Abstract
Ionizing radiation (IR) exposure is inevitable in our modern society and can lead to a variety of deleterious effects including cancer and birth defects. A reliable, reproducible and sensitive assessment of exposure to IR and the individual response to that exposure would provide much needed information for the optimal treatment of each donor examined. We have developed a diagnostic test for IR exposure based on detection of the phosphorylated form of variant histone H2AX (γ-H2AX), which occurs specifically at sites of DNA double-strand breaks (DSBs). The cell responds to a nascent DSB through the phosphorylation of thousands of H2AX molecules flanking the damaged site. This highly amplified response can be visualized as a γ-H2AX focus in the chromatin that can be detected in situ with the appropriate antibody. Here we assess the usability of γ-H2AX focus formation as a possible biodosimeter for human exposure to IR using peripheral blood lymphocytes irradiated ex vivo and three-dimensional artificial models of human skin biopsies. In both systems, the tissues were exposed to 0.2–5 Gy, doses of IR that might be realistically encountered in various scenarios such as cancer radiotherapies or accidental exposure to radiation. Since the γ-H2AX response is maximal 30 minutes after exposure and declines over a period of hours as the cells repair the damage, we examined the time limitations of the useful detectibility of γ-H2AX foci. We report that a linear response proportional to the initial radiation dose was obtained 48 hours and 24 hours after exposure in blood samples and skin cells respectively. Thus, detection of γ-H2AX formation to monitor DNA damage in minimally invasive blood and skin tests could be useful tools to determine radiation dose exposure and analyze its effects on humans.
1. Introduction
Exposure to ionizing radiation (IR) can have deleterious or therapeutic effects depending on the situation. Potentially damaging exposure may come from cosmic rays, the sun and radioactive substances on the ground, during high altitude journeys, or in space. In addition, there is the threat of radiobiological terrorism. Therapeutic exposure may come from treatments using IR for diagnostic purposes and for treatment of various diseases. For these reasons, there is an increasing need for quick and reliable bioassays to evaluate risk for individuals exposed to IR (Cucinotta and Durante, 2006; Marchetti et al., 2006). In addition to optimizing IR treatments for disease, a safe and effective bioassay that can rapidly and accurately distinguish individuals who need therapy from those who do not is essential to allow health professionals to prevent, mitigate, and treat the immediate and long-term medical effects of IR exposure from occupational, accidental and premeditated occurances. The most damaging lesion introduced by IR into cells is the DNA double strand break (DSB). Cells respond rapidly and massively to nascent breaks in order to locate them in the chromatin and repair the damage as quickly and accurately as possible, since erroneously repaired breaks can lead to cancer and cell death. In proliferating cells, the cell cycle is arrested during repair. Both these processes, repair and cell cycle arrest, involve the activation of large numbers of proteins and induction of genes associated with cell cycle and growth control (Schmidt-Ullrich et al., 2000). Among the proteins involved in the early steps of the cellular response in sensing DNA damage and controlling progression through the cell cycle are H2AX, ATM, CDKN1A, and TP53, which have been proposed as the top group of candidate biodosimeters (Marchetti et al., 2006). An immediate result of IR exposure is the DNA double-strand break (DSB), that can be detected with an antibody to γ-H2AX, a phosphorylated form of the histone H2A variant H2AX which forms rapidly at the sites of DNA DSBs (Pilch et al, 2003; Redon et al, 2002; Sedelnikova et al., 2003). In vitro studies show that γ-H2AX formation peaks at 15–30 min after IR proportionally to the dose, and then declines over several hours (Cucinotta et al, 2008; Rogakou et al, 1999; Sedelnikova et al., 2004). γ-H2AX is required for the concentration and stabilization of DNA repair proteins and plays a role in both non-homologous end-joining (NHEJ) and homologous recombination (HR) repair pathways (Celeste et al, 2003; Shrivastav et al., 2008). The ratio of DNA DSBs to visible γ-H2AX foci is close to 1:1, which forms the basis of a sensitive quantitative method for detection of DNA DSBs in mammalian cells (Rothkamm and Lobrich, 2003; Sedelnikova et al., 2002).
Diagnostic information concerning exposure to IR can be obtained from changes in the γ-H2AX level in peripheral lymphocytes (Lobrich et al, 2005; Rothkamm et al., 2007) and epidermal skin cells obtained by biopsy (Qvarnstrom et al., 2004). In this study, we examined peripheral lymphocytes and the EpiDermFT full thickness skin model (EFT-300), which consists of organized layers analogous to those found in skin in vivo. In particular, we assessed the diagnostic window when γ-H2AX foci can yield useful information on IR exposure since γ-H2AX focus formation is maximal at 30 minutes after IR and decreases afterward. This study shows that γ-H2AX formation in skin or blood could be a robust biodosimetric measure of IR in humans during the first 48 hours after exposure.
2. Materials and Methods
2.1 Cell systems
Blood samples were collected in heparin-coated tubes and obtained from healthy donors, men and women 29–72 years old, at the NIH blood bank in accordance with NIH regulations. Lymphocytes were isolated from whole blood samples as previously described (Sedelnikova et al., 2008). To avoid stress-induced DNA damage post-isolation, the whole blood samples were processed for IR within 30 min post-blood draw. The EpiDermFT full thickness skin systems (EFT-300) were obtained from MatTek (Ashland, MA). The system consists of normal, human-derived epidermal keratinocytes and normal, human-derived dermal fibroblasts which have been grown on a semipermeable membrane to form a multilayered, highly differentiated model of the human dermis and epidermis (Fig. 1A). The tissues were cultured at 37 °C, 5% CO2, 20% O2, on specially prepared cell culture inserts using serum free medium, according to the manufacturer’s instructions. The surfaces of the tissues were exposed to air to stimulate differentiation.
