Abstract
Introduction
While testicular germ cell tumors (TGCTs) are the most common malignancy in young men, germ cell tumors in women are uncommon. Familial clustering, epidemiologic evidence of increased risk with family or personal history of TGCT, and associations with genitourinary tract anomalies suggest an underlying genetic predisposition to TGCT, but traditional linkage studies have yet to identify a highlypenetrant TGCT cancer susceptibility gene. In this paper, we investigate the familial occurrence of testicular and ovarian germ cell tumors.
Methods
We report a family in which a TGCT and an ovarian germ cell tumor (OGCT) occurred in two siblings, summarize the existing literature on familial occurrences of OGCT, either alone or in combination with extragonadal or TGCTs, and compare the incidence of familial and sporadic testicular and ovarian GCTs. Sporadic GCT data were obtained from the US Surveillance Epidemiology and End Results (SEER) registry.
Results
We identified 16 reports of OGCT occurring in conjunction with either ovarian, testicular or extragonadal GCT. In these familial cases, the mean age at onset of female dysgerminoma was younger than that noted in the general population (age 17 vs age 24, p=0.01). In SEER, the incidence of TGCT was 15 times higher than that of OGCT. Histologic distributions in males and females showed distinctly different patterns.
Discussion
Although the incidence of OGCTs in the general population is quite low, its occurrence in multiple members of the same family and in families with TGCT suggests that a gene conferring susceptibility to GCTs may exist in some families.
Keywords: ovarian germ cell tumor, familial, epidemiology, testicular germ cell tumor, genetic predisposition, SEER
INTRODUCTION
Germ cell tumors (GCTs) arise from embryonic germ cells that fail to properly differentiate and, instead, undergo malignant transformation (1). GCTs are classified by anatomic site, patient age at diagnosis, and histologic subtype. More than 90% of GCTs develop in the gonads; the remainder are extragonadal, and distributed along the midline of the body. The locations of extragonadal GCTs reflect the anatomic path traveled by germ cell precursors during embryologic development, including the mediastinum, retroperitoneum and, less frequently, central nervous system sites, such as the pineal gland, neurohypophysis, and sacrococcygeal region (2). Furthermore, the histologic subtypes of malignant GCTs reflect the stages of embryologic development. They are divided into seminoma (called dysgerminoma in the ovaries) and nonseminoma. The nonseminoma GCTs include embryonal carcinoma, teratoma, yolk sac tumor, choriocarcinoma, and mixed GCTs (2, 3).
Malignant GCTs occur much more frequently in males than females, with a 15-fold difference in incidence rates (4). Consequently, the etiology and epidemiology of testicular GCT (TGCT) are more extensively studied than ovarian GCT (OGCT). The familial clustering of testicular cancer is well documented; large epidemiologic studies suggest a 4-fold increase in risk of TGCT in sons of men with TGCT, and an 8-10-fold increase in risk among men with an affected brother (5-7). The association of TGCT with congenital urogenital abnormalities further supports an underlying genetic predisposition. Skakkebaek et al have proposed the term testicular dysgenesis syndrome (TDS), which ranges from structural abnormalities such as cryptorchidism and hypospadias to the extreme manifestation of testicular cancer, and suggests that these entities are causally related (8). Unfortunately, specific high-penetrance susceptibility genes predisposing to TGCT have not been identified (9).
Adult malignant TGCTs and OGCTs have similar embryologic, pathologic, and cytogenetic features, and thus may share common mechanisms (1, 3, 10, 11). Since few cases of familial malignant OGCT have been described (12-19), we reviewed reported cases to explore the hypothesis that gonadal GCTs might share a common genetic predisposition, and to expand our understanding of whether a family history of testicular and/or extragonadal germ cell tumors might also predispose to ovarian germ cell tumors. We present a family in which TGCT and OGCT occurred in two siblings, briefly review the existing literature on familial occurrences of OGCT, either alone or in combination with TGCT, and compare the descriptive epidemiology of OGCT and TGCT.
