of aerosolized ribavirin in the treatment of asymptomatic pediatric marrow transplant patients testing positive for RSV. Bone Marrow Transplantation, 24, 661–664. [DOI] [PubMed] [Google Scholar]
- Allander, T. , Tammi, M.T. , Eriksson, M. , Bjerkner, A. , Tiveljung‐Lindell, A. & Andersson, B. (2005) Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proceedings of the National Academy of Sciences of the United States of America, 102, 12891–12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, J.C. , Singh, K.K. , Spector, S.A. & Sawyer, M.H. (2006) Human bocavirus: prevalence and clinical spectrum at a children’s hospital. Clinical Infectious Diseases, 43, 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan, T. , Fassas, A.B. , Desikan, R. , Siegel, D. , Munshi, N. , Mehta, J. , Singhal, S. , Barlogie, B. & Anaissie, E. (1999) Patients with multiple myeloma may safely undergo autologous transplantation despite ongoing RSV infection and no ribavirin therapy. Bone Marrow Transplantation, 24, 505–509. [DOI] [PubMed] [Google Scholar]
- Baldwin, A. , Kingman, H. , Darville, M. , Foot, A.B. , Grier, D. , Cornish, J.M. , Goulden, N. , Oakhill, A. , Pamphilon, D.H. , Steward, C.G. & Marks, D.I. (2000) Outcome and clinical course of 100 patients with adenovirus infection following bone marrow transplantation. Bone Marrow Transplantation, 26, 1333–1338. [DOI] [PubMed] [Google Scholar]
- Boeckh, M. (2004) Other viruses after hematopoietic cell transplantation In: Thomas’ Hematopoietic Cell Transplantation, 3rd edn (eds by Blume K.G., Forman S.J. & Appelbaum F.R.), pp. 757–768. Blackwell Publishing, Oxford, UK. [Google Scholar]
- Boeckh, M. , Berrey, M.M. , Bowden, R.A. , Crawford, S.W. , Balsley, J. & Corey, L. (2001) Phase 1 evaluation of the respiratory syncytial virus‐specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. Journal of Infectious Diseases, 184, 350–354. [DOI] [PubMed] [Google Scholar]
- Boeckh, M. , Gooley, T. , Englund, J. , Chien, J. , Crawford, S.W. , Bowden, R. & Corey, L. (2004) RSV infection in HCT recipients: risk factors for acquisition and lower tract disease, and impact on mortality. Blood, 104, 57a (abstract 187). [Google Scholar]
- Boeckh, M. , Englund, J. , Li, Y. , Miller, C. , Cross, A. , Fernandez, H. , Kuypers, J. , Kim, H. , Gnann, J. & Whitley, R. (2007) Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clinical Infectious Diseases, 44, 245–249. [DOI] [PubMed] [Google Scholar]
- Boivin, G. , Abed, Y. , Pelletier, G. , Ruel, L. , Moisan, D. , Cote, S. , Peret, T.C. , Erdman, D.D. & Anderson, L.J. (2002) Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory‐tract infections in all age groups. Journal of Infectious Diseases, 186, 1330–1334. [DOI] [PubMed] [Google Scholar]
- Bordigoni, P. , Carret, A.S. , Venard, V. , Witz, F. & Le Faou, A. (2001) Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clinical Infectious Diseases, 32, 1290–1297. [DOI] [PubMed] [Google Scholar]
- Bowden, R.A. (1997) Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. American Journal of Medicine, 102, 27–30; discussion 42–23. [DOI] [PubMed] [Google Scholar]
- Bright, R.A. , Shay, D.K. , Shu, B. , Cox, N.J. & Klimov, A.I. (2006) Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. Jama, 295, 891–894. [DOI] [PubMed] [Google Scholar]
- Bruno, B. , Gooley, T. , Hackman, R.C. , Davis, C. , Corey, L. & Boeckh, M. (2003) Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biology of Blood and Marrow Transplantation, 9, 341–352. [DOI] [PubMed] [Google Scholar]