Figure 1.
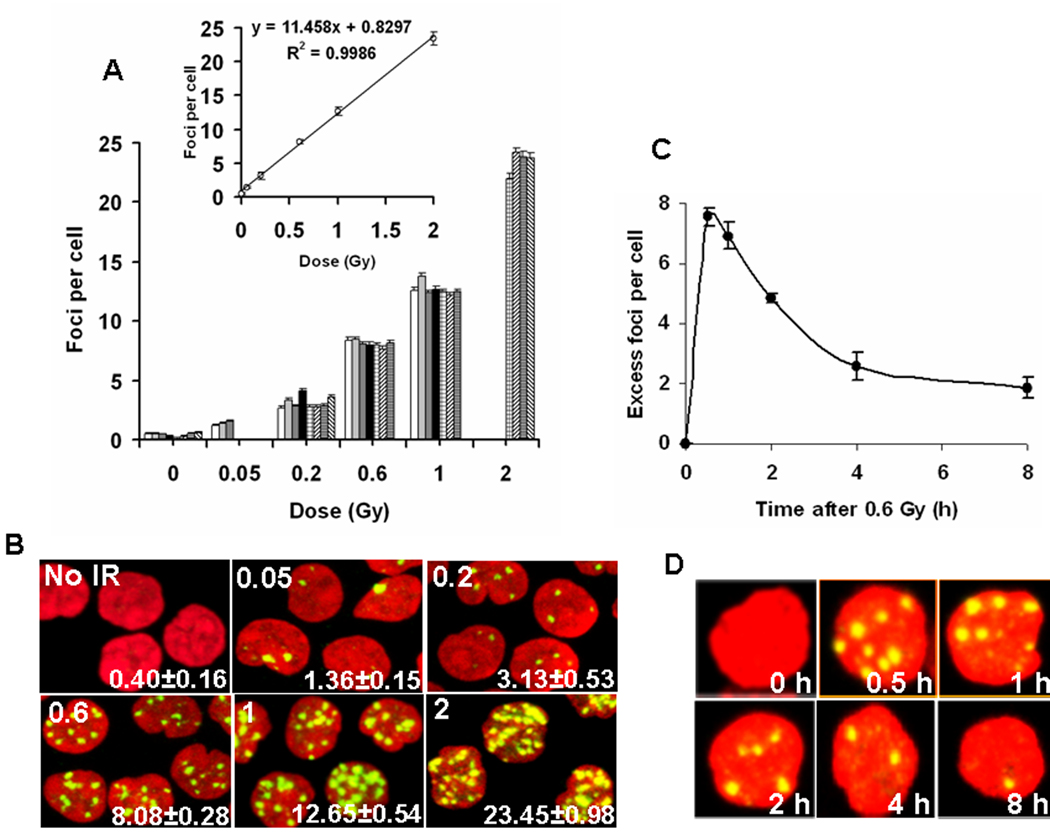
DNA damage in peripheral blood lymphocytes exposed to IR ex vivo. (A) (Main graph) Incidence of γ-H2AX foci in blood taken from eight individual donors and irradiated ex vivo. Lymphocytes were purified 30 minutes post-IR, stained for γ-H2AX detection and then γ-H2AX foci were counted. Each donor is noted with a differently patterned bar. Error bars indicate standard errors. (Inset) Average numbers of γ-H2AX foci per cell were determined. Error bars indicate standard deviations (n=8). (B) Representative images of irradiated lymphocytes used for panel A. The irradiation dose (Gy) is shown in the top left corner and the number of γ-H2AX foci per cell is shown in the lower right corner (average ±standard error). Green, γ-H2AX; red, DNA stained with PI. (C) Kinetics of γ-H2AX focal loss in lymphocytes after 0.6 Gy IR. Blood samples were irradiated with 0.6 Gy and incubated at 37°C. At indicated times lymphocytes were isolated and stained for γ-H2AX detection. Error bars indicate standard deviations (n=3). (D) Representative images of irradiated lymphocytes used for panel C. Green, γ-H2AX; red, DNA stained with propidium iodide (PI).
2.2 Irradiation and processing
The anticoagulated whole blood samples and the skin tissue models were exposed to 0.2–5 Gy γ-IR in a Mark-1 γ-irradiator (JL Shepherd & Associates, San Fernando, CA); control samples were sham-irradiated. The tissues were frozen or fixed by formaldehyde exposure and paraffin-embedded after incubation at 37 °C for various times post-exposure, sectioned perpendicularly to the tissue surface, so the sections contain both fibroblast and keratinocyte layers.
The irradiated or sham-irradiated blood samples were treated the same way. After irradiation and different periods of incubation at 37°C, the lymphocytes were separated and prepared for immunocytochemistry as previously described (Sedelnikova et al, 2008). For immunocytochemistry, PBS was replaced with PBS containing 0.5% Tween-20 and 0.1% Triton X-100 (Bio-Rad, Hercules, CA) for blocking and antibody incubation.
Formalin-fixed paraffin-embedded tissue sections were deparaffinized through xylene and re-hydrated through graded alcohols. Endogenous peroxide was blocked with 0.6% H2O2 in methanol, and antigen was retrieved using a microwave processor for 10 min at 100°C (retrieval solution was obtained from Biogenex, San Ramon, CA). After cooling and washing, biotin-conjugated mouse monoclonal anti-γ-H2AX antibody (Millipore, Billerica, MA) and avidin-biotin enzyme complex (Vectashield Elite ABC Kit, Vector Laboratories, Burlingame, CA) were applied, and the peroxidase reaction was retrieved by 3,3’ diamino-benzidine-tetrahydrochloride (DAB) (Sigma-Aldrich, St. Louis, MO). The sections were counterstained with hematoxilin, viewed and photographed with Olympus microscope and an Olympus DP70 camera (Olympus America Inc., Center Valley, PA).