METHODS
We initiated the Multidisciplinary Etiologic Study of Familial GCTs to identify possible testicular cancer susceptibility genes, and to more precisely characterize the clinical phenotype of, and psychosocial issues associated with, the familial testicular cancer syndrome. Families with two or more cases of documented GCT in blood relatives (≥1 being testicular in origin), or a single family member with bilateral testicular cancer, were eligible for the study. Study participants completed detailed family history, medical, epidemiologic and psychosocial/behavioral questionnaires, and provided a blood sample. A subset of families traveled to the National Institutes of Health Clinical Center for a more detailed history and physical examination, laboratory testing, semen analysis (males aged ≥18 with at least one remaining testicle), ultrasound imaging of the testes or ovaries, and computerized tomography (CT) of the abdomen or ultrasound of the kidneys. This study has been reviewed and approved by the National Cancer Institute (NCI) Institutional Review Board (NCI Protocol 02-C-0178), and all participants provided written informed consent.
The literature review on familial occurrence of OGCT is based upon a MEDLINE, SCOPUS, and WEB OF SCIENCE search of all languages, using the search terms “hereditary,” “familial germ cell tumor, ” “ovarian germ cell tumor” and “ovarian dysgerminoma,” alone or in combination, to identify previously-reported familial clusters of OGCT and TGCT. Cases with germline chromosome abnormalities or documented gonadal dysgenesis (a known developmental risk factor for GCT) were not included in this review (20).
We also compared the US general population incidence of male and female germ cell tumors by histologic subtype using Surveillance, Epidemiology, and End Results (SEER 9 Regs Limited-Use, Nov 2006 (1973-2004) Database) (21). Graphs were created in EXCEL from SEER*Stat-generated incidence rates. Age-adjusted incidence rates for females and males were calculated, compared by age at diagnosis and histological subtypes, and plotted by 5-year age groups. The histology (ICD) codes (22) used were dysgerminoma/seminoma (9060-9064), teratoma (9080, 9082-9084, 9090-9091), embryonal carcinoma (9070), yolk sac tumor (9071), choriocarcinoma (9100-9103), and mixed GCT (ICD 9072, 9081, 9085). Mixed GCT are defined as tumors comprised of 2 or more histologic types of malignant GCTs, including polyembryoma, and teratocarcinoma (3, 21). Benign GCTs, e.g., mature ovarian teratomas in females and spermatocytic seminomas in males, were excluded from these analyses. Comparisons of mean age at onset for familial versus sporadic cases were performed using the Wilcoxon Sum Rank test in SAS version 9.1.3.
RESULTS
Case Report
During the course of the Familial Germ Cell Tumor Study, we encountered a family of Ashkenazi Jewish origin in which a brother and sister were diagnosed with a malignant germ cell tumor. The proband was a 28-year old male diagnosed with stage IIC TGCT at age 25. He underwent a left radical orchiectomy. Pathology revealed a 3.5 cm mixed germ cell tumor, predominantly composed of embryonal carcinoma with a minor seminoma component; intratubular germ cell neoplasia was also noted. Tumor markers were not elevated. He received four cycles of etoposide and cisplatin chemotherapy. A post-treatment CT scan revealed a residual <1 cm mass; a retroperitoneal lymph node dissection showed no viable tumor. At the time of this evaluation (three years after diagnosis), physical exam and laboratory evaluations (including a complete blood count, comprehensive metabolic panel, tumor markers and serum sex steroid levels) were all within normal limits. Right testicular ultrasound showed only a small, clinically insignificant hydrocele. Semen analysis showed no evidence of infertility.
The proband’s sister presented at age 26 with a 14.5 × 9.7 × 13.5 cm complex mass in the right adnexa on CT scan. She underwent a right salpingo-oophorectomy. Pathology showed immature neural elements and occasional immature chondroid mesenchymal tissue, yielding a diagnosis of Stage IA immature teratoma. Tumor markers were not elevated. Post-operatively, she was treated with three cycles of bleomycin, etoposide, and cisplatin chemotherapy. Subsequent imaging studies have shown no evidence of disease recurrence. When evaluated at the NCI (5 years after diagnosis), physical exam and laboratory values were normal. Transvaginal ultrasound showed a 1.5 cm left ovarian cyst, unchanged from previous imaging studies. Of note, the patient had given birth to two healthy children subsequent to her OGCT diagnosis and treatment. A detailed review of the extended family revealed a history of benign fibroid tumors in the siblings’ mother and maternal aunt. Their maternal grandmother was reported to have had an “ovarian growth” (unknown age). A maternal great aunt with colon cancer was the only other relative with malignancy.