- Bruno, B. , Zager, R.A. , Boeckh, M.J. , Gooley, T.A. , Myerson, D.H. , Huang, M.L. & Hackman, R.C. (2004) Adenovirus nephritis in hematopoietic stem‐cell transplantation. Transplantation, 77, 1049–1057. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control; Prevention; Infectious Diseases Society of America; American Society of Blood; Marrow Transplantation . (2000) Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Biology of Blood and Marrow Transplantation, 6, 659–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti, S. , Collingham, K.E. , Fegan, C.D. & Milligan, D.W. (1999) Fulminant adenovirus hepatitis following unrelated bone marrow transplantation: failure of intravenous ribavirin therapy. Bone Marrow Transplantation, 23, 1209–1211. [DOI] [PubMed] [Google Scholar]
- Chakrabarti, S. , Collingham, K.E. , Holder, K. , Oyaide, S. , Pillay, D. & Milligan, D.W. (2000a) Parainfluenza virus type 3 infections in hematopoetic stem cell transplant recipients: response to ribavirin therapy. Clinical Infectious Diseases, 31, 1516–1518. [DOI] [PubMed] [Google Scholar]
- Chakrabarti, S. , Collingham, K.E. , Fegan, C.D. , Pillay, D. & Milligan, D.W. (2000b) Adenovirus infections following haematopoietic cell transplantation: is there a role for adoptive immunotherapy? [In Process Citation]. Bone Marrow Transplantation, 26, 305–307. [DOI] [PubMed] [Google Scholar]
- Chakrabarti, S. , Avivi, I. , Mackinnon, S. , Ward, K. , Kottaridis, P.D. , Osman, H. , Waldmann, H. , Hale, G. , Fegan, C.D. , Yong, K. , Goldstone, A.H. , Linch, D.C. & Milligan, D.W. (2002) Respiratory virus infections in transplant recipients after reduced‐intensity conditioning with Campath‐1H: high incidence but low mortality. British Journal Haematology, 119, 1125–1132. [DOI] [PubMed] [Google Scholar]
- Chavez‐Bueno, S. , Mejias, A. , Merryman, R.A. , Ahmad, N. , Jafri, H.S. & Ramilo, O. (2007) Intravenous palivizumab and ribavirin combination for respiratory syncytial virus disease in high‐risk pediatric patients. Pediatric Infectious Disease Journal, 26, 1089–1093. [DOI] [PubMed] [Google Scholar]
- Chemaly, R.F. , Ghosh, S. , Bodey, G.P. , Rohatgi, N. , Safdar, A. , Keating, M.J. , Champlin, R.E. , Aguilera, E.A. , Tarrand, J.J. & Raad, I.I. (2006) Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore), 85, 278–287. [DOI] [PubMed] [Google Scholar]
- Chiu, C.Y. , Alizadeh, A.A. , Rouskin, S. , Merker, J.D. , Yeh, E. , Yagi, S. , Schnurr, D. , Patterson, B.K. , Ganem, D. & Derisi, J.L. (2007) Diagnosis of a critical respiratory illness caused by human metapneumovirus by use of a pan‐virus microarray. Journal of Clinical Microbiology, 45, 2340–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, J.Y. , Han, T.H. , Kim, C.K. & Kim, S.W. (2006) Bocavirus infection in hospitalized children, South Korea. Emerging Infectious Diseases, 12, 1254–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez, K.J. , Erdman, D.D. , Peret, T.C. , Gill, V.J. , Childs, R. , Barrett, A.J. & Bennett, J.E. (2001) Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. Journal of Infectious Diseases, 184, 1093–1097. [DOI] [PubMed] [Google Scholar]
- Cortez, K. , Murphy, B.R. , Almeida, K.N. , Beeler, J. , Levandowski, R.A. , Gill, V.J. , Childs, R.W. , Barrett, A.J. , Smolskis, M. & Bennett, J.E. (2002) Immune‐globulin prophylaxis of respiratory syncytial virus infection in patients undergoing stem‐cell transplantation. Journal of Infectious Diseases, 186, 834–838. [DOI] [PubMed] [Google Scholar]
