Abstract
Background
Characterization of the behavioral correlates of neuromorphometry and neurochemistry in older adults has important implications for an improved understanding of the aging process. The objective of this study was to test the hypothesis that a measure of hippocampal neuronal metabolism was associated with verbal memory in nondemented older adults after controlling for hippocampal volume.
Methods
4-T MRI, proton magnetic resonance spectroscopy (1H MRS), and neuropsychological assessment were conducted in 48 older adults (23 women; mean age 81 years). Average hippocampal N-acetyl aspartate/creatine ratios (NAA/Cr) and hippocampal volumes were obtained. Neuropsychological evaluation included tests of verbal memory (Buschke and Grober Free and Cued Selective Reminding Test–Immediate Recall [FCSRT-IR], Wechsler Memory Scale–Revised Logical Memory subtest) and attention and executive function (Trail Making Test Parts A and B).
Results
Linear regression analysis indicated that after adjusting for age, hippocampal NAA/Cr was a significant predictor of FCSRT-IR performance (β = 0.38, p = 0.01, R 2 = 0.21). Hippocampal volume was also a significant predictor of FCSRT-IR performance after adjusting for age and midsagittal area (β = 0.47, p = 0.01, R 2 = 0.24). In a combined model, hippocampal NAA/Cr (β = 0.33, p = 0.03) and volume (β = 0.35, p = 0.03) were independent predictors of FCSRT-IR performance, accounting for 30% of the variance in memory.
Conclusions
These findings indicate that nondemented older adults with smaller hippocampal volumes and lower levels of hippocampal N-acetyl aspartate/creatine ratio metabolites perform more poorly on a test of verbal memory. The integrity of both the structure and metabolism of the hippocampus may underlie verbal memory function in nondemented elderly.
With advancing age, healthy adults exhibit decline in verbal memory abilities1,2 and hippocampal volumetric decreases (e.g., see references 3–6) that are modestly associated (for review, see reference 7). Additionally, metabolic markers of aging have been identified using proton MRS, which probes neuronal chemical function through examination of neurotransmitters. Decreased N-acetyl aspartate and its ratio to creatine (NAA/Cr) is a robust age-associated finding in the hippocampus.8–10
Because changes in neuronal metabolism precede cell death and volume loss, hippocampal NAA/Cr measures may supplement volumetric measures for the prediction of memory performance. One study8 examining this relationship reported that normal aging is associated with changes in hippocampal structure and neurochemistry, and that these changes may impact performance on tasks of spatial memory. The goal of the current study is to expand these findings by evaluating magnetic resonance (MR-)–derived hippocampal volumes and hippocampal NAA/Cr metabolites as predictors of verbal memory performance in a community-based sample of healthy older adults. We hypothesized that anatomic (hippocampal volume) and metabolic (hippocampal NAA/Cr) measures would be independent predictors of verbal memory performance in nondemented elderly.
METHODS
Participants
Between February 2004 and August 2006, a subset of 48 older adults was drawn from the Einstein Aging Study (EAS), a community-based sample of individuals over the age of 70 years residing in the Bronx, New York. Because the primary objective of this investigation was to examine MR-derived predictors of memory function in nondemented elderly, the subset was systematically selected to obtain a range of memory performance on the Buschke and Grober Free and Cued Selective Reminding Test–Immediate Recall (FCSRT-IR; discussed further below) in older adults who did not meet diagnostic criteria for dementia based on the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition.11 In the absence of contrain-dications to MRI, all individuals with mild memory impairment (free recall FCSRT-IR ≤ 24, n = 14) and a random sample of individuals with normal memory (free recall FCSRT-IR > 24, n = 34) were invited to participate in the current study. FCSRT-IR free recall impairment cut-scores were derived from prior analyses.12,13 Of the 48 participants in the study, 8 reported a subjective memory complaint as determined by a positive response to the item “Do you feel that you have more problems with memory than most?” from the Geriatric Depression Scale.14 Of these participants with memory complaints, 6 exhibited memory within normal limits, and 2 exhibited mild memory impairment; there-fore, 2 of the 48 participants in this sample met diagnostic criteria for amnestic mild cognitive impairment (MCI).15
The study design and methods of the EAS are described elsewhere.16 Briefly, potential participants were recruited through systematic sampling from Medicare or voter registration lists for Bronx County. Participants were excluded who reported severe sensory loss or medical conditions that would interfere with completion of neuropsychological assessment, were non-English speakers, or who were institutionalized. General cognitive status was assessed with the Blessed Information–Memory–Concentration test (BIMC17). Participants were evaluated at a diagnostic case conference attended by a study neurologist, neuropsychologist, and social worker.
