Abstract
The earliest stage in the development of neuronal polarity is characterized by extension of undifferentiated “minor processes” (MPs), which subsequently differentiate into the axon and dendrites. We investigated the role of the myosin II motor protein in MP extension using forebrain and hippocampal neuron cultures. Chronic treatment of neurons with the myosin II ATPase inhibitor blebbistatin increased MP length, which was also seen in myosin IIB knockouts. Through live-cell imaging we demonstrate that myosin II inhibition triggers rapid minor process extension to a maximum length range. Myosin II activity is determined by phosphorylation of its regulatory light chains (rMLC), mediated by myosin light chain kinase (MLCK) or RhoA-kinase (ROCK). Pharmacological inhibition of MLCK or ROCK increased MP length moderately, with combined inhibition of these kinases resulting in an additive increase in MP length similar to the effect of direct inhibition of myosin II. Selective inhibition of RhoA signaling upstream of ROCK, with cell-permeable C3 transferase, increased both the length and number of MPs. To determine whether myosin II affected development of neuronal polarity, MP differentiation was examined in cultures treated with direct or indirect myosin II inhibitors. Significantly, inhibition of myosin II, MLCK, or ROCK accelerated the development of neuronal polarity. Increased myosin II activity, through constitutively active MLCK or RhoA, decreased both the length and number of MPs and, consequently, delayed or abolished the development of neuronal polarity. Together, these data indicate that myosin II negatively regulates MP extension, and the developmental time course for axonogenesis.
Keywords: myosin II, myosin light chain kinase, Rho-kinase, minor process, polarity, neuronal development
Establishment of appropriate functional connectivity in the nervous system requires regulated development of axons and dendrites, generating and maintaining neuronal polarity. For hippocampal and forebrain neurons, polarity arises in vitro through a well-characterized sequence of morphological changes (Craig and Banker, 1994; Bradke and Dotti, 2000a, b; Heidemann et al., 2003; Dehmelt and Halpain, 2004; Arimura and Kaibuchi, 2007). Following attachment to a permissive substrate, these neurons extend broad actin-rich lamellipodia and filopodia (Stage I) which then segment and condense into multiple undifferentiated neurites, termed minor processes (Stage II). Through asymmetric growth, one minor process becomes significantly longer than the others, eventually attaining an axonal phenotype (StageIII), while the remaining minor processes subsequently differentiate into dendrites (Stage IV). Although the stereotyped sequence of morphogenesis is known, the cellular and molecular mechanisms governing the establishment of neuronal polarity are not fully understood.
Myosin II is a mechanoenzyme that generates cellular contractile forces through interaction with actin filaments and regulates various aspects of the cytoskeleton and cellular morphology (Wylie and Chanter, 2001, 2003; Brown and Bridgman, 2004; Chantler and Wylie, 2003; Conti and Adelstein, 2008). Neurons express both myosin heavy chain isoforms, IIA and IIB. A third isoform, IIC, has been described recently, but is expressed only by certain neuronal populations and at low levels during development (Golomb et al., 2004). Each heavy chain associates with two light chains, separated into essential and regulatory functional subtypes. Binding of the essential chain to the heavy chain neck region is necessary for myosin to be operative, while the regulatory myosin light chain (rMLC) directly controls myosin II activity in a phosphorylation-dependent manner. Accordingly, when rMLC is phosphorylated at the S19 residue, myosin II is able to generate contractile forces against actin filaments. In neurons, three major regulatory kinases and one phosphatase are known to determine rMLC phosphorylation levels, and thus myosin II-based contractility (Amano et al., 2000; Bresnik, 1999; Ng and Luo, 2004). Myosin light chain kinase (MLCK) is activated by Ca2+-calmodulin and phosphorylates the rMLC. RhoA-kinase (ROCK) is activated by the upstream RhoA-GTPase and, in turn, phosphorylates rMLC and inhibits myosin light chain phosphatase (MLCP). The contribution of myosin II to the development of neuronal polarity through regulation by its upstream kinases is not known.
Our studies reveal the significance of myosin II activity during the earliest stage in the development of neuronal polarity. We show that myosin II activity antagonizes the extension of minor processes, mediated through activation of both MLCK and ROCK. Through live-cell imaging we demonstrate that myosin II inhibition triggers rapid minor process extension to a maximum length range. Finally, we show that myosin II regulates axonal differentiation, influencing the time course of axonogenesis without altering characteristic neuronal polarity. Together, our data suggest a model in which the relative level of myosin II activity, and thus contractility, inhibits minor process extension, and in turn regulates the time-course of the development of neuronal polarity.
MATERIALS AND METHODS
Cell Culture
For most experiments, forebrain neuron cultures were prepared from embryonic day 8 (E8) chickens using modifications to previously published methods (Heidemann et al., 2003). Briefly, the superficial portions of chick forebrains were isolated and dissociated with trypsin-EDTA (Gibco, Invitrogen Corp., Carlsbad CA) followed by incubation in calcium-magnesium free saline with gentle mechanical agitation. Cultures were plated at 10 × 104 cells per German glass coverslip (pre-coated with 0.1 mg/mL poly-DL-Lysine; Sigma-Aldrich, Inc., St. Louis MO), and maintained in M199 medium (Gibco, Invitrogen Corp.) supplemented with N9 at 37°C/5% CO2. For additional experiments, hippocampal neuron cultures were prepared from embryonic day 20 (E20) rats or E13.5–18 mice using published protocols (Goslin and Banker, 1989). E20 rat hippocampal neurons were plated at 6 × 104 cells per 25 mm poly-L-lysine-coated coverslip in 6-well cell culture plates (BD Falcon, San Jose CA) and maintained in serum-free Neurobasal medium (Gibco, Invitrogen Corp.) supplemented with B27 and L-glutamine. Myosin IIB knockout mouse embryos were identified from a combination of a PCR assay and immunoblots.
Drug Treatment
Forebrain cultures were maintained in M199 medium or M199 medium supplemented with 50 µM (S)-(−)-Blebbistatin (Toronto Research Chemicals, Inc., North York, ON, Canada), 500 nM ML-7 (Biomol Research Laboratories Inc., Plymouth Meeting PA), 10 µM Y-27632 (CN Biosciences supplied by Calbiochem-Novabiochem Corp, San Diego, CA), a combination of ML-7 and Y-27632, or DMSO vehicle. For F-actin disruption experiments, forebrain neurons were treated 30 minutes after plating with 4 µM latrunculin-A (Biomol Research Laboratories Inc.), or 6 µM jasplakinolide (Molecular Probes, Inc., Eugene OR). For hippocampal neuron cultures, filtered blebbistatin (50 µM) or DMSO was added in Neurobasal medium at the time of plating (for E20 rat experiments) or between 45 min and 12 hrs after plating (at 100 µM for E13.5–18 mouse experiments). Hippocampal neurons maintained for over 5 DIV received glial conditioned medium with added blebbistatin or DMSO. For dynamic microtubule disruption experiments, hippocampal neurons were maintained for 2 DIV and treated with 150 nM nocodazole for 24 hours prior to fixation.
C3-Transferase Treatment
Cell-permeable C3-transferase exoenzyme (Cytoskeleton, Inc., Denver CO), a selective inhibitor of RhoA GTPase, was reconstituted at 0.2 mg/mL in 50% glycerol/sterile water. Following incubation of E8 chicken forebrain neurons for 2 DIV at 37°C/5% CO2 in serum-containing M199, this medium was removed and the cultures gently washed three times in pre-equilibrated serum-free M199. Subsequently, cultures were treated with 5 µg/mL C3-transferase in serum-free M199 for 4 h at 37°C/5% CO2, conditions optimized to yield a robust phenotype based on manufacturer’s biochemical data from Rho activity assays.
Transfections
Electroporation-mediated DNA transfection
EYFP-tagged cDNA constructs encoding wild type myosin light chain kinase (P-IRES-EYFP-WT-MLCK) or constitutively active MLCK (P-IRES-EYFP-CA-MLCK) (Kim et al., 2002; generous gift of Dr. Mark F. A. VanBerkum, Wayne State University) were prepared using standard methods (Qiagen HiSpeed Plasmid Purification Midi Kit, Qiagen, Valencia CA) and electroporated into dissociated E8 chicken forebrain neurons using the Amaxa Nucleofector and the Chicken Neuron Nucleofector Kit for Chicken Hippocampal Neurons (Amaxa Biosystems, Gaithersburg MD). pmaxGFP™ plasmid (Amaxa Biosystems) was used as a positive control. For each reaction, 1.6 × 106 neurons were suspended in 100 µl volume transfection medium supplemented with 10 µg of plasmid DNA, and transfected using the G003 setting of the electroporator. Forebrain neurons were then transferred into fresh M199 medium, re-counted, and plated at 10 × 104 cells per coverslip immediately following transfection.
Peptide-mediated protein transfection
The constitutively active L63RhoA protein was delivered to forebrain neurons using the Chariot™ peptide as previously described (Gallo et al., 2002). Briefly, 1 µg purified L63RhoA protein (Cytoskeleton, Inc.) or BSA inert control protein was mixed with 6 µL of sonicated cell-permeable peptide Chariot™ (ActiveMotif Inc., Carlsbad CA), and incubated for 30 minutes. Forebrain neuron cultures that had been maintained for 2 DIV were then transfected with the Chariot™ peptide-protein complex for 8 hours prior to fixation and analysis of morphometric changes.
Electrophoresis and Immunoblotting
E8 chick forebrain cultures were maintained for 1–1.5 DIV and lysed at 4° C with the addition of 10 mM Tris pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM NaVO4, 0.1% SDS, 0.5% SDS, and 1% Triton-X 100 with protease and phosphatase inhibitor cocktails (Sigma-Aldrich, Inc.). Whole cell lysates were sedimented and the supernatant concentrated using YM-3 microcon centrifugal filter devices (Millipore Corp., Bedford MA) immediately prior to quantifying total lysate protein with the Bio-Rad Quick Start ™ Bradford Protein Assay (Bio-Rad Laboratories, Inc. Hercules CA). Protein samples solubilized in NuPAGE® LDS sample buffer and reducing agent (Invitrogen Corp.) were separated electrophoretically through a 12% SDS polyacrylamide gel and transferred onto 0.45 µm Immobilon-P PVDF membranes (Millipore Corp.) using a semidry blotting apparatus (Bio-Rad Laboratories). Blots were blocked overnight at 4˚C with 5% non-fat dried milk in TBS and 0.05% Tween-20 (5% TBST). Blots then were incubated for 2 h at RT in 2% TBST containing rabbit polyclonal antibodies against nonmuscle myosin II heavy chain A (MHC-A) or B (MHC-B) at 1:5,000 (Covance Research Products, Berkeley, CA). After washing, blots were incubated for 1 h at RT with secondary peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H+L) antibodies at 1:50,000 in 2% TBST (Jackson Immunoresearch Laboratories, Inc. West Grove, PA). Immunoreactive bands were detected with the Amersham ECL Plus Western Blotting Detection System (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) and exposed to Kodak Biomax XAR film. Subsequently, the membranes were stripped, blocked, and re-probed with mouse monoclonal (clone AC-15) anti-β-actin antibodies (Sigma-Aldrich, Inc.) at 1:5,000 (2% TBST) followed by peroxidase-conjugated AffiniPure goat anti-mouse IgG (H+L) secondary antibodies at 1:50,000 (2% TBST) (Jackson Immunoresearch Laboratories, Inc.).
