Abstract
The bacterial or host determinants of lethality associated with respiratory Francisella infections are currently unknown. No exo- or endotoxins that contribute to the severity of this disease have been identified. However, a deregulated host immune response upon infection is characterized by an initial 36- to 48-h delay followed by a rapid and excessive inflammatory response prior to death at 72–120 h. Here, we extend these findings by comparing host immune responses between sublethal and lethal respiratory infections of mice with an attenuated transposon mutant (Mut) of F. novicida (F.n.) strain U112 (sublethal) versus the wild-type (WT) strain (lethal). Infection with WT bacteria, but not the Mut, was characterized by sustained bacteremia and systemic dissemination of the pathogen with temporal increases in bacterial burdens in liver and spleen. Severe pathology with large foci of infiltrates associated with extensive tissue damage was evident in WT-infected lungs, and Mut-infected mice displayed much reduced pathology with intact lung architecture. Similar to other experimental models of severe sepsis, WT- but not the Mut-infected mice exhibited a robust increase in numbers of Gr1+ and CD11b+ cells, while displaying a significant depletion of αβ T cells. Further, a dramatic up-regulation of multiple cytokines and chemokines was observed only in lethal WT infection. In addition, an earlier and larger increased expression of S100A9, a known mediator of sepsis, was observed in WT-infected mice. Taken together, these results show that a hyperinflammatory host immune response, culminating in severe sepsis, is responsible for the lethal outcome of respiratory tularemia.
Keywords: tularemia, 58 kDa mutant, hypercytokinemia, bacteremia
Introduction
F.n. is a Gram-negative coccobacillus, which causes tularemia. Francisella tularensis subsp. tularensis or Type A strains have been listed by the Center for Disease Control as category A bioterrorism agents, owing to their extremely low infectious dose and easy mode of dispersal [1]. Although clinical manifestation of the disease varies with the route of infection and the organs affected, respiratory infections with F. tularensis subsp. tularensis present the deadliest form of disease, resulting in 30–40% mortality with an infectious dose of one to 10 organisms [2, 3]. The murine model organism F.n. mimics this condition by leading to severe lung pathology and death of the experimental host within 3–5 days [4].
The search for potential virulence factors of Francisella has led to identification of several proteins, including those contained within a Francisella pathogenicity island, which are required by the organism to replicate within the host cells [5,6,7]. However, no exo- or endotoxin activity was attributed to these proteins, which could be directly responsible for the lethality of Francisella infections [8]. Nevertheless, it is likely that bacterial and host determinants are involved in the disease severity associated with this infection, although any direct evidence in this regard is limited.
Recent genome sequence analysis of the virulent prototype strain SchuS4 led to identification of a family of five hypothetical proteins unique to Francisella [9]. A spontaneous mutant of a type A strain of Francisella was found to harbor a defective version of one of these genes (FTT_0918) that encodes for a 58-kDa hypothetical protein, and this mutant was found to be attenuated for infection in mice via the intradermal route [10]. In the current study, we have used an attenuated transposon mutant of F.n. (Mut) lacking a homologue of this protein (locus tag FTN_0444) and have found that it replicates in vivo but causes only a mild pulmonary infection. We hypothesized that a comparison of infections elicited by WT and Mut organisms would identify host responses that lead to mortality.
Host innate immune responses to Francisella strains are highly dependent on the route of infection. Whereas an intradermal infection with Type B LVS or virulent Type A strain results in early production (1–2 days p.i.) of inflammatory cytokines such as IFN-γ, TNF-α, and IL-1β [11, 12], infection via the respiratory route with virulent Type A strain or with F.n. does not elicit detectable amounts of these signature proinflammatory cytokines until 3 days p.i. [4, 13,14,15]. Studies from our laboratory have shown that during murine respiratory tularemia, this initial period of delay in the innate immune response is followed by bacteremia and an excessive up-regulation of multiple host immune mediators [4]. Such disproportionate immune responses are typically associated with severe sepsis, which causes multiple organ failure and mortality in various disease models [16,17,18].
In this study, we compared the pulmonary immune responses elicited upon infection with the Mut and the WT strain U112. Respiratory infection with Mut lacked evidence of sustained bacteremia and failed to elicit hypercytokinemia found in the WT infection, even when bacterial burdens in lungs were similar. The results indicate a causal relationship of severe sepsis with lethality in respiratory tularemia.
MATERIALS AND METHODS
Bacterial strains and mice
The WT F.n. strain U112 and the transposon mutants lacking the 58-kDa protein (locus tag FTN_0444) were kindly provided by Dr. Larry Gallagher (University of Washington, Seattle, WA, USA) as a two-allele set [19]. The bacteria were grown on TSA medium at 37°C. After an overnight growth, the bacteria were harvested and suspended in a freezing medium (250 mM sucrose, 10 mM sodium phosphate, pH 7.2, and 5 mM glutamic acid). Stocks were aliquoted and frozen at −80°C for further use.
All of the in vivo experiments were performed using 6- to 8-week-old female C57BL/6 purchased from Charles River Laboratories (Wilmington, MA, USA). The animal use protocols were approved by the Institutional Animal Care and Usage Committee at University of Texas at San Antonio (San Antonio, TX, USA) and followed federal guidelines.
Antibodies
IF staining and FACS analysis of cellular infiltrates were performed using R-PE-conjugated hamster anti-mouse αβ TCR-β chain mAb (BD PharMingen, Franklin Lakes, NJ, USA) for αβ T cells, a purified rat anti-mouse Gr1 mAb, clone Ly-6G (Clone Accurate Chemical, Westbury, NY, USA), followed by the secondary antibody RRX-conjugated Affipure goat anti-rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for IF staining and an APC-conjugated antibody for FACS analysis of PMN cells. An R-PE-conjugated rat anti-mouse CD11b mAb (BD PharMingen) was used for monocytes. Appropriate isotype control antibodies were used for FACS analysis. For detection of CD4+ and CD8+ cells by IF staining, rat anti-mouse CD4 mAb or rat anti-mouse CD8 mAb (R&D Systems), followed by Alexa 488-conjugated chicken anti-goat antibody (Molecular Probes, Eugene, OR, USA), was used. For FACS analysis, an APC-Cy7-conjugated rat anti-mouse CD4 mAb (BD PharMingen) for CD4+ T cells and an Alexa-647-conjugated rat anti-mouse CD8 antibody (BD PharMingen) for CD8+ T cells were used. A goat anti-S100A9 antibody (R&D Systems, Minneapolis, MN, USA), followed by a Cy3 conjugated affipure F(ab′)2 fragment rabbit anti-goat antibody (Jackson ImmunoResearch Laboratories), was used for S100A9 detection in frozen lung sections. For detection by immunoblotting, a HRP-conjugated donkey anti-goat antibody (Jackson ImmunoResearch Laboratories) was used as a secondary antibody.
Infection of mice
Mice were anesthetized with a mixture of ketamine hydrochloride and xylazine (30 mg/ml ketamine, 4 mg/ml xylazine in PBS) and were infected intranasally with 3 × 102 CFUs of the WT or the Mut bacteria in 20 μl PBS. In our hands, this dose of WT bacteria resulted in a 100% mortality rate by 96–120 h p.i. For some experiments, mice were infected with 2 × 105 CFUs of the Mut bacteria in 20 μl PBS. The mock-inoculated mice received 20 μl PBS intranasally.
Survival and bacterial burden
Mice were monitored daily for signs of disease, which typically included piloerection, hunched gait, lethargy, and eye discharge. The survival was recorded for up to 3 weeks p.i. In some experiments, the mice infected with WT and various doses of Mut bacteria were killed at 6, 24, 72, and 120 h p.i., and blood was collected before perfusing the mice with sterile PBS. The lungs, liver, and spleen were harvested after perfusion and homogenized aseptically in cold PBS with Complete™ protease inhibitor cocktail (Roche Diagnostics, Germany). For the bacterial burden analyses, the homogenates were serially diluted in sterile PBS and plated on TSA. CFU counts/organ were calculated after incubating the plates at 37°C overnight. The blood collected from animals was plated similarly to determine CFU counts.
Multi-analyte profile analysis
The lung homogenates were centrifuged at 2000 × g for 15 min to clear cellular debris, and the supernatants were frozen immediately at −80°C. The biomarker levels in lung homogenates were determined commercially by Rules-Based Medicine (Austin, TX, USA) using a multiplexed flow-based system: mouse MAP™ (multi-analyte profiles) analysis technology.
Histological and IF staining
For histological and IF staining, frozen lung tissues were processed as described previously [4]. The images were acquired using a Leica DMR epifluorescent microscope (Leica Microsystems, Wetzlar, Germany) with an attached cooled charged-coupled device (CCD) SPOT RT camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA). The images were processed and analyzed using Adobe Photoshop 7.0 (Adobe Software, Mountain View, CA, USA).