Magnetic resonance imaging
MRI was performed on a Varian-Magnex 4-T imaging system at the Gruss Magnetic Resonance Research Center at the Albert Einstein College of Medicine, Bronx, New York. T1-weighted inversion recovery gradient echo images were acquired with 1.5-mm slice thickness, field of view (FOV) = 240, and 160 × 160 resolution, resulting in pixel resolution of 1.5 × 1.5 × 1.5 mm3. Using planum temporale geometry, 120 slices were taken parallel to the planum temporale, resulting in 180-mm coronal–axial coverage. The images were reformatted orthogonal to the planum temporale, and volumetric measurements of the hippocampus were performed.18,19 The limit of the posterior hippocampal tail was identified from the oblique coronal image, wherein the crus of the fornix was visualized. Anteriorly, the pes hippocampus was disarticulated from the amygdala by identification of the alveus, or by extending the horizontal line defined by the uncal recess of the temporal horn. Laterally and superiorly, the temporal horn CSF demarcated the hippocampus. Medially, the uncal and ambient cistern were used. Inferiorly, the subiculum was separated from the parahippocampal gray matter with a straight line. The intracranial midsagittal area was obtained to account for individual differences in head size.20 Volumetrics were obtained by a single trained rater with high intrarater reliability (intraclass correlation coefficient = 0.99).
Magnetic resonance spectroscopy
All data were acquired at 4-T using a Varian INOVA console and quadrature head coil using a modified LASER sequence (10-mm thickness, 80 × 100-mm in-plane FOV selection) in combination with two dimensions of phase encoding (24 × 24, FOV = 192 × 192 mm, 19.2 minutes) angulating the plane along the temporal pole.21 The magnetic field homogeneity was adjusted for each subject.22 To provide reproducible selection criteria, hippocampal voxels were reconstructed using a voxel shifting method.23,24 Centers of reconstructed spectroscopic voxels were defined from structural images using anatomically defined criteria. Hippocampal boundaries were manually delineated on anatomic images, and a midline between the medial and lateral boundaries was calculated. Four loci—one positioned at the level of the aqueduct along the midline, two anterior loci, and one posterior locus—were selected by translating along the midline in 9-mm increments. These coordinates were used in voxel shifting reconstruction to provide spectroscopic voxels centered over these loci. Spectral data were obtained using a convolution difference of 250 Hz followed by 3 Hz of gaussian broadening and Fourier transformation in the spectral domain. Data were fit using gaussian line shapes, and the NAA/Cr ratio was determined by using the ratios of the resonance areas.
Neuropsychological evaluation
Based on the hypotheses of the current study, two verbal memory variables of interest were selected from the EAS comprehensive neuropsychological battery: free recall from the FCSRT-IR25,26 and immediate recall from the Wechsler Memory Scale–Revised Logical Memory subtest (LM).27 The FCSRT-IR is a verbal memory task that controls attention and strategy use to maximize learning and provide a measurement of immediate memory that is not confounded by deficits in other cognitive abilities. In the first part of the task, participants name 16 objects that are displayed individually. They are then presented with the same 16 objects and asked to identify each object following a categorical prompt. In the free recall condition, a measure of self-organized retrieval, the participant is immediately asked to recall the 16 objects. If the participant does not correctly recall an object, he or she is provided with a category cue to test cued recall. There are a total of three free and cued recall trials; scores range from 0 to 48.
LM27 is a verbal memory test in which participants are read two contextually related short stories and asked to recall story details. Scores range from 0 to 50.
The Trail Making Test28 was examined to determine the specificity of cognitive findings. TMTA is a measure of attention involving psychomotor speed and visual scanning, whereas TMTB is a measure of executive function involving set shifting and concept formation. Scores for TMTA and TMTB are provided as seconds to task completion.
Statistical analyses
Age, education, and sex, were examined as potential covariates using Pearson product–moment and Spearman rank correlation coefficients. Ethnicity was examined as a potential covariate using analysis of variance. Pearson correlation coefficients were used to examine relationships among verbal memory performance, hippocampal volume, and hippocampal NAA/Cr.
A series of linear regression analyses were performed to examine MR-derived predictors of verbal memory performance. The first linear regression examined the effect of hippocampal volume on verbal memory performance with age and midsagittal area as covariates. The second linear regression analysis examined the effect of hippocampal NAA/Cr on verbal memory performance with age as a covariate. The third linear regression analysis examined the effect of both hippocampal neurochemistry and volume on verbal memory performance with age as a covariate. Linear regressions were repeated with measures of attention and executive function as dependent variables to examine specificity of the findings.
Secondary analyses were performed to examine the potentially spurious inclusion of individuals with mild memory impairment in our sample. Discriminant function analysis was used to determine the extent to which memory group membership could be predicted by hippocampal volume and NAA/Cr values. Pearson correlation coefficients were performed to explore the relationship between memory and MR-derived variables of interest in individuals exhibiting normal memory and individuals exhibiting mild memory impairment.
RESULTS
Sample characteristics
Sample demographics, verbal memory performance, and hippocampal measurements are presented in table 1. Sex and ethnicity characteristics were consistent with those of both the larger EAS cohort and the US Census Bureau data for Bronx County. Participants performed in the average range on verbal memory tests.29,30 There were no ethnicity differences on the MR-derived or memory variables of interest.