Frozen sections were dried, fixed in 2% paraformaldehyde for 20 minutes at room temperature, permeabilized with 1% Triton X-100 and processed for immunocytochemistry as previously described (Rogakou, et al, 1999).
Immunostaining for frozen sections, and lymphocyte preparations were performed using the primary mouse monoclonal anti-γ-H2AX antibody (Abcam Inc., Cambridge, MA) and the secondary goat anti-mouse Alexa-488-conjugated IgG (Invitrogen, Eugene, OR). Nuclei were stained with propidium iodide (PI). Laser scanning confocal microscopy was performed with a Nikon PCM 2000 (Nikon, Inc., Augusta, GA). The foci were visually counted in 50–100 cells.
3. Results
3.1 Lymphocytes
For a biodosimeter to be useful, it should yield similar values for different individuals. Lymphocytes are a useful model to evaluate the effects of IR exposure on an organism, as they can be obtained minimally invasively and under standard conditions from human blood donors. We collected blood samples from individuals and exposed them to increasing doses of radiation (0.02–5 Gy) ex vivo. After different recovery times, lymphocytes were isolated and stained for γ-H2AX. Thirty minutes after exposure, the lymphocytes from eight individuals exhibited a similar response to IR over the range of 0.05 to 2.0 Gy (Fig.1A, B) as well as a linear relationship between the number of γ-H2AX foci and the IR dose. These results show that different donors, independent of age or gender, respond in a very similar manner to IR exposure. The numbers of γ-H2AX foci are known to decrease with increasing time 30 minutes post-exposure. The extent of this decrease was quantified for lymphocytes taken from three individuals and irradiated with 0.6 Gy ex vivo (Fig. 1C, D). The number of foci decreased to one-half their maximum about 2.5 hours post IR or about 2 hours after the maximum. However, the rate of decrease slowed so that 8 hours post IR, over 20% of the γ-H2AX foci remain. Since DNA DSBs induced by IR are heterogeneous and usually accompanied by other DNA lesions, some breaks may be more difficult to repair than others. It may be that those remaining 8 hours post-IR represent breaks that are difficult to rejoin (Goodarzi et al., 2008).
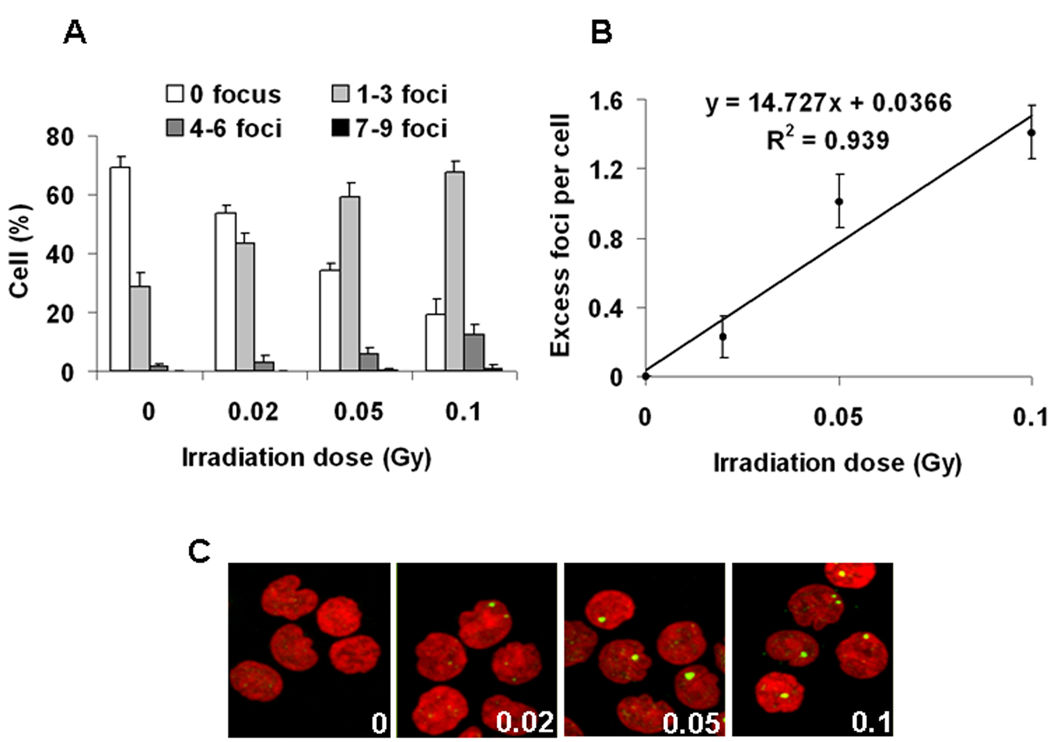
The response of lymphocytes to low doses of IR was further examined near the lower limit of detection (Fig. 2A–C). Doses as low as 0.02 Gy are detectable, and, as shown for radiation doses up to 2 Gy (Fig 1A), the data followed a linear relationship between dose and the γ-H2AX signal (Fig 2B). The average number of foci per cell per Gy was about 14.7 foci per cell (Fig 2B) compared to 11.5 foci per cell per Gy when measured over a greater dose ranges (Fig. 1A). This is presumably due to increasing numbers of overlapping foci at the higher doses of radiation.
Figure 2.
DNA damage in human peripheral blood lymphocytes exposed ex vivo to low IR doses. (A) Focal distribution in lymphocytes 30 minutes after exposure to 0, 0.02, 0.05 and 0.1 Gy. (B) Average numbers of excess (background subtraction) γ-H2AX foci per cell as determined 30 minutes after 0.02, 0.05 and 0.1 Gy. Error bars indicate standard deviations (n=3). (C) Representative images of irradiated lymphocytes used for panel A. The irradiation dose (Gy) is shown in the lower right corner. Green, γ-H2AX; red, DNA stained with PI.
