Summary
Immune responses to pathogens occur within the context of current and previous infections. Cross protection refers to the phenomena where infection with a particular pathogen provides enhanced resistance to a subsequent unrelated pathogen in an antigen independent manner. Proposed mechanisms of antigen-independent cross protection have involved the secretion of IFN-γ, which activates macrophages thus providing enhanced innate immunity against the secondary viral or bacterial pathogen. Here we provide evidence that a primary infection with the chronic respiratory pathogen, Mycoplasma pulmonis, provides a novel form of cross protection against a secondary infection with Listeria monocytogenes that is not mediated by IFN-γ, but instead relies upon IL-17 and mobilization of neutrophils. Mice infected with M. pulmonis have enhanced clearance of L. monocytogenes from the spleen and liver which is associated with increased numbers of Gr-1+CD11b+ cells and higher levels of IL-17. This enhanced clearance of L. monocytogenes was absent in mice depleted of Gr-1+ cells or in mice deficient in the IL-17 receptor. Additionally, both the IL-17 receptor and neutrophils were essential for optimal clearance of M. pulmonis. Thus, a natural component of the immune response directed against M. pulmonis was able to enhance clearance of L. monocytogenes.
Keywords: Bacterial infection, cytokine, cytokine receptor, neutrophil
Introduction
Innate and adaptive immune responses against pathogens are extremely complex, involving multiple cell types and effector molecules. Innate immune responses are initiated by bacterial, viral, fungal or parasitic elements that are conserved across organisms, while adaptive immune responses develop against specific antigenic epitopes contained within these pathogens. Activation of innate and adaptive immune cell populations is transiently induced through interactions with the specific pathogen. Thus, immune responses are generally self-regulating and specifically directed against the particular pathogen encountered. However, in certain circumstances, immune responses against a given pathogen can also act on a completely unrelated pathogen. These “cross protective” responses can enhance the clearance of pathogens in an antigen non-specific fashion, but can in some situations involve adaptive immune cell populations.
Previously described mechanisms of cross protection involve the production of IFN-γ which in turn can activate macrophages, generating a heightened state of innate immunity to a secondary pathogen. Classical cross protection is offered by IFN-γ secreting effector lymphocytes responding to a primary pathogen. Early studies suggest that macrophages activated during a primary viral or bacterial infection could respond to an unrelated viral or bacterial pathogen, thus providing increased protection compared to naive animals [1;2]. This form of cross protection is effective while the primary pathogen persists, and then subsides when the effector lymphocytes differentiate into memory cells [1–5]. In contrast, latency-induced cross protection involves the secretion of IFN-γ in response to latent viral proteins which can provide protection for up to three months following the initial encounter with the primary viral pathogen [3]. Macrophage-dependent control of the secondary bacterial infection was shown to be the mechanism of this enhanced protection. A third method of cross protection describes a mechanism by which memory T cell populations can provide protection against a new secondary pathogen, long after the primary pathogen has been cleared from the system. In this memory-induced model of cross protection, memory CD8+ T cells secrete IFN-γ in an antigen-independent manner in response to IL-12 and IL-18 which are innately secreted during the initial immune response to a secondary infection [6;7]. In this scenario, the T cells that developed into memory cells during the adaptive immune response to a primary viral pathogen now function to heighten the innate immune response to a secondary bacterial pathogen. All of these methods of cross protection involve an increase in IFN-γ production which can lead to macrophage activation. Here, we propose a novel mechanism of cross protection that instead is dependent on IL-17 production and granulocyte mobilization.
Production of IL-17 occurs in a wide variety of chronic inflammatory conditions, and mice lacking or showing reduced IL-17 production have decreased susceptibility to the development of autoimmune diseases [8–11]. Furthermore, IL-17 is produced during a number of bacterial, viral, and fungal infections [12–19]. The necessity of IL-17 in the clearance of multiple pathogens was determined using different experimental approaches that negate IL-17 production or signaling [12;13;15;20;21]. The ability of IL-17 to mobilize neutrophils is thought to be the principal reason this cytokine is important for protection against infectious pathogens. IL-17 is known to induce the mobilization of neutrophils through the production of cytokines and chemokines such as IL-6, G-CSF, GM-CSF, CXCL1, CXCL2, and CXCL8 [9]. To our knowledge, it is yet to be shown that this immune pathway is utilized as a mechanism of cross protection.
Mycoplasma pulmonis is an atypical bacterium, due to the absence of a cell wall, that induces a chronic respiratory infection in rodents that is characterized by neutrophilic inflammation and lymphocyte infiltration. This rodent model is similar to the human disease induced by Mycoplasma pneumoniae [22;23], which is the leading cause of pneumonia worldwide [24–26]. Multiple cell types, cytokines, and chemokines are involved in the immune response to mycoplasma infections along the respiratory tract. We recently found increased IL-17 mRNA expression in the lungs of M. pulmonis infected mice [27]. Consistent with this, another report found that IL-17 protein and mRNA levels were increased during M. pneumoniae infection in mice [28]. Furthermore, IL-17 levels were associated with neutrophil recruitment during M. pneumoniae infection. Multiple studies report increased numbers of neutrophils in the lungs and bronchoalveolar lavage during both M. pulmonis [29–32] and M. pneumoniae infection [28;33;34]. Collectively, these data suggest that the production of IL-17 during mycoplasma infections may induce the mobilization of neutrophils.
Listeria monocytogenes is a gram-positive intracellular bacterium that is a common source of contamination in both raw and processed foods that can lead to septicemia and death in susceptible individuals [35]. L. monocytogenes primarily replicates within the spleen and liver following intravenous or oral inoculation. While the sterilizing clearance of L. monocytogenes is dependent on T cells, the initial immune response that limits bacterial numbers involves macrophages and neutrophils. Depletion of neutrophils, with an anti-Gr-1 antibody, resulted in impaired clearance of L. monocytogenes [36–38]. Likewise, depletion of macrophages [39;40] or prevention of accumulation of CD11b expressing cells [41–43] both increase susceptibility to L. monocytogenes infection. While IFN-γ is an essential cytokine for the clearance of L. monocytogenes during natural infection [44;45], IL-17 production has not yet been determined to be a natural requirement of the immune response against Listeria monocytogenes. However, mice deficient in leukocyte function-associated antigen-1 (LFA-1, or CD11a/CD18) have decreased susceptibility to L. monocytogenes that was associated with increased serum levels of IL-17 and G-CSF [46]. This increase in IL-17 was associated with enhanced neutrophil recruitment to the liver. Thus, the production of IL-17 during L. monocytogenes infection appears to increase resistance to L. monocytogenes by amplifying neutrophil mobilization.
The current study investigates the impact of a chronic respiratory infection with M. pulmonis on a subsequent L. monocytogenes infection. The investigation of immune responses to pathogens in the context of other infections is an important topic of research. Individuals are likely to simultaneously encounter multiple pathogens, and the interactions between the immune responses against those different pathogens may be a determining factor in susceptibility to the infections. Furthermore, subclinical chronic diseases that may go undetected in the population may impact subsequent acute infections. The pre-existing immune environment during chronic infections with pathogens such as M. pulmonis and M. pneumoniae could influence the developing immune response to infection with an unrelated pathogen. In this study, we provide evidence that chronic infection with the pulmonary pathogen, M. pulmonis, provides a yet undiscovered method of cross protection against L. monocytogenes infection that is dependent on the IL-17 receptor and granulocytes. Furthermore, we show that the IL-17 receptor and neutrophils are required for optimal clearance of M. pulmonis.
