Abstract
Polyamines are essential metabolites in eukaryotes participating in a variety of proliferative processes, and in trypanosomatid protozoa play an additional role in the synthesis of the critical thiol trypanothione. Whereas the polyamine biosynthesis arising from L-ornithine has been well studied in protozoa, the metabolic origin(s) of L-ornithine have received less attention. Arginase (EC 3.5.3.1) catalyzes the enzymatic hydrolysis of L-arginine to L-ornithine and urea, and we tested the role of arginase in polyamine synthesis by the generation of an arg− knockout in Leishmania major by double targeted gene replacement. This mutant lacked arginase activity and required the nutritional provision of polyamines or L-ornithine for growth. A complemented line (arg−/+ARG) expressing arginase from a multicopy expression vector showed 30-fold elevation of arginase activity, similar polyamine and ornithine levels as the wild-type, and resistance to the inhibitors α-difluoromethylornithine (DFMO) and Nω-hydroxy-L-arginine (NOHA). This established that arginase is the major route of polyamine synthesis in promastigotes cultured in vitro. The arg− parasites retained the ability to differentiate normally to the infective metacyclic stage, and were able to induce progressive disease following inoculation into susceptible BALB/c mice, albeit less efficiently than WT parasites. These data suggest that the infective amastigote form of Leishmania, which normally resides within an acidified parasitophorous vacuole, can survive in vivo through salvage of host polyamines and/or other molecules, aided by the tendency of acidic compartments to concentrate basic metabolites. This may thus contribute to the relative resistance of Leishmania to ornithine decarboxylase (ODC) inhibitors. The availability of infective, viable, arginase-deficient parasites should prove useful in dissecting the role of L-arginine metabolism in both pro- and anti-parasitic responses involving host nitric oxide synthase, which requires L-arginine to generate NO.
Keywords: polyamines, L-arginine, null mutant, infections, nitric oxide
1. Introduction
L-arginine is a critical metabolite for living organisms, which additionally plays key and sometimes opposing roles in host-pathogen interactions. Constitutive functions include its requirement for protein synthesis, and as an intermediate (through L-ornithine) leading to essential polyamine synthesis [1]. As one of the substrates of nitric oxide synthases (NOS), L-arginine plays a critical role through the activity of inducible NOS in generating NO required for the control of many parasites, including Leishmania [2]. Conversely, L-arginine consumption through the activity of arginase deprives NOS of substrate and mitigates NO production [3]. The opposing roles for L-arginine can be seen by the fact that immune cells typically synthesize NOS or arginase, but not both, depending on their role in host defense and pathology [4, 5]. Superimposed upon this dynamic is the fact that many pathogens acquire L-arginine from the host and synthesize arginase themselves to obtain L-ornithine. While the role of the host arginase has received considerable study in leishmaniasis [6, 7], less attention has been paid to the roles of similar activities encoded by the parasite in these processes. In this work we focus on the role of arginase in the trypanosomatid protozoan Leishmania major, the agent of Old World cutaneous leishmaniasis.
Arginase (ARG, EC 3.5.3.1) catalyzes the enzymatic hydrolysis of L-arginine to L-L-ornithine and urea. Two arginase isoforms (arginase I and II) have been identified in mammals which conserve high structural and kinetic homologies, but differing on subcellular localization, tissue distribution, regulation of expression and immunological reactivity [8, 9]. Arginase I is a cytosolic enzyme that functions primarily in the urea cycle, whereas arginase II is a mitochondrial protein potentially involved in proline, glutamate and polyamine synthesis. Both arginases I and II are expressed in macrophages, however arginase I is induced in resident macrophages by T-helper type 2 lymphocytes (Th2) cytokine stimulation, and up-regulation of both arginases is inhibited by Th1 cytokines and regulate polyamine synthesis [10]. Leishmania parasites contain a single arginase gene (ARG), whose biochemical properties and expression has been studied in L. amazonensis and L. mexicana [11–13]. Studies of an arginase null mutant L. mexicana showed these were auxotrophic for polyamines, an essential metabolite for parasite growth [12, 14] attributed largely to their role in the synthesis of the essential cofactor trypanothione [15–17]. L. mexicana and L. amazonensis arginases (ARG) are compartmentalized in glycosomes, although localization to this site does not appear to be essential for its role in polyamine synthesis [12, 13]. Further studies showed that the L. mexicana arg− mutant retained infectivity to mice, albeit with somewhat attenuated survival attributed at least in part to increased host NO production and parasite killing, presumably due to increased L-arginine availability [18].
In this work we begin a similar analysis of the role of parasite arginase in the immune response to L. major. While similarly an agent of cutaneous leishmaniasis, the underlying biology of L. major vs. L. mexicana differ in many respects. Members of the Old World Leishmania reside within ‘tight’ parasitophorous vacuoles typically bearing a single amastigotes, whereas New World Leishmania including L. mexicana reside in ‘spacious’ vacuoles typically bearing many amastigotes [19]. Similarly the immunological mechanisms of host defense differ considerably between these groups [20]. Lastly, even when the biochemical role is conserved in evolution, the consequences of genetic ablation can differ greatly; for example, loss of the Golgi GDP-mannose transporter LPG2 gives a strong ‘persistence without pathology’ phenotype in L. major, while amastigote virulence is unaffected in L. mexicana [21]. Thus, we describe here the generation and characterization of arg− mutants of L. major, as a prelude to understanding its role in this important group of parasites.
2. Materials and Methods
2.1. Parasite Culture
L. major LV39c5 (LV39cl5; RHO/SU/59/P) promastigotes were cultured at 26°C in M199 supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS) or 1% (w/v) bovine serum albumin (BSA) fraction V (Sigma) where indicated.
2.2. Cloning of L. major ARG gene and molecular targeting constructs
The 990 L. major ARG ORF (LmjF35.1480; www.genedb.org) fragment was amplified by PCR from genomic DNA using the primers SMB1970 and SMB1971 (Table 1). The resulting fragment was digested with SmaI and BamHI and inserted into the similarly digested expression vector pXG [22], yielding pXG-ARG (B5014). Southern blot hybridizations was performed as described [23], using either the ARG ORF amplicon described above, or a targeting-fragment flanking probe prepared by PCR using LV39c5 genomic DNA template and oligonucleotides SMB1972 and SMB1973 (Table 1).
Table 1.
