Abstract
Signals from stressed cells and the enteric microbiota activate macrophages and dendritic cells and mediate intestinal inflammation. HMGB1 serves as an immunogenic stimuli causing release of inflammatory cytokines by myeloid cells. Ethyl pyruvate inhibits secretion of HMGB1 and improves survival in models of endotoxemia and hemorrhagic shock. We reasoned that ethyl pyruvate may be protective in colitis, which involves similar inflammatory pathways. In IL-10−/− mice with established chronic colitis, ethyl pyruvate administration ameliorated colitis and reduced intestinal cytokine production. IL-10−/− mice demonstrated increased intestinal HMGB1 expression and decreased expression of RAGE compared with wild-type mice. Fecal HMGB1 levels were decreased in ethyl pyruvate-treated mice. Furthermore, ethyl pyruvate induced HO-1 expression in intestinal tissue. In TNBS-induced colitis, intrarectal administration of ethyl pyruvate resulted in amelioration of colitis and reduced intestinal cytokine production. In LPS-activated murine macrophages, ethyl pyruvate decreased expression of IL-12 p40 and NO production but did not affect IL-10 levels. Ethyl pyruvate did not inhibit nuclear translocation of NF-κB family members but attenuated NF-κB DNA binding. Additionally, ethyl pyruvate induced HO-1 mRNA and protein expression and HO-1 promoter activation. Moreover, ethyl pyruvate prevented nuclear-to-cytoplasmic translocation of HMGB1. In conclusion, the HMGB1/RAGE pathway has pathophysiologic and diagnostic significance in experimental colitis. Ethyl pyruvate and other strategies to inhibit HMGB1 release and function represent promising interventions in chronic inflammatory diseases.
Keywords: inflammation, innate immunity, inflammatory bowel disease
Introduction
Although etiologies of the IBDs remain unclear, chronic gut inflammation results from excessive inflammatory responses [1] to the enteric microbiota [2]. Therapeutic strategies that dampen excessive innate immune activation and cytokine production may ameliorate IBD. HMGB1 is a nonhistone, chromatin-associated nuclear protein that has emerged as a prototypical DAMP molecule with important intra- and extracellular roles [3]. HMGB1 is actively secreted by immunostimulated macrophages [4] and enterocytes [5] and is released by necrotic but not apoptotic cells [6]. Extracellular HMGB1 induces inflammatory mediators [7] and has been implicated in cytokine-induced pathologies, such as endotoxemia, ischemia and reperfusion, and arthritis [8, 9]. HMGB1 also alters intestinal epithelial cell permeability [10]. There are several defined receptors for HMGB1 including RAGE [11], TLR2, and TLR4 [12, 13]. RAGE is activated by multiple DAMPs, including S100 family members that are increased in human IBD [14], and therefore, is an attractive target for therapeutic intervention [15].
Ethyl pyruvate is a simple aliphatic ester of the metabolic intermediate pyruvate [4]. Ethyl pyruvate is an effective anti-inflammatory agent in a variety of in vitro and in vivo model systems [4]. Ethyl pyruvate administration ameliorates organ damage and improves survival in animal models of mesenteric ischemia and reperfusion, hemorrhagic shock, endotoxemia, and sepsis [16,17,18]. Importantly, many of the anti-inflammatory effects of ethyl pyruvate in acute inflammation may be related to inhibition of HMGB1 secretion [17].
Taken together, these observations suggest that ethyl pyruvate may also have a role as a therapeutic agent in chronic inflammatory diseases, such as the IBDs. Here, we show that local and systemic ethyl pyruvate administration in murine colitis models ameliorates intestinal inflammation. The anti-inflammatory effects of ethyl pyruvate are pleiotropic and include inhibition of intestinal HMGB1 secretion. Thus, ethyl pyruvate and other strategies to inhibit HMGB1 release and function are promising interventions in chronic inflammatory diseases.
MATERIALS AND METHODS
Mice
An IL-10−/− mouse colony (C57BL/6) was maintained in accordance with guidelines from the American Association for Laboratory Animal Care. The Institutional Animal Care and Use Committees at the University of Pittsburgh (PA, USA) and the University of North Carolina (Chapel Hill, NC, USA) approved research protocols. Ten-week-old male and female IL-10−/− mice were grouped randomly and treated with sterile, lactated Ringer’s solution alone (vehicle) or 40 mg/kg ethyl pyruvate (Acros Organics, Belgium) in lactated Ringer’s solution in a total volume of 500 μl i.p. every other day for 2 weeks. Female BALB/c mice (12–13 weeks old) were anesthetized lightly with isoflurane and then administered 2.5 mg TNBS (Sigma Chemical Co., St. Louis, MO, USA) in 50% ethanol (total volume, 100 μl) intrarectally via a PE20 polyethylene-tubing catheter (Becton Dickinson, San Jose, CA, USA) equipped with a 1-ml syringe. Mice were grouped randomly, and lactated Ringer’s solution (vehicle) or 40 mg/kg ethyl pyruvate dissolved in lactated Ringer’s solution was delivered intrarectally at 4 h, 2 days, and 4 days after TNBS administration. After 5 days, animals were killed.
Intestinal tissue explant cultures
Colons were isolated from individual mice, cut open longitudinally, and feces were collected for ELISA. Colonic tissue fragments (0.05 g dry weight) were processed for explant cultures as described previously [19]. Supernatants were collected after 24 h, assayed for spontaneous cytokine production via ELISA, and normalized to dry gut weight.
Histologic evaluation
Colonic tissue was fixed in 10% buffered formalin and embedded in paraffin. Sections (5 μm-thick) were stained with H&E. Colitis scores were determined for each high-powered field (100× magnification) viewed by a pathologist (A. R. Sepulveda) blinded to the treatment groups. For IL-10−/− mice, colitis scores were calculated as described previously [19]. For TNBS colitis, the degree of inflammation was graded from 0 to 3 (0, no signs of inflammation; 1, increased mucosal neutrophils or lymphocytes present; 2, increased mucosal and submucosal or transmural neutrophils or lymphocytes present; 3, mucosal regenerative features with crypt distortion and increased crypt proliferation or ulcers or erosions) and represented as the mean sum of 10 fields.
Fecal HMGB1 and RAGE assessments
Fecal samples from colons of killed mice were extracted in PBS by rotation at 4°C for 48 h and analyzed for HMGB1 and RAGE concentration by sandwich ELISAs. The RAGE ELISA was purchased from R&D Systems (Minneapolis, MN, USA). HMGB1 and RAGE protein concentrations were normalized to total fecal protein concentrations (Coomassie protein assay kit, Pierce, Rockford, IL, USA).
HMGB1 ELISA
A specific ELISA that detects human and murine HMGB1 was developed in the laboratory of M. T. Lotze. The detection antibody is a rabbit polyclonal anti-HMGB1 purified using affinity chromatography (Sigma Chemical Co.). To generate this antibody, rabbits were immunized with the HMGB1 peptide sequence KPDAAKKGVVKAEKSC.
Assay.
Three commercially available capture antibodies were tested, and the anti-HMGB1 capture antibody MAB1690 (R&D Systems) performed best. Immuno Maxisorp 96-well plates (Nunc, Rochester, NY, USA; Cat. No. 439454) are coated with 1 ug/ml anti-HMGB1 antibody MAB1690 (R&D Systems) at 50 ul/well in PBS. The plates are incubated, covered, and blocked overnight with 4% BSA (Spectrum, Rancho Dominguez, CA, USA; Cat. No. 9048-46-8) in PBS.
Standard preparation.