Immunocytochemistry
Forebrain neuronal cultures were fixed with 0.25% gluteraldehyde, immunostained, and mounted on slides with NOFADE medium as previously described (Louden et al., 2006), with antibody combinations as follows. Myosin II heavy chain isoforms were detected using rabbit polyclonal antibodies against nonmuscle myosin II heavy chain A or B at 1:200 (Covance Research Products) followed by rhodamine labeled secondary antibody at 1:400 (Jackson Immunoreserach Laboratories, Inc.). Omission of the primary antibody resulted in no detectable staining. Detection of phosphorylated regulatory myosin light chains (rMCLP) was performed using antibodies raised to chicken light chains phosphorylated at serine 19 (generous gift of Dr. H. F. Yee, UCSF) and the volumetric marker CellTracker (Molecular Probes) using previously reported ratiometric methods (Loudon et al., 2006). Transfection constructs tagged with GFP or YFP were detected using rabbit polyclonal antibodies to GFP at 1:500 (Abcam Inc., Cambridge, MA) and FITC-conjugated goat anti-rabbit secondary antibodies at 1:100 (Jackson Immunoresearch Laboratories, Inc.). L63RhoA protein is histidine-tagged, and was detected using mouse monoclonal IgG1 antibodies (clone 3H2201) to 6X His tag at 1:100 (Abcam Inc.), followed by TRITC-conjugated goat anti-mouse antibodies at 1:400 (Sigma-Aldrich, Inc). Cytoskeletal characterization was performed with one or more of the following antibody combinations: mouse anti-MLCK monoclonal antibodies at 1:100 (Sigma Aldrich) and FITC-conjugated goat anti-mouse secondary antibodies at 1:100 (Jackson Immunoresearch Laboratories, Inc.); mouse anti-tau-1 monoclonal IgG2a antibody (MAB3420) at 1:50 (Chemicon International, Temecula CA) and TRITC-conjugated goat anti-mouse secondary antibodies at 1:100 (Sigma-Aldrich, Inc.); goat anti-ROCK-1(C-19) or ROCK-1(K-18) polyclonal antibodies at 1:50 overnight (Santa Cruz Biotechnology, Inc., Santa Cruz CA) and Alexa Fluor 488 donkey anti-goat secondary antibodies at 1:100 (Invitrogen Corporation). For determination of morphology, cultures were double-labeled for F-actin, using rhodamine or FITC-conjugated phalloidin at 8/100 µL of staining solution (Molecular Probes), and α-Tubulin, using monoclonal FITC-conjugated antibodies (DM1A clone) at 1:100 (Sigma-Aldrich, Inc.). For actin immunostaining in jasplakinolide experiments, mouse monoclonal (clone AC-15) anti-β-actin antibodies (Sigma-Aldrich, Inc.) were used at 1:200 followed by goat anti-mouse IgG (TRITC) secondary antibodies at 1:400 (Jackson Immunoresearch Laboratories, Inc.). For hippocampal neuron experiments, cultures were fixed in pre-warmed (37°C) 4% PFA in 0.1M cacodylate buffer for 20 min, followed by permeabilization with 0.2% Triton-X-100 and immunostaining for myosin IIA and IIB heavy chain isoforms (Covance Research Products), tyrosinated tubulin (Accurate Chemical and Scientific Corporation, Westbury NY), tau (tau-1, Sigma-Aldrich, Inc. or tau-1 clone MAB3420 Chemicon International,), MAP-2 (Chemicon International, clone MAB364), or phosphorylated neurofilaments (Covance Research Products, clone SMI 312). Immunostaining for morphometrics was performed as described for forebrain neuron cultures.
Imaging
Fluorescence microscopy
For fixed and immunostained forebrain cultures, images were acquired with a Zeiss Axiovert 200M inverted microscope (Carl Zeiss, Inc., Gottingen, Germany), equipped with an Orca-ER CCD camera (Hamamatsu Inc., Bridgewater NJ). 20x EC plan-neofluar and 100 x plan-neofluar 1.3 oil immersion objectives (Carl Zeiss, Inc.) were used to collect multichannel fluorescent images using the 6D multidimensional acquisition mode of Zeiss AxioVision Software (release 4.5). Data was captured as 16 bit greyscale or pseudocolored digital images (1280 × 1008 pixels) for analysis with Zeiss AxioVision software, with Adobe Photoshop (Version 7.0) used to construct multifield montages. Fixed and immunostained mouse hippocampal cultures were imaged with either an Olympus Fluoview confocal microscope or standard Olympus IX70 fluorescence microscope (Olympus America Inc., Center Valley PA) equipped with a Cooke Sensicam CCD camera (Applied Scientific Instrumentation, Eugene OR), while rat hippocampal cultures were imaged with a Zeiss Axiovert 200M inverted microscope (Carl Zeiss, Inc.), equipped with an Orca-ER CCD camera (Hamamatsu Inc.).
Phase contrast videomicroscopy
Time-lapse phase contrast imaging of neuronal cultures was performed on the Zeiss Axiovert 200M inverted microscope described above. E8 forebrain neuron cultures were prepared as described previously, plated on poly-DL-Lysine coated glass coverslip (0.25 mm #1, Bellco Biotechnology) microwell dishes (10–12 mm diameter), and maintained in F12 complete medium (Gibco Invitrogen Corp.) at 37–39 °C using the Tempcontrol 37-2 digital regulator coupled to a three-plate insert heated-stage and objective heater (Carl Zeiss, Inc.). Time lapse images were acquired with a 100x plan-neofluar 1.3 oil-immersion objective and digital image stacks were captured for up to 90 minutes at 15 second interframe intervals using Zeiss AxioVision Software. Quantitative analysis of minor process dynamics over each time series was performed using the interactive measurement module of the AxioVision Software (curve spline tool). Minor process extension rates were determined by measuring the initial and final neurite lengths between fixed time points, and expressed as distance in µm over the elapsed time.
Morphometric Analysis
Chick forebrain neuron cultures were fixed at 2 DIV and the effects of myosin II inhibitors (blebbistatin, ML-7, Y27632, or C-3 transferase) or activators (constitutively active MLCK or L63RhoA) on minor process extension and the development of polarity were examined. Using fluorescence microscopy, a minimum of 12–16 fields (at 20X) were acquired for analysis of each treatment by forced sampling of the coverslip area. Each identifiable neuron in these high-contrast images was binned into previously described morphological subtype categories: “stage 0,” “stage I,” “stage II,” or “stage III” based on structural features (Craig and Banker, 1994; Bradke and Dotti, 2000a). For stage II neurons, the lengths of all minor processes that could be completely and unambiguously traced were measured as the longest continuous segment path using calibrated AxioVision software curve spine tool. To assess the development of polarity, the proportion of each morphological stage was expressed as a percentage of the total neuronal population for a given treatment condition, obtained from a minimum of three separate experiments that were pooled following ANOVA tests. Statistical comparison of the distribution of morphological stages was performed between treatment populations using the Welch T-test that does not assume equal variances. Similar morphological analyses were performed with hippocampal neuron cultures fixed between 1 and 3 DIV.
RESULTS
Myosin IIA and IIB heavy chain isoforms are differentially expressed at the earliest stage in the development of neuronal polarity
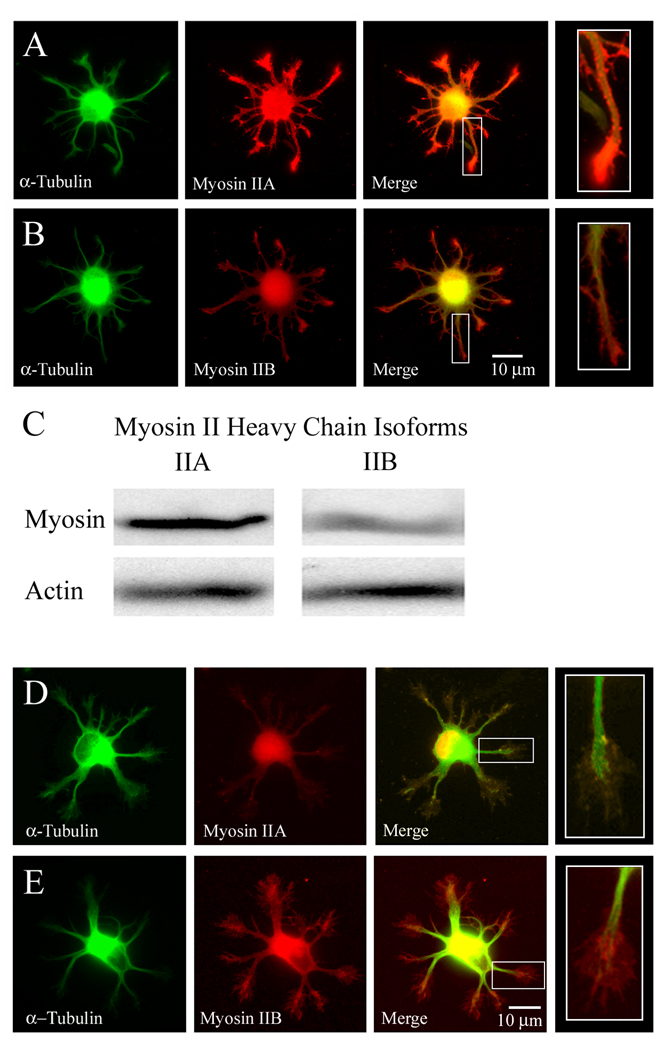
To begin to investigate the role of myosin activity in the extension of minor processes, we first examined the expression and subcellular distribution of myosin II heavy chain isoforms in two CNS neuronal populations developing in vitro. Embryonic chick forebrain neuron cultures were fixed at 2 days in vitro (DIV) and stained with isoform specific antibodies to myosin IIA or myosin IIB, and with phalloidin to reveal F-actin or DM1A–FITC to reveal α-tubulin. While both isoforms were distributed throughout the soma and minor processes of stage II neurons, immunocytochemical staining levels were robust for myosin IIA, while myosin IIB staining levels were significantly lower (Figure 1A–B). The subcellular localization patterns for myosin IIA and IIB were similar, organized as small puncta throughout the minor processes and often enriched at the growth cones, particularly for myosin IIA. Semi-quantitative Western blot analysis of forebrain neuron cultures at 1–1.5 DIV confirmed that myosin IIA reactivity predominates at early developmental stages (Figure 1C). We next sought to determine whether similar myosin IIA and IIB expression patterns were seen in stage II rat and mouse hippocampal neurons, which develop through a sequence of morphogenesis similar to that of forebrain neurons. In contrast with forebrain neurons, however, hippocampal neurons (3 DIV) showed higher levels of myosin IIB staining relative to IIA (Figure 1D–E), which was distributed throughout minor processes and concentrated at growth cones. Myosin IIA levels remained low at all stages of rat hippocampal neuron development, while myosin IIB levels likewise remained low during chick forebrain neuron development (Supplemental Figure S1). Together, these data show that myosin IIA and IIB isoforms are expressed from the earliest stage in the development of neuronal polarity for two CNS populations. The contrasting isoform predominance may reflect species-specific or neuronal type-specific differences in myosin II expression or antibody reactivity.
Figure 1.
Myosin IIA and IIB heavy chain isoforms are differentially expressed in embryonic neurons during early developmental stages. (A–B) Subcellular distribution of myosin II isoforms in stage II chick forebrain neurons at 2 DIV, immunostained for α-tubulin (green) and myosin IIA or myosin IIB heavy chains (red) using isoform-specific polyclonal antibodies. Selected minor processes depicted as enlarged insets. (A) Minor processes stain robustly for myosin IIA, distributed as small puncta throughout their length and often enriched within tips characteristic of elaborated growth cones. (B) In contrast, myosin IIB staining is uniform but faint within minor processes. (C) Western blot of E8 chick forebrain extract prepared from 1–1.5 DIV cultures showing relative levels of myosin IIA (lane 1) and IIB (lane 2) heavy chain isoform proteins. (D–E) Subcellular distribution of myosin II isoforms in stage II rat hippocampal neurons at 3 DIV, immunostained for α-tubulin (green) and myosin IIA or myosin IIB heavy chains (red). Selected minor processes depicted as enlarged insets. Myosin IIB staining predominates in developing minor processes (E), while myosin IIA levels are barely detectible (D).
Direct inhibition of myosin II activity triggers increased minor process outgrowth
In order to determine the role of myosin II activity in the outgrowth of minor processes, we directly inhibited myosin activation using blebbistatin (50 µM), a specific myosin II ATPase blocker (Straight et al., 2003; Allingham et al., 2005). Following chronic bath application of blebbistatin or DMSO vehicle for 2 DIV, forebrain cultures were fixed and immunostained to reveal microtubules, and the number and length of minor processes was determined. Inhibition of myosin II increased the average minor process length by 76% over the DMSO control, also resulting in clock-wise looping neurites (Figure 2A–B). Live-cell imaging of stage II forebrain neuron dynamics immediately before and after acute blebbistatin treatment revealed rapid-onset increases in minor process elongation (Figure 2C). Interestingly, the number of minor processes extended was reduced by 24% with chronic myosin inhibition as compared to vehicle controls (Figure 2D). This suggests a possible homeostatic inhibitory control exerted by myosin II activity, such that the blebbistatin-triggered elongation of minor processes was balanced by a reduction in the number of processes initiated or stabilized.