Results
Chronic infection with M. pulmonis facilitates clearance of L. monocytogenes
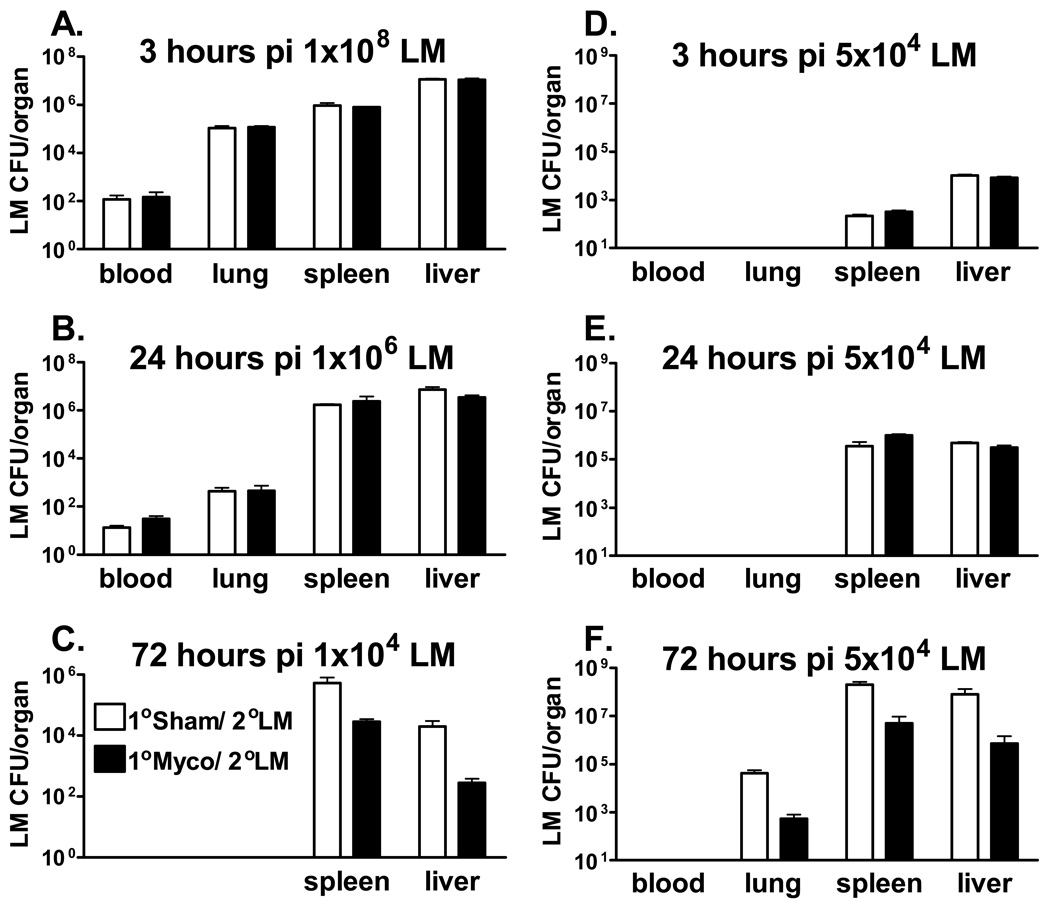
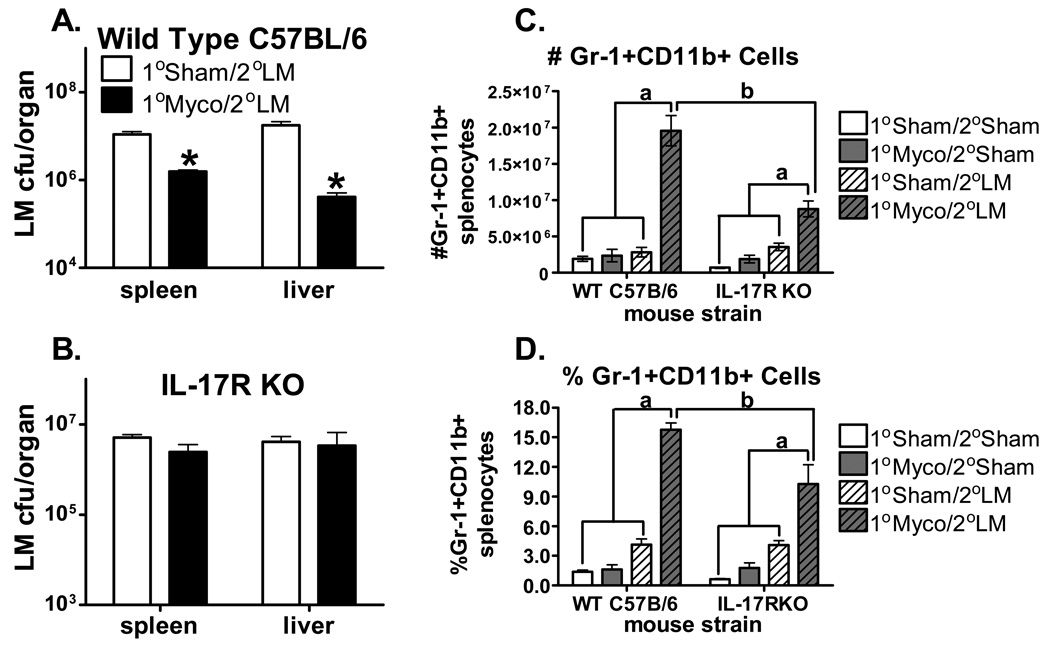
To investigate the impact of chronic M. pulmonis infection on the course of a subsequent, unrelated, bacterial infection, C57BL/6 mice were first inoculated with M. pulmonis or mycoplasma broth medium (sham). Seventeen days later, half of the broth control and M. pulmonis inoculated mice were innoculated with L. monocytogenes or phosphate buffered saline (PBS). Bacterial CFU counts were enumerated in the spleen and liver at day 3 post-infection (p.i.) with L. monocytogenes. As seen in Figure 1C, prior infection with M. pulmonis (Myco) significantly decreased L. monocytogenes (LM) CFU counts, independent of the organ assayed, p ≤ 0.05. While C57BL/6 mice are known to have a greater resistance to M. pulmonis infection[47], this phenomena was also observed in a strain of mice that is highly susceptible to M. pulmonis infection: BALB/c mice displayed the same pattern of prior infection with M. pulmonis reducing L. monocytogenes CFU counts (data not shown). Thus, a primary infection with M. pulmonis provided protection against a secondary infection with L. monocytogenes in two strains of mice that vary in their susceptibility to M. pulmonis infection.
Figure 1.
Prior Infection with M. Pulmonis Confers Resistance Against L. Monocytogenes Infection. C57BL/6 mice were i.v. infected with 1×108 (A), 1×106 (B), 1×104 (C), or 5×104 (D, E, F) CFUs of L. monocytogenes (LM) 17 days after nasal-pulmonary inoculation with mycoplasma broth medium (1°Sham/2°LM) or M. pulmonis (1°Myco/2°LM). At 3 hrs (A & D), 24 hrs (B & E), or 72 hrs (C & F) p.i., L. monocytogenes CFU counts were assessed in the indicated tissues. A two-way ANOVA detected a significant effect of M. pulmonis infection, such that M. pulmonis infection decreased L. monocytogenes CFU counts independent of organ assayed at 72 hrs p.i., p ≤ 0.05 (C &F). However, two-way ANOVAs did not detect any significant differences in any tissue assayed at 3 and 24 hrs p.i., p > 0.05 (A, B, D, E). These data are representative of 3 independent experiments. All data are expressed as the mean ± SEM (n = 4/group).
The reduced bacterial burden observed in the spleen and liver could have been a result of decreased bacterial spread of L. monocytogenes to these organs. Due to the upregulation of neutrophils and macrophages during M. pulmonis infection [28;31;32;48], enhanced phagocytosis of L. monocytogenes immediately following i.v. innoculation could have occurred in the blood and lungs of mice with the chronic respiratory M. pulmonis infection, prior to L. monocytogenes reaching the spleen or liver. To test this hypothesis, L. monocytogenes CFU counts were assayed in the blood, lung, spleen, and liver at earlier time points p.i. with L. monocytogenes. In order to observe detectable levels of L. monocytogenes in the blood and lungs at these earlier time points, higher inoculation doses of L. monocytogenes were used. Increases in L. monocytogenes CFU counts in the blood and lungs were not observed at either 3 hrs p.i. or 24 hrs p.i. with L. monocytogenes (Figures 1A and 1B), indicating that L. monocytogenes was not being engulfed by cells in either of these tissues to a greater degree in M. pulmonis infected mice, p ≥ 0.05. Furthermore, chronic M. pulmonis infection did not facilitate the clearance of L. monocytogenes in the spleen and liver at these early time points, p ≥ 0.05.
If the reduction of CFU counts in the spleen and liver at day 3 p.i. with L. monocytogenes was mediated by a decreased bacterial spread to these organs, there should be reduced CFU counts in these organs from the onset of infection. However, this was not observed at either 3 or 24 hrs p.i. with higher inoculation doses. To determine if the early time point or the higher inoculation dose led to the lack of cross-protection observed, CFU counts were enumerated at 3, 24, and 72 hrs p.i. using the same inoculation dose of L. monocytogenes (Figures 1D, 1E, and 1F). The enhanced clearance of L. monocytogenes offered by M. pulmonis was again only evident at 72 hrs p.i. with L. monocytogenes (Figure 1F), p ≤ 0.05. Thus, M. pulmonis infection facilitated clearance of L. monocytogenes is mediated by a more complex immune mechanism than an increase in phagocytosis and trapping of L. monocytogenes in the lungs or peripheral blood of mice with a pulmonary mycoplasma infection. Enhanced protection against L. monocytogenes appears to occur following bacterial spread to the spleen and liver. The pre-existing immune environment induced by a chronic respiratory infection determined the subsequent development of the immune response against L. monocytogenes. Therefore, an interaction between the long-lasting immune response to M. pulmonis and the immune response being mounted against an acute L. monocytogenes infection was necessary to reduce the bacterial burden in L. monocytogenes infected mice.