Summary of Existing Literature
Prior to this report, eight studies on families with both OGCT and testicular or extragonadal GCT have been reported (Table 1) (17, 23-27) by other investigators. Six represent familial cases of ovarian and testicular germ cell tumors, and two are cases of ovarian and extragonadal germ cell tumors. Additionally, eight kindreds with >1 malignant GCT in females have been documented (Table 2) (12-19). Affected sister-brother and sister-sister pairs account for 5/9 (56%) of the male and female GCT families and 4/8 (50%) of the OGCT families, respectively.
Table 1.
Summary of Literature on Familial Germ Cell Malignancy in Males and Females
| Reference | Affected | Diagnosis | Age at diagnosis |
Other Cancers in family (age at diagnosis) |
|---|---|---|---|---|
| Trentini GP (1974) | Subject (F)* | Dysgerminoma | 13 |
Maternal uncle: Cancer, unknown (2); Maternal uncle: Stomach(35); Maternal uncle: Tongue (42); 3 Paternal cousins once removed: Lung(?) |
| Brother | Embryonal carcinoma | 22 | ||
| Yule SM (1994) | Subject (F) | Dysgerminoma | 13 |
Aunt: stomach (?); Cousin: uterine(?) |
| Father | Seminoma | 38 | ||
| Huddart RA (1996) | Subject (M)* | Seminoma | 51 | |
| Brother | Teratoma | 28 | ||
| Cousin (F) | Yolk Sac tumor | 32 | ||
| Huddart RA (1996) | Subject (M) | Retroperitoneal Teratoma | 26 | |
| Sister | Dysgerminoma | 45 | ||
| Akyuz C (1997) | Subject (F) | Dysgerminoma | 9 | |
| Brother | Mediastinal Embryonal carcinoma | 15 | ||
| Stettner AR (1999) | Subject (M) | Teratoma | 49 | First cousin (F): breast (47) |
| First cousin once removed (F) |
Immature Teratoma | 25 | ||
| Stettner AR (1999) | Subject (F) | Dysgerminoma | 27 | |
| Paternal Uncle | Seminoma | 22 | ||
| Galani E (2005) | Subject (F) | Immature Teratoma | 12 | |
| Sister | Dysgerminoma | 22 | ||
| Brother | Mixed germ cell tumor: Embryonal carcinoma/ Seminoma |
27 | ||
| NCI Family (current report) |
Subject (M) | Mixed germ cell tumor: Embryonal carcinoma/ Seminoma |
25 | Maternal great aunt: colon (?) |
| Sister | Immature teratoma | 27 |
F = female; M = male
Table 2.
Summary of Literature of Familial Germ Cell Malignancy in Females
| Reference | Affected | Diagnosis | Age at diagnosis |
Other Cancers in family (age at diagnosis) |
|---|---|---|---|---|
| Jackson SM (1967) | Subject* | Mixed germ cell tumor: Chorionepithelioma/ Dysgerminoma |
26 | |
| Daughter | Dysgerminoma | 12 | ||
| Manka I (1970) | Subject | Dysgerminoma | 16 | |
| Sister | Dysgerminoma | 14 | ||
| Talerman A (1973) | Subject | Mixed germ cell tumor: Dysgerminoma/ Teratocarcinoma |
20 | |
| Sister | Dysgerminoma | 20 | ||
| Weinblatt M (1991) | Subject | Mixed GCT: Teratoma/Embryonal carcinoma |
10 | Mother: ?ovarian (17); Sister: embryonal rhabdomyosarcoma of the neck (15) |
| Sister | Embryonal carcinoma | 11 | ||
| Blake KI (1993) | Subject | Dysgerminoma | 8 | Paternal great aunt: Breast (66); Maternal great grandmother: Breast (70); Paternal great aunt: carcinoma, unknown primary (60) |
| Sister | Mixed GCT: Choriocarcinoma/Yolk sac tumor |
11 | ||
| Poremba C (1993) | Subject | Intracranial immature teratoma | 38 weeks | |
| Mother | Immature teratoma | 27 | ||
| Mandel M (1994) | Subject | Yolk sac tumor | 8 | Sister: Langerhans’ histiocytosis (2) |
| Sister | Dysgerminoma | 12 | ||
| Paternal grandmother |
Embryonal carcinoma | 65 | ||
| Stettner AR (1999) | Subject | Mixed GCT: Yolk sac tumor/ Teratocarcinoma |
26 | Mother: Breast (31) |
| Maternal Aunt | Immature teratoma | 47 | ||
| Maternal Cousin |
Mixed GCT: Choriocarcinoma/ Immature teratoma/Yolk sac tumor |
15 |
Maternal Grandmother reported to have ovarian cancer with unknown histology or age of diagnosis
In a majority of these families with multiple cases, histology was discordant between the affected family members (Table 1). Pure dysgerminoma was the most common GCT among the female cases (12/28, 43%) with age of occurrence from childhood to early adulthood (range: 8-27, except one case at 45). Teratoma and mixed GCT (6 cases each) were the second most common histology types. Seminoma was the most common TGCT histology in male relatives (3/10, 30%), with an older age at onset (range 22-51). In the cases reviewed, clinically malignant teratoma occurred only in postpubertal males (range 26-49), while in females the age range was much broader (from the fetal period to 47 years of age).