Oligonucleotides used in this work
| Oligo Number |
Sequencea | Purposed |
|---|---|---|
| SMB1966 | GCTCTAGACCACGCACCGCATGTTTCACGCG | ARG 5’ flank (F) |
| SMB1967 | GGACTAGTGATGAACGGGGGCGGGTGGTGGTG | ARG 5’ flank (R) |
| SMB1977 | CGGGATCCATGTGTGTGTGTGTGCGCGC | ARG 3’ flank (F) |
| SMB1974 | GGAATTCACATCCCCCACTGCTGCCAATCCA | ARG 3’ flank (R) |
| SMB1289 | CGCCTCACTAGTATGAAAAAG-CCTGAACTC | HYG ORF (F) |
| SMB1594 | CGGGATCCCTATTCCTTTGCCCTCGGACGA | HYG ORF (F) |
| SMB1568 | TTAGATGGATCCTCAGGCACCGGGCTTGCG | PAC ORF (R) |
| SMB1569 | TATGATACTAGTATGACCGAGTACAAGCCCAC | PAC ORF (F) |
| SMB1972 | ACTCGACGCGTTCCTGTGGCAAGG | external flanking probe (F) |
| SMB1973 | CATGCTCGCGCACGCGCGTTTCG | external flanking probe (R) |
| SMB1970 | TCCCCCGGGCCACCATGGAGCACGTGCAGCAGTACAAGTTCb | ARG add-back (F) |
| SMB1971 | CGCGGATCCCTACAGCTTGGCGTCCTTACGCGGGG | ARG add-back (R) |
| SMB1992 | CGAAACTACAAAAAAAAGAGGGTGCAAGTCTGCTGTTTTTCATTCAGTCTCATCGGCACCCACCCAACCAATATTCGAAGCCACCACCACCCATGAAAAAGCCTGAACTCc | 92-bp from 5’flank and 18-bp from HYG (F) |
| SMB1993 | CGCACAGCTAAAAATGAGGCCGTTTCGTCACATACATACATACGCACTGACAGACACGCTTGAAGGTAGTGCGCGCGCACACACACACACATCTATTCCTTTGCCCTCGGc | 92-bp from 3’flank and 18-bp from HYG (R) |
| SMB2016 | CGAAACTACAAAAAAAAGAGGGTGCAAGTCTGCTGTTTTTCATTCAGTCTCATCGGCACCCACCCAACCAATATTCGAAGCCACCACCACCCATGACCGAGTACAAGCCCc | 92-bp from 5’ flank and 18-bp from PAC (F) |
| SMB2017 | CGCACAGCTAAAAATGAGGCCGTTTCGTCACATACATACATACGCACTGACAGACACGCTTGAAGGTAGTGCGCGCGCACACACACACACATTCAGGCACCGGGCTTGCGc | 92-bp from 5’ flank and 18-bp from PAC (F) |
| SMB2000 | CGAAACTACAAAAAAAAGAGGGTG | Re-amplified Long Oligo targeting(F) |
| SMB2001 | CGCACAGCTAAAAATGAGGCCGTT | Re-amplified Long Oligo targeting (R) |
Underlined sequence indicates restriction site;
Bold sequence indicates optimized translation initiation sequence;
Underlined and italicized sequence indicates 18-bp corresponding to antibiotic selection marker (HYG or PAC);
Orientation of primers - F, forward; R, reverse.
The 5’ ARG flanking region was PCR amplified from LV39c5 genomic DNA using oligonucleotides SMB1966 and SMB1967 (see Table I). The 1.0 kb amplicon was digested with XbaI and SpeI and inserted into similarly digested pBluescript SK. The 3’ ARG flanking region was PCR amplified from LV39c5 genomic DNA using the oligonucleotides SMB1968 and SMB1969. The 1.0 kb amplicon was digested with BamHI and EcoRI and inserted into the construct above which had been digested similarly, yielding an intermediate into which drug markers could be inserted between the 5’ and 3’ ARG flanking regions.
The HYG ORF was obtained by PCR amplification using pXG63HYG (B3318) template DNA with the oligonucleotides SMB1289 and SMB1594. The 1.0 kb amplicon was digested with SpeI and BamHI and inserted into the intermediate construct above, yielding pBluescript SK 5’-arg-HYG-3’arg (B5012). The PAC ORF was obtained by PCR amplification with oligonucleotides SMB1569 and SMB1568 using pXGPAC (B3325) template DNA. The 600 bp amplicon was cut with SpeI and BamHI and cloned into the intermediate construct above, yielding pBluescript SK 5’-arg-PAC-3’arg (B5013).
2.3. Long oligonucleotide fragment targeting
We generated targeting fragments by direct PCR as follows. The forward primer consisted of 92 nt of the 5’ ARG flanking sequence joined to 18 nt of the HYG resistance marker ORF, while the reverse primer consisted of 92 nt of reverse complement of the 3’ 92 nt ARG flanking sequence, joined to the last 18 nt of the HYG resistance marker (SMB1992, and SMB1993). These were used with pXG63HYG DNA template in a PCR reaction (50 mM KCl, 10 mM Tris-HCl, pH 9.0, 1% (v/v) Triton X-100, 2.5 mM MgCl, 200µM of each dNTP, 50 pmol of each long oligonucleotide primer, and 2.5 units of Taq DNA polymerase in a final volume of 50 µl). A 1239-bp product was generated after 17 cycles of denaturation at 95°C for 1 min, annealing at 50 °C for 1 min, and extension at 72 °C for 1 min. The expected 1239 bp PCR product was obtained and purified after electrophoresis on 2% low melting point agarose TAE gels. To obtain sufficient DNA for transfection, the purified DNA fragment was used as template for PCR re-amplification with shorter terminal 24 nt primers (SMB2000 and SMB2001), and this product was used for electroporation after purification.
2.4. Generation of arg− null mutants in L. major
Transfection was performed by the high voltage electroporation method [24]. Briefly, L. major WT promastigotes were grown up to 5 × 106 cells per ml, washed in cold cytomix (120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4, 25 mM Hepes pH 7.6, 2 mM EDTA, 5 mM MgCl2) and resuspended in the same solution at 1 × 108 cells per ml. 0.5 ml aliquots were electroporated twice in 0.4 cm electrode gap cuvettes (1.5 kV, 25 µF using a Bio-Rad Gene Pulser II apparatus), transferred to 10 ml of M199 plus 10% FCS and incubated at 26°C for 8 h in absence of antibiotics. They were then collected by centrifugation and resuspended in 100 µl of fresh M199 plus 10% FCS prior to plating on semisolid M199 medium containing antibiotics.
Targeting fragments for ARG were linearized with XbaI+EcoRI, (2.9 kb for HYG cassette and 2.6 kb for PAC) and purified before transfection into L. major LV39c5. For replacement of the first ARG allele, WT promastigotes were electroporated with 5 µg of the linear 2.9-kb XbaI-EcoRV ARG:HYG targeting fragment, and transfectants obtained following plating on semisolid medium containing 30 µg/ml HYG. Numerous clonal lines were obtained and verified. In contrast electroporations of the 1239-bp PCR fragment from the long-oligonucleotide procedure yielded only a single colony. These heterozygous replacements (formally ARG/ Δarg::HYG) are collectively referred to as +/HYG.
Several +/HYG heterozygotes were subjected to transfection with 5 µg of the 2.6-kb XbaI-EcoRV ARG::PAC fragment and plating on semisolid media containing 30 µg/ml hygromycin B and 10 µg/ml puromycin and 1 mM putrescine in order to circumvent its auxotrophy. Numerous clonal lines were obtained and Successful replacements of arg alleles in three were confirmed by Southern blot analysis; these Δarg::HYG/Δarg::PAC lines are referred to as arg−.
To restore ARG expression, several independent arg− mutants were transfected with pXG-ARG and plated on semisolid media containing 10 µg/ml G418. Several clonal lines were obtained (Δarg::HYG/Δarg::PAC [pXG-ARG]) were obtained, referred to as arg− /+pXG-ARG. Three independent arg− null clones, as well as their respective add-backs were used in the studies reported here, with similar results (data not shown).
2.5. Arginase Activity
Arginase activity was measured using a micromethod previously described [25]. Leishmania cell pellets (2 ×108 promastigotes) were washed twice with PBS, and resuspended in 0.5 ml of 10 mM MnCl2, 50 mM Tris-HCl, pH 7.5 containing 5 µg pepstatin, 5 µg aprotinin and 5 µg antipain as protease inhibitors, sonicated on ice (15W, 10s) and centrifuged at 10.000 g for 20 min at 4°C. The enzyme was then activated for 10 min at 55°C, and L-arginine hydrolysis was initiated by the addition of 25 µl of 0.5 M L-arginine, pH 9.7 to a 25 µl aliquot of the previously activated lysate. Incubation was performed at 37° C for 60 min and the reaction stopped by the addition of 400 µl of an acid mixture containing H2SO4, H3PO4 and H2O (1:3:7). After the addition of 25 µl 9% (w/v) α-isonitrosopropiophenone (ISPF) (dissolved in 100% ethanol), the samples were heating up to 100°C for 45 min and standing in the dark for 10 min. The urea formed was colorimetrically quantified at 540 nm. A calibration curve was prepared with increasing amounts of urea between 1.5 and 30 µg. In this case to 100 µl of urea solution at the appropriate concentrations, 400 µl of the acid mixture and 25 µl ISPF were added, and the procedure followed as describe above.