Figure 2.
Inhibition of myosin II activity increases process length but decreases process number. (A) Forebrain neuron cultures were treated with 50 µM blebbistatin or DMSO vehicle control for 2 DIV, then fixed and immunostained for α-tubulin (green) and F-actin (red) to reveal minor processes. (B) Quantification of minor process length showed an increase with blebbistatin treatment (n = 289 processes), relative to DMSO controls (n = 297; mean = 19 ± 0.5 µm). (C) Still frames from phase-contrast live-cell imaging of stage II forebrain neurons immediately before (time 0) and one hour after (time 60) treatment with blebbistatin or DMSO vehicle. Minor processes exposed to blebbistatin extended rapidly and steadily (arrowheads indicate position of growth cones prior to treatment) while DMSO treated processes exhibited no net growth. (D) Quantification of minor process number showed a reduction with blebbistatin treatment (n = 304 neurons) as compared to DMSO controls (n = 344; mean = 6.5 ± 0.1). (E) Rat hippocampal neuron cultures were treated with 50 µM blebbistatin or DMSO vehicle control for 2 DIV, then fixed and immunostained for α-tubulin (green) and F-actin (red) to reveal neurites. (F) Quantification of murine neurite length showed an increase with blebbistatin exposure (n = 20 neurons), knockout of myosin IIB gene expression (n = 24), or blebbistatin treatment of myosin IIB knockouts (n = 24), relative to wild type DMSO controls (n = 38; mean = 123.8 ± 9.7 µm). Data are presented normalized to control values. **p ≤ 0.01, ***p ≤ 0.0001, ****p ≤ 0.00001, Welch t-test. Error bars indicate SEM.
In developing hippocampal cultures composed of Stage II and III neurons, the lengths of axons, dendrites, and minor processes were increased by 82% following 2 days of chronic blebbistatin treatment from the time of plating (100µM), relative to DMSO controls (Figure 2E–F). Unlike chicken forebrain neurons, in rat hippocampal neurons expression of the myosin IIB isoform predominates. Therefore, we examined whether genetic ablation of myosin IIB would produce effects on minor process outgrowth mirroring pharmacological inhibition of myosin II. Indeed, hippocampal cultures from myosin IIB knockout (KO) mice exhibited an average neurite length that was 67% greater than wild type controls. Moreover, the magnitude of increase was similar between wildtype neurons and either DMSO-treated KO neurons or KO neurons treated with blebbistatin (Figure 2F), as would be predicted if neurite outgrowth at these early stages is chiefly regulated by myosin IIB activity in this neuronal type. No differences in neurite number were seen between hippocampal culture treatment conditions (data not shown). Taken together, our data reveal a significant role for myosin II in regulating the outgrowth of minor processes at the earliest stages of morphogenesis for two CNS neuronal cell types, in two species, suggesting a novel function for myosin II in mediating neuronal development.
Since substratum has been shown to influence the role of myosin II in axon extension (Gallo, 2004; Ketschek et al., 2007), we also investigated the role of myosin II in minor process outgrowth on a more biologically relevant substrate, laminin. While the majority of forebrain neurons cultured on laminin display unipolar (25%) or bipolar (50%) forms, 7% attain stage II morphology (Supplemental Figure S2A). Inhibition of myosin II with blebbistatin treatment increased the average minor process length of these neurons over controls by ∼ 28% on laminin (LN), as compared to ∼ 71% for neurons maintained on poly-DL-lysine (PL) or PL+LN (Supplemental Figure S2B). The lower magnitude of process lengthening for blebbistatin treated neurons cultured on laminin is likely to reflect the greater contribution of myosin II to adhesion relative to microtubule advance on integrin-mediated substrata as compared to polycationic poly-DL-lysine (Gallo, 2004; Ketschek et al., 2007). Moreover, recent studies have shown that matrix rigidity negatively regulates neurite extension, mediated through receptor-like protein tyrosine phosphatase α (RPTPα) triggered clustering of integrin and focal contact proteins in response to fibronectin but not laminin (Kostic et al., 2007). This suggests that rigidity responses on laminin involve alternative pathways, possibly involving myosin II activity.
Combined MLCK and ROCK activation drives myosin II activity underlying the control of minor process outgrowth
Our data show that myosin II activity negatively regulates minor process outgrowth. In order to determine the relative contribution of the two major regulators of rMLC phosphorylation, MLCK and ROCK, we analyzed the distribution and function of these kinases in developing minor processes of chicken forebrain neurons. Both MLCK and ROCK are expressed by forebrain neurons from the earliest stages of morphogenesis (data not shown) and are distributed throughout the minor processes of stage II neurons, often enriched at the tips (Figure 3A–B). To begin to determine the relative contribution of MLCK or ROCK in the regulation of myosin II in minor process growth dynamics, we treated embryonic forebrain neurons for 2 days with saturating doses of the MLCK inhibitor, ML-7 (500 nM), an inhibitor of ROCK, Y-27632 (10 µM), or a combination of these drugs, and examined morphological changes in fixed and immunostained preparations. Saturating doses were determined from dose response assays (data not shown; range for ML-7: 250 nM – 2 µM; range for Y-27632: 1 – 25 µM). The minor processes of Stage II neurons treated with ML-7 were 22% longer than vehicle controls, while treatment with Y-27632 resulted in minor processes which were 43% longer than controls (Figure 3C). Treatment with both ML-7 and Y-27632 resulted in minor processes that were 85% longer than controls (Figure 3C). Thus, ML-7 and Y-27632 individually produced increases in minor process length, and in combination produced additive effects on outgrowth that are similar to that seen with direct inhibition of myosin II by blebbistatin (76% increase), suggesting both kinases regulated myosin II activity in minor processes. The RhoA GTPase is the upstream regulator of ROCK and additional pathways (Ng and Luo, 2004; Koh, 2006). To examine the contribution of RhoA activity, we treated 2 DIV forebrain neurons for 4 hr with the C3-transferase exoenzyme, a selective inhibitor of Rho-family GTPases. As predicted, RhoA inhibition upstream of ROCK also promoted an increase in minor process lengths, which were 29% longer than controls (Figure 3D). In addition, C-3 transferase treatment resulted in a 40% increase in the number of minor processes extended, as compared to mock-treated controls, reflecting distinct contributions for Rho family GTPases and downstream ROCK in regulating minor process outgrowth (Figure 3E). The discrepancy between the magnitude of minor process length increases with Y-27632 and C3 transferase treatment is most likely to reflect the duration of treatment, which was 2 days and 4 hours, respectively, due to technical limitations of using the cell permeable protein C3. It is also possible that these growth differences may be due to the fact that RhoA regulates additional pathways independent of ROCK. Regardless, both inhibition of RhoA and ROCK promoted increased lengthening of minor processes.
Figure 3.
Combined MLCK and ROCK activity regulates minor process growth. (A) Forebrain neurons fixed at 2 DIV and immunostained for MLCK (green) and phalloidin-stained for F-actin (red). MLCK staining is evident within the soma and throughout minor processes of stage II neurons, often appearing enriched in areas also enriched for F-actin. (B) Forebrain neurons fixed at 2 DIV and immunostained for ROCK (green) and phalloidin-stained for F-actin (red). ROCK levels are highest within the soma and more diffuse within minor processes, also appearing to co-localize with regions of high F-actin content. (C) Quantification of minor process length following inhibition of MLCK or ROCK. Forebrain neurons were maintained for 2 DIV in the presence of MLCK inhibitor (ML-7, 500 nM), Rho Kinase inhibitor (Y-27632, 10 µM), both ML-7 and Y-27632, or DMSO vehicle. Chronic treatment with ML-7 (n = 622 processes) or Y-27632 (n = 526) increased minor process length relative to DMSO controls (n = 586), while combined treatment with ML-7 and Y-27632 (M+Y, n = 554) produced an increase in length similar to that produced with blebbistatin treatment. (D–E) Forebrain neuron cultures at 2 DIV were treated with the RhoA inhibitor, cell-permeable C3-transferase, for 4 hours prior to fixation. Both the length (D) and number (E) of minor processes were increased following exposure to C-3 transferase (n = 1385 processes, 369 neurons), relative to mock-treated controls (n =1697 processes, mean = 23.8 ± 0.2 µm; n = 343 neurons, mean = 9.2 ± 0.15 processes). Data are presented normalized to the control values. ****p ≤ 0.00001, Welch t-test. Error bars indicate SEM.
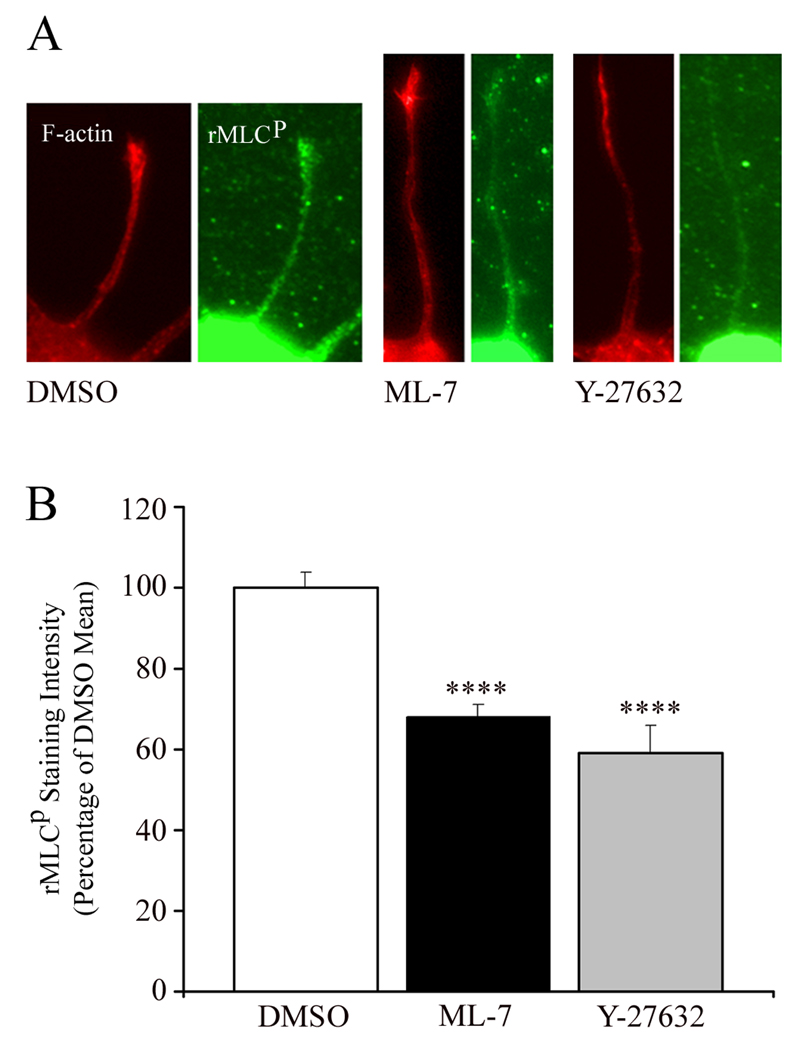
Finally, we sought to confirm directly that pharmacological inhibition of MCLK or ROCK decreases rMLC activation, underlying the observed changes in minor process morphology. Following chronic treatment of forebrain neurons with ML-7 or Y-27632, we examined the levels of rMLC phosphorylation in fixed preparations immunostained for rMLC-p, as previously reported (Loudon et al., 2006). Consistent with the partial effects of ML-7 and Y-27632 on minor process length changes, both treatments partially decreased the staining intensity of rMLC-p (Figure 4A–B). Taken together, this data indicates that both MLCK and ROCK partially regulate myosin II activity in developing minor processes and contribute to controlling their extension.
Figure 4.
Both MLCK and ROCK partially regulate myosin II activity through myosin light chain phosphorylation. (A) Immunodetection of phosphorylated regulatory myosin light chains (rMLC-p). Forebrain neurons were maintained for 2 DIV in the presence of MLCK inhibitor (ML-7, 500 nM), Rho Kinase inhibitor (Y-27632, 10 µM), or DMSO vehicle control. Neurons were loaded with 2.5 µM of the volumetric fluorescent reporter CellTracker, then fixed and immunostained with an antibody to rMLC-p (green) and phalloidin-stained for F-actin (red) to reveal minor processes. Relative to the DMSO mean rMLC-p staining intensity, both ML-7 and Y-27632 reduced staining within minor processes. (B) Quantification of rMLC-p staining intensity was performed using previously published ratiometric methods (Louden et al., 2006), and revealed partial reduction in rMLC-p staining with ML-7 (n = 168 processes) or Y-27632 (n = 136) as compared to DMSO (n = 136). Data are presented normalized to the DMSO control values. ****p ≤ 0.00001, Welch t-test. Error bars indicate SEM.
