Abstract
Pancreatic lipase-related protein 2 (PLRP2) is induced by IL-4 in vitro in cytotoxic T lymphocyte (CTL) clones and CTLs from immunized wild-type (WT) PLRP2+/+ are more cytotoxic than PLRP2−/− CTLs, suggesting to previous investigators that the lipase PLRP2 might support CTL functions. Here, we further evaluate PLRP2 in CTLs. We found that PLRP2 was optimally induced in splenocytes by 3.5 × 10−8 M IL-4 by day 6 after activation and was restricted to CD8+ T cells. PLRP2 mRNA was detected inconsistently (and at low levels) after activation in the presence of IL-2. Cytotoxicity in 4 h 51Cr assays of WT CTLs was ∼3-fold the activity of PLRP2−/− CTLs cultured with IL-4 and, with IL-2, was unexpectedly ∼2 fold the activity of PLRP2−/− CTLs. Thus, PLRP2 gene ablation affected short-term (perforin-dependent) cytotoxicity, even under the IL-2 conditions. Other variables failed to account for the reduced cytotoxicity. Granzyme B levels, activation markers, and CD8+ T cell frequencies were similar for WT vs. PLRP2−/− CTLs (with either cytokine). Addition of rPLRP2 to IL-4 induced PLRP2−/− CTLs (or to cytotoxic granule extracts) failed to increase lysis, suggesting that the missing mediator is more than released PLRP2. Cytotoxicity of WT and PLRP2−/− CTLs was similar in 2-day tumor survival assays with IL-4, which can be mediated by perforin-independent mechanisms. We conclude that extracellular PLRP2 lipase is unable to directly augment the cytotoxicity that was lost by PLRP2 ablation and that after reevaluation, the question of what is PLRP2’s role in CD8 T cells is still unanswered.
Keywords: CD8, pe T cellsrforin, eGFP-P815
Introduction
Digestive lipases are produced in the pancreas and secreted into the gut where the enzymes hydrolyze triglycerides and phospholipids to generate fatty acids that are then transported from the gut into the body for subsequent utilization. There are four members of the pancreatic lipase family, the classical pancreatic triglyceride lipase (PTL) and the more recently characterized pancreatic lipase-related proteins (PLRP1, PLRP2, as well as the newly recognized human PLRP3). PTL hydrolyzes triglycerides into fatty acids, while PLRP2 hydrolyzes multiple substrates, including triglycerides, phospholipids, and galactolipids [1,2,3,4,5]. In addition to its hydrolysis of multiple lipid substrates, PLRP2 has activity in the absence of colipase, a pancreatic protein, which is required for PTL activity [4,5,6,7].
While previous studies of pancreatic lipases have mainly focused on their role in digestion, other cells also express PLRP2. Gene expression of PLRP2 was previously reported in cytotoxic T lymphocyte cell lines when stimulated with IL-4 [8] and in Paneth cells, which are secretory epithelial cells in the intestinal crypts [9]. Both T and Paneth cells promote antimicrobial resistance, suggesting that PLRP2 may participate in protective responses against invading pathogens. In studies involving PLRP2−/− mice, T cells taken ex vivo 10 days after immunization with EL4 tumor cells had reduced EL-4 specific cytotoxicity when compared with that of wild-type (WT) T cells [10]. Increased WT cytotoxic activity was also found after in vitro restimulation in cultures supplemented with IL-2 [10]. In both approaches, the CTLs’ phenotype, the frequencies of the cytotoxic CD8 T cells in the population, and the extent of their development of cytotoxic granules were undefined, raising the possibility that these variables rather than PLRP2 deficiency might account for the differences in cytotoxic activity.
In this paper, we sought to account for the effect of PLRP2 deficiency and in the process eliminated several reasonable possibilities. We present the first evidence of PLRP2 protein expression in fresh isolated T cells cultured with IL-4. We found PLRP2 expression was localized to the CD8+ T cell subset, which advances the need to further evaluate the role of PLRP2 in CTL activity. The addition of the cytokine, IFN-γ (to polarize T cells toward type 1 effectors) had little effect on the expression of PLRP2 induced by the type 2 cytokine IL-4. Our investigation found that WT CTLs (cultured with either IL-2 or IL-4) demonstrated more cytotoxicity than PLPR2−/− CTLs in short-term 51Cr assays where perforin is essential, suggesting that the lipase might augment a perforin-dependent mechanism. The short-term lysis by IL-4-induced PLRP2−/− CTLs (or by cytotoxic granule extracts) was unchanged by the addition of recombinant PLRP2, which suggests that any extracellular PLRP2 lipase has a function other than to damage to target cells. In contrast, cytotoxicity of WT vs. PLRP2−/− CTLs was similar in 2-day tumor survival assays, regardless of the cytokine used to induce the CTLs. We interpret the results to mean that the gene ablation that included PLRP2 has a highly reproducible impact on rapid cytotoxic T-cell functions and that extracellular PLRP2 lipase is unlikely to mediate direct cytotoxicity.
MATERIALS AND METHODS
Cytokines
Recombinant mouse IL-2 was purchased from BD Biosciences (5×107 units/mg; 1.7×1011 units/mmol, lot 25873) or eBioscience (1×107 units/mg, 1.7×1011 units/mmol, lot E011701; San Diego, CA, USA). Recombinant mouse IL-4 was purchased from BD Biosciences (2.5×107 units/mg; 3.5×1011 units/mmole, lots 11129 and 33301; San Jose, CA, USA). Recombinant mouse IFN-γ was from PeproTech (1×107 units /mg; 1.5 ×108 units/mmol; Rocky Hill, NJ, USA).