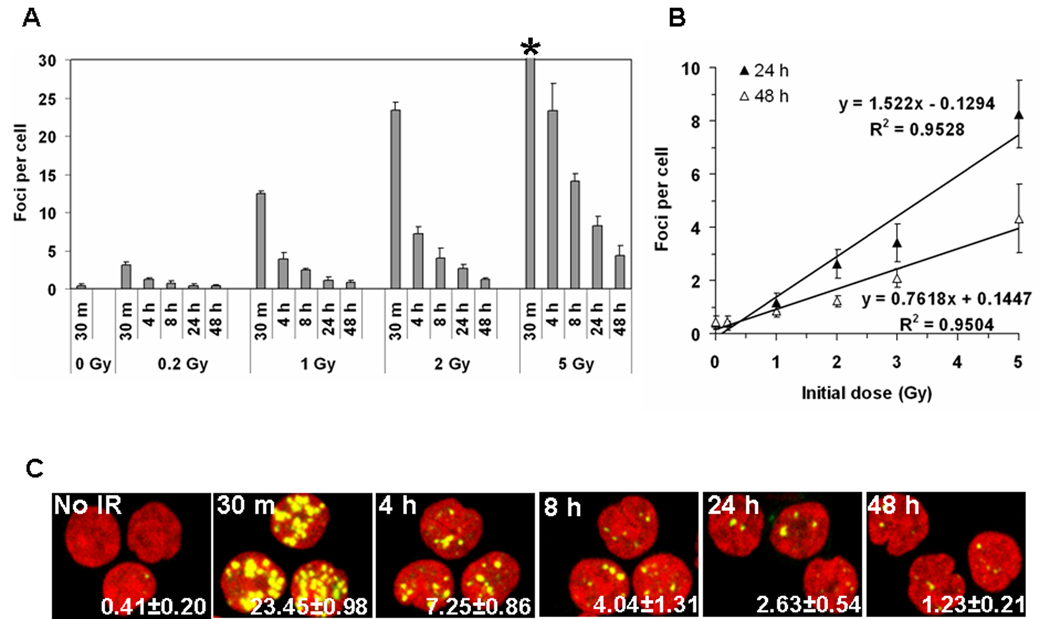
Clearly γ-H2AX focal numbers in lymphocytes provide a good measure of radiation exposure with clear reproducibility and a high sensitivity at times soon after exposure with over 20% of the maximum number of γ-H2AX foci still present at 8 hours post exposure (Fig 1C). The persistence of γ-H2AX foci in irradiated lymphocytes was further investigated for times up to 48 hours after IR exposure (Fig 3A–C). At 0.2 Gy exposure, the numbers of γ-H2AX foci returned to values indistinguishable from the control lymphocytes (Fig. 3A). However, for doses greater than 1 Gy, a substantial response was detected in the lymphocytes for up to 2 days after exposure (Fig. 3B). The foci numbers were 13% at 1 day and 6.6% at 2 days after exposure compared to that at 30 minutes after exposure (Fig. 1A) and the responses were linearly dependent on the initial exposure (Fig. 4B). Measurements such as these could be useful in this time and dose range when measures of lymphocyte depletion may be ambiguous.
Figure 3.
Kinetics of long-term γ-H2AX focal loss in blood from five individuals after IR (A) The incidence of γ-H2AX foci in lymphocytes irradiated with 0, 0.2, 1.0, 2.0, and 5.0 Gy taken at the noted times post-exposure. (*) The foci 30 minutes after 5 Gy were too numerous to count. (B) The incidence of γ-H2AX foci at 24 hours and 48 hours post-IR exposure is shown. Error bars signify standard deviations (n=5). (C) Representative images of irradiated lymphocytes used for panel A. Time post-IR is shown in the top left corner and the number of γ-H2AX foci per cell is shown in the lower right corner (average±standard error). Green, γ-H2AX; red, DNA stained with PI.
Figure 4.
