Abstract
Bmx non-receptor tyrosine kinase has an established role in endothelial and lymphocyte signaling, however its role in the heart is unknown. To determine whether Bmx participates in cardiac growth, we subjected mice deficient in the molecule (Bmx KO mice) to transverse aortic constriction (TAC). In comparison to WT mice, which progressively developed massive hypertrophy following TAC, Bmx KO mice were resistant to TAC-induced cardiac growth at the organ and cell level. Loss of Bmx preserved cardiac ejection fraction and decreased mortality following TAC. These findings are the first to demonstrate a necessary role for the Tec family of tyrosine kinases in the heart and reveal a novel regulator (Bmx) of pressure overload-induced hypertrophic growth.
Keywords: tyrosine kinase, cardiac hypertrophy, signal transduction
Introduction
Bmx (also known as Etk) is a member of the Tec family of non-receptor tyrosine kinases that has a critical role in B-cell development and proliferation.1,2 The Tec family was originally discovered by the observation that mutations in the Btk family member induce x-chromosome linked agammaglobulinemia (XLA),3,4 an immunological disorder characterized by impaired antibody production and B-lymphocyte development. Although structurally similar to Btk, Bmx does not participate in XLA. Bmx has been shown to regulate wound healing in the epidermis5 and, more recently, the response of skeletal muscle to prolonged ischemia.6
Very little is known about the functional significance of Bmx or other members of the Tec family in the heart. An early study documented expression of Bmx mRNA in the endocardium and vasculature of the adult myocardium,7 but it was only recently that reports suggested Bmx may be activated by nitric oxide8 or ischemic preconditioning9 in the heart. While non-receptor tyrosine kinases have been functionally implicated in cardiac phenotype, the role of the Tec family is unknown. In the present study, we demonstrate that absence of Bmx function prevents the hypertrophic response of the myocardium to pressure overload at the anatomical, functional and cell level. Our investigation uses genetic tools and physiologic analyses to demonstrate a necessary role of this family of tyrosine kinase in cardiac function during stress.
Materials & Methods
Please see the detailed Materials and Methods section in the online supplement available at http://circres.ahajournals.org for a description of the mouse model of transverse aortic banding, the Bmx KO mouse line, assessment of cardiac function by echocardiography, histology, gene expression analyses and western blotting.
Results
To determine whether Bmx non-receptor tyrosine kinase is involved in cardiac signaling, we examined basal cardiac phenotype in homozygous Bmx KO mice.5 As monitored by echocardiography, these mice manifest no abnormalities in cardiac function at baseline resting conditions.
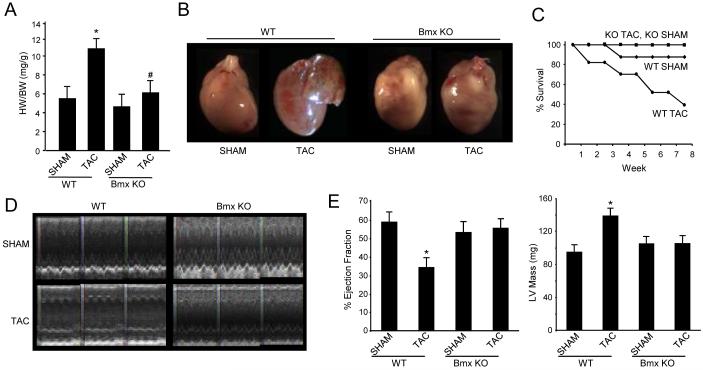
Given that Bmx has been implicated in growth and proliferative processes in non-cardiac cells, we sought to examine the role of this protein in cardiac growth following pressure overload. TAC surgery was performed on adult male Bmx KO or strain-matched balb/c WT controls after which mice were allowed to recover for up to 8 weeks with weekly monitoring of cardiac parameters by ECHO (efficacy of TAC operation was determined by evaluation of carotid pressure gradient, data not shown). In WT mice, TAC caused robust hypertrophy at 8 weeks following the surgery, as evidenced by increased heart weight to body weight (Fig. 1A) or heart weight to tibia length (Online Fig. I A) ratios in TAC as compared to SHAM WT mice. Gross images also demonstrate changes in cardiac morphology, notably left atrial distention in TAC operated WT mice (Fig. 1B). Remarkably, Bmx KO mice were resistant to pressure overload-induced hypertrophic growth even after 8 weeks of stress (Figs. 1A, 1B and Online Fig. I A), demonstrating a previously unrecognized role for Bmx signaling in the heart and implicating the molecule as a necessary component of cardiac hypertrophy following stress.