Animal use
The animal protocols for this study were approved by the University of Nevada, Reno Animal Care and Use Committee.
Cell culture
Splenocytes were harvested from the spleens of Balb/c mice wild-type (Jackson Laboratories, Bar Harbor, ME, USA), Balb/c PLRP2−/− mice [10], and their littermate controls from Dr. Mark Lowe, bred at the University of Pittsburgh; or Balb/c Pfn−/− originally bred onto the Balb/c background by Dr. M. J. Smyth [11] and maintained at the National Cancer Institute (Frederick, MD, USA). For in vitro cultures, T cells were cultured at 5 × 105 cells/ml in T -75 flasks (Sarstedt) with complete RPMI-1640 media (Sigma Chemical Co, St. Louis, MO, USA) containing 10% fetal calf serum (HyClone, Logan, UT, USA), 10 mM HEPES buffer (Fisher Scientific, Waltham, MA, USA), 1% Pen-Strep (Sigma), 24 mM sodium bicarbonate (Fisher Scientific), 25 μM 2-mercaptoethanol (Sigma), and 2.5 μg/ml concanavalin A (conA) (Sigma), with the varying cytokine conditions indicated in the results. Following an initial 3-day culture with conA and cytokines, the T cells were washed to remove the mitogen conA and recultured in complete tissue culture medium with renewal of the respective cytokines. Cells were recultured again in fresh cytokines (500 U/ml) on day 5 if they were maintained longer. EGFP-P815 mastocytoma cells [12] were maintained in Dulbecco’s modified Eagle medium, DMEM, (Gibco, Grand Island, NY, USA) with 180 mM sodium bicarbonate (Fisher) and 10% FBS (HyClone). Cell cultures were incubated at 37°C and 5% CO2. CT.4R cells were generously given by Dr. William Paul at the National Institutes of Health [13] and grown in RPMI-1640 media containing 10% fetal calf serum, 1% Pen-Strep (Sigma), 24 mM sodium bicarbonate (Fisher), 25 μM 2-mercaptoethanol (Sigma), (2 mM) l-glutamine (Sigma), 100 mM sodium pyruvate (Sigma), and 500 U/ml mouse recombinant IL-4.
RNA extraction and analysis
Gene expression was monitored by nonquantitative and quantitative RT-PCR.
Nonquantitative RT-PCR.
T cells were counted and extracted for total RNA using TRIzol (Sigma). The extracts were flash frozen in liquid nitrogen for storage and to protect against RNase activity. The RNA was dissolved in nuclease-free water and the concentration determined with a NanoDrop ND-1000 Spectrophotometer. Reverse transcription was performed with the Ambion retroscript kit (Ambion, Austin, TX, USA). Half to 1.0 μg of total RNA was transcribed with oligo-dT primers following the manufacturer’s directions. Briefly, the RNA and primers were heated at 80°C for 3 min and placed on ice. A master mix of deoxynucleotides, RT buffer, RNase inhibitor, and reverse transcriptase was added according to the directions in a final volume of 20 μl, and the reaction was incubated in a Robocycler (Stratagene, La Jolla, CA, USA) for 1 cycle at 80°C for 3 min and 1 cycle at 44°C for 60 min followed by 92°C for 10 min. The product was amplified by PCR in an Applied Biosystems 9800 Fast Thermal Cycler. 0.4 to 1.0 μl was added to 10 μl of GeneAmp Fast PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The primer pair to amplify mouse PLRP2 was 5′-ATCTTGGTCGCTCTGTACGGATGTAACG-3′ and 5′-GTCTTTAGCGCGTTGCAGTGTGA-3′. The primer pair to amplify mouse cyclophilin, used as an internal control to monitor differences in initial amounts of RNA amplified, was 5′-ATGGTCAACCCCACCGTGTT-3′ and 5′-CGTGTGAAGTCACCACCCT-3′. PLRP2 was amplified with 34 cycles of 94°C for 15 s and 67°C for 15 s and cyclophilin was amplified with 30 cycles of 94°C for 15 s and 65°C for 15 s. The products were separated by electrophoresis in a 2% agarose gel using 1× TBE buffer. The bands were stained with ethidium bromide, and digital pictures were taken.
TaqMan quantitative PCR.