M. pulmonis and L. monocytogenes co-infection increases Gr-1+CD11b+ cells
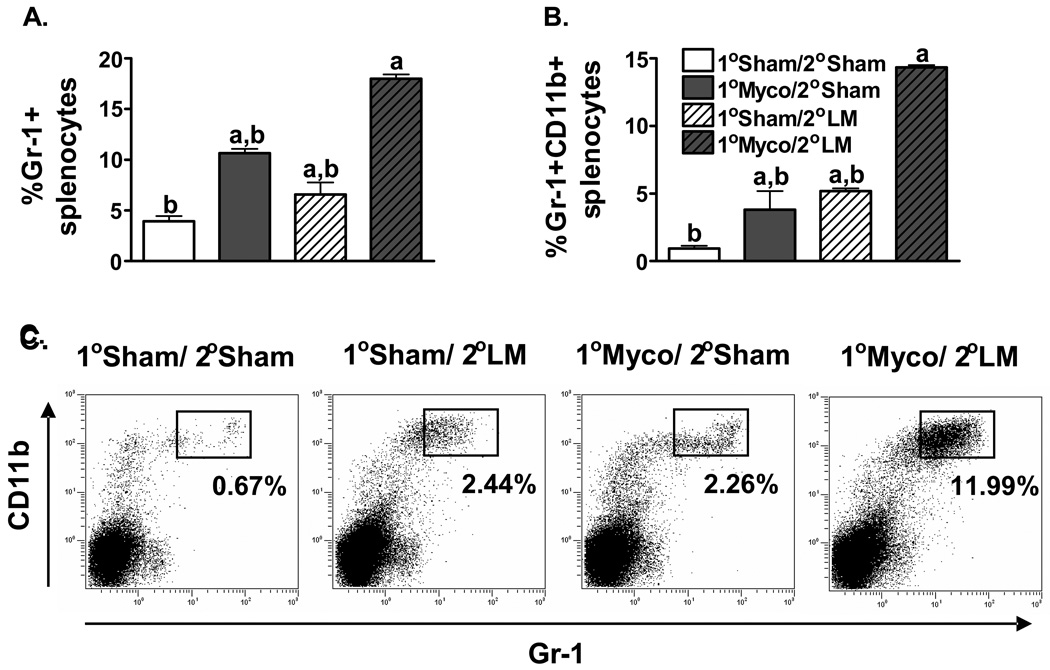
Flow cytometric analysis of the immune cell populations in the spleen was performed to investigate which cell types might be mediating the chronic M. pulmonis infection induced facilitated clearance of L. monocytogenes. Two-way ANOVAs found no detectable differences between L. monocytogenes infected mice and mice infected with M. pulmonis followed by L. monocytogenes in the percentage of CD4+ T cells, CD8+ T cells, NK cells, NK-T cells, or macrophages/monocytes (data not shown). However, there was a significant increase in the percentage of granulocytes (Gr-1+) in the spleens of mice co-infected with M. pulmonis and L. monocytogenes (Figure 2A), p ≤ 0.05. Given that CD11b is expressed on activated granulocytes [46;49], the cell surface expression of Gr-1 and CD11b was analyzed in splenocytes from 1) 1°broth/ 2°PBS, 2) 1°broth/ 2°L. monocytogenes, 3) 1°M. pulmonis/ 2°PBS, and 4) 1°M. pulmonis/ 2°L. monocytogenes infected C57BL/6 mice. As shown in Figures 2B and 2C, while both M. pulmonis and L. monocytogenes infection alone induced an increase in the percentage of Gr-1+CD11b+ cells in the spleen, the interaction between the immune responses against these pathogens led to a substantially greater increase in this cell population, p ≤ 0.05. These data suggest that neutrophils may be involved in the cross protection offered by M. pulmonis against L. monocytogenes.
Figure 2.
Gr-1+CD11b+ Cells Increase During Co-Infection with L. Monocytogenes and M. Pulmonis. At day 17 p.i. with M. pulmonis, C57BL/6 mice were inoculated with 5×104 CFUs of L. monocytogenes or PBS. At day 3 p.i. with L. monocytogenes, splenocytes were analyzed for expression of Gr-1 and CD11b in broth/PBS (1°Sham/2°Sham), M. pulmonis/PBS (1° Myco/2°Sham), broth/L. monocytogenes (1°Sham/2°LM), and M. pulmonis/L. monocytogenes (1° Myco/2°LM) inoculated mice. A two-way ANOVA detected a significant increase in the percentage of both Gr-1+ (A) and Gr-1+CD11b+ (B) splenocytes in response to both L. monocytogenes and M. pulmonis infection. Furthermore, co-infection with M. pulmonis and L. monocytogenes induced a greater increase in the percentage of these cell populations than infection with either pathogen alone (A, B, C). a, denotes a significant difference from the 1°Sham/ 2°Sham group, p ≤ 0.05. b, denotes a significant difference from the 1°Myco/ 2°LM group, p ≤ 0.05. These data are representative of 3 independent experiments. All data are expressed as the mean ± SEM (n = 4/group). Representative flow cytometry dot plots of Gr-1 and CD11b expression are presented (C).
IL-17, but not IFN-γ, production is increased during chronic M. pulmonis infection
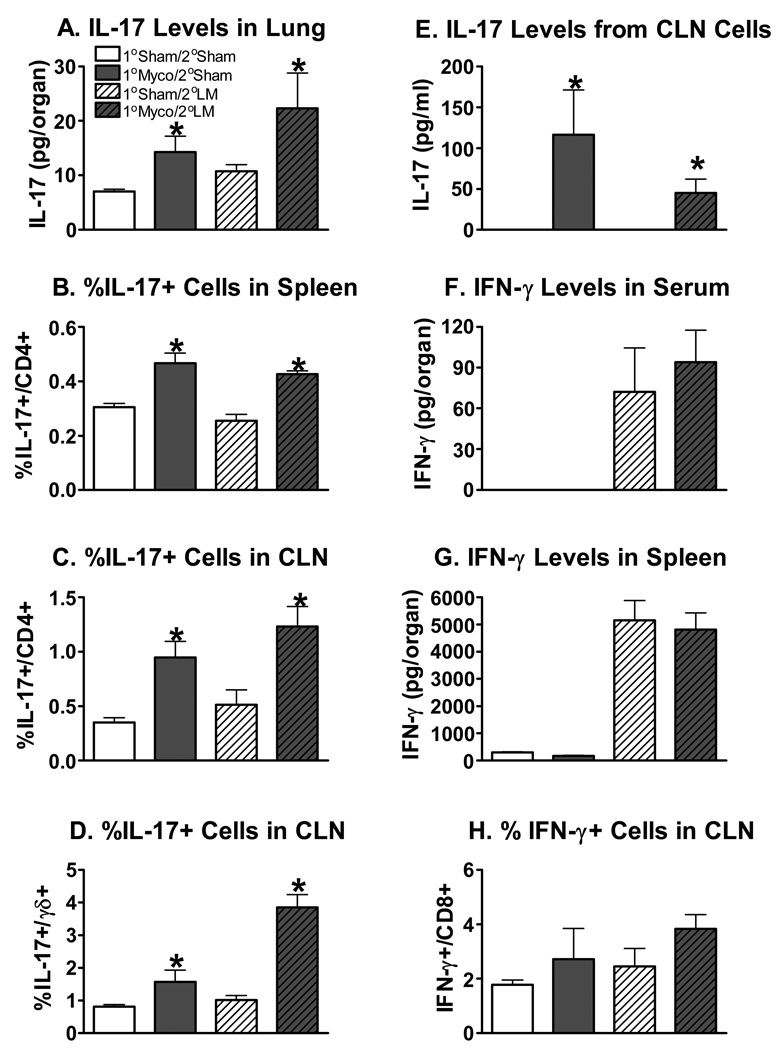
While IFN-γ is known to be essential for the clearance of L. monocytogenes [44;45], in certain circumstances IL-17 has also been shown to increase resistance to this pathogen [46]. IL-17 has not yet been shown to be required for resistance to L. monocytogenes [50], but increased production of IL-17 in LFA deficient mice has been found to amplify the neutrophil recruitment leading to facilitated clearance of this pathogen [46]. Therefore, the possibility existed that either IFN-γ or IL-17 could be mediating the M. pulmonis induced enhanced clearance of L. monocytogenes. To investigate this, we performed experiments to measure both of the cytokines in this co-infection model. At day 17 p.i. with M. pulmonis or broth, C57BL/6 mice were infected with L. monocytogenes or PBS. At day 3 p.i. with L. monocytogenes, whole lung homogenates and sera were collected to analyze IL-17 with the Luminex assay. As seen in Figure 3A, M. pulmonis infection significantly increased IL-17 levels in the lungs of L. monocytogenes infected and uninfected mice, p ≤ 0.05. A two-way ANOVA detected no significant differences in the level of IL-17 in the serum (data not shown), p ≥ 0.05. Thus, while a local increase in IL-17 was observed in the lung, a systemic increase in IL-17 in response to M. pulmonis infection was not detected.
Figure 3.
Infection with M. Pulmonis Increases IL-17, but not IFN-γ, Levels in L. Monocytogenes Infected Mice. At day 17 p.i. with M. pulmonis or broth, C57BL/6 mice were infected with L. monocytogenes or PBS. The concentration of IL-17 in lung homogenates from day 3 p.i. with L. monocytogenes was analyzed using a Luminex suspension array (A). A two-way ANOVA detected a significant effect of M. pulmonis infection, such that prior infection with M. pulmonis increased IL-17 levels in the lungs. Intracellular cytokine staining was used to determine the percentage of IL-17 producing CD4+ T cells in the spleen (B) and cervical lymph nodes (CLN) (C) and the percentage of IL-17 producing γδ+ T cells (D) in the CLN of uninfected (1°Sham/ 2°Sham), singly infected (1°Myco/ 2°Sham and 1°Sham/2°LM), and co-infected (1°Myco/ 2°LM) mice. Again, a two-way ANOVA detected a significant effect of M. pulmonis infection, such that prior infection with M. pulmonis increased the percentage of IL-17 producing cells (B, C, D). Supernatants from CLN cells cultured for two days with M. pulmonis membrane antigen were analyzed using ELISA for the concentration of IL-17 produced (E). A Kruskal-Wallis test detected that both the 1°Myco/2°Sham and 1°Myco/2°LM had increased IL-17 levels (p ≤ 0.05), and no other differences were significant, p ≥ 0.05. The concentration of IFN-γ in serum (F) and spleen homogenates (G) from day 3 p.i. with L. monocytogenes was analyzed using a Luminex suspension array. A two-way ANOVA determined that L. monocytogenes increased IFN-γ levels in both tissues, but M. pulmonis infection did not impact levels of this cytokine during L. monocytogenes infection. Likewise, two-way ANOVAs conducted on flow cytometry data did not detect a significant effect of M. pulmonis infection on the percentage of IFN-γ producing cells during L. monocytogenes infection (H), p ≥ 0.05. These data are representative of 2 independent experiments. All data are expressed as the mean ± SEM (n = 3–4/group). An * denotes the 1° Myco groups differ from the 1° Sham groups (p ≤ 0.05).