Flow cytometry analysis
Lungs were harvested from mice at 72 h p.i. after perfusion with PBS and treated with collagenase to obtain lung cells as described previously [20]. To prepare samples for flow cytometry analysis, cells were first treated with FcR-blocking antibodies for 15 min at 4°C, followed by staining with fluorescently conjugated antibodies at 4°C for 15 min. Flow cytometry was performed using a FACSArray™ (Becton Dickinson, San Diego, CA, USA) FACS analysis machine equipped with 532 and 635 nm lasers. Cells were stained singly or doubly for lymphoid-specific markers or singly for myeloid-specific markers. The lymphoid-specific markers that were analyzed included αβ TCR, CD4, and CD8, and myeloid-specific markers were CD11b and Gr1. Appropriate isotype control antibodies were used to determine the levels of background staining. FlowJo (Tree Star, Ashland, OR, USA) software was used to analyze the FACS data.
Immunoblotting
Equal amounts of lung homogenates were run on 12% SDS-PAGE and transferred to PVDF membranes, which were blocked with 5% skim milk in PBS for 1 h and then incubated with appropriate dilution of goat anti-S100A9 antibody in 1% milk in PBS plus 0.05% Tween 20. The membranes were then incubated with donkey anti-goat IgG conjugated to HRP for 1 h. The signal was detected by ECL (Amersham Biosciences, Piscataway, NJ, USA). Total pixel intensity of the S100A9 protein band in each sample was quantified using the IPLab 4.0 imaging software (BD Biosciences, Rockville, MD, USA). The same blots were probed with anti-GAPDH antibody to ascertain equal loading of samples in all lanes.
Statistical analysis
Statistical comparison between FACS data of different experimental groups was performed by Student’s t-test using Sigma Plot 8.0.
RESULTS
In vitro characterization of the 58-kDa mutant
The 58-kDa mutant was provided as a two-allele set with transposon insertions at positions 962 (Mut) and 349 (Mut-allele 2) relative to the 1671-nucleotide open reading frame. The insertion positions were confirmed by PCR amplification as described in the F.n. transposon mutant collection resource (http://francisella.org/transposons.htm). Our preliminary studies showed that transposon insertion resulted in lack of expression of the 58-kDa hypothetical protein in Mut and Mut-allele 2 bacteria (Supplemental Fig. 1A). Comparison of in vitro growth kinetics of mutant and WT bacteria in the murine macrophage cell line J774A.1 showed attenuated growth of mutant bacteria within host cells (Supplemental Fig. 1B). The attenuated growth of mutant bacteria was a result of the host environment, as the mutant bacteria grew at rates similar to WT bacteria in permissive medium (trypticase soy broth, Supplemental Fig. 1C). Further, a phagosomal integrity assay, performed as described before [21], showed that although the Mut bacteria were able to escape from a phagosome, their initial localization in the phagosome as well as their egress into the host cell cytosol were delayed in comparison with the WT bacteria (Supplemental Fig. 1D). As both mutant alleles behaved similarly, all subsequent experiments were carried out with Mut bacteria only.
The 58-kDa transposon mutant is highly attenuated for infection in vivo
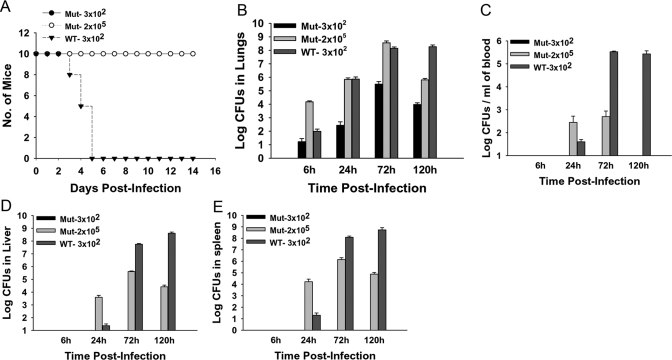
Mice infected intranasally with 3 × 102 CFUs (lethal dose 100%) of the WT bacteria displayed overt signs of infection characterized by piloerection and lethargy by 72 h p.i. By 96–120 h p.i., the condition of the mice deteriorated, showing severe eye discharge and hunched gait, and the mice usually died within a few hours. The Mut strain, on the other hand, was highly attenuated for infection in vivo, as mice infected intranasally with a similar dose of Mut bacteria survived the infection with no signs of illness at any of the times p.i. (Fig. 1A).
Figure 1.
The 58-kDa mutant is highly attenuated in vivo. Mice were inoculated intranasally with 3 × 102 CFUs of the WT or 3 × 102 or 2 × 105 CFUs of Mut bacteria in 20 μl PBS. Mock control mice received 20 μl PBS alone. (A) Survival of the mice was recorded daily over a period of 3 weeks. WT-infected mice showed disease symptoms by 72 h and became moribund and succumbed to infection by 120 h p.i., whereas mutant-infected mice remained healthy throughout the infection period; n = 10/group for this representative of three experiments. (B) Bacterial burden was enumerated in lungs harvested from Mut- and WT-infected mice at 6, 24, 72, and 120 h p.i. Mice infected with 3 × 102 CFUs of the Mut bacteria displayed lower bacterial loads than those infected with similar doses of the WT bacteria at all times p.i.-tested. Bacterial loads in mice infected with 2 × 105 CFUs of Mut bacteria were higher than WT-infected mice at 6, 24, and 72 h p.i. By 120 h p.i., the bacterial burden was reduced in Mut-infected mice, whereas it remained high in WT-infected mice at that time; n = 3–5/group. A Representative of three independent experiments is shown. Bacterial burden in (C) blood, (D) liver, and (E) spleen of mice infected with 3 × 102 CFUs of WT or 2 × 105 CFUs of Mut bacteria. No bacterial loads were detected in these organs from mice infected with 3 × 102 CFUs of Mut bacteria. n = 3–5/group. A Representative of three independent experiments is shown.
Intranasal infection with the Mut results in bacterial replication without bacteremia
To test bacterial burdens, lungs, liver, spleen, and blood from mice infected with 3 × 102 CFUs of WT or Mut, bacteria were collected at various times p.i., homogenized, and plated on TSA plates. Although the Mut bacteria replicated in the lungs of mice over time, the mice infected with WT bacteria harbored 10- to 10,000-fold more organisms in lungs as compared with those infected with a similar dose of mutant bacteria throughout the duration of the infection period (Fig. 1B). The mice infected with Mut bacteria did not display any symptoms of disease, even when their lungs harbored 3.1 × 105 ± 1.6 × 105 CFUs at 72 h p.i. By 120 h p.i., the numbers of bacteria in Mut-infected lungs dropped (9.5×103±3.3×103 CFUs), suggesting a resolution of the infection. On the other hand, the bacterial burden in WT-infected mice lungs remained high at 120 h p.i. (1.8×108±6.7×107), the time when the mice were moribund or had succumbed to the infection.
To analyze bacterial dissemination to other organs, bacterial burden analyses were carried out in liver, spleen, and blood harvested from animals infected with WT or Mut bacteria. We did not recover any bacteria from liver, spleen, or blood harvested from mice infected with 3 × 102 CFUs of the Mut bacteria at any time p.i. (Fig. 1, C–E), indicating the absence of bacteremia and systemic dissemination. The mice infected with 3 × 102 CFUs of WT bacteria exhibited bacteremia and systemic dissemination, as indicated by recovery of bacteria in spleen, liver, and blood at 24 h p.i. The bacterial burden increased rapidly at 72 h and remained high at 120 h p.i., a time when the mice were moribund or had succumbed to infection.
To exclude the possibility that improved survival of Mut-infected mice was a result of a reduced bacterial burden in lungs compared with WT-infected mice, survival and bacterial burden in organs of mice infected with a dose of 2 × 105 CFUs of Mut bacteria was assessed. The mice survived with no apparent illness, even after inoculation with a 600-fold higher dose of the Mut versus WT organism (Fig. 1A). At 6 h p.i., the bacterial burden in lungs of high-dose Mut-infected mice was greater than WT (1.5×104±2.6×103 Mut bacteria vs. 0.98×102±0.44×102 WT bacteria), reflecting a higher number of mutant bacteria reaching the organ initially (Fig. 1B). At 72 h p.i., the bacterial burden in lungs was similar in WT-infected mice compared with the higher dose Mut-infected mice (3.6×108±1.4×107 Mut bacteria vs. 1.4×108±3.9×107 WT bacteria), but only WT-infected mice showed the characteristic symptoms of disease. By 120 h p.i., the numbers of bacteria in Mut-infected lungs dropped (6.4×105±1.8×105 in Mut-infected mice vs. 1.8×108±6.7×107 in WT-infected mice), suggesting a resolution of the infection (Fig. 1B).