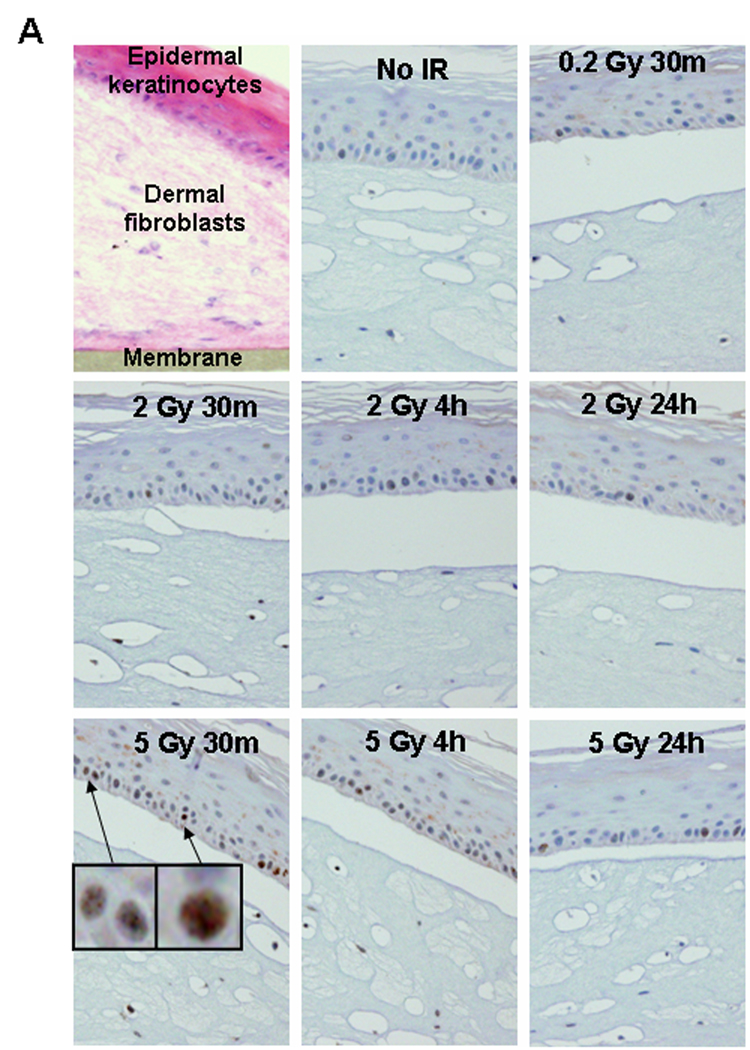
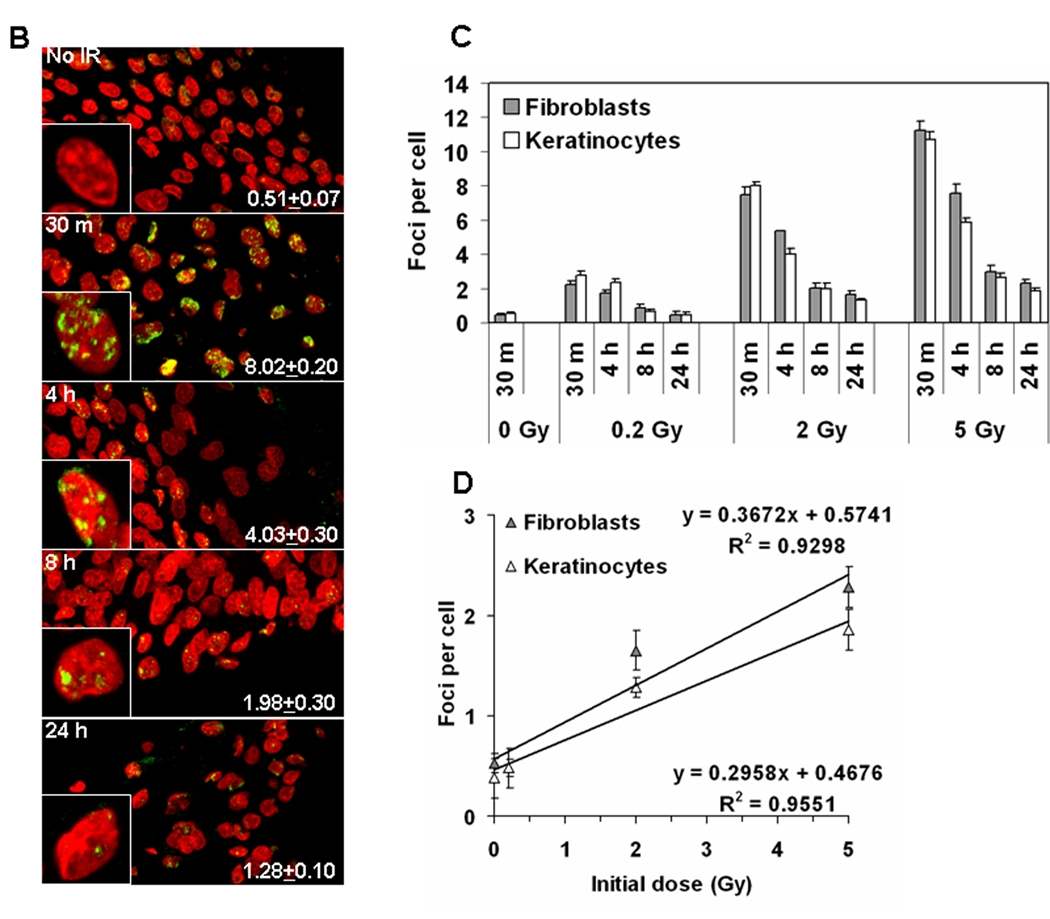
IR-induced DNA damage in artificial human skin. (A) Peroxidase staining in paraffin-embedded tissues. The EpiDermFT is a “full thickness” highly differentiated artificial skin consisting of epidermal keratinocyte and dermal fibroblast layers, corresponding to dermis and epidermis of normal human skin. The tissues were formalin-fixed and paraffin-embedded at various times after radiation exposure, and sectioned perpendicularly to their surface. (Upper left panel) Sections stained with hematoxilin and eosine. The tissue is 300 µm high grown on a 6 mm diameter cellulose membrane. Blue staining indicates cell nuclei while pink staining indicates cytoplasm. (Other panels) γ-H2AX staining in tissues irradiated with 0, 0.2, 2.0, and 5.0 Gy and fixed at various times post-IR. Blue staining is cell nuclei while brown (peroxidase) staining indicates γ-H2AX formation. Two patterns of positive reaction, punctate staining and a whole-nucleus staining (insets), were observed in 2 Gy- and 5 Gy-irradiated samples. At 24 hours post-IR some residual staining was observed. 0.2 Gy-irradiated samples exhibited no or negligible staining. All images are shown at 20 × magnification. (B) Immunostaining of γ-H2AX in frozen tissue sections. Representative images showing the presence of γ-H2AX foci in the keratinocyte EpiDermFT layer irradiated with 0 or 2 Gy. Green, γ-H2AX; red, DNA stained with propidium iodide (PI). Insets show representative single cell images. Average numbers of foci per cell ± standard error are shown in the right bottom corner of the images. (C) Quantitative measure of the incidence of γ-H2AX foci in fibroblasts (grey bars) and keratinocytes (white bars) irradiated with 0, 0.2, 2.0, and 5.0 Gy at various times post-exposure. (D) γ-H2AX focal numbers at 24 hours post-exposure are proportional to the initial dose of IR received in both fibroblasts and keratinocytes. Error bars signify standard errors.
