Abstract
Hepatic lymphocytes are enriched in NK and NKT cells that play important roles in antiviral and antitumor defenses and in the pathogenesis of chronic liver disease. In this review, we discuss the differential distribution of NK and NKT cells in mouse, rat, and human livers, the ultrastructural similarities and differences between liver NK and NKT cells, and the regulation of liver NK and NKT cells in a variety of murine liver injury models. We also summarize recent findings about the role of NK and NKT cells in liver injury, fibrosis, and repair. In general, NK and NKT cells accelerate liver injury by producing proinflammatory cytokines and killing hepatocytes. NK cells inhibit liver fibrosis via killing early-activated and senescent-activated stellate cells and producing IFN-γ. In regulating liver fibrosis, NKT cells appear to be less important than NK cells as a result of hepatic NKT cell tolerance. NK cells inhibit liver regeneration by producing IFN-γ and killing hepatocytes; however, the role of NK cells on the proliferation of liver progenitor cells and the role of NKT cells in liver regeneration have been controversial. The emerging roles of NK/NKT cells in chronic human liver disease will also be discussed. Understanding the role of NK and NKT cells in the pathogenesis of chronic liver disease may help us design better therapies to treat patients with this disease.
Keywords: NK, NKT, liver, poly I:C
Introduction
In 1976, Wisse et al. [1] first described “pit cells”, a new type of rat liver sinusoidal cell so named because they contained highly characteristic cytoplasmic granules resembling fruit pits. Now known as liver-specific NK cells, these cells show different immunophenotypical, morphological, and functional characteristics from peripheral NK cells [2,3,4,5,6]. Liver NK cells are identifiable by characteristic NK cell-specific markers using flow cytometry (see Table 1). In general, DX5 or NK1.1 markers (NK1.1 is only found in certain strains such as C57BL/6 mice) have been widely used to identify mouse NK cells (CD3−DX5+ or CD3−NK1.1+) [7]. Rat NK cells are distinguished by NKR-P1A (CD3−NKR-P1A+). Human NK cells are identifiable by CD56 (adhesion molecule mediating homotypic adhesion) cell markers and can be divided into two major populations: CD56dimCD3− and CD56brightCD3− cells. Although CD56dimCD3− cells account for 90% of human NK cells and are more cytotoxic against tumor cells, the CD56brightCD3− cells account for the remaining 10% of human NK cells and are more efficient at producing cytokines [8, 9]. Some NK cells also express DC markers, which produce substantial amounts of IFN-γ and share phenotypic and functional properties of DCs and NK cells. These cells were named as NKDC (NK1.1+CD11c+CD3− in C57BL/6 mice) [10,11,12,13] or IKDC (CD11cintB220+CD49+MHC II+ in C57BL/6 mice) cells [14, 15]. However, recent studies suggest that these cells represent a subset of activated NK cells [16,17,18], and their antigen-presenting functions have been questioned [16]. In addition, flow cytometric analyses have detected a population of cells expressing NK and T cell markers, which were referred as NKT cells. Now, it is known that NKT cells are a heterogeneous group of T lymphocytes that recognize the lipid antigens presented by the nonclassical MHC class I-like molecule CD1 [19,20,21]. The human CD1 family consists of five distinct isoforms, including CD1a, -b, -c, -d, and -e, whereas mice only express CD1d [22]. The CD1d-dependent NKT cells can be broadly divided into two types of cells including type I and type II NKT cells, which are described briefly in Table 2. The best way to identify the CD1d-depdent NKT cells is to use tetramers of CD1d loaded with α-GalCer. NK1.1 and CD3 have also been widely used to detect mouse NKT cells in C57BL/6 mice. Human NKT cells express αβ or γδ TCR and various NKRs, including CD16, CD56, CD69, CD161, and others, and are identifiable by using CD56 and CD3 markers. CD56+CD3+ cells in the human liver were also named as hepatic NT cells, as the phenotype of this population is associated most strikingly with the human liver [23].
TABLE 1.
Differential Distribution of Hepatic NK and NKT Cells in Mice, Rats, and Humans
| Species | NK cells | NKT cells |
|---|---|---|
| Mice | 5–10% (NK1.1+CD3+ or DX5+) | 30–40% (NK1.1+CD3+) |
| 20–30% CD1-dependent NKT | ||
| Rats | 25–35% (NKRP1A+CD3−) | <10% (NKRP1A+CD3+) |
| <1% CD1d-dependent NKT | ||
| Humans | 20–30% (CD56+CD3−) | 10–25% (CD56+CD3+) |
| <1% CD1d-dependent NKT |
TABLE 2.
Murine NKT Cells
| Type I (invariant NKT:iNKT) | Type II NKT | |
|---|---|---|
| CD1d | Dependent | Dependent |
| TCRs | Invariant αβTCR encoded by the Vα14 in mice (Vα24 in humans) and Jα18 | Diverse TCRs |
| Other names | Classical NKT, invariant NKT, Vα14 iNKT | Nonclassical NKT |
| Percentage | >95% NKT | <5% NKT |
| Stimulator | α-GalCer, OCH | Sulfatide |
| Knockout (KO) mice | Deficient in CD1dKO and Jα18KO mice | Deficient in CD1dKO but not in Jα18KO mice |
| Cytokine production | IFN-γ, IL-4, IL-13 | IL-4, IL-13, IL-10, IFN-γ |
| Liver injury | iNKT contributes to liver injury induced by Con A, α-GalCer, and ischemia/reperfusion but protects against liver injury induced by CCl4 and bile duct ligation. | Type II NKT protects against Con A-induced liver injury. |
OCH, A sphingosine-truncated analog of α-GalCer.
NK cells are abundant in liver lymphocytes and play an important role in first-line, innate defense against viral infection and tumor transformation [24, 25]. The functionalities of NK cells are believed to be mediated via production of cytokines such as IFN-γ or direct killing of target cells. The cytotoxicity of NK cells against target cells is determined by an imbalance between the effects of inhibitory and stimulatory receptors expressed on NK cells with their corresponding ligands expressed on target cells [26,27,28,29]. The inhibitory receptors include Ig-like killer inhibitory receptor and Ly-49A and CD94/NKG2 receptors that recognize MHC class I molecules expressed on target cells and subsequently inactivate NK cell functions. The stimulatory receptors include NKp46, NKp30, and NKp44, collectively referred to as natural cytotoxicity receptors, NKG2D, and DNAX accessory molecule-1 (CD226) [26,27,28,29]. Among them, the NKG2D receptor is the best defined and is recognized by MICA and UL16-binding proteins expressed on human target cells. Mouse NKG2D receptors are activated by RAE-1, histocompatibility 60, and mouse UL16-binding protein-like transcript 1 [26,27,28,29].
A large population of liver NK cells also expresses DC markers [11,12,13, 30]. For example, in naïve C57BL/6 mice, about one-third of liver NK cells (NK1.1+CD3−) expresses CD11c (NKDC: NK1.1+CD11c+CD3− cells), which displayed enhanced cytotoxicity against tumor cells and a greater IFN-γ response compared with CD11c–NK cells [11,12,13]. Liver NKDCs can also cause T cell alloproliferation when stimulated by CpG motifs or adenovirus [13]. Thus, liver NKDCs likely play important roles in innate and adaptive responses against bacterial and viral infections and tumor transformation [12, 13].
Liver lymphocytes are also enriched in NKT cells, accounting for 20–35% of mouse liver lymphocytes and 10–15% of rat and human liver lymphocytes [31,32,33,34]. NKT cells have been shown to play an important role in regulating innate and adaptive immunity via production of a variety of cytokines, including IFN-γ and IL-4 [19,20,21]. Increasing evidence suggests that different NKT cell subsets may exert distinct, even opposing functions (see Table 2). The important roles of NK and NKT cells in contributing to the pathogenesis of human liver disease have been documented in several excellent reviews [35,36,37,38,39,40,41,42]. In this review, we will discuss the distinguishing characteristics of mouse liver NK/NKT cells and discuss findings about the roles of NK/NKT cells in liver injury, fibrosis, and repair in rodent models. We will also discuss briefly the potential roles of NK/NKT cells in the pathogenesis of viral hepatitis, nonalcoholic steatohepatitis, alcoholic liver disease, autoimmune liver disease, and HCC.
LIVER NK AND NKT CELLS
Differential distribution of NK and NKT cells in mouse, rat, and human livers
The distribution of NK and NKT cells is different in the livers of mice, rats, and humans (Table 1). In general, flow cytometry data show that lymphocytes from mouse liver have more NKT cells, and rat and human liver lymphocytes have more NK cells. Findings from the α-GalCer injection experiments provide further evidence that the higher number of NKT cells is found in mouse livers, and rat livers contain the lower number of NKT cells. Injection of α-GalCer, a NKT cell activator, caused significant liver injury in mice, as demonstrated by elevated serum ALT levels [43, 44], and the same treatment elevated serum ALT levels only slightly in rats (O. Park and B. Gao, unpublished data). These data suggest that rats are less sensitive to α-GalCer-induced NKT-mediated liver injury. In human liver lymphocytes, the percentage of NKT (CD56+CD3+) cells is highly variable, ranging from 5% to 25% [23], and CD1d-dependent NKT cells were found to be about <1% of human liver lymphocytes [32, 45]. Several phase I/II clinical trials revealed that injection of α-GalCer did not show any signs of liver injury in humans [46, 47], suggesting that human liver lymphocytes may contain a low percentage of NKT cells.
Ultrastructural similarity and difference between liver NK and NKT cells
NK and NKT cells, along with a heterogeneous subset of cytotoxic T cells, comprise a morphologically distinct cell population, which was referred to as the large granular lymphocytes (LGLs). Compared with small nongranular T- and B-lymphocytes, LGLs are generally medium to large in size with more cytoplasm and smaller nuclear:cytoplasmic ratio. LGLs display an eccentrically located, rounded, or kidney-shaped nucleus with dispersed chromatin. The most distinguishing characteristic features of LGLs are the membrane-bound osmiophilic granules existing in variable number, shape, and density. The granules contain cytotoxic effectors that contribute to the ability of LGLs to kill other cells. Although displaying all of the morphological features of LGLs, NK cells also possess their own specific ultrastructural features that can be used to identify NK cells within tissue sections. These include well-developed cytoplasmic organelles such as Golgi apparatus, RER and mitochondria, as well as the presence of centrioles, microtubules, multivesicular bodies, rod-cored vesicles, and glycogen. Compared with NK cells from the peripheral blood and spleen, liver-associated NK cells contain a higher number but smaller size of granules with higher cytotoxicity against tumors [2].
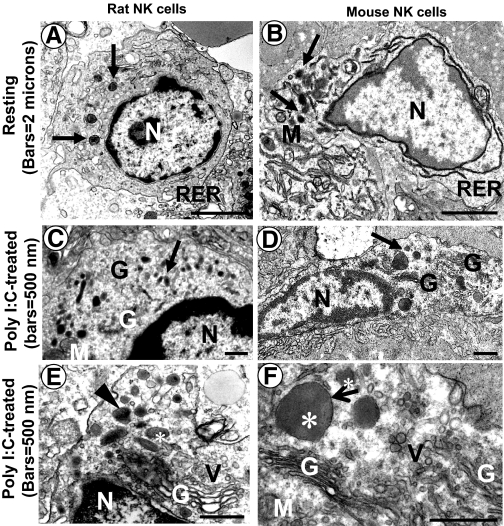
Electron microscopic analyses show liver NK cells from mice and rats are quite similar in their appearance, except that rat NK cells contain a higher number of granules per cell than mouse NK cells (see Fig. 1) [4, 5]. They are both located in the hepatic sinusoids, often adhering to the endothelial cells. Monocytes and Kupffer cells in the liver can be distinguished from NK cells by their nuclear morphology, long microvilli, and cytoplasmic inclusions containing phagocytosed material. Administration with poly I:C enlarged the Golgi apparatus significantly and increased numbers of granules and Golgi-derived vesicles in liver NK cells, reflecting their activation and increase of lytic activity (Fig. 1) [4, 5, 48]. Interestingly, electron microscopic analyses showed that injection of poly I:C increased numbers of liver NK cells in mice but not in rats [4, 5], which was also confirmed by flow cytometric analyses showing that poly I:C injection induced accumulation of NK cells in mouse livers [49,50,51,52,53] but not in rat livers (O. Park and B. Gao, unpublished data). In contrast, poly I:C treatment induced NKT cell accumulation (NKR-P1AmediumCD3+ cells) in rat livers (O. Park and B. Gao, unpublished data).
Figure 1.
Electron microscopic photographs of liver NK cells from normal Sprague-Dawley rats (A), C57BL/6 mice (B), and poly I:C-treated rats (C and E) and mice (D and F). (A and B) NK cells from both species are quite similar in their appearance; they exhibit abundant cytoplasm rich in mitochondria, RER, and Golgi apparatus. Arrows indicate the membrane-bound granules. (C and D) Mice or rats were treated with poly I:C (5 μg/g) for 12 h. poly I:C-activated NK cells display a significantly higher number of the electron-dense granules (arrows), an enlarged Golgi apparatus, and numerous Golgi-derived vesicles. (E and F) Higher magnification of the cytoplasm of poly I:C-activated NK cells. Note the expanded Golgi apparatus, numerous Golgi-derived vesicles, and electron-dense granules (asterisks) surrounded by membrane (F, small arrow). Some granules in rat liver NK cells but not mouse NK cells have heavily electron-opaque inclusions (E, arrowhead). N, Nucleus; G, Golgi apparatus; V, vesicles; M, mitochondria. Original bars, 2 μ (A and B); 500 nm (C–F).