Stock rHMGB1 (from Kevin Tracey, Feinstein Institute for Medical Research, Manhasset, NY, USA) is diluted to 1000 ng/ml in 4% BSA in PBS. Serial dilutions (1:2) are performed along the first row of the plate to obtain 12 standard concentrations, ranging from 1000 ng/ml to 0 ng/ml rHMGB1. Standard (50 ul/well) is incubated on the plate for 1 h at room temperature.
Sample preparation.
Fecal extracts were prepared as above. Extract (50 μl; undiluted) was added per well and incubated for 1 h at room temperature. Each sample is run in triplicate. Interassay variability between triplicate samples was uniformly <10%. Intrassay variability was assessed by including a control extract sample in all ELISAs, and variability was also <10%.
Detection antibody.
The detection antibody (rabbit polyclonal anti-HMGB1) is diluted in 4% BSA in PBS to 1 ug/ml. Fifty microliters per well is incubated on the plate for 1 h at room temperature. Subsequently, PBS + 0.2% Tween-20 (FisherBiotech, Australia; Cat. No. 9005-64-5) is incubated on the plate for 10 min at room temperature at 200 ul/well. Three 10-min washes are performed, and then the plates are rinsed with 200 ul/well PBS.
Secondary antibody.
Goat anti-rabbit HRP (Jackson Laboratories, Bar Harbor, ME, USA; Cat. No. 111-035-003) is diluted 1:2500 in 4% BSA in PBS, and 50 ul/well is incubated on the plate for 30 min at room temperature. Subsequently, 200 ul/well PBS + 0.2% Tween-20 is incubated on the plate for 10 min at room temperature. Three 10-min washes are performed, and then the plates are rinsed with 200 ul/well PBS three times.
Development.
Plates are developed using the Pierce HRP ELISA kit (Cat. No. 34021). Equal parts tetramethyl benzidine substrate and hydrogen peroxide are added together and 100 ul/well incubated on the plate at room temperature. After 2–5 min, when the highest standard turns dark blue, 100 ul/well 0.18 M sulfuric acid (J. T. Baker, Phillipsburg, NJ, USA; Cat. No. CAS 7664-93-9) is added to stop the reaction. Plates are read at 450 nm on a Tecan Safire plate reader.
Immunohistochemistry
Colonic tissue sections were fixed in 2% paraformaldehyde in PBS and then incubated in 30% sucrose in PBS at 4°C overnight. Samples were snap-frozen in isopentane and cut into 6 μm-thick frozen sections and placed on microscope slides. Sections were washed in 0.5% BSA in PBS (wash buffer) three times to remove sucrose and then blocked with 2% BSA in PBS for 45 min. Tissue sections were stained with rabbit anti-HMGB1 (BD PharMingen, San Diego, CA, USA) or rabbit anti-RAGE (from Tim Oury, University of Pittsburgh) [20] polyclonal antibodies at a 1:500 dilution for 1 h at room temperature. Samples were then incubated for 1 h at room temperature with secondary antibody (1:500 dilution of goat anti-rabbit Alexa 488, Molecular Probes, Eugene, OR, USA). Cells were costained with rhodamine phalloidin (1:500 dilution, Molecular Probes) to visualize F-actin and Draq5 (Biostatus Ltd., Leicestershire, UK) to visualize nuclei. Rabbit nonimmune serum or secondary antibody alone was used as controls, and nonspecific immunostaining was not observed (data not shown). Slides were viewed on an Olympus Flouview 1000 confocal microscope (Olympus America, Melville, NY, USA).
Murine macrophages
BM-derived macrophages were isolated from femurs of C57BL/6 mice as described previously [19]. Cells were seeded at 4 × 106 cells/well for RNA, 2 × 105 cells/well for ELISA, or 25 × 106 cells/plate for nuclear extracts, pretreated with ethyl pyruvate, dissolved in media, and subsequently stimulated with 100 ng/ml-repurified LPS [5] for times indicated in the figure legends.
Real-time RT-PCR
Total RNA was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was derived from 1.5 μg total RNA by RT using Superscript II (Invitrogen). Real-time RT-PCR was performed using specific primers for IL-12 p40, HO-1, and GAPDH, as described [19].
Cytokine ELISAs and NO assay
Murine IL-12 p40, TNF, and IL-10 immunoassay kits were used according to the manufacturer’s instructions (BD PharMingen). The stable NO metabolite, nitrite, was measured using the Griess reagent as described previously [21]. Values were measured using a plate reader and SOFTMax Pro v4.8 software (Molecular Devices, Sunnyvale, CA, USA).
Nuclear extracts and DNA binding assay (EMSA)
Nuclear extracts were isolated from stimulated BM-derived macrophages using the NE/PER reagents by following the manufacturer’s protocol (Pierce). Protein concentration was determined using the Bradford assay. EMSAs were performed as described previously [22]. For supershift assays, 5 μg nuclear extract was incubated with 2 μg anti-p65, anti-c-Rel, or anti-p50 polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 45 min at room temperature prior to addition of the 32P-labeled probe.
Western blots
Western blot analyses were performed on nuclear extracts as described [23]. Anti-p65, anti-c-Rel, anti-p50, and anti-Nrf2 were obtained from Santa Cruz Biotechnology; anti-HO-1 was from StressGen (San Diego, CA, USA); and anti-actin was from Sigma Chemical Co.
Luciferase assays
HEK293 cells stably transfected with a multimerized NF-κB DNA-binding element-luciferase reporter gene were seeded at 2 × 105 cells/well. Cells were pretreated for 1 h with ethyl pyruvate dissolved in media and activated for 2 h with 10 ng/ml rhTNF (R&D Systems). RAW264.7 cells were seeded at 2.5 × 105 cells/well and transfected with pHO15luc, a luciferase reporter downstream of a 15-kb mouse HO-1 promoter fragment, or pHO15lucΔE1, a luciferase reporter lacking the E1 enhancer (provided by Dr. Jawed Alam, Louisiana State University Medical Center, New Orleans, LA, USA). Cells were pretreated for 1 h with ethyl pyruvate in media and stimulated for 36 h with 1000 ng/ml LPS. The cells were lysed in reporter lysis buffer, and luciferase activity was measured with a luciferase assay system (Promega, Madison, WI, USA) using a Turner Designs Luminometer TD20/20. In RAW264.7 cells, transfection efficiency was assessed by β-galactosidase activity in the same cell lysates as reported previously [24].
HMGB1 translocation experiments
RAW264.7 cells were transfected with a HMGB1-GFP fusion protein expression vector using SuperFect (Qiagen, Valencia, CA, USA) as described previously [24]. After overnight culture, cells were stimulated with LPS (1 μg/ml) and/or ethyl pyruvate (10 mM). Twenty-four hours later, cells were fixed in 4% paraformaldehyde. Nuclei were stained with DAPI. GFP expression and cellular localization were visualized by fluorescence microscopy (Olympus IX70).
LPMC isolation
LPMCs were isolated from mouse colons by an enzymatic method as described previously [25]. LPMCs (1×106) were incubated with 1 μg rabbit polyclonal anti-RAGE antibody (ab37647, Abcam, Cambridge, MA, USA) for 1 h at 4°C. After washing, cells were stained with PE-conjugated anti-rabbit IgG (Abcam), an APC-conjugated anti-mouse F4/80 antibody (eBioscience, San Diego, CA, USA), and fixed in 4% parafolmaldehyde solution. Cells were analyzed using CyAn (Dako, Denmark) and Summit Software, Version 4.3 (Dako).