2.6. Polyamine determination
Putrescine and spermidine were identified and quantified by high performance liquid chromatography (HPLC) using a prederivatization method previously described [26]. Cells were harvested and washed twice with PBS pH 7.4 and the last pellet was disintegrated with 5% (w/v) trichloroacetic acid overnight. The extracts were centrifuged (10.000g, 15 min) and 200 µl supernatant were neutralized with 200 µl of a saturated NaHCO3 solution. The mixture was dansylated at 50°C overnight with 400 µl of a solution containing 20 mg dansyl chloride/ml in acetone (HPLC grade). The polyamines were extracted twice with toluene (HPLC grade), evaporated under nitrogen stream and the residue dissolved in 1 ml of acetonitrile (HPLC grade), prior analysis by HPLC on a C18 reverse-phase column. Dansyl polyamines were detected with a fluorescence spectrophotometer (excitation wavelength 350 nm, emission wavelength 495 nm) and the peak areas and retention times were recorded and calculated by a PC Integration Pack Programme. The quantification of polyamines was performed by means of the internal standard method (2-hydroxydiaminopropane). Polyamines were expressed as nmol per 107 promastigotes.
2.7. L-arginine and L-ornithine determination
Amino acid derivatization and analysis was performed using the following methods: HPLC for the L-ornithine analyses according to the AccQTag method using N-hydroxysuccinimidyl-6-aminoquinoyl carbamate (Waters Chromatography) [27]. The mobile phase was pumped at 1.0 ml/min and consisted of solvent A (140 mM sodium acetate, 5.6 mM triethylamine, pH 4.62 in 50 % phosphoric acid solution), solvent B (acetonitrile:water, 3:2). L-ornithine was separated with a 4 µm C18 reverse-phase column and detected by UV at 248 nm. The quantification of L-ornithine has been done using an external calibration curve.
Amino acids were determined from a precipitated with 10 % (w/v) trichloroacetic acid using a Biochron 20 automatic analyzer (Pharmacia LKB). Amino acids were determined by derivatization with ninhydrin and measurement of absorbance at 570 nm [28].
2.8. Mouse Infection
Virulence of L. major wild-type and genetically manipulated strains was assessed after inoculation on BALB/c mice footpads [29]. Groups were injected s.c. into the footpad with 105 stationary phase promastigotes or 104 amastigotes purified from mouse footpad infections [30]. Infections were monitored by comparing the thickness of the injected and uninjected footpads with a Vernier calliper and parasites were enumerated in the infected tissue by limiting-dilution.
3. Results
3.1. Targeted replacement of the L. major arginase (ARG) gene
We generated a null mutant of the annotated arginase gene of L. major (LmjF35.1480). While gene targeting occurs predominantly by homologous recombination in this species, two rounds are required as Leishmania chromosomes are primarily disomic, and promastigotes lack a sexual cycle when grown in vitro. As loss of arginase was expected to confer polyamine auxotrophy, in the second round parasites were maintained in 1 mM putrescine-supplemented media.
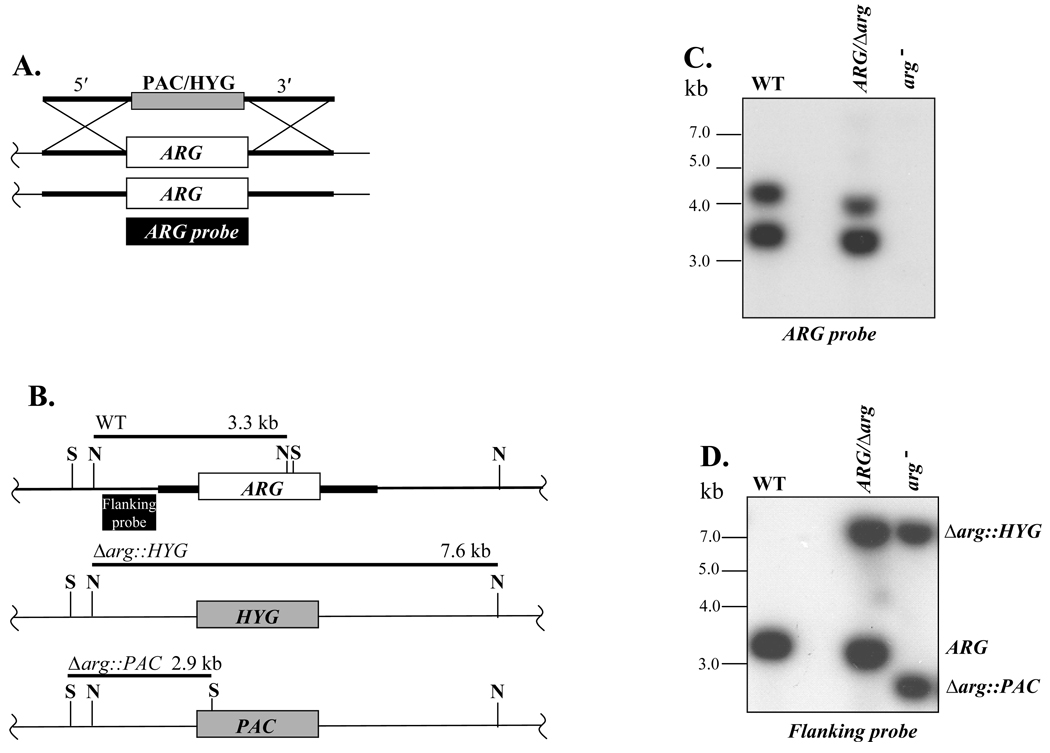
Parasites were transfected first with a targeting fragment with a hygromycin resistance (HYG) cassette replacing the ARG ORF, and subsequently with a targeting fragment with a similar fragment containing puromycin resistance (PAC) cassette (Fig. 1A). Clonal transfectant lines were recovered at each step by plating on semisolid media containing selective drugs, and doubly drug resistant parasites were readily obtained in the second round. Southern blot analysis confirmed that these parasites had undergone the planned replacement. Hybridization with an external probe (EP) yielded only the 3.3-kb NsiI WT fragment in WT DNA, the WT 3.3 kb plus the 7 kb HYG replacement fragment in the +/HYG heterozygotes, and loss of the WT fragment accompanied by the presence of the planned 7 kb HYG and 2.9 kb PAC replacement fragments in the doubly drug resistant lines (Fig. 1B). Similarly, Southern blot analysis with an ARG probe showed the ARG gene had been ablated in the doubly-drug resistant parasites (Fig. 1C). These Δarg::HYG/Δarg::PAC lines are referred to as arg−. To restore ARG expression, we introduced ARG on the multicopy episomal vector pXG, yielding add-back lines termed arg−/+ARG (Methods).
Fig. 1. Generation of an arg− null mutant Leishmania major.
A, Replacement strategy for ARG. The HYG/PAC targeting fragments are shown above chromosomal ARG locus. The ARG ORF probe used for Southern analysis in panel C is indicated. B, Structure of WT and planned Δarg::HYG and Δarg::PAC HYG replacement alleles. The ARG, PAC and HYG ORFs are shown as boxes, and the ~1 kb flanking sequences from the targeting fragment depicted in panel A is shown as a heavy line. The size of predicted restriction fragments and the probe used in panel D are shown. S, and N, SalI and NsiI sites, respectively. C, Autoradiogram showing Southern blot analysis with the ARG probe in WT, heterozygous and null arg− mutant; DNA was digested with NsiI alone (WT) or combined with SalI (mutants). D, Autoradiogram showing Southern blot analysis using the flanking probe depicted in panel B. DNAs were digested as described in panel C.