Intracellular cytokine staining and flow cytometry were used to evaluate the production of IL-17 from splenocytes and cervical lymph node cells. At day 17 p.i. with M. pulmonis or broth, C57BL/6 mice were infected with L. monocytogenes or PBS. At day 3 p.i. with L. monocytogenes, splenocytes and cervical lymph node cells from uninfected, singly infected, or co-infected mice were harvested and cultured overnight with or without M. pulmonis antigen. Cells were subsequently stained with antibodies directed against CD4, CD8, γδ T cell receptor, and intracellular IL-17. Two-way ANOVAs determined that M. pulmonis infection increased the percentage of CD4+ T cells that produced IL-17 in L. monocytogenes infected and uninfected mice in both the spleen and cervical lymph node, p ≤ 0.05. However, CD4+ T cells from mice infected with L. monocytogenes alone did not produce IL-17 above background levels seen in uninfected mice in the spleen or cervical lymph node, p ≥ 0.05. Furthermore, the greatest degree of IL-17 production was seen in cells isolated from M. pulmonis mice that were stimulated with M. pulmonis antigen (Figures 3B and 3C). In unstimulated cultures, IL-17 producing cells from M. pulmonis infected mice could also be detected, but far fewer existed than with M. pulmonis antigen stimulation (data not shown). Appreciable percentages of CD8+ T cells and γδ+ T cells that produced IL-17 were not detected in the spleen (data not shown). However, in the cervical lymph node, M. pulmonis infection also increased the percentage of γδ+ T cells that produced IL-17, p ≤ 0.05 (Figure 3D). Collectively, these data suggest that IL-17 is being specifically produced in response to M. pulmonis infection by CD4+ and γδ+ T cells.
In order to quantify the amount of IL-17 being produced by the cervical lymph node cells, supernatants from these cultures were analyzed using an enzyme-linked immunosorbent assay (ELISA). To this end, cervical lymph node cells from uninfected, singly infected, or co-infected mice were harvested and cultured for two days with or without M. pulmonis antigen or heat-killed L. monocytogenes. There were no detectable levels of IL-17 from any unstimulated cultures or from cultures stimulated with heat-killed L. monocytogenes (data not shown). However, M. pulmonis antigen stimulated the secretion of IL-17 in cell cultures from M. pulmonis infected mice (1°Myco/2°Sham and 1°Myco/2°LM groups), p ≤ 0.05 (Figure 3E). No IL-17 was detected in cultures of cells isolated from mice not infected with M. pulmonis. Thus, IL-17 production was dependent on M. pulmonis infection, and was observed only in response to M. pulmonis antigen stimulation.
In order to determine if a previous infection with M. pulmonis induced increased IFN-γ production upon infection with L. monocytogenes, IFN-γ levels were measured. At day 17 p.i. with M. pulmonis or broth, C57BL/6 mice were infected with L. monocytogenes or PBS. At day 3 p.i. with L. monocytogenes, sera and whole spleen homogenates were collected to analyze IFN-γ with the Luminex assay. M. pulmonis infection alone did not lead to production of IFN-γ, which is not unexpected at this late time point in the C57BL/6 strain of mice [27]. Importantly, a two-way ANOVA determined that mice infected with L. monocytogenes or co-infected with M. pulmonis and L. monocytogenes did not differ in the amount of IFN-γ present in the serum or spleen (Figures 3F and 3G), p ≥ 0.05. Levels of IFN-γ in the lung were not detected above background (data not shown). In addition, flow cytometry data analyzing IFN-γ production in the cervical lymph nodes did not show a significant increase of IFN-γ secreting CD8 T or CD4 T cells under any condition measured (Figure 3H and data not shown). IFN-γ producing γδ+ T cells were not detectable in the CLN (data not shown).
Thus, while prior infection with M. pulmonis did not induce changes in IFN-γ production during a subsequent L. monocytogenes infection, it did increase the level of IL-17 that was produced during L. monocytogenes infection. Collectively, these data suggest that heightened levels of IL-17 during M. pulmonis infection could be responsible for increased neutrophil mobilization and the higher numbers of Gr-1+CD11b+ cells observed during the secondary L. monocytogenes infection.
Gr-1 depletion eliminates co-infection facilitated clearance of L. monocytogenes
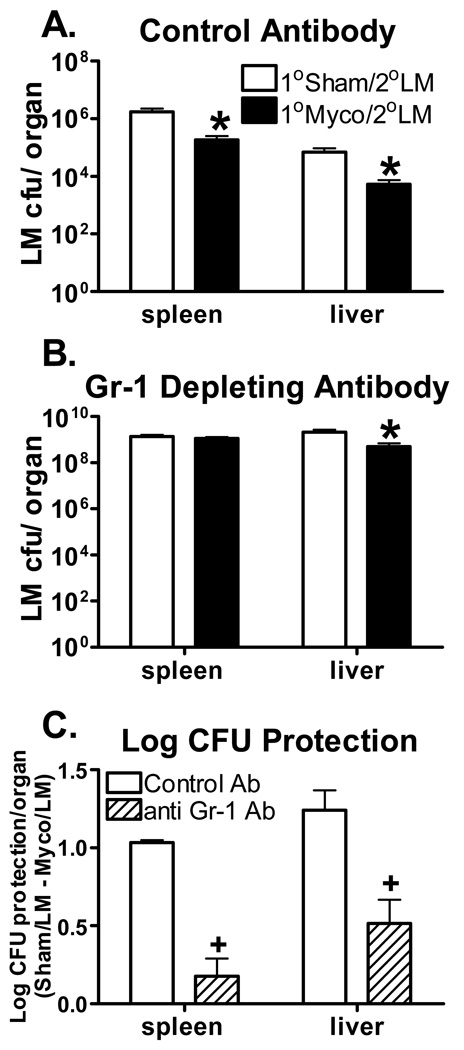
Infiltration of granulocytes into infected spleen and liver is essential for early anti-Listeria defense [37;38;41]. Thus, the necessity of this increased granulocyte population in the facilitated clearance of L. monocytogenes induced by M. pulmonis infection was investigated using a Gr-1 depleting antibody. At day 16 p.i. with M. pulmonis or broth, C57BL/6 mice were injected with anti-Gr-1 or control antibody. Mice were infected with 1×104 CFUs of L. monocytogenes 24 hrs later, and the L. monocytogenes CFU counts in the spleen and liver were enumerated at day 3 p.i. with L. monocytogenes. A lower inoculation dose of L. monocytogenes was used in this experiment because previous studies have shown that depletion of granulocytes results in increased susceptibility to L. monocytogenes [36–38]. As seen in Figure 4, depletion of Gr-1+ cells completely abrogated the M. pulmonis induced facilitated clearance of L. monocytogenes in the spleen and significantly reduced it in the liver, p ≤ 0.05. Therefore, the presence of granulocytes appears to mediate the cross protection that M. pulmonis infection offers against L. monocytogenes infection.
Figure 4.
Granulocyte Depletion Diminishes Resistance to L. Monocytogenes Conferred by M. Pulmonis. At day 16 p.i. with M. pulmonis or broth, mice were injected with the control antibody (A) or Gr-1 depleting antibody (B). Twenty-four hrs following depletion, mice were infected with 1×104 CFUs of L. monocytogenes. L. monocytogenes CFU counts were enumerated in the spleen and liver at day 3 p.i. with L. monocytogenes. All data are expressed as the mean ± SEM (n = 6/group). A two-way ANOVA detected a significant effect of M. pulmonis infection in the spleen and liver of mice that received the control antibody, such that M. pulmonis infection decreased L. monocytogenes CFU counts. While a significant effect of M. pulmonis was still detected in the liver of Gr-1 depleted mice, there was no decrease in CFUs observed in the spleen. * , denotes 1°Sham/2°LM differ from 1°Myco/2°LM (p ≤ 0.05). Log CFU protection conferred by M. pulmonis infection against L. monocytogenes was calculated by subtracting the average log bacterial burden in the spleen and livers of M. pulmonis infected mice from the average bacterial burden in the same organs of broth inoculated mice (C). Data presented are derived from two independent experiments with four to six mice in each group per experiment. A two-way ANOVA detected a significant effect of Gr-1 depletion, such that Gr-1 depleted mice had decreased log CFU protection in both the spleen and liver as compared to mice that received the control antibody. +, denotes the control antibody differs from the anti Gr-1 antibody (p ≤ 0.05).
The IL-17 receptor mediates co-infection induced CFU decrease and Gr-1+CD11b+ cell increase
IL-17 is known to induce the mobilization of neutrophils through the production of cytokines and chemokines [9], and mice deficient in the IL-17 receptor have a reduced granulocyte response during respiratory infection [13]. Upregulation of IL-17 occurs in the lungs and bronchoalveolar lavage of mice infected with M. pulmonis and M. pneuomoniae [27;28]. To investigate whether the ability to respond to IL-17 produced during chronic M. pulmonis infection underlies the increases in granulocytes and the enhanced clearance of L. monocytogenes, IL-17 receptor deficient mice were utilized. As seen in Figure 5A and 5B, the enhanced clearance of L. monocytogenes induced by M. pulmonis infection is completely absent in IL-17 receptor deficient mice as compared to wild type C57BL/6 mice infected with L. monocytogenes. Furthermore, the increased number (Figure 5C) and percent (Figure 5D) of Gr-1+CD11b+ cells in the spleens of mice co-infected with M. pulmonis and L. monocytogenes is markedly reduced in the IL-17 receptor deficient mice. Wild type C57BL/6 mice have a significantly greater number and percentage of Gr-1+CD11b+ cells in the spleen when co-infected with M. pulmonis and L. monocytogenes as compared to uninfected, M. pulmonis infected, or L. monocytogenes infected mice, p ≤ 0.05. While the co-infection induced increase in this cell population is still observable in the IL-17 receptor deficient mice, it is significantly reduced as compared to wild type C57BL/6 mice, p ≤ 0.05. Thus, the IL-17 receptor mediated not only the cross protection offered by M. pulmonis against a secondary L. monocytogenes infection, but also the increased Gr-1+CD11b+ population of cells observed in the co-infected mice. This data suggests that IL-17 produced during M. pulmonis infection was able to amplify the neutrophil mobilization during L. monocytogenes infection, thus facilitating the clearance of L. monocytogenes.