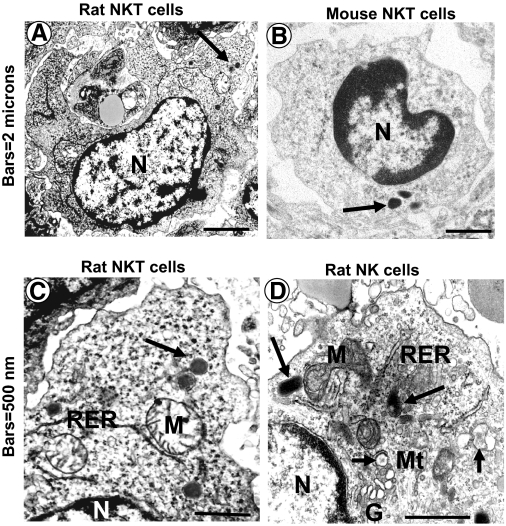
Although the ultrastructure of liver NK cells has been well documented [4, 5], the ultrastructure of liver NKT cells has been unveiled only recently [54,55,56]. By analyzing purified mouse liver NKT cells (TCRint+), investigators found that the ultrastructure of purified liver NKT cells is similar to that of liver NK cells with some differences. Although liver NK and NKT cells contain a low nuclear:cytoplasmic ratio and electron-dense granules surrounded by membrane, NKT cells appear as less-mature cells, displaying only a few organelles, including sparse mitochondria and single, short profiles of the RER (Fig. 2) [54,55,56].
Figure 2.
Ultrastructure of hepatic large granular lymphocytes that appear to consist of two different cell types, NKT (A–C) and NK (D) cells from normal Sprague-Dawley rats and C57BL/6 mice. Both cell types show a high cytoplasmic:nuclear ratio and electron-dense granules (arrows) surrounded by membrane but can be distinguished by their subsets of cytoplasmic organelles, which are found in the cytoplasm of NK cells but not in NKT cells, which (A–C) are large granular lymphocytes showing sparse mitochondria, only a few profiles of the RER, and absence of the Golgi apparatus. NK cells contain well-developed RER and Golgi area, numerous lucent or nearly lucent granules (small arrow), and microtubules. Note that ultrastructural features of the rat NKT cell (A) are similar to the mouse counterpart (B). Mt, Microtubles. Original bars, 2 μ (A and B); 500 nm (C and D).
Similarity and difference between liver and peripheral NK cells
It is well documented that bone marrow-derived blood NK cells migrate into the liver and differentiate further into liver-specific NK cells [57]. The latter shows markedly different immunophenotypical, morphological, and functional characteristics from peripheral and spleen NK cells (Table 3) [2,3,4,5,6]. Compared with peripheral NK cells, liver NK cells display higher cytotoxicity against tumor cells, possess a higher number of red-cored vesicles and granules, and express higher levels of TRAIL, perforin, granzyme B, and others [6, 51, 58, 59]. Emerging evidence suggests that liver NK cells are similar to IL-2-activated spleen NK cells, as both of them express a similar pattern of mRNAs and proteins, express high levels of cytotoxic effectors, and possess high levels of cytotoxicity [6, 51, 58,59,60]. Recent studies have shown that liver NK cells contain a high percentage of NKDC or IKDC [11,12,13, 61], which has been suggested to represent a subset of activated NK cells [16,17,18] and likely contribute to the higher cytotoxicity of liver NK cells reported in earlier publications [6, 51, 58, 59]. At present, the mechanisms attributed to the difference between peripheral and liver NK cells remain largely unknown. It is plausible that peripheral NK cells migrate into the liver and are stimulated by other liver cells such as Kupffer cells [62] and possibly hepatocytes [63], followed by differentiating into NKDC, which represent activated NK cells in the liver. Further studies are needed to clarify the detailed mechanisms underlying this process.
TABLE 3.
Similarity and Difference between Liver and Peripheral NK Cells in Mice
| Liver NK cells | Peripheral and spleen NK cells | Refs. | |
|---|---|---|---|
| Surface marker | NK 1.1+CD3−; DX5+ in mice | NK1.1+CD3−; DX5+ in mice | |
| CD11c | About 30% liver NK cells express CD11c (NKDC) | Less than 5% peripheral and spleen NK cells express CD11c | [11,12,13, 30] |
| TRAIL | High in mouse and rat liver NK cells but not in human liver NK cells | Low in peripheral and spleen NK cells | [51, 58] |
| IL-2 stimulation of TRAIL | IL-2 induction of TRAIL in human liver NK cells | IL-2 does not induce TRAIL expression in human peripheral NK cells | [51, 58] |
| Perforin and granzyme B | High in rat liver NK cells | Low in rat spleen NK cells | [59] |
| Electron micrographs | More rod-cored vesicles | Fewer rod-cored vesicles | [6] |
| More but smaller granules | Fewer but large granules | ||
| Cytotoxicity against tumor | High | Low | [58, 59] |
NK/NKT cell physiologic functions in a normal, healthy liver
NK and NKT cells, along with Kupffer cells, sinusoidal endothelial cells, and stellate cells are the major components of liver sinusoid. The liver receives dual blood supply with 25% from the hepatic artery and 75% from the gut via the portal vein. The latter is enriched in bacterial products, toxins, and food antigens. The blood from the hepatic artery and portal vein mixes in the liver sinusoids, followed by flowing back to the heart through the hepatic vein. The liver not only filters large amounts of blood (∼25% of cardiac output/min), but it also has a unique microvasculature (such as fenestration of sinusoidal endothelial cells) that traps pathogens, waste molecules, and circulating tumor cells. Indeed, the liver is the second most common site for tumor metastasis (after the lymph nodes) from virtually any primary malignant neoplasm. Kupffer cells are mainly responsible for elimination of pathogens and insoluble molecules, and sinusoidal endothelial cells contribute to remove soluble molecules from the circulation [33, 64, 65]. The enrichment and constitutive activation of NK and NKT cells in the sinusoid of a normal, healthy liver [6, 51, 58, 59] likely play a key role in immune surveillance to remove circulating tumor cells from the body. This notion is supported by evidence showing that depletion of NK/NKT cells markedly enhances tumor metastases in the liver, and augmentation of NK/NKT cells attenuates it [66,67,68]. In addition, NK and NKT cells can rapidly produce copious amounts of cytokines after activation, which make them able to respond quickly and subsequently help to remove invading pathogens, toxins, and food antigens from the portal venous blood via modulation of adaptive immune responses. Thus, NK/NKT cells together with Kupffer cells and sinusoidal endothelial cells in liver sinusoid form a strong, innate immune defense system that plays a key role in elimination of pathogens, waste molecules, toxins, and circulating tumor cells from the circulation.
Regulation of hepatic NK and NKT cells in mice
The number of NK and NKT cells changes significantly in various models of liver diseases (Table 4). Mice infected with several viral strains induce significant accumulation of NK cells in the liver. These include lymphocytic choriomeningitis virus, cytopathic hepatotropic viruses, mouse hepatitis virus, and MCMV [69,70,71,72]. The induction of NK cell accumulation by MCMV in the liver is dependent on IFN-α/β and MIP-1α [72]. Administration of double-stranded RNA poly I:C in mice, which mimics viral infection, induces a similar level of accumulation of NK cells in the liver. Such induction is partially dependent on Kupffer cells, IL-12, IFN-γ, STAT1, VCAM-1, and others [49,50,51,52,53]. Similarly, mice treated with IFN-γ resulted in NK cell accumulation in the liver and increased NKG2D and TRAIL expression on liver NK cells, which is also dependent on STAT1 signaling [49, 53].
TABLE 4.
Regulation of Hepatic NK/NKT Cells in Mice
| Factors affect NK/NKT | NK | NKTa | NK and NKT functions | References |
|---|---|---|---|---|
| Viral infection | ↑↑↑ | ↓↓↓ | NK and NKT cells contribute to antiviral effects | [69,70,71,72] |
| poly I:C injection | ↑↑↑ | ↓↓ | NK cells contribute to liver injury | [49,50,51,52,53] |
| IFN-γ injection | ↑↑ | ↓↓ | Induces NKG2D and TRAIL on NK cells | [49, 53] |
| IFN-α injection | ↑↑ | ↑ | Induces NKG2D and TRAIL on NK cells | b |
| IL-12/IL-18 injection | ↑↑↑ | ↓↓ | Increase NK cytotoxicity agonist tumor cells | [73,74,75] |
| ConA injection | ↑ | ↓↓↓ | NKT but not NK cells contribute to liver injury | [76] |
| Injection of α-GalCer | ↑ | ↓↓↓ | NKT but not NK cells contribute to liver injury | [43, 77, 78] |
| Injection of CCl4 | ↑ | ↓↓↓ | NKT cells play diverse roles in liver injury induced by CCl4 | [79, 80] |
| Nicotinamide adenine dinucleotide (NAD) | ↓↓ | Engagement of P2X(7) receptors on NKT cells contributes to autoimmune hepatitis | [41, 81] | |
| High-fat diet feeding | ↓↓↓ | NKT inhibits TNF-α production | [82, 83] | |
| Leptin or leptin receptor-deficient mice | ↓↓ | ↓↓ | NKT inhibits proinflammatory cytokine production | [84,85,86,87,88] |
| Stress | ↑ | ↑↑↑ | NK but not NKT cytotoxicity decreased | [89] |
| Partial hepatectomy | ↑ | ↑↑↑ | Elevated IFN-γ expression on NK/NKT cells | [90,91,92,93,94] |
| Ischemia/reperfusion | ↑ | ↑↑↑ | NKT contributes to liver injury | [95, 96] |
| Liver transplantation | ↓ | ↑↑↑ | Accumulation of NKT/NK cells post-transplantation | [97] |
| Injection of sulfatide | ↑↑↑ | Sulfatide activates type II NKT cells, resulting in recruiting iNKT into the mouse liver. | [98] |
The decline of NKT cells is a result of down-regulation of surface markers or activation-induced cell death.
Barbara Jaruga, Rui Sun, and B. Gao, unpublished data.
IFN-α is the primary choice of treatment for viral hepatitis, which affects half a billion people worldwide. It is generally believed that the antiviral effects of IFN-α in viral hepatitis infection are mediated via the direct inhibition of hepatitis virus replication in hepatocytes through activation of the STAT1 signaling pathway [99,100,101]. The immuoregulatory effects of IFN-α may also contribute to the antiviral effects of IFN-α in viral hepatitis; however, the underlying mechanisms remain obscure. Treating mice with IFN-α induces NK cell accumulation in the liver [72] and induces expression of NKG2D, TRAIL, FasL, granzyme B, and IFN-γ on liver lymphocytes (B. Jaruga, R. Sun, and B. Gao, unpublished data). Such NK cell activation by IFN-α likely contributes to the antiviral effect of IFN-α therapy in viral hepatitis, as NK cells play an important role in antiviral defenses against HCV (see below). In addition, injection of IL-12 and/or IL-18 induced NK cell accumulation in the liver, which contributes to liver injury and inhibition of tumor cell growth [73,74,75].
In contrast to NK cell accumulation in the liver under conditions mentioned above, the percentage or total number of NKT cells decreased. A decline in NKT cells in the liver is also observed in leptin-deficient mice [84] or after treatment with several bacterial species [102], hepatotoxins [79, 80], high-fat diet feeding [82, 83], NAD [81], and NKT activators such as Con A [76] or α-GalCer in mice [43, 77, 78]. On the contrary, many stress-related conditions result in an accumulation of NKT cells in the liver, including constraint stress [89], partial hepatectomy [90,91,92,93], liver ischemia/reperfusion [95, 96], and liver transplantation [97]. In addition, injection of the type II NKT cell activator, sulfatide, also induces accumulation of type I iNKT cells in the liver [98]. These recruited iNKT cells are anergic and protect against Con A-induced liver injury [98].
The mechanisms underlying enrichment and accumulation of NK cells in the liver under varying conditions mentioned above remain largely unknown. Several potential mechanisms have been suggested. First, the liver contains a large number of Kupffer cells, which likely play an important role in enrichment of liver NK cells. This notion is supported by the fact that in vivo depletion of Kupffer cells reduces the number of liver NK cells, and the conditioned medium from Kupffer cells enhances the viability, tumor-cytotoxic activity, and adherence of liver NK cells to sinusoidal endothelial cells [62]. Second, hepatocytes have been shown to promote expansion and differentiation of NK cells from stem cell precursors [63], contributing to NK cell accumulation in the liver. Third, many chemokines and adhesive molecules have been shown to play an important role in NK recruitment and trafficking to the liver. For example, blocking of CD2, CD11a, CD18, and CD54 antigens on blood large granular lymphocytes and/or liver endothelium reduced the number of NK cells in the liver [103]. Treatment of mice with poly I:C enhances expression of a variety of chemokines and adhesive molecules and preferentially induces accumulation of NK cells in the liver. However, similar pattern expression of these molecules was also observed in the lung and spleen after poly I:C treatment (B. Jaruga and B.Gao, unpublished data), suggesting that induction of the chemokines and adhesive molecules may not fully explain poly I:C-mediated preferential induction of NK cell accumulation in the liver.