3.2 Skin
Skin is another tissue which can be obtained by minimally invasive procedures in order to estimate DNA damage in humans following radiation exposure. We used the artificial skin model, EpiDermFT, which closely parallels human skin in morphology, growth and metabolic profile, thus providing a useful in vitro means to assess dermal sensitivity to IR (Belyakov et al, 2005; Boelsma et al., 2000; Sedelnikova et al, 2007; Zhao et al, 1999).
Morphologically, EpiDermFT consists of organized dermal and epidermal layers (Fig. 4A, upper left panel). Two protocols, immunoperoxidase staining of paraffin sections and immunofluorescence in frozen sections, were compared to examine H2AX phosphorylation in EpiDermFT pieces fixed at several times after exposure to various doses of IR. Embedding in paraffin protects the morphology of the tissues, which can be stored indefinitely without changes in signal intensity. Results, obtained with sections of paraffin-embedded tissue stained for γ-H2AX using horseradish peroxidase, are shown in Fig. 4A. Clearly, the sensitivity of this method is poor compared to the results obtained with lymphocytes (compare 2 Gy at 30 minutes for peroxidase (Fig 4A) with that for lymphocytes (Fig. 1B)). Also little if any increase in γ-H2AX staining was observed compared to no IR control at 30 minutes post-0.2 Gy IR, while this dose was easily measured in lymphocytes. Two patterns of staining were observed, a punctated staining and a pan-nuclear staining (inserts in the 5 Gy 30 minutes image). This type of staining is difficult to quantify, but it may give useful information about overall skin response to IR within a detectable range. The residual γ-H2AX signal is still detectable as brown peroxidase staining in cell nuclei 24 hours post-IR with 2 and 5 Gy, which is lacking in the no IR control (Fig 4A). The presence of this residual staining is a valuable marker of past IR exposure.
Paraffin skin sections are also poor subjects for immunofluorescence due to poor epitope exposure and autofluorescence. On the other hand frozen tissue sections are amenable to immunofluorescent staining, but the tissue morphology is only poorly retained (Fig. 4B). Frozen sections yield discrete γ-H2AX foci which are countable and which yield better sensitivity compared to peroxidase staining in the paraffin sections (Fig. 4C). A response is apparent in the frozen sections of both keratinocyte and fibroblast layers with only 0.2 Gy at 30 minutes after exposure (compare Fig. 4C with Fig. 4A, 0.2 Gy 30 minutes). γ-H2AX foci were still detected 24 hours after 2 –5 Gy and with a linear relationship between the remaining γ-H2AX foci and the initial dose applied to the skin model, independent of cell type (Fig. 4D). The presence of γ-H2AX foci at this time indicates that such an assay may still be relevant to triage procedures after external IR exposure. The rate of focal loss among the two different cell types in the skin model was similar at any given dose they received, implying a similar rate of DNA repair.
While EpiDermFT tissues yield increasing numbers of γ-H2AX foci with increasing irradiation, the sensitivity is substantially lower than that observed with lymphocytes (compare Fig. 3A and B with Fig. 4C and D). For example, at 24 hours after 5 Gy exposure, approximately 2 γ-H2AX foci per cell were found in the skin sections compared to about 8 in lymphocytes, a 4-fold difference. Several factors may account for the observed differences in sensitivity. First, lymphocytes are a much easier tissue to analyze and yield cleaner images with less background noise and with fewer variations in focal intensities. In addition, the tissue sections, whether paraffin embedded or frozen, often contain only fractions of nuclei. These issues increase the difficulty in distinguishing focal overlaps, especially with increasing doses of radiation, similar to that observed by Qvarnstrom et al (2004) in skin biopsies.
4. Discussion
Measurement of γ-H2AX focus numbers or of overall γ-H2AX levels has many of the characteristics of a useful biomarker for exposure of individuals to IR Uses may include the assessment of clinical treatments involving chemo- and radiotherapeutic protocols (Sedelnikova and Bonner, 2006). Several reports utilizing focus formation as a sensitive quantitative method for in vivo detection of DSBs in clinical samples have been described including the use of skin biopsies from prostate cancer patients receiving radiotherapy (Qvarnstrom et al., 2004), and of whole blood lymphocytes from patients with benign or malignant tumors of various organs undergoing computer tomography examination of the chest and/or abdomen (Lobrich, et al., 2005; Rothkamm, et al, 2007).
Since γ-H2AX focal formation is a cellular response to DSB formation, numbers of foci decrease as DSBs are rejoined. DNA DSBs are repaired with biphasic kinetics, a rapid phase lasting several hours followed by a slower phase (Sedelnikova, et al., 2008). These kinetics may be due to the fact that IR produces DSBs that vary from simple to more complex structures (Goodhead, 1994; Nikjoo et al, 2001). We can hypothesize that this non-uniformity in DNA damage would results in a delay in the repair of the more complex DSBs. While γ-H2AX measurements can be performed soon after radiation, there are scenarios when samples for analysis may not be obtained for many hours after IR exposure. Thus our demonstration that γ-H2AX focal measurements can yield linear and reproducible results in samples taken up to 48 hours after exposure broadens the potential usefulness of such an assay.
