Abstract
Parkinson’s disease (PD) is the eponym attached to the most prevalent neurodegenerative movement disorder of adults, derived from observations of an early nineteenth century physician and paleontologist, James Parkinson, and is now recognized to encompass much more than a movement disorder clinically or dopamine neuron death pathologically. Most PD (~90%) is sporadic (sPD), is associated with mitochondrial deficiencies and has been studied in cell and animal models arising from use of mitochondrial toxins that unfortunately have not predicted clinical efficacy to slow disease progression in humans. We have extensively studied the cytoplasmic hybrid (“cybrid”) model of sPD in which donor mtDNA’s are introduced into and expressed in neural tumor cells with identical nuclear genetic and environmental backgrounds. sPD cybrids demonstrate many abnormalities in which increased oxidative stress drives downstream antioxidant response and cell death activating signaling pathways. sPD cybrids regulate mitochondrial ETC genes and gene ontology families like sPD brain. sPD cybrids spontaneously form Lewy bodies and Lewy neurites, linking mtDNA expression to neuropathology, and demonstrate impaired organelle transport in processes and reduced mitochondrial respiration. Our recent studies show that near-infrered laser light therapy normalizes mitochondrial movement and can stimulate respiration in sPD cybrid neurons, and mitochondrial gene therapy can restore respiration and stimulate mitochondrial ETC gene and protein expression. sPD cybrids have provided multiple lines of circumstantial evidence linking mtDNA to sPD pathogenesis and can serve as platforms for therapy development. sPD cybrid models can be improved by use of non-tumor human stem cell-derived neural precursor cells and by introduction of postmortem brain mtDNA to test its causality directly.
Introduction: The Changing View of Parkinson’s Disease in the 21st Century
In 1817 James Parkinson published his monograph “An Essay on the Shaking Palsy” in which he described the clinical characteristics of six persons, three of whom he had examined to varying degrees and three of whom he had simply observed. Parkinson was quite a talented observer, having published over a decade earlier the first of three volumes of his 1200 page treatise “Organic Remains of a Former World” that revealed his detailed drawings and examination of his extensive, personal fossil collection. In addition to being a leading paleontologist of his time, Parkinson’s enduring description of the phenomenology of a movement disorder led to the eponym attaching his name to it, initially by Charcot and subsequently by Gowers at the end of the 19th century. “Parkinson’s disease” is the most prevalent neurodegenerative movement disorder of adults; but as we have learned, there are many mechanisms that can produce similar clinical phenotypes, and the phenotypes include much more than a movement disorder.
The view of Parkinson’s disease as a progressive nigrostriatal dopamine deficiency state was based on extensive pathological study of the nigrostriatal pathway in postmortem tissues combined with the successful clinical pharmacology of dopamine replacement to relieve symptoms. While still true today, we now understand that it is incomplete, and that Parkinson’s disease pathologically is much more than a focal degeneration of dopamine neurons. Other monoaminergic neurons degenerate to varying degrees, providing mechanisms for appearance of problematic “secondary” symptoms such as depression and apathy. A comprehensive view of Parkinson’s as a progressive protein aggregation synucleinopathy that initiates in gut enteric neurons, progresses to dorsal vagal neurons and then advances rostrally into nigra, limbic system and cortex has been advanced by the Braak group (Bertrand, et al., 2004, Braak, et al., 2001, Braak, et al., 2006, Braak, et al., 2003, Braak, et al., 2004, Braak, et al., 1999). This overview of Parkinson’s combines extensive preclinical pathology with an understanding of later mood, psychiatric and cognitive deficits that plague most advanced stage patients, who ironically have survived to develop these disabilities because of the successes of dopamine replacement therapies in treating their motor deficits.
The causes of sporadic Parkinson’s disease remain a mystery
Although several autosomal gene mutations (Belin and Westerlund, 2008, Gupta, et al., 2008) have recently been associated with clinical syndromes that mimic sporadically occurring PD to varying degrees, including some that are clinically identical, these genetic mutations are not present in sporadic PD, which still accounts for ~90% of cases. Further, the molecular mechanisms for neurodegeneration in these autosomal parkinsonian syndromes are not clear, as transgenic mice expressing mutant versions of these human PD genes consistently fail to yield significant dopamine neurodegeneration. However, some transgenic models yield animals with neuronal inclusions and subtle sensori-motor impairments, suggesting impairment of dopaminergic synaptic function. (Chesselet, 2008)
Although a common set of core findings are used to diagnose PD, there is no reason to assume that the similar clinical phenotypes of sporadic PD necessitate a common etiology. For example, variations in ages of onset and disease progression suggest that multiple interacting processes produce the clinical phenotypes. If the Braak formulation is correct, then pathogenesis begins years before involvement of nigral dopamine neurons, and pathogenesis may involve different molecular events at different disease stages. To further complicate the analysis, one must consider whether pathogenesis is focused on dysfunction and death of nigral dopamine neurons, death of other biogenic amine neurons, protein aggregation pathology, or some comprehensive explanation of all these events. Each may have its own molecular origins, or they may share common mechanisms.