The decline in liver NKT cells under varying conditions mentioned above may be a result of activation-induced cell death or loss of NKT cell marker expression (such as NK1.1 or CD3) or both [70, 104,105,106]. NKT cell accumulation in the liver after surgery or stress may be caused by sympathetic nerve activation [93]. In addition, the CXCR6 and its ligand CXCL16 have been shown to play an important role in recruitment of liver NKT cells. It was shown that NKT cells express the CXCR6 chemokine receptor, and sinusoidal endothelial cells express the CXCR6 ligand CXCL16 chemokine. Disruption of the CXCR6 results in a selective and severe reduction of CD1d-reactive NKT cells in the liver, providing conclusive evidence for an important role of CXCR6 and CXCL16 in inducing NKT cell enrichment in the liver [107, 108].
NK/NKT CELLS IN LIVER INJURY
NK and NKT cells in the liver play a key role in innate defenses against viral infection and tumor transformation. Recent evidence suggests that activation of NK or NKT cells also contributes to the development and progression of liver injury in a variety of rodent models [38, 109]. For example, NK cells contribute to liver injury induced by poly I:C [51, 52, 110], aeruginosa exotoxin A [111], carrageenan [112], and adenoviruses [113, 114], and iNKT cells participate in liver injury induced by Con A [76], α-GalCer [43], CpG-oligodeoxynucleotide [115], ischemia/reperfusion [95], and alcohol administration [116, 117]. In contrast, iNKT cells may protect against acute liver inflammation and injury induced by CCl4 [80] and bile duct ligation [118]. Interestingly, the solvent DMSO that is used to dissolve drugs was recently shown to activate NK and NKT cells and subsequently enhance acetaminophen-induced hepatotoxicity [119,120,121]. These studies suggest that NK and NKT cells may not contribute to the pathogenesis of drug-induced liver injury, but activation of NK and NKT cells by other factors such as DMSO, poly I:C, or viral infection may accelerate drug-induced hepatotoxicity. Indeed, Dr. Ju’s group [122] reported recently that activation of NK cells after treatment with poly I:C accelerated halothane-induced liver injury significantly. In addition, clinical studies show that patients with HCV infection, which is often associated with NK and NKT cell activation, are at increased risk of acetaminophen-induced liver injury [123]. As the roles of NK and NKT cells in liver injury in rodent models and patients with liver diseases have been reviewed extensively previously [35,36,37,38,39,40,41, 109], we will summarize the potential mechanisms underlying NK or NKT-mediated liver injury here.
Several mechanisms have been implicated in NK cell-mediated liver injury. First, NK cells can be activated by poly I:C or cytokines. Injection of poly I:C induces NK cell accumulation and activation in the liver. Activated NK cells kill hepatocytes by releasing TRAIL [51, 124, 125] and/or granzyme B [114, 126], leading to liver injury. Second, NK cells can also be activated by target cells that express up-regulated NK cell-activating ligands or decreased NK cell-inhibitory ligands, thereby inducing hepatocyte death. For example, it has been shown that NK cells are activated by RAE-1, an NK cell-activating ligand up-regulated on hepatocytes from HBV transgenic mice [127] and from bile duct-ligated mice [124] and on Kupffer cells from poly I:C/D-galactosmine-treated mice [110]. Consequently, these activated NK cells kill hepatocytes and induce hepatocellular damage. In addition, blocking NK cell-inhibitory ligand MHC class I molecules on rat hepatocytes with the OX18 antibody markedly enhanced the sensitivity of hepatocytes to NK cell-mediated killing [128]. Lastly, activated NK cells are able to produce a variety of proinflammatory cytokines such as IFN-γ, which can contribute to the pathogenesis of liver injury [90, 129, 130].
NKT cells can be activated by at least three different pathways. First, several cytokines have been shown to activate hepatic NKT cells, which subsequently enhances liver injury. For example, IL-12 treatment induces accumulation of NKT cells in the liver and enhances liver injury after partial hepatectomy [92]. Liver injury mediated by IL-12 after partial hepatectomy was not observed in iNKT-deficient mice, suggesting that IL-12 activation of NKT contributes to liver injury in this model [92]. Second, it is well established that activation of NKT cells by Con A or lipid antigen α-GalCer is able to induce liver injury [43, 76, 77]. Third, NKT cells that express the NK cell stimulatory receptor NKG2D can be activated by target cells that express elevated NKG2D ligands and consequently induce acute liver injury in HBV transgenic mice [131]. In contrast, activation of NKG2A, an inhibitory receptor, negatively regulates iNKT cell activation and subsequently ameliorates liver injury [132]. In addition, IL-6 and IL-15 can inhibit NKT cell function and subsequently prevent Con A-induced NKT-mediated hepatitis [133, 134].
The most important mechanism underlying NKT cell-mediated liver injury is likely the production of a variety of proinflammatory cytokines, such as IFN-γ, IL-4, IL-5, IL-13, and TNF-α [21, 43, 135,136,137]. Another important mechanism responsible for NKT cell-mediated hepatotoxicity is via NKT cell killing of hepatocytes [43, 76, 133, 135]. Unlike NK cells that kill target cells by releasing TRAIL and granzyme B, NKT cells kill hepatocytes by releasing FasL [76]. In contrast to the strong activation of iNKT cells by cytokines and ligands that induce liver injury, natural and weak activation of iNKT cells may protect against acute liver inflammation and injury induced by CCl4 [80] and bile duct ligation [118]. The protective effect of iNKT cells in CCl4 is partially a result of inhibition of stellate cell activation [80], and the mechanism by which iNKT cells protect against liver injury induced by bile duct ligation is unknown [118].
A single injection of Con A or α-GalCer induces NKT cell-mediated acute liver injury in mice [43, 76]; however, such treatment induces NKT cell tolerance and renders the mice resistant to subsequent NKT cell-mediated liver injury [77, 78, 138]. Chronic treatment with CCl4 results in hepatic NKT cell depletion and induces comparable liver inflammation and injury in wild-type and Jα18 KO mice [80], suggesting that NKT cells may play a limited role in chronic liver inflammation and injury as a result of hepatic NKT cell depletion and tolerance.
NK/NKT CELLS IN LIVER FIBROSIS
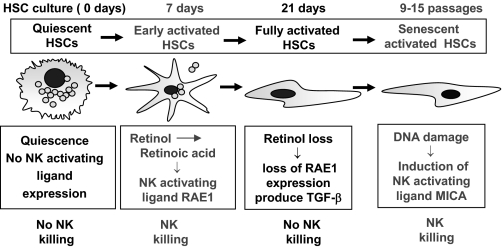
Regardless of etiology, any form of chronic liver injury can lead to liver fibrosis that is associated with accumulation of collagen in the liver [139,140,141]. Emerging evidence suggests that hepatic stellate cells (also known as fat-storing cells or Ito cells) play a key role in liver fibrogenesis. Hepatic stellate cells are quiescent in normal, healthy livers and contain 70% of the body’s vitamin A stores. During liver injury or the in vitro culturing process on plastic dishes, hepatic stellate cells become activated and differentiate into myofibroblast cells that are characterized by a loss of vitamin A and enhanced collagen expression. Activated stellate cells can senesce further during chronic liver injury or after 9–15 culturing passages in vitro [142]. Activation of stellate cells is controlled by a wide variety of cytokines, growth factors, and reactive oxygen species. Among them, TGF-β and platelet-derived growth factor are the two most important cytokines that stimulate stellate cell transformation and proliferation, respectively, and IFN-γ is one of the more important cytokines to block stellate cell activation [139,140,141]. In addition, immune cells can play an important role in regulating liver fibrosis. Notably, CD8 T cells promote liver fibrosis [143], NK cells inhibit it [49, 144,145,146,147], and NKT cells play a diverse role in regulating liver fibrosis [80]. Here, we summarize recent findings about the role of NK/NKT cells in liver fibrosis.
Recent findings from several laboratories, including our own, clearly suggest that NK cells inhibit liver fibrosis by killing activated stellate cells directly [49, 144,145,146]. These studies suggest that NK cells selectively kill early-activated or senescent-activated hepatic stellate cells, and quiescent or fully-activated stellate cells are resistant to such killing (Fig. 3). This is because early-activated and senescent-activated stellate cells express increased levels of NK cell-activating ligands (RAE-1 in mice; MICA in human), and quiescent and fully-activated stellate cells do not [49, 145]. Induction of NK cell-activating ligands in early-activated and senescent-activated stellate cells is likely a result of retinoic acid production [148] and DNA damage in these cells [145, 149], respectively. Moreover, down-regulation of the inhibitory NK cell ligand [144] and up-regulation of TRAIL receptors on activated stellate cells [150, 151] also contribute to the susceptibility of these cells to NK cell killing [49]. In addition, production of IFN-γ, a hallmark of NK cell activation, is another important mechanism contributing to the antifibrotic effects of NK cells. IFN-γ not only inhibits stellate cell activation directly [53, 152, 153] but also amplifies NK cell cytotoxicity against stellate cells via up-regulation of NKG2D and TRAIL expression on NK cells [53, 154]. Finally, NK cell activity negatively correlates with grade of liver fibrosis in patients with HCV infection, suggesting that NK cells may inhibit liver fibrosis in patients [155]. Thus, activation of NK cells could be a novel, therapeutic target to treat liver fibrosis [156].
Figure 3.
Interaction between NK cells and HSCs. NK cells kill early-activated and senescent-activated HSCs but not quiescent and fully-activated HSCs. The top panel shows the cultured HSCs. In early-activated HSCs, retinol is metabolized into retinoic acid, which then induces expression of RAE-1 (NK cell-activating ligand) in mice. Up-regulated RAE-1 activates NK cell killing of early-activated HSCs. Fully-activated HSCs lose the retinoic acid, do not express RAE-1, and are resistant to NK cell killing. In senescent-activated human HSCs, DNA damage induces expression of MICA (NK cell-activating ligand). MICA then activates NK cells, which then kill senescent-activated HSCs.
Similar to NK cells, iNKT cells can also inhibit hepatic stellate cell activation by killing activated hepatic stellate cells directly and producing IFN-γ [80]. However, in iNKT-deficient mice, chronic challenge with CCl4 induced liver fibrosis that was only a slightly higher grade than from wild-type mice at 2 weeks but not 4 weeks postchallenge, suggesting that iNKT cells may play a role in inhibiting liver fibrosis in the earlier stages but not later stages of liver fibrosis [80]. This may be a result of hepatic iNKT cell depletion during chronic liver injury. A single injection of α-GalCer inhibits bleomycine-induced lung fibrosis [157] and does not inhibit but rather enhances CCl4-induced acute liver fibrosis, although such injection elevates serum IFN-γ levels and enhances iNKT cell killing of activated HSCs [80]. This may be because a single dose of α-GalCer injection accelerates CCl4-induced liver injury significantly, which enhances liver fibrosis and dominates the inhibitory effect of α-GalCer on liver fibrosis, leading to stimulatory effects by a single α-GalCer injection on liver fibrosis induced by acute CCl4 treatment [80]. In contrast, chronic α-GalCer treatment has little effect on CCl4-induced chronic liver injury and fibrosis [80], which may be a result of hepatic iNKT cell depletion during chronic liver injury and long-term iNKT cell anergy and tolerance after α-GalCer stimulation [77, 78]. The iNKT cells also produce profibrotic cytokines such as IL-4 and IL-13, which was elevated in patients with chronic HBV infection [158], suggesting that iNKT cells may contribute to the progression of liver fibrosis in these patients.
NK/NKT CELLS IN LIVER REGENERATION
The liver has great ability to regenerate after tissue loss or injury, which is controlled by a wide variety of cytokines, growth factors, and hormones [159,160,161]. The most widely used model to study liver regeneration is two-thirds partial hepatectomy, in which the remnant hepatocytes can quickly proliferate, regenerate, and return to its original mass within 10–14 days in rodents. Recent evidence suggests that NK and NKT cells, which accumulate in the remnant liver after partial hepatectomy, regulate liver regeneration. Depletion of NK cells by anti-anti-asialo GM1 antibody or inhibition of NK cells by immunosuppressive drugs enhances liver regeneration [90, 162, 163], and activation of NK cells by poly I:C or viral infection inhibits liver regeneration [90]. Recently, we have demonstrated that NK cells are activated after allogeneic transplantation, which likely contributes to liver injury and inhibits hepatocyte proliferation [164]. The inhibitory effect of NK cells on liver regeneration is likely a result of NK cell production of IFN-γ, which subsequently induces hepatocyte cell-cycle arrest and apoptosis [90, 129]. NK cells may also kill regenerating hepatocytes [165].
Although NKT cells accumulate in the liver after partial hepatectomy, liver regeneration is comparable between NKT-deficient mice (CD1d KO and β2 microglobulin KO) and wild-type controls [90], suggesting that NKT cells may play a minor role in liver regeneration under normal conditions in the partial hepatectomy model. However, in HBV transgenic mice, NKT cells are activated, and depletion of NKT cells significantly enhances liver regeneration induced by partial hepatectomy [94]. In addition, activation of NKT cells by IL-12 or α-GalCer enhances liver injury during liver regeneration after partial hepatectomy [92]. Surprisingly, the effects of NKT cells on liver regeneration were not investigated in this study [92]. Thus, activation of NKT cells likely contributes to liver injury and inhibits liver regeneration via production of IFN-γ. In contrast, Nakashima et al. [166] reported recently that mice treated with α-GalCer 36 h after partial hepatectomy enhanced hepatocyte mitosis 44 h after partial hepatectomy. It is not clear whether injection of α-GalCer on or near the early time-points of partial hepatectomy inhibits or accelerates liver regeneration.