Table 1.
Sample demographics, verbal memory performance, and hippocampal measurements
| Total sample, n = 48 |
Normal memory, n = 34 |
Mild memory impairment, n = 14 |
|
|---|---|---|---|
| Age, mean (SD), y | 81.18 (5.47) | 80.37 (5.77) | 83.14 (4.2) |
| %Women | 47.9 | 55.9 | 28.6 |
| %White | 72.9 | 73.5 | 71.4 |
| Education, mean (SD), y | 13.08 (3.11) | 13.2 (3.50) | 12.7 (1.90) |
| %Right-handed* | 97.8 | 97.1 | 100 |
| BIMC total errors, median (range) | 2.00 (0–13) | 2.00 (0–6) | 3.5 (0–13) |
| CDR score, median (range) | 0 (0–1) | 0 (0–0.5) | 0.5 (0–1) |
| FCSRT-IR free recall score, mean (SD) | 30.06 (6.80) | 33.47 (4.65) | 21.79 (2.89) |
| FCSRT-IR total recall score, mean (SD) | 47.73 (1.22) | 47.94 (0.34) | 47.21 (2.15) |
| LM total score, mean (SD) | 18.02 (7.17) | 19.7 (7.13) | 14.43 (6.01) |
| Hippocampal volume, mean (SD)† | 4.27 (0.07) | 4.40 (0.07) | 3.92 (0.01) |
| Hippocampal NAA/Cr, mean (SD) | 1.30 (0.23) | 1.36 (0.82) | 1.17 (0.93) |
MRI volumetric data are given in cubic centimeters.
Handedness data available for n = 45.
Hippocampal volumetric data available for n = 39.
BIMC = Blessed Information–Memory–Concentration test, total error score; CDR = Clinical Dementia Rating scale; FCSRT-IR = Buschke and Grober Free and Cued Selective Reminding Test–Immediate Recall; LM = Wechsler Memory Scale–Revised Logical Memory immediate recall stories A and B; NAA/Cr = N-acetyl aspartate/creatine ratio.