- Cox, G.J. , Matsui, S.M. , Lo, R.S. , Hinds, M. , Bowden, R.A. , Hackman, R.C. , Meyer, W.G. , Mori, M. , Tarr, P.I. , Oshiro, L.S. , Ludert, J.E. , Meyers, J.D. & MacDonald, G.B. (1994) Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology, 107, 1398–1407. [DOI] [PubMed] [Google Scholar]
- Debiaggi, M. , Canducci, F. , Terulla, C. , Sampaolo, M. , Marinozzi, M.C. , Alessandrino, P.E. , Colombo, A.A. , Caldera, D. , Bragotti, L.Z. , Migliavacca, R. , Bianchi, E. , Romero, E. & Clementi, M. (2007) Long‐term study on symptomless human metapneumovirus infection in hematopoietic stem cell transplant recipients. New Microbiologica, 30, 255–258. [PubMed] [Google Scholar]
- DeVincenzo, J.P. , Hirsch, R.L. , Fuentes, R.J. & Top, Jr, F.H. (2000) Respiratory syncytial virus immune globulin treatment of lower respiratory tract infection in pediatric patients undergoing bone marrow transplantation ‐ a compassionate use experience. Bone Marrow Transplantation, 25, 161–165. [DOI] [PubMed] [Google Scholar]
- Devincenzo, J. , Cehelsky, J. , Meyers, R. , Vaishnaw, A. , Nochur, S. , Foote, K. , Wilkinson, T. , Meeking, P. , Mann, A. , Moane, E. , Oxford, J. , Studholme, R. , Dorsett, P. & Lambkin‐Williams, R. (2007) Development of a human experimental infection model of respiratory syncytial virus In: Interscience Conference on Antimicrobial Agents and Chemotherapy. Chicago, IL (September 26, 2007). [Google Scholar]
- Englund, J.A. , Piedra, P.A. , Jewell, A. , Patel, K. , Baxter, B.B. & Whimbey, E. (1996) Rapid diagnosis of respiratory syncytial virus infections in immunocompromised adults. Journal of Clinical Microbiology, 34, 1649–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund, J.A. , Boeckh, M. , Kuypers, J. , Nichols, W.G. , Hackman, R.C. , Morrow, R.A. , Fredricks, D.N. & Corey, L. (2006) Brief communication: fatal human metapneumovirus infection in stem‐cell transplant recipients. Annals of Internal Medicine, 144, 344–349. [DOI] [PubMed] [Google Scholar]
- Erard, V. , Chien, J.W. , Kim, H.W. , Nichols, W.G. , Flowers, M.E. , Martin, P.J. , Corey, L. & Boeckh, M. (2006) Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. Journal of Infectious Diseases, 193, 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erard, V. , Huang, M.L. , Ferrenberg, J. , Nguy, L. , Stevens‐Ayers, T.L. , Hackman, R.C. , Corey, L. & Boeckh, M. (2007) Quantitative real‐time polymerase chain reaction for detection of adenovirus after T cell‐replete hematopoietic cell transplantation: viral load as a marker for invasive disease. Clinical Infectious Diseases, 45, 958–965. [DOI] [PubMed] [Google Scholar]
- Feuchtinger, T. , Matthes‐Martin, S. , Richard, C. , Lion, T. , Fuhrer, M. , Hamprecht, K. , Handgretinger, R. , Peters, C. , Schuster, F.R. , Beck, R. , Schumm, M. , Lotfi, R. , Jahn, G. & Lang, P. (2006) Safe adoptive transfer of virus‐specific T‐cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. British Journal Haematology, 134, 64–76. [DOI] [PubMed] [Google Scholar]
- Flomenberg, P. , Babbitt, J. , Drobyski, W.R. , Ash, R.C. , Carrigan, D.R. , Sedmak, G.V. , McAuliffe, T. , Camitta, B. , Horowitz, M.M. , Bunin, N. & Casper, J.T. (1994) Increasing incidence of adenovirus disease in bone marrow transplant recipients. Journal of Infectious Diseases, 169, 775–781. [DOI] [PubMed] [Google Scholar]
- Foulongne, V. , Olejnik, Y. , Perez, V. , Elaerts, S. , Rodiere, M. & Segondy, M. (2006) Human bocavirus in French children. Emerging Infectious Diseases, 12, 1251–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier‐Wirth, C. & Coste, J. (2007) Fitting new technologies into the safety paradigm: use of microarrays in transfusion. Developments in Biologicals (Basel), 127, 61–70. [PubMed] [Google Scholar]
