Abstract
The composition of the ECM provides contextual cues to leukocytes in inflamed and healing tissues. One example of this is HA, where LMW-HA, generated during active inflammation, is a TLR ligand and an endogenous “danger signal,” and HMW-HA, predominant in healing or intact tissues, functions in an inverse manner. Our data suggest that HMW-HA actively promotes immune tolerance by augmenting CD4+CD25+ TReg function, and LMW-HA does not. Using a human iTReg model, we demonstrate that HMW-HA but not LMW-HA provides a costimulatory signal through cross-linking CD44 which promotes Foxp3 expression, a critical signaling molecule associated with TReg. This effect, in part, may be mediated by a role for intact HMW-HA in IL-2 production, as TReg are highly IL-2-dependent for their survival and function. We propose that HMW-HA contributes to the maintenance of immune homeostasis in uninjured tissue and effectively communicates an “all-clear” signal to down-regulate the adaptive immune system through TReg after tissue matrix integrity has been restored.
Keywords: autoimmunity, IL-2, TReg
Introduction
The inflammatory milieu is known to contribute strong activation stimuli to innate and adaptive immune responses through a variety of danger signals. However, regulatory contributions of ECM components, which potentially counterbalance the inflammatory cascade, are less understood. There is an immunologic dichotomy between intact and inflamed ECM [1, 2]. A number of ECM components exist in a nonproinflammatory form until they are fragmented or released from the matrix, at which time they become proinflammatory and chemoattractant [3,4,5,6,7]. In this context, ECM fragments have been suggested to function as endogenous “danger signals” analogous to exogenous pathogen-associated molecular pattern molecules derived from bacteria or viruses. Indeed, a number of ECM components, including fragments of versican [8], fibronectin [9], fibrinogen [10], biglycan [11], soluble heparin sulfate [12], and LMW-HA [13], are reported to signal through TLRs. Interestingly, TLR2 and/or TLR4 have been implicated in all of the aforementioned models, suggesting that common pathways for fragmentary matrix signals may exist. In addition, fragmentary ECM components promote immune activation and migration by a broad diversity of mechanisms other than TLR. These include interactions with a range of cytokines, chemokines, growth factors, MMPs, and other inflammatory mediators (reviewed in refs. [1, 14]).
Although several fragmentary ECM components are proinflammatory, the immunologic relevance of their whole parent molecules and of intact ECM in general is more ambiguous. Intact ECM has been viewed conceptually as an inert or immunologically passive foil to fragmentary ECM. However, experimental investigation is limited by the difficulty of recreating biologically plausible yet experimentally tractable model systems in vitro. Some of the salient considerations include the complex and dynamic interactions between ECM components, the extensive post-translational modification of the many ECM components, and the importance of matrix-bound soluble factors to the activity of many of these molecules in vivo.
Our recent work suggests that at least one intact ECM component, HMW-HA, may play an active role in the maintenance of immune tolerance. In this article, we discuss the role of intact HA in promoting the function of naturally occurring CD4+CD25+ TReg and present data from work in progress demonstrating that an analogous process occurs in TReg induced in vitro. In nTReg and iTReg systems, HMW-HA promotes persistence in vitro of Foxp3, a critical transcription factor for TReg. We propose a model for how intact HA may play an important role in the maintenance of immunological tolerance in uninjured or healing tissue.
HA IS AN IMMUNOLOGIC MEDIATOR
HA is an unadorned, repeating disaccharide of N-acetylglucosamine and D-glucuronic acid. It is not known to bind to cytokines or other small molecules, is not chemically modified postsynthesis, and exists in only one form: a chain of variable length ranging in molecular weight from 104 to 107 Da. Because of these features, HA has proved relatively amenable to immunologic investigation. The bioactive properties of HA vary with its size; the amounts and relative proportions of these have distinct effects on cellular metabolism and phenotype [15].
HA breakdown products predominate during injury and inflammation. LMW-HA (<15 saccharides; <3 kDa) are generated by endogenous catabolism, by bacterial hyaluronidases, and by mechanical forces and oxidative stress [16]. LMW-HA promotes angiogenesis [17], inflammation [18, 19], maturation of APCs [20, 21], and cell migration [22]. These effects of LMW-HA are mediated via MMP [23], plasminogen activator inhibitor [24], NO [25], and production of several proinflammatory cytokines, including MIP-1α, MIP-1β, MCP-1, IL-8, and IL-12 by macrophages [26,27,28,29] and IL-1β, TNF-α, and IL-12 by dendritic cells and endothelial cells [30].