Separate left and right measurements were acquired for both hippocampal NAA/Cr and volume. There were no laterality differences in hippocampal NAA/Cr [t(1,47) = 0.09, p = 0.93] or volume [t(1,38) = −0.17, p = 0.85]; therefore, all left and right variables were combined (volumes) or averaged (NAA/Cr) to reduce the number of statistical comparisons. MRI-derived hippocampal volumetric and midsagittal area measurements were available for 39 and 36 individuals due to scan acquisition limitations. There were no differences in age, education, BIMC, memory performance, or hippocampal NAA/Cr between those participants who had hippocampal volumetric data and those that did not (data not shown).
Simple correlations
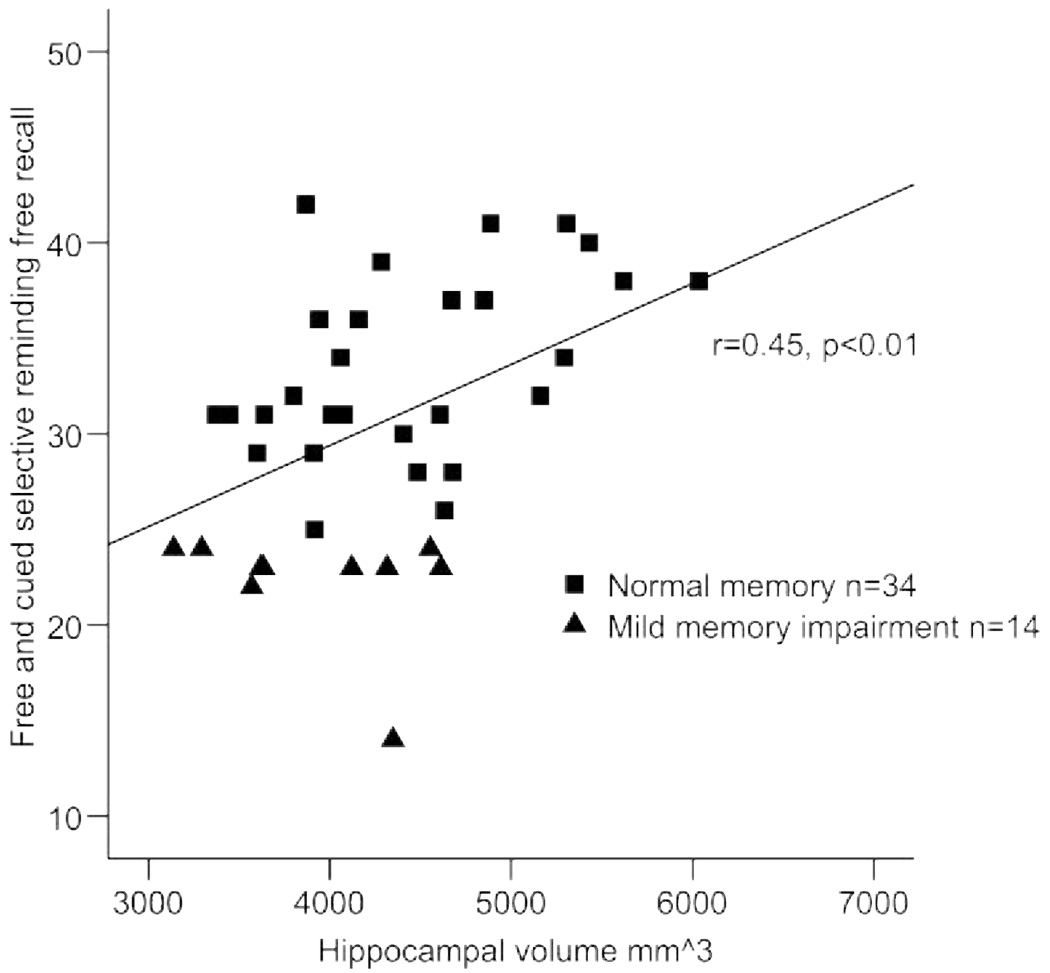
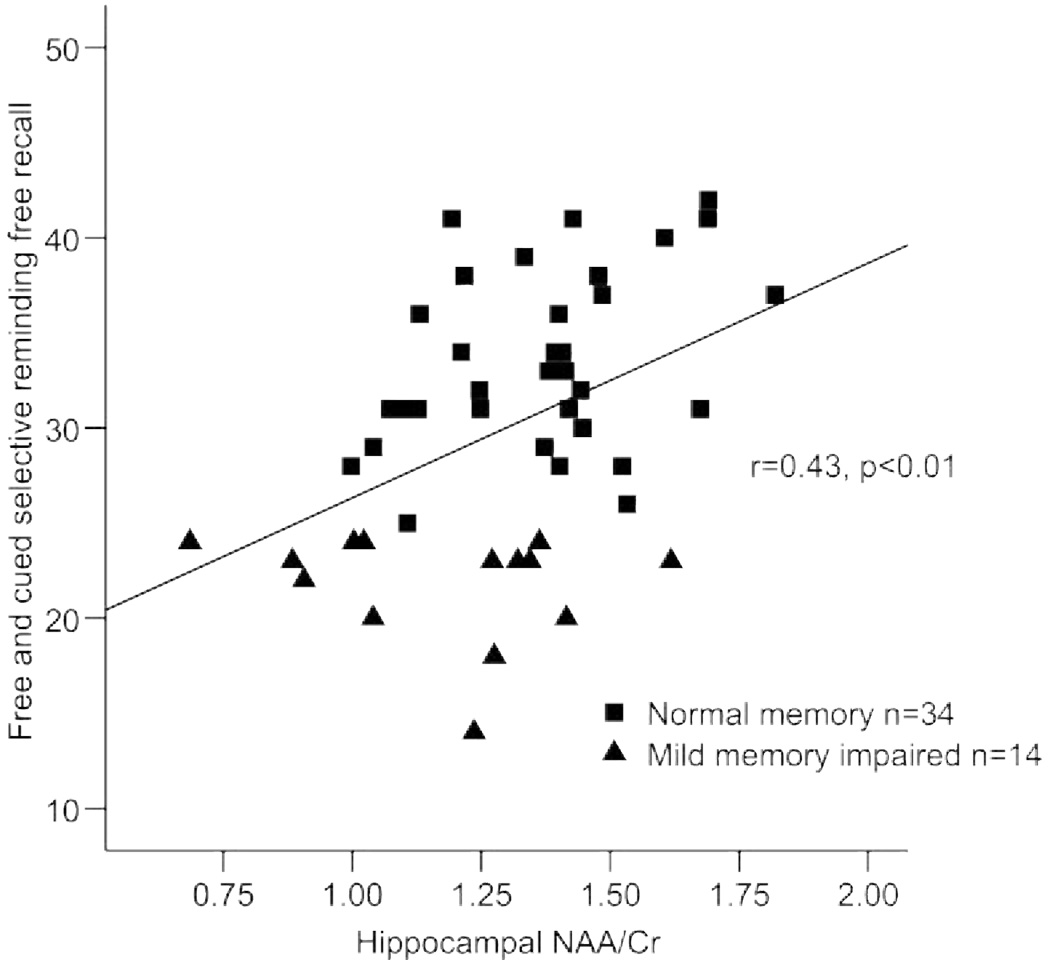
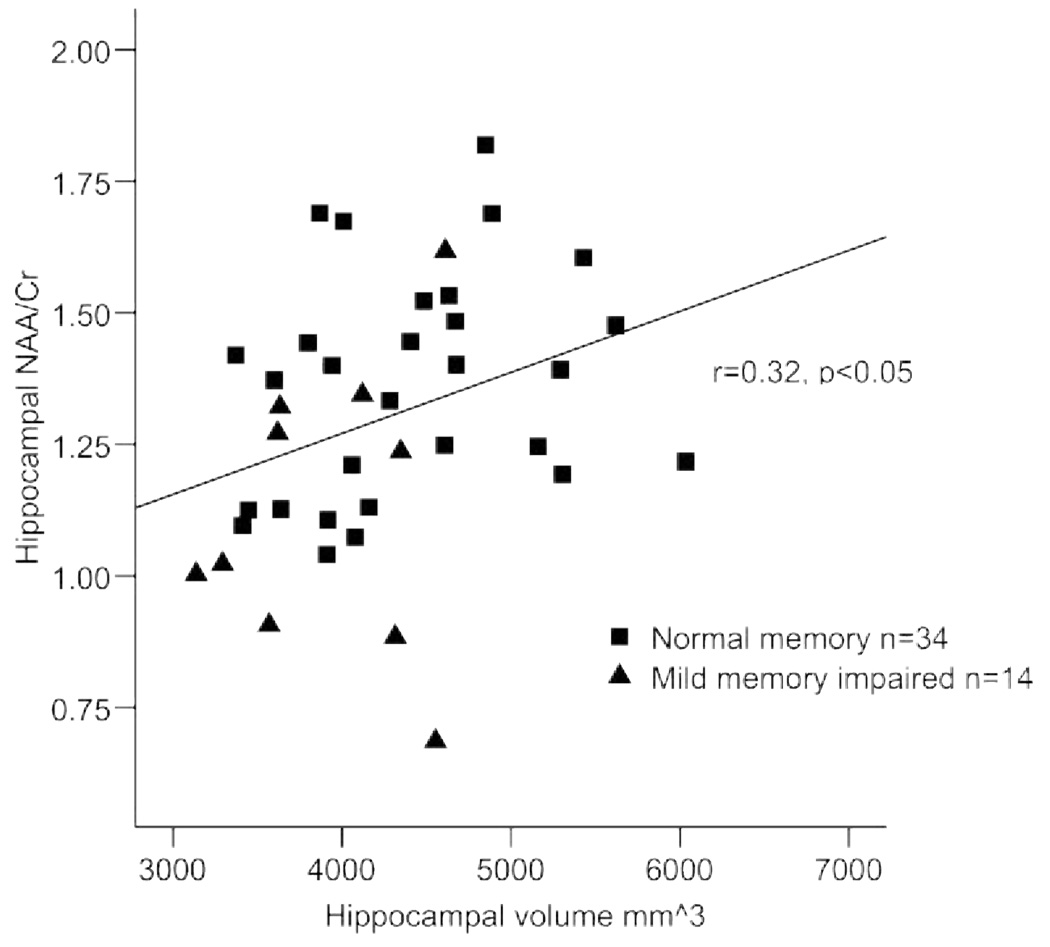
Pearson correlation coefficients revealed that age was associated with hippocampal volume (r = −0.35, p < 0.03); the strength of this relationship was similar after control of the midsagittal area (r = −0.30, p < 0.08). Individuals with smaller hippocampal volumes performed more poorly on the FCSRT-IR (r = 0.45, p < 0.01; figure 1). Individuals with lower hippocampal NAA/Cr