Figure 5.
In IL-17 Receptor Deficient Mice, M. Pulmonis Infection Does Not Facilitate Clearance of L. Monocytogenes or Increase Gr-1+CD11b+ Cells. At day 17 p.i. with M. pulmonis or broth, C57BL/6 and IL-17 receptor deficient mice were infected with L. monocytogenes or PBS. L. monocytogenes CFU counts were enumerated in the spleen and liver at day 3 p.i. with L. monocytogenes. A two-way ANOVA detected a significant effect of M. pulmonis, such that M. pulmonis decreased L. monocytogenes CFU counts in the spleen and liver of wild-type C57BL/6 mice (A). No significant effect of M. pulmonis was detected in the IL-17 receptor deficient mice (B). *, denotes that the 1°Sham/2°LM group differs from the 1°Myco/2°LM group (p ≤ 0.05). All data are expressed as the mean ± SEM and are representative of two independent experiments with four mice per group. Splenocytes from broth/ PBS (1° Sham/2° Sham), M. pulmonis/ PBS (1° Myco/2° Sham), broth/ L. monocytogenes (1° Sham/2° LM), and M. pulmonis/ L. monocytogenes (1° Myco/2° LM) mice were stained for expression of Gr-1 and CD11b. Two-way ANOVAs detected a significant effect of infection, such that mice co-infected with M. pulmonis and L. monocytogenes had an increase in the number (C) and percentage (D) of Gr-1+CD11b+ cells as compared to all other groups. While this effect was evident in wild-type C57BL/6 (WT C57B/6) and IL-17 receptor deficient (IL-17RKO) mice, it was significantly diminished in IL-17 receptor deficient mice as compared to wild type mice. a, denotes that all groups differ from 1°Myco/2°LM group (p < 0.05). b, denotes WT C57B/6 mice differ from IL-17RKO mice (p ≤ 0.05).
Gr-1+ cells are involved in the clearance of M. pulmonis
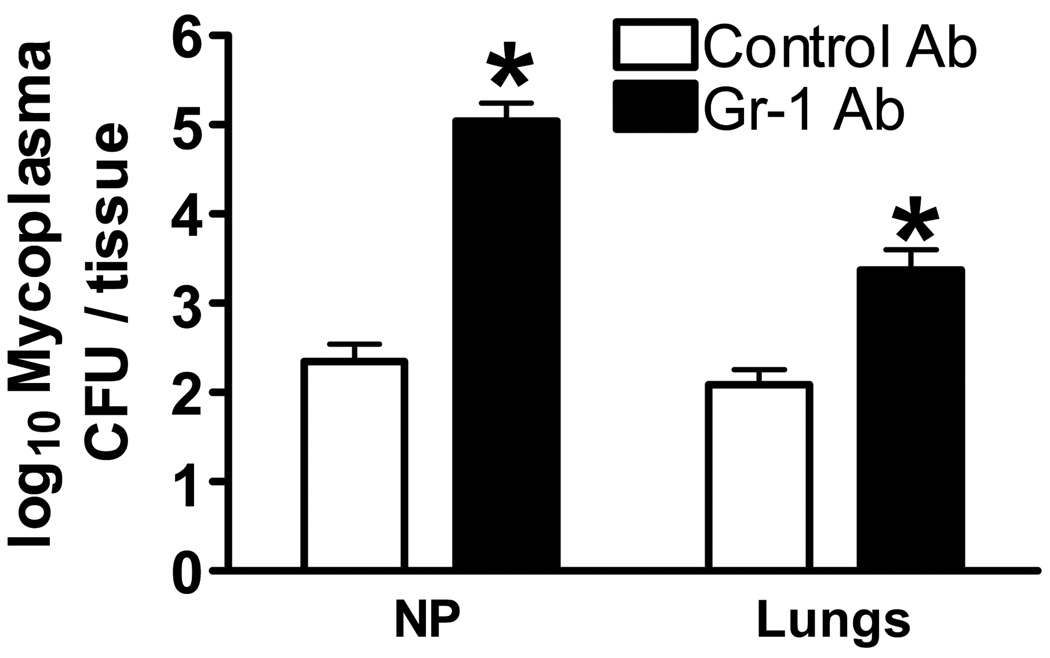
Neutrophils numbers are known to increase during M. pulmonis infection [29–32]. Here we depleted granulocytes from days 16–20 p.i. with M. pulmonis. This depletion was found to eliminate the facilitated clearance of L. monocytogenes induced by M. pulmonis. However, it was unknown whether granulocytes were involved in the clearance of M. pulmonis at this stage of infection. Indeed, Gr-1 depleted mice had higher M. pulmonis CFU counts in the lungs and nasal passages as compared to mice that had received the control antibody (Figure 6), p ≤ 0.05. Depletion of granulocytes impaired the clearance of M. pulmonis. Therefore, the upregulation of granulocytes represents an element of a normal immune response against chronic M. pulmonis infection that is able to offer cross protection against an additional pathogen.
Figure 6.
Granulocytes are Required for Optimal Clearance of M. Pulmonis From the Lungs and Nasal Passages. C57BL/6 mice were depleted of granulocytes using a Gr-1 depletion antibody from days 16 to 20 p.i. with M. pulmonis. At day 20 p.i. with M. pulmonis, CFU counts were assessed in the lungs and nasal passage wash (NP). A two-way ANOVA detected a significant effect of granulocyte depletion, such that mice given the Gr-1 depleting antibody had increased M. pulmonis CFU counts in the spleen and NP as compared to mice that received the control antibody. The means of log transformed data ± SEM are presented and are representative of two independent experiments with four mice per group. *, denotes the control antibody differs from the anti Gr-1 antibody (p ≤ 0.05).
IL-17 receptor deficient mice are more susceptible to M. pulmonis infection
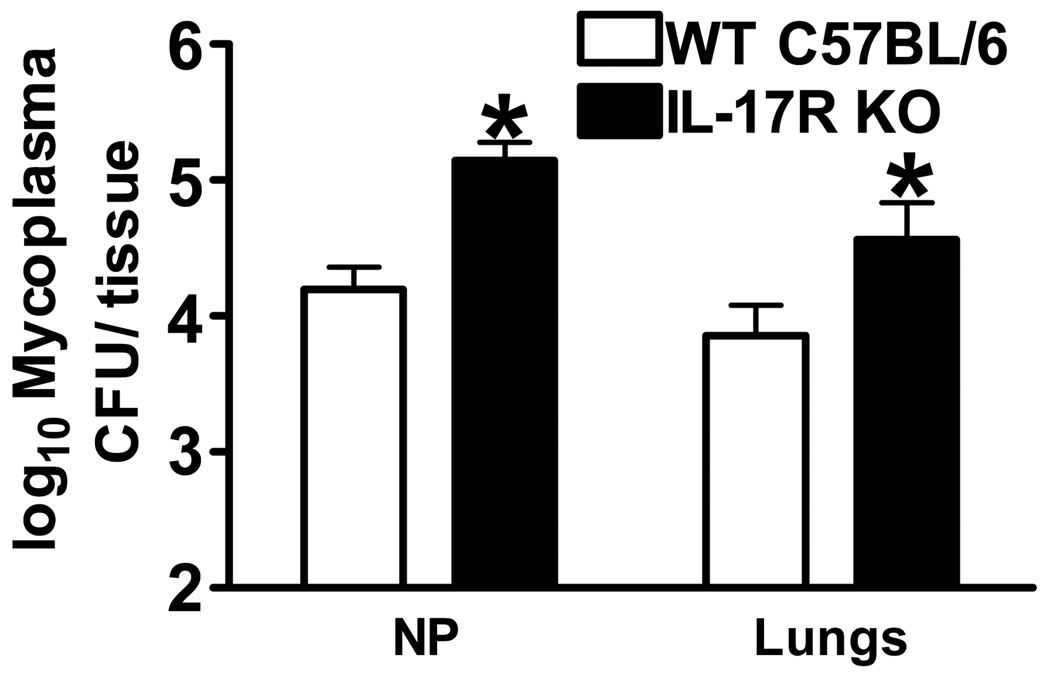
Expression of IL-17 was shown to be increased during M. pulmonis and M. pneumoniae infection [27;28], however the role that IL-17 might play in the clearance of M. pulmonis had not been investigated. To determine if the IL-17 receptor is an essential component of the immune response against chronic M. pulmonis infection, IL-17 receptor deficient mice or wild type C57BL/6 mice were inoculated intranasally with M. pulmonis or mycoplasma broth medium. At day 20 p.i. with M. pulmonis, CFU counts were enumerated in the lungs and nasal passage washes. As seen in Figure 7, IL-17 receptor deficient mice were less capable of clearing M. pulmonis from these tissues, p ≤ 0.05. Thus, the IL-17 receptor is necessary to mount an optimal immune response against M. pulmonis. This optimal immune response against M. pulmonis is then able to confer protection against a second pathogen, L. monocytogenes.
Figure 7.