Total RNA was isolated from TRIzol samples by organic extraction with chloroform (200 μl per ml of TRIzol initial) and vortexing the mixture. After 15 min at room temperature, each sample was centrifuged at 14,000 rpm for 15 min at 4°C. The aqueous phase was collected and −20°C isopropanol (500 μl per ml initial TRIzol) was added and allowed to incubate for 10 min at room temperature followed by 1 h at −80°C. The samples were next centrifuged at 14,000 rpm for 30 min at 4°C to pellet the extracted RNA. The supernatant was removed from each RNA pellet and 75% ethanol was added (1 ml per ml initial TRIzol); the mixture was then centrifuged at 14,000 rpm for 5 min at 4°C to wash the pellet. Ethanol was removed, and the remaining residual ethanol was evaporated away. The RNA was resuspended in 20 μl RNase-free H2O and then (5 units) DNase I (Promega, Madison, WI, USA) and RNase inhibitor, RNAsin (Promega), were added to each sample. The samples were incubated at 37°C to allow for degradation of DNA and then incubated at 65°C to inactivate the DNase I. The RNA concentration for each sample was determined by measuring absorbance at 260 nm using Beckman DU 650 Spectrometer (Beckman Instruments, Fullerton, CA, USA). First-strand cDNA synthesis was performed from 2 μg of each RNA sample using 250 ng random primers and nuclease-free H20 in a final volume of 12 μl. This mixture was heated to 70°C for 10 min and quickly cooled using an Applied Biosystems 2770 Thermal Cycler. Then a master mix containing 5× First Strand buffer (Invitrogen), 10 mM DTT (Invitrogen), and 0.125 mM dATP, dTTP, dGTP, and dCTP mM dNTP mix (Promega) was added to each sample to create a volume of 18 μl. Each sample was gently pipetted to mix and incubated at 25°C for 10 min followed by 2 min at 42°C. The reaction was paused and 1 unit SuperScript RT III (Invitrogen) was added to each sample, and reverse transcription reaction was carried out with 1 cycle at 50°C for 60 min and 1 cycle at 72°C for 15 min, followed by a hold at 4°C. Remnant RNA was removed with the addition of 20 units RNaseH and incubation at 37°C for 20 min. Each resulting cDNA sample was diluted 1:5 in RNase-free H2O to a final volume of 100 μl. For the TaqMan quantitative PCR assay, each reaction was performed in triplicate in a final volume of 20 μl containing a 1× concentration of TaqMan PCR Master Mix as follows: 2 μl of the cDNA were added to 10 μl TaqMan Fast Universal PCR Master Mix (2×) (Applied Biosystems) and 7 μl RNase free H2O. The 20× TaqMan FAM-dye labeled probes to detect and amplify the cDNAs were mouse PLRP2, Mm00448214_m1; mouse cyclophilin, Mm02342429_g1; and mouse perforin, Mm00812512_m1, from Applied Biosystems. For each reaction, 1 μl of primer was added to the 1× concentration of TaqMan PCR Master Mix to create the final volume of 20 μl. The sample amplification was performed on a 7500 ABI real-time PCR System (Applied Biosystems) with initiation at 95°C for 20 s, followed by 40 cycles of 95°C for 1 s and 60°C for 20 s. The polymerase chain reaction products were monitored by the increase in the fluorescence above a fixed detection threshold. Standard curves were generated for each target gene by serial diluting the cDNA of a control sample and by plotting the log of the initial copy number of the standard vs. the threshold cycle (Ct) value. Resulting Ct values from each experimental sample were fit to a standard curve and used to calculate the quantity of target genes relative to the standard curve. All samples were normalized to cyclophilin mRNA amplified from the same samples to control for variation in sample quality.
Western blot analyses
Splenocytes, grown in 500 U/ml IL-4 or IL-2, were disrupted using nitrogen bomb cavitation. The resulting cell homogenates were layered onto a 54% Percoll (Sigma) gradient and centrifuged at 45,000 g for 20 min at 4 C. The resulting density gradient was divided into six fractions. Percoll was removed by centrifugation at 145,000 g for 4 h at 4°C. Granules and vesicles were collected from above the Percoll pellet. Dry NaCl was added to bring each fraction up to a 1.15 M concentration and the samples were extracted by freeze-thawing. The fractions were run on 10% acrylamide SDS-PAGE gels (Bio-Rad). Following transfer of the proteins to a nitrocellulose sheet, each blot was probed with polyclonal chicken antibody to a recombinant fragment of human PLRP2 (Abcam ab17740). Unfortunately, the antibody that we used in Fig. 1 to PLRP2 is currently unavailable, so that we present one experiment. We also generated weak but positive results with another chicken antibody (Abcam ab37599) using extracts of unfractionated CTLs. The probed blots were developed with secondary affinity-purified donkey anti-chicken IgG labeled with IR-Dye800 and analyzed using the Odyssey infrared imaging system (LiCor Biosciences, Lincoln, NE, USA). To detect perforin, we used rabbit polyclonal anti-rat perforin (Fitzgerald Industries, Concord, MA, USA) with secondary affinity-purified goat anti-rabbit IgG labeled with AF680 (Molecular Probes, Eugene, OR, USA). The direct, LiCor fluorescent images are presented.
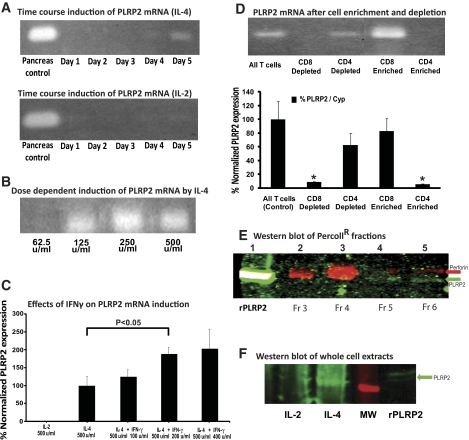
Figure 1.
