Abstract
A central focus of aging research is to determine how calorie restriction (CR) extends lifespan and delays diseases of aging. SIRT1, the mammalian ortholog of Sir2 in yeast, is a longevity factor which mediates dietary restriction in diverse species. In addition, SIRT1 plays a protective role in several models of neurodegenerative disease. We tested the role of SIRT1 in mediating the effects of CR in a mouse model of prion disease. Prion diseases are protein misfolding disorders of the central nervous system with many similarities to other neurodegenerative diseases, including deposition of aggregated protein, gliosis, and loss of synapses and neurons. We report that the onset of prion disease is delayed by CR and in the SIRT1 KO mice fed ad libitum. CR exerts no further effect on the SIRT1 KO strain, suggesting the effects of CR and SIRT1 deletion are mechanistically coupled. In conjunction, SIRT1 is downregulated in certain brain regions of CR mice. The expression of PrP mRNA and protein is reduced in the brains of CR mice and in SIRT1 knockout mice, suggesting a possible mechanism for the delayed onset of disease, as PrP levels are a critical determinant of how quickly mice succumb to prion disease. Surprisingly, CR greatly shortens the duration of clinical symptoms of prion disease and ultimately shortens lifespan of prion-inoculated mice in a manner that is independent of SIRT1. Taken together, our results suggest a more complex interplay between CR, SIRT1, and neurodegenerative diseases than previously appreciated.
Introduction
Aging research has the potential to impact a broad array of aging-related diseases (Sinclair and Guarente, 2006; Chen and Guarente, 2007). Calorie restriction (CR) is a dietary regimen that extends lifespan in a wide spectrum of species ranging from yeast to mammals (Guarente and Picard, 2005). CR also delays many diseases with seemingly different causes, such as kidney disease, cancer, autoimmune disease, metabolic syndromes, and neurodegenerative diseases including Parkinson’s and Alzheimer’s disease (Koubova and Guarente, 2003; Chen et al., 2005b; Longo and Kennedy, 2006). Mediators of CR would serve as potential targets for a CR mimetic (Baur and Sinclair, 2006).
The Sir2 gene was first identified as a longevity factor in yeast, and this function is conserved in higher organisms (Guarente and Picard, 2005). The Sir2 protein is an NAD-dependent deacetylase (Imai et al., 2000). Thus, its activity is amenable for regulation by the metabolic status of the cell, and it may serve as a prime candidate in mediating the cellular responses to CR. There is evidence that Sir2 is required for CR-induced lifespan extension in yeast and flies, although in yeast the requirement of Sir2 might depend on the specific conditions of CR (Longo and Kennedy, 2006; Chen and Guarente, 2007). SIRT1, the mammalian ortholog of the yeast Sir2, is required for the increased physical activity exhibited in CR mice (Chen et al., 2005a). In addition, transgenic mice over-expressing SIRT1 in certain tissues show some metabolic phenotypes resembling those seen in mice on a CR regimen (Bordone et al., 2007). Thus, it is relevant and critical to address whether SIRT1 mediates both CR-induced lifespan extension and the delay of aging-related diseases in mammals.
The expression of SIRT1 promotes longevity by an as yet undetermined mechanism that may involve its upregulation in many tissues of mice on a CR diet (Cohen et al., 2004). It is also thought to have a protective role in the progression of many neurodegenerative diseases. SIRT1 may serve as a downstream effector of increased NAD biosynthesis and delay axonal degeneration in a mouse model of Wallerian degeneration (Araki et al., 2004). Importantly, overexpression of SIRT1 also protects against Alzheimer’s disease, Huntington’s disease and amyotrophic lateral sclerosis in various model systems (Parker et al., 2005; Qin et al., 2006; Kim et al., 2007), consistent with its proposed neuroprotective function. However, the effect of deleting SIRT1 in a neurodegenerative disease model in a mammalian system is not known.
Prion diseases are unique among neurodegenerative diseases in that they are transmissible while still sharing commonalities with other neurodegenerative diseases such as the accumulation of aggregates of misfolded protein, a prominent astrocytic and microglial response, and loss of neurons in the central nervous system (Prusiner, 1998; Aguzzi et al., 2007). The prion protein (PrP) is an N-linked glycoprotein tethered to the cell surface via a GPI anchor. Although the normal function of PrP is poorly defined (Steele et al., 2007a), numerous lines of evidence point toward a pivotal role for PrP in the pathogenic mechanism of prion diseases (Prusiner, 1998; Aguzzi et al., 2007). In prion diseases, the normal isoform of PrP (termed PrPC) is structurally converted into PrPSc, a self-perpetuating and aggregation-prone conformation of the protein (Prusiner, 1998). The ongoing conversion of PrPC to PrPSc in neurons is required for prion toxicity (Brandner et al., 1996; Mallucci et al., 2003; Aguzzi and Heikenwalder, 2006). Yet beyond this basic observation, the pathways leading to neurotoxicity are almost completely unknown (Aguzzi et al., 2007; Steele et al., 2007b).