Pure embryonal carcinoma was observed in male relatives in 2 cases, one of which was extragonadal, both in the post-pubertal age range (ages 15 and 22); in contrast, the 2 women with this histology were diagnosed at ages 11 and 65. Pure yolk sac tumor occurred only in females (ages 8 and 32). Breast and gastric cancer were the most frequent non-GCT malignancies reported in relatives. There appeared to be no clear site-specificity, mode of inheritance, or ethnic predilection to suggest a previously-defined cancer predisposition syndrome (e.g., Li-Fraumeni syndrome).
Descriptive Epidemiology of GCTs in Males and Females (comparison of familial data to SEER data)
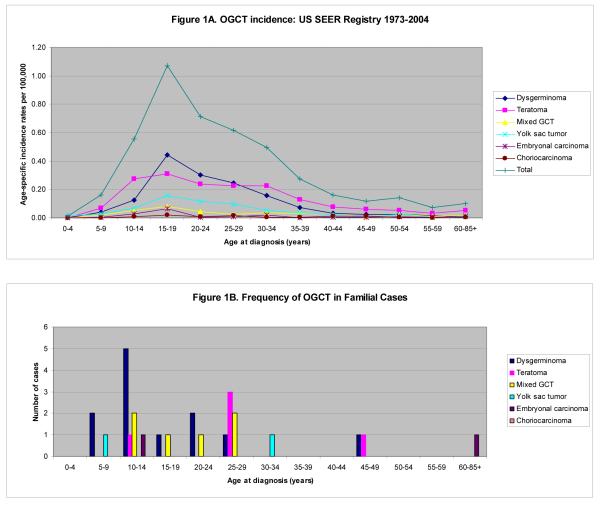
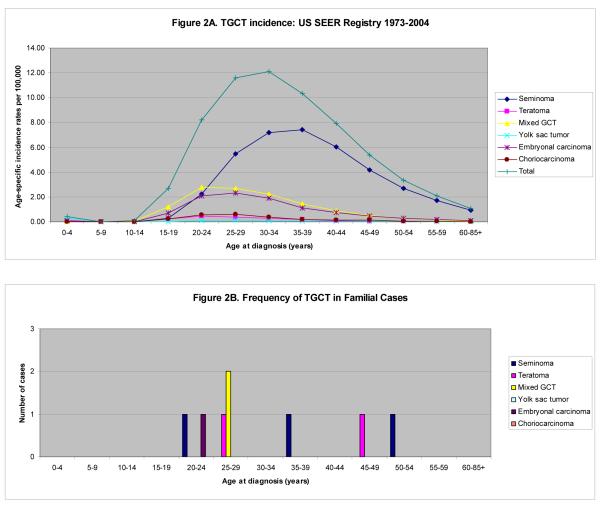
In the general population, the age-adjusted TGCT incidence rate is 4.75 per 100,000 men per year [95% confidence interval (CI) 4.68 - 4.82]. This is approximately 15 times higher than that for OGCT in women (0.33 per 100,000 women per year, 95% CI 0.31 - 0.34). Age-specific OGCT and TGCT incidence rates for all histologies as well as separated into histologic subtypes from the general US population are shown in Figures 1A and 2A, respectively. Overall, most malignant OGCTs are dysgerminomas, while the majority of malignant TGCTs are seminomas. In addition, we observe a higher proportion of embryonal carcinoma and mixed GCT of the testis as compared with the ovary.
Figure 1.
OGCT by Age and Histology: General Population, from US SEER Registry 1973-2004 (A) and Familial Case Reports (B)
Figure 2.