IL-17 Receptor Deficient Mice Have Increased Susceptibility to M. Pulmonis Infection. Wild type C57BL/6 and IL-17 receptor deficient mice were inoculated with M. pulmonis. At day 20 p.i. with M. pulmonis, CFU counts were enumerated in the lungs and NP. A two-way ANOVA detected a significant effect of mouse strain, such that IL-17 receptor deficient mice had higher M. pulmonis CFU counts in the lungs and NP as compared to wild type mice. The means of log transformed data ± SEM are presented and are representative of three independent experiments with four mice per group. *, denotes WT C57B/6 mice differ from IL-17R KO mice (p ≤ 0.05).
Discussion
Our data shows that a chronic infection with the respiratory pathogen M. pulmonis facilitated the clearance of an acute L. monocytogenes infection. The enhanced clearance of L. monocytogenes observed at day 3 p.i. in the spleen and liver, however, was not seen at earlier time-points p.i. with L. monocytogenes. This indicates that the reduced CFU counts were not attributable to decreased spread of L. monocytogenes to the spleen and liver. Likewise, the cross protection offered by M. pulmonis was not mediated by enhanced phagocytosis of L. monocytogenes in the peripheral blood or lungs of M. pulmonis infected mice, in that no increase in L. monocytogenes CFU counts existed in these tissues. Alternatively, an interaction between the established immune responses against M. pulmonis and a developing immune response against L. monocytogenes was required to facilitate the clearance of L. monocytogenes. Analysis of the cell types involved in this cross protection revealed that there was an increase in the percentage of granulocytes in the spleens of co-infected mice. Further studies using the Gr-1 depleting antibody found that the M. pulmonis induced enhanced clearance of L. monocytogenes was dependent on this granulocyte population. An increase in both IL-17 protein levels and the percentage of IL-17 producing cells was found in the M. pulmonis infected mice, while IFN-γ protein levels and IFN-γ secreting cells were not impacted by the co-infection with M. pulmonis. A deficiency in the IL-17 receptor eliminated the cross protection offered by M. pulmonis against L. monocytogenes and reduced the increase in Gr-1+CD11b+ cells observed during the co-infection. Taken together, these data show that a chronic respiratory infection with M. pulmonis facilitates clearance of systemic L. monocytogenes, and that this cross protection is dependent on the IL-17 receptor and granulocytes.
While multiple mechanisms of antigen-independent cross protection have been proposed, each of them has relied on the production of IFN-γ which in turn can activate macrophages [1–7]. The current findings suggest that a heightened state of immunity induced by IL-17 production and neutrophil mobilization can also provide an antigen-independent form of cross protection. One implication of this data is that infections which induce IL-17 production, and thus the proliferation, activation, and recruitment of neutrophils, will be able to provide cross protection against secondary infections in which neutrophils play a key role in susceptibility. Neutrophils are a primary innate immune cell population key to the initial inflammatory response and control of most pathogens. IL-17 induced inflammatory responses are utilized during infection, as well as autoimmunity. The production of IL-17 was shown to be key to the progression of multiple autoimmune conditions including Multiple Sclerosis, Rheumatoid Arthritis, psoriasis, and Crohn’s disease (for reviews see [8–11]). Thus, IL-17 inducing infections may influence the development of such autoimmune diseases, hastening the onset of symptoms or worsening the severity of autoimmune conditions. IL-17 is produced during a wide variety of infectious diseases: bacterial pathogens such as Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacteroides fragilis, Citrobacter rodentium, Escherichia coli, Bordetella pertussis, Mycobacterium bovis, Mycobacterium tuberculosis, and Mycoplasma pneumoniae; fungal pathogens such as Candida albicans, Cryptococcus neoformans, and Pneumocystis carinii; parasitic pathogens such as Toxoplasma gondii; viral infections such as vaccinia virus (for review see[17]). Infections with these pathogens may not only worsen autoimmune conditions, but also have the possibility of leading to cross protection against subsequent infections with an unrelated pathogen via IL-17 induced neutrophil proliferation, activation, and recruitment. To our knowledge, this is the first time that a novel mechanism of cross protection mediated by IL-17 secretion and neutrophil mobilization has been shown.
The current study also found that granulocytes and the IL-17 receptor are involved in the clearance of M. pulmonis from the lungs and nasal passages. Mice depleted of neutrophils with the Gr-1 antibody at days 16–20 p.i. with M. pulmonis had increased M. pulmonis CFU counts in the lungs and nasal passages. The necessity of granulocytes for the clearance of M. pulmonis had not previously been determined at this stage of infection. Neutrophils have been reported to be increased in the lungs and bronchoalveolar lavage fluids during M. pulmonis infection [29–32]. Depletion of neutrophils using cyclophosphamide was shown to increase M. pulmonis CFUs in the lungs at day 3 p.i. [31], however the impact of neutrophils on M. pulmonis clearance at later stages during infection had not been determined. In our co-infection model, we provide evidence that the mobilization of neutrophils is related to the production of IL-17. IL-17 levels in the lung were found to be increased at day 20 p.i. with M. pulmonis (Figure 3). This is consistent with previous reports that found increased IL-17 mRNA in the lungs at days 14 and 28 p.i. with M. pulmonis [27], increased IL-17 levels in the bronchoalveolar lavage at days 1, 3, and 7 p.i. with M. pneumoniae, and increased IL-17 mRNA in the lungs at 24 hrs p.i. with M. pneumoniae [28]. We extend these findings to show the necessity of the IL-17 receptor in the clearance of M. pulmonis. IL-17RKO mice had increased CFU counts in the lungs and nasal passages at day 20 p.i. with M. pulmonis. This is the first data to show that a deficiency in IL-17 signaling leads to increased susceptibility to M. pulmonis. Taken together, these data suggest that IL-17 induced neutrophil activation, proliferation or recruitment is essential to the clearance of M. pulmonis from the upper and lower respiratory tracts.
According to the data presented in this paper as well as a recently published report, IL-17RKO mice do not have impaired clearance of L. monocytogenes as compared to wild type mice at days 3 (Figure 5) or 4 p.i. [50]. However, under certain circumstances IL-17 production is capable of influencing L. monocytogenes infection. Evidence for this comes from another study: mice deficient in LFA-1 (LFA-1−/−) have increased clearance of L. monocytogenes concurrent with increased IL-17 production [46]. Furthermore, the current study confirms that IL-17 production can increase resistance to L. monocytogenes. Not only was IL-17 production increased in the animals with enhanced resistance to L. monocytogenes, but more importantly, deficiency in the IL-17R abrogated this effect. Given that IL-17 induces the secretion of multiple cytokines and chemokines that are known to induce the mobilization, proliferation, activation, and recruitment of neutrophils [9], it is not surprising that IL-17 is able to provide protection against L. monocytogenes. Neutrophils are essential for resistance to L. monocytogenes and neutrophil mobilization during L. monocytogenes infection can be amplified by the presence of IL-17 [36–38;46]. Indeed, the heightened resistance to L. monocytogenes observed during M. pulmonis infection was associated not only with higher levels of IL-17 but also greater neutrophil numbers. While IL-17 independent mechanisms of neutrophil recruitment are also utilized during L. monocytogenes infection, the cross-protection offered by M. pulmonis and the heightened granulocyte numbers during co-infection were dependent on IL-17. This is consistent with previous findings showing that LFA-1−/− mice had increased levels of IL-17, neutrophilia, and increased resistance to L. monocytogenes [46]. In the current study, we show that IL-17 production is able to induce neutrophilia that facilitates the clearance of L. monocytogenes in wild-type mice.
It is interesting to note that in the current study, a respiratory pathogen was able to enhance clearance of a secondary unrelated pathogen that resides within the spleen and liver. The chronic respiratory infection with M. pulmonis was associated with increases in IL-17 in not only the lungs and draining lymph nodes, but also the spleen. Furthermore, M. pulmonis infection worked synergistically with L. monocytogenes infection to substantially increase granulocyte numbers in the spleen. There are two potential explanations for how a respiratory pathogen such as M. pulmonis is able to modulate the immune response to L. monocytogenes within the spleen and liver. One hypothesis is that M. pulmonis disseminates to the spleen and liver, thus providing localized IL-17 and neutrophil responses to M. pulmonis, which are also able to influence the L. monocytogenes infection that is within the vicinity. As evidence against this hypothesis, a previous report [51], as well as our unpublished data at day 20 p.i. with 2×105 CFUs of M. pulmonis, found no detectable levels of M. pulmonis in the spleen. Thus, it does not appear that the IL-17 and granulocyte response within the spleen is a result of localized M. pulmonis infection within the spleen. Our preliminary data suggest low, but inconsistent levels of M. pulmonis may be recovered from the liver at day 20 p.i. with 2×105 CFUs of M. pulmonis (unpublished data), indicating that a potential local interaction between M. pulmonis specific immune responses and the clearance of L. monocytogenes is possible within this organ. Alternatively, localized M. pulmonis infection, and the subsequent M. pulmonis directed immune responses, may not be required within the spleen and liver to provide cross protection against L. monocytogenes in these tissues. Another possible mechanism is that M. pulmonis infection within the lungs and nasal passages may be influencing the development of IL-17 secreting T cells within the draining lymph nodes. These IL-17 secreting T cells could traffic into not only the lung and nasal passage tissues, but also the spleen and liver. IL-17 is known to induce the production of IL-6, G-CSF, GM-CSF, CXCL1, CXCL2, and CXCL8 [9]. Production of these cytokines and chemokines within the spleen, lymph nodes and lungs in response to IL-17, could subsequently induce the systemic mobilization of neutrophils, which would be poised and ready to respond to any infectious microorganism that they encounter. In this scenario a localized infection within the lungs would potentially be able to provide cross protection against an unrelated pathogen within other tissues in the body. IL-17 induced mobilization of neutrophils may therefore be a wide-reaching mechanism of cross protection.