Here we investigate the effects of CR and SIRT1 deletion in a mouse model of infectious prion disease. In contrast to both what has been observed with Sir2 deletion in a worm model of polyglutamine disease (Parker et al., 2005) and what would be predicted based on studies where SIRT1 is overexpressed in mouse models of neurodegeneration (Qin et al., 2006; Kim et al., 2007), SIRT1 deletion delays the onset of disease. As expected, CR delays the onset of diseases, consistent with what has been observed with other models of neurodegeneration (Duan et al., 2003; Patel et al., 2005). Both CR and SIRT1 deletion delay the onset of prion disease by mechanisms that may involve a reduction in PrP expression, a well characterized determinant of the onset and duration of prion disease (Weissmann et al., 1998). Unexpectedly, CR also greatly shortens the duration of the clinical period of the disease in a manner that is independent of SIRT1. Despite the delay in disease onset, mice on a CR diet ultimately succumb to disease slightly faster than controls on a normal diet, in contrast to what has been observed with CR and other neurodegenerative disease models (Duan et al., 2003; Patel et al., 2005). Regulation of SIRT1 expression by CR differs in various brain regions; expression of SIRT1 was upregulated by CR in the cortex and hippocampus, and downregulated in the cerebellum and midbrain. Thus, SIRT1 regulation in the brain during CR is complex, a finding which is consistent with the unexpected effects of CR and SIRT1 deletion on prion-mediated neurodegeneration.
Results
The onset of prion disease is delayed by CR and in SIRT1 knockout mice
To assess the effects of CR and SIRT1 on prion disease, we compared the response to prion infection of SIRT1 knockout mice (KO) (McBurney et al., 2003) and their wild-type (WT) littermates either fed ad libitum (AL) or on a CR diet. One month after mice were adjusted to CR, we challenged them (WT/AL, WT/CR, SIRT1 KO/AL and SIRT1 KO/CR) with 3.5 log LD50 Rocky Mountain Laboratory (RML) strain of murine prions inoculated directly into the brain.
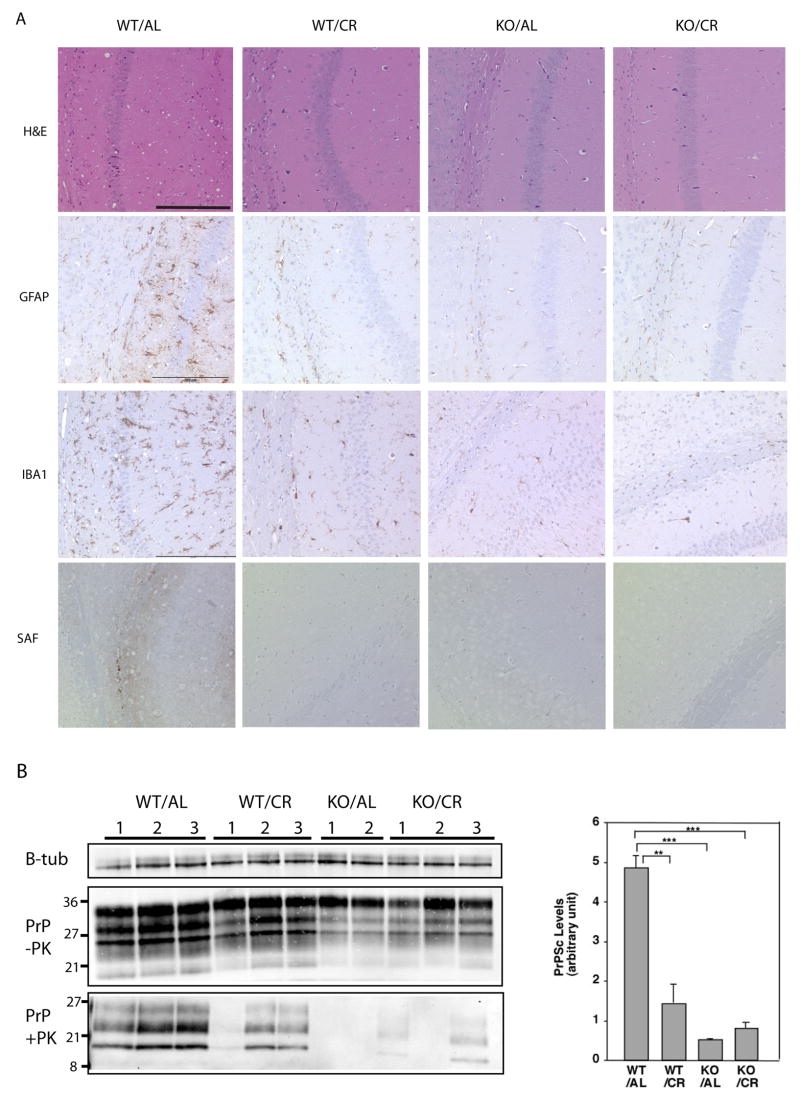
Prior to the onset of overt symptoms we sacrificed a cohort of mice at 4 months post inoculation (MPI) to conduct neuropathological analysis. RML prion strain pathology is characterized by dramatic vacuolation in white and gray matter, neuronal loss, and severe gliosis. Histological analysis for spongiform changes with hemotoxylin and eosin (HE) staining of brains taken at 4 MPI revealed vacuolation throughout the WT/AL group. However, the WT/CR mice or SIRT1 KO mice on either diet showed much less spongiform pathology (Figure 1A). In addition, immunohistochemical staining with an antibody against