In the course of these studies we tested whether targeting fragments generated by PCR with long oligonucleotides worked efficiently, as described in T. brucei [31]. Two 110 nt oligonucleotides were synthesized, bearing 92 nt of 5’ or 3’ ARG flanking sequence, joined to 18 nt of the 5’ or 3’ ends of the HYG ORF cassette respectively, and used in PCR with a HYG DNA template to generate a 1269 nt targeting fragment, which was then directly electroporated into WT parasites by standard methods. While successful the efficiency was very low, as only a single colony was obtained (which bore the expected HYG replacement; data not shown).
3.2. Growth of arg− and genetically complemented promastigotes in semidefined media: dependency on L-ornithine or polyamines
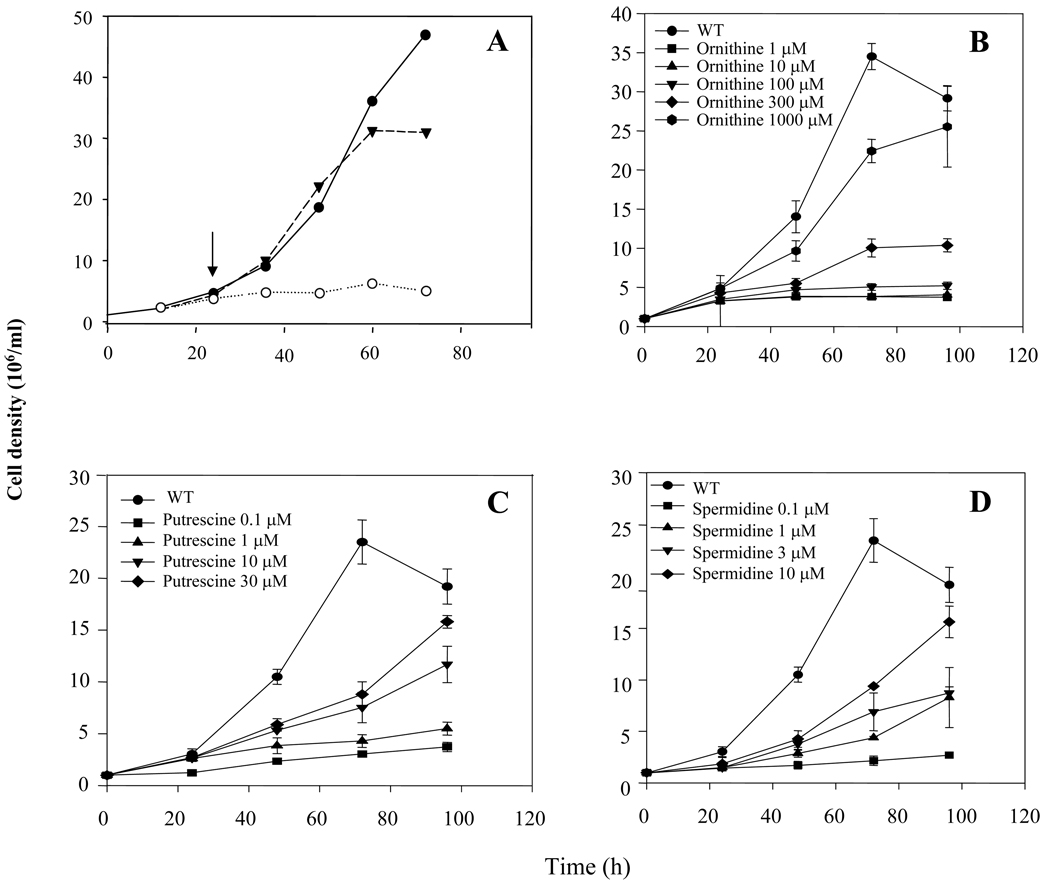
To determine the role of ARG in parasite proliferation, we tested WT and arg− promastigotes in a semidefined M199 medium, where the fetal calf serum supplement was replaced by BSA in order to eliminate serum-derived polyamines. Parasites were inoculated first in media containing 5 mM L-ornithine, and after 24 hr were washed twice and placed in media lacking the polyamine supplement (Fig. 2A, arrow). The arg− strain ceased proliferation soon after L-ornithine removal, while the WT and arg−/+ARG line grew normally (Fig. 2A).
Fig. 2. Growth of WT and arg− L. major in the presence or absence of ornithine or polyamines.
A) Cells were inoculated at 1 × 106/ml, supplementing the arg− mutant with 5 mM L-ornithine during the first 24 h. Parasites were washed twice with PBS to deplete intracellular polyamines and re-suspended in M199 medium containing 10 g/l BSA (the black arrow indicates the time that polyamine starvation took place). Cell lines are arg− (○), arg− /+ARG (▼) and WT (●). B–D) Rescue of L. major arg− growth by L-ornithine (B), putrescine (C) or spermidine (D). WT or arg− parasites were inoculated into fresh medium at 1 × 106 cells/ml as indicated, and counted on a hemacytometer at various times thereafter. Experiments were carried out in triplicate and error bars represent standard deviations.
We then tested the ability of L-ornithine, agmatine, putrescine and spermidine to rescue the growth of the arg− mutant in semi-defined M199 media containing BSA, relative to WT (Fig. 2). All metabolites were able to rescue arg− growth, with EC50s of about 1 mM L-ornithine, 500 µM agmatine (not shown), 30 µM putrescine and 20 µM spermidine. Rescue by ornithine and polyamines was expected based upon our current understanding of Leishmania polyamine synthetic pathways. Rescue by agmatine was unexpected as Leishmania is thought to lack agmatinase. The significance of this observation is uncertain however as some workers have found that commercial agmatine preparations can be contaminated by polyamines, whose presence was not ruled out in our studies. In total, these data supported the predicted role for arginase in L. major physiology and metabolism, e.g. in the provision of L-ornithine leading to the provision of essential polyamines.
3.3. Polyamine levels
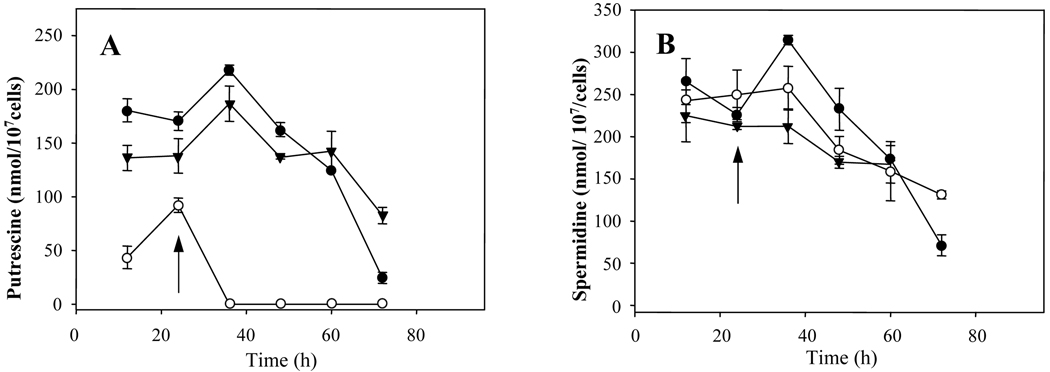
We examined polyamine levels in WT, arg− and complemented lines in parasites grown for 24 hr in the presence of 5 mM L-ornithine and then placed in L-ornithine-free media, conditions under which the arg− parasites immediately cease growth (Fig. 2A). This resulted in an immediate drop in free putrescine levels, falling from 85 nmol/107 cells in the arg− strain to below the limit of detection within 12 hr (Fig. 3A). In contrast, putrescine levels in WT and the complemented mutant were unaffected, and only began to drop as cells entered stationary phase (60–72 h; Fig. 3A) as seen previously [26]. In contrast, in all three lines free spermidine levels were not strongly affected by L-ornithine withdrawal (250 nmol / 107 promastigotes), and decreased thereafter at similar rates as the cells progressed into stationary phase (Fig 3B). As expected, spermine was not detected in any of these preparations.