Statistical analysis
Statistical significance for data subsets from experiments performed in cells was assessed by the two-tailed Student’s t-test. Statistical significance for in vivo data subsets was assessed by the Mann-Whitney U test (SPSS, Chicago, IL, USA).
RESULTS
Systemic ethyl pyruvate ameliorates colitis in IL-10−/− mice
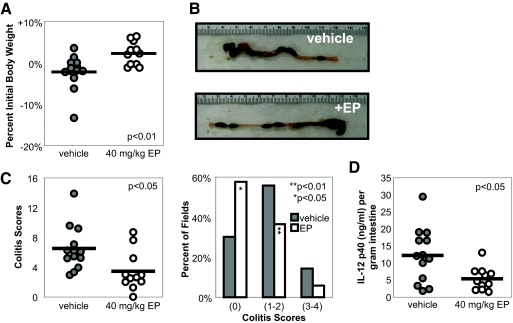
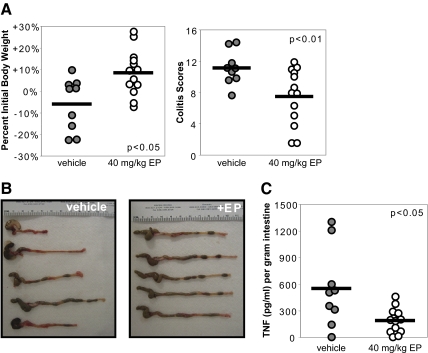
To determine whether systemic administration of ethyl pyruvate ameliorates chronic colitis in IL-10−/− mice, 10-week-old IL-10−/− mice with established colitis [19] were treated with lactated Ringer’s solution (vehicle, n=13) or 40 mg/kg ethyl pyruvate (n=11) in lactated Ringer’s solution i.p. every other day for 2 weeks. Ethyl pyruvate-treated mice demonstrated an increase in body weight compared with the vehicle treatment group (2.2% weight increase vs. 2.2% weight decrease; Fig. 1A). On gross inspection, colons from ethyl pyruvate-treated mice demonstrated improvement with increased lengths, decreased colonic wall thickening, and formed stool pellets compared with vehicle-treated mice (Fig. 1B). A 50% improvement in histological scores was observed in ethyl pyruvate-treated mice when compared with vehicle-treated mice (Fig. 1C, left) [19, 26]. Histologic scores are also presented as the percentage of fields that demonstrate no histological inflammation (colitis score of 0), mild to moderate inflammatory changes (colitis scores of 1 and 2), and severe inflammation (colitis scores of 3 and 4) to represent the spectrum of disease encountered over the entire length of the colon. Compared with the vehicle-treated group, ethyl pyruvate-treated mice had significantly more fields, demonstrating no histologic inflammation and consequently, fewer fields with inflammatory changes (Fig. 1C, right). To determine whether ethyl pyruvate alters mucosal inflammatory cytokine production, IL-12 p40 levels were measured in supernatants from colonic mucosal tissue. Intestinal explants from ethyl pyruvate-treated mice secreted significantly less IL-12 p40 compared with vehicle-treated control explants (Fig. 1D).
Figure 1.
Colitis in IL-10−/− mice is ameliorated by ethyl pyruvate administration. Ten-week-old IL-10−/− mice were treated i.p. for 2 weeks every other day with (open circles, n=11) or without (shaded circles, n=13) 40 mg/kg ethyl pyruvate (EP). (A) Ethyl pyruvate-treated mice demonstrated an overall increase in body weight versus the vehicle-treated group; P < 0.01. (B) Representative photographs of a colon from an ethyl pyruvate-treated mouse demonstrated increased length, decreased tissue thickening, and formed stool pellets compared with a vehicle-treated mouse. (C) Histologic improvement in colitis is presented as the average colitis score sum of five fields (left, P<0.05) and percent of histologic fields that demonstrate scores of 0 (no inflammation), 1–2 (mild inflammation), or 3–4 (severe inflammation; right; *, P<0.05; **, P<0.01). (D) Intestinal explants from vehicle- or ethyl pyruvate-treated mice were cultured for 24 h for measurement of spontaneous IL-12 p40 secretion by ELISA. Values were normalized to weight of intestinal explant (P<0.05).
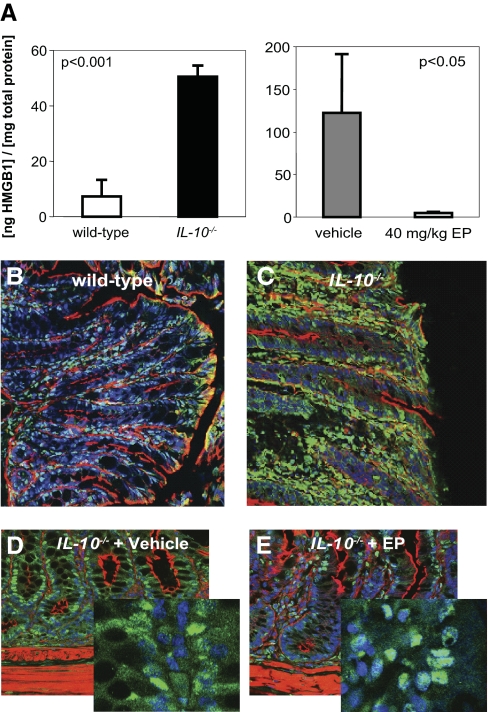
Fecal HMGB1 levels are increased in colitic IL-10−/− mice and are decreased following ethyl pyruvate treatment
HMGB1 was undetectable in the sera of wild-type or IL-10−/− mice by specific ELISA (data not shown). To investigate the effect of ethyl pyruvate on HMGB1 levels within the intestine, feces were collected at the end of the experimental protocol and assayed for HMGB1 concentration (normalized to total protein). IL-10−/− mice with active colitis (n=11, colitis score 5.2) demonstrated approximately a fivefold higher level of fecal HMGB1 (51.9±6.2 ng/mg total protein) compared with wild-type controls (n=5, colitis score 0.0, 7.2±6.0 ng/mg total protein; Fig. 2A, left). Ethyl pyruvate treatment markedly reduced fecal HMGB1 (Fig. 2A, right). There is a qualitative but not statistically significant difference in fecal HMGB1 levels in the IL-10−/− mouse cohort in the left panel of Figure 2A compared with the vehicle-treated IL-10−/− mouse cohort in the right panel. Interestingly, the mean colitis score from the IL-10−/− cohort studied in the left panel was 5.2, and the mean colitis score from the vehicle-treated IL-10−/− mice was 7.0, suggesting that fecal HMGB1 levels may correlate with the degree of intestinal inflammation. Therefore, fecal HMGB1 may be a marker of disease activity.
Figure 2.
HMGB1 expression is increased in IL-10−/− mice and decreases with ethyl pyruvate treatment. (A) Fecal HMGB1 is increased in IL-10−/− mice (right panel). Fecal samples of IL-10−/− mice (n=11, colitis score 5.2, black bar) and wild-type controls (n=5, colitis score 0.0, white bars) were analyzed for HMGB1 concentration by specific ELISA (mean±sd). Values were normalized to total fecal protein (P<0.001). Ethyl pyruvate treatment decreases fecal HMGB1 levels in IL-10−/− mice (left panel). HMGB1 levels were analyzed from vehicle (n=4; gray bar, right panel)- or ethyl pyruvate (n=4)-treated mice. Values were normalized to total protein (P<0.05). KO, Knockout. (B–E) Paraformaldehyde-fixed colons of (B) wild-type C57BL/6, (C) IL-10−/− mice, (D) IL-10−/− mice treated with vehicle, or (E) IL-10−/− mice treated with ethyl pyruvate were stained for actin (red), HMGB1 (green), and/or Draq5-nuclear stain (blue) and viewed by confocal microscopy. Each image is a representative result from at least five different mice. (B and C) Immunofluorescence demonstrates an increase in nuclear and cytoplasmic distribution of HMGB1 in the colonic epithelium of IL-10−/− mice. (D, E, and high-magnification insets) In ethyl pyruvate-treated IL-10−/− mice, compared with vehicle-treated controls, IEC immunostaining for HMGB1 was predominantly nuclear (teal-colored nuclei in the ethyl pyruvate-treated mice, representing merging of green nuclear HMGB1 immunoreactivity with the blue nuclear Draq5 stain, compared with vehicle-treated mice, where the nuclei are blue).