also exhibited poorer verbal memory performance (r = 0.43, p < 0.01; figure 2). Finally, individuals with smaller hippocampal volumes exhibited lower NAA/Cr (r = 0.32, p < 0.05; figure 3). Education and sex were not associated with the primary variables of interest, with the exception of a relationship between education and LM (r = 0.39, p < 0.01).
Figure 1.
Hippocampal volume and verbal memory performance in nondemented elderly
Figure 2.
Hippocampal N-acetyl aspartate/creatine ratio (NAA = CR) and verbal memory performance in nondemented elderly
Figure 3.
Hippocampal volume and N-acetyl aspartate/creatine ratio (NAA = CR) in nondemented elderly
Hippocampal volume and verbal memory
The model of the prediction of FCSRT-IR by hippocampal volume controlling for age and midsagittal area [F(3,32) = 3.33, p < 0.03, R 2 = 0.24] revealed a significant effect for hippocampal volume (β = 0.47, t = 2.86, df = 32, p < 0.01). The model for the prediction of LM performance was not significant.
Hippocampal NAA/Cr and verbal memory
The model of the prediction of FCSRT-IR performance by hippocampal NAA/Cr controlling for age [F(2,45) = 5.90, p < 0.01, R 2 = 0.21] revealed a significant effect for hippocampal NAA/Cr (β = 0.38, t=2.78, df = 45, p < 0.01). The model for the prediction of LM performance was not significant.