Fig. 3. Polyamine content of L. majorWT, arg− and ARG overexpressors.
This experiment was performed as described in the legend to Figure 2A. At the indicated time points aliquots were taken for determination of cellular putrescine (A) and spermidine (B) levels. The cell lines studied were arg− (○), arg−/+ARG (▼) and WT (●). Experiments were carried out by triplicate and error bars represent standard deviations.
3.4. Arginase activity and intracellular amino acids
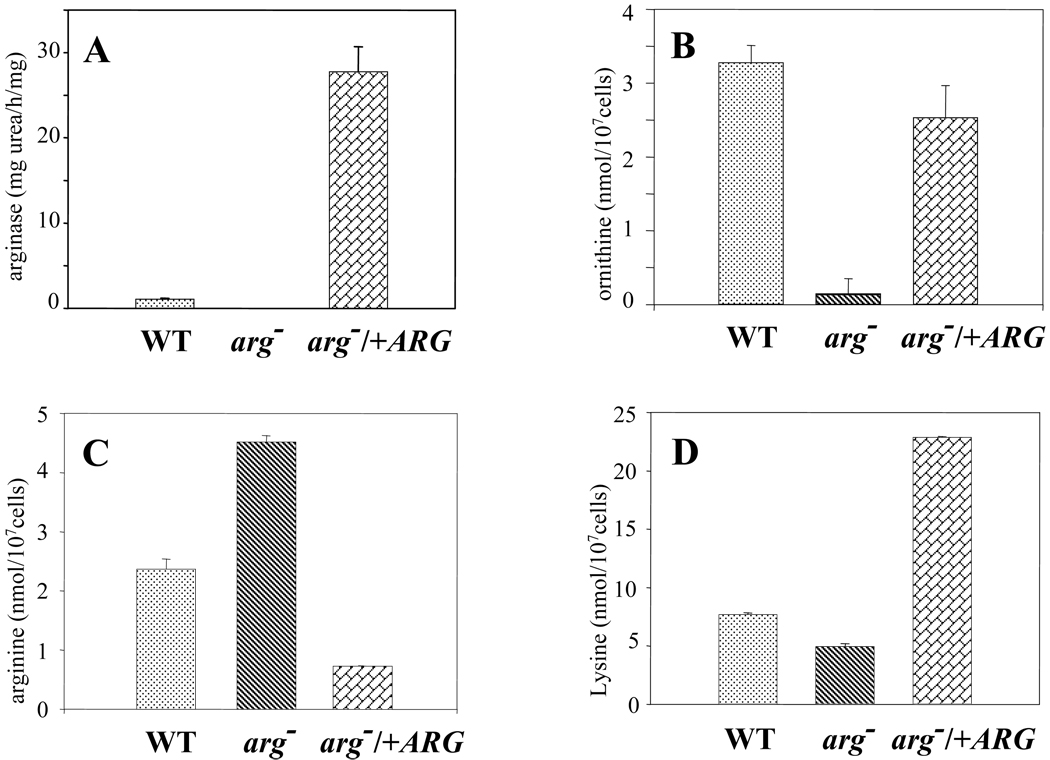
Arginase activity was determined in the WT, arg− and arg−/+ARG lines. No arginase activity was detected in the arg− null mutant, whereas the arg−/+ARG line, expressing ARG from the multi-copy episomal pXG vector, showed almost a 30-fold rise in relation to WT (Fig 4A). To confirm that the urea measured in the assay derived from arginase and not from other sources, extracts were also assayed in the presence of 100 µM Nω-hydroxy-L-arginine (NOHA), a specific competitive inhibitor; as expected, this resulted in more than a 90 % decrease in urea production (data not shown).
Fig. 4. Arginase activity (A), (B) L-ornithine, (C) L-arginine and (D) lysine levels of WT (dotted), arg− (dashed) and the genetically rescued (bricked) arg−/+ARG L. major strains.
Cultures were harvested in stationary phase (2 × 107 cells/ml), and washed twice with PBS; extracts and supernatants were then used to measure arginase activity and amino acid levels as described in Materials and Methods. Experiments were carried out by triplicate and error bars represent standard deviations.
We then determined the intracellular concentrations of the natural substrate and products L-arginine and L-ornithine in these lines. In these experiments 10 µM spermidine was added to the growth media of the arg− knockouts, in order to avoid growth inhibitory effects as well as interference with L-ornithine determination. Consistent with the lack of arginase activity revealed by enzymatic assay, L-ornithine levels were greatly reduced, to the limit of detection in the arg− strain. Interestingly L-ornithine levels were similar in the WT and complemented line (3.30 ± 0.20 and 2.55 ± 0.40 nmol/107 cells, respectively; Fig 4B) despite the massive overproduction of arginase in the arg−/+ARG line. Correspondingly, L-arginine levels were elevated nearly 2 fold in the arg− mutant, and decreased about 4 fold arg−/+ARG line. Interestingly, lysine pools were altered as a consequence of genetic manipulation of the ARG expression. Lysine levels rose from 5 nmol/107 cells in wild-type promastigotes to 20 nmol/107 cells in the genetically complemented arg−/+ARG strain, with scarce changes into the arg− null strain (Fig 4D), thus pointing to a coordinated regulation of both amino acids.
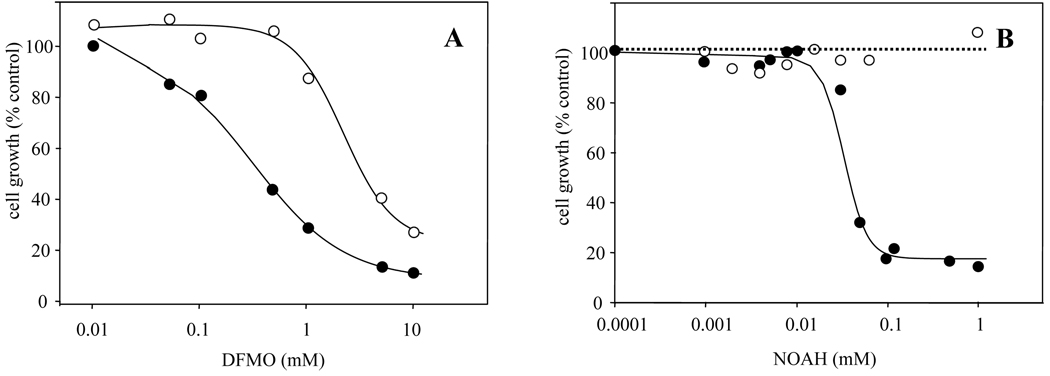
3.5. Susceptibility of arginase overexpressing promastigotes to DFMO and NOHA
DFMO is a competitive enzyme-activated inhibitor of ODC and has been used successfully as an antiparasitic drug [32]. The susceptibility of WT and the arginase overexpressing arg−/+ARG promastigotes to DFMO was measured 48 h after drug exposure (Fig. 5A). Whereas the EC50 for WT L. major was estimated to be 0.35 mM, it rose to 4 mM in the arginase-overproducer. We also examined the sensitivity of WT and ARG-overexpressing parasites to the arginase inhibitor NOHA (Fig. 5B). WT parasites showed an EC50 of 40 µM, while the ARG overexpressing parasites were completely insensitive, showing no inhibition by the highest concentration tested (1 mM).