Ethyl pyruvate modifies intestinal RAGE and HMGB1 expression
The expression and distribution of HMGB1 and RAGE were determined by immunohistochemistry in wild-type, IL-10−/−, and IL-10−/− mice treated with ethyl pyruvate. In IL-10−/− mice, HMGB1 immunoreactivity was markedly up-regulated in the colonic epithelium (Fig. 2, C compared with B). Compared with wild-type mice, where nuclear staining predominated (Fig. 2B), increased nuclear and cytoplasmic staining was evident in IL-10−/− mice. In ethyl pyruvate-treated IL-10−/− mice, compared with vehicle-treated controls, overall HMGB1 immunoreactivity was reduced, and IEC immunostaining for HMGB1 was predominantly nuclear (teal-colored nuclei in the ethyl pyruvate-treated mice, representing merging of green nuclear HMGB1 immunoreactivity with blue nuclear Draq5 stain, compared with vehicle-treated mice, where the nuclei are blue; Fig. 2, D and E, with high-magnification insets).
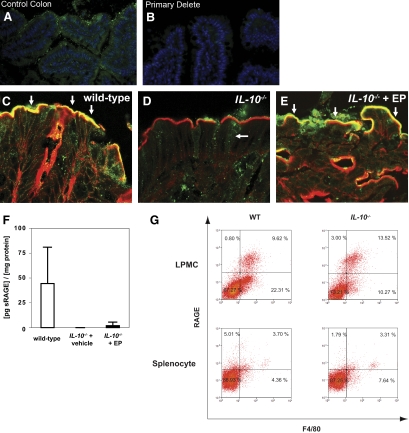
In the wild-type mouse colon, RAGE staining (green) was diffuse in the epithelium (Fig. 3A) and colocalized with actin (red) on the luminally oriented microvillus border (Fig. 3C, white arrows). Analysis of the same colonic sections with an isotype control antibody did not demonstrate specific immunoreactivity (Fig. 3B). In IL-10−/− mice, apical RAGE expression was notably down-regulated on the apical surface of the epithelium (Fig. 3D). RAGE immunoreactivity was also detected within the lamina propria (Fig. 3, A and D). In IL-10−/− mice treated with ethyl pyruvate, RAGE staining appeared similar to the wild-type intestine with prominent immunoreactivity on the apical border (Fig. 3E, white arrows). As RAGE expression has not been well-described in the intestine, protein expression was confirmed in fecal samples by ELISA. Correlating with immunohistochemistry, RAGE was detectable in fecal samples from wild-type mice but was undetectable in IL-10−/− mice with active colitis (Fig. 3E). Possibly as a result of low levels of detection (5–10 pg/ml RAGE), fecal RAGE levels did not increase significantly in IL-10−/− mice treated with ethyl pyruvate (P=0.06).
Figure 3.
Ethyl pyruvate modifies intestinal RAGE expression. Paraformaldehyde-fixed colons of (A, C) wild-type C57BL/6, (D) IL-10−/− mice, or (E) IL-10−/− mice treated with ethyl pyruvate were stained for actin (red) and RAGE (green) and viewed by confocal microscopy. (B) Absence of immunostaining of the wild-type colon with an isotype control antibody (nuclei-stained blue with Draq5). Colocalization of actin and RAGE is represented by a yellow color observed at the luminally oriented microvillus border (white arrows in C and E). Each image is a representative result from at least five different mice. Immunoflourescence demonstrates down-regulation of RAGE in the colonic epithelium of IL-10−/− mice compared with wild-type controls (C vs. D). In ethyl pyruvate-treated IL-10−/− mice (E), RAGE staining appears similar to wild-type colon. (F) Fecal extracts isolated from wild-type (n=5), IL-10−/− mice treated with vehicle (n=4), or IL-10−/− mice treated with ethyl pyruvate (ethyl pyruvate, n=4) were analyzed for RAGE by ELISA. Levels were normalized to total fecal protein. Each result represents the mean ± sd (P=0.05 for wild-type vs. IL-10, vehicle-treated; P=0.06 for wild-type vs. IL-10, ethyl pyruvate-treated). (G) LPMCs from wild-type (WT; upper panel, left) and IL-10−/− (upper panel, right) mice were stained with RAGE and F4/80 (macrophage marker) and analyzed by flow cytometry. As a control, splenocytes from wild-type (lower panel, left) and IL-10−/− (lower panel, right) mice were stained with RAGE and F4/80 for flow cytometry. Results are representative of three experiments with similar results.
To determine lamina propria cell types that express RAGE and to confirm the specificity of RAGE detection by other methods, flow cytometry was performed on colonic lamina propria mononuclear cells from wild-type and IL-10−/− mice. Virtually all RAGE expression localized to the F4/80+ macrophage population. In wild-type mice, 40% of F4/80+ lamina propria macrophages expressed RAGE, and in IL-10−/− mice, 57% of lamina propria macrophages expressed RAGE (Fig. 3G, upper panels). In comparison, RAGE expression on splenocytes is rarely detected and/or is present at extremely low levels (Fig. 3G, lower panels).
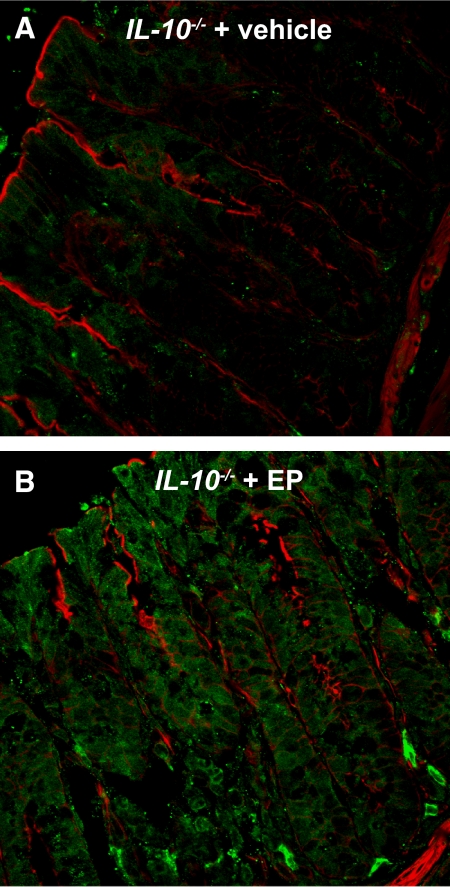
Ethyl pyruvate induces HO-1 expression in the intestinal epithelium
HO catalyzes the first and rate-limiting step in the degradation of heme to yield equimolar quantities of biliverdin, carbon monoxide, and iron [27]. We have demonstrated previously that specific induction of HO-1 ameliorates colitis in IL-10−/− mice [19]. HO-1 expression was increased throughout the epithelial crypt-villus axis in ethyl pyruvate-treated IL-10−/− mice compared with vehicle-treated controls (Fig. 4, A and B).