Figure 1. Loss of Bmx prevents pressure overload-induced hypertrophy: functional and anatomical indices.
A. Mice were sacrificed 8 weeks after TAC or SHAM surgery and heart weight to body weight (HW/BW) ratio determined (* indicates p=0.004 vs. WT SHAM for HW/BW; # indicates p=NS vs. Bmx KO SHAM; bars are SEM). B. Images of hearts from mice sacrificed 8 weeks after surgery. C. Kaplan-Meier survival curve (starting n values: WT TAC, 23; WT SHAM, 9; Bmx KO TAC, 9; Bmx KO SHAM, 7). D. M-mode ECHO images from WT and Bmx KO mice. E. Ejection fraction and left ventricular mass data obtained by ECHO at 8 weeks after SHAM or TAC surgery (* indicates p<0.01, bars are SEM; n values for all groups: WT TAC, 6; WT SHAM, 9; Bmx KO TAC, 7; Bmx KO SHAM, 9).
To evaluate the role of Bmx in functional adaptation of the myocardium to pressure overload, ECHO was used to evaluate cardiac parameters every 5-7 days following TAC or SHAM surgery. Figure 1D shows M-mode images taken 8 weeks following surgery; witness the preservation of wall motion in the TAC operated Bmx KO mice as compared with their WT counterparts (see Online Table I for additional cardiac parameters). This sustained function translated into preserved ejection fraction and fractional shortening, as well as resistance to left ventricular hypertrophy in the Bmx KO myocardium (Fig. 1E and Online Fig. I B). The functional benefits conferred by loss of Bmx also manifest as better survival following TAC (Fig. 1C). At this time it is unknown whether these benefits extend beyond 8 weeks.
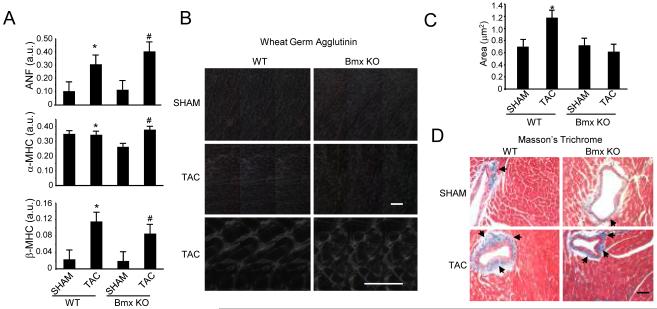
Cardiac hypertrophy induced by pressure overload or other stimuli can involve a conserved gene program that participates in reverting the myocyte to a more fetal-like state and precipitating pathological remodeling of the myocardium. We examined markers of this program in the Bmx KO myocardium and found that increases in ANF expression and aspects of the myosin heavy chain switch following TAC remain intact in the absence of Bmx (Fig. 2A). Bmx and other Tec family members have been implicated in regulating transcription in the heart10 and other cell types 11,12 however, Bmx does not appear to be required for critical aspects of the fetal gene program. These features, along with the cardiac mass data, are reminiscent of the response of the NOS3 deficient mouse to pressure overload,13 in which the loss of NOS3 protects against hypertrophic growth.
Figure 2. Altered cellular level responses in Bmx KO mice following pressure overload.
A. RT-PCR analysis of cardiac hypertrophic marker genes was performed 8 weeks after surgery (* indicates p=NS vs. WT SHAM for ANF and α-MHC and p=0.01 for β-MHC; # indicates p=0.05 vs. Bmx KO SHAM for ANF and β-MHC and p=0.04 for α-MHC; n=3 per group; 2 technical replicates; all data normalized to GAPDH). B. WGA staining to evaluate tissue morphology and cell size (Top four panels, 20X magnification; bottom two, 60X; Scale bars: 50μm). C. Quantitative data from WGA experiments (n=3 animals per group, 50 cells per animal; * indicates p=0.002 vs. WTSHAM). D. Trichrome staining to examine fibrotic deposition (20X magnification; Scale bar: 50μm).