HMW-HA (>2000 saccharides; >400 kDa) predominates in steady-state conditions and in healing tissues [31]. HMW-HA serves a variety of structural and regulatory functions in joints [32] and tissue repair [33]. HMW-HA has typically been reported to be inert or anti-inflammatory [19, 34]. HMW-HA inhibits phagocytosis by monocytes [35], limits antigen-antibody interactions [36], and has been suggested to impede lymphocyte activation [37]. HMW-HA is U.S. Food and Drug Administration-approved for injection into osteoarthritic joints and prevention of postsurgical abdominal adhesions and is under investigation in other settings including wound healing, burn treatment, and rheumatoid arthritis [38].
The best characterized HA receptor is CD44, a nearly ubiquitous cell surface receptor important in cell trafficking, activation, and apoptosis [39]. CD44 exists in multiple isoforms with distinct functional characteristics depending on alternative splicing and expression of different exons [40]. The CD44 expressed on resting T cells and monocytes is functionally inactive and binds HA only after TCR triggering or after activation by proinflammatory cytokines including TNF-α and IFN-γ [41]. Therefore, the ability of these cells to interact with HA is intrinsically related to their activation state. Signaling through CD44 improves with longer chains of HA up to 38 sugars in length [42].
CD4+CD25+ TReg CONSTRAIN ADAPTIVE IMMUNITY
CD4+CD25+ TReg are a relatively well-characterized, specialized subpopulation of T cells that expresses the transcription factor Foxp3 [43]. A variety of distinct mechanisms is involved potentially in TReg activity, and TReg cells display a range of regulatory functions [44, 45]. TReg arise during lymphocyte development from the thymus (nTReg), but it has now been shown by us and by other groups that they can also be generated in the periphery and induced ex vivo (iTReg) [46,47,48]. TReg are known to integrate diverse signals from the inflammatory milieu [49,50,51,52], including a key dependence on IL-2 to maintain viability and function. IL-2 is considered a proinflammatory cytokine, and the requirement for IL-2 fits with the model of TReg as a regulatory cell type that exists within inflammatory contexts to dampen overaggressive responses. The strength of TCR activation and the availability of IL-2 have been shown to be dominant factors in the triggering of TReg [53], consistent with the model that iTReg are a subset of T cells driven by overactivation into anergy and suppression of neighboring cells. An unaddressed question in TReg biology is how this highly IL-2-dependent cell type persists in healing or uninjured tissues, where IL-2 concentrations are presumably low.
HMW-HA PROMOTES THE FUNCTION OF NATURALLY OCCURRING CD4+CD25+ TReg
Most of the molecules that characterize TReg, including CD25 and Foxp3, are activation markers common to many highly activated T cells. The report that total Foxp3 expression and relative suppressor function are correlated with CD44 expression levels [54] fits within this paradigm. Furthermore, this study raised the possibility that CD44 interactions with HA may be integrally related to TReg function. Indeed, we reported subsequently [55] that HMW-HA promotes the suppressive function of human nTReg, and LMW-HA does not. In our follow-up work (manuscript submitted), we demonstrated that this effect is a result of a costimulatory function mediated by HMW-HA through CD44 expressed on activated TReg. Cross-linking of CD44 by HMW-HA and plate-bound anti-CD44 antibody promotes Foxp3 persistence, and neither LMW-HA nor soluble anti-CD44 antibody does so. Blocking antibodies directed against CD44 abrogate this effect. This is consistent with previous reports of cross-linking being integral to several functions attributed to CD44 [56, 57]. We found previously that TReg do not bind FITC-labeled HMW-HA unless they are preactivated via their TCR complex, at which point they up-regulate CD44v isoforms capable of binding HMW-HA [55]. The activation-induced expression of HA-binding isoforms of CD44 is correlated with Foxp3 expression and is consistent with similar activation-dependent HA binding seen in previous studies [58]. Perhaps consistent with a role for HMW-HA in promoting TReg function is the recent report that binding of HMW-HA but not LMW-HA causes the death of activated T cells [59]. Given the relationship between HA size and the stage of inflammation, the extent of CD44 cross-linking by HMW-HA could provide contextual cues to TReg and T cells regarding the inflammatory milieu.