Myosin II inhibition produces stable increases in minor process length
Using washout assays, we sought to determine whether blebbistatin treatment triggered reversible or stable growth-promoting effects. After first confirming the efficacy of blebbistatin washout using cultured chick metanephric cells, which display a robust morphological change with myosin II inhibition (Figure 5A, also see Loudon et al., 2006), we performed similar experiments using forebrain neurons maintained for 1–2 DIV. Neurons under chronic blebbistatin treatment for 48 hours attained greater minor process lengths than those treated for 24 hours and fixed, as expected (Figure 5B–C). Most significantly, minor process lengths were similar in cultures treated with blebbistatin for 24 hours and fixed at 1 DIV and those treated with blebbistatin for 24 hours followed by washout for another 24 hours prior to fixation fixed at 2 DIV (Figure 5B–C). Moreover, neurons fixed after 48 hours of chronic blebbistatin treatment exhibited significantly longer minor processes than those fixed at 2 DIV following blebbistatin washout. This suggests that the increased process outgrowth achieved within the first day of myosin II inhibition was retained following washout, representing a stable increase in length but not a permanent shift to an enhanced growth state. Although minor process length continued to increase slightly following washout, this is likely to represent base-level growth that also occurs in untreated neurons. Indeed, long-term increases in both axon and dendrite lengths were also seen in control and blebbistatin-treated hippocampal cultures maintained for up to 9 DIV (Supplemental Figure S3).
Figure 5.
Myosin II inhibition produces stable increases in minor process length. (A) Chick metanephric cell cultures were treated with blebbistatin (50 µM) for 24 or 48 hours and fixed, or treated with blebbistatin for 24 hours followed by washout for another 24 hours prior to fixation. Blebbistatin-treated cells displayed a loss of stress fibers with staining for α-tubulin (green) and F-actin (red), which was reversed following washout. (B) Blebbistatin washout was performed in forebrain neuron cultures as described for metanephric cultures, and minor process lengths were quantified (C). Minor process lengths were similar in cultures treated with blebbistatin for 24 hours and fixed at 1 DIV (n = 779 processes), or fixed at 2 DIV following washout (n = 1059), which were greater than control neuron lengths at 1 DIV (n = 789) or 2 DIV (n = 1015). The greatest lengths were attained by cultures under blebbistatin treatment for 48 hours (n = 791). Data are presented normalized to the DMSO control at 24 hours. ****p ≤ 0.00001, NS = non-significant, Welch t-test. Error bars indicate SEM.
Myosin II inhibition produces rapid increases in minor process length
To characterize the dynamics of minor process elongation triggered by myosin II inhibition, we performed live cell imaging of stage II forebrain neurons at 2 DIV before and after acute blebbistatin treatment. Within one minute of blebbistatin addition, minor processes began to extend steadily, reaching an average growth rate of 9.82 (±0.9) µm per hour, while DMSO controls instead demonstrated alternating bouts of extension and retraction with minimal to no net growth during equivalent imaging periods (Figure 6; Table 1; Supplemental Movie 1–Supplemental Movie 3). These dynamics suggest that blebbistatin relieved constraints on minor process growth normally produced by myosin II activity, allowing for rapid process elongation.
Figure 6.
Myosin II inhibition alters minor process growth dynamics. The effects of acute or chronic blebbistatin (50 µM) on minor process dynamics were determined through live cell imaging of forebrain neurons cultured for 30 minutes, 1 DIV, or 2 DIV. Acute blebbistatin treatment produced the greatest proportion of growing processes at each time point (30 minutes, n = 52 processes; 1 DIV, n = 85; 48 hours, n = 66), relative to chronic blebbistatin exposure or DMSO vehicle. Within DMSO control cultures at each time point, nearly equal numbers of processes were seen undergoing periods of growth or retraction (30 minutes, n = 71 processes; 1 DIV, n = 128; 2 DIV, n = 67). Chronic blebbistatin treatment (1 DIV, n = 141; 2 DIV, n = 40) resulted in a higher proportion of processes extending than in DMSO controls, though not as high as the proportion in acute-blebbistatin treated cultures. Data are presented as the percentage of minor processes growing (bars above the origin) or retracting (bars below the origin). *p ≤ 0.01, ***p ≤ 0.0001, Welch t-test. Error bars indicate SEM.
Table 1. Minor process growth rate (µm/hr) as a function of blebbistatin treatment.
Minor process growth rates as a function of acute and chronic blebbistatin treatment. Live-cell imaging was used to examine minor process growth dynamics in forebrain cultures within 30 min, 1 DIV, or 2 DIV of plating. Cultures were treated with blebbistatin either acutely (drug added following a 30 min reference imaging period) or chronically (from the time of plating, continuing through the imaging period), and growth rates were determined through analysis of 1 hour time-lapse imaging sequences.
| Imaging Period | 30 min | 1 DIV | 2 DIV |
|---|---|---|---|
| Acute DMSO | 0.5 ± 0.3 (71) | 0.1 ± 0.2 (128) | −0.2 ± 0.3 (67) |
| Acute Blebbistatin | 11.6 ± 2.3 (52)**** | 6.5 ± 0.6 (85)**** | 9.8 ± 0.9 (66)**** |
| Chronic Blebbistatin | --- | 2.8 ± 0.4 (141)**** | 1.4 ± 0.4 (40)* |
Minor process sample size is indicated parenthetically, and error bars indicate SEM. Comparisons between treatment and DMSO at each time point: *p ≤ 0.05, ****p ≤ 0.00001, Welch t-test.
We then compared short-term growth dynamics with minor process elongation under chronic blebbistatin treatment. We hypothesized that, if blebbistatin released internal contractile forces that served to keep minor processes within a limited length range, maximal outgrowth would occur within the first few hours of myosin II inhibition. Alternatively, if myosin II inhibition produced intracellular conditions favorable for ongoing growth, chronic blebbistatin treatment would be predicted to allow persistently increased rates of minor process extension. It was also possible that the potential for process growth might change as a function of development, so we examined minor process dynamics in forebrain neurons within 30 minutes post-plating as well as at 1 and 2 DIV. These cultures were treated with blebbistatin either acutely or chronically, and growth rates were determined through analysis of time-lapse imaging (Table 1; Supplemental Movie 1–Supplemental Movie 3). For all neuronal populations, the average minor process length increased over the developmental time course (Figure 7A); however, growth dynamics differed as a function of treatment. Minor processes were measured at the first and last frame of each hour-long time-lapse movie, and binned as “growing” or “retracting” based on the net length change. While DMSO-treated neurons displayed roughly equal proportions of growing or retracting minor processes at each developmental time point (Figure 6), the majority of minor processes in acute blebbistatin-treated cultures were seen to grow (93% ± 2% at 30 min, 89% ± 7% at 1 DIV, and 95% ± 3% at 2 DIV), while for neurons chronically exposed to blebbistatin only an intermediate 75% ± 5% (1DIV) or 68% ± 9% (2 DIV) of minor processes were observed to grow. Thus, while stage II neurons under chronic myosin II inhibition retained the capacity for ongoing minor process growth, a subset of their processes appeared to retract slightly (< 5 µm) over the imaging period examined. The average rate of minor process growth differed between acute and chronic blebbistatin treatment paradigms. Maximal growth rates were achieved at each developmental time point only following acute blebbistatin treatment, with lower rates of growth evident in chronically-treated cultures (Figure 7B; Table 1). Moreover, the average minor process length reached a maximum within one day of chronic blebbistatin treatment, while acute blebbistatin treatment continued to trigger significant process lengthening at each time point. Taken together, these findings indicate that the onset of increased growth occurs rapidly following myosin II inhibition, and suggest that the subset of minor processes observed to retract slightly under chronic blebbistatin might have previously reached an intrinsically determined maximal length. In support of this interpretation, measurement of minor processes in Stage III forebrain neurons revealed average lengths that were similar in magnitude to those at Stage II, while axons were significantly longer. (Supplemental Figure S3).
Figure 7.
Myosin II inhibition rapidly increases minor process lengthening. (A) The effects of acute or chronic blebbistatin (50 µM) treatment on minor process dynamics were examined through live cell imaging of forebrain neurons cultured for 30 minutes, 1 DIV, or 2 DIV. The change in minor process length between the first (time 0) and last (time 60) frame of each hour long experiment was quantified. At each developmental time point, acute blebbistatin treatment (30 min., n = 52 processes; 1 DIV, n = 85; 2 DIV n = 66) increased minor process length as compared to DMSO controls (30 min., n = 71; 1 DIV, n = 128; 2 DIV n = 67). Chronic blebbistatin treatment (1 DIV, n = 141; 2 DIV n = 40) produced lower magnitude increases in process length than acute treatment. (B) Normalization of minor process growth reveals that acute blebbistatin treatment produced a similar enhancement of growth at each developmental time point, while DMSO control processes exhibited no net growth. Following chronic blebbistatin treatment, increases in process growth were only seen at 1 DIV. Sample sizes indicated above. Data are presented as the percentage of the initial length. *p ≤ 0.05, ****p ≤ 0.00001, Welch t-test. Error bars indicate SEM.
Inhibition of myosin II activity accelerates the time course of the development of neuronal polarity
Pharmacological inhibition of myosin II directly, using blebbistatin, or indirectly, through inhibition of the upstream regulators MLCK or ROCK, promoted the extension of minor processes. To determine whether myosin II activity regulates the subsequent differentiation of minor processes, we examined markers of cytoskeletal maturation in cultures of forebrain or hippocampal neurons maintained for 3 DIV. At this time point, untreated or DMSO vehicle control cultures are predominantly comprised of stage II and III neurons. In blebbistatin treated cultures, both stage II and III neurons were identifiable by morphological features. Moreover, immunostaining revealed that stage III-IV neurons exhibited a single long axon, strongly positive for the dephosphorylated axonal marker tau-1 and negative for dendrite-specific MAP-2, and multiple short dendrite precursors with minimal tau and high levels of MAP-2 (Figure 8A–D). Significantly, we did not observe instances of blebbistatin-treated neurons with multiple axons, indicating that myosin II does not play a direct role in the specification of axonal identity.
Figure 8.
Inhibition of myosin II activity accelerates the development of neuronal polarity. (A–B) Mouse hippocampal cultures were maintained for 3 DIV in the presence of DMSO vehicle or blebbistatin, respectively, then fixed and immunostained to reveal the distribution of tyrosinated tubulin (green) and tau-1 (red), an axonal marker. Axon specification is not affected by blebbistatin treatment. Arrows indicate tau-1 positive axons. Note the absence of tau-1 staining in dendrites (asterisks). (C–D) Rat hippocampal cultures were maintained for 3 DIV in the presence of DMSO vehicle or blebbistatin, respectively, then fixed and immunostained to reveal the distribution of tau-1 (red), and dendrite-specific MAP-2 (green), with overlap shown as yellow. Arrows indicate single tau-1 positive axons. (E) Forebrain neurons were maintained for 2 DIV in the presence of blebbistatin (50 µM), ML-7 (500 nM), Y-27632 (10 µM), both ML-7 and Y-27632, or DMSO, and the proportion of neurons at each developmental stage was determined for each treatment population. The distribution of neurons was shifted toward more mature morphological stages by blebbistatin (n = 19 fields), and to a lesser extent by ML-7 (n = 16), Y-27632 (n = 24), or a combination of these drugs (n = 18), as compared to DMSO controls (n = 12). (F) Hippocampal neurons were cultured in the presence of blebbistatin (n = 15 fields per time point) or DMSO (n = 15) and fixed at 1, 2, and 3 DIV to determine the proportion of neurons at each developmental stage. Similar to forebrain cultures, the distribution of neurons was shifted toward more mature morphological stages by blebbistatin relative to DMSO. Data are presented as the percentage distribution. *p ≤ 0.05, **p ≤ 0.001, ***p ≤ 0.0001, ****p ≤ 0.00001, Welch t-test. Error bars indicate SEM.