The tragic1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) experience of the early 1980’s vitalized research into PD pathogenesis and led to the hypothesis that mitochondrial bioenergetic failure, particularly at complex I of the electron transport chain (ETC), could induce dopamine neuron death (Langston, 1996). Selective dopamine neuron vulnerability in MPTP models derived in part from dopamine transporter (DAT)-mediated accumulation of the proximate toxin 1-methyl-4-phenylpyridinium (MPP+), a complex I inhibitor, but a mitochondrial pathogenesis model arising from this finding was difficult to reconcile with the deficiency of complex I activity found in multiple tissues of PD patients. That generalized complex I inhibition could produce selective nigral dopamine neuron loss was then demonstrated by systemic rotenone infusion or rotenone treatment of brain slices or neural cells (Greenamyre, et al., 2001, Sherer, et al., 2002, Sherer, et al., 2003, Sherer, et al., 2003, Testa, et al., 2005). This finding suggested that the partial complex I catalytic deficiency observed in PD brain and tissues might have etiologic importance for dopamine neurodegeneration, although the degree of complex I loss in PD brain was typically ~50% or less, much below that produced by MPTP/MPP+ or rotenone.
Development of antibodies that can capture entire functional electron transport chain (ETC) complexes allowed a study of complex I isolated from PD brain mitochondria (Keeney, et al., 2006). NADH-driven electron flow was reduced, and several complex I subunits showed increased oxidative damage that could be reproduced by NADH-driven electron flow through control (CTL) brain complex I. These findings supported the developing story that PD brain experiences a complex I deficiency possibly self-generated through oxidative stress damage to its catalytic subunits. If true, this hypothesis implies an inherent inefficiency of electron transfer among complex I subunits that could result from genetic or environmental influences, or some combination.
The possible involvement of deficient mitochondrial bioenergetic function as a component of PD pathogenesis gained support most recently when two groups independently found that pigmented nigral neurons develop an age-related loss of cytochrome oxidase (CO) histochemical activity associated with increased abundance of mitochondrial DNA (mtDNA) containing significant deletions (Bender, et al., 2006, Kraytsberg, et al., 2006). The majority of mtDNA in most sampled neurons in specimens from persons in their 80’s was deleted to the extent that important complex I and complex IV genes were removed. CO(−) neurons had a greater abundance of deleted mtDNA’s, and PD brains had a 3-fold increase in abundance of CO(−) neurons (Bender, et al., 2006). So far, nigral pigmented neurons appear to be more vulnerable to accumulation of these mtDNA deletions than neurons from other regions (Bender, et al., 2008). Finally, the mtDNA deletion break points tended to occur at direct repeat sequences and were unique to each specimen, eliminating the possibility that there is a PD “common mtDNA deletion” (Reeve, et al., 2008).
All examinations of postmortem PD brains are constrained by the fact that these samples typically represent persons who died in late stages of their disease. The molecular abnormalities found may not be representative of problems earlier in pathogenesis, and the characteristics of neurons in these specimens may reflect the “survivors” and not those of neurons that have died. For these reasons representative cell and animal models are potentially of great value if they reproduce essential early disease characteristics. Optimum cell models would reproduce a number of molecular abnormalities found in PD brain, not just one or two characteristics. Although PD is much more than a dopamine neurodegeneration, progressive death of nigral dopamine neurons has eluded the transgenic animal models of PD. This consistent finding implies that other factors beyond the autosomal gene mutation are at work to produce the human disease. So far selective dopamine neurodegeneration has only been reproduced by mitochondrial toxins or chronic inflammatory stimuli such as lipopolysaccharide injections. The relevance of these stimuli to human sporadic PD remains controversial. Furthermore, manipulation of drug targets developed using dopaminergic neurotoxins in cell and animal models have not altered disease progression in human PD trials (Waldmeier, et al., 2006). This disappointing finding with two therapeutics brought to Phase II clinical trials in humans based on consistent activity in preclinical cell and animal models implies that there will be limited investments in development of future neuroprotective agents unless improved models become available.
The Cytoplasmic Hybrid (“cybrid”) Model of sPD: What is a cybrid?
Cybrid (cytoplasmic hybrid) cell lines avoid many problems associated with genetic animal and neurotoxin models of PD. Cybrid cell lines are created by fusing donated platelets containing mitochondrial DNA (mtDNA) from PD or disease-free volunteers with host mtDNA-free human SH-SY5Y neuroblastoma or NT2 teratocarcinoma cells. After fusion, host cells repopulated with platelet-derived mitochondria undergo metabolic selection to eliminate cells with incomplete repopulation (Ghosh, et al., 1999, Swerdlow, et al., 1996). Multiple replication cycles yield cybrid cell lines that have a uniform nuclear genetic and environmental background and differ from each other only in the source of their mitochondrial genes (Ghosh, et al., 1999, Swerdlow, et al., 1996). The logic behind the cybrid model is that differences among cell lines created from different groups of persons based on their having a clinical phenotype (PD, CNT, etc) derive from the donor mtDNA’s being expressed against a constant nuclear genetic and environmental background. Thus, cybrids isolate functional differences among different populations of mitochondrial genomes without defining what those differences are.