HMW-HA PROMOTES FUNCTION OF CD4+CD25+ TReg INDUCED IN VITRO
As noted previously, TReg require IL-2 to maintain viability and function. In our previously published work, we found that HMW-HA promoted Foxp3 expression by activated TReg predominantly in settings of low IL-2 [55]. Given this observation, we then asked whether HMW-HA was impacting the availability of or requirement for this key cytokine. Consistent with reports of enhanced IL-2 production by other T cell subsets and NKT cells upon CD44 costimulation [60, 61] and with reports of requirements for HA and CD44 in certain IL-2-dependent processes [62, 63], we hypothesized that CD44 cross-linking promotes modest IL-2 production and thereby provides a tonic signal necessary for TReg persistence and function.
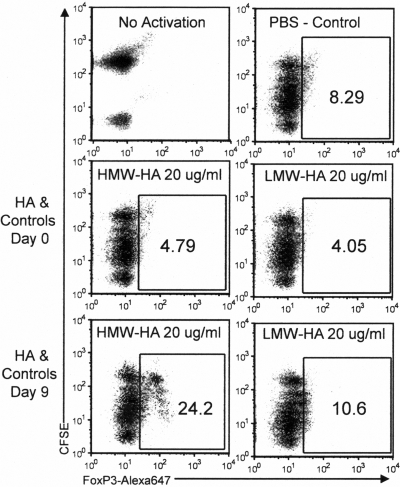
To examine the role of HMW-HA on TReg IL-2 interactions, we used a human iTReg model described previously [46] with minor variations. The induction of functional Foxp3+CD25+ TReg from CD4+CD25– precursors is known to be highly dependent on the availability of IL-2 [64, 65]. This model is pertinent to the role of the ECM in TReg physiology, as the induction of TReg from similar populations of cells is thought to occur in vivo in peripheral tissues, presumably where ECM integrity is most relevant. We found that HMW-HA but not LMW-HA promotes persistence of Foxp3 expression by such iTReg populations (Fig. 1). These effects were most pronounced when HMW-HA treatment occurs late but not early in induction cultures, when expression of activation markers and production of endogenous IL-2 could be expected to wane. Although soluble HMW-HA was used in the particular experiment shown in Figure 1, we find that the magnitude of the salutary effect on Foxp3 expression is typically most pronounced when plate-bound HMW-HA is used. This effect on Foxp3 expression was approximately equivalent to the addition of exogenous IL-2 added at 20 IU/ml (Fig. 2A). Upon sorting these cells on the basis of CD25 expression on Day 10 of culture, iTReg induced with HMW-HA or exogenous IL-2 treatment on Day 7 were found to suppress proliferative responses of autologous T cells better than control iTReg (Fig. 2B).
Figure 1.
HMW-HA treatment added late in induction culture promotes Foxp3 expression by iTReg. CFSE-labeled CD4+CD25– cells were activated and treated on Day 0 or Day 9 with HMW-HA or LMW-HA. Flow cytometry for Foxp3 expression on Day 10 is shown.
Figure 2.
HMW-HA treatment produces effects on iTReg number and function analogous to IL-2 addition. (A) Percentage of CD25+ cells positive for Foxp3 upon addition of HMW-HA or IL-2 at 20 IU/ml on Day 7 of the induction culture. Significance was determined via a Student’s t-test. ns, Not significant. (B) TReg suppression assay using the top 5% of CD25-expressing cells sorted from each induction condition and autologous CFSE-labeled CD4+CD25– T cells as the responder population. Data are expressed as percent suppression relative to proliferative population observed in the absence of iTReg. Data are representative of three independent experiments. (C) IL-2 was assessed in cell culture supernatants drawn on Day 8 following inception of the iTReg induction culture, 1 day after washing and transfer to plates coated with HMW-HA or controls. Data are for four experiments.