When hepatocyte proliferation is inhibited, hepatic progenitor cells, also known as oval cells, can proliferate and differentiate into mature hepatoctyes and biliary epithelial cells [159,160,161]. Using the Con A plus partial hepatectomy model, Hines et al. [167] reported that NK cells inhibit oval cell proliferation. In contrast, using the choline-deficient diet-feeding model, Strick-Marchand et al. [168] reported that NK cells may stimulate oval cell expansion via production of proinflammatory cytokines (IFN-γ and TNF-α). Further studies are needed to clarify the role of NK and NKT cells in the proliferation of hepatic progenitor cells during liver regeneration.
NK/NKT CELLS IN CHRONIC LIVER DISEASES
Hepatitis viral infection, alcohol drinking, and NAFLD are the three major causes of chronic liver disease worldwide. The mechanisms underlying the pathogenesis of these liver disorders are not fully understood. However, increasing evidence suggests that NK and NKT cells play an important role in the pathogenesis of viral hepatitis, nonalcoholic steatohepatitis, and alcoholic liver disease, which will be discussed below. We will also discuss briefly the potential roles of NK and NKT cells in autoimmune immune liver disease and liver cancer.
HCV and HBV infection
The important function of NK cells in antiviral defenses against a range of viral infections has been well documented in animal models and in humans with NK cell defects [169]. However, the role of NK cells in HCV infection is less clear as a result of a lack of suitable small-animal models for HCV infection. Many lines of evidence from in vitro cell culture experiments and clinical studies suggest that NK cells play an important role in controlling HCV infection, especially in the early stages of infection. By coculturing HCV-infected hepatocytes with NK cells, several groups have demonstrated that NK cells can inhibit HCV infection and replication in hepatocytes [170,171,172]. Although there are no reports showing that individuals with NK cell defects are more susceptible to HCV infection, many clinical studies have revealed that NK cell functions correlate positively with spontaneous recovery from acute HCV infection [173] or effective IFN-α therapy in patients with chronic HCV infection [174,175,176].
It is generally believed that NK cells are activated during the early stages of HCV infection by type I IFNs and DCs [177, 178]. The activated NK cells can inhibit HCV infection by killing HCV-infected hepatocytes directly [172], secreting cytokines such as IFN-γ that inhibit HCV replication [171, 179], and stimulating adaptive immune responses [35, 36]. Thus, NK cell activation likely plays a critical role in controlling HCV infection in the early stages. However, emerging evidence suggests that HCV can evade NK cell surveillance via multiple mechanisms, which is likely one reason resulting in persistent infection in the majority of patients [36]. First, the HCV-truncated HCV E2 protein inhibits NK cell function by binding to CD81 on NK cells [180, 181], but this finding has been challenged by a recent study showing that exposure to infectious HCV virions did not affect NK cell function [182]. Second, HCV infection dysregulates the expression of NK cell inhibitory and stimulatory ligands on hepatocytes, resulting in resistance of HCV-infected hepatocytes to NK cell killing [183, 184]. Third, chronic HCV infection alters expression of NK cell receptors on NK cells and subsequently reduces NK cytolytic activity [185] and the ability of NK cells to produce cytokines and activate DCs [186]. Lastly, numerous studies have reported that NK cells are depleted significantly in patients with chronic HCV infection [155, 176, 187, 188].
Many markers have been used to identify human NKT cells including CD56+CD3+(also called NT cells) CD161+, and Vα24TCR+ cells. Similar to NK cells, NKT cells can also secrete IFN-γ to inhibit HCV replication in hepatocytes [189], and their activity correlates positively with the outcome of acute HCV infection [190] and the efficacy of IFN-α treatment in chronic HCV infection [176]. This suggests that NKT cells probably play a role in controlling HCV infection, particularly in the early stages of infection. Numerous studies have reported that NKT cells are depleted significantly during chronic HCV infection [176, 190,191,192], which likely contributes to a failure to resolve HCV infection. A recent clinical trial reported that treatment with iNKT activator α-GalCer was safe and showed immunomodulatory effects in IFN-α-refractory HCV patients but had no significant effect on HCV-RNA levels [47]. Thus, further studies are required to clarify the therapeutic application of iNKT activators in HCV infection.
Elevation and activation of NK and NKT cells have been observed in the early stages of HBV infection in humans [193, 194], suggesting that NK and NKT cells play a role in controlling the early stages of HBV infection in humans. Interestingly, activation of NK and NKT cells has also been observed in the early stages of HBV infection in woodchucks [195], and induction of innate immune activation-associated antiviral gene expression was not observed in the early stages of HBV infection in chimpanzees [196]. A lack of an early innate immune response may be an unusual feature of HBV infection in chimpanzees. The inhibitory effect of NK and NKT cells on HBV infection is likely mediated via secretion of IFN-γ that inhibits HBV replication and stimulation of adaptive immune responses [37, 197,198,199].
In contrast to the containment of HBV and HCV replication by NK and NKT cells, these mediators may also contribute to liver injury during chronic hepatitis viral infection via several mechanisms mentioned above. These include directly lysing hepatocytes, producing cytokines that recruit inflammatory cells, inducing hepatocyte apoptosis, and inhibiting hepatocyte proliferation [94, 125, 127, 131, 200, 201].
NAFLD
NAFLD affects 10–20% of the population in developed countries and is increasing in prevalence with the rise of diabetes and obesity [202, 203]. The molecular mechanisms underlying the pathogenesis of NAFLD remain largely unknown. Recent studies conducted primarily by the laboratories of Drs. Diehl and Li and co-workers [84,85,86,87] suggest that NKT cells may have a protective effect in animal models of NAFLD. Leptin-deficiency, high-fat diet consumption, and feeding on a sucrose diet cause NAFLD associated with reduction of hepatic NKT cells. This reduction may be a result of reduced hepatic CD1d expression and increased NKT apoptosis caused by reduced production of norepinephrine and IL-15. Depletion of NKT cells promotes proinflammatory polarization of hepatic cytokine production that sensitizes the liver to LPS toxicity [84], and elevation of hepatic NKT cells by probiotic treatment or adoptive transfer improved NAFLD [82, 204]. Findings from clinical studies of NKT cells in patients with NAFLD have been controversial. Xu et al. [205] reported that peripheral NKT cells are depleted in patients with NAFLD and correlated negatively with disease severity, and Tajiri et al. [206] reported that hepatic NKT cells are increased in NAFLD and may promote liver injury. In contrast to NKT cells, the role of NK cells in the pathogenesis in NAFLD has not been explored. It has been reported that the function of NK cells is suppressed in leptin receptor-deficient obese mice [88]; however, whether NK cells contribute to the pathogenesis of NAFLD remains unknown.
Alcoholic liver disease
The spectrum of alcoholic liver disease includes fatty liver, hepatitis, fibrosis, cirrhosis, and HCC. Alcoholic hepatitis is characterized by hepatic steatosis and infiltration of inflammatory cells (predominant neutrophils). There is no evidence suggesting that NK cells are involved in alcoholic liver injury. In contrast, NKT cells appear to contribute to such injury because NKT deficiency delays while activation of NKT cells accelerates alcoholic liver injury [116, 117]. In addition, it has been well documented that NK functions are suppressed in alcoholic cirrhosis patients [207], and ethanol feeding inhibits NK cell functions in mice [208, 209]. Recently, we have demonstrated that chronic ethanol feeding diminished the inhibitory effect of NK/IFN-γ in liver fibrosis, which may be an important mechanism contributing to alcohol acceleration of liver fibrosis in patients with chronic HCV infection [154, 210].
Autoimmune liver diseases
NKT cells have been implicated in the pathogenesis of diverse autoimmune diseases including autoimmune liver disease [211]. It has been reported that hepatic CD1 expression and NKT cell number were elevated in patients with PBC [45, 212], a disease caused by immune-mediated destruction of bile ducts. An important role of NKT cells in the initiation of PBC was demonstrated recently in two murine models showing that NKT deficiency attenuated the development of PBC induced by overexpression of a dominant-negative TGF-βR in T cells or by infection with Novosphingobium aromaticivoran [213, 214]. Furthermore, the detrimental role of NKT cells in Con A-induced T cell hepatitis has been well documented [76]. Strictly speaking, Con A-induced hepatitis does not represent a model of autoimmune hepatitis; however, this model shows many features of autoimmune hepatitis [215]. Thus, NKT cells also likely contribute to the pathogenesis of human autoimmune hepatitis. In contrast, little is known about the role of NK cells in the pathogenesis of autoimmune liver disease, although NK cells have been implicated in other autoimmune diseases [216]. It has been reported that the cytotoxic activity of NK cells is increased, and the production of cytokines decreased in patients with PBC, suggesting that NK cells may have a role in the immunopathogenesis of PBC [217].
HCC
Many studies have reported that NK cell function is impaired in patients with liver cirrhosis [188, 207, 218], a major risk factor for developing HCC. The frequency and function of liver and peripheral NK cells have also been reported to be decreased in HCC patients [219, 220]. As the antitumor effect of NK cells has been well documented in a variety of tumor models, including metastatic liver tumors [73, 221], it is generally believed that reduction of NK cells in cirrhotic liver is associated with the progression of HCC.
Emerging evidence from animal models suggests that distinct NKT cell subsets may play opposing roles in controlling liver tumor. It is generally believed that iNKT cells can inhibit liver tumor growth via production of IFN-γ and activation of NK cells [68, 222, 223]. However, there is also evidence suggesting that CD4+NKT cells may promote liver tumor growth via production of Th2 cytokine and subsequent inhibition of tumor antigen-specific CD8+ T cell expansion [224, 225]. More detailed studies are needed to clarify the role of NKT cell subsets in HCC.
CONCLUSIONS
Liver lymphocytes are abundant in NK cells, which play beneficial roles in inhibiting viral infection, tumor cell growth, and liver fibrosis but can also play detrimental roles in stimulating liver injury and attenuating liver regeneration (hepatocyte proliferation). These effects in the liver are likely mediated by the direct killing of target cells and production of IFN-γ by NK cells. Activated NK cells can also produce many other cytokines; however, the role of these cytokines on NK cell function in the liver has not been explored. For example, a subset of IL-22-producing NK cells (NK-22) was identified recently [226, 227]. As IL-22 has been shown to protect against liver injury and promote liver regeneration [228,229,230,231], it would be interesting to identify whether IL-22-producing NK-22 cells play a beneficial role in ameliorating liver injury and regeneration via producing IL-22 in contrast to other subsets of NK cells that promote liver injury and inhibit regeneration via producing IFN-γ. Although it is clear that NK cell activation inhibits hepatocyte proliferation by producing IFN-γ, the effects of NK/IFN-γ on liver progenitor cell (oval cell) proliferation remain obscure and should be investigated further.
NKT cells are a heterogeneous population, which are enriched in liver lymphocytes and play a diverse role in acute liver injury, fibrosis, and regeneration. Different subsets of NKT cells or different degrees of NKT activation may play opposing roles in acute liver injury. For example, injection of a type I iNKT activator, α-GalCer, induces liver injury [43], and injection of a type II NKT activator, sulfatide, prevents T cell-mediated liver injury [98]. Furthermore, strong activation of type I iNKT cells by α-GalCer enhances, and natural and weak activation of type I iNKT cells prevents CCl4-induced inflammation and injury [80]. The role of NKT cells in chronic liver injury and fibrosis may be limited because of hepatic NKT cell depletion and tolerance [80]. The findings about the role of NKT cells in liver regeneration have been controversial [94, 166]. It is plausible that different states of NKT cell activation may play opposing roles in liver regeneration (hepatocyte proliferation). The complex roles of NKT cells in liver disease may be because there are several subtypes of NKT cells that can produce a wide array of cytokines, including Th1 (IFN-γ) cytokines, Th2 (IL-4, IL-13) cytokines, and Th17 (IL-17 and IL-22) cytokines.
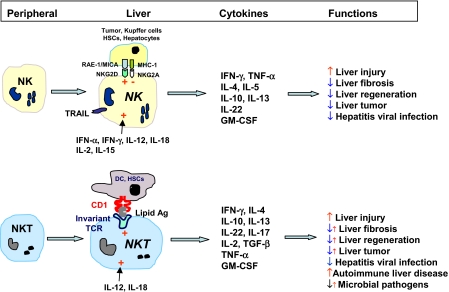
In summary, hepatic NK and NKT cells have many similar functions after activation, such as producing large amounts of cytokines and killing viral-infected cells and tumor cells, and play many similar roles in liver injury, fibrosis, and regeneration (Fig. 4). Although the roles of liver NK and NKT cells have been studied extensively in animal models, many aspects of their functions in human liver disease remain to be unveiled (Fig. 4). Further characterization of the functions of NK/NKT cells in human liver disease may help us design better strategies to treat patients with this disease.
Figure 4.
Similar and differential functions of NK and NKT cells in liver disease. Peripheral NK cells enter the liver and differentiate into liver-specific NK cells, which express TRAIL and have enhanced cytotoxicity. NK cells in the liver can be activated by a variety of cytokine or by NKG2D ligands that are expressed on tumor cells, activated Kupffer cells and stellate cells, and damaged hepatocytes. Immature NKT cells enter the liver and differentiate into liver NKT cells, which can be activated by several cytokines or lipid antigens presented by CD1d on DCs and hepatic stellate cells. Activated NK and NKT cells produce copious amounts of cytokines, playing important roles in antiviral and antitumor defenses in liver injury, fibrosis, and repair. The role of NKT cells in autoimmune liver disease has been well documented, and the role of NK cells remains in this disease to be uncovered. +, Activation; −, inhibition; ↑, promotion; ↓, inhibition; ↓↑, dual role as a result of different subsets or different degree activation of NKT cells.