Bacterial dissemination to other organs could be detected in mice inoculated with the 600-fold higher dose of Mut (Fig. 1, C–E). These mice displayed bacterial burdens higher than that in WT-infected mice in spleen, liver, and blood at 24 h p.i. By 72 h p.i., the numbers of mutant bacteria recovered from these tissues were 2–3 logs less than those of WT bacteria at the same time-point. By 120 h p.i., the number of mutant bacteria decreased in liver and spleen, and importantly, no bacteria were recovered in blood at that time p.i.
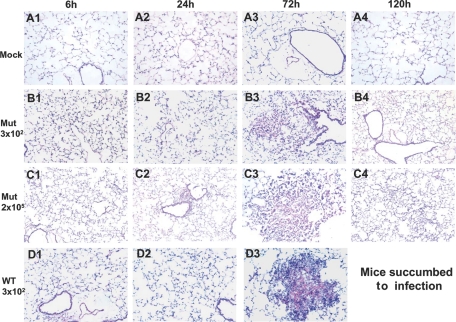
Infection with Mut bacteria is associated with reduced lung pathology
Because of the absence of overt symptoms of disease in mice infected with the mutant, we compared the pathological outcome in the lungs of mice infected with Mut and WT bacteria. H&E-stained lung sections of mock control mice displayed clear air spaces with no cellular infiltration at any time p.i. (Fig. 2, A1–A4). The WT-infected animals showed a characteristic delayed response, where cellular infiltration was limited until 72 h p.i. (Fig. 2, D1 and D2). However, by this time-point, significant cellular infiltration and extensive pathology were evident in the lung tissue with massive cell death occurring in the center of large granuloma-like areas of infiltration (Fig. 2, D3). In contrast, the lungs of mice infected with the same dose of mutant bacteria showed some areas of hypercellularity by 6 h p.i. (Fig. 2, B1). However, such hypercellularity was not apparent at 24 h p.i. (Fig. 2, B2). At 72 h p.i., the lungs from Mut-infected mice were characterized by infiltration of mononuclear cells, but the areas of infiltration were substantially smaller and fewer in number compared with WT-infected lungs (Fig. 2, B3). The cells in these areas appeared viable, plus the center of these smaller areas of infiltration lacked evidence of cellular debris, characteristic of cell death that was observed in WT-infected lung lesions. By 120 h p.i., the cellular infiltration had resolved, and lungs looked clear (Fig. 2, B4).
Figure 2.
The Mut-infected mice lungs display reduced pathology. Mice were infected intranasally with 3 × 102 CFUs of the WT or 3 × 102 or 2 × 105 CFUs of Mut bacteria in 20 μl PBS. Mock control mice received 20 μl PBS alone. At 6, 24, 72, and 120 h p.i., the lungs of mice were isolated, sectioned, and stained with H&E. (A1–A4) Representative sections from lungs of mock mice at 6, 24, 72, and 120 h p.i., respectively. (B1–B4) Representative lung sections from mice infected with 3 × 102 CFUs of Mut at 6, 24, 72, and 120 h p.i. (C1–C4) Representative lung sections of mice infected with 2 × 105 CFUs of Mut at 6, 24, 72, and 120 h p.i. (D1–D3) Representative lung sections of mice infected with 3 × 102 CFUs of WT at 6, 24, and 72 h p.i. Data are from one representative experiment of three performed (n=3 for each group/experiment). Original magnification, ×200.
The lungs of mice infected with the higher dose (2×105 CFUs) of Mut displayed defined areas of cellular infiltrates by 24 h p.i. (Fig. 2, C2). At 72 h p.i., these areas of infiltration grew larger but lacked evidence of cell death and debris found in WT-infected animals (Fig. 2, C3). By 120 h p.i., the areas of infiltration appeared to resolve with only some hypercellularity evident (Fig. 2, C4). Thus, mice infected with Mut bacteria displayed reduced lung pathology in comparison with the WT-infected mice, which were independent of the bacterial burden in lungs.
Altered kinetics of cellular infiltrates in the lungs of mice infected with the Mut bacteria
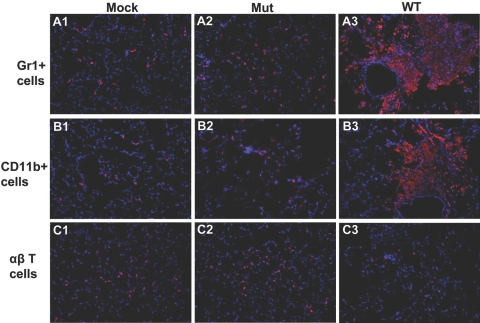
To examine the types of cells infiltrating the lungs of mutant and WT-infected mice, in situ IF staining and FACS analysis were performed on lungs for granulocytes (Gr1+), monocytes (CD11b+), and αβ T cells. These cell types were selected, owing to their role in Francisella pathogenesis as described earlier [22].
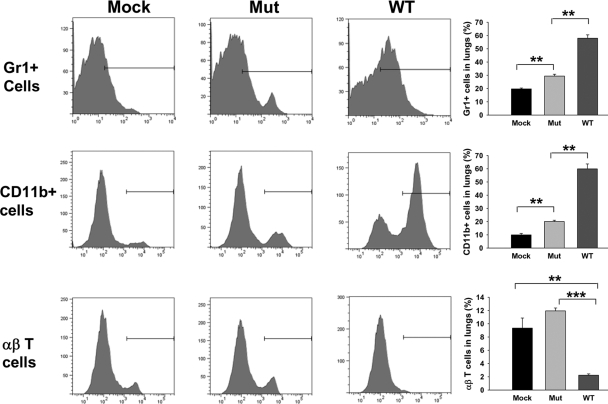
Recruitment of Gr1+ cells in lungs of WT- and Mut-infected lungs showed an initial delay of 48 h (data not shown), followed by substantially increased numbers in the WT-infected lungs at 72 h p.i., particularly in perivascular and peribronchial lesion areas (Fig. 3, A3). Mut-infected lungs, on the other hand, showed a more moderate increase in the number of Gr1+ cells at 72 h p.i. (Fig. 3, A2) These cells appeared activated and enlarged and were seen dispersed throughout the lung parenchyma, unlike the WT-infected lungs, where these Gr1+ cells were concentrated in lesion areas. The infiltration of Gr1+ cells was quantified by flow cytometry analysis, which confirmed the results of IF staining (Fig. 4, top row). The Mut-infected mice showed a significant increase in the number of Gr1+ cells as compared with mock (P<0.003). WT-infected animals, on the other hand, exhibited a robust increase in the number of Gr1+ cells, which was significantly higher than those in mock or Mut-infected mice (P<0.0005; Fig. 4, top row).
Figure 3.
Cellular infiltration in lungs of mice infected with the mutant is altered. Mice were infected intranasally with 3 × 102 CFUs of the WT or Mut bacteria in 20 μl PBS. Mock control mice received 20 μl PBS alone. At 72 h p.i., the lungs from mice were isolated and sectioned, and in situ IF staining was performed. (A1–A3) A purified rat anti-mouse Gr1 mAb (clone Ly-6G) and a RRX-conjugated Affipure goat anti-rat IgG were used to visualize the PMN cells. A1 represents Gr1+ cells in mock control mice; A2 shows the Gr1+ staining in Mut-infected mice, and A3 shows staining of Gr1+ cells in WT-infected mice. (B1–B3) CD11b+ myeloid cells (red) were visualized using an R-PE-conjugated CD11b mAb. B1 shows CD11b+ cells in mock lung; B2 and B3 represent CD11b+ staining in Mut- and WT-infected lungs, respectively. (C1–C3) R-PE-conjugated anti-mouse αβ TCR-β chain mAb was used to stain αβ T cells (red). C1 displays αβ T cells in mock animals, and C2 and C3 represent αβ T cell staining in Mut- and WT-infected mice lungs, respectively. Nuclei (blue) were stained with 4′6′-diamidino-2-phenylindole-dilactate. Images are from one representative experiment of three performed (n=3–4 mice/group for each experiment). Original magnification, ×200.
Figure 4.
Flow cytometry analysis of cellular infiltrates in Mut- and WT-infected lungs. Lung cells were isolated by collagenase treatment from mock control mice and mice infected intranasally with 3 × 102 CFUs of the WT or Mut bacteria at 72 h p.i. The cells were stained with (top row) anti-Gr1-APC; (middle row) anti-CD11b-PE, or (bottom row) anti-αβ TCR-β chain-PE. Appropriate isotype-matched negative controls were used to set the gates. The positively stained cells were expressed as percent of total lung cell population. Total number of lung cells (×106) in this representative experiment was 6.66 ± 0.88 in mock, 7.5 ± 2.84 in Mut-infected, and 10.66 ± 1.33 in WT-infected animals. An average of percent positive cells from three mice from a representative of three experiments is shown. **, P < 0.005; ***, P < 0.0001.