Human SH-SY5Y neuroblastoma and NT2 teratocarcinoma cells have been widely used for studies of neurodegenerative disease because they express neuronal characteristics and do not require transfection to express human proteins. PD cybrid cell lines have been successfully used to explore the contribution of mitochondrial dysfunction and mtDNA gene mutations to PD pathogenesis. Because PD cybrids can be generated from patients at all stages of the disease, they provide a window into early stages of PD pathogenesis not available from pathology samples.
What have Parkinson’s SH-SY5Y and NT2 cybrids revealed about sporadic PD?
Cybrid studies have revealed that PD mitochondrial genes are detrimental to cell survival in ways that correlate with changes seen in PD brain.
Complex I deficiency, oxidative stress
PD cybrid lines exhibit- 1) decreased complex I catalytic activity (Esteves, et al., 2008, Gu, et al., 1998, Swerdlow, et al., 1996), 2) increased production of ROS (Esteves, et al., 2008, Swerdlow, et al., 1996) and oxidized proteins (Esteves, et al., 2008), 3) altered intracellular calcium homeostasis (Sheehan, et al., 1997), 4) increased levels of antioxidant enzymes (Cassarino, et al., 1997), 5) increased nuclear translocation of the transcription factor NFκβ (Cassarino, et al., 2000) increased levels of survival-promoting proteins Bcl-2 and Bcl-XL (Veech, et al., 2000), 7) increased loss of viability, PARP cleavage, less mitochondrial cytochrome c and increased caspase 3 activity, more LDH release and activation of the stress kinases p38 and JNK (Esteves, et al., 2008, Onyango, et al., 2005), 8) less ATP and more depolarized mitochondria (Esteves, et al., 2008). PD cybrids also showed increased alpha synuclein oligomerization and depolymerization of tubulin (Esteves, et al., 2008). Elevated oxidative stress and alpha synuclein oligomers were reduced by the antioxidants coenzyme Q10 (Coq10) and reduced glutathione (GSH), whereas only CoQ10 restored complex I activity, ATP levels and polymerized tubulin (Esteves, et al., 2008). All of these findings implicate expression of sPD mtDNA as producing cybrids that are burdened by oxidative stress and activation of compensatory systems that fail to protect completely. Further, the findings with antioxidants, particularly CoQ10, suggest that such therapeutic strategies hold great promise towards improving both electron transport and protein aggregation abnormalities.
Mitochondrial morphological abnormalities
In 2000 we reported (Trimmer, et al., 2000) that rounded, swollen mitochondria were detectable in PD cybrids at the light microscope level by staining with the mitochondria specific dye, MitoTrackerCMXRos (Molecular Probes). By electron microscopy, the mitochondria in PD cybrid lines were morphologically abnormal. In addition to being swollen rather than rod-like, PD cybrid mitochondria had a pale mitochondrial matrix with few cristae and in some cases expressed intramitochondrial inclusions. These features were not found in the mitochondria from CNT cybrids. Morphometric analysis showed that there was a statistically significant increase in the number of large mitochondria (0.26–0.65μm) in PD cybrids compared to CNT cybrids (Trimmer et al, 2000).
Cybrid Lewy bodies and Lewy neurites
Morphological examination of PD cybrid cell lines also revealed the existence of fibrillar and vesicular inclusions (cybrid Lewy bodies) that faithfully replicate the essential antigenic and structural features of Lewy bodies in PD brain (Trimmer, et al., 2004). The size of the cybrid Lewy bodies is comparable to the size of Lewy bodies in the brain and the size of the fibrils in the fibrillar and concentric cybrid Lewy bodies matches the size of fibrils found in Lewy bodies in PD brain (Trimmer, et al., 2004, Wakabayashi, et al., 2007). Cybrid Lewy bodies are present in a small percentage of the PD cybrid cells not unlike the 4–5% expression level for Lewy bodies determined by neuropathogists. Since these inclusions rarely form in SH-SY5Y host cells or cybrids made from disease-free control donors, PD mitochondrial genes are sufficient to drive the formation of these Lewy body-like inclusions.
PD cybrid lines in SH-SY5Y host cells can be differentiated into process-bearing dopaminergic neurons by exposure to very low levels of staurosporine (Borland, et al., 2009). Neuronal cells generated from SH-SY5Y lines exhibit morphologically distinct axonal and dendritic processes. Cybrid neurons express neuronal markers such as synaptophysin, tyrosine hydroxylase, dopamine transporter, VMAT, and the D2 dopamine receptor. Differentiated PD cybrid neurons spontaneously exhibit cybrid Lewy bodies as well as α-synuclein positive neuritic swellings (cybrid Lewy neurites) that resemble Lewy neurite pathology PD brain (Borland, et al., 2009). The cybrid Lewy bodies and cybrid Lewy neurites produced by PD cybrid cells in culture are formed without the need for exogenous proteasomal, lysosomal inhibition or α-synuclein over-expression. PD cybrid cell lines and the inclusions they generate are currently the only human models available to study these key neuropathological inclusions and their role in PD pathogenesis.
Reduced mitochondrial transport velocities
The damaged neurites of dopaminergic neurons are a prominent feature of early Parkinson’s disease (PD) pathogenesis. One hypothesis for the morphological dystrophy of neurites in sporadic PD is axonal transport failure. Reduced axonal transport deprives the cell body of vital trophic factors and deprives axonal terminals of synaptic vesicles and organelles like mitochondria. PD cybrids show a CoQ10-sensitive depolymerization of tubulin that could account for microtubule dysfunction and impaired organelle transport. (Esteves, et al., 2008).