- Gavin, P.J. & Katz, B.Z. (2002) Intravenous ribavirin treatment for severe adenovirus disease in immunocompromised children. Pediatrics, 110, e9. [DOI] [PubMed] [Google Scholar]
- Gerna, G. , Campanini, G. , Rovida, F. , Percivalle, E. , Sarasini, A. , Marchi, A. & Baldanti, F. (2006) Genetic variability of human coronavirus OC43‐, 229E‐, and NL63‐like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients. Journal of Medical Virology, 78, 938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, S. , Champlin, R. , Couch, R. , Englund, J. , Raad, I. , Malik, S. , Luna, M. & Whimbey, E. (1999) Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients [see comments]. Clinical Infectious Diseases, 29, 528–532. [DOI] [PubMed] [Google Scholar]
- Gutman, J.A. , Peck, A.J. , Kuypers, J. & Boeckh, M. (2007) Rhinovirus as a cause of fatal lower respiratory tract infection in adult stem cell transplantation patients: a report of two cases. Bone Marrow Transplantation, 40, 809–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale, G.A. , Heslop, H.E. , Krance, R.A. , Brenner, M.A. , Jayawardene, D. , Srivastava, D.K. & Patrick, C.C. (1999) Adenovirus infection after pediatric bone marrow transplantation. Bone Marrow Transplantation, 23, 277–282. [DOI] [PubMed] [Google Scholar]
- Harrington, R.D. , Hooton, T.M. , Hackman, R.C. , Storch, G.A. , Osborne, B. , Gleaves, C.A. , Benson, A. & Meyers, J.D. (1992) An outbreak of respiratory syncytial virus in a bone marrow transplant center. Journal of Infectious Diseases, 165, 987–993. [DOI] [PubMed] [Google Scholar]
- Hayden, F.G. , Osterhaus, A.D. , Treanor, J.J. , Fleming, D.M. , Aoki, F.Y. , Nicholson, K.G. , Bohnen, A.M. , Hirst, H.M. , Keene, O. & Wightman, K. (1997) Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group [see comments]. New England Journal of Medicine, 337, 874–880. [DOI] [PubMed] [Google Scholar]
- Hindiyeh, M. , Hillyard, D.R. & Carroll, K.C. (2001) Evaluation of the Prodesse Hexaplex multiplex PCR assay for direct detection of seven respiratory viruses in clinical specimens. American Journal of Clinical Pathology, 116, 218–224. [DOI] [PubMed] [Google Scholar]
- Van Den Hoogen, B.G. , De Jong, J.C. , Groen, J. , Kuiken, T. , De Groot, R. , Fouchier, R.A. & Osterhaus, A.D. (2001) A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nature Medicine, 7, 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, M.S. (2001) Adenovirus In: Fields Virology (eds by Knipe D.M. & Howley P.M.), pp. 2301–2326. Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- Howard, D.S. , Phillips, I.G. , Reece, D.E. , Munn, R.K. , Henslee‐Downey, J. , Pittard, M. , Barker, M. & Pomeroy, C. (1999) Adenovirus infections in hematopoietic stem cell transplant recipients. Clinical Infectious Diseases, 29, 1494–1501. [DOI] [PubMed] [Google Scholar]
- Ison, M.G. , Hayden, F.G. , Kaiser, L. , Corey, L. & Boeckh, M. (2003) Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clinical Infectious Diseases, 36, 1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, J.L. , Peterson, C. & Lesho, E. (1997) A meta‐analysis of zinc salts lozenges and the common cold. Archives of Internal Medicine, 157, 2373–2376. [PubMed] [Google Scholar]
- Johny, A.A. , Clark, A. , Price, N. , Carrington, D. , Oakhill, A. & Marks, D.I. (2002) The use of zanamivir to treat influenza A and B infection after allogeneic stem cell transplantation. Bone Marrow Transplantation, 29, 113–115. [DOI] [PubMed] [Google Scholar]