glial fibrillary acidic protein (GFAP) and a microglial marker ionized calcium-binding adaptor molecule 1 (IBA1) indicated that gliosis was much more advanced in WT mice fed AL than in other groups (Figure 1A). While both CR and SIRT1 deletion alleviated spongiosis and gliosis, there was no further improvement in SIRT1 KO mice on CR, suggesting that the effects of CR and SIRT1 depletion may be mechanistically coupled.
Figure 1.
The onset of prion disease is delayed in calorie restricted mice and SIRT1 knockout mice. Wild type (WT) mice and SIRT1 KO mice fed AL or on CR diets were injected with RML prions. Brains were removed 4 months post inoculation. (A) Brain sections were stained with hematoxylin and eosin to visualize vacuolation, anti-GFAP to visualize gliosis, anti-IBA1 staining to visualize microglia, and anti-PrP (SAF) on formic acid treated samples to visualize aggregates of PrP. Scale bars correspond to 200um. (B) Brain homogenates were subjected to proteinase-K digestion. Proteinase resistant PrP was detected by western blotting with anti-PrP antibody. β-tubulin (from undigested homogenates) was used as a loading control. The level of PK-resistant PrP was quantitated using ImageJ software.
The formation of proteinase K (PK)-resistant PrP is a classic surrogate marker for prion disease and typically correlates well with disease state in RML inoculated mice (Steele et al., 2007b). We assayed the amount of PK-resistant PrP accumulation in these mouse brains at 4 MPI by subjecting brain homogenates to PK digestion, SDS-PAGE, and immunodetection of the three glycoforms (di-, mono-, and unglycosylated) of PrP by immunoblotting (Figure 1B). The amount of PK-resistant PrP that had accumulated in the brains of WT/CR, SIRT1 KO/AL, and SIRT1 KO/CR mice was reduced in comparison to WT/AL (Figure 1B).
During prion disease, PrP forms dense protein deposits in the brain that can be visualized by immunostaining for PrP after treatment of tissue sections with formic acid, which removes PrPC but spares PrP aggregates. Prion aggregate staining was clearly detected in WT/AL brains at 4 MPI, but was not apparent in WT/CR, SIRT1 KO/AL, or SIRT1 KO/CR brain sections at 4 MPI (Figure 1A, “SAF”). The higher amount of PK-resistant PrP in WT/AL mice is consistent with the more severe pathology observed in these mice. We also harvested the brains of all four groups of mice when death was imminent. Similar levels of PK-resistant PrP were detected in terminal samples taken from WT/AL, WT/CR, SIRT1 KO/AL, and SIRT1 KO/CR (Supplemental Figure 1B). Thus, while CR and SIRT1 deletion suppress prion aggregation levels in pre-symptomatic mice, all groups of mice arrive at a comparable level of prion deposition at death.