Differentiated PD cybrid neuronal cells have provided a unique model to study the role of axonal transport loss in sporadic PD. We also exposed cultures of staurosporine-differentiated SH-SY5Y neuronal cells to rotenone, a pesticide which causes neuropathological changes that resemble PD. We measured the velocity of MitoTrackerCMX-Ros-labeled mitochondria in these two sporadic PD models. The axonal transport of mitochondria was significantly reduced in the processes of PD cybrids and in rotenone-treated SH-SY5Y cells (Borland, et al., 2009); Trimmer et al, in press). These results support the hypothesis that reduced axonal transport plays a critical role in the early pathogenesis of sporadic PD.
Respiration, gene expression and mtDNA analyses
Respiration, the consumption of oxygen by the mitochondrial ETC, cannot be reliably assayed in mitochondria from frozen post-mortem brain tissue, in contrast to maximal enzymatic activities of individual ETC complexes (complex I, complex IV, etc) that are routinely assayed. The distinction between these two assays is important, as respiration results from the coordinated and complex interaction of substrates and ETC complexes in a highly regulated fashion, whereas ETC complex catalytic activity assays only maximal potential electron transfer rate within a particular ETC complex. There is no a priori reason why the two are necessarily related.
Because cybrids are living cells, it is feasible to study their respiratory properties and relate these to genotypic and biochemical properties of their ETC assembly. In addition, respiration in cybrids can be examined in intact cells metabolizing glucose, where metabolic substrate control systems are intact (so-called “high-resolution respirometry” (Hutter, et al., 2006)), or in isolated mitochondria, where individual ETC complex-specific substrates are provided. We have examined both types of respiration in PD/CNT cybrid lines and compared the respiration rates to mtDNA gene and gene expression levels and levels of ETC proteins.
We found at most a modest correlation between respiration rates of intact cybrid cells compared to that of their isolated mitochondria. This was surprising but implies that isolated mitochondria when removed from the internal substrate and feedback controls of intact cells show altered respiratory properties.
We did find good correlations among intact cell respiration rates and mtDNA gene levels but surprisingly not with ETC protein levels. The antibodies we used to assay ETC proteins are all directed against nuclear genome encoded proteins, except for that against CO subunit 2 that is mtDNA encoded. This suggests the possibility that mtDNA encoded ETC subunits may be rate limiting for respiration.
We have begun to search for the nature of mtDNA differences that could explain differences between sPD and CNT cybrid lines. In terms of absolute mtDNA gene copy numbers, we have found that sPD cybrids show deficiencies of mtDNA genes compared to CNT for multiple mtDNA genes. This finding implies a deficiency of mitochondrial biogenesis in the sPD lines, the origins of which are not clear at this time.
In terms of mtDNA mutations we have used the Surveyor® nuclease approach to search for heteroplasmic mutations. Surveyor is a plant-derived, mismatch-cleaving nuclease that is our hands can detect down to ~2% heteroplasmy using automated electrophoresis for separation and measurement of DNA bands. We followed the approach described by Bannwarth, et al (Bannwarth, et al., 2005, Bannwarth, et al., 2006) to amplify overlapping ~2 kbase regions of the mtDNA heavy circle, followed by Surveyor treatment. Although we found differences in the apparent abundance of heteroplasmies across the mitochondrial genome, there were no obvious patterns that distinguished sPD from CNT cybrids (in review).
These results have only limited interpretations for several reasons. First, the Surveyor approach in the absence of sequencing of specific heteroplasmies does not define specific mutations, only whether heteroplasmies can be detected or not. Second, even if specific mutations were discovered, the biological importance of those mutations is not clear as to whether they would alter ETC function. Third, we routinely observed loss of untreated amplicon intensities following Surveyor treatment that was not accounted for in the Surveyor treated lanes. This suggests that there are multiple low abundance heteroplasmies that are cut by Surveyor but not detectable on our gel system.
We have also used nested PCR to search for mtDNA deletions in cybrids that would be located in the major arc, similar to the location of the ~5 kbase “common deletion” and the variably sized deletions described in mtDNA’s from PD nigral neurons (Bender, et al., 2006, Reeve, et al., 2008). To date we have detected multiple, variably sized (5–7 kbase) deletions, many of which have direct repeat break points (B. Miller, personal communication). Although there is much analysis of the deletions to be done, our preliminary findings suggest that PD cybrids will show mtDNA deletions that are similar to those found in vivo in PD brain neurons.