Induction of PLRP2 by IL-4. Splenic T cells were activated with conA in media containing differing concentrations of murine r-IL-2 or r-IL-4. (A–D) Induction of mRNA for PLRP2. (A) Time course of induction with IL-4 and lack of induction with IL-2. Over a 5-day time course, cells were cultured with 250 U/ml of cytokine and collected. PLRP2 expression was determined by RT-PCR. Expression of PLRP2 was first detected on day 4 and increased on day 5 in samples induced with IL-4. PLRP2 expression was barely detectable on day 5 in cells cultured with IL-2. Concurrent amplification of the constitutively expressed gene for cyclophilin indicated that the starting amounts of cDNA were similar (not illustrated). (B) Dose-response with IL-4. Expression of PLPR2 responded to IL-4 in a dose-dependent manner observed on day 6. The lowest dose for expression of PLRP2 was 125 U/ml (3.5×10−10 M) r-IL-4. (C) Marginal effect of IFN-γ on induction of PLRP2 mRNA. Splenocytes were induced with IL-4 alone or IL-4 plus increasing concentration of IFN-γ. (The cells given 400 U/ml IFN-γ without other cytokines died by day 3.) PLPR2 expression was monitored on day 5 using TaqMan quantitative RT-PCR. IFN-γ actually appeared to increase the level of PLRP2 expression (by about twofold). The P values were determined by a two-tailed t test. P < 0.05 for the addition of 200 units/ml IFN-γ. The P value for comparison of IL-4 alone vs. IL-4 plus 500 units of IFN-γ was 0.12. D. CD8+ T cells preferentially express PLRP2 mRNA. To identify the T cell subset expressing PLRP2, we used magnetic bead enrichment and depletion of CD4 and CD8 T cells. The cells were induced with IL-4 and separated on day 5. RNA extracts of these cells were tested using both RT-PCR (gel detection) and TaqMan quantitative RT-PCR (bar graph) to determine the levels of PLRP2 expression. PLRP2 mRNA was normalized to cyclophilin to control for variation in sample quality and was detected in the starting cells and in two samples, CD8+ cells and cells depleted of CD4+ T cells. Data are expressed as the % change from unfractionated cells. When paired t tests were made between any of the 3 positive sets of cells and the CD8-depleted or the CD4-enriched cells, the P values were <0.05, indicated by asterisks. The 100% value was the Q RT-PCR ratio of PLRP2 mRNA to cyclophilin mRNA for the control unfractionated IL-4-induced WT cells. The actual average cycle time to PCR threshold (Ct) values for these control unfractionated IL-4-induced WT cells were 18.0 for cyclophilin and 27.0 for PLRP2, indicating that there was actually 29 (512-fold) more cyclophilin mRNA than PLRP2 mRNA in these cells. For perspective, their perforin mRNA was also in low quantity (see Fig. 5), and the actual average cycle time to PCR threshold (Ct) values were 19.7 for cyclophilin and 28.7 for perforin, indicating that there was 29 (512-fold) more cyclophilin mRNA than perforin mRNA. Thus both perforin and PLRP2 mRNAs are in low quantity in IL-4 induced cells. For the high PLRP2 IL-4-induced long-term cell line CT.4R [8], there was sixfold more PLRP2 mRNA than for the enriched CD8 T cells illustrated here. (E) Detection of PLRP2 protein by immunoblots. Whole cell extracts from day 6 T cells induced with IL-4 were fractionated over a Percoll gradient. The proteins in the different fractions were loaded and separated on a 10% SDS-PAGE gel. The resulting blot was developed with polyclonal antibodies to perforin (imaging data presented in red, 680 nm) and to PLRP2 (imaging data presented in green, 800 nm). Perforin was enriched in the dense Percoll fraction 4 (lane 3), while PLRP2 was in the lightest fraction 6 (lane 5). (F) Detection of PLRP2 protein in whole cell extracts. Whole cell extracts were made from day 6 T cells induced with either IL-4 or IL-2. Cell extracts were loaded onto 7.5% SDS-PAGE gels. The resulting blot was developed with polyclonal anti-PLRP2 and imaged at 800 nm.
3H-Triglyceride lipase assay
Lipase activity was determined by release of fatty acid from 3H-labeled triolein. An assay solution containing 30 mM Tris-HCl (Fisher), 1 mM CaCl2 (Sigma) at pH 8.5 and 0.32 mM total triolein with 0.312 mM unlabeled (from 1.016 M stock, Sigma) mixed with 3H-triolein (from 0.1 mM stock, specific activity 52.6 Ci/mmol, Perkin Elmer (Waltham, MA, USA) at a 50:1 ratio. The micelles were incubated with recombinant human PLRP2, which was found to have specific activity 193 moles FA produced per mole of lipase per minute (a generous gift from Dr. F. Carriere). Assays were incubated at 37°C and stopped at various times. Fatty acids were extracted using chloroform/methanol/heptane (14.5 parts/12 parts/10 parts) (Fisher) and made basic with a pH 10 carbonate solution. The aqueous phase was formed by centrifugation at 2600 rpm (1040 g) for 5 min at room temperature. From the top phase, 200 μl were collected, mixed with 500 μl of scintillation cocktail (MicroScint-20 Perkin Elmer), and counted in a Beckman scintillation counter for 3 min per sample.
DiFMU-octanoate lipase assay for granzyme effects on PLRP2
The effects of cytotoxic granule enzymes on recombinant (r-) human PLRP2 were assessed using a sensitive fluorogenic assay, with RNK-16 granule extracts as a source of granzymes. The granule extracts were mixed with 1 μg of rPLRP2, preincubated at room temperature for 10 min, and run for 60 min at 37°C. The PLRP2 lipase activity was monitored by the generation of fluorescence from the DiFMU octanoate substrate [14] (Invitrogen) using a synergy HT multi-mode microplate reader (BioTek, Winooski, VT, USA).
Cytotoxicity assays
51Cr release assays.
T cell lysis was detected using redirected lysis assays with P815 mastocytoma cells as radiolabeled targets. T cells were redirected to the Fc receptor-bearing P815 cells with 1 μg/ml anti-mouse CD3 mAb (clone 145-2C11) (eBioscience). P815 target cells were labeled with Na51CrO4 (Perkin Elmer) [15] and assays run in quadruplicate with 10,000 radiolabeled cells per well for 4 h. In experiments where recombinant PLRP2 was added to the CTLs, 1 μg/ml final PLRP2 was added at the beginning of each 4 h assay. The data are displayed as the averages of the quadruplicate wells with the standard deviations. Specific release was calculated by the formula:
 |
For comparison of cytotoxicity, lytic units (LU) per 10 million splenocytes units are used, calculated as one lytic unit as the number of cells required to lyse 50% of the target cells, using regression analyses of the cytotoxicity at different E:T ratios [16]. In some experiments, cytotoxicity was mediated by rat natural killer-16 cytotoxic granule extracts, with or without 1 μg/ml r-PLRP2 in the assay.