Figure 4.
Ethyl pyruvate induces intestinal HO-1 in IL-10−/− mice. Immunofluorescence with a specific HO-1 antibody demonstrates an increase in HO-1 staining in the colonic epithelium of ethyl pyruvate-treated IL-10−/− mice. Paraformaldehyde-fixed colonic sections of (A) vehicle- or (B) 40 mg/kg ethyl pyruvate-treated IL-10−/− mice were stained for actin (red) and HO-1 (green) and viewed by confocal microscopy. Each image is a representative result from at least five different mice.
Local delivery of ethyl pyruvate ameliorates TNBS-induced colitis
To determine whether ethyl pyruvate can ameliorate inflammation through direct mucosal effects, colitis was induced in BALB/c mice by intrarectal administration of TNBS. Mice were treated with vehicle (n=14) or 40 mg/kg ethyl pyruvate (n=14) by intrarectal delivery 4 h, 2 days, and 4 days after the administration of TNBS, and the experiment was terminated on Day 5. Mice were killed if they lost 20% or more of body weight. On Day 7, 67% (9/14) of vehicle-treated mice survived, and 93% (13/14) of ethyl pyruvate-treated mice survived (P=0.06). Surviving mice treated with ethyl pyruvate (n=13) demonstrated an increase in body weight compared with vehicle-treated mice (n=9; 8.5% weight increase vs. 6.1% weight loss, P<0.05; Fig. 5A, left). Ethyl pyruvate treatment resulted in a significant improvement in histological scores (Fig. 5A, right). Gross inspection of the intestines showed increased colon length, less colonic wall thickening, and increased stool pellets in ethyl pyruvate-treated mice (Fig. 5B). Direct mucosal ethyl pyruvate administration reduced intestinal TNF production significantly when compared with vehicle treatment (Fig. 5C).
Figure 5.
Local ethyl pyruvate administration ameliorates TNBS-induced colitis. Lactated Ringer’s solution with (open circles, n=13) or without (shaded circles, n=9) 40 mg/kg ethyl pyruvate was given intrarectally at 4 h, 2 days, and 4 days after TNBS administration and mice killed on Day 5. (A) Ethyl pyruvate-treated mice demonstrated an increase in body weight versus the vehicle-treated group (left panel; P<0.05). Colitis scores were significantly lower in the ethyl pyruvate-treated mice (right panel; P<0.01). (B) Representative photographs of colons from ethyl pyruvate-treated mice demonstrated increased lengths, decreased tissue thickening, and formed stool pellets compared with vehicle-treated mice. (C) Intestinal explants from vehicle or ethyl pyruvate-treated mice were cultured for 24 h and spontaneous TNF secretion measured by ELISA. Values were normalized to weight of intestinal explants (P<0.05).
Ethyl pyruvate inhibits IL-12 p40 and NO production in LPS-stimulated murine macrophages
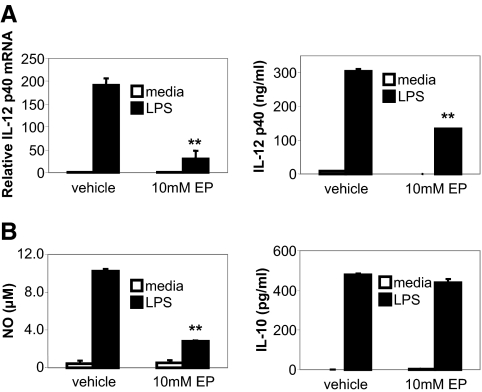
To study anti-inflammatory effects of ethyl pyruvate in cells, cytokine expression in ethyl pyruvate-treated murine macrophages was determined. Preincubation of BM-derived macrophages with ethyl pyruvate inhibited LPS-stimulated IL-12 p40 mRNA (Fig. 6A, left) and protein production (Fig. 6A, right), without affecting cell viability (>95% by Trypan blue exclusion). Also, LPS-stimulated NO levels were inhibited by ethyl pyruvate (Fig. 6B, left). Ethyl pyruvate did not affect the production of IL-10 from LPS-activated macrophages (Fig. 6B, right).
Figure 6.
Ethyl pyruvate inhibits LPS-induced IL-12 p40 and NO production in murine macrophages. BM-derived murine macrophages were incubated with 10 mM ethyl pyruvate for 1 h prior to activation with 100 ng/ml LPS. (A) Cells were harvested at 4 h for mRNA, and real-time RT-PCR assay for IL-12 p40 was performed using GAPDH as an internal control. Real-time RT-PCR was performed in duplicate and repeated three times (mean±sd). Fold induction is compared with values in vehicle/media samples (=1). Supernatants were harvested at 24 h and assayed for IL-12 p40 (A, right panel), NO (B, left panel), and IL-10 (B, right panel). Experiments were performed in duplicate and repeated three times. A representative result is shown (mean±sd). **, P < 0.01, compared with LPS-stimulated samples without ethyl pyruvate.
Ethyl pyruvate inhibits DNA binding but not nuclear translocation of NF-κB
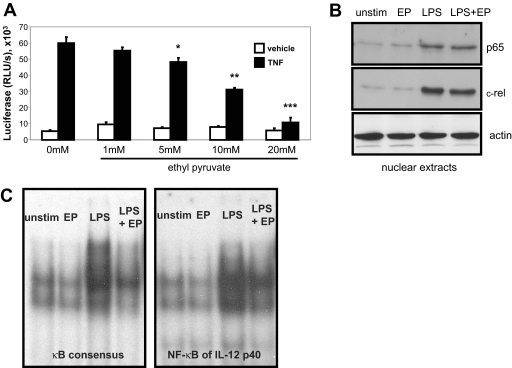
Ethyl pyruvate did not inhibit LPS-stimulated activation of MAPKs p38, JNK, or ERK (data not shown). However, ethyl pyruvate effectively abrogates TNF-induced NF-κB activity in a dose-dependent manner in the HEK293 cell line stably transfected with a multimerized NF-κB luciferase reporter gene (Fig. 7A). Stimulation of BM-derived macrophages with LPS results in increased nuclear expression of NF-κB subunits p65 and c-Rel compared with nuclear extracts from unstimulated cells. Ethyl pyruvate pretreatment of cells did not inhibit nuclear NF-κB localization (Fig. 7B).
Figure 7.
Ethyl pyruvate inhibits NF-κB activity and DNA binding. (A) TNF-induced NF-κB activation is inhibited by ethyl pyruvate. HEK293 cells stably transfected with a multimerized NF-κB DNA-binding element-luciferase reporter were preincubated for 1 h with an increasing dose of ethyl pyruvate. Cells were stimulated subsequently for 2 h with 10 ng/ml rhTNF. Cell lysates were analyzed for luciferase activity. Results are expressed as relative light units (RLU)/s. Experiments were performed in triplicate and repeated three times. A representative result is shown (mean±sd). *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with TNF-stimulated samples without ethyl pyruvate. (B) Nuclear translocation of NF-κB is not inhibited by ethyl pyruvate. BM-derived murine macrophages were incubated with 10 mM ethyl pyruvate for 1 h before stimulation with 100 ng/ml LPS for 4 h. NF-κB protein expression of p65 and c-Rel in nuclear extracts were determined by Western blot. Actin was used as a loading control. This result is representative of three independent experiments. (C) Ethyl pyruvate attenuates NF-κB DNA binding. BM-derived murine macrophages were incubated with 10 mM ethyl pyruvate for 1 h before stimulation with 100 ng/ml LPS for 4 h. Nuclear extracts were incubated with a radiolabeled probe containing a murine Igκ NF-κB consensus DNA-binding element (left) or the NF-κB DNA-binding element of the IL-12 p40 promoter (right). This result is representative of three independent experiments.