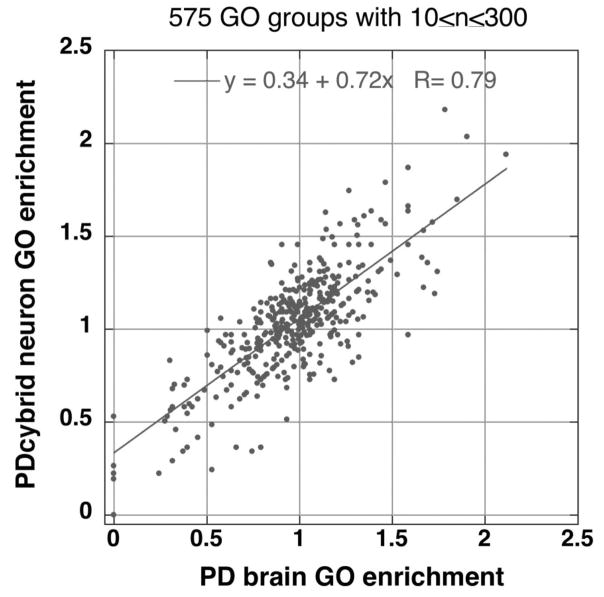
Finally we examined mitochondrial and nuclear ETC gene expression using RT-qPCR and general nuclear gene expression with microarrays to compare sPD and CNT cybrids to sPD and CNT brain samples. In our RT-qPCR studies we observed very high correlations among relative expressions of mtDNA-encoded and nuclear DNA-encoded ETC genes between sPD cybrids and sPD brains (Borland, et al., 2009). In our gene microarray studies we found a high-correlation among Gene Ontology group enrichments in sPD cybrids compared to sPD brain (Figure 1). Taken together, these gene expression studies showed that on average, sPD cybrids were regulating their ETC gene expressions very similar to sPD brains and that both cybrids and brains were activating similar gene programs and showing similar biological “intent”, though not necessarily by altering expressions of identical gene members of those programs.
Figure 1.
Correlation between relative enrichment levels for 575 gene ontology (GO) groups derived from PD/CTL expression ratios in 10 PD brain samples compared to 9 PD cybrid neuron samples. GO families with members >10 and <300 in size were used.
Use of sPD Cybrids as Translational Platforms for Therapy Development
Near infrared light irradiation
Light therapy (LT) with coherent laser light or light emitting diodes in the far red to near-infrared spectrum (600–860nm) modulates numerous biological processes and is currently being used worldwide to treat a wide range of human neurological conditions (Byrnes, et al., 2005, Lampl, et al., 2007, Oron, et al., 2007). The distance near-infrared light penetrates and transmits through skin, fat, muscle, bone and organs depends on the wavelength being used, but the distance is sufficient to penetrate the brain when delivered through the scalp and skull (Lampl, et al., 2007). LT causes changes at the cellular level by interacting with photo-acceptors and stimulating primary molecular processes that result in long-term detectable biological effects. The primary photo-acceptor in mitochondria appears to be cytochrome c oxidase (complex IV) (Karu, et al., 2004). Mitochondria change their oxygen utilization properties, and accelerate electron transfer leading to secondary biochemical reactions in response to absorption of photons. These mitochondrial changes continue to occur long after LT has stopped (Karu, 2008). Complex IV is routinely inhibited by nitric oxide (NO). LT can overcome NO inhibition of complex IV and increase the breakdown of NO (Borutaite, et al., 2000). Lt also stimulates oxygen utilization, and ATP synthesis thereby increasing energy metabolism and improving cell survival (Borutaite, et al., 2000). LT increases activity of mtETC complexes I, II and IV, reverses inhibition of complex I by peroxynitrite and increases the ADP/ATP translocator, mitochondrial membrane potential, mitochondrial DNA and RNA replication and protein synthesis (Borutaite, et al., 2000, Greco, et al., 1989, Passarella, et al., 1988, Vacca, et al., 1993, Yu, et al., 1997).
After exposure of differentiated PD cybrid neuronal cells and rotenone-exposed SH-SY5Y cells to a low energy laser treatment (LLLT, 50mW/cm2 for 40 seconds, 810nm diode laser), the velocity of mitochondrial movement by axonal transport was increased and restored to control levels for several hours after completion of the LT (Trimmer et al, in press). LT has also been shown to reverse the cellular changes induced by MPP+ and rotenone in both cell and animal models (Liang, et al., 2008, Rojas, et al., 2008). In conclusion, reduced axonal transport of mitochondria is present in two models of sporadic PD pathogenesis and can be ameliorated by the application of a low energy laser treatment. Because laser LT is already in clinical trials for stroke, in light of these encouraging cybrid results it is feasible now for study in sPD.
Mitochondrial gene therapy
In light of the findings of abundant deleted mtDNA’s in PD nigral neurons and reduced mtDNA gene levels in sPD cybrids, a strategy to augment mitochondrial genomes to restore bioenergetic capacity in sPD brain is worthy of consideration. We have developed a technology that utilizes an engineered human mitochondrial transcription factor A (TFAM) protein that includes a protein transduction domain (PTD) to facilitate passage across plasma membranes, and a mitochondrial localization sequence/signal (MLS) to target mitochondria. The resulting TFAM with its “mitochondrial transduction domain” (MTD=PTD+MLS, MTD−TFAM) binds mtDNA and translocates it rapidly to mitochondria inside cells. A single treatment of sPD cybrid cells with MTD−TFAM complexed with human mtDNA is capable of restoring respiration and increases many fold mtDNA levels and gene expression as well as ETC protein levels. These desirable outcomes appear to result from stimulation of mitochondrial biogenesis, as levels of both the mitochondrial biogenesis transcriptional coactivator PGC-1α gene expression and mitochondrially imported 25 kDa TFAM protein are increased weeks after treatment. These encouraging findings in the cybrid cell model of sPD support development of this mitochondrial gene therapy approach for therapeutic use in humans.