There was little difference in the number of CD11b+ myeloid cells in WT- or Mut-infected lungs when compared with the mock control mice at 6 h and 24 h p.i. (data not shown). However, the number of these cells was increased substantially at 72 h p.i. in WT-infected lungs, and similar to Gr1+ cells, CD11b+ cells were also concentrated in the perivascular and peribronchial lesion areas (Fig. 3, B3). Morphology of these cells suggested extensive cell death indicated by the amorphous staining pattern in lesion areas. The Mut-infected lungs, on the other hand, displayed a more moderate increase of CD11b+ cells at 72 h p.i. (Fig. 3, B2). These cells were spread throughout the lung parenchyma with slightly higher numbers in areas of hypercellularity, as large, defined lesions were absent in Mut-infected lungs. Importantly, the cells appeared healthy, as indicated by the well-defined cellular morphology of the stained cells (Fig. 3, B2). Flow cytometry analysis of CD11b+ cells was performed on total lung cells from mock or Mut- or WT-infected mice (Fig. 4, middle row). The quantitative analysis showed 21.4% ± 0.2% CD11b+ cells in Mut-infected lungs versus 62.0% ± 2.3% CD11b+ cells in WT-infected lungs, confirming a highly significant increase in the number of CD11b+ cells in WT-infected mice in comparison with their Mut-infected counterparts (P<0.0004).
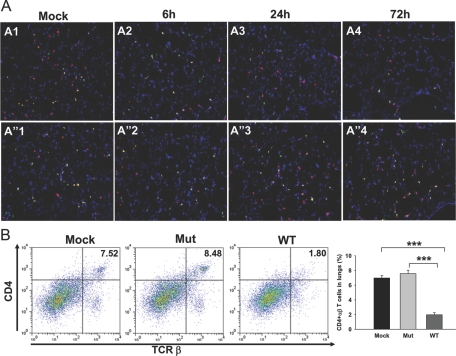
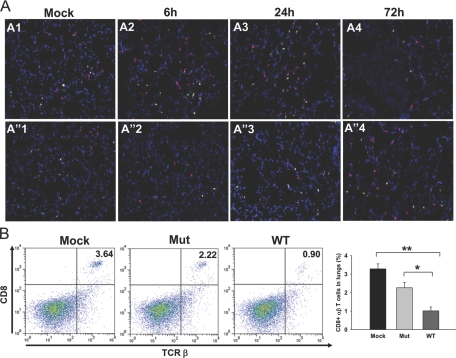
The recruitment of αβ T cells followed an initial delay of 48 h, similar to that observed with Gr1+ and CD11b+ cells (data not shown). Intriguingly, at 72 h p.i., the numbers of αβ T cells were significantly lower in WT-infected lungs as compared with those in Mut-infected lungs (Fig. 3, C3). The αβ T cell numbers in WT-infected lungs at that time-point were even lower than those in mock lungs, indicating an active depletion of these cells upon infection (Fig. 3, C1 and C3). Flow cytometry analysis of total lung cells showed that the number of αβ T cells in WT-infected lungs was 2.2% ± 0.19% of total cells versus 9.3% ± 1.5% of total cells in mock lungs (P<0.003) and 11.9% ± 0.43 in Mut-infected lungs (P<0.00002), depicting a significant loss of αβ T cells in mouse lungs upon respiratory infection with WT F.n. (Fig. 4 bottom row). The absolute numbers of αβ T cells in lungs of mock (0.60×106±0.06×106) and Mut (0.98×106±0.21×106)- and WT (0.21×106±0.01×106)-infected mice reflected similar depletion of these cells from lungs upon WT infection. As CD4+ and CD8+ cells have been shown to be important for protection against Francisella infection (reviewed in ref. [22]), frozen lung sections from mock control or WT- or Mut-infected animals were stained simultaneously for αβ T cells and CD4 or αβ T cells and CD8 T cell markers. Mock control lungs displayed a majority of the αβ T cells to be CD4+ (Fig. 5A, A1, and A”1) and fewer cells to be CD8+ (Fig. 6A, A1, and A”1). The Mut-infected mice showed a marginal increase in the number of CD4+ αβ T cells at 72 h p.i. (Fig. 5A, A”4) in comparison with mock control lungs, and the number of CD8+ αβ T cells was reduced slightly in comparison with mock lungs (Fig. 6A, A”4), although these changes were not significant. As the majority of αβ T cells in mock and Mut-infected lungs were CD4+, it is likely that CD4+ αβ T cells were preferentially depleted in WT-infected lungs at 72 h p.i. (Figs. 5A, A4, and 6A, A4). FACS analysis further confirmed the results of IF staining, where the number of CD4+ αβ T cells in mock and Mut-infected lungs was higher than the CD8+ αβ T cells (Figs. 5B and 6B). The depletion of CD4+ T cells was more pronounced than CD8+ T cells in WT-infected lungs (Figs. 5B and 6B). The absolute numbers of CD4+ T cells in mock (0.49×106±0.01×106), Mut (0.66×106±0.04×106), and WT (0.17×106±0.06×106) as well as CD8+ T cells in mock (0.19×106±0.01×106), Mut (0.18×106±0.02×106), and WT (0.09×106±0.006×106) reflected similar results.
Figure 5.
CD4+ αβ T cells are depleted from WT-infected mice lungs. (A) In situ IF staining was performed on frozen lung sections from mock control or mice infected intranasally with 3 × 102 CFUs of the WT or Mut bacteria at 72 h p.i. The sections were stained with anti-αβ TCR-β chain-PE (red) and anti-CD4 followed by Alexa-488-conjugated secondary antibody (green). A1 and A”1 represent mock control mice. A2–A4 are lungs from mice infected with WT at 6, 24, and 72 h p.i., respectively. A”2–A”4 represent lungs from Mut-infected mice at 6, 24, and 72 h p.i., respectively. Representative images from two independent experiments, each with three to four mice/group, are shown. Original magnification, ×100. (B) Lung cells from mock and Mut- and WT-infected mice were analyzed for CD4+ αβ T cells by FACS at 72 h p.i. The cells were stained simultaneously with anti-αβ TCR-β chain-PE and anti-CD4-APC-Cy7. Lung cells stained singly for the αβ TCR-β chain or CD4 were used to set the gates. The double-positive cells are expressed as percent of total lung cell population. Total number of lung cells (×106) in this representative experiment was 5.70 ± 1.27 in mock, 8.7 ± 0.76 in Mut-infected, and 11.83 ± 0.49 in WT-infected animals. An average of percent positive cells from three mice from a representative of two experiments is shown. ***, P < 0.0001.
Figure 6.
Infiltration of CD8+ T cells in lungs after infection with Mut or WT bacteria. In situ IF staining was performed on frozen lung sections from mock control or mice infected intranasally with 3 × 102 CFUs of the WT or Mut bacteria at 72 h p.i. The sections were stained with anti-αβ TCR-β chain-PE (red) and anti-CD8 followed by Alexa-488-conjugated secondary antibody (green). (A, A1 and A”1) Mock control mice. (A, A2–A4) Lungs from mice infected with WT at 6, 24, and 72 h p.i., respectively. (A, A”2–A”4) Lungs from Mut-infected mice at 6, 24, and 72 h p.i., respectively. Representative images are from two independent experiments, each with three to four mice. Original magnification, ×200. (B) Lung cells from mock and Mut- and WT-infected mice were analyzed for CD8+ αβ T cells by FACS at 72 h p.i. The cells were stained simultaneously with anti-αβ TCR-β chain-PE and anti-CD8-Alexa-647. Lung cells stained singly for αβ TCR-β chain or CD8 were used to set the gates. The double-positive cells are expressed as percent of total lung cell population. Total number of lung cells (×106) in this representative experiment was 5.70 ± 1.27 in mock, 8.7 ± 0.76 in Mut-infected, and 11.83 ± 0.49 in WT-infected animals. An average of percent positive cells from three mice from a representative of two experiments is shown. *, P <0.05; **, P < 0.005.
Hypercytokinemia is absent in lungs of Mut-infected mice, but the kinetics of cytokine up-regulation is similar to WT
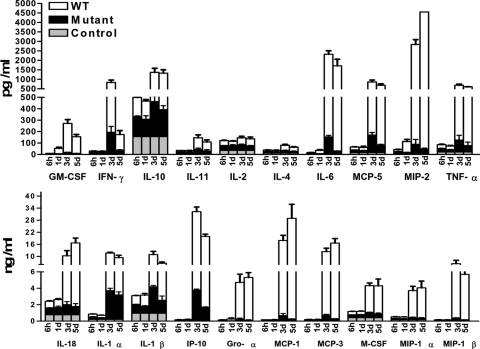
Using a multiplex assay designed for measurement of rodent inflammatory biomarkers at the protein level in tissue samples, quantitative analyses of several cytokines, chemokines, and metabolites were performed on lung homogenates to obtain an overview of the pulmonary immune response in mice infected with WT and Mut bacteria. Consistent with the absence of any cellular infiltration at 6 h and 24 h p.i., WT F.n.-infected mice exhibited no increase in the production of characteristic proinflammatory cytokines until 72 h p.i. (Fig. 7 and Supplemental Table 1), including cytokines associated with the inflammasome [23]. However, by 72 h p.i., levels of almost all of the biomarkers tested were up-regulated by 10- to 500-fold in WT-infected lungs (Fig. 7). In contrast, Mut-infected mice displayed the same kinetics of cytokine up-regulation but at much lower levels (Fig. 7 and Supplemental Table 1). The highly elevated concentrations of IL-6, KC/Gro-α (CXCL1), MCP-1 (CCL2), and IP-10 (CXCL10) in WT-infected mice compared with Mut (Fig. 7) are of particular interest, as these cytokines at excessive levels correlate with disease severity, sepsis, and mortality [24, 25].