TGCT by Age and Histology: General Population from US SEER Registry 1973-2004 (A) and Familial Case Reports (B)
Prepubertal GCTs are more common in females than males; teratoma, dysgerminoma, and yolk sac tumor are the most common histologies. We observe an increase in OGCT incidence into the late teens, and a steady decline thereafter. In contrast, the incidence of TGCT in males begins to increase around puberty (ages 10-14) and peaks in the early- to mid-thirties. Figures 1B and 2B show the frequency of familial OGCT and TGCT cases by histology, respectively. The mean age at diagnosis among familial cases of ovarian dysgerminoma was significantly lower than that seen in the SEER population (mean age 17.1 years vs. 24.1 years, p=0.01). This difference was not seen for other histologic subtypes among females or for males. Teratomas appeared to occur less frequently among familial OGCT cases and more frequently among familial TGCT cases than in the general population. Seminomas and dysgerminomas were the most frequent familial GCTs; seminoma is also the most common histology seen among males in the general population, while teratoma is the most common among females.
DISCUSSION
In this report, we have presented a family in which TGCT and OGCT occurred in a brother and sister, and summarized the existing literature on familial occurrences of OGCT. In addition, we compared the incidence of GCTs between these familial cases and the general population. We found evidence of familial OGCT clustering, both site-specific and in combination with TGCT, and noted distinct histologic patterns for OGCT and TGCT, by age. We also compared the familial cases to incidence data from the SEER registry, and within the constraints of the small number of familial cases available for analysis, observed differences in histology and age-at-diagnosis for OGCT and TGCT. These findings support the hypothesis that OGCT may be an additional, albeit rare, manifestation of the familial gonadal tumor syndrome.
Familial clustering, epidemiologic evidence of increased risk with family or personal history, and association with genitourinary tract anomalies all provide evidence of an underlying genetic predisposition to testicular cancer (5, 6, 28). However, in part because families with many affected individuals are exceedingly rare, traditional linkage studies have yet to identify a TGCT specific cancer susceptibility gene (9). Although the incidence of OGCT in the general population is low, its occurrence in multiple-case site-specific families and in families with TGCT cases is further support that a gene conferring susceptibility to GCTs may exist in some families.
The fact that GCTs of the testes and ovaries share similar histologic features is consistent with the hypothesis that they arise from the same progenitor cell and also suggests the existence of a possible common susceptibility gene to GCT. The earliest precursors of human germ cells are termed primordial germ cells (PGCs); they constitute the reproductive cells which develop into sperm in males and eggs in females. For a germ cell to develop into a GCT, it must overcome the normal cellular regulatory processes and revert to differentiation pathways that mimic embryogenesis. The histologic subtypes of GCTs reflect the direction and degree of germ cell differentiation (1, 3). Differences in both incidence rates and histologic distribution between male and female GCTs may be due to differences in the timing of gametogenesis.
Mammalian PGCs arise from a pluripotent cell population during weeks 5-6 of embryologic development. PGCs migrate under the control of the KIT stem-cell-factor signaling pathway, along the midline of the body to the genital ridge. During migration, PGCs are actively proliferating, and express factors such as tissue non-specific alkaline phosphatase (TNAP) and the POU domain transcription factor, OCT3/4. Once they reach the developing gonads, normal PGCs stop proliferating and begin to differentiate into either sperm or eggs (1, 3, 29). It is believed that during the migratory journey, some germ cells may become trapped along their migratory path, failing to reach the genital ridge. Such ectopic PGCs should undergo apoptosis but, occasionally, extragonadal germ cells may survive and remain at risk of tumorigenesis.
In females, oogenesis takes place primarily during prenatal development. Oogonia develop from PGCs during a series of 20 to 30 mitoses and, by the third intrauterine month, most have developed into primary oocytes and have entered prophase of meiosis I. There are over 2 million oocytes at birth, but most undergo programmed cell death, leaving only several hundred to mature. Meiosis I resumes only when a mature primary oocyte is released during ovulation, and meiosis II is completed at fertilization.