How can the cybrid model be improved?
Creation of cybrids from multi-potential neural precursor cells and brain mtDNA
There are at least two major limitations of the cybrid cell model as it presently exists. First, the host cells are tumor cells, and their neoplastic phenotypes undoubtedly influence their cell cycle progression (mature neurons do not enter the cell cycle) and mitochondrial biogenesis. Although both SH-SY5Y and NT2 cells can be differentiated into non-dividing neurons, they are not primary neurons. Second, cybrids are made from mesodermal sources of mtDNA such as platelets or enucleated fibroblasts. It is unknown the degree to which these peripheral mtDNA’s residing in replaceable cells reflect brain mtDNA’s existing in non-mitotic cells that have existed for decades.
Given these limitations, it is remarkable that sPD cybrids produce phenotypes that resemble sPD brain to any degree. That the resemblances noted above exist at all speaks to the high probability that PD pathogenesis in the brain is derived in part from expression of systemic mtDNA present in brain and peripheral tissues.
A major improvement in the cybrid model could result from use of human multi-potential neural cells, such as neural progenitor cells (NPC’s) that can be propagated and differentiated into neurons, oligodendroglia or astrocytes (Dhara, et al., 2008, Rao, et al., 2008, Shin, et al., 2006). We are presently exploring how to use NPC’s that have their endogenous mtDNA reduced >90% acutely by treatment with reverse transcriptase inhibitors (RTI) like dideoxycytidine (ddC). RTI drugs have as a major side effect the consequences of inhibiting DNA polymerase-γ that is specific for replication of mtDNA and can be used for acute mtDNA reduction.
A second area of improvement would come from introduction of brain mtDNA. If possible, this approach would provide a direct test of mtDNA causality in bringing about sPD alterations, such as Lewy body formation. With our mitochondrial gene therapy we are in a position to introduce mtDNA of any source, including postmortem brain. We are presently pursuing that possibility but are at too early a stage to report any results.
Summary
Since its introduction by us in 1996, the sPD cybrid model has contributed much compelling circumstantial information implicating mtDNA as a pathogenic factor in sPD pathogenesis. sPD cybrids show many abnormalities in bioenergetics, oxidative stress, calcium and stress pathway signaling and cell death pathways/survival. They spontaneously form Lewy bodies, perhaps one of their most compelling properties supporting the role of mtDNA in sPD pathogenesis. They resemble sPD brain in other important ways, including how they regulate both mtDNA and nDNA-mediated ETC gene expression.
sPD cybrids have slowed mitochondrial transport that is normalized by near-IR laser light therapy. They also show impaired respiration and reduced mtDNA copy numbers that are normalized by mitochondrial gene therapy. In these manners sPD cybrids are serving as translational platforms for development of novel experimental therapies.
sPD cybrids as they are currently made have at least two substantial limitations related to the use of tumor cell hosts and mesodermal mitotic sources of mtDNA’s. Improvements in human stem cell technologies have yielded neural precursor cells that can be propagated and differentiated into the three major CNS cell types, potentially eliminating the use of neural tumor cells as hosts. The mitochondrial gene therapy technology allows introduction into mitochondria of any exogenous mtDNA. Currently we are attempting to introduce and propagate human brain sPD mtDNA’s. If successful, the resulting cybrid cells will be expressing mtDNA’s from pathologically confirmed disease brains inside neural precursor cells that can be differentiated into neurons and glia. Theoretically, this could result in a “brain in a dish” model for sPD and related diseases. Such a model system would almost certainly provide additional insights into pathogenesis and serve as more reliable therapy development platforms.
Acknowledgments
The authors’ work has been supported by the National Institutes of Health, PhotoThera, Inc., the American Parkinson Disease Association, the Parkinson Disease Foundation, the Commonwealth of Virginia ADRAF, and the D. Loy Stewart Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bannwarth S, Procaccio V, Paquis-Flucklinger V. Surveyor Nuclease: a new strategy for a rapid identification of heteroplasmic mitochondrial DNA mutations in patients with respiratory chain defects. Hum Mutat. 2005;25:575–582. doi: 10.1002/humu.20177. [DOI] [PubMed] [Google Scholar]
- 2.Bannwarth S, Procaccio V, Paquis-Flucklinger V. Rapid identification of unknown heteroplasmic mutations across the entire human mitochondrial genome with mismatch-specific Surveyor Nuclease. Nat Protoc. 2006;1:2037–2047. doi: 10.1038/nprot.2006.318. [DOI] [PubMed] [Google Scholar]