Hippocampal NAA/Cr, hippocampal volume, and verbal memory
The model of the prediction of FCSRT-IR performance by hippocampal NAA/Cr and volume (F(3,35) = 5.05, p < 0.01, R 2 = 0.30) revealed significant effects for both hippocampal NAA/Cr (β = 0.33, t = 2.22, df = 35, p < 0.03) and volume (β = 0.35, t = 2.21, df = 35, p < 0.03). The model for the prediction of LM performance was not significant. Table 2 depicts a summary of all three regression models for the prediction of FCSRT-IR.
Table 2.
Regression models for the effect of age, hippocampal volume, and hippocampal NAA/Cr on Free and Cued Selective Reminding Test free recall performance
| Model no. | Predictor | R2 | Standardized β | p Value |
|---|---|---|---|---|
| 1 | Age | 0.24 | 0.00 | 0.99 |
| Midsagittal area | 0.10 | 0.55 | ||
| Hippocampal volume | 0.47 | 0.01† | ||
| 2 | Age | 0.21 | −0.17 | 0.23 |
| Hippocampal NAA/Cr | 0.38 | 0.01† | ||
| 3 | Age | 0.30 | 0.01 | 0.96 |
| Hippocampal volume | 0.35 | 0.03* | ||
| Hippocampal NAA/Cr | 0.33 | 0.03* |
Significant at the p < 0.05 level.
Significant at the p < 0.01 level.
NAA/Cr = N-acetyl aspartate/creatine ratio.
Specificity of memory findings
Replication of analyses with measures of attention and executive function as dependent variables revealed nonsignificant models.
Memory impairment
As described previously, 34 participants exhibited normal memory performance and 14 participants exhibited mild memory impairment. Of the 14 mildly memory-impaired individuals, 2 reported a memory complaint and met diagnostic criteria for amnestic MCI.15 Linear regressions were repeated with the removal of the MCI participants, and results remained unchanged. Discriminant function analysis revealed a significant model for the prediction of memory group by hippocampal volume and NAA/Cr (Wilks lambda = 0.80, p < 0.02). The unstandardized canonical discriminant function coefficient was 3.46 for hippocampal NAA/Cr and 0.001 for hippocampal volume with a constant of −7.39. Classification results indicated that 71.8% of individuals were correctly classified by memory group by hippocampal volume and NAA/Cr: 72.4% of the participants with normal memory and 70.0% of the participants with mild memory impairment. Pearson correlation analyses indicated that poorer verbal memory performance was associated with lower hippocampal NAA/Cr (r = 0.38, p < 0.03) and smaller hippocampal volumes (r = 0.42, p < 0.03) in elderly individuals with normal memory performance. However, there was no relationship between memory performance and hippocampal NAA/Cr (r = −0.19, p < 0.51) or volume (r = −0.35, p < 0.40) in participants with mild memory impairment.
DISCUSSION
The goal of this study was to examine hippocampal neuromorphometric and neurochemical predictors of verbal memory performance in a well-characterized sample of nondemented older adults. Our findings indicate that poorer performance on a cued list-learning verbal memory test was associated with smaller hippocampal volumes and lower levels of hippocampal NAA/Cr. When considered in concert, both measurements of hippocampal volume and neurochemistry were independent predictors of verbal memory that accounted for 30% of the variance in memory performance. This finding suggests that both the structural and metabolic integrity of the hippocampus support declarative memory abilities in nondemented elderly and that each of these biologic measures uniquely strengthens the ability to predict verbal memory performance. Further, these results seem to be specific to verbal memory performance as measured by the FCSRT-IR, because hippocampal volume and NAA/Cr were not significantly related to other cognitive measures of attention or executive function.
Investigations of the relationship between MRI-derived hippocampal volumes and memory performance have yielded modest associations in healthy older adult samples (for review, see references 7 and 31). In our sample of nondemented older adults, we demonstrate a relationship between hippocampal volume and performance on FCSRT-IR; individuals with smaller hippocampal volumes exhibited poorer free recall on a test of verbal memory. In addition, several studies have reported relationships between hippocampal metabolic markers of neuronal integrity and performance on navigational tasks.8 Previous studies have also demonstrated that medial temporal lobe metabolic markers are correlated with lower verbal memory performance in individuals with mild memory impairment32 and increase after memory training in healthy older adults.33 Our findings indicated that nondemented older adults with lower NAA/Cr exhibited poorer free recall on a test of verbal memory.
Previous studies10,34 have reported relationships between brain metabolites and brain volume in the elderly, yet only one8 has specifically examined the relationship among age-related memory performance and the neurochemistry and volume of the hippocampus. The authors reported that older adults demonstrated decreased volumes, lower NAA/Cr, and poorer performance on tests of spatial memory and configural learning. Better performance on cognitive tasks was significantly associated with hippocampal volume and NAA/Cr. In addition, NAA/Cr was a significant predictor of spatial memory, whereas NAA/Cr and hippocampal volume were significant predictors of configural learning. In the current study, we examined a larger sample of older adults and performance on a verbal memory measure and found that both hippocampal volume and hippocampal NAA/Cr are significant predictors of performance on a verbal memory task. These findings both support and extend those of the aforementioned study,8 suggesting that both hippocampal structure and function support declarative memory processes in nondemented older adults.