- Kehl, S.C. , Henrickson, K.J. , Hua, W. & Fan, J. (2001) Evaluation of the Hexaplex assay for detection of respiratory viruses in children. Journal of Clinical Microbiology, 39, 1696–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, N. , Widmer, A.F. , Decker, M. , Steffen, I. , Halter, J. , Heim, D. , Weisser, M. , Gratwohl, A. , Fluckiger, U. & Hirsch, H.H. (2008) Respiratory syncytial virus infection in patients with hematological diseases: single‐center study and review of the literature. Clinical Infectious Diseases, 46, 402–412. [DOI] [PubMed] [Google Scholar]
- Kim, Y.J. , Boeckh, M. & Englund, J.A. (2007) Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Seminars in Respiratory and Critical Care Medicine, 28, 222–242. [DOI] [PubMed] [Google Scholar]
- Kumar, D. , Erdman, D. , Keshavjee, S. , Peret, T. , Tellier, R. , Hadjiliadis, D. , Johnson, G. , Ayers, M. , Siegal, D. & Humar, A. (2005) Clinical impact of community‐acquired respiratory viruses on bronchiolitis obliterans after lung transplant. American Journal of Transplantation, 5, 2031–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, W.M. , Grindle, K. , Pappas, T. , Marshall, D.J. , Moser, M.J. , Beaty, E.L. , Shult, P.A. , Prudent, J.R. & Gern, J.E. (2007) High‐throughput, sensitive, and accurate multiplex PCR‐microsphere flow cytometry system for large‐scale comprehensive detection of respiratory viruses. Journal of Clinical Microbiology, 45, 2626–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen, A.M. , Myers, G.D. , Sili, U. , Huls, M.H. , Weiss, H. , Leung, K.S. , Carrum, G. , Krance, R.A. , Chang, C.C. , Molldrem, J.J. , Gee, A.P. , Brenner, M.K. , Heslop, H.E. , Rooney, C.M. & Bollard, C.M. (2006) Monoculture‐derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nature Medicine, 12, 1160–1166. [DOI] [PubMed] [Google Scholar]
- Legrand, F. , Berrebi, D. , Houhou, N. , Freymuth, F. , Faye, A. , Duval, M. , Mougenot, J.F. , Peuchmaur, M. & Vilmer, E. (2001) Early diagnosis of adenovirus infection and treatment with cidofovir after bone marrow transplantation in children. Bone Marrow Transplantation, 27, 621–626. [DOI] [PubMed] [Google Scholar]
- Lewinsohn, D.M. , Bowden, R.A. , Mattson, D. & Crawford, S.W. (1996) Phase I study of intravenous ribavirin treatment of respiratory syncytial virus pneumonia after marrow transplantation. Antimicrobial Agents and Chemotherapy, 40, 2555–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles, W.C. , Cushing, H. , Holt, S. , Bryan, C. & Hackman, R.C. (1993) Severe adenoviral nephritis following bone marrow transplantation: successful treatment with intravenous ribavirin [see comments]. Bone Marrow Transplantation, 12, 409–412. [PubMed] [Google Scholar]
- Linde, K. , Barrett, B. , Wolkart, K. , Bauer, R. & Melchart, D. (2006). Echinacea for preventing and treating the common cold. Cochrane Database of Systematic Reviews, CD000530. [DOI] [PubMed] [Google Scholar]
- Lion, T. , Baumgartinger, R. , Watzinger, F. , Matthes‐Martin, S. , Suda, M. , Preuner, S. , Futterknecht, B. , Lawitschka, A. , Peters, C. , Potschger, U. & Gadner, H. (2003) Molecular monitoring of adenovirus in peripheral blood after allogeneic bone marrow transplantation permits early diagnosis of disseminated disease. Blood, 102, 1114–1120. [DOI] [PubMed] [Google Scholar]
- Liu, H.H. , Cao, X. , Yang, Y. , Liu, M.G. & Wang, Y.F. (2006) Array‐based nano‐amplification technique was applied in detection of hepatitis E virus. Journal of Biochemistry and Molecular Biology, 39, 247–252. [DOI] [PubMed] [Google Scholar]