- 3.Belin AC, Westerlund M. Parkinson’s disease: a genetic perspective. FEBS J. 2008;275:1377–1383. doi: 10.1111/j.1742-4658.2008.06301.x. [DOI] [PubMed] [Google Scholar]
- 4.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 5.Bender A, Schwarzkopf RM, McMillan A, Krishnan KJ, Rieder G, Neumann M, Elstner M, Turnbull DM, Klopstock T. Dopaminergic midbrain neurons are the prime target for mitochondrial DNA deletions. J Neurol. 2008 doi: 10.1007/s00415-008-0892-9. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand E, Lechowicz W, Szpak GM, Lewandowska E, Dymecki J, Wierzba-Bobrowicz T. Limbic neuropathology in idiopathic Parkinson’s disease with concomitant dementia. Folia Neuropathol. 2004;42:141– 150. [PubMed] [Google Scholar]
- 7.Borland MK, Mohanakumar KP, Rubinstein JD, Keeney PM, Xie J, Capaldi R, Dunham LD, Trimmer PA, Bennett JP., Jr Relationships among molecular genetic and respiratory properties of Parkinson’s disease cybrid cells show similarities to Parkinson’s brain tissues. Biochim Biophys Acta. 2009;1792:68–74. doi: 10.1016/j.bbadis.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borutaite V, Budriunaite A, Brown GC. Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim Biophys Acta. 2000;1459:405–412. doi: 10.1016/s0005-2728(00)00178-x. [DOI] [PubMed] [Google Scholar]
- 9.Braak E, Sandmann-Keil D, Rub U, Gai WP, de Vos RA, Steur EN, Arai K, Braak H. alpha-synuclein immunopositive Parkinson’s disease- related inclusion bodies in lower brain stem nuclei. Acta Neuropathol (Berl) 2001;101:195–201. doi: 10.1007/s004010000247. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha- synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology relate. 2003 doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 13.Braak H, Sandmann-Keil D, Gai W, Braak E. Extensive axonal Lewy neurites in Parkinson’s disease: a novel pathological feature revealed by alpha-synuclein immunocytochemistry. Neurosci Lett. 1999;265:67–69. doi: 10.1016/s0304-3940(99)00208-6. [DOI] [PubMed] [Google Scholar]
- 14.Byrnes KR, Waynant RW, Ilev IK, Wu X, Barna L, Smith K, Heckert R, Gerst H, Anders JJ. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med. 2005;36:171–185. doi: 10.1002/lsm.20143. [DOI] [PubMed] [Google Scholar]
- 15.Cassarino DS, Fall CP, Swerdlow RH, Smith TS, Halvorsen EM, Miller SW, Parks JP, Parker WD, Jr, Bennett JP., Jr Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson’s disease. Biochim Biophys Acta. 1997;1362:77–86. doi: 10.1016/s0925-4439(97)00070-7. [DOI] [PubMed] [Google Scholar]
- 16.Cassarino DS, Halvorsen EM, Swerdlow RH, Abramova NN, Parker WD, Jr, Sturgill TW, Bennett JP., Jr Interaction among mitochondria, mitogen-activated protein kinases, and nuclear factor-kappaB in cellular models of Parkinson’s disease. J Neurochem. 2000;74:1384–1392. doi: 10.1046/j.1471-4159.2000.0741384.x. [DOI] [PubMed] [Google Scholar]
- 17.Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson’s disease? Exp Neurol. 2008;209:22–27. doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhara SK, Hasneen K, Machacek DW, Boyd NL, Rao RR, Stice SL. Human neural progenitor cells derived from embryonic stem cells in feeder-free cultures. Differentiation. 2008;76:454–464. doi: 10.1111/j.1432-0436.2007.00256.x. [DOI] [PubMed] [Google Scholar]
- 19.Esteves AR, Arduino DM, Swerdlow RH, Oliveira CR, Cardoso SM. Oxidative Stress involvement in alpha-synuclein oligomerization in Parkinsons disease cybrids. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2247. [DOI] [PubMed] [Google Scholar]
- 20.Esteves AR, Domingues AF, Ferreira IL, Januario C, Swerdlow RH, Oliveira CR, Cardoso SM. Mitochondrial function in Parkinson’s disease cybrids containing an nt2 neuron-like nuclear background. Mitochondrion. 2008;8:219–228. doi: 10.1016/j.mito.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh SS, Swerdlow RH, Miller SW, Sheeman B, Parker WD, Jr, Davis RE. Use of cytoplasmic hybrid cell lines for elucidating the role of mitochondrial dysfunction in Alzheimer’s disease and Parkinson’s disease. Ann N Y Acad Sci. 1999;893:176–191. doi: 10.1111/j.1749-6632.1999.tb07825.x. [DOI] [PubMed] [Google Scholar]
- 22.Greco M, Guida G, Perlino E, Marra E, Quagliariello E. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428–1434. doi: 10.1016/0006-291x(89)91138-8. [DOI] [PubMed] [Google Scholar]
- 23.Greenamyre JT, Sherer TB, Betarbet R, Panov AV. Complex I and Parkinson’s disease. IUBMB Life. 2001;52:135–141. doi: 10.1080/15216540152845939. [DOI] [PubMed] [Google Scholar]
- 24.Gu M, Cooper JM, Taanman JW, Schapira AH. Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Ann Neurol. 1998;44:177–186. doi: 10.1002/ana.410440207. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Dawson VL, Dawson TM. What causes cell death in Parkinson’s disease? Ann Neurol. 2008;64(Suppl 2):S3–15. doi: 10.1002/ana.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutter E, Unterluggauer H, Garedew A, Jansen-Durr P, Gnaiger E. High-resolution respirometry--a modern tool in aging research. Exp Gerontol. 2006;41:103–109. doi: 10.1016/j.exger.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Karu TI. Mitochondrial Signaling in Mammalian Cells Activated by Red and Near-IR Radiation. Photochem Photobiol. 2008;84:1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 28.Karu TI, Pyatibrat LV, Afanasyeva NI. A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation. Photochem Photobiol. 2004;80:366–372. doi: 10.1562/2004-03-25-RA-123. [DOI] [PubMed] [Google Scholar]