Additional discussion should be given to the specificity of our finding that hippocampal volume and NAA/Cr were related to performance on one measure of immediate verbal memory, FCSRT-IR, but not another, LM. This finding may be unexpected given the classic view of the hippocampus as supportive of delayed memory rather than immediate memory performance (e.g., see reference 35). The FCSRT-IR is a unique memory test that uses controlled attention and information processing to maximize learning.36,37 FCSRT performance has been shown to have high discriminative validity for the diagnosis of dementia (e.g., see reference 38) and for prediction of the development of future dementia.12 LM, conversely, uses an oral presentation of a short story to examine the contribution of a meaningful context to an individual’s ability to learn and retrieve verbal information.27,37 Although both tasks are measures of immediate declarative verbal memory, the FCSRT-IR provides a measure of memory that is not confounded by potential difficulties in attention or executive function. It is notable that we observed consistently significant findings in performance on free recall of the FCSRT-IR, but not LM, because this may provide additional information regarding the complex nature of hippocampal structure and function relationships. Our findings suggest that hippocampal volume and NAA/Cr are predictors of performance on a cued list-learning verbal memory task that controls for attention and strategy use, but not of performance on a verbal memory test of contextually related information.
There are several limitations to the current study. Our MR scanning protocol focused on the examination of hippocampal volumetry and NAA/Cr. Thus, the neuroanatomic specificity of our findings cannot be determined with the design of our study. Similarly, our sample was drawn from a larger study of communitydwelling older adults, and we were not able to examine the relationship among our variables of interest across the entire adult lifespan or in individuals with clinical diagnoses (e.g., dementia). An important consideration for future investigations is that it is possible that the observed associations may vary as a function of both age and cognitive status. Finally, our sample comprised elderly individuals with both normal memory performance and mild memory impairment. Additional analyses examining potential confounds associated with the clinical heterogeneity of our sample indicated that whereas significant associations between verbal memory performance and MR-derived hippocampal measures remained evident in individuals with normal memory, there were no relationships between these variables in the memory-impaired group. It is possible that the lack of a relationship between these variables in the latter group is due to truncated ascertainment of group membership, which likely resulted in diminished variability in memory performance. Nonetheless, this pattern of results lends confidence to the interpretation of our findings in the larger sample of nondemented elderly and suggests that the observed relationships were not simply an artifact of memory group status. Additional investigation is imperative to further characterize the complex relationships between memory performance and its biologic substrates across the spectrum of healthy, preclinical, and pathologic aging.
ACKNOWLEDGMENT
The authors thank Charlotte Magnotta for assistance with participant recruitment; Danielle Coyle, Betty Forro, Alicia Gomez, and Mary Joan Sebastian for assistance with neuropsychological assessment; Cynthia Pan for assistance with hippocampus volumetric quantification; and all of the study participants who gave their time in support of this research.
Supported by National Institute on Aging Grant AG03949.
GLOSSARY
- BIMC
Blessed Information-Memory-Concentration test
- CDR
Clinical Dementia Rating scale
- EAS
Einstein Aging Study
- FCSRT-IR
Buschke and Grober Free and Cued Selective Reminding Test–Immediate Recall
- FOV
field of view
- LM
Wechsler Memory Scale–Revised Logical Memory subtest
- MCI
mild cognitive impairment
- MR
magnetic resonance
- MRS
magnetic resonance spectroscopy
- NAA/Cr
N-acetyl aspartate/creatine ratio
- TMTA
Trail Making Test Part A
- TMTB
Trail Making Test Part B
Footnotes
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Verhaeghen P, Marcoen A. More or less the same? A memorability analysis on episodic memory tasks in young and older adults. J Gerontol. 1993;48:P172–P178. doi: 10.1093/geronj/48.4.p172. [DOI] [PubMed] [Google Scholar]
- 2.Keefover RW. Aging and cognition. Neurol Clin. 1998;16:635–648. doi: 10.1016/s0733-8619(05)70085-2. [DOI] [PubMed] [Google Scholar]
- 3.Raz N. The aging brain observed in vivo: differential changes and their modifiers. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York: Oxford University Press; 2004. pp. 17–55. [Google Scholar]
- 4.Walhovd KB, Fjell AM, Reinvang I, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- 6.Liu RS, Lemieux L, Bell GS, et al. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage. 2003;20:22–33. doi: 10.1016/s1053-8119(03)00219-2. [DOI] [PubMed] [Google Scholar]