In males, PGCs differentiate into spermatogonia during the second and third trimesters of intrauterine development. After birth, they undergo multiple rounds of mitotic division and differentiation in the seminiferous tubules, which retain a population of spermatogonia that have the capacity for self-renewal (2). Male germ cells undergo mitotic arrest within the seminiferous tubules; meiosis is specifically inhibited in fetal and childhood periods. At the onset of puberty, spermatogenesis begins, and diploid spermatagonia undergo meiosis I and II to form haploid spermatozoa. Ultimately, an estimated 1012 spermatozoa are produced during a lifetime, requiring several hundred successive mitoses. Progression to malignant GCT seems to coincide with the onset of puberty in both sexes. The difference in incidence of GCTs between the two sexes might be explained by the lower number of germ cells in the ovaries compared with the testes at this time.
The different histologic subtypes of GCTs closely mirror the patterns of embryologic germ cell development. Testicular seminomas and ovarian dysgerminomas arise from pluripotent germ cells which resemble undifferentiated PGCs. Nonseminomas, such as embryonal carcinoma, yolk sac tumor, choriocarcinoma, and immature teratoma, are more differentiated forms of GCTs, resembling cells that are further along in embryologic development (1). Embryonal carcinomas are unique in that they are both differentiated and have embryonic characteristics, but are derived from pluripotent cells, like seminomas (30, 31).
There are distinct differences in the distribution of the various histologic subtypes of GCTs in males and females. The behaviour of the tumors, and teratomas in particular, are also different in males and females. In the testes, the behavior of teratomas depends on whether the patient is prepubertal or postpubertal: teratomas in the former are benign, whereas in the latter they are malignant, whether mature or immature. In contrast, in females immature teratomas are always considered malignant and mature teratomas are always considered benign. These differences can be rationalized by a pathogenetic model in which ovarian teratomas and prepubertal testicular teratomas are derived from benign germ cells, while postpubertal testicular teratomas are derived from a malignant germ cell that develops into a nonteratomatous form of GCT, which in turn differentiates into teratomatous elements (3). This may also explain the higher prevalence of mixed GCTs in males compared with females.
Similar somatic genetic abnormalities have been shown to occur in OGCTs and TGCTs. Chromosomal aberrations are frequent in both tumor types; the most common is the presence of isochromosome 12p (1, 32). Interestingly, linkage studies in multiple-case TGCT families have identified a possible susceptibility gene locus on chromosome 12 (9). Faulkner demonstrated loss of heterozygosity of polymorphic genetic markers in the same genomic regions in both OGCT and TGCT, suggesting that these regions may contain tumor suppressor genes that are important in the development of germ cell tumors in both males and females (33). Kraggerud et al. examined DNA copy number changes in malignant ovarian germ cell tumors and concluded that ovarian dysgerminomas and endodermal sinus tumors shared similar genetic pathways to testicular germ cell tumors (32). There is also an increased incidence of GCTs in several congenital disorders involving gonadal dysgenesis, including trisomy 21 (33, 34). Increased and prolonged expression of stem cell-related proteins (OCT3/4, KIT, NANOG) in the gonadal tissues of trisomy 21 patients suggests that a delay in fetal GC differentiation is a key factor in the development of GCTs (35). Interestingly, somatic mutations in stem cell-related proteins (OCT3/4, KIT) are seen in both OGCTs and TGCTs.
Our case report and literature review reported herein support the possibility that the same biologic pathways are involved in the initiation of both male and female GCTs. An underlying genetic susceptibility, in association with hormonal or environmental exposures, may be required to develop malignancy (28, 33, 36). Further research based on families affected by both OGCT and TGCT cases may be helpful in defining the biological pathways involved in germ cell tumorigenesis.
ACKNOWLEDGEMENTS
This work was funded by the Intramural Research Program of the National Cancer Institute, and supported by resource contracts with Westat (NO2-CP-11019-50 and NO2-CP-65504-50). The authors would like to acknowledge the contributions of Claudia Soho, Jennifer Loud, and June Peters to the CGB Familial Testicular Cancer study. We also offer special thanks to our study participants, whose commitment has made this research possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Condensed Abstract: While testicular germ cell tumors (TGCTs) are the most common malignancy in young men, germ cell tumors in women are uncommon, and little is known about the familial occurrence of this rare disease. In this report, we describe the existing literature and a novel case of familial occurrence of testicular and ovarian germ cell tumors, and compare these to their sporadic counterparts; our data suggest a genetic susceptibility to germ cell tumors in both men and women.