These effects on Foxp3 persistence and iTReg viability are consistent with HMW-HA, impacting the availability of or requirement for IL-2. Although our data do not rule out ancillary mechanisms, we find that HMW-HA but not LMW-HA treatment of cells in an iTReg induction culture promotes production of modest levels of IL-2 (Fig. 2C). We do not see a similar induction of IFN-γ (data not shown). Given the established role of IL-2 in TReg function and viability, we next asked whether HMW-HA promoted viability of iTReg populations. This does appear to be the case, as HMW-HA on Day 7 of induction culture indeed resulted in a larger percentage of viable cells on Day 10 of culture, as ascertained by staining for the apoptotic marker annexin V and the necrosis marker 7-amino-actinomycin (unpublished data). This is consistent with a previous report of a role for HA in cell survival [66].
A MODEL FOR HOW HMW-HA MIGHT PROMOTE THE MAINTENANCE OF IMMUNE TOLERANCE
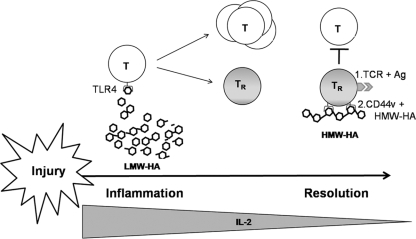
We interpret the data reported here and in our related work as supporting the following model (Fig. 3). Upon activation in the setting of injury, a fraction of T cells is induced to become TReg. During the active immune response phase, the activity of these is thought to be inhibited by IL-6 (and other proinflammatory mediators) produced by subsets of activated T cells and macrophages. LMW-HA is likely one of these mediators, as it has been shown to promote IL-6 production, and HMW-HA does not [67]. Upon resolution of the injury response, the concentration of IL-2 produced by activated T cells diminishes. At the same time, tissue repair mechanisms begin to synthesize intact HMW-HA. As the concentration of ambient IL-2 wanes, the molar balance of HA molecules shifts in favor of HMW-HA forms capable of cross-linking CD44v isoforms. CD44 cross-linking by HMW-HA provides a second costimulatory signal in the setting of a primary antigen-specific signal through the TCR. Together, these two signals promote TReg activation, including low-level production of IL-2. Alternative sources of IL-2 in this context are also possible, as availability is likely to be limiting as the inflammatory response wanes. We propose that the HMW-HA molecular composition of the ECM thereby conveys a tissue integrity signal that functions as the opposite of a danger signal, in that adaptive TReg receive an important secondary signal in situ, promoting immune tolerance. This mechanism may be one way in which viable TReg populations persist in healing or uninjured tissues to bring inflammatory processes to a close and maintain homeostasis. The intermolecular interactions described here, between ECM and the adaptive T lymphocyte, may represent just one of many such pathways for providing contextual clues linking tissue status to the regulation of the immune response.
Figure 3.
A model for how HA size might contribute to immune regulation and the maintenance of immune tolerance. Upon injury, T cells proliferate in response to LMW-HA and other proinflammatory cues. A fraction of activated T cells (T) becomes TReg (TR). As the concentration of ambient IL-2 wanes with the resolution of inflammation, the molar balance of HA molecules shifts in favor of HMW-HA forms capable of cross-linking CD44v isoforms. CD44 cross-linking by HMW-HA provides a second costimulatory signal in the setting of a primary antigen-specific signal through the TCR; together, these two signals promote production of IL-2 and thereby maintain TReg viability and function. The persistence of iTReg populations in peripheral tissues is likely to be important to the maintenance of immune tolerance.
ACKNOWLEDGMENTS
This work was supported by grants from NIH (DK46635, HL18645, and DK53004) and Juvenile Diabetes Research Foundation (The Center for Translational Research at BRI). P. L. B. is supported by NIH K-08 grant DK080178-01 and a NIH Loan Repayment Program grant. We thank Nathan Standifer and Alice Long for their helpful comments and suggestions.
DISCLOSURE
The authors have no conflicting financial interests.