- Ljungman, P. (2001) Respiratory virus infections in stem cell transplant patients: the European experience. Biology of Blood and Marrow Transplantation, 7(Suppl), 5S–7S. [DOI] [PubMed] [Google Scholar]
- Ljungman, P. , Gleaves, C.A. & Meyers, J.D. (1989) Respiratory virus infection in immunocompromised patients. Bone Marrow Transplantation, 4, 35–40. [PubMed] [Google Scholar]
- Ljungman, P. , Andersson, J. , Aschan, J. , Barkholt, L. , Ehrnst, A. , Johansson, M. & Weiland, O. (1993) Influenza A in immunocompromised patients. Clinical Infectious Diseases, 17, 244–247. [DOI] [PubMed] [Google Scholar]
- Ljungman, P. , Ward, K.N. , Crooks, B.N. , Parker, A. , Martino, R. , Shaw, P.J. , Brinch, L. , Brune, M. , De La Camara, R. , Dekker, A. , Pauksen, K. , Russell, N. , Schwarer, A.P. & Cordonnier, C. (2001) Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplantation, 28, 479–484. [DOI] [PubMed] [Google Scholar]
- Ljungman, P. , Ribaud, P. , Eyrich, M. , Matthes‐Martin, S. , Einsele, H. , Bleakley, M. , Machaczka, M. , Bierings, M. , Bosi, A. , Gratecos, N. & Cordonnier, C. (2003) Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplantation, 31, 481–486. [DOI] [PubMed] [Google Scholar]
- Machado, C.M. , Cardoso, M.R. , Da Rocha, I.F. , Boas, L.S. , Dulley, F.L. & Pannuti, C.S. (2005) The benefit of influenza vaccination after bone marrow transplantation. Bone Marrow Transplantation, 36, 897–900. [DOI] [PubMed] [Google Scholar]
- Mahony, J. , Chong, S. , Merante, F. , Yaghoubian, S. , Sinha, T. , Lisle, C. & Janeczko, R. (2007) Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead‐based assay. Journal of Clinical Microbiology, 45, 2965–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, A. , Russell, V. , Eastick, K. , Leadbetter, G.H. , Hallam, N. , Templeton, K. & Simmonds, P. (2006) Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. Journal of Infectious Diseases, 194, 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino, R. , Ramila, E. , Rabella, N. , Munoz, J.M. , Peyret, M. , Portos, J.M. , Laborda, R. & Sierra, J. (2003) Respiratory virus infections in adults with hematologic malignancies: a prospective study. Clinical Infectious Diseases, 36, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naesens, L. , Lenaerts, L. , Andrei, G. , Snoeck, R. , Van Beers, D. , Holy, A. , Balzarini, J. & De Clercq, E. (2005) Antiadenovirus activities of several classes of nucleoside and nucleotide analogues. Antimicrobial Agents and Chemotherapy, 49, 1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neofytos, D. , Ojha, A. , Mookerjee, B. , Wagner, J. , Filicko, J. , Ferber, A. , Dessain, S. , Grosso, D. , Brunner, J. , Flomenberg, N. & Flomenberg, P. (2007) Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biology of Blood and Marrow Transplantation, 13, 74–81. [DOI] [PubMed] [Google Scholar]
- Nichols, W.G. , Gooley, T. & Boeckh, M. (2001a) Community‐acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biology of Blood and Marrow Transplantation, 7(Suppl), 11S–15S. [DOI] [PubMed] [Google Scholar]
- Nichols, W.G. , Corey, L. , Gooley, T. , Davis, C. & Boeckh, M. (2001b) Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood, 98, 573–578. [DOI] [PubMed] [Google Scholar]
- Nichols, W.G. , Guthrie, K.A. , Corey, L. & Boeckh, M. (2004a) Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clinical Infectious Diseases, 39, 1300–1306. [DOI] [PubMed] [Google Scholar]
- Nichols, W.G. , Erdman, D.D. , Han, A. , Zukerman, C. , Corey, L. & Boeckh, M. (2004b) Prolonged outbreak of human parainfluenza virus 3 infection in a stem cell transplant outpatient department: insights from molecular epidemiologic analysis. Biology of Blood and Marrow Transplantation, 10, 58–64. [DOI] [PubMed] [Google Scholar]