Figure 7.
Hypercytokinemia is absent from lungs of Mut-infected mice. The lungs from mock control and mice infected intranasally with 3 × 102 CFUs of Mut or WT bacteria were harvested at 6 h, 1 day, 3 days, and 5 days p.i., homogenized in PBS with protease inhibitors, and analyzed for rodent multi-analyte profile (Rules-Based Medicine). Results shown are average of three infected and mock control mice from two to three independent experiments.
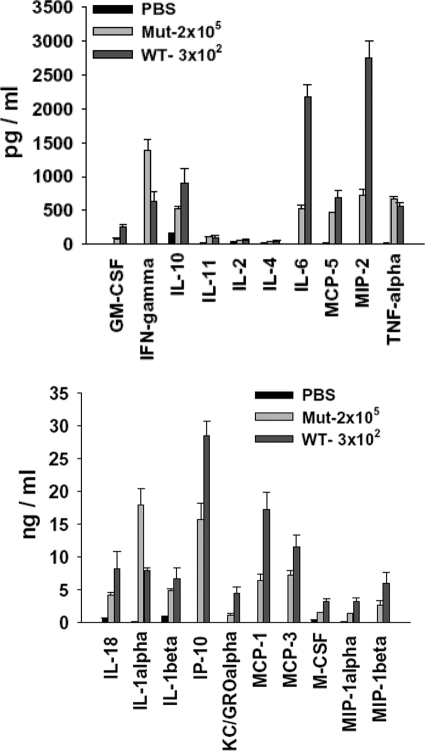
To rule out the possibility that reduced bacterial burden in lungs of Mut-infected mice might be contributing to the lower levels of immune mediators tested, lung homogenates from mice infected with 2 × 105 CFUs of mutant bacteria at 72 h p.i. were analyzed by multiplex assay. The levels of severe sepsis-associated mediators such as IL-6, MCP-1, IP-10, and KC/Gro-α were still lower, even upon infection with a high dose of mutant bacteria as compared with the WT (Fig. 8). Intriguingly, IFN-γ, IL-1α, and TNF-α were up-regulated at higher levels in these mice as compared with the WT, indicating differential up-regulation of certain host mediators (Fig. 8).
Figure 8.
Bacterial load in lungs does not contribute to the absence of hypercytokinemia in Mut-infected mice. The lungs from mice infected with 2 × 105 CFUs of Mut or 3 × 102 CFUs of WT bacteria were harvested at 72 h p.i., homogenized, and analyzed for rodent multi-analyte profile (Rules-Based Medicine). Results shown are average of three infected mice from two independent experiments for Mut and three independent experiments for WT.
S100A9, a severe sepsis mediator, is expressed at much lower levels in mutant-infected lungs
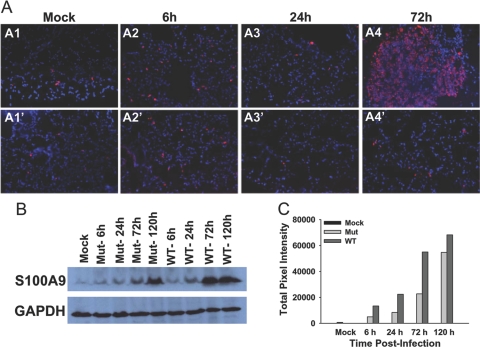
S100A9 is a damage-associated mediator of severe sepsis, which has been shown to be up-regulated during murine respiratory tularemia [4]. We sought to compare the expression of this protein during respiratory infection with Mut and WT bacteria. Consistent with bacteremia and up-regulation of sepsis-associated cytokines, the WT-infected lungs displayed up-regulated levels of S100A9 at 72 h p.i. with little or no change at 6 h and 24 h p.i. in comparison with mock-infected mice (Fig. 9A, A1–A4). The increased expression of this protein was associated with areas of lesions as well as the lung parenchyma in WT-infected mice. In contrast, the mutant-infected lungs showed little or no change in expression of S100A9 in comparison with mock control mice lungs at all the times p.i.-tested (Fig. 9A, A1‘–A4’). The expression of S100A9 was also tested by Western blot analysis of lung homogenates from Mut- and WT-infected mice. As observed with the IF staining, WT-infected lung homogenates at 72 h and 120 h p.i. showed a substantial increase in amount of S100A9 while displaying only marginal changes at 6 h and 24 h p.i., as compared with mock control levels (Fig. 9B). Mut-infected lungs also showed an increase in expression of S100A9 at 72 h p.i., albeit at much lower levels than in the WT-infected counterparts at that time (Fig. 9B). However, at 120 h p.i., Mut-infected lungs exhibited a further increased expression of this protein, approaching the level in WT-infected lungs at this time-point. The same blots were probed with anti-GAPDH antibody, which confirmed similar loading of samples in all lanes (Fig. 9B). The S100A9 band intensities were quantified in terms of total pixel intensities using the imaging software IPLab 4.0. Figure 9C represents a numerical approximation of the observations made by IF staining and immunoblotting.
Figure 9.
S100A9 expression is up-regulated in the lungs of WT-infected mice. (A) S100A9 expression was assessed using in situ IF staining of lung sections from mice infected with 3 × 102 CFUs of Mut or WT bacteria. (A1–A4) A1 depicts S100A9 expression in mock control; A2–A4 represent images of WT-infected lungs at 6, 24, and 72 h p.i., respectively. (A1’–A4’) A1’ shows S100A9 expression in mock lung; A2’–A4’ show Mut-infected lungs at 6, 24, and 72 h p.i., respectively. Images are from one representative experiment of two performed (n=3–4 mice/group). Original magnification, ×200. (B) Lung homogenates from mock control (Lane 1) and from mice infected with 3 × 102 CFUs of Mut at 6, 24, 72, and 120 h p.i. (Lanes 2–5, respectively) or WT bacteria (Lanes 6–9) were run on polyacrylamide gels, transferred to PVDF membranes, and probed with anti-S100A9 antibody. Immunoblotting with anti-GAPDH antibody was performed to monitor equal loading of samples. Data represent at least three independent experiments. (C) A plot of total pixel intensity of the S100A9 band in each lane of one representative blot is depicted. The pixel intensities were quantified using IPLab 4.0 imaging software.
DISCUSSION
The cause of extreme virulence and high mortality associated with respiratory tularemia is unknown. A 30-kb region of the genome comprising a Francisella pathogenicity island has been identified [26], but the genes of the pathogenicity island have been shown to be required mostly for the intracellular survival of the bacterium rather than explaining the high virulence of F. tularensis [7, 27, 28]. Bacterial or host factor(s) possibly contributing to disease severity are yet to be identified. The current study demonstrates that lethal pulmonary infection with F.n. results in hypercytokinemia or production of overwhelming levels of immune mediators reminiscent of severe sepsis. On the other hand, infection with a mutant strain of F.n., defective in causing lethal infection, elicits much reduced levels of these immune mediators. These results suggest that an unbridled immune response resulting from severe sepsis contributes to disease severity in murine respiratory tularemia.
The 58-kDa protein belongs to a family of five hypothetical proteins, which are unique to Francisella having no homologies to any known proteins [9]. A spontaneous mutant of a Type A strain harboring a defective version of this 58-kDa protein has been shown previously to be attenuated for infection in mice via the normally lethal intradermal route [10]. In the present study, mice infected intranasally with a transposon mutant of F.n. lacking this protein survived the infection. In addition, “Allele-2” of the 58-kDa mutant with transposon insertion at a different site in the gene was similarly attenuated. Moreover, the transposon insertion did not have any apparent polar effects, as another transposon mutant lacking the downstream gene FTN_0445 of this apparent two-gene operon was virulent for infection in mice (Supplemental Fig. 2). These data provide strong evidence that the null mutation of the 58-kDa protein alone is responsible for the attenuation. A range of bioinformatics tools failed to identify any motifs that might indicate any possible function of this protein. Thus, the role of this protein in Francisella pathogenesis needs to be determined experimentally.