ACKNOWLEDGMENTS
The intramural program of National Institute on Alcohol Abuse and Alcoholism/National Institutes of Health (NIAAA/NIH) supported work from B.G.’s lab reviewed here. The authors thank the previous and current members in B. G.’s lab at NIAAA/NIH who contributed to the work summarized here and also thank Dr. S. Tao Cheng and the Electron Microscopy Facility staff at the National Institute of Neurological Disorders and Stroke (NINDS) for providing their facility and use of their electron microscopy.
Footnotes
Abbreviations: α-GalCer=α-galactosylceramide, ALT=alanine aminotransaminase, DC=dendritic cell, FasL=Fas ligand, HCC=hepatocellular carcinoma, HCV=hepatitis C virus, HSC=hepatic stellate cell, IKDC=IFN-γ-producing killer cells, iNKT=invariant NKT cells, KO=knockout, LGL=large granular lymphocyte, MCMV=murine cytomegalovirus, MICA=MHC class I chain-related gene A, NAFLD=nonalcoholic fatty liver disease, NT cell=natural T cell, PBC=primary biliary cirrhosis, poly I:C=polyinosinic:polycytidylic acid, RAE-1=retinoic acid early-inducible gene 1, RER=rough endoplasmic reticulum
References
- Wisse E, van't Noordende J M, van der Meulen J, Daems W T. The pit cell: description of a new type of cell occurring in rat liver sinusoids and peripheral blood. Cell Tissue Res. 1976;173:423–435. doi: 10.1007/BF00224305. [DOI] [PubMed] [Google Scholar]
- Wisse E, Luo D, Vermijlen D, Kanellopoulou C, De Zanger R, Braet F. On the function of pit cells, the liver-specific natural killer cells. Semin Liver Dis. 1997;17:265–286. doi: 10.1055/s-2007-1007204. [DOI] [PubMed] [Google Scholar]
- Bouwens L, Wisse E. Pit cells in the liver. Liver. 1992;12:3–9. doi: 10.1111/j.1600-0676.1992.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Bouwens L, Remels L, Baekeland M, Van Bossuyt H, Wisse E. Large granular lymphocytes or “pit cells” from rat liver: isolation, ultrastructural characterization and natural killer activity. Eur J Immunol. 1987;17:37–42. doi: 10.1002/eji.1830170107. [DOI] [PubMed] [Google Scholar]
- Bouwens L. Isolation and characteristics of hepatic NK cells. Bouwens L, editor. Austin, TX, USA: R. G. Landes Co.; NK Cells in the Liver. 1995 [Google Scholar]
- Vanderkerken K, Bouwens L, Wisse E. Characterization of a phenotypically and functionally distinct subset of large granular lymphocytes (pit cells) in rat liver sinusoids. Hepatology. 1990;12:70–75. doi: 10.1002/hep.1840120112. [DOI] [PubMed] [Google Scholar]
- Koo G C, Dumont F J, Tutt M, Hackett J, Jr, Kumar V. The NK-1.1(−) mouse: a model to study differentiation of murine NK cells. J Immunol. 1986;137:3742–3747. [PubMed] [Google Scholar]
- Caligiuri M A. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann D, Jahreis A, Wolfe T, Hughes A, Coon B, van Stipdonk M J, Prilliman K R, Schoenberger S P, von Herrath M G. CD40L blockade prevents autoimmune diabetes by induction of bitypic NK/DC regulatory cells. Immunity. 2002;16:403–415. doi: 10.1016/s1074-7613(02)00290-x. [DOI] [PubMed] [Google Scholar]
- Pillarisetty V G, Katz S C, Bleier J I, Shah A B, Dematteo R P. Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-γ via autocrine IL-12. J Immunol. 2005;174:2612–2618. doi: 10.4049/jimmunol.174.5.2612. [DOI] [PubMed] [Google Scholar]
- Plitas G, Chaudhry U I, Kingham T P, Raab J R, DeMatteo R P. NK dendritic cells are innate immune responders to Listeria monocytogenes infection. J Immunol. 2007;178:4411–4416. doi: 10.4049/jimmunol.178.7.4411. [DOI] [PubMed] [Google Scholar]
- Burt B M, Plitas G, Stableford J A, Nguyen H M, Bamboat Z M, Pillarisetty V G, DeMatteo R P. CD11c identifies a subset of murine liver natural killer cells that responds to adenoviral hepatitis. J Leukoc Biol. 2008;84:1039–1046. doi: 10.1189/jlb.0408256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C W, Crafton E, Fan H N, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky T W, Stins M F, Lanier L L, Pardoll D M, Housseau F. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- Taieb J, Chaput N, Menard C, Apetoh L, Ullrich E, Bonmort M, Pequignot M, Casares N, Terme M, Flament C, Opolon P, Lecluse Y, Metivier D, Tomasello E, Vivier E, Ghiringhelli F, Martin F, Klatzmann D, Poynard T, Tursz T, Raposo G, Yagita H, Ryffel B, Kroemer G, Zitvogel L. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- Caminschi I, Ahmet F, Heger K, Brady J, Nutt S L, Vremec D, Pietersz S, Lahoud M H, Schofield L, Hansen D S, O'Keeffe M, Smyth M J, Bedoui S, Davey G M, Villadangos J A, Heath W R, Shortman K. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J Exp Med. 2007;204:2579–2590. doi: 10.1084/jem.20071351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich C A, Lesjean-Pottier S, Hasan M, Richard-Le Goff O, Corcuff E, Mandelboim O, Di Santo J P. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J Exp Med. 2007;204:2569–2578. doi: 10.1084/jem.20071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius A L, Barchet W, Cella M, Colonna M. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J Exp Med. 2007;204:2561–2568. doi: 10.1084/jem.20070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J Exp Med. 2005;202:1623–1626. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Savage P B, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner M B. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Doherty D G, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Lanier L L. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- Lanier L L. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lanier L L. Natural killer cell receptor signaling. Curr Opin Immunol. 2003;15:308–314. doi: 10.1016/s0952-7915(03)00039-6. [DOI] [PubMed] [Google Scholar]
- Vivier E, Nunes J A, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- Ortaldo J R, Young H A. Mouse Ly49 NK receptors: balancing activation and inhibition. Mol Immunol. 2005;42:445–450. doi: 10.1016/j.molimm.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Lian Z X, Okada T, He X S, Kita H, Liu Y J, Ansari A A, Kikuchi K, Ikehara S, Gershwin M E. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J Immunol. 2003;170:2323–2330. doi: 10.4049/jimmunol.170.5.2323. [DOI] [PubMed] [Google Scholar]
- Exley M A, Koziel M J. To be or not to be NKT: natural killer T cells in the liver. Hepatology. 2004;40:1033–1040. doi: 10.1002/hep.20433. [DOI] [PubMed] [Google Scholar]
- Kenna T, Mason L G, Porcelli S A, Koezuka Y, Hegarty J E, O'Farrelly C, Doherty D G. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J Immunol. 2003;171:1775–1779. doi: 10.4049/jimmunol.171.4.1775. [DOI] [PubMed] [Google Scholar]
- Gao B, Jeong W I, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Alvarez F. Role of NK and NKT cells in the immunopathogenesis of HCV-induced hepatitis. J Leukoc Biol. 2004;76:743–759. doi: 10.1189/jlb.0304197. [DOI] [PubMed] [Google Scholar]
- Golden-Mason L, Rosen H R. Natural killer cells: primary target for hepatitis C virus immune evasion strategies? Liver Transpl. 2006;12:363–372. doi: 10.1002/lt.20708. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wei H, Gao B, Hu Z, Zheng S, Tian Z. Activation and function of hepatic NK cells in hepatitis B infection: an underinvestigated innate immune response. J Viral Hepat. 2005;12:38–45. doi: 10.1111/j.1365-2893.2005.00543.x. [DOI] [PubMed] [Google Scholar]
- Notas G, Kisseleva T, Brenner D. NK and NKT cells in liver injury and fibrosis. Clin Immunol. 2009;130:16–26. doi: 10.1016/j.clim.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Swain M G. Hepatic NKT cells: friend or foe? Clin Sci (Lond) 2008;114:457–466. doi: 10.1042/CS20070328. [DOI] [PubMed] [Google Scholar]
- Ajuebor M N. Role of NKT cells in the digestive system. I. Invariant NKT cells and liver diseases: is there strength in numbers? Am J Physiol Gastrointest Liver Physiol. 2007;293:G651–G656. doi: 10.1152/ajpgi.00298.2007. [DOI] [PubMed] [Google Scholar]
- Dennert G, Aswad F. The role of NKT cells in animal models of autoimmune hepatitis. Crit Rev Immunol. 2006;26:453–473. doi: 10.1615/critrevimmunol.v26.i5.50. [DOI] [PubMed] [Google Scholar]
- Yamagiwa S, Kamimura H, Ichida T. Natural killer cell receptors and their ligands in liver diseases. Med Mol Morphol. 2009;42:1–8. doi: 10.1007/s00795-008-0434-7. [DOI] [PubMed] [Google Scholar]
- Biburger M, Tiegs G. α-Galactosylceramide-induced liver injury in mice is mediated by TNF-α but independent of Kupffer cells. J Immunol. 2005;175:1540–1550. doi: 10.4049/jimmunol.175.3.1540. [DOI] [PubMed] [Google Scholar]
- Osman Y, Kawamura T, Naito T, Takeda K, Van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of α-galactosylceramide. Eur J Immunol. 2000;30:1919–1928. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kita H, Naidenko O V, Kronenberg M, Ansari A A, Rogers P, He X S, Koning F, Mikayama T, Van De Water J, Coppel R L, Kaplan M, Gershwin M E. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, Shimizu N, Horiguchi S, Okamoto Y, Fujii S, Taniguchi M, Fujisawa T, Nakayama T. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12:6079–6086. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- Veldt B J, van der Vliet H J, von Blomberg B M, van Vlierberghe H, Gerken G, Nishi N, Hayashi K, Scheper R J, de Knegt R J, van den Eertwegh A J, Janssen H L, van Nieuwkerk C M. Randomized placebo controlled phase I/II trial of α-galactosylceramide for the treatment of chronic hepatitis C. J Hepatol. 2007;47:356–365. doi: 10.1016/j.jhep.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Carpen O, Virtanen I, Saksela E. The cytotoxic activity of human natural killer cells requires an intact secretory apparatus. Cell Immunol. 1981;58:97–106. doi: 10.1016/0008-8749(81)90152-0. [DOI] [PubMed] [Google Scholar]
- Radaeva S, Sun R, Jaruga B, Nguyen V T, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun R, Wei H, Dong Z, Gao B, Tian Z. Poly I:C prevents T cell-mediated hepatitis via an NK-dependent mechanism. J Hepatol. 2006;44:446–454. doi: 10.1016/j.jhep.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Ochi M, Ohdan H, Mitsuta H, Onoe T, Tokita D, Hara H, Ishiyama K, Zhou W, Tanaka Y, Asahara T. Liver NK cells expressing TRAIL are toxic against self hepatocytes in mice. Hepatology. 2004;39:1321–1331. doi: 10.1002/hep.20204. [DOI] [PubMed] [Google Scholar]
- Dong Z, Wei H, Sun R, Hu Z, Gao B, Tian Z. Involvement of natural killer cells in poly I:C-induced liver injury. J Hepatol. 2004;41:966–973. doi: 10.1016/j.jhep.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Jeong W I, Park O, Radaeva S, Gao B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology. 2006;44:1441–1451. doi: 10.1002/hep.21419. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Kaneda K, Seki S, Nakajima Y. Pit cells as liver-associated natural killer cells: morphology and function. Med Electron Microsc. 2004;37:29–36. doi: 10.1007/s00795-003-0229-9. [DOI] [PubMed] [Google Scholar]
- Daibata M, Matsuo Y, Machida H, Taguchi T, Ohtsuki Y, Taguchi H. Differential gene-expression profiling in the leukemia cell lines derived from indolent and aggressive phases of CD56+ T-cell large granular lymphocyte leukemia. Int J Cancer. 2004;108:845–851. doi: 10.1002/ijc.11647. [DOI] [PubMed] [Google Scholar]
- Van der Vliet H J, Pinedo H M, von Blomberg B M, van den Eertwegh A J, Scheper R J, Giaccone G. Natural killer T cells. Lancet Oncol. 2002;3:574. doi: 10.1016/s1470-2045(02)00850-1. [DOI] [PubMed] [Google Scholar]
- Vanderkerken K, Bouwens L, De Neve W, Van den Berg K, Baekeland M, Delens N, Wisse E. Origin and differentiation of hepatic natural killer cells (pit cells) Hepatology. 1993;18:919–925. doi: 10.1002/hep.1840180425. [DOI] [PubMed] [Google Scholar]
- Ishiyama K, Ohdan H, Ohira M, Mitsuta H, Arihiro K, Asahara T. Difference in cytotoxicity against hepatocellular carcinoma between liver and periphery natural killer cells in humans. Hepatology. 2006;43:362–372. doi: 10.1002/hep.21035. [DOI] [PubMed] [Google Scholar]
- Vermijlen D, Luo D, Froelich C J, Medema J P, Kummer J A, Willems E, Braet F, Wisse E. Hepatic natural killer cells exclusively kill splenic/blood natural killer-resistant tumor cells by the perforin/granzyme pathway. J Leukoc Biol. 2002;72:668–676. [PubMed] [Google Scholar]
- Luo D, Vanderkerken K, Chen M C, Vermijlen D, Asosingh K, Willems E, Triantis V, Eizirik D L, Kuppen P J, Wisse E. Rat hepatic natural killer cells (pit cells) express mRNA and protein similar to in vitro interleukin-2 activated spleen natural killer cells. Cell Immunol. 2001;210:41–48. doi: 10.1006/cimm.2001.1803. [DOI] [PubMed] [Google Scholar]