- Nichols, W.G. , Peck‐Campbell, A.J. & Boeckh, M. (2008) Antivirals for respiratory infections other than influenza: impact and therapeutic advances. Clinical Microbiology Reviews, 21, 274–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, K.G. , Aoki, F.Y. , Osterhaus, A.D. , Trottier, S. , Carewicz, O. , Mercier, C.H. , Rode, A. , Kinnersley, N. & Ward, P. (2000) Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet, 355, 1845–1850. [DOI] [PubMed] [Google Scholar]
- Nolte, F.S. , Marshall, D.J. , Rasberry, C. , Schievelbein, S. , Banks, G.G. , Storch, G.A. , Arens, M.Q. , Buller, R.S. & Prudent, J.R. (2007) MultiCode‐PLx system for multiplexed detection of seventeen respiratory viruses. Journal of Clinical Microbiology, 45, 2779–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patick, A.K. (2006) Rhinovirus chemotherapy. Antiviral Research, 71, 391–396 Epub 2006 Apr 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck, A.J. , Corey, L. & Boeckh, M. (2004) Pretransplantation respiratory syncytial virus infection: impact of a strategy to delay transplantation. Clinical Infectious Diseases, 39, 673–680. [DOI] [PubMed] [Google Scholar]
- Peck, A.J. , Englund, J.A. , Kuypers, J. , Guthrie, K.A. , Corey, L. , Morrow, R. , Hackman, R.C. , Cent, A. & Boeckh, M. (2007) Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood, 110, 1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raad, I. , Abbas, J. & Whimbey, E. (1997) Infection control of nosocomial respiratory viral disease in the immunocompromised host. American Journal of Medicine, 102, 48–52 discussion 53–54. [DOI] [PubMed] [Google Scholar]
- Regn, S. , Raffegerst, S. , Chen, X. , Schendel, D. , Kolb, H.J. & Roskrow, M. (2001) Ex vivo generation of cytotoxic T lymphocytes specific for one or two distinct viruses for the prophylaxis of patients receiving an allogeneic bone marrow transplant. Bone Marrow Transplantation, 27, 53–64. [DOI] [PubMed] [Google Scholar]
- Schenk, T. , Strahm, B. , Kontny, U. , Hufnagel, M. , Neumann‐Haefelin, D. & Falcone, V. (2007) Disseminated bocavirus infection after stem cell transplant. Emerging Infectious Diseases, 13, 1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer, J. , Kirby, K. , Sandmaier, B. , Maloney, D. , Storb, R. , Corey, L. & Boeckh, M. (2006) Community Acquired Respiratory Virus (CRV) Infection after Myeloablative Versus Non‐myeloablative (NM) Hematopoietic Cell Transplantation (HCT) In: Infectious Disease Society of America Annual Meeting, pp. 183, Toronto, Ontario, Canada. [Google Scholar]
- Schilham, M.W. , Claas, E.C. , Van Zaane, W. , Heemskerk, B. , Vossen, J.M. , Lankester, A.C. , Toes, R.E. , Echavarria, M. , Kroes, A.C. & Van Tol, M.J. (2002) High levels of adenovirus DNA in serum correlate with fatal outcome of adenovirus infection in children after allogeneic stem‐cell transplantation. Clinical Infectious Diseases, 35, 526–532. [DOI] [PubMed] [Google Scholar]
- Schleuning, M. , Buxbaum‐Conradi, H. , Jager, G. & Kolb, H.J. (2004) Intravenous ribavirin for eradication of respiratory syncytial virus (RSV) and adenovirus isolates from the respiratory and/or gastrointestinal tract in recipients of allogeneic hematopoietic stem cell transplants. The Hematology Journal, 5, 135–144. [DOI] [PubMed] [Google Scholar]