Since myosin II inhibition increased minor process extension, it was possible that myosin II might also regulate the time course for the development of neuronal polarity. To examine this possibility, we cultured forebrain neurons for 2 DIV in the presence of blebbistatin, ML-7, Y-27632, or combined ML-7 and Y-27632, as previously described. Inhibition of myosin II, MLCK, or ROCK shifted the distribution of neurons toward more mature morphological stages, as compared to DMSO controls (Figure 8E). Interestingly, the combined treatment of ML-7 and Y-27632 did not result in a shift in population distribution comparable to blebbistatin treatment, as might be predicted from the additive contribution of MLCK and ROCK to minor process extension (Figure 3C). While approximately 70% of blebbistatin-treated neurons had attained stage III morphologies at this time point, only about 50% of ML-7, Y-27632, or ML-7 + Y-27632 treated neurons had reached stage III. This discrepancy may be due to divergent downstream signaling effects mediated by these separate kinases. A similar increase in the proportion of more mature stage III neurons with blebbistatin treatment was also evident in hippocampal neuron cultures maintained for up to 3 DIV (Figure 8F). Together, these findings indicate that while myosin II activity does not directly influence the specification of axonal identity, it does regulate the onset of polarity by restraining minor process extension and thus the time course for differentiation of the axon.
Increased myosin II activity restricts minor process extension and slows the development of neuronal polarity
Inhibition of myosin II, MLCK, or ROCK increased minor process extension and accelerated the development of polarity for forebrain neurons without altering the characteristic sequence of morphogenesis. We predicted that increased activity of MLCK or ROCK would instead generate increased adhesion or contractile forces within minor processes and restrict their extension. To begin to examine this hypothesis, we prepared plasmid cDNAs encoding enhanced yellow fluorescence protein (EYFP), EYFP-fused wild type MLCK (WT-MLCK), or EYFP-fused constitutively active MLCK (CA-MLCK), with pmaxEGFP used as a control. These plasmid constructs were then electroporated into freshly dissociated chicken forebrain neurons, which were plated as monolayers and allowed to develop for 2 days prior to fixation and immunodetection of EGFP and the EYFP reporter. Forebrain neurons transfected with EYFP-CA-MLCK remained predominantly at immature developmental stages, extending only stunted filopodial buds or short minor processes (Figure 9A). Indeed, morphological comparisons revealed that constitutively active MLCK significantly restricted the length of minor processes to 57% of WT-MLCK expressing neurons, and also reduced the number of process extended by 26% (Figure 9B–C). Although expression of WT-MLCK formally constitutes overexpression of MLCK, the overall phenotype of Stage II WT-MLCK neurons closely resembled P-IRES-EYFP or pmaxEGFP controls, possibly due to intrinsic constraints on MLCK activation (Kim et al., 2002).
Figure 9.
Increased myosin II activity restricts minor process extension. (A) forebrain neurons were transfected with EGFP cDNA, or EYFP-tagged cDNA constructs encoding wild type myosin light chain kinase (WT-MLCK) or constitutively active MLCK (CA-MLCK), then fixed at 2 DIV and immunostained for EGFP/EYFP (green) to reveal minor processes. (B–C) Minor process length and number were decreased in stage II forebrain neurons expressing CA-MLCK (n = 228 processes, 53 neurons) as compared to wild type controls (n = 304 processes; n = 52 neurons). (D) forebrain neuron cultures were peptide-transfected at 2 DIV with constitutively active RhoA protein (L63RhoA) or inert BSA control protein for 8 hours prior to fixation and immunostaining for the His-tag reporter, or α-tubulin (green) and F-actin (red). (E–F) Minor process length and number were decreased in stage II forebrain neurons following transfection with L63RhoA (n = 569 processes, 111 neurons) as compared to BSA peptide-transfected controls (n = 887 processes, 129 neurons). Data are presented normalized to the corresponding WT-MLCK or BSA control values. ****p ≤ 0.00001, Welch t-test. Error bars indicate SEM.
In order to determine the effects of RhoA activation, constitutively active His-tagged L63RhoA protein was delivered to forebrains neurons after 2 DIV by Chariot-peptide mediated transfection, as previously reported (Gallo et al., 2002). Previously, constitutively active L63RhoA was shown to activate ROCK signaling downstream, increasing phosphorylation of rMLC and myosin II activity indirectly (Gallo et al., 2002; Gallagher et al., 2004). Following an 8 hr incubation, transfected cultures were fixed and immunostained for the His-tag reporter and either α-tubulin or F-actin, to reveal morphological characteristics. Similar to CA-MLCK transfectants, forebrain neurons transfected with L63RhoA protein also displayed shorter and fewer minor processes as compared to BSA-transfected controls (Figure 9D–F). Since L63RhoA was introduced into neurons with established processes, the decrease in minor process number may represent the retraction of processes existing prior to delivery of the constitutively-active RhoA protein, rather than a failure to extend minor processes from the initial time of plating, which is likely to underlie the effects of CA-MLCK. Alternatively, ongoing process growth from BSA-transfected neurons, but stalled growth from L63RhoA transfectants, could result in these minor process length and number differences.
Since both the length and number of minor processes were reduced with constitutively active MLCK and RhoA, it follows that the time course for the development of polarity might be delayed as a secondary effect. To test this prediction, we transfected forebrain neurons with WT-MLCK, CA-MLCK, or EGFP immediately before plating, to produce continuous activation of MLCK before the earliest stages of neuronal differentiation commenced. Following 2 days in culture, transfected neurons were fixed and immunostained for EGFP or the EYFP reporter, and the number of neurons at each developmental stage was determined for each transfectant population, based on morphology. The majority of CA-MLCK transfected neurons were blocked at the earliest stages of differentiation, with (36%) remaining attached to the substrate but rounded, while (52%) had developed reduced lamellipodia, attaining stage I. Only (12%) reached stage II, displaying very short unbranched minor processes, and no stage III neurons were detected (Figure 10). The population of WT-MLCK transfectants was shifted toward a larger proportion of stage I and II neurons, likely due to increased myosin II activity resulting from MLCK overexpression. These findings are consistent with the observation that myosin II inhibition promoted minor process extension and the development of neuronal polarity, together showing that the relative level of myosin II activation can regulate minor process growth and differentiation.
Figure 10.
Increased myosin II activity inhibits the development of neuronal polarity. Quantification of morphological subtypes in forebrain neurons electroporated with control EGFP cDNA or EYFP-tagged constructs encoding wild type myosin light chain kinase (WT-MLCK) or constitutively active MLCK (CA-MLCK). Cultures were fixed at 2 DIV and immunostained for EGFP/EYFP (similarly detected with anti-EGFP antibody) to reveal morphology. All transfected neurons were scored as Stage 0, I, II, or III, and the proportion at each developmental stage was determined for each transfectant population. Development of polarity was delayed or inhibited in neuronal populations expressing CA-MLCK (n = 138 neurons) relative to both WT-MLCK (n = 393) and EGFP (n = 300) expressing populations. Data are presented as the percentage distribution. *p ≤ 0.05, ****p ≤ 0.00001 as compared to EGFP control, Welch t-test. Error bars indicate SEM.
Microtubule Dynamics Partially Mediate Process Length Increases Following Myosin II Inhibition
Myosin II inhibition rapidly increased minor process lengths. Process extension is a complex phenomenon involving both F-actin and microtubule based mechanisms. Microtubule polymerization and dynamics are required for process extension (Dent and Gertler, 2003) and may thus be reflective of a rate-limiting step in ongoing growth. In order to determine whether microtubule dynamics are required for the increase in minor process length induced by inhibition of myosin II, mouse hippocampal cultures were treated with either blebbistatin or blebbistatin and 150 nM nocodazole, a concentration that attenuates microtubule dynamics without causing microtubule depolymerization (Rochlin et al., 1996). Nocodazole prevented the increase in minor process length induced by blebbistatin, indicating that microtubule dynamics are required for the blebbistatin-induced increase in process length (Figure 11A).
Figure 11.
Role of microtubule dynamics and actin filaments in minor process extension. (A) Microtubule dynamics are necessary for minor process length increases following myosin II inhibition. Hippocampal neurons were maintained for 2 DIV and treated with blebbistatin (100 µM), stabilizing concentrations of nocodazole (150 nM), or both blebbistatin and nocodazole for 24 hours prior to fixation. Blebbistatin treatment (n = 12 processes) produced an increase in the length of neurites over controls (n = 11, mean = 137.1 ± 25.65 µm), which was dampened by concurrent nocodazole treatment (n = 13). Nocodazole treatment alone (n = 15) diminished neurite lengths relative to controls. Data are presented normalized to the DMSO control at 24 hours. (B) F-actin disruption promotes minor process length. Forebrain neuron cultures were treated 30 minutes after plating with 50 µM blebbistatin, F-actin-depolymerizing concentrations of latrunculin A (4 µM), latrunculin plus blebbistatin, or DMSO vehicle control for 2 DIV, then fixed and immunostained for α-tubulin to reveal minor processes. Deplymerization of F-actin with latruculin (n = 560 processes) increased minor process lengths as compared to DMSO controls (n = 709), which were further increased with concurrent blebbistatin treatment. Latrunculin dampened the increase in minor process extension produced by blebbistatin treatment alone (n = 1068). **p ≤ 0.01, ***p ≤ 0.0001, Welch t-test, with paired comparisons. Error bars indicate SEM.
Depolymerization or stabilization of F-actin alters neuronal development
Myosin II requires F-actin in order to generate contractile forces. Consistent with this expectation, actin disruption with F-actin depolymerizing concentrations of latrunculin A increased minor process lengths in forebrain neurons, as compared to DMSO controls (Figure 11B; Supplemental Figure S4). This is likely to reflect reduced actomyosin-based steric hindrance of microtubule penetration into distal minor process regions, either by transport or ongoing polymerization. Latrunculin-enhanced minor process extension was further potentiated with concurrent myosin II inhibition, but failed to reach the magnitude of extension produced by blebbistatin alone (Figure 11B; Supplemental Figure S4). Although latrunculin depolymerizes F-actin, disorganized F-actin puncta were observed along minor process shafts and distal tips, similar to those seen in previous studies of DRG axons treated with depolymerizing concentrations of cytochalasin B (Marsh and Letourneau 1984; Letourneau et al., 1987). The slight increase in process lengths induced by blebbistatin in latrunculin-treated cultures may be attributable to myosin II acting on latrunculin-induced F-actin puncta. Indeed, Verkhovsky et al. (1997) have demonstrated that actin depolymerizing agents cause the myosin II driven formation of F-actin “asters” which appear similar to the puncta observed in neurons following latrunculin treatment. Thus, the latrunculin induced asters/puncta may be providing a slight degree of inhibition on process extension. Consistent with this notion, in cultures treated with blebbistatin and latrunculin we did not observe F-actin puncta/asters as in cultures treated with latrunculin alone (Supplemental Figure S4).
Depolymerization of F-actin and stabilization of F-actin also altered the development of neuronal morphology (Supplemental Figure S5). Consistent with the requirement of F-actin based filopodia for initial process formation (Dent et al., 2007), latrunculin treatment largely prevented the development of stage II and II neurons. Similarly, stabilization of F-actin using jasplakinolide also blocked the majority of neurons at stage I of development. While inhibition of myosin II using blebbistatin did not alter the effect of latrunculin, it resulted in an increase in the proportion of stage II and stage III neurons in cultures treated with jasplakinolide. This result is consistent with the role of myosin II in negatively regulating neuronal development by generating contractile forces against F-actin.
DISCUSSION
Neurons are polarized cells that typically elaborate a single axon and multiple dendrites through a stereotyped developmental sequence involving cytoskeletal reorganization. While a variety of extracellular cues have long been known to influence the development of neuronal polarity (Arimura and Kaibuchi, 2007), the underlying intracellular mechanisms have only recently begun to emerge. Previous studies have revealed roles for myosin II activity in filopodia extension, neurite growth, axonal pathfinding and dendritic spine maturation (Wylie and Chantler, 2001; Chantler and Wylie, 2003; Brown and Bridgman, 2004; Kim and Chang 2004; Medeiros et al., 2006; Ryu et al., 2006; Dent et al., 2007; Rösner et al., 2007; Kubo et al., 2008); however, this is the first study examining the function of myosin II during early stages of neuronal morphogenesis and the development of polarity. Collectively, our data reveal a critical role for myosin II in negatively regulating the time course for minor process extension in two CNS cell types, and in turn the sequential development of neuronal polarity. However, we did not find evidence that myosin II is involved in the specification of axonal identity, as neurons continued to exhibit a single axon.