- Burt B M, Plitas G, Nguyen H M, Stableford J A, Bamboat Z M, Dematteo R P. Circulating HLA-DR(+) natural killer cells have potent lytic ability and weak antigen-presenting cell function. Hum Immunol. 2008;69:469–474. doi: 10.1016/j.humimm.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkerken K, Bouwens L, Van Rooijen N, Van den Berg K, Baekeland M, Wisse E. The role of Kupffer cells in the differentiation process of hepatic natural killer cells. Hepatology. 1995;22:283–290. [PubMed] [Google Scholar]
- Bordoni V, Alonzi T, Agrati C, Poccia F, Borsellino G, Mancino G, Fimia G M, Piacentini M, Fantoni A, Tripodi M. Murine hepatocyte cell lines promote expansion and differentiation of NK cells from stem cell precursors. Hepatology. 2004;39:1508–1516. doi: 10.1002/hep.20234. [DOI] [PubMed] [Google Scholar]
- Lovdal T, Andersen E, Brech A, Berg T. Fc receptor mediated endocytosis of small soluble immunoglobulin G immune complexes in Kupffer and endothelial cells from rat liver. J Cell Sci. 2000;113:3255–3266. doi: 10.1242/jcs.113.18.3255. [DOI] [PubMed] [Google Scholar]
- Smedsrod B. Clearance function of scavenger endothelial cells. Comp Hepatol. 2004;3:S22. doi: 10.1186/1476-5926-2-S1-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltrout R H, Herberman R B, Zhang S R, Chirigos M A, Ortaldo J R, Green K M, Jr, Talmadge J E. Role of organ-associated NK cells in decreased formation of experimental metastases in lung and liver. J Immunol. 1985;134:4267–4275. [PubMed] [Google Scholar]
- Wiltrout R H, Denn A C, III, Reynolds C W. Augmentation of organ-associated NK activity by BRMs: association of NK activity with mononuclear cell infiltration. Pathol Immunopathol Res. 1986;5:219–233. doi: 10.1159/000157015. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Takehara T, Tatsumi T, Kanto T, Suzuki T, Jinushi M, Sugimoto Y, Sasaki Y, Hori M, Hayashi N. CD1d-mediated stimulation of natural killer T cells selectively activates hepatic natural killer cells to eliminate experimentally disseminated hepatoma cells in murine liver. Int J Cancer. 2003;106:81–89. doi: 10.1002/ijc.11163. [DOI] [PubMed] [Google Scholar]
- McIntyre K W, Welsh R M. Accumulation of natural killer and cytotoxic T large granular lymphocytes in the liver during virus infection. J Exp Med. 1986;164:1667–1681. doi: 10.1084/jem.164.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs J A, Cho S, Roberts T J, Sriram V, Zhang J, Xu M, Brutkiewicz R R. Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus. J Virol. 2001;75:10746–10754. doi: 10.1128/JVI.75.22.10746-10754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Mather T P, Orange J S, Biron C A. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1α (MIP-1α)-dependent pathways. J Exp Med. 1998;187:1–14. doi: 10.1084/jem.187.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Mather T P, Lewis C A, Biron C A. Type I interferons regulate inflammatory cell trafficking and macrophage inflammatory protein 1α delivery to the liver. J Clin Invest. 2002;110:321–330. doi: 10.1172/JCI15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subleski J J, Hall V L, Back T C, Ortaldo J R, Wiltrout R H. Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Res. 2006;66:11005–11012. doi: 10.1158/0008-5472.CAN-06-0811. [DOI] [PubMed] [Google Scholar]
- Fogler W E, Volker K, Watanabe M, Wigginton J M, Roessler P, Brunda M J, Ortaldo J R, Wiltrout R H. Recruitment of hepatic NK cells by IL-12 is dependent on IFN-γ and VCAM-1 and is rapidly down-regulated by a mechanism involving T cells and expression of Fas. J Immunol. 1998;161:6014–6021. [PubMed] [Google Scholar]
- Chang C J, Chen Y H, Huang K W, Cheng H W, Chan S F, Tai K F, Hwang L H. Combined GM-CSF and IL-12 gene therapy synergistically suppresses the growth of orthotopic liver tumors. Hepatology. 2007;45:746–754. doi: 10.1002/hep.21560. [DOI] [PubMed] [Google Scholar]
- Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci USA. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biburger M, Tiegs G. Activation-induced NKT cell hyporesponsiveness protects from α-galactosylceramide hepatitis and is independent of active transregulatory factors. J Leukoc Biol. 2008;84:264–279. doi: 10.1189/jlb.0607352. [DOI] [PubMed] [Google Scholar]
- Parekh V V, Wilson M T, Olivares-Villagomez D, Singh A K, Wu L, Wang C R, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi Y, Arai K, Moroda T, Kawamura T, Umezu H, Naito M, Ohtsuka K, Hasegawa K, Takahashi-Iwanaga H, Iwanaga T. Supportive cellular elements for hepatic T cell differentiation: T cells expressing intermediate levels of the T cell receptor are cytotoxic against syngeneic hepatoma, and are lost after hepatocyte damage. Eur J Immunol. 1995;25:3452–3459. doi: 10.1002/eji.1830251237. [DOI] [PubMed] [Google Scholar]
- Park O, Wang L, Jeong W, Wang H, Lian Z, Gershwin M, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H, Aswad F, Minagawa M, Govindarajan S, Dennert G. P2X7 receptors regulate NKT cells in autoimmune hepatitis. J Immunol. 2006;176:2152–2160. doi: 10.4049/jimmunol.176.4.2152. [DOI] [PubMed] [Google Scholar]
- Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Iwabuchi K, Iwata D, Miyazaki A, Kon Y, Niino M, Kikuchi S, Yanagawa Y, Kaer L V, Sasaki H, Onoe K. Effect of high fat diet on NKT cell function and NKT cell-mediated regulation of Th1 responses. Scand J Immunol. 2008;67:230–237. doi: 10.1111/j.1365-3083.2007.02062.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Oben J A, Yang S, Lin H, Stafford E A, Soloski M J, Thomas S A, Diehl A M. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004;40:434–441. doi: 10.1002/hep.20320. [DOI] [PubMed] [Google Scholar]
- Li Z, Soloski M J, Diehl A M. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- Li Z, Lin H, Yang S, Diehl A M. Murine leptin deficiency alters Kupffer cell production of cytokines that regulate the innate immune system. Gastroenterology. 2002;123:1304–1310. doi: 10.1053/gast.2002.35997. [DOI] [PubMed] [Google Scholar]
- Yang L, Jhaveri R, Huang J, Qi Y, Diehl A M. Endoplasmic reticulum stress, hepatocyte CD1d and NKT cell abnormalities in murine fatty livers. Lab Invest. 2007;87:927–937. doi: 10.1038/labinvest.3700603. [DOI] [PubMed] [Google Scholar]
- Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. 2002;298:297–302. doi: 10.1016/s0006-291x(02)02462-2. [DOI] [PubMed] [Google Scholar]
- Oya H, Kawamura T, Shimizu T, Bannai M, Kawamura H, Minagawa M, Watanabe H, Hatakeyama K, Abo T. The differential effect of stress on natural killer T (NKT) and NK cell function. Clin Exp Immunol. 2000;121:384–390. doi: 10.1046/j.1365-2249.2000.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Gao B. Negative regulation of liver regeneration by innate immunity (natural killer cells/interferon-γ) Gastroenterology. 2004;127:1525–1539. doi: 10.1053/j.gastro.2004.08.055. [DOI] [PubMed] [Google Scholar]
- Kato T, Sato Y, Takahashi S, Kawamura H, Hatakeyama K, Abo T. Involvement of natural killer T cells and granulocytes in the inflammation induced by partial hepatectomy. J Hepatol. 2004;40:285–290. doi: 10.1016/j.jhep.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Ito H, Ando K, Nakayama T, Taniguchi M, Ezaki T, Saito K, Takemura M, Sekikawa K, Imawari M, Seishima M, Moriwaki H. Role of Vα 14 NKT cells in the development of impaired liver regeneration in vivo. Hepatology. 2003;38:1116–1124. doi: 10.1053/jhep.2003.50471. [DOI] [PubMed] [Google Scholar]
- Minagawa M, Oya H, Yamamoto S, Shimizu T, Bannai M, Kawamura H, Hatakeyama K, Abo T. Intensive expansion of natural killer T cells in the early phase of hepatocyte regeneration after partial hepatectomy in mice and its association with sympathetic nerve activation. Hepatology. 2000;31:907–915. doi: 10.1053/he.2000.5850. [DOI] [PubMed] [Google Scholar]
- Dong Z, Zhang J, Sun R, Wei H, Tian Z. Impairment of liver regeneration correlates with activated hepatic NKT cells in HBV transgenic mice. Hepatology. 2007;45:1400–1412. doi: 10.1002/hep.21597. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Kawamura H, Nagura T, Kato T, Naito T, Kameyama H, Hatakeyama K, Abo T. Association of NKT cells and granulocytes with liver injury after reperfusion of the portal vein. Cell Immunol. 2005;234:31–38. doi: 10.1016/j.cellimm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Lappas C M, Day Y J, Marshall M A, Engelhard V H, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara H, Nagasaki K, Hsieh C L, Ogura Y, Esquivel C O, Martinez O M, Krams S M. IFN-γ, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses. Am J Transplant. 2005;5:2094–2103. doi: 10.1111/j.1600-6143.2005.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder R C, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld J J, Hoofnagle J H. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- Gao B, Hong F, Radaeva S. Host factors and failure of interferon-α treatment in hepatitis C virus. Hepatology. 2004;39:880–890. doi: 10.1002/hep.20139. [DOI] [PubMed] [Google Scholar]
- Radaeva S, Jaruga B, Hong F, Kim W H, Fan S, Cai H, Strom S, Liu Y, El-Assal O, Gao B. Interferon-α activates multiple STAT signals and down-regulates c-Met in primary human hepatocytes. Gastroenterology. 2002;122:1020–1034. doi: 10.1053/gast.2002.32388. [DOI] [PubMed] [Google Scholar]
- Kim S, Lalani S, Parekh V V, Vincent T L, Wu L, Van Kaer L. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. J Clin Invest. 2008;118:2301–2315. doi: 10.1172/JCI33071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Vanderkerken K, Bouwens L, Kuppen P J, Baekeland M, Seynaeve C, Wisse E. The role of adhesion molecules in the recruitment of hepatic natural killer cells (pit cells) in rat liver. Hepatology. 1996;24:1475–1480. doi: 10.1002/hep.510240629. [DOI] [PubMed] [Google Scholar]
- Harada M, Seino K I, Wakao H, Sakata S, Ishizuka Y, Ito T, Kojo S, Nakayama T, Taniguchi M. Down-regulation of the invariant Vα14 antigen receptor in NKT cells upon activation. Int Immunol. 2004;16:241–247. doi: 10.1093/intimm/dxh023. [DOI] [PubMed] [Google Scholar]
- Wilson M T, Johansson C, Olivares-Villagomez D, Singh A K, Stanic A K, Wang C R, Joyce S, Wick M J, Van Kaer L. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100:10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K, Haschemi A, Yegutkin G G, Candinas D, Exley M, Robson S C. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Cameron T O, Sidobre S, Manlongat N, Kronenberg M, Briskin M J, Dustin M L, Littman D R. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanov E, Veinotte L, Cullen R, Chamberlain E, Butcher E C, Johnston B. Critical role for the chemokine receptor CXCR6 in homeostasis and activation of CD1d-restricted NKT cells. J Immunol. 2008;181:81–91. doi: 10.4049/jimmunol.181.1.81. [DOI] [PubMed] [Google Scholar]
- Dong Z, Wei H M, Sun R, Tian Z G. The roles of innate immune cells in liver injury and regeneration. Cell Mol Immunol. 2007;4:241–252. [PubMed] [Google Scholar]
- Hou X, Zhou R, Wei H, Sun R, Tian Z. NKG2D-Rae 1 recognition between natural killer (NK) cells and Kupffer cells in a novel murine NK cell-dependent fulminant hepatitis. Hepatology. 2009;49:940–949. doi: 10.1002/hep.22725. [DOI] [PubMed] [Google Scholar]
- Muhlen K A, Schumann J, Wittke F, Stenger S, Van Rooijen N, Van Kaer L, Tiegs G. NK cells, but not NKT cells, are involved in Pseudomonas aeruginosa exotoxin A-induced hepatotoxicity in mice. J Immunol. 2004;172:3034–3041. doi: 10.4049/jimmunol.172.5.3034. [DOI] [PubMed] [Google Scholar]
- Abe T, Kawamura H, Kawabe S, Watanabe H, Gejyo F, Abo T. Liver injury due to sequential activation of natural killer cells and natural killer T cells by carrageenan. J Hepatol. 2002;36:614–623. doi: 10.1016/s0168-8278(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Chen Q, Wei H, Sun R, Zhang J, Tian Z. Therapeutic RNA silencing of Cys-X3-Cys chemokine ligand 1 gene prevents mice from adenovirus vector-induced acute liver injury. Hepatology. 2008;47:648–658. doi: 10.1002/hep.21993. [DOI] [PubMed] [Google Scholar]
- Stout-Delgado H W, Getachew Y, Rogers T E, Miller B C, Thiele D L. The role of serpinb9/serine protease inhibitor 6 in preventing granzyme B-dependent hepatotoxicity. Hepatology. 2007;46:1530–1540. doi: 10.1002/hep.21820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata T, Kinoshita M, Inatsu A, Habu Y, Nakashima H, Shinomiya N, Seki S. Functional alterations of liver innate immunity of mice with aging in response to CpG-oligodeoxynucleotide. Hepatology. 2008;48:1586–1597. doi: 10.1002/hep.22489. [DOI] [PubMed] [Google Scholar]