After initial uptake by the host cells, Francisella transiently resides in a phagosome before escaping into the host cell cytosol, where replication of bacteria occurs. As cytosolic escape is essential for growth of Francisella within the host cells, we determined whether absence of the 58-kDa protein affected this property of F.n. Although several mutants defective in intracellular growth fail to escape the phagosome [29, 30], the 58-kDa transposon Mut in the current study was able to escape the phagosome. However, the process of initial localization in phagosome as well as the egress into the cytosol were delayed in comparison with the WT bacteria. Other studies in our laboratory show that entry of Mut bacteria into macrophages as well as into alveolar epithelial cells occurs by mechanisms different than that of WT bacteria (unpublished data). The fact that absence of this protein affects the growth of bacteria in the host cells and not so much in permissive media in vitro suggests an important function of this protein in host-pathogen interactions in Francisella infection. Regardless, our laboratory has been using this 58-kDa/FTN_0444 attenuated mutant as an important tool to elucidate host-pathogen interactions that contribute to disease severity in respiratory tularemia.
Intranasal infection with the WT F.n. resulted in extremely high bacterial burdens in lungs, liver, and spleen of infected mice. The infection also resulted in bacteremia, which is a hallmark of severe sepsis [31]. Blood-borne bacteria have been widely implicated in the pathogenesis of severe sepsis and septic shock, leading to widespread tissue injury, shock, and death [32]. Bacteremia, in the case of respiratory pathogens, is closely associated with the ability of pathogens to evade pulmonary immune responses and to disseminate away from the site of initial entry, which makes pneumonic infections a leading cause of sepsis [18, 33]. Additionally, a study of patients with bacteremia revealed that the mortality was significantly higher in patients with bacteremia, arising from pulmonary or abdominal infection than from any other route [31, 34]. Studies from our and other laboratories have shown that upon pulmonary infection, Francisella can disseminate to systemic organs, resulting in bacteremia and multiple organ failure [4, 35, 36]. The absence of bacteremia from low-dose Mut-infected mice or of sustained bacteremia in the high-dose Mut mice suggests that the ability of WT F.n. to remain blood-borne contributes to the mortality associated with this infection.
The initial delay in cellular infiltration as well as the production of inflammatory cytokines in WT- and Mut-infected mice correlated with earlier observations that F. tularensis may cause an initial active suppression of host immune responses in lungs [13]. Alternatively, it may hide from innate immune responses by using cell surface receptors otherwise involved in tolerance induction and immune suppression [37]. Pathogens as diverse as Mycobacterium and HIV target these receptors to enter host immune cells, thereby escaping immune activation [38, 39]. Francisella has also been shown to use mannose receptor 1 to gain entry into murine macrophages [40, 41]. However, how such interactions affect the immunological outcome of the disease is currently unknown. Nonetheless, this initial delay has been postulated as a virulence mechanism of Francisella infection [13, 14]. Whether this initial delay predisposes the host to subsequent severe sepsis leading to death is questionable in light of the similar delay observed with the sublethal infection with the mutant in the current study. Clearly, other mechanisms are contributing to the extreme virulence of this organism. An interesting possibility is that the hypothetical 58-kDa protein may have an important role in eliciting a hyperactive host response, resulting in tissue damage, or it is involved in tissue damage directly as a toxin or a toxin regulator. We are currently performing proteomic analyses of the Mut and WT bacteria to test these hypotheses.
Following an initial 2-day delay, a massive influx of inflammatory cells was observed in lungs of WT-infected mice. This infiltration constitutes cells of myeloid as well as lymphoid origin [4, 22]. CD11b+ cells are thought to be the primary cell type for infection and replication of Francisella, and these cells represent the predominant subset in the lesions of mice infected with Francisella [42, 43]. Increased numbers of these cells may be the cause of increased pathology and cell death in the lungs of WT-infected mice, as macrophages infected with the LVS strain have been shown to undergo apoptosis in vitro [44, 45]. Also, it would be of interest to see whether there are differences in the ability of mutant and WT bacteria to infect CD11b+ cells, as Mut-infected lungs displayed much reduced numbers of these cells and carried less of a bacterial burden.
The depletion of αβ T cells at later time-points of infection from lungs of WT-infected mice is intriguing, as Mut-infected mouse lungs were not depleted of these cells. In Francisella infections, the majority of studies involving T cell-mediated immune responses has been performed using a LVS (Type B attenuated mutant) murine infection model using sublethal intradermal infections in which T cell-deficient mice succumb to overwhelming bacterial burdens [22]. Following vaccination with LVS, CD4+ and CD8+ T cells appear to be required for protection against a respiratory challenge with virulent Type A F. tularensis [46, 47]. The effector T cell mechanisms that control the infection mainly involve IFN-γ and/or TNF-α [47,48,49], but bacterial killing is partially mediated by NO produced by activated macrophages [50, 51]. However, no studies have been performed to date showing the status of T cells in lungs of mice following primary pulmonary infection with a lethal dose of F. tularensis. Nevertheless, a low-dose aerosol exposure of mice was shown to induce thymic atrophy and thymic depletion of CD4+CD8+ thymocytes [52]. Additionally, a sublethal intranasal infection of LVS in mice elicits a significantly lower number of IFN-γ+ CD4+ T cells in lungs compared with mice infected via the intradermal route [53]. Taken together, specific T cell depletion in respiratory tularemia may well be a virulence mechanism contributing to the inability of the host to transition from an innate to adaptive immune response, which is known to depend on IFN-γ-producing T cells. Also, T cell depletion from WT F.n.-infected mouse lungs is in line with previous studies depicting loss of T cells during sepsis in experimental models as well as in trauma patients [54, 55]. This defect in the onset of adaptive immunity constituting Th-1 response in WT-infected mice seems to be contributing to lethality of the infection, as Mut-infected mice, exhibiting higher numbers of CD4+ T cells, do much better. The mechanism of T cell depletion during murine respiratory tularemia is a topic of current research in our laboratory.
An overwhelming increase in levels of inflammatory cytokines was observed in WT-infected mice following a 2-day delay. These levels are similar to what has been observed in cases of severe sepsis, where hypercytokinemia, an uncontrolled immune response, leads to multiple organ failure [56]. In contrast, Mut-infected mice exhibited cytokine up-regulation at much lower levels. This may be an indication that a more regulated immune response takes place in the Mut-infected lungs. The levels of severe sepsis-associated cytokines IL-6, IL-8, MCP-1, and IP-10 [24, 25] were much lower in mice infected with even 2 × 105 CFUs of mutant bacteria and harboring bacterial burdens higher than those in WT-infected mice. On the other hand, IFN-γ and TNF-α were produced in a higher amount in these mice. As for many intracellular pathogens, TNF-α and IFN-γ are the prototype cytokines associated with protective Th1 responses during Francisella infection [57, 58]. These cytokines play an important role in generation of effector molecules such as reactive oxygen species and nitrogen species, which are responsible for killing of intracellular microbes. In the current study, the amount of these cytokines elicited upon infection with lower doses (3×102 CFUs) of Mut bacteria appears to be sufficient to clear the infection from lungs. However, when the bacterial burden is increased upon infection with higher doses of Mut bacteria (2×105 CFUs), a selective amplification of the levels of these cytokines occurs, which likely controls the bacterial replication and ultimately clears the infection. Such results emphasize the need for an adequate and appropriate innate immune response. In contrast, in WT mice with similarly high bacterial burdens in lungs, the lower amounts of these cytokines seem inadequate to control bacterial replication, possibly contributing to bacteremia. A recent study describing the immunologic parameters of host death versus survival in a murine pulmonary F. tularensis LVS model indicated IL-6, MIP-2, and MCP-1 as immune markers of mortality, as they were detected only in the lungs of moribund mice but not in those of mice that survived the disease, and IL-1β was detected only in surviving mice [35]. However, in our studies, the mice infected with the Mut bacteria survived the infection, but at the same time, these cytokines were produced at levels significantly higher than the control mice, albeit in much lower amounts that those in the WT-infected mice. Thus, the excessive amounts of the immune mediators produced in response to the WT infection seem to play a major role in the lethal outcome of the disease.
The S100A9, a pleiotropic inflammatory marker protein, is reported to induce neutrophil chemotaxis [59], to mediate apoptosis [60], to activate the NF-κB pathway [61], and to modulate NADPH oxidase activity by its property to bind arachidonic acid [62]. Previous studies from our laboratory have shown up-regulation of this protein in mice during F.n. infection [4]. In the current study, the reduced levels of this inflammatory marker in Mut-infected mice compared with WT-infected mice at 72 h p.i. correlate with reduced inflammation in lungs of Mut-infected mice. However, at 120 h p.i., the levels in Mut-infected lungs approached that of WT-infected mice. This indicates that S100A9 may be playing a direct role in protection and remodeling of tissue following resolution of infection. Indeed, it has been shown to exhibit protective roles such as antimicrobial activity [63], regulation of matrix metalloproteinase activity [64, 65], and wound repair [66, 67].