Cytometric tumor outgrowth assays
On day 5, CTLs were plated out with eGFP-P815 s [12] at specified effector to target ratios, in triplicate wells for each condition, in the presence of 1 μg/ml anti-CD3 (clone 4-2C11) (eBioscience), or with 1 μg/ml of the IgG control antibody, Golden Syrian hamster anti-mouse IgG (eBioscience). The assay was incubated at 37°C with 5% humidity for 48 h. Upon completion of the 48-h incubation, cells were harvested and measured with a Beckman Coulter XL/MCL Flow Cytometer. Live and dead eGFP-P815s were distinguished from one another based on GFP fluorescence and forward scatter properties. Because dead cells can disintegrate into debris and become undetectable, we counted live cells in constant volumes that were determined using cell count beads (Polymer Labs, Amherst, MA, USA) that were standardized with BD Biosciences TruCount tubes. The beads were added to each sample and used to normalize the samples to a constant volume per sample. The data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR, USA). The data are displayed as the averages of the triplicates with the standard deviations.
T-cell separation
Anti-CD4 and anti-CD8 magnetic particles were purchased from BD Biosciences (cat. #551539 and #551516). Cells were placed into 4-ml tubes (Falcon cat. #352052) and incubated with beads at a concentration of 50 μl per 107 cells. The cells were incubated at 4°C for 20 min, and then each tube was placed in the magnetic apparatus (BD Biosciences iMagnet) for 10 min at 4°C. Supernatant containing the negatively enriched cells was carefully removed and placed in a separate tube. The tubes containing the negatively enriched cells where further enriched through 3 repetitions of the magnetic separation described above. Cells were stained and assayed by flow cytometry, as described below.
Flow cytometry analyses of cellular proteins
Cells were harvested and epitopes were detected with the following mAbs: PE-Cy7-anti-mouse CD3 IgG (clone 145-2C11, eBioscience), PE-Cy5-anti-mouse CD4 IgG2b (clone GK1.5, eBioscience), and FITC-anti-mouse CD8 IgG2a (clone 53-6.7, eBioscience), PE-anti-mouse CD25 IgG1L (clone PC61.5, eBioscience), PE-anti-mouse CD44 IgG2b (clone IM7, eBioscience), PE-anti-mouse CD45 IgG2b (clone 30-F11, eBioscience) and PE-anti-mouse CD137 Syrian hamster IgG (clone 17B5, eBioscience). After external antibody staining, the cells were washed, fixed, and permeabilized (IntraPrep Kit, Beckman Coulter). Grz B was detected with PE anti-human Grz B mAb (clone GB-12; Caltag Laboratories Burlingame, CA, USA) that cross-reacts with mouse Grz B and fails to react with Grz B−/− T cells (data not shown).
Intracellular cytokine staining
T cells after culture for 5 days were stimulated with 80 ng/ml PMA (Sigma) and 800 μg/ml ionomycin (Sigma) in the presence of the Golgi plug used at a concentration of 1:1000 as per manufacturer’s instructions (BD Biosciences) for 4 h. Following this incubation, cells were washed with PBS containing 1% FBS. The resulting cells were fixed and permeabilized using the Beckman Coulter IntraPrep intracellular staining kit, and staining was carried out per manufacturer’s instructions (Beckman Coulter). IFN-γ, IL-4, and IL-5 was detected using AF647-anti mouse IFN-γ mAb (clone XMG1.2, eBioscience), PE-anti-mouse IL-4 (clone 11B11, eBioscience) and PE-anti-mouse IL-5 (clone TRFK5, eBioscience), respectively. Samples were measured with a Beckman Coulter FC500 flow cytometer. The data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Statistical analyses
Results are expressed as mean ± sd of the collected data. Statistical evaluations of the data were performed with Excel and Sigma plot software. The differences in mean values were evaluated by Student’s t test. Differences were considered significant if the P was <0.05.
RESULTS
IL-4 induced pancreatic lipase-related protein 2 in CD8+ T cells
Grusby et al. [8] found that pancreatic lipase-related protein 2 (PLRP2) mRNA expression was induced in long-term CD8 T cell lines cultured with IL-4. To set the foundation for examination of the role of the PLRP2 lipase in CTL killing, we first wanted to validate that the PLRP2 mRNA and the protein are present in the CTLs that we will later examine for function. We first determined PLRP2 induction by IL-4 in freshly isolated T cells by performing a time course at substantial concentrations of IL-4. Induction of PLRP2 mRNA in splenocytes stimulated with the lectin concanavalin A and cultured with exogenous IL-4 was first detected on day 4 in the presence of 250 U/ml IL-4, with increased PLRP2 mRNA expression on day 5 (Fig. 1A). Incubation with 500 U/ml IL-4 resulted in earlier detection of PLRP2 mRNA, starting on day 3 without significantly increasing the mRNA expression days 4 and 5 (data not shown). Then, we performed a dose-response for expression of PLRP2 mRNA on day 6. The lowest IL-4 concentration able to induce PLRP2 was 125 U/ml (3.5 × 10−10 M, 5 ng/ml of this lot of IL-4) (Fig. 1B). The PLRP2 mRNA response increased in a dose-dependent manner, plateauing at 250 U and higher IL-4. For physiological perspective, 2 ng of IL-4 can be produced by fewer than 1.5 × 106 memory Th2 cells in culture [17], and in vivo, mast cells and eosinophils also produce IL-4.