- Minagawa M, Deng Q, Liu Z X, Tsukamoto H, Dennert G. Activated natural killer T cells induce liver injury by Fas and tumor necrosis factor-α during alcohol consumption. Gastroenterology. 2004;126:1387–1399. doi: 10.1053/j.gastro.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Jaruga B, Hong F, Kim W H, Sun R, Fan S, Gao B. Chronic alcohol consumption accelerates liver injury in T cell-mediated hepatitis: alcohol disregulation of NF-κB and STAT3 signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2004;287:G471–G479. doi: 10.1152/ajpgi.00018.2004. [DOI] [PubMed] [Google Scholar]
- Wintermeyer P, Cheng C, Gehring S, Hoffman B L, Holub M, Brossay L, Gregory S. Invariant natural killer T cells suppresses the neutrophil inflammatory response in a mouse model of cholestatic liver damage. Gastroenterology. 2009;136:1048–1059. doi: 10.1053/j.gastro.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z X, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760–1774. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Masson M J, Carpenter L D, Graf M L, Pohl L R. Pathogenic role of natural killer T and natural killer cells in acetaminophen-induced liver injury in mice is dependent on the presence of dimethyl sulfoxide. Hepatology. 2008;48:889–897. doi: 10.1002/hep.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Innate immunity and acetaminophen-induced liver injury: why so many controversies? Hepatology. 2008;48:699–701. doi: 10.1002/hep.22556. [DOI] [PubMed] [Google Scholar]
- Cheng L, You Q, Yin H, Holt M, Franklin C, Ju C. Effect of polyI:C cotreatment on halothane-induced liver injury in mice. Hepatology. 2009;49:215–226. doi: 10.1002/hep.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen G C, Sam J, Thuluvath P J. Hepatitis C is a predictor of acute liver injury among hospitalizations for acetaminophen overdose in the United States: a nationwide analysis. Hepatology. 2008;48:1336–1341. doi: 10.1002/hep.22536. [DOI] [PubMed] [Google Scholar]
- Kahraman A, Barreyro F J, Bronk S F, Werneburg N W, Mott J L, Akazawa Y, Masuoka H C, Howe C L, Gores G J. TRAIL mediates liver injury by the innate immune system in the bile duct-ligated mouse. Hepatology. 2008;47:1317–1330. doi: 10.1002/hep.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy P T, Lampertico P, Das A, Lopes A R, Borrow P, Williams K, Humphreys E, Afford S, Adams D H, Bertoletti A, Maini M K. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout-Delgado H W, Getachew Y, Miller B C, Thiele D L. Intrahepatic lymphocyte expression of dipeptidyl peptidase I-processed granzyme B and perforin induces hepatocyte expression of serine proteinase inhibitor 6 (Serpinb9/SPI-6) J Immunol. 2007;179:6561–6567. doi: 10.4049/jimmunol.179.10.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wei H, Sun R, Dong Z, Zhang J, Tian Z. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology. 2007;46:706–715. doi: 10.1002/hep.21872. [DOI] [PubMed] [Google Scholar]
- Blom W M, De Bont H J, Meijerman I, Kuppen P J, Mulder G J, Nagelkerke J F. Interleukin-2-activated natural killer cells can induce both apoptosis and necrosis in rat hepatocytes. Hepatology. 1999;29:785–792. doi: 10.1002/hep.510290303. [DOI] [PubMed] [Google Scholar]
- Sun R, Park O, Horiguchi N, Kulkarni S, Jeong W I, Sun H Y, Radaeva S, Gao B. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice. Hepatology. 2006;44:955–966. doi: 10.1002/hep.21344. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sun R, Jiang W, Wei H, Tian Z. Liver-specific HBsAg transgenic mice are over-sensitive to poly(I:C)-induced liver injury in NK cell- and IFN-γ-dependent manner. J Hepatol. 2007;47:183–190. doi: 10.1016/j.jhep.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Vilarinho S, Ogasawara K, Nishimura S, Lanier L L, Baron J L. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci USA. 2007;104:18187–18192. doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Takeda K, Kaneda H, Matsumoto H, Hayakawa Y, Raulet D H, Ikarashi Y, Kronenberg M, Yagita H, Kinoshita K, Abo T, Okumura K, Smyth M J. NKG2A inhibits invariant NKT cell activation in hepatic injury. J Immunol. 2009;182:250–258. doi: 10.4049/jimmunol.182.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Tian Z, Kulkarni S, Gao B. IL-6 prevents T cell-mediated hepatitis via inhibition of NKT cells in CD4+ T cell- and STAT3-dependent manners. J Immunol. 2004;172:5648–5655. doi: 10.4049/jimmunol.172.9.5648. [DOI] [PubMed] [Google Scholar]
- Li B, Sun R, Wei H, Gao B, Tian Z. Interleukin-15 prevents concanavalin A-induced liver injury in mice via NKT cell-dependent mechanism. Hepatology. 2006;43:1211–1219. doi: 10.1002/hep.21174. [DOI] [PubMed] [Google Scholar]
- Jaruga B, Hong F, Sun R, Radaeva S, Gao B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J Immunol. 2003;171:3233–3244. doi: 10.4049/jimmunol.171.6.3233. [DOI] [PubMed] [Google Scholar]
- Louis H, Le Moine A, Flamand V, Nagy N, Quertinmont E, Paulart F, Abramowicz D, Le Moine O, Goldman M, Deviere J. Critical role of interleukin 5 and eosinophils in concanavalin A-induced hepatitis in mice. Gastroenterology. 2002;122:2001–2010. doi: 10.1053/gast.2002.33620. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, Taniguchi M. Augmentation of Vα14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45:475–485. doi: 10.1002/hep.21498. [DOI] [PubMed] [Google Scholar]
- Bataller R, Brenner D A. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S L. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S L, Rockey D C, Bissell D M. Hepatic fibrosis 2006: report of the Third AASLD Single Topic Conference. Hepatology. 2007;45:242–249. doi: 10.1002/hep.21459. [DOI] [PubMed] [Google Scholar]
- Schnabl B, Purbeck C A, Choi Y H, Hagedorn C H, Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–664. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- Safadi R, Ohta M, Alvarez C E, Fiel M I, Bansal M, Mehal W Z, Friedman S L. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127:870–882. doi: 10.1053/j.gastro.2004.04.062. [DOI] [PubMed] [Google Scholar]
- Melhem A, Muhanna N, Bishara A, Alvarez C E, Ilan Y, Bishara T, Horani A, Nassar M, Friedman S L, Safadi R. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins R A, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe S W. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhanna N, Doron S, Wald O, Horani A, Eid A, Pappo O, Friedman S L, Safadi R. Activation of hepatic stellate cells after phagocytosis of lymphocytes: a novel pathway of fibrogenesis. Hepatology. 2008;48:963–977. doi: 10.1002/hep.22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehal W Z. Activation-induced cell death of hepatic stellate cells by the innate immune system. Gastroenterology. 2006;130:600–603. doi: 10.1053/j.gastro.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Radaeva S, Wang L, Radaev S, Jeong W I, Park O, Gao B. Retinoic acid signaling sensitizes hepatic stellate cells to NK cell killing via upregulation of NK cell activating ligand RAE1. Am J Physiol Gastrointest Liver Physiol. 2007;293:G809–G816. doi: 10.1152/ajpgi.00212.2007. [DOI] [PubMed] [Google Scholar]
- Gasser S, Orsulic S, Brown E J, Raulet D H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taimr P, Higuchi H, Kocova E, Rippe R A, Friedman S, Gores G J. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology. 2003;37:87–95. doi: 10.1053/jhep.2003.50002. [DOI] [PubMed] [Google Scholar]
- Fischer R, Cariers A, Reinehr R, Haussinger D. Caspase 9-dependent killing of hepatic stellate cells by activated Kupffer cells. Gastroenterology. 2002;123:845–861. doi: 10.1053/gast.2002.35384. [DOI] [PubMed] [Google Scholar]
- Rockey D C, Chung J J. Interferon γ inhibits lipocyte activation and extracellular matrix mRNA expression during experimental liver injury: implications for treatment of hepatic fibrosis. J Investig Med. 1994;42:660–670. [PubMed] [Google Scholar]
- Baroni G S, D'Ambrosio L, Curto P, Casini A, Mancini R, Jezequel A M, Benedetti A. Interferon γ decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology. 1996;23:1189–1199. doi: 10.1002/hep.510230538. [DOI] [PubMed] [Google Scholar]
- Jeong W I, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-γ contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima C, Paschal D M, Wang C C, Yoshihara C S, Wood B L, Yeo A E, Emerson S S, Shuhart M C, Gretch D R. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573–580. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- Gao B, Radaeva S, Jeong W. Activation of natural killer cells inhibits liver fibrosis: a novel strategy to treat liver fibrosis. Expert Rev Gastroenterol Hepatol. 2007;1:173–180. doi: 10.1586/17474124.1.1.173. [DOI] [PubMed] [Google Scholar]
- Kimura T, Ishii Y, Morishima Y, Shibuya A, Shibuya K, Taniguchi M, Mochizuki M, Hegab A E, Sakamoto T, Nomura A, Sekizawa K. Treatment with α-galactosylceramide attenuates the development of bleomycin-induced pulmonary fibrosis. J Immunol. 2004;172:5782–5789. doi: 10.4049/jimmunol.172.9.5782. [DOI] [PubMed] [Google Scholar]
- De Lalla C, Galli G, Aldrighetti L, Romeo R, Mariani M, Monno A, Nuti S, Colombo M, Callea F, Porcelli S A, Panina-Bordignon P, Abrignani S, Casorati G, Dellabona P. Production of profibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J Immunol. 2004;173:1417–1425. doi: 10.4049/jimmunol.173.2.1417. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G K, DeFrances M. Liver regeneration. Adv Biochem Eng Biotechnol. 2005;93:101–134. doi: 10.1007/b99968. [DOI] [PubMed] [Google Scholar]
- Fausto N, Campbell J S, Riehle K J. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- Tamura F, Masuhara A, Sakaida I, Fukumoto E, Nakamura T, Okita K. FK506 promotes liver regeneration by suppressing natural killer cell activity. J Gastroenterol Hepatol. 1998;13:703–708. doi: 10.1111/j.1440-1746.1998.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Francavilla A, Starzl T E, Barone M, Zeng Q H, Porter K A, Zeevi A, Markus P M, van den Brink M R, Todo S. Studies on mechanisms of augmentation of liver regeneration by cyclosporine and FK 506. Hepatology. 1991;14:140–143. doi: 10.1002/hep.1840140123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Zheng S S, Park O, Wang H, Sun Z, Gao B. Activation of innate immunity (NK/IFN-γ) in rat allogeneic liver transplantation: contribution to liver injury and suppression of hepatocyte proliferation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1070–G1077. doi: 10.1152/ajpgi.00554.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Abo T, Sugawara S, Kanno A, Kumagai K. Age-related variation in the proportion and activity of murine liver natural killer cells and their cytotoxicity against regenerating hepatocytes. J Immunol. 1988;141:315–323. [PubMed] [Google Scholar]
- Nakashima H, Inui T, Habu Y, Kinoshita M, Nagao S, Kawaguchi A, Miura S, Shinomiya N, Yagita H, Seki S. Activation of mouse natural killer T cells accelerates liver regeneration after partial hepatectomy. Gastroenterology. 2006;131:1573–1583. doi: 10.1053/j.gastro.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Hines I N, Kremer M, Isayama F, Perry A W, Milton R J, Black A L, Byrd C L, Wheeler M D. Impaired liver regeneration and increased oval cell numbers following T cell-mediated hepatitis. Hepatology. 2007;46:229–241. doi: 10.1002/hep.21674. [DOI] [PubMed] [Google Scholar]
- Strick-Marchand H, Masse G X, Weiss M C, Di Santo J P. Lymphocytes support oval cell-dependent liver regeneration. J Immunol. 2008;181:2764–2771. doi: 10.4049/jimmunol.181.4.2764. [DOI] [PubMed] [Google Scholar]
- Lee S H, Miyagi T, Biron C A. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28:252–259. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang T, Ho C, Orange J S, Douglas S D, Ho W Z. Natural killer cells inhibit hepatitis C virus expression. J Leukoc Biol. 2004;76:1171–1179. doi: 10.1189/jlb.0604372. [DOI] [PubMed] [Google Scholar]
- Wang S H, Huang C X, Ye L, Wang X, Song L, Wang Y J, Liang H, Huang X Y, Ho W Z. Natural killer cells suppress full cycle HCV infection of human hepatocytes. J Viral Hepat. 2008;15:855–864. doi: 10.1111/j.1365-2893.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Bost A, Glass J I, Tan S L. Cytokine-activated natural killer cells exert direct killing of hepatoma cells harboring hepatitis C virus replicons. J Interferon Cytokine Res. 2006;26:854–865. doi: 10.1089/jir.2006.26.854. [DOI] [PubMed] [Google Scholar]
- Khakoo S I, Thio C L, Martin M P, Brooks C R, Gao X, Astemborski J, Cheng J, Goedert J J, Vlahov D, Hilgartner M, Cox S, Little A M, Alexander G J, Cramp M E, O'Brien S J, Rosenberg W M, Thomas D L, Carrington M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- Wozniakowska-Gesicka T, Wisniewska-Ligier M, Zeman K, Banasik M. [Prognostic value of natural killer cells monitoring in the course of IFN-α therapy in children with chronic hepatitis C] Pol Merkur Lekarski. 2000;8:376–377. [PubMed] [Google Scholar]