Supplementary Material
Footnotes
Abbreviations: BRI=Benaroya Research Institute at Virginia Mason, ECM=extracellular matrix, Foxp3=forkhead box p3, HA=hyaluronan, HMW=high molecular weight, iTReg=induced TReg, LMW=low molecular weight, MMP=matrix metalloproteinase, NIH=National Institutes of Health, nTReg=natural TReg, TReg=regulatory T cell
References
- Adair-Kirk T L, Senior R M. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labat-Robert J, Robert L. The effect of cell-matrix interactions and aging on the malignant process. Adv Cancer Res. 2007;98:221–259. doi: 10.1016/S0065-230X(06)98007-5. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Adams D H, Hubscher S, Hirano H, Siebenlist U, Shaw S. T-cell adhesion by proteoglycan-immobilized cytokine MIP-1 β. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Thomas A H, Edelman E R, Stultz C M. Collagen fragments modulate innate immunity. Exp Biol Med (Maywood) 2007;232:406–411. [PubMed] [Google Scholar]
- Barilla M L, Carsons S E. Fibronectin fragments and their role in inflammatory arthritis. Semin Arthritis Rheum. 2000;29:252–265. doi: 10.1016/s0049-0172(00)80012-8. [DOI] [PubMed] [Google Scholar]
- Gray A J, Bishop J E, Reeves J T, Mecham R P, Laurent G J. Partially degraded fibrinogen stimulates fibroblast proliferation in vitro. Am J Respir Cell Mol Biol. 1995;12:684–690. doi: 10.1165/ajrcmb.12.6.7766431. [DOI] [PubMed] [Google Scholar]
- Powell J D, Horton M R. Threat matrix: low molecular weight hyaluronan (HA) as a danger signal. Immunol Res. 2005;31:207–218. doi: 10.1385/IR:31:3:207. [DOI] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin W W, Descargues P, Grivennikov S, Kim Y, Luo J L, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura Y, Watari M, Jerud E S, Young D W, Ishizaka S T, Rose J, Chow J C, Strauss J F., III The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- Kuhns D B, Priel D A, Gallin J I. Induction of human monocyte interleukin (IL)-8 by fibrinogen through the Toll-like receptor pathway. Inflammation. 2007;30:178–188. doi: 10.1007/s10753-007-9035-1. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Babelova A, Kiss E, Hausser H J, Baliova M, Krzyzankova M, Marsche G, Young M F, Mihalik D, Götte M, Malle E, Schaefer R M, Gröne H J. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G B, Brunn G J, Kodaira Y, Platt J L. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon J C. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor H, Vaday G G, Lider O. Modulation of leukocyte behavior by an inflamed extracellular matrix. Dev Immunol. 2000;7:227–238. doi: 10.1155/2000/51902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R, Asari A A, Sugahara K N. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Ågren U M, Tammi R H, Tammi M I. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic Biol Med. 1997;23:996–1001. doi: 10.1016/s0891-5849(97)00098-1. [DOI] [PubMed] [Google Scholar]
- West D C, Hampson I N, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- Powell J D, Horton M R. Threat matrix: low molecular weight hyaluronan (HA) as a danger signal. Immunol Res. 2005;31:207–218. doi: 10.1385/IR:31:3:207. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich G D, Mascarenhas M M, Garg H G, Quinn D A, Homer R J, Goldstein D R, Bucala R, Lee P J, Medzhitov R, Noble P W. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Li L, Kamiryo M, Asteriou T, Moustakas A, Yamashita H, Heldin P. Hyaluronan fragments induce endothelial cell differentiation in a CD44– and CXCL1/GRO1-dependent manner. J Biol Chem. 2005;280:24195–24204. doi: 10.1074/jbc.M411913200. [DOI] [PubMed] [Google Scholar]
- Termeer C C, Hennies J, Voith U, Ahrens T, Weiss J M, Prehm P, Simon J C. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol. 2000;165:1863–1870. doi: 10.4049/jimmunol.165.4.1863. [DOI] [PubMed] [Google Scholar]
- Sugahara K N, Murai T, Nishinakamura H, Kawashima H, Saya H, Miyasaka M. Hyaluronan oligosaccharides induce CD44 cleavage and promote cell migration in CD44-expressing tumor cells. J Biol Chem. 2003;278:32259–32265. doi: 10.1074/jbc.M300347200. [DOI] [PubMed] [Google Scholar]