- 7.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Driscoll I, Hamilton DA, Petropoulos H, et al. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- 9.Angelie E, Bonmartin A, Boudraa A, Gonnaud PM, Mallet JJ, Sappey-Marinier D. Regional differences and metabolic changes in normal aging of the human brain: proton MR spectroscopic imaging study. AJNR Am J Neuroradiol. 2001;22:119–127. [PMC free article] [PubMed] [Google Scholar]
- 10.Schuff N, Amend DL, Knowlton R, Norman D, Fein G, Weiner MW. Age-related metabolite changes and volume loss in the hippocampus by magnetic resonance spectroscopy and imaging. Neurobiol Aging. 1999;20:279–285. doi: 10.1016/s0197-4580(99)00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 12.Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54:827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- 13.Grober E, Kawas C. Learning and retention in preclinical and early Alzheimer’s disease. Psychol Aging. 1997;12:183–188. doi: 10.1037//0882-7974.12.1.183. [DOI] [PubMed] [Google Scholar]
- 14.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome [erratum appears in Arch Neurol 1999;56:760] Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 16.Lipton RB, Katz MJ, Kuslansky G, et al. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc. 2003;51:1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- 17.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 18.Jack CR, Jr, Petersen RC, O’Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 19.Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson KJ, Wardlaw JM, Edmond CL, Deary IJ, Maclullich AM. Intracranial area: a validated method for estimating intracranial volume. J Neuroimaging. 2005;15:76–78. doi: 10.1177/1051228404270243. [DOI] [PubMed] [Google Scholar]
- 21.Hetherington HP, Pan JW, Spencer DD. 1H and 31P spectroscopy and bioenergetics in the lateralization of seizures in temporal lobe epilepsy. J Magn Reson Imaging. 2002;16:477–483. doi: 10.1002/jmri.10177. [DOI] [PubMed] [Google Scholar]
- 22.Hetherington HP, Pan JW, Mason GF, et al. Quantitative 1H spectroscopic imaging of human brain at 4.1 T using image segmentation. Magn Reson Med. 1996;36:21–29. doi: 10.1002/mrm.1910360106. [DOI] [PubMed] [Google Scholar]
- 23.Twieg DB, Meyerhoff DJ, Hubesch B. Phosphorus-31 magnetic resonance spectroscopy in humans by spectroscopic imaging: localized spectroscopy and metabolite imaging. Magn Reson Med. 1989;12:291–305. doi: 10.1002/mrm.1910120302. [DOI] [PubMed] [Google Scholar]
- 24.Hetherington HP, Kim JH, Pan JW, Spencer DD. 1H and 31P spectroscopic imaging of epilepsy: spectroscopic and histologic correlations. Epilepsia. 2004;45 suppl 4:17–23. doi: 10.1111/j.0013-9580.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 25.Grober E, Buschke H. Genuine memory deficits in dementia. Developmental Neuropsychol. 1987;3:13–36. [Google Scholar]
- 26.Buschke H. Cued recall in amnesia. J Clin Neuropsychol. 1984;6:433–440. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- 27.Wechsler D. Wechsler Memory Scale–Revised Manual. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- 28.Battery AIT. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant Generals Office; 1944. [Google Scholar]
- 29.Holtzer R, Goldin Y, Zimmerman ME. Comparison of robust versus conventional norms of neuropsychological tests in aging. J Int Neuropsychol Soc. 2007;13:4. [Google Scholar]
- 30.Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ. Mayo’s older Americans normative studies: Age- and IQ-adjusted norms for the Wechsler Memory Scale–Revised. Clin Neuropsychol. 2005;19:378–463. doi: 10.1080/13854040590945201. [DOI] [PubMed] [Google Scholar]
- 31.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartres-Faz D, Junque C, Clemente IC, et al. Relationship among (1)H-magnetic resonance spectroscopy, brain volumetry and genetic polymorphisms in humans with memory impairment. Neurosci Lett. 2002;327:177–180. doi: 10.1016/s0304-3940(02)00424-x. [DOI] [PubMed] [Google Scholar]
- 33.Valenzuela MJ, Jones M, Wen W, et al. Memory training alters hippocampal neurochemistry in healthy elderly. Neuroreport. 2003;14:1333–1337. doi: 10.1097/01.wnr.0000077548.91466.05. [DOI] [PubMed] [Google Scholar]
- 34.Spencer DC, Zitzelberger T, Spielman D, Kaye J. MRS in relation to hippocampal volume in the oldest old. Neurology. 2003;60:1194–1196. doi: 10.1212/01.wnl.0000052822.22994.86. [DOI] [PubMed] [Google Scholar]
- 35.Cave CB, Squire LR. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus. 1992;2:151–163. doi: 10.1002/hipo.450020207. [DOI] [PubMed] [Google Scholar]
- 36.Buschke H, Sliwinski M, Kuslansky G, Lipton RB. Aging, encoding specificity, and memory change in the Double Memory Test. J Int Neuropsychol Soc. 1995;1:483–493. doi: 10.1017/s1355617700000576. [DOI] [PubMed] [Google Scholar]
- 37.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- 38.Tounsi H, Deweer B, Ergis AM, et al. Sensitivity to semantic cuing: an index of episodic memory dysfunction in early Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13:38–46. doi: 10.1097/00002093-199903000-00006. [DOI] [PubMed] [Google Scholar]