- Bonavita M S, Franco A, Paroli M, Santilio I, Benvenuto R, De Petrillo G, Levrero M, Perrone A, Balsano C, Barnaba V. Normalization of depressed natural killer activity after interferon-α therapy is associated with a low frequency of relapse in patients with chronic hepatitis C. Int J Tissue React. 1993;15:11–16. [PubMed] [Google Scholar]
- Yamagiwa S, Matsuda Y, Ichida T, Honda Y, Takamura M, Sugahara S, Ishikawa T, Ohkoshi S, Sato Y, Aoyagi Y. Sustained response to interferon-α plus ribavirin therapy for chronic hepatitis C is closely associated with increased dynamism of intrahepatic natural killer and natural killer T cells. Hepatol Res. 2008;38:664–672. doi: 10.1111/j.1872-034X.2008.00317.x. [DOI] [PubMed] [Google Scholar]
- Shin E C, Seifert U, Kato T, Rice C M, Feinstone S M, Kloetzel P M, Rehermann B. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J Clin Invest. 2006;116:3006–3014. doi: 10.1172/JCI29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara T, Shingai M, Matsumoto M, Wakita T, Seya T. Hepatitis C virus-infected hepatocytes extrinsically modulate dendritic cell maturation to activate T cells and natural killer cells. Hepatology. 2008;48:48–58. doi: 10.1002/hep.22337. [DOI] [PubMed] [Google Scholar]
- Frese M, Schwarzle V, Barth K, Krieger N, Lohmann V, Mihm S, Haller O, Bartenschlager R. Interferon-γ inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology. 2002;35:694–703. doi: 10.1053/jhep.2002.31770. [DOI] [PubMed] [Google Scholar]
- Crotta S, Stilla A, Wack A, D'Andrea A, Nuti S, D'Oro U, Mosca M, Filliponi F, Brunetto R M, Bonino F, Abrignani S, Valiante N M. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C T, Klimpel G R. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–49. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J C, Shiina M, Ahlenstiel G, Rehermann B. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49:12–21. doi: 10.1002/hep.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzer K, Falk C S, Encke J, Eichhorst S T, Ulsenheimer A, Seliger B, Krammer P H. Upregulation of major histocompatibility complex class I on liver cells by hepatitis C virus core protein via p53 and TAP1 impairs natural killer cell cytotoxicity. J Virol. 2003;77:8299–8309. doi: 10.1128/JVI.77.15.8299-8309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C, He X, Ma H, Hou N, Wei C, Song T, Zhang Y, Sun L, Ma Q, Zhong H. Hepatitis C virus infection downregulates the ligands of the activating receptor NKG2D. Cell Mol Immunol. 2008;5:475–478. doi: 10.1038/cmi.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, Kanazawa Y, Hiramatsu N, Hayashi N. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072–6081. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- Golden-Mason L, Madrigal-Estebas L, McGrath E, Conroy M J, Ryan E J, Hegarty J E, O'Farrelly C, Doherty D G. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008;57:1121–1128. doi: 10.1136/gut.2007.130963. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi N, Seki S, Hatsuse K, Ohkawa T, Koike Y, Aihara T, Habu Y, Nakagawa R, Ami K, Hiraide H, Mochizuki H. Decrease of CD56(+)T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32:962–969. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Wang X, Wang S, Wang Y, Song L, Hou W, Zhou L, Li H, Ho W. CD56+ T cells inhibit hepatitis C virus replication in human hepatocytes. Hepatology. 2009;49:753–762. doi: 10.1002/hep.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L, Castelblanco N, O'Farrelly C, Rosen H R. Phenotypic and functional changes of cytotoxic CD56pos natural T cells determine outcome of acute hepatitis C virus infection. J Virol. 2007;81:9292–9298. doi: 10.1128/JVI.00834-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Gadola S, Meier U, Young N T, Harcourt G, Karadimitris A, Coumi N, Brown D, Dusheiko G, Cerundolo V, Klenerman P. Frequency and phenotype of circulating Vα24/Vβ11 double-positive natural killer T cells during hepatitis C virus infection. J Virol. 2003;77:2251–2257. doi: 10.1128/JVI.77.3.2251-2257.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deignan T, Curry M P, Doherty D G, Golden-Mason L, Volkov Y, Norris S, Nolan N, Traynor O, McEntee G, Hegarty J E, O'Farrelly C. Decrease in hepatic CD56(+) T cells and V α 24(+) natural killer T cells in chronic hepatitis C viral infection. J Hepatol. 2002;37:101–108. doi: 10.1016/s0168-8278(02)00072-7. [DOI] [PubMed] [Google Scholar]
- Webster G J, Reignat S, Maini M K, Whalley S A, Ogg G S, King A, Brown D, Amlot P L, Williams R, Vergani D, Dusheiko G M, Bertoletti A. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 2000;32:1117–1124. doi: 10.1053/jhep.2000.19324. [DOI] [PubMed] [Google Scholar]
- Fisicaro P, Valdatta C, Boni C, Massari M, Mori C, Zerbini A, Orlandini A, Sacchelli L, Missale G, Ferrari C. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut. 2009;58:974–982. doi: 10.1136/gut.2008.163600. [DOI] [PubMed] [Google Scholar]
- Guy C S, Mulrooney-Cousins P M, Churchill N D, Michalak T I. Intrahepatic expression of genes affiliated with innate and adaptive immune responses immediately after invasion and during acute infection with woodchuck hepadnavirus. J Virol. 2008;82:8579–8591. doi: 10.1128/JVI.01022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S, Thimme R, Purcell R H, Chisari F V. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci USA. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Kakimi K, Wieland S, Guidotti L G, Chisari F V. Interleukin-18 inhibits hepatitis B virus replication in the livers of transgenic mice. J Virol. 2002;76:10702–10707. doi: 10.1128/JVI.76.21.10702-10707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimi K, Guidotti L G, Koezuka Y, Chisari F V. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengers D, Sille F C, Derkow K, Besra G S, Janssen H L, Schott E, Boes M. Critical role for CD1d-restricted invariant NKT cells in stimulating intrahepatic CD8 T-cell responses to liver antigen. Gastroenterology. 2008;134:2132–2143. doi: 10.1053/j.gastro.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J L, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16:583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- Malhi H, Gores G J. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres D M, Harrison S A. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- Elinav E, Pappo O, Sklair-Levy M, Margalit M, Shibolet O, Gomori M, Alper R, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Adoptive transfer of regulatory NKT lymphocytes ameliorates non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice and is associated with intrahepatic CD8 trapping. J Pathol. 2006;209:121–128. doi: 10.1002/path.1950. [DOI] [PubMed] [Google Scholar]
- Xu C F, Yu C H, Li Y M, Xu L, Du J, Shen Z. Association of the frequency of peripheral natural killer T cells with nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13:4504–4508. doi: 10.3748/wjg.v13.i33.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri K, Shimizu Y, Tsuneyama K, Sugiyama T. Role of liver-infiltrating CD3+CD56+ natural killer T cells in the pathogenesis of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2009;21:673–680. doi: 10.1097/MEG.0b013e32831bc3d6. [DOI] [PubMed] [Google Scholar]
- Laso F J, Madruga J I, Giron J A, Lopez A, Ciudad J, San Miguel J F, Alvarez-Mon M, Orfao A. Decreased natural killer cytotoxic activity in chronic alcoholism is associated with alcohol liver disease but not active ethanol consumption. Hepatology. 1997;25:1096–1100. doi: 10.1002/hep.510250508. [DOI] [PubMed] [Google Scholar]
- Pan H N, Sun R, Jaruga B, Hong F, Kim W H, Gao B. Chronic ethanol consumption inhibits hepatic natural killer cell activity and accelerates murine cytomegalovirus-induced hepatitis. Alcohol Clin Exp Res. 2006;30:1615–1623. doi: 10.1111/j.1530-0277.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteel G E. Silencing a killer among us: ethanol impairs immune surveillance of activated stellate cells by natural killer cells. Gastroenterology. 2008;134:351–353. doi: 10.1053/j.gastro.2007.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med. 2009;9:4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- Tsuneyama K, Yasoshima M, Harada K, Hiramatsu K, Gershwin M E, Nakanuma Y. Increased CD1d expression on small bile duct epithelium and epithelioid granuloma in livers in primary biliary cirrhosis. Hepatology. 1998;28:620–623. doi: 10.1002/hep.510280303. [DOI] [PubMed] [Google Scholar]
- Chuang Y H, Lian Z X, Yang G X, Shu S A, Moritoki Y, Ridgway W M, Ansari A A, Kronenberg M, Flavell R A, Gao B, Gershwin M E. Natural killer T cells exacerbate liver injury in a transforming growth factor β receptor II dominant-negative mouse model of primary biliary cirrhosis. Hepatology. 2008;47:571–580. doi: 10.1002/hep.22052. [DOI] [PubMed] [Google Scholar]
- Mattner J, Savage P B, Leung P, Oertelt S S, Wang V, Trivedi O, Scanlon S T, Pendem K, Teyton L, Hart J, Ridgway W M, Wicker L S, Gershwin M E, Bendelac A. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–315. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs G. Cellular and cytokine-mediated mechanisms of inflammation and its modulation in immune-mediated liver injury. Z Gastroenterol. 2007;45:63–70. doi: 10.1055/s-2006-927397. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhang J, Tian Z. The regulatory effect of natural killer cells: do “NK-reg cells” exist? Cell Mol Immunol. 2006;3:241–254. [PubMed] [Google Scholar]
- Chuang Y H, Lian Z X, Tsuneyama K, Chiang B L, Ansari A A, Coppel R L, Eric Gershwin M. Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J Autoimmun. 2006;26:232–240. doi: 10.1016/j.jaut.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Saibara T, Maeda T, Miyazaki M, Onishi S, Yamamoto Y. Depressed immune function in patients with cirrhosis before emergence of hepatocellular carcinoma. Hepatology. 1993;18:315–319. [PubMed] [Google Scholar]
- Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, Tien P, Wang F S. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Chuang W L, Liu H W, Chang W Y. Natural killer cell activity in patients with hepatocellular carcinoma relative to early development and tumor invasion. Cancer. 1990;65:926–930. doi: 10.1002/1097-0142(19900215)65:4<926::aid-cncr2820650418>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wallace M E, Smyth M J. The role of natural killer cells in tumor control—effectors and regulators of adaptive immunity. Springer Semin Immunopathol. 2005;27:49–64. doi: 10.1007/s00281-004-0195-x. [DOI] [PubMed] [Google Scholar]
- Tatsumi T, Takehara T, Yamaguchi S, Sasakawa A, Sakamori R, Ohkawa K, Kohga K, Uemura A, Hayashi N. Intrahepatic delivery of α-galactosylceramide-pulsed dendritic cells suppresses liver tumor. Hepatology. 2007;45:22–30. doi: 10.1002/hep.21447. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Takehara T, Tatsumi T, Suzuki T, Jinushi M, Kanazawa Y, Hiramatsu N, Kanto T, Tsuji S, Hori M, Hayashi N. Concanavalin A injection activates intrahepatic innate immune cells to provoke an antitumor effect in murine liver. Hepatology. 2004;40:1190–1196. doi: 10.1002/hep.20447. [DOI] [PubMed] [Google Scholar]
- Crowe N Y, Coquet J M, Berzins S P, Kyparissoudis K, Keating R, Pellicci D G, Hayakawa Y, Godfrey D I, Smyth M J. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricard G, Cesson V, Devevre E, Bouzourene H, Barbey C, Rufer N, Im J S, Alves P M, Martinet O, Halkic N, Cerottini J C, Romero P, Porcelli S A, Macdonald H R, Speiser D E. Enrichment of human CD4+ V(α)24/Vβ11 invariant NKT cells in intrahepatic malignant tumors. J Immunol. 2009;182:5140–5151. doi: 10.4049/jimmunol.0711086. [DOI] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz J K, Doherty J M, Mills J C, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk K, Witte E, Hoffmann U, Doecke W D, Endesfelder S, Asadullah K, Sterry W, Volk H D, Wittig B M, Sabat R. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J Immunol. 2007;178:5973–5981. doi: 10.4049/jimmunol.178.9.5973. [DOI] [PubMed] [Google Scholar]
- Radaeva S, Sun R, Pan H N, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol. 2004;1:43–49. [PubMed] [Google Scholar]
- Brand S, Dambacher J, Beigel F, Zitzmann K, Heeg M H, Weiss T S, Prufer T, Olszak T, Steib C J, Storr M, Goke B, Diepolder H, Bilzer M, Thasler W E, Auernhammer C J. IL-22-mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1019–G1028. doi: 10.1152/ajpgi.00239.2006. [DOI] [PubMed] [Google Scholar]
- Zenewicz L A, Yancopoulos G D, Valenzuela D M, Murphy A J, Karow M, Flavell R A. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]