- 29.Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- 31.Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38:1843– 1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 32.Langston JW. The etiology of Parkinson’s disease with emphasis on the MPTP story. Neurology. 1996;47:S153–160. doi: 10.1212/wnl.47.6_suppl_3.153s. [DOI] [PubMed] [Google Scholar]
- 33.Liang HL, Whelan HT, Eells JT, Wong-Riley MT. Near- infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. Neuroscience. 2008;153:963–974. doi: 10.1016/j.neuroscience.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onyango IG, Tuttle JB, Bennett JP., Jr Activation of p38 and N-acetylcysteine-sensitive c-Jun NH2-terminal kinase signaling cascades is required for induction of apoptosis in Parkinson’s disease cybrids. Mol Cell Neurosci. 2005;28:452–461. doi: 10.1016/j.mcn.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Oron A, Oron U, Streeter J, De TL, Alexandrovich A, Trembovler V, Shohami E. low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma. 2007;24:651–656. doi: 10.1089/neu.2006.0198. [DOI] [PubMed] [Google Scholar]
- 36.Passarella S, Ostuni A, Atlante A, Quagliariello E. Increase in the ADP/ATP exchange in rat liver mitochondria irradiated in vitro by helium-neon laser. Biochem Biophys Res Commun. 1988;156:978–986. doi: 10.1016/s0006-291x(88)80940-9. [DOI] [PubMed] [Google Scholar]
- 37.Rao RC, Boyd J, Padmanabhan R, Chenoweth JG, McKay RD. Efficient Serum-Free Derivation of Oligodendrocyte Precursors from Neural Stem Cell-Enriched Cultures. Stem Cells. 2008 doi: 10.1634/stemcells.2007-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeve AK, Krishnan KJ, Elson JL, Morris CM, Bender A, Lightowlers RN, Turnbull DM. Nature of mitochondrial DNA deletions in substantia nigra neurons. Am J Hum Genet. 2008;82:228–235. doi: 10.1016/j.ajhg.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rojas JC, Lee J, John JM, Gonzalez-Lima F. Neuroprotective Effects of Near-Infrared Light in an In Vivo Model of Mitochondrial Optic Neuropathy. J Neurosci. 2008;28:13511–13521. doi: 10.1523/JNEUROSCI.3457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheehan JP, Swerdlow RH, Parker WD, Miller SW, Davis RE. :1221–1233. doi: 10.1046/j.1471-4159.1997.68031221.x. [DOI] [PubMed] [Google Scholar]
- 41.Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- 44.Shin S, Mitalipova M, Noggle S, Tibbitts D, Venable A, Rao R, Stice SL. Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells. 2006;24:125–138. doi: 10.1634/stemcells.2004-0150. [DOI] [PubMed] [Google Scholar]
- 45.Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Jr, Davis RE, Parker WD., Jr Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 46.Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134:109–118. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Trimmer PA, Borland MK, Keeney PM, Bennett JP, Jr, Parker WD., Jr Parkinson’s disease transgenic mitochondrial cybrids generate Lewy inclusion bodies. J Neurochem. 2004;88:800–812. doi: 10.1046/j.1471-4159.2003.02168.x. [DOI] [PubMed] [Google Scholar]
- 48.Trimmer PA, Swerdlow RH, Parks JK, Keeney P, Bennett JP, Jr, Miller SW, Davis RE, Parker WD., Jr Abnormal mitochondrial morphology in sporadic Parkinson’s and Alzheimer’s disease cybrid cell lines. Exp Neurol. 2000;162:37–50. doi: 10.1006/exnr.2000.7333. [DOI] [PubMed] [Google Scholar]
- 49.Vacca RA, Marra E, Quagliariello E, Greco M. Activation of mitochondrial DNA replication by He-Ne laser irradiation. Biochem Biophys Res Commun. 1993;195:704–709. doi: 10.1006/bbrc.1993.2102. [DOI] [PubMed] [Google Scholar]
- 50.Veech GA, Dennis J, Keeney PM, Fall CP, Swerdlow RH, Parker WD, Jr, Bennett JP., Jr Disrupted mitochondrial electron transport function increases expression of anti-apoptotic bcl-2 and bcl-X(L) proteins in SH-SY5Y neuroblastoma and in Parkinson disease cybrid cells through oxidative stress. J Neurosci Res. 2000;61:693–700. doi: 10.1002/1097-4547(20000915)61:6<693::AID-JNR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 51.Wakabayashi K, Tanji K, Mori F, Takahashi H. The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology. 2007;27:494–506. doi: 10.1111/j.1440-1789.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 52.Waldmeier P, Bozyczko-Coyne D, Williams M, Vaught JL. Recent clinical failures in Parkinson’s disease with apoptosis inhibitors. 2006 doi: 10.1016/j.bcp.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 53.Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol. 1997;66:866–871. doi: 10.1111/j.1751-1097.1997.tb03239.x. [DOI] [PubMed] [Google Scholar]