In lymphocytes fixed 30 minutes after exposure to IR, we measured 12.6 foci per cell per Gy using Cs137 γ-radiation compared to ∼9 foci per cell per Gy reported using 220 kVp X-rays (Scherthan et al., 2008) and 14.7 foci per cell per Gy reported using 150 kV X-rays (Rothkamm, et al., 2007). Similarly, we measured ∼0.2 and 1.4 foci per cell for 0.02 and 0.1 Gy respectively using Cs137 γ-radiation while ∼0.2 and ∼1 foci per cell were observed using 90kV X-rays (Lobrich, et al, 2005). These results show a general agreement among investigators. The differences observed could be due to various technical issues and/or variations in focus counting by different investigators. The estimate of 15 foci per lymphocyte after 1 Gy irradiation is a low number compared to estimates usually seen with cells in culture (Kato et al., 2006; Leatherbarrow et al, 2006; Yasui, 2004). Fewer γ-H2AX foci forming in lymphocytes could have several explanations. First, G0 lymphocytes possess less genetic material than dividing cells in culture (i.e. we expect 1.5 times more DNA on average in a mix of G1, S and G2/M cells, and even higher in cancer cell lines which frequently exhibit genome amplification). As post-mitotic lymphocytes contain less DNA than most cell lines used in laboratories, we can expect less DSB formation than in cells with higher DNA content for the same radiation dose. In fact, a previous study showed that twice as many γ-H2AX foci are observed in metaphase cells relative to G1 cells at any given dose (Kato et al, 2008). Second, it is known that higher order chromatin structure influences the distribution of DNA DSBs. For example, heterochromatin is refractory to γ-H2AX foci formation (Goodarzi, et al, 2008). Post-mitotic cells have fewer actively transcribed genes and much of the rest of their genome forms compact blocks of heterochromatin (Francastel et al., 2000). High levels of heterochromatin in post-mitotic lymphocytes could account for the lower number of γ-H2AX foci observed.
However, frozen sections of artificial skin yield substantially lower numbers of foci per cell than lymphocytes. At 30 minutes after 2 Gy exposure, we counted ∼8 foci per cell in fibroblasts and keratinocytes in the artificial skin compared to ∼25 in lymphocytes. The difference was also similar 24 hours after 5 Gy exposure when we counted ∼2 foci per cell in the artificial skin sections compared to ∼8 foci per cell in lymphocytes. Several factors may help explain these differences. In lymphocytes, the whole nuclear volume was analyzed for γ-H2AX. In the skin sections, however, it was not possible to analyze whole nuclear volumes as sections may include only part of a nucleus. In contrast to the lymphocytes where foci intensities were bright and foci easy to discriminate, the skin cells contained smaller foci structures making focal discrimination more difficult. Finally, DSB repair kinetics could differ between cell types (Leatherbarrow, et al., 2006; Sedelnikova, et al, 2008). In addition, frozen sections show higher background and greater variations in foci intensities. Similar observations were made by Qvarnstrom et al (2004) when analyzing skin biopsies from patients undergoing radiation treatments. These authors used digital image analysis performing various mathematical transformations and quantified γ-H2AX foci as a function of DNA content instead of per cell.
Our results indicate a high degree of reproducibility between the individuals we examined with respect to γ-H2AX focus numbers per cell per Gy. However, there may be individual differences in radiosensitivity due to deficiencies in DSB repair for example in patients with genomic instability syndromes such as ataxia telangiectasia, Nijmegen breakage syndrome, Werner syndrome and others (Lobrich, et al, 2005; Porcedda et al, 2006; Sedelnikova, et al., 2008). While these individual variations have to be taken into account when assessing DNA damage measured by γ-H2AX, focus formation may also be useful in determining which individuals have deficiencies. For example Porcedda (2008) utilized the ex vivo γ-H2AX response of lymphocytes to correctly identify all 4 ataxia telangiectasia individuals in a pool of 19. Therefore, γ-H2AX may help determine the DSB repair capacity of patients in order to "individualize" treatment planning.
Since numbers of γ-H2AX foci per cell depend on both the dose received and the time elapsed as well as cell type, ideally a clinician would only have to know the approximate time since the exposure to be able to accurately assess, using blood or skin samples, the IR-induced damage as well as the appropriate treatment course. Skin cells as well as lymphocytes displayed a linear dose response that easily allows the calculation of standards for IR exposure estimates given the time since exposure. Peripheral lymphocytes also undergo decreases in number after individual exposure to IR which can also yield diagnostic information on the extent of exposure, primarily at doses above 3 Gy where lymphocyte numbers decrease below the normal range after two days. Thus, the persistence of substantial numbers of γ-H2AX foci for 48 hours after IR exposures greater than 1 Gy means that this technique may also be used even when there is a subsequent delay in sample collection from the time of exposure. The persistence of γ-H2AX foci for up to 48 hours could be linked to the formation of complex DSB structures. Such DSBs would necessitate more time for repair than simple DSB structures and would accumulate with increasing radiation doses, explaining the linear relationship between residual foci and the irradiation dose received by the biological samples.
In conclusion, radiation exposure risk to human health can be more effectively assessed with a rapid assay of individual exposure. The ability to measure γ-H2AX, along with other appropriate markers, will enable clinicians to more quickly provide effective treatments for persons exposed to IR. Developing high throughput assays for γ-H2AX detection will make this method even more useful.