Materials and Methods
Mice
C57BL/6 and BALB/c mice, tested to be virus- and mycoplasma-free, were obtained from Harlan Sprague-Dawley (Indianapolis, IN) or Taconic (Germantown, NY). IL-17 receptor knockout mice (IL-17RKO) backcrossed on a C57BL/6 genetic background have previously been described [13]. Female mice between 6 to 12 weeks of age were housed with food and water ad libitum in sterile microisolator cages with sterile bedding at the University of North Texas Health Science Center AAALAC accredited animal facility. All animal studies were performed under the approval of the Institutional Animal Care and Use Committee at the University of North Texas Health Science Center.
Pathogens and Infections
The UAB CT strain of M. pulmonis was used in all experiments. Stock cultures were grown, as previously described [52], in mycoplasma broth medium and frozen at −80°C. For innoculation, thawed aliquots were diluted to 2×105 CFU/ 20 µl. Before infection, mice were anesthetized with an intramuscular injection of ketamine-xylazine, and nasal-pulmonary innoculations of 20 µl of diluted mycoplasma in mycoplasma broth medium were given for experimental infections. Control mice were inoculated with 20 µl sterile mycoplasma broth medium.
L. monocytogenes 10403 serotype 1 was grown on brain-heart infusion (BHI) agar plates (BD Bacto, Sparks, MD), and virulent stocks were maintained by repeated passage through C57BL/6 mice. For infection of mice, log-phase cultures of L. monocytogenes grown in BHI broth were washed twice and diluted in phosphate buffered saline (PBS) to the desired concentration. L. monocytogenes or PBS was injected i.v. into the lateral tail vein at day 17 p.i. with M. pulmonis. Unless otherwise indicated, mice were innoculated with approximately 2.5×104 L. monocytogenes.
Neutrophil Depletion
Neutrophils were depleted using a Gr-1 depleting antibody (RB6-8C5, BioXCell, West Lebanon, NH) one day prior to infection with L. monocytogenes. At day 16 p.i. with M. pulmonis or broth, mice were injected with 200 µg anti-Gr-1 or isotype control antibody. Mice were infected with L. monocytogenes 24 hrs later, and sacrificed at day 3 p.i. with L. monocytogenes. Successful depletion of the Gr-1+ cells was confirmed using flow cytometry in the spleen and peripheral blood on the day of sacrifice (data not shown).
Quantification of Bacterial Growth In Vivo
The number of M. pulmonis CFUs in the lungs and nasal passages were determined as previously described [53]. Briefly, lungs were minced and placed in mycoplasma broth medium. Nasal passages were flushed with 1 ml of mycoplasma broth medium. The samples were sonicated (Vibra cell sonicator; Sonics & Materials/Vibro Cell, Newtown, CT) for 1 min at 50 amplitudes without pulsing. After sonication, serial dilutions (1:10) were prepared, and 20 µl of each dilution was plated onto mycoplasma agar medium. After 7 days of incubation at 37°C, colonies were counted, and the M. pulmonis CFUs recovered from each tissue were calculated.
To determine L. monocytogenes CFUs, the spleen, liver, and lungs from infected mice were homogenized in sterile ddH2O. Whole blood was collected into tubes containing Hank’s balanced salt solution (HBSS) supplemented with 5% FCS and 0.67 mg/ml heparin and was centrifuged at 12,000 rpm for 3 min. Following the removal of the supernatant, the pellet was resuspended in sterile ddH2O. Serial dilutions (1:10) of all of the tissues were prepared, and 50 µl of each dilution was plated on BHI agar plates. After overnight incubation at 37°C, colonies were counted, and the L. monocytogenes CFUs recovered from each tissue were calculated.
Preparation of M. pulmonis Antigen
A crude preparation of M. pulmonis membranes was used for in vitro stimulation and was prepared as previously described [54]. Briefly, M. pulmonis was grown at 37°C in mycoplasma broth medium. Cells were then centrifuged at 10,000 rpm for 20 min, and the pellets were washed and suspended in 2M glycerol. For the subsequent cell lysis, the suspension of cells was sonicated and then forced through a 27-gauge needle into cold sterile distilled water. The unlysed organisms were removed by another round of high speed centrifugation. Supernatants were then centrifuged at 20,000 rpm for 1 hr to obtain mycoplasma membrane preparation. Protein concentration of the membrane preparation, resuspended in sterile PBS, was determined by Bradford assay assay (Bio-Rad, Hercules, CA), and the membranes were stored at −80°C until further use. A final concentration of 5 µg/ml of M. pulmonis membrane preparation was used for in vitro stimulation.
In Vitro Antigen Stimulation
Cervical lymph nodes and spleens were ground between glass slides and red blood cells were subsequently lysed with Tris ammonium chloride (pH 7.2). The resulting cells were then cultured overnight in complete RPMI medium supplemented with 10% FCS (Atlanta Biologicals, Nocross, GA), l-glutamine, vitamins, penicillin/streptomycin, non-essential amino acids, and sodium pyruvate. All supplements were from Invitrogen-Gibco (Carlsbad, CA). Cell culture was performed at 37°C in humidified air containing 5% CO2. Cell cultures were either stimulated overnight with or without M. pulmonis membrane antigen, with 50 ng/ml Phorbol 12-Myristate 13- Acetate (PMA; Sigma-Aldrich, St. Louis, MO), and 500 ng/ml Ionomycin (EMD, Gibbstown, NJ) for the last 5 hrs of the culture and analyzed with flow cytometry, or were stimulated with M. pulmonis membrane antigen or heat-killed L. monocytogenes (MOI 50:1) for two days and the filtered culture supernatants were analyzed using ELISA.
Antibodies and Cell Staining
For cell staining, the following antibodies from BD PharMingen (San Diego, CA) were used: anti-Gr-1 FITC (RB6-8C5), anti-CD11b PE-Cy7 (M1/70), anti-CD3e FITC (145-2C11), anti-CD4 PE-Cy7 (RM4-5), anti-CD8α FITC (53-6.7), anti-NK1.1 PE (PK136), anti-γδ T cell receptor (GL3) FITC, anti-CD16/CD32 (2.4G2), anti-IFN-γ APC (XMG1.2), and anti-IL-17 PE (TC11-18H10). To accomplish intracellular cytokine staining, GolgiPlug containing Brefeldin A (BFA) (BD PharMingen), was added 4 hrs prior to the harvest of the cell cultures. Staining of the cells involved incubation at 4°C for 15 min in staining buffer (PBS + 2% FCS + 0.1% sodium azide) with saturating amounts of the cell-surface antibodies and anti-CD16/CD32 to block Fc receptors. To measure intracellular cytokines, cells were fixed and permeabilized at 4°C for 20 min using the intracellular cytokine staining kit from BD PharMingen. After washing in permeabilization wash buffer, the cells were incubated in saturating amounts of anti-IFN-γ and anti-IL-17 at 4°C for 20 min. Data was acquired and analyzed using a Beckman Coulter Cytomics FC500 (Fullerton, CA).
Luminex Suspension Array
Levels of IL-17 and IFN-γ in the lungs, spleen, and serum were measured using a LINCOplex™ mouse cytokine kit (LINCO Research, Inc., Saint Charles, MO). Lungs and spleen were homogenized using a PRO200 homogenizer (PRO Scientific, Oxford, CT) in PBS containing a protease inhibitor cocktail (Roche, Switzerland). Serum was obtained by removing the supernatant from whole blood following centrifugation at 12,000 rpm for 30 min. IL-17 and IFN-γ concentrations were assessed in the samples according to the protocol provided in the LINCOplex™ kit. Samples were read using a Bio-Plex 100 system (Bio-Rad, Hercules, CA). Cytokine levels were determined by comparison with standard curves generated from the murine recombinant cytokines provided in the LINCOplex™ kit and analyzed using a Bio-Plex Manager software (Bio-Rad).
ELISA
An ELISA was conducted on cell culture supernatants using plate bound purified anti-IL-17 (BD Pharmingen, clone TC11-18H10, 2 µg/ml) capture antibody and biotinylated anti-IL-17 (BD Pharmingen, clone TC11-8H4.1, 2 µg/ml) detection antibody, with quantification by reference to rIL-17 standard (R&D Systems). IL-17 concentrations were measured at 450nm on an EL808 instrument (BioTek).
Statistical Analyses
Analyses of variances (ANOVAs) were conducted on the normally distributed data. Bonferroni t-tests and Tukey-Kramer analyses were used for post-hoc analyses. Kruskal-Wallis tests with Dunn’s multiple comparison post-hoc tests were used to analyze nonparametric data. M. pulmonis CFU data was log transformed prior to analysis, and is represented as such in the figures. A p value of 0.05 or less was considered significant in all cases.
Acknowledgements
The authors would like to thank Joshua Balch and Nicole Dobbs for their excellent technical assistance and Dena Johnson for her critical review of the manuscript. Flow cytometry was performed in the Flow Cytometry and Laser Capture Microdissection Core Facility at The University of North Texas Health Science Center. This research was funded by NIH AI064592 (to R.E.B.) and NIH AI042075 (to J.W.S.).