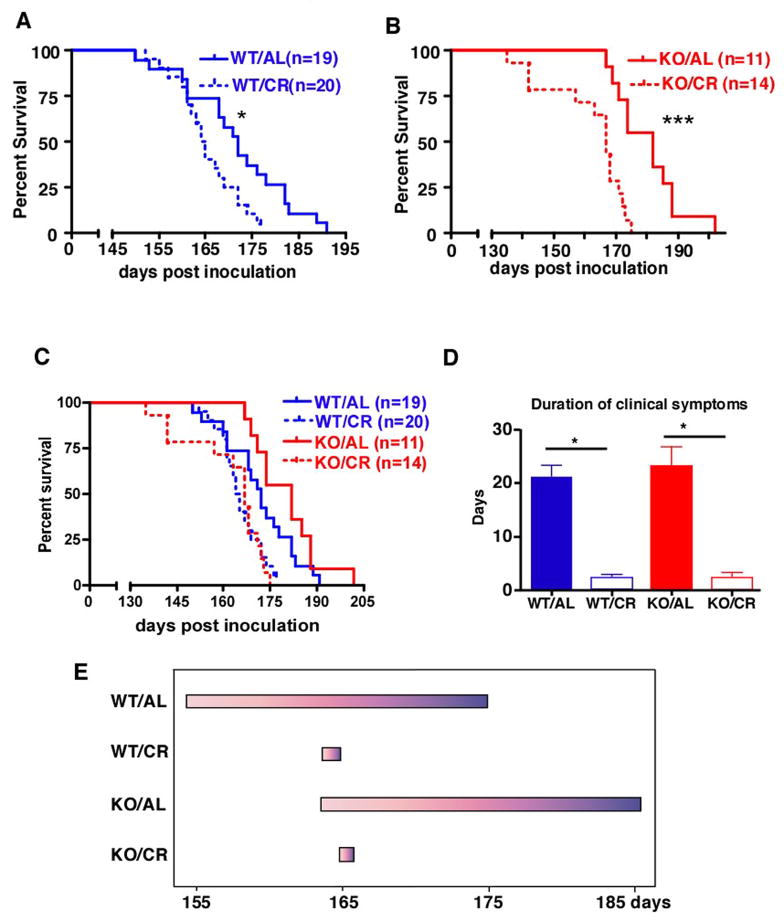
The effects of calorie restriction and SIRT1 deletion on the lifespan of mice with prion disease
Prion diseases are characterized by a long asymptomatic period followed by a rapid deterioration. At a gross level, mice inoculated with RML strain of prions develop ataxia and imbalance, lose weight, and show hunched posture (Kingsbury et al., 1983). Mice were monitored for behavioral changes daily. Prion inoculated WT/AL mice first showed symptoms of disease at 155 days post inoculation and survived for an average of 20 days before reaching the terminal stage of the disease (Figure 2). Both the onset of clinical symptoms and the death of SIRT1 KO mice fed AL were delayed for about 10 days when compared to WT controls (Figure 2). WT/CR mice also showed a similar delay in the onset of clinical symptoms when compared to WT mice fed AL. The onset of symptoms in SIRT1 KO mice on the CR diet was not further delayed. These results are consistent with the idea that CR delays the onset of prion disease via SIRT1, perhaps by reducing levels of this sirtuin in some critical brain region(s).
Figure 2.
The effect of CR and SIRT1 knockout on the lifespan of mice with prion disease. (A) The survival of WT mice fed AL or CR and injected intracranially with RML prions (P=0.012, log rank test). (B) The survival of SIRT1 KO mice fed AL or CR injected intracranially with RML prion (P=0.002, log rank test). (C) The survival of WT and SIRT1 KO mice fed AL or CR injected intracranially with RML prions (combination of panels A and B). (D) The duration of clinical symptoms was shortened by CR independently of SIRT1. (E) Summary of the progression of prion disease for WT and SIRT1 KO fed AL or on CR. The beginning of each bar represents the onset of clinical symptoms and the end of each bar represents the terminal stage of the disease.
Surprisingly, even though CR delayed the onset of prion disease, the CR mice had a shorter lifespan (median survival of 164.5 days for WT/CR and 167 for SIRT1 KO/CR), compared to mice fed AL (median survival of 172 days for WT/AL and 182 for SIRT1 KO/AL) (Figure 2A–C). This was because CR mice had a strikingly short duration of clinical symptoms (on average 2 days) (Figure 2D). Moreover, many CR mice remained apparently healthy and active until they experienced sudden death. The shortened duration of clinical symptoms in CR mice was SIRT1 independent, as it was also observed in SIRT1 KO mice on CR (Figure 2D). The sudden death of CR mice cannot be because they are less tolerant to neuronal loss, since a similar degree of brain pathology in terms of spongiosis and gliosis was observed in the terminal brains of WT/AL, WT/CR, and SIRT1 KO/CR mice (SIRT1 KO/AL was not performed) (Supplemental Figure 1A). Since the onset of disease occurs later in CR mice, yet they arrive at the same level of brain pathology as AL mice in a much shorter time, we infer that prion pathogenesis after the onset of symptoms is accelerated in CR mice.