We also assessed whether IL-2 induced CTLs would lack PLRP2. In 3 of 4 nonquantitative RT-PCR experiments, faint signals were observed in cells cultured with only IL-2 (illustrated, Fig. 1A). Further investigation of PLRP2 using quantitative TaqMan RT-PCR (with a different primer pair) failed to detect any signal in IL-2-induced CTLs in 3 out of 3 experiments (Fig. 1C). We consider it possible that occasional PLRP2 expression could be supported by endogenous IL-4 being produced by activated CD4+ T cells within the cultures. Our findings from these PCR assays clearly indicate that CTLs generated with IL-2 have low or no PLRP2 mRNA. Thus, the IL-2 cultured WT CTLs are used later in the paper as controls to probe whether the PLRP2 gene ablation influenced parameters other than PLRP2.
To determine whether PLRP2 expression was influenced by cytokines other than IL-2 and IL-4, the type 1 helper T cell cytokine IFN-γ was added to the IL-4-induced splenocytes. High concentrations of IFN-γ support type 1 and suppress type 2 lymphocyte responses even when the type 2-polarizing cytokine IL-4 is present [18,19,20,21]. Splenocytes were activated with conA and induced with IL-4 alone or with IL-4 combined with increasing concentrations of IFN-γ. (Cells were also given IFN-γ without other cytokines, but they died by day 3.) On day 5, as expected, PLRP2 expression was observed in the cells induced with IL-4 alone. For analysis of the effects of IFN-γ, the PLRP2 expression of the IL-4-induced CTLs without IFN-γ was set as the 100% control. The additions of IFN-γ had a slight enhancing effect on the expression of PLRP2 (Fig. 1C). The data show that although PLRP2 is induced by the type 2 cytokine IL-4, its expression remains unaffected by IFN-γ. Thus, the presence of type 1 IFN-γ-secreting CD4+ T helper cells would be unlikely to counteract the effects of IL-4 provided by type 2 CD4+ T helper cells or by mast cells.
In previous studies, only CD8+ (and not CD4+) T cell lines expressed PLRP2 mRNA [8], but the studies lacked cytotoxic CD4+ CTL lines. To test whether it is only the CD8+ subset that can express PLRP2 or whether all CTLs have PLRP2 expression, we examined CD4+ CTLs. We stimulated cells with conA, a mitogen that activates CD4+ cytotoxic T cells, as well as CD8+ cytotoxic T cells. ConA-activated CTLs were cultured with IL-4 and were separated on day 6 into CD4+ or CD8+ by magnetic bead enrichment. The degree of cellular enrichment was assessed by flow cytometry. In the experiment illustrated (Fig. 1D), the starting population was 34% CD4+ 53% CD8+; the CD4+-enriched cells were 96% CD4+ 1.5% CD8+, and the CD4-depleted cells were 1% CD4+ 95% CD8+, while the CD8+-enriched cells were 2% CD4+ 78% CD8+ and the CD8-depleted cells were 69.4% CD4+ 9% CD8+. The semiquantitative PCR analyses indicated that only the populations with CD8+ T cells were PLRP2-positive and that the CD4+-enriched T cells lacked PLRP2 (Fig. 1D). In additional experiments, >95% of the IL-4 cultured CD4+ T cells contained granzyme B, a cytotoxic granule protein, and marker of cytotoxic function, albeit in 3- to 7.5-fold lower mean fluorescent intensities than in CD8+ cells (results not illustrated). The CD4+-enriched IL-4 cultured T cells were also cytotoxic (though several-fold less so than the IL-2 cultured CTLs, not illustrated). In summary, these results show that only the CD8+ T cell subset expresses PLRP2 and that CD4+ CTLs lack PLRP2. To verify that the PLRP2 mRNA was translated into protein, we probed for PLRP2 using immunoblots of splenocytes that were grown in 500 U/ml IL-4, disrupted, and fractionated into Percoll fractions of different densities. The cell proteins were run on SDS-PAGE gels. The blots were probed for PLRP2 using chicken antibodies elicited with a human PLRP2 protein fragment (Abcam ab17740), antibodies that cross-react with mouse PLRP2. Antibodies to perforin indicate the fractions enriched for cytotoxic granule proteins. An immunoreactive band, corresponding to PLRP2 at 52 kDa, was observed in the least dense fractions (Fig. 1E), while perforin was located in denser cell fractions. Specificity controls with nonimmune chicken IgY were negative (data not shown). PLRP2 was undetectable in IL-2 cultured cells, as confirmed by immunoblots of whole cell extracts from IL-2- and IL-4-induced CTLs (Fig. 1F). Thus, PLRP2 is expressed as a lymphocyte protein.
We also addressed if the CTLs of this study were polarized to a type 2 phenotype by the IL-4 [22,23,24]. We used intracellular IL-4 and IL-5 as markers of type 2 CTLs and simultaneously measured intracellular IFN-γ, which is the hallmark of type 1 polarized CD4+ T cells. Only 2-3% of the IL-4 induced CD8+ T cells expressed IL-4 or IL-5 (and not IFN-γ), while about ∼60% of CD8+ cells expressed high levels of IFN-γ (but not IL-4 or IL-5) and that few cells produced all three cytokines (Supplemental Fig. 1). The CD4+ T cells within the cultures served as positive controls for intracellular IL-4 and IL-5, with about ∼25-30% of CD4+ cells staining (Supplemental Fig. 1). Similar intracellular IL-4, IL-5, and IFN-γ staining occurred when splenocytes were cultured with IL-2 instead of IL-4 (data not shown). In summary, the PLRP2+ CD8+ T cells lack the IL-4 and IL-5 phenotype that would classify them as type 2 CTLs (Tc2 cells).