- Horton M R, Shapiro S, Bao C, Lowenstein C J, Noble P W. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J Immunol. 1999;162:4171–4176. [PubMed] [Google Scholar]
- Horton M R, Olman M A, Bao C, White K E, Choi A M, Chin B Y, Noble P W, Lowenstein C J. Regulation of plasminogen activator inhibitor-1 and urokinase by hyaluronan fragments in mouse macrophages. Am J Physiol Lung Cell Mol Physiol. 2000;279:L707–L715. doi: 10.1152/ajplung.2000.279.4.L707. [DOI] [PubMed] [Google Scholar]
- McKee C M, Lowenstein C J, Horton M R, Wu J, Bao C, Chin B Y, Choi A M, Noble P W. Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear factor κB-dependent mechanism. J Biol Chem. 1997;272:8013–8018. doi: 10.1074/jbc.272.12.8013. [DOI] [PubMed] [Google Scholar]
- Hodge-Dufour J, Noble P W, Horton M R, Bao C, Wysoka M, Burdick M D, Strieter R M, Trinchieri G, Puré E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol. 1997;159:2492–2500. [PubMed] [Google Scholar]
- Horton M R, Burdick M D, Strieter R M, Bao C, Noble P W. Regulation of hyaluronan-induced chemokine gene expression by IL-10 and IFN-γ in mouse macrophages. J Immunol. 1998;160:3023–3030. [PubMed] [Google Scholar]
- Horton M R, McKee C M, Bao C, Liao F, Farber J M, Hodge-DuFour J, Puré E, Oliver B L, Wright T M, Noble P W. Hyaluronan fragments synergize with interferon-γ to induce the C-X-C chemokines MIG and interferon-inducible protein-10 in mouse macrophages. J Biol Chem. 1998;273:35088–35094. doi: 10.1074/jbc.273.52.35088. [DOI] [PubMed] [Google Scholar]
- McKee C M, Penno M B, Cowman M, Burdick M D, Strieter R M, Bao C, Noble P W. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon J C. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent T C, Fraser J R. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- Brown T J, Laurent U B, Fraser J R. Turnover of hyaluronan in synovial joints: elimination of labeled hyaluronan from the knee joint of the rabbit. Exp Physiol. 1991;76:125–134. doi: 10.1113/expphysiol.1991.sp003474. [DOI] [PubMed] [Google Scholar]
- Noble P W. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- Deed R, Rooney P, Kumar P, Norton J D, Smith J, Freemont A J, Kumar S. Early-response gene signaling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer. 1997;71:251–256. doi: 10.1002/(sici)1097-0215(19970410)71:2<251::aid-ijc21>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Forrester J V, Balazs E A. Inhibition of phagocytosis by high molecular weight hyaluronate. Immunology. 1980;40:435–446. [PMC free article] [PubMed] [Google Scholar]
- Delmage J M, Powars D R, Jaynes P K, Allerton S E. The selective suppression of immunogenicity by hyaluronic acid. Ann Clin Lab Sci. 1986;16:303–310. [PubMed] [Google Scholar]
- Day A J, de la Motte C A. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005;26:637–643. doi: 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kogan G, Soltes L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007;29:17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- Sherman L, Sleeman J, Herrlich P, Ponta H. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol. 1994;6:726–733. doi: 10.1016/0955-0674(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Borland G, Ross J A, Guy K. Forms and functions of CD44. Immunology. 1998;93:139–148. doi: 10.1046/j.1365-2567.1998.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pure E, Cuff C A. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- Lesley J, Hascall V C, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–26975. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, Puig P E, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay J Y, Bernard J, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-β-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M R, Kasprowicz D J, Gersuk V H, Benard A, Van Landeghen M, Buckner J H, Ziegler S F. Induction of Foxp3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Camara N, Lombardi G, Lechler R I. Induction of allopeptide-specific human CD4+CD25+ regulatory T cells ex vivo. Blood. 2003;102:2180–2186. doi: 10.1182/blood-2003-04-1164. [DOI] [PubMed] [Google Scholar]