Fig. 5. Inhibition of L. major WT (○) and arginase overproducing (arg−/+ARG) (●) strains to (A) the ODC inhibitor α-DFMO and (B) the arginase inhibitor NOHA.
Cell density was measured by counting using a hemocytometer. Experiments were carried out by triplicate and error bars represent standard deviations.
3.6. The L. major arg− null mutant is able to induce infection in vivo
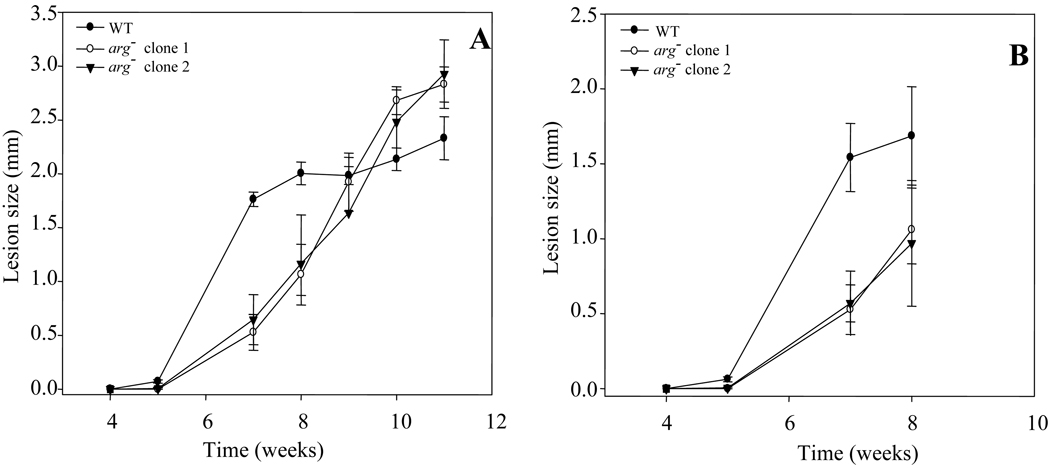
To probe the role of arginase in vivo, we tested the ability of parasites to induce lesion formation in susceptible BALB/c mice following inoculation of metacyclic parasites into the footpad. In these experiments we tested the infective promastigotes grown in culture as well as amastigotes purified from footpad infections. Several tests showed that the arg− mutant produced comparable levels of metacyclics as WT, as assayed by the formation of parasites unreactive with peanut agglutinin in stationary phase (data not shown) [33]. Inoculation of 105 stationary phase WT parasites produced visible footpad lesions within 5th week which progressed rapidly thereafter. In contrast, lesion emergence in the arg− infected mice was delayed until 7th week post-inoculation and progressed somewhat more slowly thereafter (Fig. 6A). After extended periods (>10 weeks) the arg− infections showed increased pathology than WT, although the difference was not significant. Similar results were obtained following inoculation of 104 amastigotes purified from infected mice (Fig. 6B). As in the initial phases of infection lesion formation lags behind parasite replication due to a period of ‘silent’ replication [34], we determined the parasitemia in a small number of mice by limiting dilution assays. At the 7 week time point in the metacyclic infection experiment shown in Fig. 6A, WT infections yielded 1.2 × 107 parasites (n = 2), while the arg− parasites yielded 7.2 × 106 parasites (n = 4), about 60% of WT, which is statistically insignificant.
Fig. 6. Time-course of L. major infections in BALB/c mice.
The increase in footpad thickness was monitored after inoculation of (A) 1 × 105 stationary phase promastigotes or (B) 104 purified amastigotes purified from the mouse lesions shown in panel A. Each point indicates the average increase in footpad thickness for four mice inoculated with WT L. major (●) or five mice inoculated with arg− clone 1 (○) or arg− clone 2 (▼); error bars represent standard deviations.
Thus despite an absolute requirement for arginase in promastigotes grown under defined conditions (Fig. 2A), loss of arginase has a relatively modest effect on mouse infectivity, at least in the susceptible BALB/c infection model. Elsewhere we show that restoration of ARG expression to the arg− mutant results in full restoration of lesion progression and parasitemia (H.M. Muleme, R.M. Reguera, A. Palfi, R. Azinwi, S.M. Beverley and J.E. Uzonna, submitted).
4. Discussion
In this report we have generated and characterized the properties of an arginase null mutant in L. major, generated by two rounds of homologous gene replacement in the presence of 1 mM putrescine (Fig. 1). Under these conditions, arg− null mutants were readily recovered. In the course of these studies we tested whether targeting constructs bearing just 92 nt of flanking sequence, generated using a ‘long oligonucleotide’ PCR protocol successful in other organisms could yield replacements in L. major. The efficiency was poor, as only a single line colony was obtained in several trials, vs. hundreds in similar experiments with longer constructs bearing ~1 kb flanking sequences. These results are consistent with results obtained in L. tarentolae previously [35], with an efficiency far less than seen in similar experiments with T. brucei [31],
Biochemical assays showed a complete absence of arginase enzymatic activity in the arg− lines (Fig. 4A), establishing the L. major ARG gene (LmjF35.1480) as the only source for this activity in cultured promastigotes. When cultured in semi-defined media in vitro, the arg− parasites fail to grow unless supplemented with L-ornithine, putrescine or spermidine (Fig. 2). We also determined the consequences to upstream and downstream metabolites in these studies. Following L-ornithine withdrawal, putrescine levels dropped rapidly in the arg− parasites (Fig. 3A), although spermidine levels showed little change from WT (Fig. 3B). As expected, arg− parasites showed greatly reduced levels of the product L-ornithine, and conversely elevated levels of the substrate L-arginine (Fig. 4B, 4C). For the most part, the phenotypes of the arg− mutant were in all cases returned to WT upon restoration of ARG expression in complemented arg−/+ARG parasites, establishing the specificity of the targeted null mutant. However, some differences in the complemented lines were noted, attributed to the fact that the use of episomal expression vectors resulted in arginase overexpression (Fig. 4A). In the arg−/+ARG line, L-arginine levels were reduced 4-fold, however L-ornithine levels did not rise, and in fact were about 20% lower (Fig. 4B, 4C). The lack of concordance between arginase overexpression and L-arginine levels in the complemented line might be explained as a cellular adaptation of the amino acid transporter to new metabolic needs, or excretion of L-ornithine into the media. Another unexpected finding was that lysine levels were elevated about 4-fold in the arg−/+ARG overexpressor, suggesting that there may be some link between the control of L-arginine and lysine synthesis.
These data thus establish that in common with L. mexicana, L. major ARG is the primary source for L-ornithine required for essential polyamine synthesis. In this respect Leishmania species differ from T. cruzi, which lack L-ornithine decarboxylase and orthologs of the Leishmania arginases, and thus must acquire essential polyamines by salvage [36–38]. In addition to the essential roles that polyamines play in all organisms, trypanosomatids require polyamines for the synthesis of trypanothione, which plays critical roles in maintaining cellular redox balance and defense against oxidative stress [15, 39].
As expected, introduction of ARG on the multicopy expression vector pXG resulted in arginase overexpression, about 30-fold higher than WT. This provided another tool to study ARG function within the parasite, beyond their usefulness as a control for restoration of arg− mutant phenotypes. Correspondingly, the ARG overproducer was highly resistant to the arginase inhibitor NOHA, showing no inhibition at concentrations more than 25-fold the WT EC50 (Fig. 5). Similarly, the ARG overproducer was more than 10-fold resistant to the specific ODC inhibitor DFMO. Potentially this could arise through increased conversion of L-arginine into L-ornithine in the ARG overproducer, which could then compete with DFMO for binding to the ODC active site. While the data showed that L-ornithine levels were not elevated in the ARG overproducer (Fig. 4B), increased flux through this pathway might account for this finding.