- Sivaprakasam, P. , Carr, T.F. , Coussons, M. , Khalid, T. , Bailey, A.S. , Guiver, M. , Mutton, K.J. , Turner, A.J. , Grainger, J.D. & Wynn, R.F. (2007) Improved outcome from invasive adenovirus infection in pediatric patients after hemopoietic stem cell transplantation using intensive clinical surveillance and early intervention. Journal of Pediatric Hematology/oncology, 29, 81–85. [DOI] [PubMed] [Google Scholar]
- Small, T.N. , Casson, A. , Malak, S.F. , Boulad, F. , Kiehn, T.E. , Stiles, J. , Ushay, H.M. & Sepkowitz, K.A. (2002) Respiratory syncytial virus infection following hematopoietic stem cell transplantation. Bone Marrow Transplantation, 29, 321–327. [DOI] [PubMed] [Google Scholar]
- Sparrelid, E. , Ljungman, P. , Ekelof‐Andstrom, E. , Aschan, J. , Ringden, O. , Winiarski, J. , Wahlin, B. & Andersson, J. (1997) Ribavirin therapy in bone marrow transplant recipients with viral respiratory tract infections. Bone Marrow Transplantation, 19, 905–908. [DOI] [PubMed] [Google Scholar]
- Turner, R.B. , Bauer, R. , Woelkart, K. , Hulsey, T.C. & Gangemi, J.D. (2005) An evaluation of Echinacea angustifolia in experimental rhinovirus infections. New England Journal of Medicine, 353, 341–348. [DOI] [PubMed] [Google Scholar]
- Uchio, E. , Fuchigami, A. , Kadonosono, K. , Hayashi, A. , Ishiko, H. , Aoki, K. & Ohno, S. (2007) Anti‐adenoviral effect of anti‐HIV agents in vitro in serotypes inducing keratoconjunctivitis. Graefes Archive for Clinical and Experimental Ophthalmology, 245, 1319–1325. [DOI] [PubMed] [Google Scholar]
- Vu, D. , Peck, A.J. , Nichols, W.G. , Varley, C. , Englund, J.A. , Corey, L. & Boeckh, M. (2007) Safety and tolerability of oseltamivir prophylaxis in hematopoietic stem cell transplant recipients: a retrospective case‐control study. Clinical Infectious Diseases, 45, 187–193. [DOI] [PubMed] [Google Scholar]
- Wasserman, R. , August, C.S. & Plotkin, S.A. (1988) Viral infections in pediatric bone marrow transplant patients. Pediatric Infectious Disease Journal, 7, 109–115. [DOI] [PubMed] [Google Scholar]
- Whimbey, E. , Vartivarian, S.E. , Champlin, R.E. , Elting, L.S. , Luna, M. & Bodey, G.P. (1993) Parainfluenza virus infection in adult bone marrow transplant recipients. European Journal of Clinical Microbiology and Infectious Diseases, 12, 699–701. [DOI] [PubMed] [Google Scholar]
- Whimbey, E. , Couch, R.B. , Englund, J.A. , Andreeff, M. , Goodrich, J.M. , Raad, I.I. , Lewis, V. , Mirza, N. , Luna, M.A. , Baxter, B. , Tarrand, J.J. & Bodey, G.P. (1995) Respiratory syncytial virus pneumonia in hospitalized adult patients with leukemia. Clinical Infectious Diseases, 21, 376–379. [DOI] [PubMed] [Google Scholar]
- Whimbey, E. , Champlin, R.E. , Couch, R.B. , Englund, J.A. , Goodrich, J.M. , Raad, I. , Przepiorka, D. , Lewis, V.A. , Mirza, N. , Yousuf, H. , Tarrand, J.J. & Bodey, G.P. (1996) Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clinical Infectious Diseases, 22, 778–782. [DOI] [PubMed] [Google Scholar]
- Wyde, P.R. , Chetty, S.N. , Jewell, A.M. , Boivin, G. & Piedra, P.A. (2003) Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral Research, 60, 51–59. [DOI] [PubMed] [Google Scholar]
- Yusuf, U. , Hale, G.A. , Carr, J. , Gu, Z. , Benaim, E. , Woodard, P. , Kasow, K.A. , Horwitz, E.M. , Leung, W. , Srivastava, D.K. , Handgretinger, R. & Hayden, R.T. (2006) Cidofovir for the treatment of adenoviral infection in pediatric hematopoietic stem cell transplant patients. Transplantation, 81, 1398–1404. [DOI] [PubMed] [Google Scholar]
- Zambon, M. , Bull, T. , Sadler, C.J. , Goldman, J.M. & Ward, K.N. (1998) Molecular epidemiology of two consecutive outbreaks of parainfluenza 3 in a bone marrow transplant unit. Journal of Clinical Microbiology, 36, 2289–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]