PrP expression is down-regulated in both calorie restricted and SIRT1 knockout mice
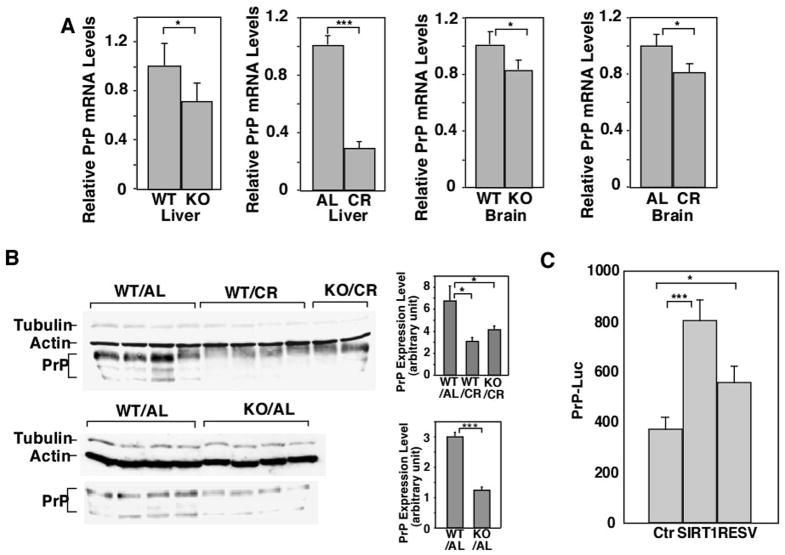
The initial observation that prion accumulation was suppressed at 4 MPI in CR mice and SIRT1 KO mice prompted us to test whether PrP expression was reduced in these mice—a known mechanism of altering the progression of prion disease (Weissmann et al., 1998). Indeed, both PrP mRNA and protein expression were reduced in the brains of SIRT1 KO mice compared to their WT controls (Figure 3A, B). Similarly, PrP levels were reduced in the brains of CR mice compared to AL controls (Figure 3A, B). Thus, decreased PrP gene expression in CR mice and SIRT1 KO mice may delay the onset of disease in these mice.
Figure 3.
PrP expression is downregulated in CR mice and SIRT1 knockout mice. (A) PrP mRNA in brain and liver and (B) PrP protein levels in brain are downregulated in CR mice and SIRT1 KO mice. PrP mRNA and protein expression levels were compared between WT mice and SIRT1 KO mice fed AL, or WT mice fed AL or calorie restricted. mRNA levels were quantified by real time PCR and relative abundance of mRNA was obtained by normalization to cyclophilin levels (n=4). Protein levels were quantified by western blotting with anti-PrP antibody SAF83. Both tubulin and actin were used as loading controls. (C) SIRT1 promotes the expression of the PrP promoter. 293T cells were transfected with a luciferase reporter driven by the PrP promoter, with or without addition of a SIRT1 construct and/or resveratrol. The expression from the reporter construct was quantified by a luciferase assay.
SIRT1 is a transcriptional cofactor, which can either positively or negatively regulate transcription depending on the context or gene in question (Brunet et al., 2004). Since PrP expression is downregulated in SIRT1 KO mice, we examined whether SIRT1 directly regulates the PrP promoter using a luciferase reporter assay. We transiently transfected 293T cells with a construct through which luciferase expression is driven by a modified PrP promoter (Cabral et al., 2002). Both overexpression of SIRT1 and resveratrol, a polyphenolic SIRT1-activating compound (Howitz et al., 2003), enhanced the expression from the PrP promoter in 293T cells (Figure 3C). Thus, the decreased PrP level in SIRT1 KO mice may be due to a direct effect of SIRT1 on the PrP promoter.