- Knoechel B, Lohr J, Kahn E, Bluestone J A, Abbas A K. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhu J, Yang Y. Protection against autoimmunity in nonlymphopenic hosts by CD4+ CD25+ regulatory T cells is antigen-specific and requires IL-10 and TGF-β. J Immunol. 2005;175:4283–4291. doi: 10.4049/jimmunol.175.7.4283. [DOI] [PubMed] [Google Scholar]
- Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Suciu-Foca N, Manavalan J S, Cortesini R. Generation and function of antigen-specific suppressor and regulatory T cells. Transpl Immunol. 2003;11:235–244. doi: 10.1016/S0966-3274(03)00052-2. [DOI] [PubMed] [Google Scholar]
- Walker M R, Carson B D, Nepom G T, Ziegler S F, Buckner J H. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25– cells. Proc Natl Acad Sci USA. 2005;102:4103–4108. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firan M, Dhillon S, Estess P, Siegelman M H. Suppressor activity and potency among regulatory T cells is discriminated by functionally active CD44. Blood. 2006;107:619–627. doi: 10.1182/blood-2005-06-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollyky P L, Lord J D, Masewicz S A, Evanko S P, Buckner J H, Wight T N, Nepom G T. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–747. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Fujii K, Nakano K, Tanaka Y. Crosslinking of CD44 on human osteoblastic cells upregulates ICAM-1 and VCAM-1. FEBS Lett. 2003;539:45–50. doi: 10.1016/s0014-5793(03)00182-0. [DOI] [PubMed] [Google Scholar]
- Wyant T L, Fisher M T, McKallip R J, Nagarkatti P S, Nagarkatti M, Conrad D H. Mouse B cell activation is inhibited by CD44 cross-linking. Immunol Invest. 2005;34:399–416. doi: 10.1080/08820130500265406. [DOI] [PubMed] [Google Scholar]
- Lesley J, Howes N, Perschl A, Hyman R. Hyaluronan binding function of CD44 is transiently activated on T cells during an in vivo immune response. J Exp Med. 1994;180:383–387. doi: 10.1084/jem.180.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Johnson P. Hyaluronan induces cell death in activated T cells through CD44. J Immunol. 2008;181:7044–7054. doi: 10.4049/jimmunol.181.10.7044. [DOI] [PubMed] [Google Scholar]
- Huet S, Groux H, Caillou B, Valentin H, Prieur A M, Bernard A. CD44 contributes to T cell activation. J Immunol. 1989;143:798–801. [PubMed] [Google Scholar]
- Larkin J, Renukaradhya G J, Sriram V, Du W, Gervay-Hague J, Brutkiewicz R R. CD44 differentially activates mouse NK T cells and conventional T cells. J Immunol. 2006;177:268–279. doi: 10.4049/jimmunol.177.1.268. [DOI] [PubMed] [Google Scholar]
- Guan H, Nagarkatti P S, Nagarkatti M. Blockade of hyaluronan inhibits IL-2-induced vascular leak syndrome and maintains effectiveness of IL-2 treatment for metastatic melanoma. J Immunol. 2007;179:3715–3723. doi: 10.4049/jimmunol.179.6.3715. [DOI] [PubMed] [Google Scholar]
- Mahaffey C L, Mummert M E. Hyaluronan synthesis is required for IL-2 mediated T cell proliferation. J Immunol. 2007;179:8191–8199. doi: 10.4049/jimmunol.179.12.8191. [DOI] [PubMed] [Google Scholar]
- Buckner J H, Ziegler S F. Regulating the immune system: the induction of regulatory T cells in the periphery. Arthritis Res Ther. 2004;6:215–222. doi: 10.1186/ar1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S A, Buckner J H. Combination of rapamycin and IL-2 increases de novo induction of human CD4(+)CD25(+)FOXP3(+) T cells. J Autoimmun. 2008;30:293–302. doi: 10.1016/j.jaut.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Obeid L M, Hannun Y A, Minamisawa S, Berger F G, Markwald R R, Toole B P, Ghatak S. Hyaluronan constitutively regulates activation of COX-2-mediated cell survival activity in intestinal epithelial and colon carcinoma cells. J Biol Chem. 2008;283:14335–14344. doi: 10.1074/jbc.M703811200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki H, Hirohata S, Miyoshi T, Takahashi K, Ogawa H, Shinohata R, Demircan K, Kusachi S, Yamamoto K, Ninomiya Y. Hyaluronan receptors involved in cytokine induction in monocytes. Glycobiology. 2009;19:83–92. doi: 10.1093/glycob/cwn109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.