Acknowledgements
We thank Dr. Asako Nakamura, NCI, for critical reading of the manuscript, Irina Kareva and Ksenia Gouliaeva, NCI, for data entry and statistical analysis, and Brian Tabb, SAIC, NCI-Frederick, for help with sectioning and staining the EpiDermFT tissues. This study was funded by the NIAID Radiation/Nuclear Countermeasures Program and the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belyakov OV, Mitchell SA, Parikh D, Randers-Pehrson G, Marino SA, Amundson SA, Geard CR, Brenner DJ. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc Natl Acad Sci U S A. 2005;102:14203–14208. doi: 10.1073/pnas.0505020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelsma E, Gibbs S, Faller C, Ponec M. Characterization and comparison of reconstructed skin models: morphological and immunohistochemical evaluation. Acta Derm Venereol. 2000;80:82–88. [PubMed] [Google Scholar]
- Cucinotta FA, Pluth JM, Anderson JA, Harper JV, O'Neill P. Biochemical kinetics model of DSB repair and induction of gamma-H2AX foci by non-homologous end joining. Radiat Res. 2008;169:214–222. doi: 10.1667/RR1035.1. [DOI] [PubMed] [Google Scholar]
- Francastel C, Schubeler D, Martin DI, Groudine M. Nuclear compartmentalization and gene activity. Nat Rev Mol Cell Biol. 2000;1:137–143. doi: 10.1038/35040083. [DOI] [PubMed] [Google Scholar]
- Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M, Jeggo PA. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31:167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Goodhead DT. Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int J Radiat Biol. 1994;65:7–17. doi: 10.1080/09553009414550021. [DOI] [PubMed] [Google Scholar]
- Kato TA, Nagasawa H, Weil MM, Little JB, Bedford JS. Levels of gamma-H2AX Foci after low-dose-rate irradiation reveal a DNA DSB rejoining defect in cells from human ATM heterozygotes in two at families and in another apparently normal individual. Radiat Res. 2006;166:443–453. doi: 10.1667/RR3604.1. [DOI] [PubMed] [Google Scholar]
- Kato TA, Okayasu R, Bedford JS. Comparison of the induction and disappearance of DNA double strand breaks and gamma-H2AX foci after irradiation of chromosomes in G1-phase or in condensed metaphase cells. Mutat Res. 2008;639:108–112. doi: 10.1016/j.mrfmmm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Leatherbarrow EL, Harper JV, Cucinotta FA, O'Neill P. Induction and quantification of gamma-H2AX foci following low and high LET-irradiation. Int J Radiat Biol. 2006;82:111–118. doi: 10.1080/09553000600599783. [DOI] [PubMed] [Google Scholar]
- Lobrich M, Rief N, Kuhne M, Heckmann M, Fleckenstein J, Rube C, Uder M. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci U S A. 2005;102:8984–8989. doi: 10.1073/pnas.0501895102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti F, Coleman MA, Jones IM, Wyrobek AJ. Candidate protein biodosimeters of human exposure to ionizing radiation. Int J Radiat Biol. 2006;82:605–639. doi: 10.1080/09553000600930103. [DOI] [PubMed] [Google Scholar]
- Nikjoo H, O'Neill P, Wilson WE, Goodhead DT. Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiat Res. 2001;156:577–583. doi: 10.1667/0033-7587(2001)156[0577:cafdts]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Pilch DR, Sedelnikova OA, Redon C, Celeste A, Nussenzweig A, Bonner WM. Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem Cell Biol. 2003;81:123–129. doi: 10.1139/o03-042. [DOI] [PubMed] [Google Scholar]
- Porcedda P, Turinetto V, Lantelme E, Fontanella E, Chrzanowska K, Ragona R, De Marchi M, Delia D, Giachino C. Impaired elimination of DNA double-strand break-containing lymphocytes in ataxia telangiectasia and Nijmegen breakage syndrome. DNA Repair (Amst) 2006;5:904–913. doi: 10.1016/j.dnarep.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Qvarnstrom OF, Simonsson M, Johansson KA, Nyman J, Turesson I. DNA double strand break quantification in skin biopsies. Radiother Oncol. 2004;72:311–317. doi: 10.1016/j.radonc.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkamm K, Balroop S, Shekhdar J, Fernie P, Goh V. Leukocyte DNA damage after multi-detector row CT: a quantitative biomarker of low-level radiation exposure. Radiology. 2007;242:244–251. doi: 10.1148/radiol.2421060171. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci U S A. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan H, Hieber L, Braselmann H, Meineke V, Zitzelsberger H. Accumulation of DSBs in gamma-H2AX domains fuel chromosomal aberrations. Biochem Biophys Res Commun. 2008;371:694–697. doi: 10.1016/j.bbrc.2008.04.127. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Dent P, Grant S, Mikkelsen RB, Valerie K. Signal transduction and cellular radiation responses. Radiat Res. 2000;153:245–257. doi: 10.1667/0033-7587(2000)153[0245:stacrr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Bonner WM. GammaH2AX in cancer cells: a potential biomarker for cancer diagnostics, prediction and recurrence. Cell Cycle. 2006;5:2909–2913. doi: 10.4161/cc.5.24.3569. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Horikawa I, Redon C, Nakamura A, Zimonjic DB, Popescu NC, Bonner WM. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell. 2008;7:89–100. doi: 10.1111/j.1474-9726.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Nakamura A, Kovalchuk O, Koturbash I, Mitchell SA, Marino SA, Brenner DJ, Bonner WM. DNA double-strand breaks form in bystander cells after microbeam irradiation of three-dimensional human tissue models. Cancer Res. 2007;67:4295–4302. doi: 10.1158/0008-5472.CAN-06-4442. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Pilch DR, Redon C, Bonner WM. Histone H2AX in DNA damage and repair. Cancer Biol Ther. 2003;2:233–235. doi: 10.4161/cbt.2.3.373. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res. 2002;158:486–492. doi: 10.1667/0033-7587(2002)158[0486:qdoiid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yasui LS. GammaH2AX foci induced by gamma rays and 125idU decay. Int J Radiat Biol. 2004;80:895–903. doi: 10.1080/09553000400017556. [DOI] [PubMed] [Google Scholar]
- Zhao JF, Zhang YJ, Kubilus J, Jin XH, Santella RM, Athar M, Wang ZY, Bickers DR. Reconstituted 3-dimensional human skin as a novel in vitro model for studies of carcinogenesis. Biochem Biophys Res Commun. 1999;254:49–53. doi: 10.1006/bbrc.1998.9821. [DOI] [PubMed] [Google Scholar]