We then tested whether the arg− L. major was able to cause disease in the susceptible BALB/c mouse model. While the mutant parasites were able to generate disease, pathology emerged somewhat less rapidly (Fig. 6) accompanied by (at best) a slight reduction in parasitemia. Since arg− parasites require L-ornithine or polyamines in vitro for growth, these data suggest that either the arg− parasites elaborates an alternative ARG activity, or that sufficient L-ornithine and/or polyamines are available to Leishmania amastigotes to support growth. Current data provide no support for the first alternative [12]. In contrast, the levels of L-ornithine and polyamines available in vivo are known to be substantial [40], and several Leishmania species express potent arginine and polyamine transporters able to salvage these metabolites from external sources [41–44]. Interestingly the residence of Leishmania within an acidified parasitophorous vacuole would be expected to favor the accumulation of basic nutrients such as polyamines and L-ornithine, as well as L-arginine. Thus the ability of arg− L. major and L. mexicana [18] to survive in the mammalian host seems likely to arise from the availability of metabolites able to bypass the arginase deficiency.
Previous studies have shown that administration of arginase inhibitors inhibits Leishmania survival in animal models, albeit partially, and the methods used could not readily establish whether the effect arose from inhibition of host, parasite or both arginases [6, 45]. These findings are consistent with our data showing that lack of parasite arginase similarly results in only partial inhibition of parasite replication and pathology in mouse infections (Fig. 6). These data suggest that broad inhibition of arginase activity alone will be insufficient to achieve therapeutically useful control of leishmaniasis. Potentially, combined inhibition of arginase with downstream enzymes leading to polyamine synthesis could result in improved therapeutic responses.
While not essential for amastigote survival in the mammalian host, arg− infections were not identical to those caused by WT parasites, as the disease pathology in BALB/c mice was delayed regardless of whether infections were initiated with promastigotes or amastigotes (Fig. 6). We recently have obtained similar findings in the resistant C57BL6 mouse model, a more relevant model of human disease, where we additionally showed that restoration of ARG expression returned the infection profile to that of WT (H.M. Muleme, R.M. Reguera, A. Palfi, R. Azinwi, S.M. Beverley and J.E. Uzonna, submitted). Similarly, L. mexicana arg− mutants were shown to exhibit delayed lesion formation recently as well [18]. These studies document the differences in lesion progression and pathology between WT and arg− mutants, seen in comparisons involving two Leishmania species inhabiting parasitophorous vacuoles that differ considerably. While in this work we have stressed the nutritional consequences of polyamine deprivation arising from arginase deficiency, another important role of arginase activity is in depriving inducible NOS of the arginine substrate required for generation of NO needed for the control of leishmaniasis [7, 10, 46]. Studies of the mouse infections by the arg− L. mexicana described by Gaur et al [18] or by the arg− L. major described here and reported elsewhere (H.M. Muleme, R.M. Reguera, A. Palfi, R. Azinwi, S.M. Beverley and J.E. Uzonna, submitted), indeed show that infections by arg− parasites are accompanied by increased NOS – dependent NO synthesis, consistent with a role for parasite arginase in depriving host cells of arginine.
Acknowledgements
We thank A. Fairlamb, S. Roberts and B. Ullman for discussions and/or sharing preliminary data. This work was supported by NIH Grant 21903 (SMB) and in part by Instituto de Salud Carlos III (grant PI06302 and RICET) from Ministerio de Salud y Consumo from the Spanish Kingdom, and by Junta de Castilla y León grant LE002A/08 to RMR.
Abbreviations
- NO
nitric oxide
- NOS
nitric oxide synthase
- ODC
ornithine decarboxylase
- DFMO
α-difluomethylornithine
- NOHA
Nω-hydroxy-L-arginine
- IL
interleukin
- FCS
foetal calf serum
- BSA
bovine serum albumin
- TCA
trichloracetic acid
- ORF
open reading frame
- HYG
hygromycin B
- PAC
puromycin
- ISPF
α-isonitrosopropiophenone
- HPLC
high performance liquid chromatography
- EP
external probe
References
- 1.Morris SM., Jr Arginine: beyond protein. Am J Clin Nutr. 2006;83:508S–512S. doi: 10.1093/ajcn/83.2.508S. [DOI] [PubMed] [Google Scholar]
- 2.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 3.Modolell M, Corraliza IM, Link F, Soler G, Eichmann K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur J Immunol. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- 4.Peranzoni E, Marigo I, Dolcetti L, Ugel S, Sonda N, Taschin E, Mantelli B, Bronte V, Zanovello P. Role of arginine metabolism in immunity and immunopathology. Immunobiology. 2007;212:795–812. doi: 10.1016/j.imbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 6.Iniesta V, Gomez-Nieto LC, Corraliza I. The inhibition of arginase by N(omega)-hydroxyl-arginine controls the growth of Leishmania inside macrophages. J Exp Med. 2001;193:777–784. doi: 10.1084/jem.193.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanasen N, Soong L. L-arginine metabolism and its impact on host immunity against Leishmania infection. Immunol Res. 2008;41:15–25. doi: 10.1007/s12026-007-8012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol. 1996;114:107–132. doi: 10.1016/0305-0491(95)02138-8. [DOI] [PubMed] [Google Scholar]
- 9.Ash DE. Structure and function of arginases. J Nutr. 2004;134:2760S–2767S. doi: 10.1093/jn/134.10.2760S. [DOI] [PubMed] [Google Scholar]
- 10.Mori M, Gotoh T. Arginine metabolic enzymes, nitric oxide and infection. J Nutr. 2004;134:2820S–2825S. doi: 10.1093/jn/134.10.2820S. [DOI] [PubMed] [Google Scholar]
- 11.da Silva ER, Castilho TM, Pioker FC, Tomich de Paula Silva CH, Floeter-Winter LM. Genomic organisation and transcription characterisation of the gene encoding Leishmania (Leishmania) amazonensis arginase and its protein structure prediction. Int J Parasitol. 2002;32:727–737. doi: 10.1016/s0020-7519(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 12.Roberts SC, Tancer MJ, Polinsky MR, Gibson KM, Heby O, Ullman B. Arginase plays a pivotal role in polyamine precursor metabolism in Leishmania. Characterization of gene deletion mutants. J Biol Chem. 2004;279:23668–23678. doi: 10.1074/jbc.M402042200. [DOI] [PubMed] [Google Scholar]
- 13.da Silva ER, da Silva MF, Fischer H, Mortara RA, Mayer MG, Framesqui K, Silber AM, Floeter-Winter LM. Biochemical and biophysical properties of a highly active recombinant arginase from Leishmania (Leishmania) amazonensis and subcellular localization of native enzyme. Mol Biochem Parasitol. 2008;159:104–111. doi: 10.1016/j.molbiopara.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Heby O, Roberts SC, Ullman B. Polyamine biosynthetic enzymes as drug targets in parasitic protozoa. Biochem Soc Trans. 2003;31:415–419. doi: 10.1042/bst0310415. [DOI] [PubMed] [Google Scholar]