Having demonstrated that loss of Bmx renders mice resistant to the morphological changes associated with pressure overload and linking this to preserved function of the cardiac pump, we next sought to examine cellular level changes in the wake of TAC and the role of Bmx. Ventricular sections were analyzed by Masson’s trichrome and wheat germ agglutinin (WGA) staining. WGA demarcates plasma membrane and was thus used for the dual purpose of evaluating changes in gross myocyte morphology (which were not observed, Fig. 2B) as well as for calculating myocyte size. This latter index revealed that, akin to the organ and functional data, absence of Bmx function prevents hypertrophic growth at the myocyte level (Fig. 2B and 2C). Histological analyses of cardiac sections by trichrome identified regions of fibrotic deposition, in particular around macrovasculature, following TAC that appeared unaltered by the loss of Bmx (Fig. 2D). We also used TUNEL staining to examine apoptosis in the hearts of WT and Bmx KO mice and observed no difference in the degree of TUNEL positivity between these groups (data not shown).
To investigate local signaling mechanisms through which Bmx is necessary for pressure overload hypertrophy, we examined known regulators of the protein also implicated in cardiac growth. In WT mice, we observed increased Bmx activation (as detected by phosphorylation on tyrosine 40, Online Fig. I C) as well as a trend towards increased total Bmx protein following TAC. Other investigators have implicated altered association of Bmx with membranous fractions,14,15 as well as caspase-dependent degradation of the protein,16 as means of regulation; the contribution of these and other processes to available active Bmx in the basal myocardium or following TAC are unknown at this time. Because Akt is a known regulator of cardiac growth17 and has been implicated in Bmx signaling in non-cardiac cells,12,18 we examined Akt total protein and phosphorylation following TAC. Absence of Bmx function did not alter total Akt following TAC (Online Fig. I E). Likewise, proximal (PDK) and distal (GSK3β) signaling partners of Akt were ostensibly unaffected by the loss of Bmx. Furthermore, the behavior of Akt phosphorylation (at either T308 or S473) as detected by western blotting was not significantly changed between the normal and Bmx deficient hearts.
Discussion
This study provides the first definitive evidence for a role of the Tec family of tyrosine kinases in cardiac phenotype using genetic tools and physiologic analyses. We demonstrate that Bmx is a necessary component of the morphological, functional and cell level responses of the heart to pressure overload. Whether Bmx plays a role in other forms of cardiac hypertrophy, such as that induced by neural/hormonal stimuli, will be examined in future studies. A recent study showed that Bmx activity is necessary for acute and prolonged responses to ischemia in the rodent hindlimb.6 The short term recovery required Bmx signaling not in the skeletal muscle, but rather in bone marrow-derived cells. Given the increasingly recognized role of non-cardiac cells in injury responses in the myocardium, we recognize that some of the deleterious affects Bmx exerts in the WT animal may arise from cells other than cardiomyocytes.
As mentioned previously, the Bmx KO phenotype following TAC shares several features with the NOS3 KO, including resistance to hypertrophic growth, preserved cardiac function and undisturbed fetal gene activation.13 It is reasonable to hypothesize that Bmx modulates signaling via NOS3 during pressure overload; however, additional details regarding the relationship between these signaling systems will require further experimentation. The Bmx KO mice do not display altered cardiac development, indicating that while Bmx is indispensable in the response of the adult heart to pressure overload, it appears to not be required for normal embryonic cardiac development. These findings lend credence to task-specific roles for different signaling kinase family members and support the function of Bmx as a stress-activated isoform of the Tec family.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the NIH (TMV and YW), AHA (TMV and YW), Laubisch Endowment at UCLA (TMV and YW) and Helsinki University Hospital Funds (KA).
Footnotes
Disclosures: None.