REFERENCES
- 1.Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5(3):210–22. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- 2.Houldsworth J, Korkola JE, Bosl GJ, Chaganti RS. Biology and genetics of adult male germ cell tumors. J Clin Oncol. 2006;24(35):5512–8. doi: 10.1200/JCO.2006.08.4285. [DOI] [PubMed] [Google Scholar]
- 3.Ulbright TM. Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod Pathol. 2005;18(Suppl 2):S61–79. doi: 10.1038/modpathol.3800310. [DOI] [PubMed] [Google Scholar]
- 4.Moller H, Evans H. Epidemiology of gonadal germ cell cancer in males and females. Apmis. 2003;111(1):43–6. doi: 10.1034/j.1600-0463.2003.11101071.x. discussion 46-8. [DOI] [PubMed] [Google Scholar]
- 5.Heimdal K, Olsson H, Tretli S, Flodgren P, Borresen AL, Fossa SD. Familial testicular cancer in Norway and southern Sweden. Br J Cancer. 1996;73(7):964–9. doi: 10.1038/bjc.1996.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemminki K, Li X. Familial risk in testicular cancer as a clue to a heritable and environmental aetiology. Br J Cancer. 2004;90(9):1765–70. doi: 10.1038/sj.bjc.6601714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westergaard T, Olsen JH, Frisch M, Kroman N, Nielsen JW, Melbye M. Cancer risk in fathers and brothers of testicular cancer patients in Denmark. A population-based study. Int J Cancer. 1996;66(5):627–31. doi: 10.1002/(SICI)1097-0215(19960529)66:5<627::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16(5):972–8. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 9.Crockford GP, Linger R, Hockley S, Dudakia D, Johnson L, Huddart R, et al. Genome-wide linkage screen for testicular germ cell tumour susceptibility loci. Hum Mol Genet. 2006;15(3):443–51. doi: 10.1093/hmg/ddi459. [DOI] [PubMed] [Google Scholar]
- 10.Hoei-Hansen CE, Kraggerud SM, Abeler VM, Kaern J, Rajpert-De Meyts E, Lothe RA. Ovarian dysgerminomas are characterised by frequent KIT mutations and abundant expression of pluripotency markers. Mol Cancer. 2007;6:12. doi: 10.1186/1476-4598-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth LM, Talerman A. Recent advances in the pathology and classification of ovarian germ cell tumors. Int J Gynecol Pathol. 2006;25(4):305–20. doi: 10.1097/01.pgp.0000225844.59621.9d. [DOI] [PubMed] [Google Scholar]
- 12.Blake KI, Gerrard MP. Malignant germ cell tumours in two siblings. Med Pediatr Oncol. 1993;21(4):299–300. doi: 10.1002/mpo.2950210413. [DOI] [PubMed] [Google Scholar]
- 13.Jackson SM. Ovarian dysgerminoma in three generations? J Med Genet. 1967;4(2):112–3. doi: 10.1136/jmg.4.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandel M, Toren A, Kende G, Neuman Y, Kenet G, Rechavi G. Familial clustering of malignant germ cell tumors and Langerhans’ histiocytosis. Cancer. 1994;73(7):1980–3. doi: 10.1002/1097-0142(19940401)73:7<1980::aid-cncr2820730732>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Manka I, Klauber E. Ovarian dysgerminoma in sisters. Bratisl Lek Listy. 1970;53(5):581–3. [PubMed] [Google Scholar]
- 16.Poremba C, Dockhorn-Dworniczak B, Merritt V, Li CY, Heidl G, Tauber PF, et al. Immature teratomas of different origin carried by a pregnant mother and her fetus. Diagn Mol Pathol. 1993;2(2):131–6. [PubMed] [Google Scholar]
- 17.Stettner AR, Hartenbach EM, Schink JC, Huddart R, Becker J, Pauli R, et al. Familial ovarian germ cell cancer: report and review. Am J Med Genet. 1999;84(1):43–6. doi: 10.1002/(sici)1096-8628(19990507)84:1<43::aid-ajmg9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Talerman A. Gonadoblastoma and dysgerminoma in two siblings with dysgenetic gonads. Obstet Gynecol. 1971;38(3):416–26. [PubMed] [Google Scholar]