Gene ablation of PLRP2 reduced the rapid cytotoxicity of T cells
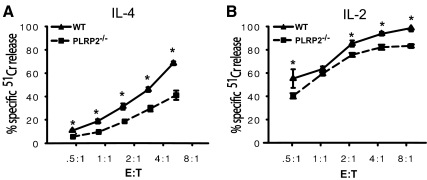
To test whether PLRP2 gene ablation affects killing mediated by CTLs representing a polyclonal spectrum of naturally occurring lymphocytes, we performed 51Cr release assays using T cells from PLRP2 knockout and wild-type littermate Balb/c mice. Splenic T cells were activated by the polyclonal activator concanavalin A. IL-4 induced CTLs from the WT mice had 3.5-fold the lytic activity of the PLRP2−/− CTLs (Fig. 2A). Unexpectedly (because of their lack of PLRP2), IL-2 induced WT CTLs had ∼2-fold the lytic activity of the PLRP2−/− CTLs (Fig. 2B). The IL-2 results agree with previous findings by M. Lowe et al. [10], who found that WT splenic CTLs restimulated in vitro with only exogenous IL-2 had higher activity than PLRP2−/− CTLs. It should be noted that the activity of both IL-2 and IL-4 induced CTLs in these 51Cr release assays is more than 95% perforin-dependent (unpublished data). Thus, the differential between WT and PLRP2−/− CTLs is highly reproducible, but, in the light of our mRNA findings, perplexing for the IL-2-supported CTLs and inconsistent with the previous hypothesis that the PLRP2 lipase has a direct role in cytotoxicity [8,9,10].
Figure 2.
CTLs from WT PLRP2+/+ mice have more cytotoxic activity than PLRP2−/− CTLs in short-term killing assays. The CTLs were induced with 500 U/ml of either mouse IL-2 or IL-4 for 5 days in tissue culture. Cytotoxicity in both assays was redirected with monoclonal anti-CD3 antibody. The data are displayed as the averages with bars bracketing the standard deviations. (A–B) 51Cr release assays. The killing by IL-4- and IL-2-induced PLRP2−/− or wild-type CTLs was tested using short-term 4 h 51Cr release by redirected lysis of P815 target cells. (A) The cytotoxic activity of IL-4-induced CTLs. Cytotoxicity mediated by PLRP+ WT mice was 3.5-fold the activity of PLRP2−/− CTLs, based on lytic units, which compare the numbers of different CTLs required to mediate equivalent levels of killing. Here, the LU50 values were 386/107 T cells for the WT and 110 for the PLRP2−/− CTLs. These data are representative of 5 experiments. (B) The cytotoxic activity of IL-2 induced CTLs. The cytotoxicity from wild-type mice was 1.6-fold the cytotoxic activity of CTLs generated from PLRP2−/− mice, as indicated by the increased number of knockout cells needed to generate equivalent cytotoxicity, based on lytic units. Here, the LU50 values were 2048/107 T cells for the WT and 1276 for the PLRP2−/− CTLs.
Ablation of PLRP2 was without effect on long-term CTL-mediated apoptosis
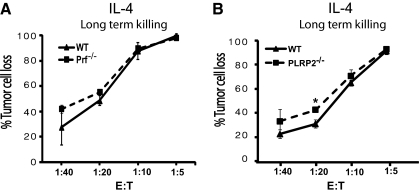
We questioned whether the PLRP2-associated cytotoxic activity that was observed in the 4-h assays would also be detected in longer assays that monitor apoptosis. We monitored tumor cell survival after 48 h using flow cytometry with fluorescent eGFP P815 cells as ‘target’ cells. In this assay, low effector CTL to target ratios are used in redirected lysis of eGFP-P815 cells and the lytic activity is detected by the loss of viable “green” P815 cells [12]. These assays monitor cell death (rather than reduced P815 cell division) because at the higher E:T ratios, fewer target cells were recovered than were initially plated. Furthermore, when the P815 cells failed to survive, debris from dead cells was detected by flow cytometry. The lytic activity was similar for WT and perforin−/− C57BL/6 mice with both IL-2 and IL-4-activated CTLs (see Fig. 3A for the IL-4 CTLs), which indicates that these long-term assays can detect perforin-independent apoptosis. Interestingly, the activities of PLRP2+/+- and PLPR2−/− IL-4-induced CTLs were similar (Fig. 3B). In other experiments, we observed that PLRP2+/+ WT and PLPR2−/− IL-4-induced CTLs expressed FasL and TRAIL by TaqMan RT-PCR (not illustrated). These observations suggest that PLRP2 gene ablation affects only short-term, perforin-dependent mechanisms.
Figure 3.
Long term cytotoxicity mediated by IL-4-induced CTLs can utilize perforin-independent mechanisms and was effective without PLRP2. P815 tumor cell survival was monitored after 48 h of incubation of CTL effector cells with eGFP-P815 s and quantified by flow cytometry. The effector cells were induced with IL-4 as in Fig. 2. (A) Perforin-independent cytotoxicity can account for the long-term CTL activity. The LU50/107 T cells was 22,222 for the WT cells and 28,529 for the Pfn−/− CTLs. (B) Long-term cytotoxicity was effective without PLRP2. IL-4-induced PLRP2+/+ and PLRP2−/− CTLs displayed similar cytotoxic activity in eGFP-P815 long-term 48-h killing assays, monitoring redirected lysis at very low E:T ratios. The LU50/107 T cells was 14,077 for the WT cells and 17,856 for the PLRP2−/− CTLs. (Note that the lowest E:T is one effector to 40 targets in this assay.) These data are representative of 2 experiments.