Growth cone dynamics are mediated through myosin IIA and IIB, together regulating adhesion to the ECM and retrograde F-actin flow, which generate the traction forces necessary for extension as actin polymerizes at the leading edge. Consequently, the rate of retrograde flow is inversely proportional to the rate of growth cone advance (Dent and Gertler, 2003). As predicted from this relationship, we found that decreasing myosin II activity allows rapid extension of minor processes, presumably by relieving contractile force. Since we observed that myosin IIA predominated in chick forebrain neurons, while IIB levels were highest in mouse and rat hippocampal neurons, it is interesting to note that total myosin II inhibition with blebbistatin produced similar increases in minor process length for both neuronal types. This suggests that both myosin II isoforms perform the same cellular function in these separate populations.
Through loss and gain of function experiments we determined that both major regulators of rMLC phosphorylation, MLCK and ROCK, partially regulate myosin II activity, together controlling minor process extension. In contrast, myosin II regulation appears to be mediated chiefly by ROCK in DRG growth cones (Loudon et al., 2006). These results, in combination with recent studies in non-neuronal cells, indicate that MLCK and ROCK can perform distinct and non-redundant roles in regulating myosin II function according to cell type and the subcellular distribution of myosin II (Beningo et al., 2006; Totsukawa et al., 2004). Upstream of ROCK, C3-transferase mediated inhibition of RhoA increased minor process extension, while constitutively active L63RhoA diminished process outgrowth. This is in agreement with earlier studies demonstrating that RhoA-mediated ROCK signaling negatively regulates process outgrowth for both hippocampal and cerebellar primary neuron populations (Bito et al., 2000; Da Silva et al., 2003). Since the RhoA regulatory pathway is complex, it is possible that, in addition to mediating changes in myosin II activity through ROCK, other cytoskeletal elements might be affected through separate pathways (Luo, 2000; Birkenfeld et al., 2001; Da Silva et al., 2003; Govek et al., 2005). Additional experiments will be necessary to determine whether cross-talk occurs between the various signaling pathways involved in myosin II regulation within the developing CNS.
A variety of intracellular effectors are involved in establishing or maintaining neuronal polarity, including PI-3 kinase, Rap1B, Par3/Par6/aPKC complex, GSK-3β, CRIMP-2, LKB1 and downstream SAD kinases (Nishimura et al., 2005; Wiggin et al., 2005; Solecki et al., 2006; Yoshimura et al., 2006; Arimura and Kaibuchi, 2007; Barnes et al., 2007; Shelly et al., 2007). Ultimately, these signaling cascades converge at the level of the cytoskeleton to generate localized structural rearrangement through the mechanism of symmetry breaking. During the establishment of neuronal polarity, symmetry breaking events occur first at the Stage I-II transition, triggering minor process extension from the spherical neuronal cell body, and second, at the Stage II-III transition, allowing one process to lengthen significantly and establish an axonal phenotype (Bradke and Dotti, 2000a,b; Da Silva and Dotti, 2002). Our data suggest that myosin II may be responsible for maintaining the neuronal sphere in newly postmitotic neurons, generating contractile forces against cortical actin to produce a barrier against cytoskeletal changes that trigger minor process outgrowth. According to this model, stochastic or signal-transduction mediated local changes in either F-actin stability or myosin II activity could alter actomyosin-based forces sufficiently to trigger minor process outgrowth. Indeed, recent studies showed that loss of all Ena/VASP actin regulatory proteins prevented F-actin bundling and filopodia formation which, in turn, abolished cortical neurite outgrowth (Kwiatkowski et al., 2007; Dent et al., 2007). Interestingly, neurite outgrowth could be rescued in the Ena/Vasp null background by inhibition of myosin II activity (Dent et al., 2007). Our data show that in neurons with a normal complement of functional actin regulatory proteins, changes in myosin II activity alone can account for regulation of minor process outgrowth. Most striking was the stalled development at stage 0, or I, and lack of minor processes in all but 12% of forebrain neurons transfected with CA-MLCK. Of those few transfected neurons that attained stage II, processes were extremely stunted, in agreement with prior findings in cultured hippocampal neurons transfected with a constitutively active MLCK construct (Kim and Chang, 2004). Together, these findings suggest that a shift in the balance of actomyosin-based cytoskeletal forces may be both necessary and sufficient for symmetry breaking during the early stages of process outgrowth.
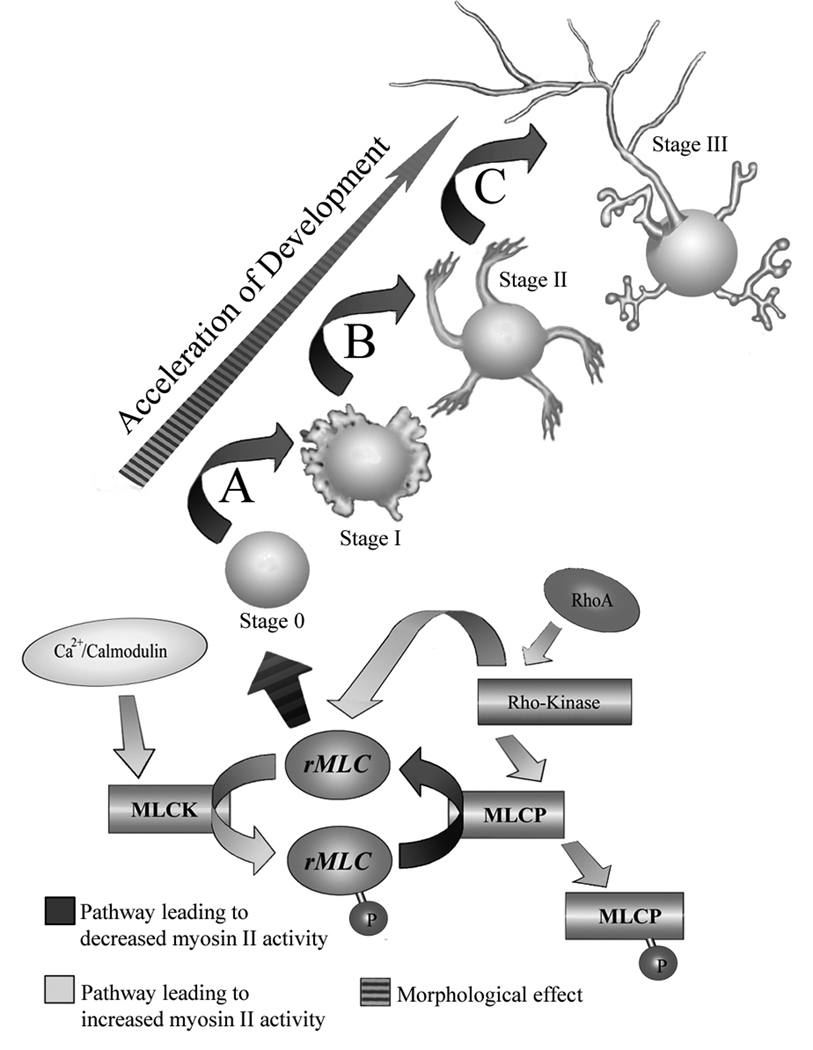
Mechanically applied tension can influence the development of neuronal polarity by inducing axonal differentiation (Chada et al., 1997; Lamoureux et al., 2002), so it was possible that myosin II could similarly drive the symmetry breaking events underlying the stage II-III transition through its motor activity. However, we found that increasing actomyosin-based contractility, through constitutively active MLCK expression, retarded the development of polarity. Instead, blebbistatin-mediated relief of myosin II contractile force triggered steady process elongation that led to accelerated development of neuronal polarity without directly specifying axonal identity. We found that minor process elongation rates peak rapidly after acute blebbistatin exposure, with significant reduction of growth rates seen in Stage II neurons during chronic myosin II inhibition over longer developmental periods. Moreover, minor processes eventually appear to reach a limiting length, seen in both stage II and III neurons in culture, with or without endogenous myosin II activity. Considered together, these findings indicate a mechanism whereby myosin II activity regulates the time course for attainment of an intrinsically determined maximal length range for minor processes, after which yet uncharacterized mechanisms may be required for establishing the positive feedback signaling that is thought to promote selective elongation of one process during axon specification (Figure 12). This interpretation is consistent with previous live-cell imaging studies of hippocampal neurons in which minor processes elongated to ∼20 µm before stalling for several hours prior to the differentiation of one process into a fast-growing axon (Dehmelt et al., 2003).
Figure 12.
Model of the proposed mechanism for myosin II regulation during the development of neuronal polarity. In the newly post-mitotic neuron, actomyosin-based contractility maintains cellular integrity and constrains cytoskeletal dynamics. (A) Local signaling leading to changes in F-actin stability or decreases in myosin II activity trigger symmetry breaking and filopodial extension from the neuronal sphere, which may be potentiated through decreases in myosin II-mediated kinking and severing of actin bundles. (B) Ongoing myosin II activity regulates the time course for minor process growth, until an intrinsically determined maximum length is attained, (C) after which as yet uncharacterized cytoskeletal changes trigger symmetry breaking events that underlie axonal specification.
It will be necessary to correlate our data with observation of cortical neurons developing in vivo, which generate axons and dendrites within the context of their migration away from the ventricular zone to target regions (Metin et al., 2006). As such, neurons first elaborate leading and trailing processes in a bipolar migratory phase, transform into a multipolar morphology during a period of growth arrest upon reaching the subventricular zone, and then again restructure to attain a bipolar morphology as a new leading process extends and becomes the apical dendrite while the trailing process (formerly the leading process) becomes the axon (Noctor et al., 2004; LoTurco and Bai, 2006). Thus, the direction of migration may establish the plane of neuronal polarity and future location of the emerging axon (Calderon de Anda et al., 2008). Myosin II activity is known to be critical for the mechanism of neuronal migration in vivo (Bellion et al., 2005), and defects in the adhesion and migration of postmitotic cortical neurons have been suggested to underlie the ventricular malformations of myosin IIB knockout mice (Tullio et al., 2001; Ma et al., 2007; Bao et al., 2007), though development of individual neurons in these knockouts remains to be examined. It will be important to determine whether spatial and temporal regulation of myosin II plays a role in the localized accumulation of specific cytoskeletal elements during the development of neuronal polarity.
Supplementary Material
Levels of myosin II reactivity in hippocampal neurons and forebrain neurons developing in vitro. (A) Hippocampal neuron cultures were maintained for 2 DIV prior to fixation and immunostaining for α-tubulin (green) and the myosin IIA isoform (red); staining overlap is shown as yellow. Stage I neuron elaborating a thin lamellipodial veil, showing faint myosin IIA staining within the soma, as pictured in single channel grayscale. Stage II neuron extending minor processes displaying nearly undetectable levels of myosin IIA. Stage III neuron extending a nascent axon as well as dendrite precursors, all of which demonstrate extremely low levels of myosin IIA expression. (B) Forebrain neurons were cultured for 2 DIV prior to fixation and immunostaining for α-tubulin (green) and the myosin IIB isoform (red); staining overlap is shown as yellow. Similarly low levels of myosin IIB staining are evident at each developmental stage.
Myosin II inhibition increases minor process length in forebrain neurons cultured on poly-DL-lysine or laminin. (A) Forebrain neurons were maintained for 2 DIV on glass coverslips pre-coated with poly-DL-lysine (0.1 mg/mL), laminin (25 µg/mL; Invitrogen), or poly-DL-lysine overlaid with laminin, and the proportion of neuronal morphologies was determined for each treatment population. The distribution of neurons was shifted toward a larger proportion of unipolar and bipolar morphologies on laminin (n = 18 fields, 2253 neurons), as compared to more mature morphological stages on poly-DL-lysine alone (n = 14, 15365) or overlaid with laminin (n = 12, 10063). (B) Forebrain neurons cultured on poly-DL-lysine, laminin, or poly-DL-lysine overlaid with laminin were treated with 50 µM blebbistatin or DMSO vehicle control for 2 DIV, then fixed and immunostained for α-tubulin and F-actin to reveal minor processes of stage II neurons. Quantification of minor process length showed an increase with blebbistatin treatment relative to DMSO controls, with greater increases achieved on poly-DL-lysine (n = 891 DMSO processes, 745 Bleb processes), or poly-DL-lysine plus laminin (n = 446, 361), as compared to laminin alone (n = 77, 163). **p ≤ 0.01, ***p ≤ 0.0001, Welch t-test. Error bars indicate SEM.