- 19.Weinblatt M, Kochen J. an unusual family cancer syndrome manifested in young siblings. Cancer. 1991;68(5):1068–70. doi: 10.1002/1097-0142(19910901)68:5<1068::aid-cncr2820680526>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Sternberg WH, Barclay DL, Kloepfer HW. Familial XY gonadal dysgenesis. N Engl J Med. 1968;278(13):695–700. doi: 10.1056/NEJM196803282781302. [DOI] [PubMed] [Google Scholar]
- 21.SEER 9 Surveillance E, and End Results (SEER) Program . SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2006 Sub (1973-2004) - Linked To County Attributes - Total U.S., 1969-2004 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; ( www.seer.cancer.gov) released April 2007, based on the November 2006 submission. [Google Scholar]
- 22.International classification of diseases for oncology. 3rd ed World Health Organization; Geneva: 2000. [Google Scholar]
- 23.Akyuz C, Koseoglu V, Gogus S, Balci S, Buyukpamukcu M. Germ cell tumours in a brother and sister. Acta Paediatr. 1997;86(6):668–9. doi: 10.1111/j.1651-2227.1997.tb08954.x. [DOI] [PubMed] [Google Scholar]
- 24.Galani E, Alamanis C, Dimopoulos MA. Familial female and male germ cell cancer. A new syndrome? Gynecol Oncol. 2005;96(1):254–5. doi: 10.1016/j.ygyno.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 25.Huddart RA, Thompson C, Houlston R, Huddart RA, Nicholls EJ, Horwich A. Familial predisposition to both male and female germ cell tumours? J Med Genet. 1996;33(1):86. doi: 10.1136/jmg.33.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trentini GP, Palmieri B. An unusual case of gonadic germinal tumor in a brother and sister. Cancer. 1974;33(1):250–5. doi: 10.1002/1097-0142(197401)33:1<250::aid-cncr2820330138>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Yule SM, Dawes PJ, Malcolm AJ, Pearson AD. Occurrence of seminoma and dysgerminoma in father and daughter. Pediatr Hematol Oncol. 1994;11(2):211–3. doi: 10.3109/08880019409141659. [DOI] [PubMed] [Google Scholar]
- 28.Lindor NGM. Hereditary Neoplastic Syndromes. In: SDaF J, editor. Cancer Epidemiology and Prevention. Oxford University Press; New York: 2006. [Google Scholar]
- 29.Donovan PJ, de Miguel MP. Turning germ cells into stem cells. Curr Opin Genet Dev. 2003;13(5):463–71. doi: 10.1016/j.gde.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Chaganti RS, Houldsworth J. Genetics and biology of adult human male germ cell tumors. Cancer Res. 2000;60(6):1475–82. [PubMed] [Google Scholar]
- 31.Skotheim RI, Lind GE, Monni O, Nesland JM, Abeler VM, Fossa SD, et al. Differentiation of human embryonal carcinomas in vitro and in vivo reveals expression profiles relevant to normal development. Cancer Res. 2005;65(13):5588–98. doi: 10.1158/0008-5472.CAN-05-0153. [DOI] [PubMed] [Google Scholar]
- 32.Kraggerud SM, Szymanska J, Abeler VM, Kaern J, Eknaes M, Heim S, et al. DNA copy number changes in malignant ovarian germ cell tumors. Cancer Res. 2000;60(11):3025–30. [PubMed] [Google Scholar]
- 33.Rapley E. Susceptibility alleles for testicular germ cell tumour: a review. Int J Androl. 2007;30(4):242–50. doi: 10.1111/j.1365-2605.2007.00778.x. discussion 250. [DOI] [PubMed] [Google Scholar]
- 34.Satge D, Honore L, Sasco AJ, Vekemans M, Chompret A, Rethore MO. An ovarian dysgerminoma in Down syndrome. Hypothesis about the association. Int J Gynecol Cancer. 2006;16(Suppl 1):375–9. doi: 10.1111/j.1525-1438.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 35.Cools M, Honecker F, Stoop H, Veltman JD, de Krijger RR, Steyerberg E, et al. Maturation delay of germ cells in fetuses with trisomy 21 results in increased risk for the development of testicular germ cell tumors. Hum Pathol. 2006;37(1):101–11. doi: 10.1016/j.humpath.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Weir HK, Marrett LD, Kreiger N, Darlington GA, Sugar L. Pre-natal and peri-natal exposures and risk of testicular germ-cell cancer. Int J Cancer. 2000;87(3):438–43. doi: 10.1002/1097-0215(20000801)87:3<438::aid-ijc20>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]