The current study indicates that severe sepsis, characterized by deregulated activation of host immune responses in conjunction with bacteremia at later stages of infection, seems to be the cause of host death. The 58-kDa mutant presents a powerful tool to dissect molecular host-pathogen interactions during respiratory tularemia as well as other respiratory infections, which is critical given the high mortality rate of patients with sepsis following infections with virulent respiratory pathogens.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants 1P01A10157986, NS35974, and AI 59703 to J. M. T. We gratefully acknowledge Drs. Colin Manoil, Larry Gallagher, and Elizabeth Ramage (University of Washington) for providing the transposon mutant. We thank Mr. Umamahesh Gundra for technical help with FACS.
DISCLOSURE
The authors have no financial conflict of interest.
Supplementary Material
Footnotes
Abbreviations: APC=allophycocyanin, F.n.=Francisella novicida, IF=immunofluorescence, IP-10=IFN-inducible protein 10, KC=keratinocyte-derived chemokine, LVS=live attenuated strain, Mut=mutant, p.i.=postinfection, PMN=polymorphonuclear leukocyte, PVDF=polyvinylidene difluoride, RRX=Rhodamine Red-X, subsp.=subspecies, TSA=trypticase soy agar, WT=wild-type
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
References
- Dennis D T, Inglesby T V, Henderson D A, Bartlett J G, Ascher M S, Eitzen E, Fine A D, Friedlander A M, Hauer J, Layton M, Lillibridge S R, McDade J E, Osterholm M T, O'Toole T, Parker G, Perl T M, Russell P K, Tonat K, Working Group on Civilian Biodefense Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- McLendon M K, Apicella M A, Allen L A. Francisella tularensis: taxonomy, genetics, and immunopathogenesis of a potential agent of biowarfare. Annu Rev Microbiol. 2006;60:167–185. doi: 10.1146/annurev.micro.60.080805.142126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci. 2007;1105:1–29. doi: 10.1196/annals.1409.009. [DOI] [PubMed] [Google Scholar]
- Mares C A, Ojeda S S, Morris E G, Li Q, Teale J M. Initial delay in the immune response to Francisella tularensis is followed by hypercytokinemia characteristic of severe sepsis and correlating with upregulation and release of damage-associated molecular patterns. Infect Immun. 2008;76:3001–3010. doi: 10.1128/IAI.00215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra N P, Soni S, Reilly T J, Liu J, Klose K E, Gunn J S. Combined deletion of four Francisella novicida acid phosphatases attenuates virulence and macrophage vacuolar escape. Infect Immun. 2008;76:3690–3699. doi: 10.1128/IAI.00262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T M, Casey M S, Becker R H, Dorsey C W, Glass E M, Maltsev N, Zahrt T C, Frank D W. Identification of Francisella tularensis Himar1-based transposon mutants defective for replication in macrophages. Infect Immun. 2007;75:5376–5389. doi: 10.1128/IAI.00238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron G S, Nano F E. MglA and MglB are required for the intramacrophage growth of Francisella novicida. Mol Microbiol. 1998;29:247–259. doi: 10.1046/j.1365-2958.1998.00926.x. [DOI] [PubMed] [Google Scholar]
- Wayne Conlan J, Oyston P C. Vaccines against Francisella tularensis. Ann N Y Acad Sci. 2007;1105:325–350. doi: 10.1196/annals.1409.012. [DOI] [PubMed] [Google Scholar]
- Larsson P, Oyston P C, Chain P, Chu M C, Duffield M, Fuxelius H H, Garcia E, Halltorp G, Johansson D, Isherwood K E, Karp P D, Larsson E, Liu Y, Michell S, Prior J, Prior R, Malfatti S, Sjöstedt A, Svensson K, Thompson N, Vergez L, Wagg J K, Wren B W, Lindler L E, Andersson S G, Forsman M, Titball R W. The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat Genet. 2005;37:153–159. doi: 10.1038/ng1499. [DOI] [PubMed] [Google Scholar]
- Twine S, Byström M, Chen W, Forsman M, Golovliov I, Johansson A, Kelly J, Lindgren H, Svensson K, Zingmark C, Conlan W, Sjöstedt A. A mutant of Francisella tularensis strain SCHU S4 lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect Immun. 2005;73:8345–8352. doi: 10.1128/IAI.73.12.8345-8352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark S, Sunnemark D, Bucht A, Sjostedt A. Rapid local expression of interleukin-12, tumor necrosis factor α, and γ interferon after cutaneous Francisella tularensis infection in tularemia-immune mice. Infect Immun. 1999;67:1789–1797. doi: 10.1128/iai.67.4.1789-1797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovliov I, Kuoppa K, Sjostedt A, Tarnvik A, Sandstrom G. Cytokine expression in the liver of mice infected with a highly virulent strain of Francisella tularensis. FEMS Immunol Med Microbiol. 1996;13:239–244. doi: 10.1111/j.1574-695X.1996.tb00244.x. [DOI] [PubMed] [Google Scholar]
- Bosio C M, Bielefeldt-Ohmann H, Belisle J T. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J Immunol. 2007;178:4538–4547. doi: 10.4049/jimmunol.178.7.4538. [DOI] [PubMed] [Google Scholar]
- Lembo A, Pelletier M, Iyer R, Timko M, Dudda J C, West T E, Wilson C B, Hajjar A M, Skerrett S J. Administration of a synthetic TLR4 agonist protects mice from pneumonic tularemia. J Immunol. 2008;180:7574–7581. doi: 10.4049/jimmunol.180.11.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett N S, Olmos S, Durrant D M, Metzger D W. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect Immun. 2005;73:2306–2311. doi: 10.1128/IAI.73.4.2306-2311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus D C, Linde-Zwirble W T, Lidicker J, Clermont G, Carcillo J, Pinsky M R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Dremsizov T, Clermont G, Kellum J A, Kalassian K G, Fine M J, Angus D C. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest. 2006;129:968–978. doi: 10.1378/chest.129.4.968. [DOI] [PubMed] [Google Scholar]
- Knapp S, Schultz M J, van der Poll T. Pneumonia models and innate immunity to respiratory bacterial pathogens. Shock. 2005;24:12–18. doi: 10.1097/01.shk.0000191385.41689.f3. [DOI] [PubMed] [Google Scholar]
- Gallagher L A, Ramage E, Jacobs M A, Kaul R, Brittnacher M, Manoil C. A comprehensive transposon mutant library of Francisella novicida, a bioweapon surrogate. Proc Natl Acad Sci USA. 2007;104:1009–1014. doi: 10.1073/pnas.0606713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Juarrero M, Orme I M. Characterization of murine lung dendritic cells infected with Mycobacterium tuberculosis. Infect Immun. 2001;69:1127–1133. doi: 10.1128/IAI.69.2.1127-1133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin A, Scott D W, Thompson J A, Mann B J. Identification of an essential Francisella tularensis subsp. tularensis virulence factor. Infect Immun. 2009;77:152–161. doi: 10.1128/IAI.01113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins K L, Cowley S C, Bosio C M. Innate and adaptive immunity to Francisella. Ann N Y Acad Sci. 2007;1105:284–324. doi: 10.1196/annals.1409.014. [DOI] [PubMed] [Google Scholar]
- Weiss D S, Henry T, Monack D M. Francisella tularensis: activation of the inflammasome. Ann N Y Acad Sci. 2007;1105:219–237. doi: 10.1196/annals.1409.005. [DOI] [PubMed] [Google Scholar]
- Gomes R N, Figueiredo R T, Bozza F A, Pacheco P, Amancio R T, Laranjeira A P, Castro-Faria-Neto H C, Bozza P T, Bozza M T. Increased susceptibility to septic and endotoxic shock in monocyte chemoattractant protein 1/cc chemokine ligand 2-deficient mice correlates with reduced interleukin 10 and enhanced macrophage migration inhibitory factor production. Shock. 2006;26:457–463. doi: 10.1097/01.shk.0000228801.56223.92. [DOI] [PubMed] [Google Scholar]
- Herzum I, Renz H. Inflammatory markers in SIRS, sepsis and septic shock. Curr Med Chem. 2008;15:581–587. doi: 10.2174/092986708783769704. [DOI] [PubMed] [Google Scholar]
- Nano F E, Zhang N, Cowley S C, Klose K E, Cheung K K, Roberts M J, Ludu J S, Letendre G W, Meierovics A I, Stephens G, Elkins K L. A Francisella tularensis pathogenicity island required for intramacrophage growth. J Bacteriol. 2004;186:6430–6436. doi: 10.1128/JB.186.19.6430-6436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C G, Cowley S C, Cheung K K, Nano F E. The identification of five genetic loci of Francisella novicida associated with intracellular growth. FEMS Microbiol Lett. 2002;215:53–56. doi: 10.1111/j.1574-6968.2002.tb11369.x. [DOI] [PubMed] [Google Scholar]
- Golovliov I, Sjostedt A, Mokrievich A, Pavlov V. A method for allelic replacement in Francisella tularensis. FEMS Microbiol Lett. 2003;222:273–280. doi: 10.1016/S0378-1097(03)00313-6. [DOI] [PubMed] [Google Scholar]
- Santic M, Molmeret M, Klose K E, Jones S, Kwaik Y A. The Francisella tularensis pathogenicity island protein IglC and its regulator MglA are essential for modulating phagosome biogenesis and subsequent bacterial escape into the cytoplasm. Cell Microbiol. 2005;7:969–979. doi: 10.1111/j.1462-5822.2005.00526.x. [DOI] [PubMed] [Google Scholar]