Abbreviations
- p.i.
post-infection
- LM
Listeria monocytogenes
- Myco
Mycoplasma pulmonis
- BFA
Brefeldin A
- CLN
cervical lymph node
- NP
nasal passage wash
Footnotes
Conflict of Interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Mackaness GB. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J.Exp.Med. 1969;129:973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackaness GB. The immunological basis of acquired cellular resistance. J.Exp.Med. 1964;120:105–120. [PubMed] [Google Scholar]
- 3.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 4.Pasquetto V, Guidotti LG, Kakimi K, Tsuji M, Chisari FV. Host-virus interactions during malaria infection in hepatitis B virus transgenic mice. J.Exp.Med. 2000;192:529–536. doi: 10.1084/jem.192.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams AE, Edwards L, Humphreys IR, Snelgrove R, Rae A, Rappuoli R, Hussell T. Innate imprinting by the modified heat-labile toxin of Escherichia coli (LTK63) provides generic protection against lung infectious disease. J.Immunol. 2004;173:7435–7443. doi: 10.4049/jimmunol.173.12.7435. [DOI] [PubMed] [Google Scholar]
- 6.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J.Exp.Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg RE, Crossley E, Murray S, Forman J. Relative contributions of NK and CD8 T cells to IFN-gamma mediated innate immune protection against Listeria monocytogenes. J.Immunol. 2005;175:1751–1757. doi: 10.4049/jimmunol.175.3.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr.Opin.Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr.Opin.Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Chung DR, Kasper DL, Panzo RJ, Chitnis T, Grusby MJ, Sayegh MH, Tzianabos AO. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J.Immunol. 2003;170:1958–1963. doi: 10.4049/jimmunol.170.4.1958. [DOI] [PubMed] [Google Scholar]
- 13.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J.Exp.Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am.J. Respir.Cell. Mol.Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 15.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J.Infect.Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 16.Kohyama S, Ohno S, Isoda A, Moriya O, Belladonna ML, Hayashi H, Iwakura Y, Yoshimoto T, Akatsuka T, Matsui M. IL-23 enhances host defense against vaccinia virus infection via a mechanism partly involving IL-17. J.Immunol. 2007;179:3917–3925. doi: 10.4049/jimmunol.179.6.3917. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol.Immunol. 2007;51:1139–1147. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 18.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J.Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 19.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J.Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J.Exp.Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect.Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassell GH. Derrick Edward Award Lecture. The pathogenic potential of mycoplasmas: Mycoplasma pulmonis as a model. Rev.Infect.Dis. 1982;(4 Suppl):S18–S34. doi: 10.1093/clinids/4.supplement_1.s18. [DOI] [PubMed] [Google Scholar]
- 23.Simecka JW. Immune responses following mycoplasma infection. In: Blanchard A, Browning G, editors. Mycoplasmas. Molecular biology, pathogenicity, and strategies for control. Norfolk, U.K.: Horizon Bioscience; 2005. pp. 485–534. [Google Scholar]
- 24.Foy HM. Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clin.Infect.Dis. 1993;17 Suppl 1:S37–S46. doi: 10.1093/clinids/17.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- 25.Foy HM, Kenny GE, Cooney MK, Allan ID. Long-term epidemiology of infections with Mycoplasma pneumoniae. J. Infect.Dis. 1979;139:681–687. doi: 10.1093/infdis/139.6.681. [DOI] [PubMed] [Google Scholar]
- 26.Chan ED, Welsh CH. Fulminant Mycoplasma pneumoniae pneumonia. West. J. Med. 1995;162:133–142. [PMC free article] [PubMed] [Google Scholar]
- 27.Sun X, Jones HP, Hodge LM, Simecka JW. Cytokine and chemokine transcription profile during Mycoplasma pulmonis infection in susceptible and resistant strains of mice: Macrophage Inflammatory Protein 1{beta} (CCL4) and Monocyte Chemoattractant Protein 2 (CCL8) and accumulation of CCR5+ Th Cells. Infect.Immun. 2006;74:5943–5954. doi: 10.1128/IAI.00082-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes.Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Zhang D, Lyubynska N, Wolters PJ, Killeen NP, Baluk P, McDonald DM, Hawgood S, Caughey GH. Mast cells protect mice from Mycoplasma pneumonia. Am.J. Respir.Crit. Care Med. 2006;173:219–225. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Zhang D, Zhang H, Wolters PJ, Killeen NP, Sullivan BM, Locksley RM, Lowell CA, Caughey GH. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J.Exp.Med. 2006;203:2907–2917. doi: 10.1084/jem.20061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hickman-Davis JM, Lindsey JR, Matalon S. Cyclophosphamide decreases nitrotyrosine formation and inhibits nitric oxide production by alveolar macrophages in mycoplasmosis. Infect.Immun. 2001;69:6401–6410. doi: 10.1128/IAI.69.10.6401-6410.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker RF, Davis JK, Blalock DK, Thorp RB, Simecka JW, Cassell GH. Pulmonary clearance of Mycoplasma pulmonis in C57BL/6N and C3H/HeN mice. Infect.Immun. 1987;55:2631–2635. doi: 10.1128/iai.55.11.2631-2635.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin RJ, Chu HW, Honour JM, Harbeck RJ. Airway inflammation and bronchial hyperresponsiveness after Mycoplasma pneumoniae infection in a murine model. Am.J. Respir.Cell. Mol.Biol. 2001;24:577–582. doi: 10.1165/ajrcmb.24.5.4315. [DOI] [PubMed] [Google Scholar]
- 34.Chu HW, Breed R, Rino JG, Harbeck RJ, Sills MR, Martin RJ. Repeated respiratory Mycoplasma pneumoniae infections in mice: effect of host genetic background. Microbes.Infect. 2006;8:1764–1772. doi: 10.1016/j.micinf.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Pamer EG. Immune responses to Listeria monocytogenes. Nat.Rev.Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 36.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J.Exp.Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czuprynski CJ, Brown JF, Maroushek N, Wagner RD, Steinberg H. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J.Immunol. 1994;152:1836–1846. [PubMed] [Google Scholar]
- 38.Rogers HW, Unanue ER. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect.Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samsom JN, Annema A, Groeneveld PH, van RN, Langermans JA, van FR. Elimination of resident macrophages from the livers and spleens of immune mice impairs acquired resistance against a secondary Listeria monocytogenes infection. Infect.Immun. 1997;65:986–993. doi: 10.1128/iai.65.3.986-993.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinto AJ, Stewart D, van RN, Morahan PS. Selective depletion of liver and splenic macrophages using liposomes encapsulating the drug dichloromethylene diphosphonate: effects on antimicrobial resistance. J.Leukoc.Biol. 1991;49:579–586. doi: 10.1002/jlb.49.6.579. [DOI] [PubMed] [Google Scholar]
- 41.Conlan JW, North RJ. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J.Exp.Med. 1991;174:741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conlan JW, North RJ. Monoclonal antibody NIMP-R10 directed against the CD11b chain of the type 3 complement receptor can substitute for monoclonal antibody 5C6 to exacerbate listeriosis by preventing the focusing of myelomonocytic cells at infectious foci in the liver. J.Leukoc.Biol. 1992;52:130–132. doi: 10.1002/jlb.52.1.130. [DOI] [PubMed] [Google Scholar]
- 43.Rosen H, Gordon S, North RJ. Exacerbation of murine listeriosis by a monoclonal-antibody specific for the type-3 complement receptor of myelomonocytic cells - absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J.Exp.Med. 1989;170:27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 45.Harty JT, Schreiber RD, Bevan MJ. CD8 T cells can protect against an intracellular bacterium in an interferon gamma-independent fashion. Proc.Natl.Acad.Sci.U.S.A. 1992;89:11612–11616. doi: 10.1073/pnas.89.23.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyamoto M, Emoto M, Emoto Y, Brinkmann V, Yoshizawa I, Seiler P, Aichele P, Kita E, Kaufmann SH. Neutrophilia in LFA-1-deficient mice confers resistance to listeriosis: possible contribution of granulocyte-colony-stimulating factor and IL-17. J.Immunol. 2003;170:5228–5234. doi: 10.4049/jimmunol.170.10.5228. [DOI] [PubMed] [Google Scholar]
- 47.Cartner SC, Simecka JW, Briles DE, Cassell GH, Lindsey JR. Resistance to mycoplasmal lung disease in mice is a complex genetic trait. Infect.Immun. 1996;64:5326–5331. doi: 10.1128/iai.64.12.5326-5331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hickman-Davis JM, Michalek SM, Gibbs-Erwin J, Lindsey JR. Depletion of alveolar macrophages exacerbates respiratory mycoplasmosis in mycoplasma-resistant C57BL mice but not mycoplasma-susceptible C3H mice. Infect.Immun. 1997;65:2278–2282. doi: 10.1128/iai.65.6.2278-2282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 50.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat.Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cartner SC, Lindsey JR, Gibbs-Erwin J, Cassell GH, Simecka JW. Roles of innate and adaptive immunity in respiratory mycoplasmosis. Infect.Immun. 1998;66:3485–3491. doi: 10.1128/iai.66.8.3485-3491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davidson MK, Davis JK, Lindsey JR, Cassell GH. Clearance of different strains of Mycoplasma pulmonis from the respiratory tract of C3H/HeN mice. Infect.Immun. 1988;56:2163–2168. doi: 10.1128/iai.56.8.2163-2168.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolard MD, Hodge LM, Jones HP, Schoeb TR, Simecka JW. The upper and lower respiratory tracts differ in their requirement of IFN-gamma and IL-4 in controlling respiratory mycoplasma infection and disease. J.Immunol. 2004;172:6875–6883. doi: 10.4049/jimmunol.172.11.6875. [DOI] [PubMed] [Google Scholar]
- 54.Simecka JW, Cassell GH. Serum antibody and cellular responses in LEW and F344 rats after immunization with Mycoplasma pulmonis antigens. Infect.Immun. 1987;55:731–735. doi: 10.1128/iai.55.3.731-735.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]