References
- 1.Tamagnone L, Lahtinen I, Mustonen T, Virtaneva K, Francis F, Muscatelli F, Alitalo R, Smith CI, Larsson C, Alitalo K. BMX, a novel nonreceptor tyrosine kinase gene of the BTK/ITK/TEC/TXK family located in chromosome Xp22.2. Oncogene. 1994;9:3683–8. [PubMed] [Google Scholar]
- 2.Qiu Y, Robinson D, Pretlow TG, Kung HJ. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3 ′-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci. 1998;95:3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, Sparkes RS, Kubagawa H, Mohandas T, Quan S, Belmont JW, Cooper MD, Conley ME, Witte ON. Deficient Expression of a B-Cell Cytoplasmic Tyrosine Kinase in Human X-Linked Agammaglobulinemia. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 4.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–33. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 5.Rajantie I, Ekman N, Iljin K, Arighi E, Gunji Y, Kaukonen J, Palotie A, Dewerchin M, Carmeliet P, Alitalo K. Bmx tyrosine kinase has a redundant function downstream of angiopoietin and vascular endothelial growth factor receptors in arterial endothelium. Mol Cell Biol. 2001;21:4647–4655. doi: 10.1128/MCB.21.14.4647-4655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y, Luo Y, Tang SB, Rajantie I, Salven P, Heil M, Zhang R, Luo DH, Li XH, Chi HB, Yu J, Carmeliet P, Schaper W, Sinusas AJ, Sessa WC, Alitalo K, Min W. Critical function of Bmx/Etk in ischemia-mediated arteriogenesis and angiogenesis. J Clin Invest. 2006;116:2344–2355. doi: 10.1172/JCI28123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekman N, Lymboussaki A, Vastrik I, Sarvas K, Kaipainen A, Alitalo K. Bmx tyrosine kinase is specifically expressed in the endocardium and the endothelium of large arteries. Circulation. 1997;96:1729–1732. doi: 10.1161/01.cir.96.6.1729. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Ping P, Wang GW, Lu M, Pantaleon D, Tang XL, Bolli R, Vondriska TM. Bmx, a member of the Tec family of nonreceptor tyrosine kinases, is a novel participant in pharmacological cardioprotection. Am J Physiol. 2004;287:H2364–6. doi: 10.1152/ajpheart.00416.2004. [DOI] [PubMed] [Google Scholar]
- 9.Mathur P, Kaga S, Zhan LJ, Das DK, Maulik N. Potential candidates for ischemic preconditioning-associated vascular growth pathways revealed by antibody array. Am J Physiol. 2005;288:H3006–H3010. doi: 10.1152/ajpheart.01203.2004. [DOI] [PubMed] [Google Scholar]
- 10.Willey CD, Palanisamy AP, Johnston RK, Mani SK, Shiraishi H, Tuxworth WJ, Zile MR, Balasubramanian S, Kuppuswamy D. STAT3 activation in pressure-overloaded feline myocardium: role for integrins and the tyrosine kinase BMX. Int J Biol Sci. 2008;4:184–99. doi: 10.7150/ijbs.4.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saharinen P, Ekman N, Sarvas K, Parker P, Alitalo K, Silvennoinen O. The Bmx tyrosine kinase induces activation of the Stat signaling pathway, which is specifically inhibited by protein kinase Cdelta. Blood. 1997;90:4341–53. [PubMed] [Google Scholar]
- 12.Chau CH, Clavijo CA, Deng HT, Zhang QZ, Kim KJ, Qiu Y, Le AD, Ann DK. Etk/Bmx mediates expression of stress-induced adaptive genes VEGF, PAI-1, and iNOS via multiple signaling cascades in different cell systems. Am J Physiol. 2005;289:C444–C454. doi: 10.1152/ajpcell.00410.2004. [DOI] [PubMed] [Google Scholar]
- 13.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–31. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vargas L, Nore BF, Berglof A, Heinonen JE, Mattsson PT, Smith CI, Mohamed AJ. Functional interaction of caveolin-1 with Bruton’s tyrosine kinase and Bmx. J Biol Chem. 2002;277:9351–7. doi: 10.1074/jbc.M108537200. [DOI] [PubMed] [Google Scholar]
- 15.Schwartzberg PL, Finkelstein LD, Readinger JA. TEC-family kinases: regulators of T-helper-cell differentiation. Nat Rev Immunol. 2005;5:284–95. doi: 10.1038/nri1591. [DOI] [PubMed] [Google Scholar]
- 16.Wu YM, Huang CL, Kung HJ, Huang CY. Proteolytic activation of ETK/Bmx tyrosine kinase by caspases. J Biol Chem. 2001;276:17672–8. doi: 10.1074/jbc.M010964200. [DOI] [PubMed] [Google Scholar]
- 17.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–65. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 18.Zhang R, Xu Y, Ekman N, Wu Z, Wu J, Alitalo K, Min W. Etk/Bmx transactivates vascular endothelial growth factor 2 and recruits phosphatidylinositol 3-kinase to mediate the tumor necrosis factor-induced angiogenic pathway. J Biol Chem. 2003;278:51267–76. doi: 10.1074/jbc.M310678200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.