Myosin II inhibition produces long-term increases in the length of both axons and dendrites. (A–D) Embryonic hippocampal cultures were maintained for up to 9 DIV, with 50% of medium exchanged every 3 days, prior to fixation and immunostaining for tyrosinated tubulin (green) and phosphorylated neurofilaments (red) to identify axons. Pseudo-color merged images of multiple neurons reveal axons as yellow (arrowheads) while dendrites are green. (A) Wild type + DMSO, (B) Wild type + Bleb, (C) Myosin IIB knockout + DMSO, (D) Myosin IIB knockout + Bleb. Although axon specification was not affected by myosin II inhibition, both axons and dendrites continue to grow longer at later stages of development. (E) Embryonic forebrain or hippocampal cultures were maintained for 2 DIV prior to fixation and immunostaining to reveal neurites. Quantification of stage III forebrain neurites showed an increase in both minor process and axon lengths with chronic blebbistatin exposure (n = 97 neurons), relative to DMSO controls (n = 99; mp mean = 18.5 ± 0.2 µm, axon mean = 87.7 ± 2.6 µm). Similarly, measurement of stage III hippocampal neurites revealed increases in both minor process (maturing dendrite) and axon lengths with ongoing blebbistatin treatment (n = 105 neurons) as compared to DMSO controls (n = 104; mp mean = 21.0 ± 0.3 µm, axon mean = 167.8 ± 5.9 µm). Data are presented normalized to control values. ***p ≤ 0.0001, ****p ≤ 0.00001, Welch t-test. Error bars indicate SEM.
F-Actin disruption changes minor process morphology. Forebrain neuron cultures were treated 30 minutes after plating, allowing neuron-substrate adhesion, with 50 µM blebbistatin, F-actin-depolymerizing concentrations of latrunculin A (4 µM), latrunculin plus blebbistatin, or DMSO vehicle control for 2 DIV, then fixed and immunostained for α-tubulin (green) and F-actin (red) to reveal minor processes. Both DMSO and blebbistatin treated neurons displayed a dense array of microtubules and F-actin distributed throughout minor processes, often concentrated distally. Blebbistatin-treated neurons generated longer, curving processes with more condensed tips as compared to DMSO controls. Latrunculin treatment produced curving minor processes with regions of splayed microtubules (arrowhead and inset) and disorganized F-actin puncta (arrowhead and inset). Latrunculin plus blebbistatin treatment produced straight and thin elongated processes, with small regions of F-actin enrichment frequently observed at minor process tips (arrowheads) but largely excluded from the process shafts. Note that F-actin image is saturated to reveal thin minor processes.
F-actin disruption shifts the distribution of forebrain morphologies to more immature stages. Forebrain neuron cultures were treated 30 minutes after plating with 50 µM blebbistatin, 4 µM latrunculin A, latrunculin plus blebbistatin, 6 µM jasplakinolide, jasplakinolide plus blebbistatin, or DMSO vehicle control for 2 DIV, then fixed and immunostained for α-tubulin. The proportion of neuronal morphologies was determined for each treatment population. The distribution of neurons was shifted toward a larger proportion of stage I, unipolar, and bipolar morphologies with F-actin disruption produced by latrunculin (n = 1152 neurons), latrunculin plus blebbistatin (n = 3040), jasplakinolide (n = 1935), or jasplakinolide plus blebbistatin (n = 2872), as compared to more mature morphological stages attained by blebbistatin (n = 2144) or DMSO (n = 2487). Blebbistatin added to latrunculin did not change the distribution of neuronal morphologies seen with latrunculin alone, while blebbistatin addition to jasplakinolide increased the proportion of stage II and III neurons observed relative to jasplakinolide treatment alone.
Minor process dynamics at 1 DIV following acute DMSO treatment. Forebrain neurons were plated and allowed to develop for 1 DIV prior to imaging. Following a 30 minute reference imaging period, cultures were treated with DMSO, and observed by time-lapse video microscopy for one hour of development, using 15 second interframe interval capture rates. No differences in process dynamics were seen before and after DMSO addition. Minor processes remain dynamic, exhibiting bouts of extension and retraction with slight overall advance during the imaging period. Similar dynamics were also evident in forebrain cultures allowed to develop for 2 DIV prior to DMSO addition and imaging.
Minor process dynamics at 1 DIV following acute blebbistatin treatment. Forebrain neurons were plated and allowed to develop for 1 DIV prior to imaging. Following a 30 minute reference imaging period, cultures were treated with blebbistatin, and observed by time-lapse video microscopy for one hour of development, using 15 second interframe interval capture rates. Within one minute of blebbistatin addition, the majority of minor processes begin to extend rapidly and steadily, advancing significantly during the imaging period. Similar dynamics were also evident in forebrain cultures allowed to develop for 2 DIV prior to blebbistatin addition and imaging.
Minor process dynamics at 1 DIV following chronic blebbistatin treatment. Forebrain neurons were plated in blebbistatin-containing medium and allowed to develop for 1 DIV prior to imaging. Cultures then were observed by time-lapse video microscopy for one hour of development, with continued blebbistatin treatment, using 15 second interframe interval capture rates. While slight minor process elongation occurs within the imaging period, growth rates are significantly lower than those observed following acute blebbistatin treatment, and close to growth rates in DMSO treated cultures. Similar dynamics were also evident in forebrain cultures allowed to develop with blebbistatin treatment for 2 DIV prior to imaging.
Acknowledgements
Supported by NIH NS048090 (Gallo), NS026150 (Bridgman), and NRSA Postdoctoral Training Grant # 8102122 (Kollins)
The authors wish to acknowledge Dr. Robert Loudon for preparing subcloned WT and CA-MLCK constructs, Jennifer Huang for preliminary substratum data, and Grady Phillips for providing technical support.
REFERENCES
- Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- Bao J, Ma X, Liu C, Adelstein R. Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice. J Biol Chem. 2007;282:22102–22111. doi: 10.1074/jbc.M702731200. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Bellion A, Baudoin JP, Alvarez C, Bornens M, Metin C. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J Neurosci. 2005;25:5691–5699. doi: 10.1523/JNEUROSCI.1030-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Hamao K, Dembo M, Wang YL, Hosoya H. Traction forces of fibroblasts are regulated by the Rho-dependent kinase but not by the myosin light chain kinase. Arch Biochem Biophys. 2006;456:224–231. doi: 10.1016/j.abb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenfeld J, Betz H, Roth D. Inhibition of neurite extension by overexpression of individual domains of LIM kinase 1. J Neurochem. 2001;78:924–927. doi: 10.1046/j.1471-4159.2001.00500.x. [DOI] [PubMed] [Google Scholar]
- Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron. 2000;26:431–441. doi: 10.1016/s0896-6273(00)81175-7. [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr Opin Neurobiol. 2000a;10:574–581. doi: 10.1016/s0959-4388(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. Changes in membrane trafficking and actin dynamics during axon formation in cultured hippocampal neurons. Microsc Res Tech. 2000b;48:3–11. doi: 10.1002/(SICI)1097-0029(20000101)48:1<3::AID-JEMT2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Brown ME, Bridgman PC. Myosin function in nervous and sensory systems. J Neurobiol. 2004;58:118–130. doi: 10.1002/neu.10285. [DOI] [PubMed] [Google Scholar]
- Calderon de Anda F, Gartner A, Tsai LH, Dotti CG. Pyramidal neuron polarity axis is defined at the bipolar stage. J Cell Sci. 2008;121:178–185. doi: 10.1242/jcs.023143. [DOI] [PubMed] [Google Scholar]
- Chada S, Lamoureux P, Buxbaum RE, Heidemann SR. Cytomechanics of neurite outgrowth from chick brain neurons. J Cell Sci. 1997;110(Pt 10):1179–1186. doi: 10.1242/jcs.110.10.1179. [DOI] [PubMed] [Google Scholar]
- Chantler PD, Wylie SR. Elucidation of the separate roles of myosins IIA and IIB during neurite outgrowth, adhesion and retraction. IEE Proc Nanobiotechnol. 2003;150:111–125. doi: 10.1049/ip-nbt:20031076. [DOI] [PubMed] [Google Scholar]
- Conti MA, Adelstein RS. Nonmuscle myosin moves in new directions. J Cell Sci. 2008;121:11–18. doi: 10.1242/jcs.007112. [DOI] [PubMed] [Google Scholar]
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Da Silva JS, Dotti CG. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci. 2002;3:694–704. doi: 10.1038/nrn918. [DOI] [PubMed] [Google Scholar]
- Da Silva JS, Medina M, Zuliani C, Di Nardo A, Witke W, Dotti CG. RhoA/ROCK regulation of neuritogenesis via profilin IIa-mediated control of actin stability. J Cell Biol. 2003;162:1267–1279. doi: 10.1083/jcb.200304021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Smart FM, Ozer RS, Halpain S. The role of microtubule-assiciated protein 2c in the reorganization of MTs and lamellipodia during neurite initiation. J Neurosci. 2003;29:9479–9990. doi: 10.1523/JNEUROSCI.23-29-09479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. Actin and microtubules in neurite initiation: are MAPs the missing link? J Neurobiol. 2004;58:18–33. doi: 10.1002/neu.10284. [DOI] [PubMed] [Google Scholar]
- Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Dent EW, Kwiatkowski AV, Mebane LM, Philippar U, Barzik M, Rubinson DA, Gupton S, Van Veen JE, Furman C, Zhang J, Alberts AS, Mori S, Gertler FB. Filopodia are required for cortical neurite initiation. Nat Cell Biol. 2007;9:1347–1359. doi: 10.1038/ncb1654. [DOI] [PubMed] [Google Scholar]
- Gallagher ED, Gutowski S, Sternweis PC, Cobb MH. RhoA binds to the amino terminus of MEKK1 and regulates its kinase activity. J Biol Chem. 2004;279:1872–1877. doi: 10.1074/jbc.M309525200. [DOI] [PubMed] [Google Scholar]
- Gallo G. Myosin II activity is required for severing-induced axon retraction in vitro. Exp Neurol. 2004;189:112–121. doi: 10.1016/j.expneurol.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Gallo G, Yee HF, Jr, Letourneau PC. Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J Cell Biol. 2002;158:1219–1228. doi: 10.1083/jcb.200204140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG, Goldin E, Conti MA, Sellers JR, Adelstein RS. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J Biol Chem. 2004;279:2800–2808. doi: 10.1074/jbc.M309981200. [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol. 1989;108:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Heidemann SR, Reynolds M, Ngo K, Lamoureux P. The culture of chick forebrain neurons. Methods Cell Biol. 2003;71:51–65. doi: 10.1016/s0091-679x(03)01004-5. [DOI] [PubMed] [Google Scholar]
- Jacobson C, Schnapp B, Banker GA. A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron. 2006;49:797–804. doi: 10.1016/j.neuron.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Ketschek AR, Jones SL, Gallo G. Axon extension in the fast and slow lanes: substratum-dependent engagement of myosin II functions. Dev Neurobiol. 2007;67:1305–1320. doi: 10.1002/dneu.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Fritz JL, Seneviratne AK, VanBerkum MF. Constitutively active myosin light chain kinase alters axon guidance decisions in Drosophila embryos. Dev Biol. 2002;249:367–381. doi: 10.1006/dbio.2002.0768. [DOI] [PubMed] [Google Scholar]
- Kim Y, Chang S. Modulation of actomyosin contractility by myosin light chain phosphorylation/dephosphorylation through Rho GTPases signaling specifies axon formation in neurons. Biochem Biophys Res Commun. 2004;318:579–587. doi: 10.1016/j.bbrc.2004.04.068. [DOI] [PubMed] [Google Scholar]
- Koh CG. Rho GTPases and their regulators in neuronal functions and development. Neurosignals. 2006;15:228–237. doi: 10.1159/000101527. [DOI] [PubMed] [Google Scholar]
- Kostic A, Sap J, Sheetz M. RPTPα is required for rigidity-dependent inhibition of extension and differentiation of hippocampal neurons. J Cell Sci. 2007;120:3895–3904. doi: 10.1242/jcs.009852. [DOI] [PubMed] [Google Scholar]