To study NF-κB protein-DNA interactions, EMSAs were performed with a DNA probe containing the murine Ig κ NF-κB consensus sequence (Fig. 7C, left) or the NF-κB element of the murine IL-12 p40 promoter (Fig. 7C, right). Nuclear extracts from BM-derived macrophages treated with ethyl pyruvate showed significantly less NF-κB DNA binding compared with extracts from LPS-activated cells. Supershift experiments demonstrated the presence of NF-κB p50 and c-Rel in the DNA-binding complex (data not shown), suggesting that ethyl pyruvate may target DNA binding of p50 containing homodimers and/or heterodimers. As ethyl pyruvate attenuates NF-κB DNA binding without inhibiting nuclear translocation, a nuclear protein that restricts NF-κB DNA binding may be induced. PPAR-γ has been shown to complex with RelA and inhibit NF-κB activity [28]. However, ethyl pyruvate did not affect nuclear PPAR-γ expression in unstimulated or LPS-activated macrophages (data not shown).
Ethyl pyruvate induces HO-1 mRNA, protein, and transcriptional activity
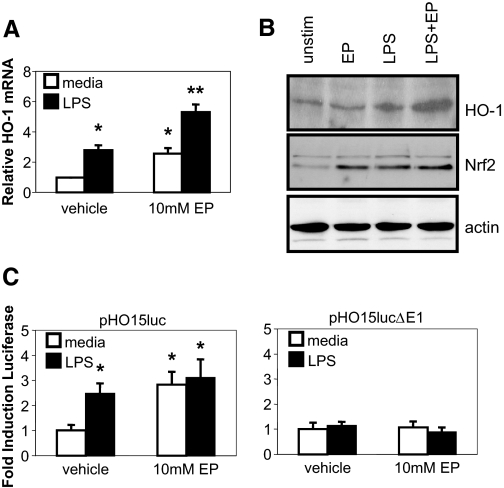
As ethyl pyruvate induces HO-1 in vivo, mechanisms of HO-1 induction were determined. Ethyl pyruvate (with or without LPS) induced HO-1 mRNA (Fig. 8A) and protein (Fig. 8B) in BM-derived macrophages. Next, transcriptional activation of the HO-1 gene by ethyl pyruvate was studied in the murine macrophage RAW264.7 cell line transfected with a luciferase reporter plasmid containing 15 kb of the murine HO-1 promoter (pHO15luc; Fig. 8C) [29]. Ethyl pyruvate alone and ethyl pyruvate plus LPS augmented HO-1 promoter activity by threefold. An enhancer region, E1, identified previously, contains an ARE important for HO-1 induction [30]. A mutant promoter construct lacking the E1 enhancer site (pHO15lucΔE1) was transfected into RAW264.7 cells. The mutant promoter was only minimally responsive to ethyl pyruvate (Fig. 8C, right). Recent evidence suggests that antioxidants, such as ethyl pyruvate, activate the ARE via activation of the transcription factor Nrf2 [31]. Ethyl pyruvate increased levels of nuclear Nrf2, correlating with increased expression of HO-1 (Fig. 8B).
Figure 8.
Ethyl pyruvate induces HO-1 mRNA, protein, and promoter activity. BM-derived macrophages were incubated with 10 mM ethyl pyruvate for 1 h prior to activation with 100 ng/ml LPS. (A) Cells were harvested at 4 h for mRNA, and real-time RT-PCR assay for HO-1 was performed using GAPDH as an internal control. Real-time RT-PCR was performed in duplicate and repeated three times (mean±sd). Fold induction is compared with values in vehicle/media samples (=1). (B) Cells were harvested at 4 h for nuclear extracts. Western blots were performed for HO-1 and Nrf2 protein. RAW264.7 cells were transiently transfected with the (C) wild-type pHO15luc or (D) pHO15lucΔE1 mutant reporter. Results are expressed as relative light units normalized to β-galactosidase activity from a cotransfected heat shock protein promoter-β-galactosidase plasmid. Fold induction of the reporter is compared with values in unstimulated cells (=1) for each group. Each result represents the mean ± sd of an experiment performed in triplicate. *, P < 0.05; **, P < 0.01, compared with unstimulated samples.
Ethyl pyruvate inhibits nuclear to cytoplasmic translocation of HMGB1 in macrophages
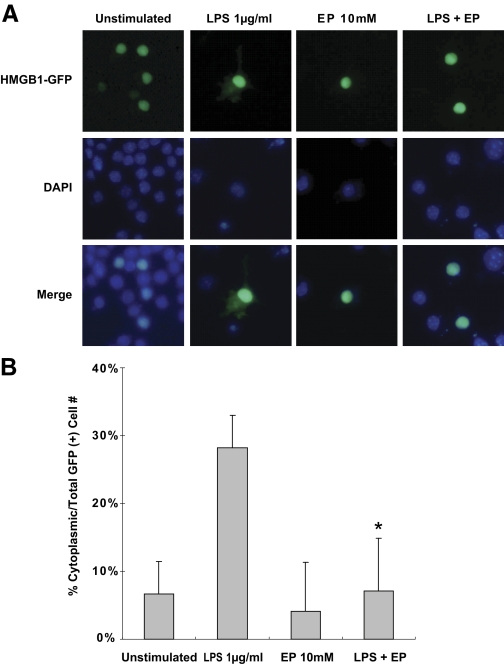
To assess whether ethyl pyruvate affects HMGB1 localization in activated macrophages, RAW 264.7 cells were transfected with a HMGB1-GFP fusion protein expression plasmid. Cells were stimulated with LPS in the presence or absence of ethyl pyruvate for 24 h and GFP localization assessed. LPS-treated cells (29%) demonstrated cytoplasmic translocation of HMGB1. Simultaneous treatment with ethyl pyruvate reduced cytoplasmic GFP expression from 29% to 9%, similar to values in unstimulated cells (Fig. 9, A and B).
Figure 9.
Ethyl pyruvate inhibits nuclear to cytoplasmic translocation of HMGB1 in macrophages. RAW 264.7 cells were transfected with a HMGB1-GFP fusion protein expression vector. After overnight culture, cells were stimulated with LPS (1 μg/ml) and/or ethyl pyruvate (10 mM) for 24 h. Nuclei were stained with DAPI. (A) Representative images from three experiments are shown, demonstrating cytoplasmic localization of HMGB1-GFP following LPS activation and inhibition of cytoplasmic translocation in LPS-activated RAW 264.7 cells treated with ethyl pyruvate. (B) Percentage of cells demonstrating cytoplasmic GFP staining (in nuclear GFP-positive cells to control for transfection efficiency) is represented. Results are expressed as mean ± sd of triplicate wells. Data were representative of three independent experiments with similar results. *, P < 0.05, versus cells stimulated with LPS alone.
DISCUSSION
Local and systemic ethyl pyruvate administration ameliorates intestinal inflammation in murine colitis models. Anti-inflammatory effects of ethyl pyruvate correlate with inhibition of intestinal HMGB1 expression and secretion. Therefore, HMGB1 likely plays an important adjuvant role in initiating and perpetuating enteric inflammation. Furthermore, HMGB1 expression is abundantly detected in the colon and may be a biomarker of disease activity. Ethyl pyruvate has pleiotropic, anti-inflammatory effects, including down-regulation of inflammatory cytokine expression, inhibition of NF-κB DNA binding, and up-regulation of HO-1.