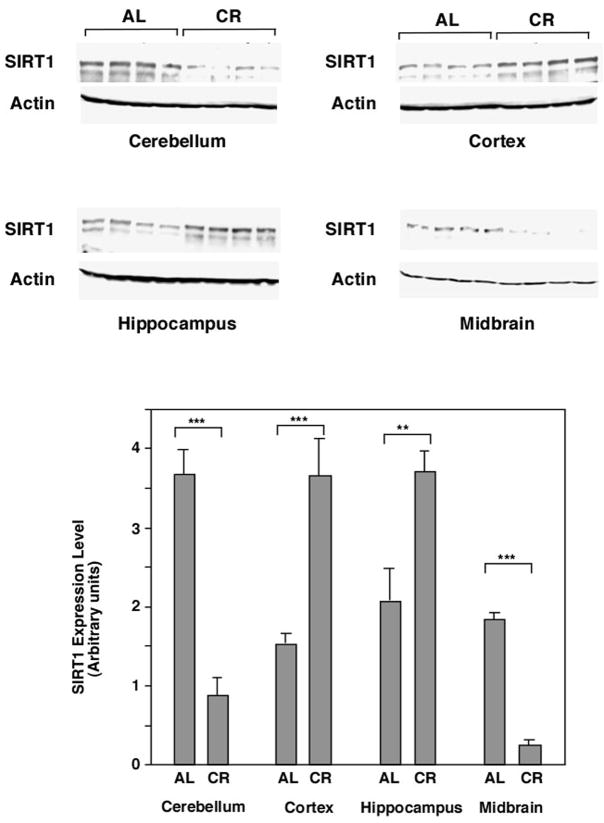
SIRT1 is downregulated in certain brain regions of calorie restricted mice
Since either CR or the deletion of SIRT1 delay disease onset and these effects are not additive, we reasoned that CR may down regulate SIRT1 in brain regions affected by prion disease. Therefore we examined SIRT1 expression in microdissected brain regions of CR mice (not infected with prions) and found that it was differentially affected in different regions. SIRT1 was upregulated in the cortex and hippocampus, but downregulated in the cerebellum and midbrain (Figure 4). The regulation of SIRT1 in other brain regions of CR mice remains to be evaluated. Perhaps one or more of the brain regions in which SIRT1 is downregulated by CR are important in the spread of prion pathogenesis.
Figure 4.
SIRT1 is downregulated in certain brain regions of CR mice. The cerebellum, cortex, hippocampus and midbrain were dissected from WT C57Bl/6 mice fed AL or on CR (provided by the National Institute on Aging). The expression of SIRT1 in different brain regions was quantified by western blotting with anti-SIRT1 antibody using ImageJ. Actin was used as a loading control.
Discussion
We propose the following mechanism for the relationship between CR, SIRT1 and prion disease: SIRT1 is downregulated in certain brain regions of CR mice, resulting in a decreased PrP expression level, PrPSc accumulation, and delayed onset of prion disease. The following evidence supports such a model: 1) Both SIRT1 deletion and CR delay the onset of prion disease, and CR has no further effect on SIRT1 KO mice (Figure 1, 2). This suggests that the effects of SIRT1 deletion and CR on the onset of prion disease are mechanistically coupled. 2) SIRT1 expression is downregulated in some brain regions during CR (Figure 4). 3) Decreased PrP expression levels were observed in CR mice and SIRT1 KO mice (Figure 3) 4). SIRT1 directly regulates the promoter of PrP (Figure 3).
As a protein deacetylase, SIRT1 regulates the activities of diverse transcription factors (Haigis and Guarente, 2006). Thus, modification of SIRT1 activity by CR has profound effects on gene expression and thus on diverse biological processes. Indeed, SIRT1 appears to be a positive regulator of PrP gene expression. Perhaps as a result of this regulation, we observed suppression of prion accumulation and the alleviation of pathology in SIRT1 KO mice that had been injected with RML prions. However, despite the dramatic suppression of prion pathology and aggregation in SIRT1 KO mice at the preclinical stage of the disease (4 MPI), SIRT1 deletion resulted in only a modest extension of lifespan (about 10 days) compared to SIRT1 WT (both on AL diets). This is likely because SIRT1 has a neuroprotective function: once prion aggregation reaches a certain threshold and becomes neurotoxic, mice with loss-of-function of SIRT1 are less tolerant to neurotoxicity and die faster. Thus, SIRT1 deletion may have two opposing effects on prion neurotoxicity— it initially suppresses PrPSc accumulation but later increasing susceptibility to neurotoxicity. Future studies will assess whether CR-induced changes in cholesterol levels, neuronal lipid composition, or insulin signaling are responsible for the decreased prion replication observed at 4 MPI. In addition, it is not clear which other deacetylases besides SIRT1 are altered in the SIRT1 knockout mouse. It is possible that compensatory up- or down-regulations may be responsible for some of the effects of SIRT1 deletion observed in this study.
SIRT1 is a positive lifespan determinant and is upregulated during CR in many tissues. However, we found that CR differentially regulates SIRT1 expression in different brain regions. SIRT1 is down-regulated by CR in the cerebellum and midbrain, and this effect may account for the initial delay in onset of infectious prion disease in CR mice. Neuroprotective effects associated with decreased sirtuin activity are not unprecedented. Recently, inactivation of SIRT2, a cytoplasmic NAD-dependent deacetylase that is prominently expressed in the brain, was reported to protect against disease in models of Parkinson’s disease (Outeiro et al., 2007). Interestingly, in our experiments the cortex and hippocampus both showed an increase in SIRT1 expression during CR. The increase of SIRT1 in the hippocampus is consistent with the proposed protective function of SIRT1 against Alzheimer’s disease (Qin et al., 2006; Kim et al., 2007), in which hippocampal dysfunction is a prominent feature.