- 15.Oza SL, Shaw MP, Wyllie S, Fairlamb AH. Trypanothione biosynthesis in Leishmania major. Mol Biochem Parasitol. 2005;139:107–116. doi: 10.1016/j.molbiopara.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Fairlamb AH, Cerami A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- 17.Krauth-Siegel LR, Comini MA, Schlecker T. The trypanothione system. Subcell Biochem. 2007;44:231–251. doi: 10.1007/978-1-4020-6051-9_11. [DOI] [PubMed] [Google Scholar]
- 18.Gaur U, Roberts SC, Dalvi RP, Corraliza I, Ullman B, Wilson ME. An effect of parasite-encoded arginase on the outcome of murine cutaneous leishmaniasis. J Immunol. 2007;179:8446–8453. doi: 10.4049/jimmunol.179.12.8446. [DOI] [PubMed] [Google Scholar]
- 19.Castro R, Scott K, Jordan T, Evans B, Craig J, Peters EL, Swier K. The ultrastructure of the parasitophorous vacuole formed by Leishmania major. J Parasitol. 2006;92:1162–1170. doi: 10.1645/GE-841R.1. [DOI] [PubMed] [Google Scholar]
- 20.Colmenares M, Kar S, Goldsmith-Pestana K, McMahon-Pratt D. Mechanisms of pathogenesis: differences amongst Leishmania species. Trans R Soc Trop Med Hyg. 2002;96 Suppl 1:S3–S7. doi: 10.1016/s0035-9203(02)90044-1. [DOI] [PubMed] [Google Scholar]
- 21.Turco SJ, Späth GF, Beverley SM. Is lipophosphoglycan a virulence factor? A surprising diversity between Leishmania species. Trends Parasitol. 2001;17:223–226. doi: 10.1016/s1471-4922(01)01895-5. [DOI] [PubMed] [Google Scholar]
- 22.Ha DS, Schwarz JK, Turco SJ, Beverley SM. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol. 1996;77:57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Russell DW. Molecular cloning : a laboratory manual. 3rd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 24.Robinson KA, Beverley SM. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol Biochem Parasitol. 2003;128:217–228. doi: 10.1016/s0166-6851(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 25.Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods. 1994;174:231–235. doi: 10.1016/0022-1759(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 26.Balana-Fouce R, Escribano MI, Alunda JM. Leishmania infantum: polyamine biosynthesis and levels during the growth of promastigotes. Int J Biochem. 1991;23:1213–1217. doi: 10.1016/0020-711x(91)90218-c. [DOI] [PubMed] [Google Scholar]
- 27.Cohen SA, Michaud DP. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem. 1993;211:279–287. doi: 10.1006/abio.1993.1270. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Capillas C, Moral A. Free amino acids and biogenic amines in red and white muscle of tuna stored in controlled atmospheres. Amino Acids. 2004;26:125–132. doi: 10.1007/s00726-003-0054-4. [DOI] [PubMed] [Google Scholar]
- 29.Titus RG, Muller I, Kimsey P, Cerny A, Behin R, Zinkernagel RM, Louis JA. Exacerbation of experimental murine cutaneous leishmaniasis with CD4+ Leishmania major-specific T cell lines or clones which secrete interferon-gamma and mediate parasite-specific delayed-type hypersensitivity. Eur J Immunol. 1991;21:559–567. doi: 10.1002/eji.1830210305. [DOI] [PubMed] [Google Scholar]
- 30.Zufferey R, Allen S, Barron T, Sullivan DR, Denny PW, Almeida IC, Smith DF, Turco SJ, Ferguson MA, Beverley SM. Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by Leishmania major. J Biol Chem. 2003;278:44708–44718. doi: 10.1074/jbc.M308063200. [DOI] [PubMed] [Google Scholar]
- 31.Shen S, Arhin GK, Ullu E, Tschudi C. In vivo epitope tagging of Trypanosoma brucei genes using a one step PCR-based strategy. Mol Biochem Parasitol. 2001;113:171–173. doi: 10.1016/s0166-6851(00)00383-2. [DOI] [PubMed] [Google Scholar]
- 32.Heby O, Persson L, Rentala M. Targeting the polyamine biosynthetic enzymes: a promising approach to therapy of African sleeping sickness, Chagas' disease, and leishmaniasis. Amino Acids. 2007;33:359–366. doi: 10.1007/s00726-007-0537-9. [DOI] [PubMed] [Google Scholar]
- 33.Sacks DL, Perkins PV. Identification of an infective stage of Leishmania promastigotes. Science. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 34.Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged "silent" phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol. 2000;165:969–977. doi: 10.4049/jimmunol.165.2.969. [DOI] [PubMed] [Google Scholar]
- 35.Papadopoulou B, Dumas C. Parameters controlling the rate of gene targeting frequency in the protozoan parasite Leishmania. Nucleic Acids Res. 1997;25:4278–4286. doi: 10.1093/nar/25.21.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrillo C, Cejas S, Huber A, Gonzalez NS, Algranati ID. Lack of arginine decarboxylase in Trypanosoma cruzi epimastigotes. J Eukaryot Microbiol. 2003;50:312–316. doi: 10.1111/j.1550-7408.2003.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 37.Ariyanayagam MR, Tetaud E, Fairlamb AH. Diamine auxotrophy in a eukaryotic parasite. Biochem Soc Trans. 1998;26:606–609. doi: 10.1042/bst0260606. [DOI] [PubMed] [Google Scholar]
- 38.Ariyanayagam MR, Fairlamb AH. Diamine auxotrophy may be a universal feature of Trypanosoma cruzi epimastigotes. Mol Biochem Parasitol. 1997;84:111–121. doi: 10.1016/s0166-6851(96)02788-0. [DOI] [PubMed] [Google Scholar]
- 39.Muller S, Liebau E, Walter RD, Krauth-Siegel RL. Thiol-based redox metabolism of protozoan parasites. Trends Parasitol. 2003;20:320–328. doi: 10.1016/s1471-4922(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 40.Sakata K, Kashiwagi K, Sharmin S, Ueda S, Irie Y, Murotani N, Igarashi K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem Biophys Res Commun. 2003;305:143–149. doi: 10.1016/s0006-291x(03)00716-2. [DOI] [PubMed] [Google Scholar]
- 41.Shaked-Mishan P, Suter-Grotemeyer M, Yoel-Almagor T, Holland N, Zilberstein D, Rentsch D. A novel high-affinity arginine transporter from the human parasitic protozoan Leishmania donovani. Mol Microbiol. 2006;60:30–38. doi: 10.1111/j.1365-2958.2006.05060.x. [DOI] [PubMed] [Google Scholar]
- 42.Hasne MP, Ullman B. Identification and characterization of a polyamine permease from the protozoan parasite Leishmania major. J Biol Chem. 2005;280:15188–15194. doi: 10.1074/jbc.M411331200. [DOI] [PubMed] [Google Scholar]
- 43.Basselin M, Coombs GH, Barrett MP. Putrescine and spermidine transport in Leishmania. Mol Biochem Parasitol. 2000;109:37–46. doi: 10.1016/s0166-6851(00)00234-6. [DOI] [PubMed] [Google Scholar]
- 44.Kandpal M, Tekwani BL. Polyamine transport systems of Leishmania donovani promastigotes. Life Sci. 1997;60:1793–1801. doi: 10.1016/s0024-3205(97)00139-2. [DOI] [PubMed] [Google Scholar]
- 45.Iniesta V, Carcelen J, Molano I, Peixoto PM, Redondo E, Parra P, Mangas M, Monroy I, Campo ML, Nieto CG, Corraliza I. Arginase I induction during Leishmania major infection mediates the development of disease. Infect Immun. 2005;73:6085–6090. doi: 10.1128/IAI.73.9.6085-6090.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vincendeau P, Gobert AP, Daulouede S, Moynet D, Mossalayi MD. Arginases in parasitic diseases. Trends Parasitol. 2003;19:9–12. doi: 10.1016/s1471-4922(02)00010-7. [DOI] [PubMed] [Google Scholar]