HMGB1 has emerged as a prototypical DAMP molecule [3]. DAMPs, like HMGB1, when released outside of the cell following tissue injury, move from a reducing to an oxidizing environment. Outside of the cell, HMGB1 may alter the redox of the extracellular environment, promoting chronic inflammation [32]. Extracellular HMGB1 induces inflammatory mediators [7, 13], augments the activity of cytokines such as IL-1β [33], and is a potent chemotactic factor [34, 35]. Moreover, in septic mice, ethyl pyruvate treatment demonstrated a significant decrease in HMGB1 levels that correlated with improved mortality [17]. In IL-10−/− mice, increased fecal levels of HMGB1 are demonstrated (Fig. 2A), suggesting apical secretion of HMGB1 from IECs and/or IEC necrosis as a consequence of chronic inflammation. Immunohistochemistry in ethyl pyruvate-treated mice suggest that ethyl pyruvate may function mechanistically in part to prevent HMGB1 release/secretion, as demonstrated previously [17]. Furthermore, treatment with ethyl pyruvate decreased fecal levels of HMGB1 (Fig. 2A), which correlated with improvement in histologic disease activity (Fig. 1, C and D). A study from Maeda et al. [36] demonstrated increased serum and colonic HMGB1 in mice with DSS-induced colitis. Moreover, anti-HMGB1 antibodies attenuated acute DSS-induced colitis, and in a model of colitis-associated colon cancer (APC/min+mice treated with DSS), anti-HMGB1 antibodies reduced tumor incidence. Therefore, HMGB1 may have pathogenic significance and diagnostically may be a marker of disease activity.
There are several defined receptors for HMGB1, including RAGE [11,12,13]. Given the prominent apical location of intestinal RAGE immunostaining, IEC RAGE may be produced in a secreted form, sRAGE. The detection of RAGE in fecal samples by ELISA could represent sRAGE, and/or it may represent membrane RAGE expressed on sloughed or necrotic IECs. As HMGB1 is secreted apically into the gastrointestinal lumen by IECs, and RAGE has an apical location, it is interesting to speculate that HMGB1-RAGE interactions on the apical surface of epithelia modulate immune responses in the gut. RAGE binds several DAMPs and therefore, is an attractive target for therapeutic intervention in IBD. A small study has demonstrated amelioration of colitis by sRAGE administration in IL-10−/− mice [37]. Furthermore, a specific antibody that recognizes carboxylated glycans on RAGE-attenuated murine colitis in an adoptive T cell transfer model [15]. Likewise, HMGB1 is a ligand for the pattern recognition receptors TLR2 and TLR4 [12, 13]. Studies have demonstrated up-regulation of intestinal TLR2 and TLR4 in human IBD [38, 39], Analyses of TLR function in murine intestine suggest dichotomous roles in maintaining homeostasis in normal hosts but perpetuating chronic inflammation in IBD [40]. It is intriguing to speculate that HMGB1 interactions with its receptors in the intestine during health and disease may determine whether an extracellular signal is protective or inflammatory.
NF-κB family members have been implicated as key activators of inflammatory responses [41, 42]. We observed substantial inhibition of NF-κB DNA binding by ethyl pyruvate and consequent inhibition of NF-κB target genes, such as IL-12 p40 and NO. Ethyl pyruvate inhibits NF-κB DNA-binding activity but not nuclear translocation. We speculate that NF-κB regulation by ethyl pyruvate may be related to its intracellular antioxidant properties. This is supported by evidence that antioxidants impair NF-κB p50 DNA binding, as p50 DNA binding can be rescued by the addition of the reducing agent, DTT [43]. This suggests that redox-sensitive amino acids are important for NF-κB DNA binding [44].
Using pharmacologic inducers of HO-1, amelioration of colitis was demonstrated in IL-10−/− mice [19]. HO-1 gene expression is induced through activation of the transcription factor Nrf2 [45]. This transcription factor is also important in antioxidant-driven HO-1 expression mediated through the E1 enhancer site that contains the ARE and the Nrf2-binding site [30, 31]. Therefore, we also speculate that ethyl pyruvate induces HO-1 expression in cells and in vivo, through mechanisms involving its antioxidant properties [46], and nuclear translocation of the redox-sensitive transcription factor Nrf2.
Anti-inflammatory effects of ethyl pyruvate are demonstrated in two different murine models of IBD using two routes of administration. Intrarectal administration of the haptenating agent TNBS has been described to result in colitis mediated by Th1 cytokines [47]. However, for short-term studies, it is more accurate to describe this model as an acute intestinal injury. Nevertheless, this model provides several advantages for testing local administration of ethyl pyruvate by the intrarectal route. First, the distribution of colonic injury parallels the distribution of locally applied therapy, as TNBS and ethyl pyruvate are given intrarectally. Furthermore, as ethyl pyruvate augments compromised intestinal barrier function [48], TNBS colitis is an ideal model, as altered barrier function is well described [49]. In comparison, IL-10−/− mice represent a spontaneously occurring model of chronic colitis. Importantly, in this study, ethyl pyruvate was administered systemically to mice with established inflammation and hence, was effective as a therapeutic rather than a preventative agent.
In summary, ethyl pyruvate, a simple aliphatic ester derivative of pyruvate, has pleiotropic, anti-inflammatory effects, including modulating HMGB1 secretion/release/receptor activation, attenuating NF-κB DNA binding, and inducing HO-1. Ethyl pyruvate demonstrated significant anti-inflammatory effects in vivo in acute and chronic murine colitis. As a lipophilic agent, ethyl pyruvate can be contemplated as a therapeutic modality that can be delivered directly to the gastrointestinal tract by the oral or intrarectal route. It is likely to be safe, as ethyl pyruvate is a common additive in beverages and confectionary products, and its safety profile has been studied extensively in animals [50, 51]. This study provides proof of concept to support further investigations of ethyl pyruvate as an anti-inflammatory modality in human IBD. Furthermore, HMGB1 may play an adjuvant role in initiating and perpetuating enteric inflammation. Targeting HMGB1 with ethyl pyruvate and other modalities could represent a novel, therapeutic approach in chronic inflammatory diseases.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants 2 RO1 DK54452 (S. E. P.), NRSA F30 ES013617 (S. H. D.), 1 PO1 CA 101944 (M. T. L.), and P30 DK34987 (Immunotechnologies Core, S.E.P.); Crohn’s and Colitis Foundation of America (CCFA) Research Fellowship Award (K. M.) and CCFA Senior Research Award (S. E. P.).