The striking shortening of the duration of clinical symptoms that we observed in CR mice is unprecedented in studies of prion diseases. While CR has a beneficial effect on almost all diseases of aging, including delaying the onset of prion disease, it is highly unusual that CR diminished the survival of prion-infected mice. This may suggest that prion-diseased animals are in high demand of energy once clinical deficits appear, and CR puts the animals closer to an energy precipice. However, CR dramatically delays all aspects of Huntington’s disease, in which weight loss and a disruption of energy balance are apparent (Duan et al., 2003). Our experiments suggest that neurodegenerative diseases may proceed through stages where a single therapeutic strategy may protect against certain symptoms of a given disease while simultaneously exacerbating other, highlighting the complexity of treating these devastating diseases.
Methods
Mouse strains, calorie restriction, and prion inoculations
All animal procedures were in accordance with the conditions set forth by the MIT animal care committee. SIRT1 KOs have been described previously (McBurney et al., 2003); these mice are maintained on an outbred genetic background consisting of CD1 and 129/Sv. 3–4 months old animals were either fed AL or subjected to a 30% CR diet, which was provided daily. Only wild type and SIRT1 KO littermates were used. For analysis of SIRT1 expression in the brain, C57Bl/6 mice on a CR diet and AL controls were purchased from the National Institutes of Aging.
For prion injections, mice were injected intracranially with 30 μl of 0.001% brain homogenate containing approximately 3.5 log LD50/30μl infectious units. Mice were assessed daily for standard prion symptoms including ataxia, imbalance, priapism (males), and hunched posture.
Proteinase K treatment and Western blotting
10% homogenates (weight/volume) of whole brain were made in PBS from tissue frozen at 4 MPI. Tissue was homogenized in a glass dounce homogenizer and sonicated, then large debris were pelleted by low speed centrifugation (~500g for 5 minutes). Homogenates were diluted to 1% in lysis buffer consisting of PBS and 1% Triton X-100 and 1% Tween 20 and treated with 50ug/ml proteinase K for 1 hour at 37°C. Antibodies used for western blottings: SAF83 (Chemicon) was used at 1:2500 dilution to detect PrP. Anti-SIRT1 (1:1000, Upstate). Western blottings were quantified with ImageJ.
Neuropathological analysis
Brains were immersion fixed in formalin and treated with 98% formic acid for 1 hour then fixed for >24 hours prior to paraffin embedding. 2 micron thick sections were cut onto positively charged glass slides and stained with hematoxylin and eosin, or immunostained. For prion aggregate staining, sections were deparaffinized and incubated for 6 minutes in 98% formic acid then washed in distilled water for 5 min. Sections were heated to 100°C in a pressure cooker in citrate buffer (pH 6.0), cooled for 3 minutes, then washed in distilled water for 5 minutes. Immunohistochemical stains were performed on an automated Nexus staining apparatus (Ventana Medical Systems) using an IVIEW DAB Detection Kit (Ventana). After incubation with protease 1 (Ventana) for 16 min, sections were incubated with anti-PrP SAF-84 (SPI bio; 1:200) for 32 min. Sections were counterstained with hematoxylin. GFAP (1:1000 for 24 min.; DAKO) immunohistochemistry for astrocytes and IBA1 (1:2500 for 32 min.; Wako Chemicals) for microglia was performed similarly, however with antigen retrieval by heating to 100°C in EDTA buffer (pH8.0) within a steamer. A blinded analysis of vacuolation and gliosis in the hippocampus, thalamus, striatum, cortex, and cerebellum was conducted.
RNA preparation and analysis
RNA was isolated from mouse tissues using trizole (Invitrogen) and was further purified with RNeasy mini kit (Qiagen). For real-time PCR analysis, cDNA were synthesized from total RNA by SuperScript III reverse transcriptase (Invitrogen) with random primers. The resulting cDNA was subjected to PCR analysis with gene-specific primers (Forward primer: aggaccgctactaccgtgaaa. Reverse primer: ctcggtgaagttctccccct) in the presence of SYBR green (Qiagen). Relative abundance of mRNA was obtained by normalization to cyclophilin levels (Forward primer: tccaaagacagcagaaaactt. Reverse primer: tcttcttgctggtcttgccatt).