- Lindgren H, Golovliov I, Baranov V, Ernst R K, Telepnev M, Sjostedt A. Factors affecting the escape of Francisella tularensis from the phagolysosome. J Med Microbiol. 2004;53:953–958. doi: 10.1099/jmm.0.45685-0. [DOI] [PubMed] [Google Scholar]
- Brun-Buisson C, Doyon F, Carlet J. Bacteremia and severe sepsis in adults: a multicenter prospective survey in ICUs and wards of 24 hospitals. French Bacteremia-Sepsis Study Group. Am J Respir Crit Care Med. 1996;154:617–624. doi: 10.1164/ajrccm.154.3.8810595. [DOI] [PubMed] [Google Scholar]
- Bone R C, Grodzin C J, Balk R A. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–243. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- Tsai K S, Grayson M H. Pulmonary defense mechanisms against pneumonia and sepsis. Curr Opin Pulm Med. 2008;14:260–265. doi: 10.1097/MCP.0b013e3282f76457. [DOI] [PubMed] [Google Scholar]
- Weinstein M P, Murphy J R, Reller L B, Lichtenstein K A. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. II. Clinical observations, with special reference to factors influencing prognosis. Rev Infect Dis. 1983;5:54–70. doi: 10.1093/clinids/5.1.54. [DOI] [PubMed] [Google Scholar]
- Chiavolini D, Alroy J, King C A, Jorth P, Weir S, Madico G, Murphy J R, Wetzler L M. Identification of immunologic and pathologic parameters of death versus survival in respiratory tularemia. Infect Immun. 2008;76:486–496. doi: 10.1128/IAI.00862-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestal C A, Malik M, Catlett S V, Savitt A G, Benach J L, Sellati T J, Furie M B. Francisella tularensis has a significant extracellular phase in infected mice. J Infect Dis. 2007;196:134–137. doi: 10.1086/518611. [DOI] [PubMed] [Google Scholar]
- Steinman R M, Hawiger D, Nussenzweig M C. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T B, Kwon D S, Torensma R, van Vliet S J, van Duijnhoven G C, Middel J, Cornelissen I L, Nottet H S, KewalRamani V N, Littman D R, Figdor C G, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T B, Van Vliet S J, Koppel E A, Sanchez-Hernandez M, Vandenbroucke-Grauls C M, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopal A, MacFarlane A S, Mohapatra N, Soni S, Gunn J S, Schlesinger L S. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect Immun. 2006;74:5114–5125. doi: 10.1128/IAI.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulert G S, Allen L A. Differential infection of mononuclear phagocytes by Francisella tularensis: role of the macrophage mannose receptor. J Leukoc Biol. 2006;80:563–571. doi: 10.1189/jlb.0306219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger C E, Forestal C A, Italo J K, Benach J L, Furie M B. The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines. J Leukoc Biol. 2005;77:893–897. doi: 10.1189/jlb.1104637. [DOI] [PubMed] [Google Scholar]
- Rasmussen J W, Cello J, Gil H, Forestal C A, Furie M B, Thanassi D G, Benach J L. Mac-1+ cells are the predominant subset in the early hepatic lesions of mice infected with Francisella tularensis. Infect Immun. 2006;74:6590–6598. doi: 10.1128/IAI.00868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X H, Golovliov I, Sjostedt A. Francisella tularensis induces cytopathogenicity and apoptosis in murine macrophages via a mechanism that requires intracellular bacterial multiplication. Infect Immun. 2001;69:4691–4694. doi: 10.1128/IAI.69.7.4691-4694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X H, Sjostedt A. Delineation of the molecular mechanisms of Francisella tularensis-induced apoptosis in murine macrophages. Infect Immun. 2003;71:4642–4646. doi: 10.1128/IAI.71.8.4642-4646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T H, Hutt J A, Garrison K A, Berliba L S, Zhou Y, Lyons C R. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun. 2005;73:2644–2654. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne Conlan J, Shen H, Kuolee R, Zhao X, Chen W. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an αβ T cell- and interferon γ- dependent mechanism. Vaccine. 2005;23:2477–2485. doi: 10.1016/j.vaccine.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Cowley S C, Elkins K L. Multiple T cell subsets control Francisella tularensis LVS intracellular growth without stimulation through macrophage interferon γ receptors. J Exp Med. 2003;198:379–389. doi: 10.1084/jem.20030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley S C, Sedgwick J D, Elkins K L. Differential requirements by CD4+ and CD8+ T cells for soluble and membrane TNF in control of Francisella tularensis live vaccine strain intramacrophage growth. J Immunol. 2007;179:7709–7719. doi: 10.4049/jimmunol.179.11.7709. [DOI] [PubMed] [Google Scholar]
- Bosio C M, Elkins K L. Susceptibility to secondary Francisella tularensis live vaccine strain infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity. Infect Immun. 2001;69:194–203. doi: 10.1128/IAI.69.1.194-203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins K L, Cooper A, Colombini S M, Cowley S C, Kieffer T L. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect Immun. 2002;70:1936–1948. doi: 10.1128/IAI.70.4.1936-1948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kuolee R, Austin J W, Shen H, Che Y, Conlan J W. Low dose aerosol infection of mice with virulent type A Francisella tularensis induces severe thymus atrophy and CD4+CD8+ thymocyte depletion. Microb Pathog. 2005;39:189–196. doi: 10.1016/j.micpath.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolard M D, Hensley L L, Kawula T H, Frelinger J A. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of γ interferon-positive T cells. Infect Immun. 2008;76:2651–2659. doi: 10.1128/IAI.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martignoni A, Tschop J, Goetzman H S, Choi L G, Reid M D, Johannigman J A, Lentsch A B, Caldwell C C. CD4-expressing cells are early mediators of the innate immune system during sepsis. Shock. 2008;29:591–597. doi: 10.1097/SHK.0b013e318157f427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R S, Tinsley K W, Swanson P E, Schmieg R E, Jr, Hui J J, Chang K C, Osborne D F, Freeman B D, Cobb J P, Buchman T G, Karl I E. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- Riedemann N C, Guo R F, Ward P A. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins K L, Rhinehart-Jones T R, Culkin S J, Yee D, Winegar R K. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun. 1996;64:3288–3293. doi: 10.1128/iai.64.8.3288-3293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiby D A, Fortier A H, Crawford R M, Schreiber R D, Nacy C A. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier P A. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170:3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- Ghavami S, Kerkhoff C, Los M, Hashemi M, Sorg C, Karami-Tehrani F. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: the role of ROS and the effect of metal ions. J Leukoc Biol. 2004;76:169–175. doi: 10.1189/jlb.0903435. [DOI] [PubMed] [Google Scholar]
- Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H, Chazin W J, Nakatani Y, Yui S, Makino H. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor κ B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther. 2006;8:R69. doi: 10.1186/ar1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff C, Nacken W, Benedyk M, Dagher M C, Sopalla C, Doussiere J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. FASEB J. 2005;19:467–469. doi: 10.1096/fj.04-2377fje. [DOI] [PubMed] [Google Scholar]
- Sohnle P G, Hunter M J, Hahn B, Chazin W J. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14) J Infect Dis. 2000;182:1272–1275. doi: 10.1086/315810. [DOI] [PubMed] [Google Scholar]
- Yong H Y, Moon A. Roles of calcium-binding proteins, S100A8 and S100A9, in invasive phenotype of human gastric cancer cells. Arch Pharm Res. 2007;30:75–81. doi: 10.1007/BF02977781. [DOI] [PubMed] [Google Scholar]
- Isaksen B, Fagerhol M K. Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol Pathol. 2001;54:289–292. doi: 10.1136/mp.54.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorey I S, Roth J, Regenbogen J, Halle J P, Bittner M, Vogl T, Kaesler S, Bugnon P, Reitmaier B, Durka S, Wöckner M, Rieger N, Konstantinow A, Wolf E, Goppelt A, Werner S. The Ca2+-binding proteins S100A8 and S100A9 are encoded by novel injury-regulated genes. J Biol Chem. 2001;276:35818–35825. doi: 10.1074/jbc.M104871200. [DOI] [PubMed] [Google Scholar]
- Wu N, Davidson J M. Migration inhibitory factor-related protein (MRP)8 and MRP14 are differentially expressed in free-electron laser and scalpel incisions. Wound Repair Regen. 2004;12:327–336. doi: 10.1111/j.1067-1927.2004.012313.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.