Footnotes
Abbreviations: APC=allophycocyanin, ARE=antioxidant response element, BM=bone marrow, DAMP=damage-associated molecular pattern, DAPI=4′-6-diamidino-2-phenylindole, DSS=dextran sodium sulfate, h=human, HEK=human embryonic kidney, HMGB1=high mobility group box 1, HO=heme oxygenase, IBD=inflammatory bowel disease, IEC=intestinal epithelial cell, IL-10−/−=IL-10-deficient, LPMC=Lamina propria mononuclear cell, Nrf2=NF-E2-related factor-2, PPAR-γ=peroxisome proliferator-activated receptor γ, RAGE=receptor for advanced glycation end products, sRAGE=soluble RAGE, TNBS=2,4,6-trinitrobenzene sulfonic acid
References
- Xavier R J, Podolsky D K. Unraveling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Sartor R B. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Czura C J, Sama A E, Tracey K J. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164:1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- Fink M P. Ethyl pyruvate: a novel anti-inflammatory agent. J Intern Med. 2007;261:349–362. doi: 10.1111/j.1365-2796.2007.01789.x. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Ma Y, Weis J H, Vogel S N, Weis J J. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi M E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Andersson U, Wang H, Palmblad K, Aveberger A C, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey K J. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsung A, Sahai R, Tanaka H, Nakao A, Fink M P, Lotze M T, Yang H, Li J, Tracey K J, Geller D A, Billiar T R. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Erlandsson-Harris H. HMGB1 is a potent trigger of arthritis. J Intern Med. 2004;255:344–350. doi: 10.1111/j.1365-2796.2003.01303.x. [DOI] [PubMed] [Google Scholar]
- Sappington P L, Yang R, Yang H, Tracey K J, Delude R L, Fink M P. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- Schmidt A M, Yan S D, Yan S F, Stern D M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Wang H, Ding A, Golenbock D T, Latz E, Czura C J, Fenton M J, Tracey K J, Yang H. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- Park J S, Svetkauskaite D, He Q, Kim J Y, Strassheim D, Ishizaka A, Abraham E. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, Roth J. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52:847–853. doi: 10.1136/gut.52.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikrishna G, Turovskaya O, Shaikh R, Newlin R, Foell D, Murch S, Kronenberg M, Freeze H H. Carboxylated glycans mediate colitis through activation of NF-κ B. J Immunol. 2005;175:5412–5422. doi: 10.4049/jimmunol.175.8.5412. [DOI] [PubMed] [Google Scholar]
- Sims C A, Wattanasirichaigoon S, Menconi M J, Ajami A M, Fink M P. Ringer’s ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med. 2001;29:1513–1518. doi: 10.1097/00003246-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura C J, Fink M P, Tracey K J. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappington P L, Fink M E, Yang R, Delude R L, Fink M P. Ethyl pyruvate provides durable protection against inflammation-induced intestinal epithelial barrier dysfunction. Shock. 2003;20:521–528. doi: 10.1097/01.shk.0000092697.10326.8b. [DOI] [PubMed] [Google Scholar]
- Hegazi R A, Rao K N, Mayle A, Sepulveda A R, Otterbein L E, Plevy S E. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med. 2005;202:1703–1713. doi: 10.1084/jem.20051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanford L E, Enghild J J, Valnickova Z, Petersen S V, Schaefer L M, Schaefer T M, Reinhart T A, Oury T D. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE) J Biol Chem. 2004;279:50019–50024. doi: 10.1074/jbc.M409782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S L, Finn A J, Dave S H, Meng F, Lowell C A, Sanghera J S, DeFranco A L. Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-β. J Leukoc Biol. 2000;67:405–414. doi: 10.1002/jlb.67.3.405. [DOI] [PubMed] [Google Scholar]
- Plevy S E, Gemberling J H, Hsu S, Dorner A J, Smale S T. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol Cell Biol. 1997;17:4572–4588. doi: 10.1128/mcb.17.8.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Zhu C, Li F, Hegazi R, He K, Babyatsky M, Bauer A J, Plevy S E. Inhibition of interleukin-12 p40 transcription and NF-κB activation by nitric oxide in murine macrophages and dendritic cells. J Biol Chem. 2004;279:10776–10783. doi: 10.1074/jbc.M313416200. [DOI] [PubMed] [Google Scholar]
- Zhu C, Gagnidze K, Gemberling J H, Plevy S E. Characterization of an activation protein-1-binding site in the murine interleukin-12 p40 promoter. Demonstration of novel functional elements by a reductionist approach. J Biol Chem. 2001;276:18519–18528. doi: 10.1074/jbc.M100440200. [DOI] [PubMed] [Google Scholar]
- Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath M R. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. 2007;2:2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- Berg D J, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach M W, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R, Marver H S, Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970;75:410–421. [PubMed] [Google Scholar]
- Kelly D, Campbell J I, King T P, Grant G, Jansson E A, Coutts A G, Pettersson S, Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- Alam J, Wicks C, Stewart D, Gong P, Touchard C, Otterbein S, Choi A M, Burow M E, Tou J. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000;275:27694–27702. doi: 10.1074/jbc.M004729200. [DOI] [PubMed] [Google Scholar]
- Gong P, Stewart D, Hu B, Li N, Cook J, Nel A, Alam J. Activation of the mouse heme oxygenase-1 gene by 15-deoxy-δ(12,14)-prostaglandin J(2) is mediated by the stress response elements and transcription factor Nrf2. Antioxid Redox Signal. 2002;4:249–257. doi: 10.1089/152308602753666307. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kang M I, Watai Y, Tong K I, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A, Lotze M T. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- Dumitriu I E, Bianchi M E, Bacci M, Manfredi A A, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol. 2007;81:84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- Palumbo R, Galvez B G, Pusterla T, De Marchis F, Cossu G, Marcu K B, Bianchi M E. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-κB activation. J Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Hikiba Y, Shibata W, Ohmae T, Yanai A, Ogura K, Yamada S, Omata M. Essential roles of high-mobility group box 1 in the development of murine colitis and colitis-associated cancer. Biochem Biophys Res Commun. 2007;360:394–400. doi: 10.1016/j.bbrc.2007.06.065. [DOI] [PubMed] [Google Scholar]
- Hofmann M A, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath M F, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt A M. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Cario E, Podolsky D K. Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann M, Kiessling S, Mestermann S, Webb G, Spottl T, Andus T, Scholmerich J, Herfarth H, Ray K, Falk W, Rogler G. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Hao L, Medzhitov R. Role of Toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Murphy T L, Cleveland M G, Kulesza P, Magram J, Murphy K M. Regulation of interleukin 12 p40 expression through an NF-κ B half-site. Mol Cell Biol. 1995;15:5258–5267. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q W, Kashiwabara Y, Nathan C. Role of transcription factor NF-κ B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- Tse H M, Milton M J, Piganelli J D. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med. 2004;36:233–247. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Han Y, Englert J A, Yang R, Delude R L, Fink M P. Ethyl pyruvate inhibits nuclear factor-κB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther. 2005;312:1097–1105. doi: 10.1124/jpet.104.079707. [DOI] [PubMed] [Google Scholar]
- Andreadi C K, Howells L M, Atherfold P A, Manson M M. Involvement of Nrf2, p38, B-Raf, and nuclear factor-κB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol. 2006;69:1033–1040. doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]
- Fink M P. Ethyl pyruvate: a novel anti-inflammatory agent. Crit Care Med. 2003;31:S51–S56. doi: 10.1097/00003246-200301001-00008. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss I J, Blumberg R S. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- Sappington P L, Han X, Yang R, Delude R L, Fink M P. Ethyl pyruvate ameliorates intestinal epithelial barrier dysfunction in endotoxemic mice and immunostimulated caco-2 enterocytic monolayers. J Pharmacol Exp Ther. 2003;304:464–476. doi: 10.1124/jpet.102.043182. [DOI] [PubMed] [Google Scholar]
- Neurath M F, Pettersson S, Meyer zum Buschenfelde K H, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-κ B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Andersen P H, Jensen N J. Mutagenic investigation of flavorings: dimethyl succinate, ethyl pyruvate and aconitic acid are negative in the Salmonella/mammalian-microsome test. Food Addit Contam. 1984;1:283–288. doi: 10.1080/02652038409385855. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations and the World Health Organization Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives. 2001 http://jecfa.ilsi.org/. [Google Scholar]