Luciferase assay
293T Cells were transfected with 10 ng of fire-fly luciferase reporter driven by the PrP promoter, 2 ng of pRL-TK (Renilla luciferase; Promega), together with 250 ng construct expressing SIRT1 (pBabe vector) or 5uM resveratrol. Luciferase activity was then measured using the Dual-Luciferase Reporter Assay System (Promega) 24 h posttransfection. The firefly luciferase activity was normalized to co-expressed Renilla luciferase activity. The experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed using GraphPad Prism. Student’s T test was used for analysis of RT-PCR, Western blotting, and behavioral data while the log rank test was used for survival data. In the Figures, statistical significance is indicated as follows: * denotes P <0.05; ** denotes P<0.01; and denotes *** P<0.001.
Supplementary Material
Pathological characterization of brains taken from terminally ill prion-inoculated mice. WT mice fed AL or on CR and SIRT1 KO mice on CR were injected with RML prions and brain tissues were collected from terminally ill mice. (A) Neuropathological analysis revealed extensive vacuolation by hematoxlyin and eosin staining, and gliosis by anti-GFAP staining in all samples in WT/AL, WT/CR, and SIRT1 KO/CR. Scale bar corresponds to 200um. (B) Brain homogenates from terminal samples were subjected to proteinase K digestion. Proteinase resistant PrP was detected by western blotting with an anti-PrP antibody, which showed strong accumulation in all samples. β-tubulin (from undigested homogenates) was used as a loading control. There were no significant differences between WT/AL, WT/CR, or SIRT1 KO/CR samples (KO/AL was not analyzed).
Acknowledgments
We are grateful to Artur Topolszki and Walker Jackson (WIBR) for assistance with prion inoculations and to Vilma Martins (Ludwig Institute for Cancer Research) for providing the luciferase reporter construct. SL is an investigator in the Howard Hughes Medical Institute, DC was supported by a Leukemia and Lymphoma Society postdoctoral fellowship (5168-06) and LG is funded by the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguzzi A, Heikenwalder M. Pathogenesis of prion diseases: current status and future outlook. Nat Rev Microbiol. 2006;4:765–775. doi: 10.1038/nrmicro1492. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol. 2007;8:552–561. doi: 10.1038/nrm2204. [DOI] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007 doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Cabral AL, Lee KS, Martins VR. Regulation of the cellular prion protein gene expression depends on chromatin conformation. J Biol Chem. 2002;277:5675–5682. doi: 10.1074/jbc.M104815200. [DOI] [PubMed] [Google Scholar]
- Chen D, Guarente L. SIR2: a potential target for calorie restriction mimetics. Trends Mol Med. 2007;13:64–71. doi: 10.1016/j.molmed.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005b;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. Embo J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury DT, Kasper KC, Stites DP, Watson JD, Hogan RN, Prusiner SB. Genetic control of scrapie and Creutzfeldt-Jakob disease in mice. J Immunol. 1983;131:491–496. [PubMed] [Google Scholar]
- Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–321. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. doi: 10.1126/science.1090187. [DOI] [PubMed] [Google Scholar]
- McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Neri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L. Unlocking the secrets of longevity genes. Sci Am. 2006;294:48–51. doi: 10.1038/scientificamerican0306-48. [DOI] [PubMed] [Google Scholar]
- Steele AD, King OD, Jackson WS, Hetz CA, Borkowski AW, Thielen P, Wollmann R, Lindquist S. Diminishing apoptosis by deletion of Bax or overexpression of Bcl-2 does not protect against infectious prion toxicity in vivo. J Neurosci. 2007b;27:13022–13027. doi: 10.1523/JNEUROSCI.3290-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AD, Lindquist S, Aguzzi A. The prion protein knockout mouse: a phenotype under challenge. Prion. 2007c;1:83–93. doi: 10.4161/pri.1.2.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C, Fischer M, Raeber A, Bueler H, Sailer A, Shmerling D, Rulicke T, Brandner S, Aguzzi A. The use of transgenic mice in the investigation of transmissible spongiform encephalopathies. Rev Sci Tech. 1998;17:278–290. doi: 10.20506/rst.17.1.1079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pathological characterization of brains taken from terminally ill prion-inoculated mice. WT mice fed AL or on CR and SIRT1 KO mice on CR were injected with RML prions and brain tissues were collected from terminally ill mice. (A) Neuropathological analysis revealed extensive vacuolation by hematoxlyin and eosin staining, and gliosis by anti-GFAP staining in all samples in WT/AL, WT/CR, and SIRT1 KO/CR. Scale bar corresponds to 200um. (B) Brain homogenates from terminal samples were subjected to proteinase K digestion. Proteinase resistant PrP was detected by western blotting with an anti-PrP antibody, which showed strong accumulation in all samples. β-tubulin (from undigested homogenates) was used as a loading control. There were no significant differences between WT/AL, WT/CR, or SIRT1 KO/CR samples (KO/AL was not analyzed).