- Kubo T, Endo M, Hata K, Taniguchi J, Kitajo K, Tomura S, Yamaguchi A, Mueller BK, Yamashita T. Myosin IIA is required for neurite outgrowth inhibition produced by repulsive guidance molecule. J Neurochem. 2008;105:113–126. doi: 10.1111/j.1471-4159.2007.05125.x. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski AV, Rubinson DA, Dent EW, Edward van Veen J, Leslie JD, Zhang J, Mebane LM, Philippar U, Pinheiro EM, Burds AA, Bronson RT, Mori S, Fassler R, Gertler FB. Ena/VASP is Required for neuritogenesis in the developing cortex. Neuron. 2007;56:441–455. doi: 10.1016/j.neuron.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Lamoureux P, Ruthel G, Buxbaum RE, Heidemann SR. Mechanical tension can specify axonal fate in hippocampal neurons. J Cell Biol. 2002;159:499–508. doi: 10.1083/jcb.200207174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC, Shattuck TA, Ressler AH. “Pull” and “push” in neurite elongation: observations on the effects of different concentrations of cytochalasin B and taxol. Cell Motil Cyto. 1987;8:193–209. doi: 10.1002/cm.970080302. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Bai J. The multipolar stage and disruptions in neuronal migration. Trends Neurosci. 2006;29:407–413. doi: 10.1016/j.tins.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Loudon RP, Silver LD, Yee HF, Jr, Gallo G. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol. 2006;66:847–867. doi: 10.1002/neu.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- Ma X, Bao J, Adelstein R. Loss of cell adhesion causes hydrocephalus in nonmuscle myosin II-B-ablated and mutated mice. Mol Biol Cell. 2007;18:2305–2312. doi: 10.1091/mbc.E07-01-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L, Letourneau PC. Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J Cell Biol. 1984;99:2041–2047. doi: 10.1083/jcb.99.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- Metin C, Baudoin JP, Rakic S, Parnavelas JG. Cell and molecular mechanisms involved in the migration of cortical interneurons. Eur J Neurosci. 2006;23:894–900. doi: 10.1111/j.1460-9568.2006.04630.x. [DOI] [PubMed] [Google Scholar]
- Ng J, Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron. 2004;44:779–793. doi: 10.1016/j.neuron.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol. 2005;7:270–277. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Rochlin MW, Wickline KM, Bridgman PC. Microtubule stability decreases axon elongation but not axoplasm production. J Neurosci. 1996;16:3236–3246. doi: 10.1523/JNEUROSCI.16-10-03236.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösner H, Moller W, Wassermann T, Mihatsch J, Blum M. Attenuation of actinomyosin II contractile activity in growth cones accelerates filopodia-guided and microtubule-based neurite elongation. Brain Res. 2007;1176:1–10. doi: 10.1016/j.brainres.2007.07.081. [DOI] [PubMed] [Google Scholar]
- Ryu J, Liu L, Wong TP, Wu DC, Burette A, Weinberg R, Wang YT, Sheng M. A critical role for myosin IIb in dendritic spine morphology and synaptic function. Neuron. 2006;49:175–182. doi: 10.1016/j.neuron.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Govek EE, Tomoda T, Hatten ME. Neuronal polarity in CNS development. Genes Dev. 2006;20:2639–2647. doi: 10.1101/gad.1462506. [DOI] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Totsukawa G, Wu Y, Sasaki Y, Hartshorne DJ, Yamakita Y, Yamashiro S, Matsumura F. Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. J Cell Biol. 2004;164:427–439. doi: 10.1083/jcb.200306172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullio AN, Bridgman PC, Tresser NJ, Chan CC, Conti MA, Adelstein RS, Hara Y. Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscule myosin II-B heavy chain. J Comp Neurol. 2001;433:62–74. doi: 10.1002/cne.1125. [DOI] [PubMed] [Google Scholar]
- Verkhovsky AB, Svitkina TM, Borisy GG. Polarity sorting of actin filaments in cytochalasin-treated fibroblasts. J Cell Sci. 1997;110:1693–1704. doi: 10.1242/jcs.110.15.1693. [DOI] [PubMed] [Google Scholar]
- Wiggin GR, Fawcett JP, Pawson T. Polarity proteins in axon specification and synaptogenesis. Dev Cell. 2005;8:803–816. doi: 10.1016/j.devcel.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Wylie SR, Chantler PD. Separate but linked functions of conventional myosins modulate adhesion and neurite outgrowth. Nat Cell Biol. 2001;3:88–92. doi: 10.1038/35050613. [DOI] [PubMed] [Google Scholar]
- Wylie SR, Chantler PD. Myosin IIA drives neurite retraction. Mol Biol Cell. 2003;14:4654–4666. doi: 10.1091/mbc.E03-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Arimura N, Kaibuchi K. Signaling networks in neuronal polarization. J Neurosci. 2006;26:10626–10630. doi: 10.1523/JNEUROSCI.3824-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Levels of myosin II reactivity in hippocampal neurons and forebrain neurons developing in vitro. (A) Hippocampal neuron cultures were maintained for 2 DIV prior to fixation and immunostaining for α-tubulin (green) and the myosin IIA isoform (red); staining overlap is shown as yellow. Stage I neuron elaborating a thin lamellipodial veil, showing faint myosin IIA staining within the soma, as pictured in single channel grayscale. Stage II neuron extending minor processes displaying nearly undetectable levels of myosin IIA. Stage III neuron extending a nascent axon as well as dendrite precursors, all of which demonstrate extremely low levels of myosin IIA expression. (B) Forebrain neurons were cultured for 2 DIV prior to fixation and immunostaining for α-tubulin (green) and the myosin IIB isoform (red); staining overlap is shown as yellow. Similarly low levels of myosin IIB staining are evident at each developmental stage.
Myosin II inhibition increases minor process length in forebrain neurons cultured on poly-DL-lysine or laminin. (A) Forebrain neurons were maintained for 2 DIV on glass coverslips pre-coated with poly-DL-lysine (0.1 mg/mL), laminin (25 µg/mL; Invitrogen), or poly-DL-lysine overlaid with laminin, and the proportion of neuronal morphologies was determined for each treatment population. The distribution of neurons was shifted toward a larger proportion of unipolar and bipolar morphologies on laminin (n = 18 fields, 2253 neurons), as compared to more mature morphological stages on poly-DL-lysine alone (n = 14, 15365) or overlaid with laminin (n = 12, 10063). (B) Forebrain neurons cultured on poly-DL-lysine, laminin, or poly-DL-lysine overlaid with laminin were treated with 50 µM blebbistatin or DMSO vehicle control for 2 DIV, then fixed and immunostained for α-tubulin and F-actin to reveal minor processes of stage II neurons. Quantification of minor process length showed an increase with blebbistatin treatment relative to DMSO controls, with greater increases achieved on poly-DL-lysine (n = 891 DMSO processes, 745 Bleb processes), or poly-DL-lysine plus laminin (n = 446, 361), as compared to laminin alone (n = 77, 163). **p ≤ 0.01, ***p ≤ 0.0001, Welch t-test. Error bars indicate SEM.
Myosin II inhibition produces long-term increases in the length of both axons and dendrites. (A–D) Embryonic hippocampal cultures were maintained for up to 9 DIV, with 50% of medium exchanged every 3 days, prior to fixation and immunostaining for tyrosinated tubulin (green) and phosphorylated neurofilaments (red) to identify axons. Pseudo-color merged images of multiple neurons reveal axons as yellow (arrowheads) while dendrites are green. (A) Wild type + DMSO, (B) Wild type + Bleb, (C) Myosin IIB knockout + DMSO, (D) Myosin IIB knockout + Bleb. Although axon specification was not affected by myosin II inhibition, both axons and dendrites continue to grow longer at later stages of development. (E) Embryonic forebrain or hippocampal cultures were maintained for 2 DIV prior to fixation and immunostaining to reveal neurites. Quantification of stage III forebrain neurites showed an increase in both minor process and axon lengths with chronic blebbistatin exposure (n = 97 neurons), relative to DMSO controls (n = 99; mp mean = 18.5 ± 0.2 µm, axon mean = 87.7 ± 2.6 µm). Similarly, measurement of stage III hippocampal neurites revealed increases in both minor process (maturing dendrite) and axon lengths with ongoing blebbistatin treatment (n = 105 neurons) as compared to DMSO controls (n = 104; mp mean = 21.0 ± 0.3 µm, axon mean = 167.8 ± 5.9 µm). Data are presented normalized to control values. ***p ≤ 0.0001, ****p ≤ 0.00001, Welch t-test. Error bars indicate SEM.
F-Actin disruption changes minor process morphology. Forebrain neuron cultures were treated 30 minutes after plating, allowing neuron-substrate adhesion, with 50 µM blebbistatin, F-actin-depolymerizing concentrations of latrunculin A (4 µM), latrunculin plus blebbistatin, or DMSO vehicle control for 2 DIV, then fixed and immunostained for α-tubulin (green) and F-actin (red) to reveal minor processes. Both DMSO and blebbistatin treated neurons displayed a dense array of microtubules and F-actin distributed throughout minor processes, often concentrated distally. Blebbistatin-treated neurons generated longer, curving processes with more condensed tips as compared to DMSO controls. Latrunculin treatment produced curving minor processes with regions of splayed microtubules (arrowhead and inset) and disorganized F-actin puncta (arrowhead and inset). Latrunculin plus blebbistatin treatment produced straight and thin elongated processes, with small regions of F-actin enrichment frequently observed at minor process tips (arrowheads) but largely excluded from the process shafts. Note that F-actin image is saturated to reveal thin minor processes.
F-actin disruption shifts the distribution of forebrain morphologies to more immature stages. Forebrain neuron cultures were treated 30 minutes after plating with 50 µM blebbistatin, 4 µM latrunculin A, latrunculin plus blebbistatin, 6 µM jasplakinolide, jasplakinolide plus blebbistatin, or DMSO vehicle control for 2 DIV, then fixed and immunostained for α-tubulin. The proportion of neuronal morphologies was determined for each treatment population. The distribution of neurons was shifted toward a larger proportion of stage I, unipolar, and bipolar morphologies with F-actin disruption produced by latrunculin (n = 1152 neurons), latrunculin plus blebbistatin (n = 3040), jasplakinolide (n = 1935), or jasplakinolide plus blebbistatin (n = 2872), as compared to more mature morphological stages attained by blebbistatin (n = 2144) or DMSO (n = 2487). Blebbistatin added to latrunculin did not change the distribution of neuronal morphologies seen with latrunculin alone, while blebbistatin addition to jasplakinolide increased the proportion of stage II and III neurons observed relative to jasplakinolide treatment alone.
Minor process dynamics at 1 DIV following acute DMSO treatment. Forebrain neurons were plated and allowed to develop for 1 DIV prior to imaging. Following a 30 minute reference imaging period, cultures were treated with DMSO, and observed by time-lapse video microscopy for one hour of development, using 15 second interframe interval capture rates. No differences in process dynamics were seen before and after DMSO addition. Minor processes remain dynamic, exhibiting bouts of extension and retraction with slight overall advance during the imaging period. Similar dynamics were also evident in forebrain cultures allowed to develop for 2 DIV prior to DMSO addition and imaging.
Minor process dynamics at 1 DIV following acute blebbistatin treatment. Forebrain neurons were plated and allowed to develop for 1 DIV prior to imaging. Following a 30 minute reference imaging period, cultures were treated with blebbistatin, and observed by time-lapse video microscopy for one hour of development, using 15 second interframe interval capture rates. Within one minute of blebbistatin addition, the majority of minor processes begin to extend rapidly and steadily, advancing significantly during the imaging period. Similar dynamics were also evident in forebrain cultures allowed to develop for 2 DIV prior to blebbistatin addition and imaging.
Minor process dynamics at 1 DIV following chronic blebbistatin treatment. Forebrain neurons were plated in blebbistatin-containing medium and allowed to develop for 1 DIV prior to imaging. Cultures then were observed by time-lapse video microscopy for one hour of development, with continued blebbistatin treatment, using 15 second interframe interval capture rates. While slight minor process elongation occurs within the imaging period, growth rates are significantly lower than those observed following acute blebbistatin treatment, and close to growth rates in DMSO treated cultures. Similar dynamics were also evident in forebrain cultures allowed to develop with blebbistatin treatment for 2 DIV prior to imaging.