PLRP2+/+ and PLRP2−/− CTLs were phenotypically indistinguishable and the CD8+ CTLs were present in similar frequencies within WT and KO cell cultures
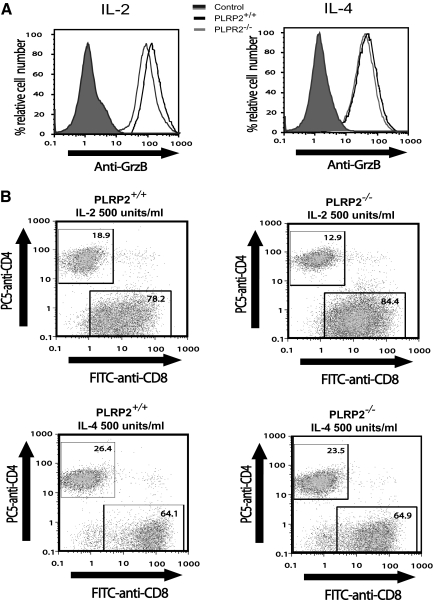
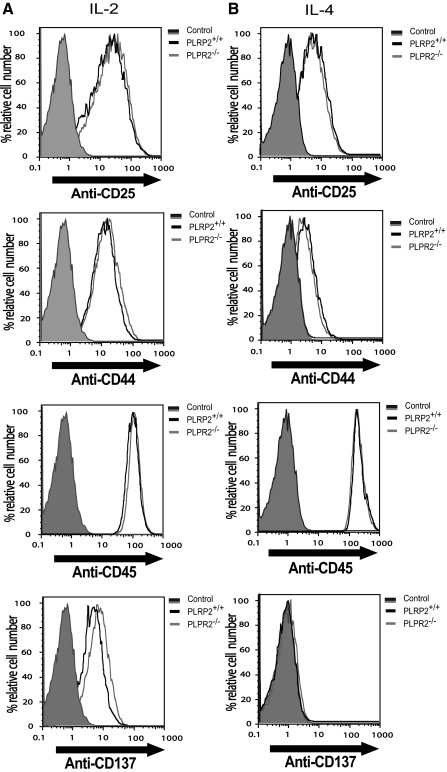
Perforin-dependent cytotoxicity is affected by the amounts of perforin per cell, the amounts of other perforin-dependent mediators per cell (such as granzyme B), and by the activation of T cells, which will affect adhesion and other T-cell surface proteins. We questioned whether the difference in the lytic ability of PLPR2−/− CTLs could be attributable to less granzyme B expression, differences in activation proteins or differences in the distribution of CD4 T cells compared with CD8 CTLs. Using flow cytometry to determine granzyme B expression, the production of granzyme B was similar in T cells from PLRP2−/− T cells when compared with T cells from WT mice (Fig. 4A). Analysis of the ratio of CD8 and CD4 cells showed similar ratios when one compares the PLRP2−/− and WT population grown under the same conditions (Fig. 4B). The activation and cell surface markers CD25, CD44, CD45, and CD137 of IL-2- and IL-4-induced CTLs from both PLRP2 WT and KO mice were virtually identical (Fig. 5A and B). Thus expression of the high-affinity IL-2 receptor CD25, the adhesion molecule for hyaluronan CD44 that is up-regulated with the memory T cell phenotype, the tyrosine phosphatase CD45 associated with the T cell receptors for antigen, and a costimulatory molecule for antigenic responses in IL-4 treated cells CD137 [25, 26], molecules with quite diverse functions were comparable. These results eliminate the possibility of a difference in CTL frequency, granule formation, or the assessed surface phenotypes being responsible for the differences in cytotoxic activities observed between PLRP2−/− and PLRP2+/+ CTLs.
Figure 4.
WT and PLRP2−/− CTLs are similar in Grz B expression and CD8 T cell frequency. IL-2- and IL-4-induced CTLs from PLRP2+/+ and PLRP2−/− mice were examined for their production of granzyme B and the relative distribution of CD4+ and CD8+ T cells after 5 days of tissue culture. (A) Granzyme B expression. Grz B of IL-2-induced CTLs generated from WT mice had a mean fluorescence index (MFI) of 160, while the MFI of the KO CTLs was 108, values that are similar. This similarity can happen when identical samples are subjected to intracellular staining for granzyme B. IL-4-induced CTLs, WT, and PLRP2−/−, also had indistinguishable levels of Grz B. These levels were lower than in the IL-2-induced cells, and had MFI values of 54 and 64. (B) The distributions of CD8 and CD4 cells were unaffected by the deficiency in PLRP2. The cells were gated on CD3+ T cells and then analyzed for cells reacting with anti-CD4 or anti-CD8 monoclonal antibodies. The CD8 distributions from IL-2-induced PLRP2+/+ vs. PLRP2−/− were 78.2% vs. 84.4%, and for CD4 the distributions were 18.9% vs. 12.9%, respectively. The IL-4 induced PLRP2+/+ vs. PLRP2−/− CD8 distributions were 64.1% vs. 64.9%, and for CD4, the distributions were 26.4% vs. 23.5%, respectively.
Figure 5.
PLRP2+/+ and PLRP2−/− CTLs are phenotypically indistinguishable by cell surface markers. Splenocytes from PLRP2+/+ and PLRP2−/− CTLs were induced with either IL-2 or IL-4 for 6 days. Surface expression of CD25, CD44, CD45, and CD137 was examined using flow cytometry, to monitor ability to up-regulate cell surface proteins that have diverse functions in CTLs. The cells displayed are CD3+CD8+ T cells. We monitored receptors (e.g., CD25 for IL-2), adhesion molecules involved in killing (CD44), a tyrosine phosphatase involved in TCR signal transduction (CD45), and a costimulatory molecule, CD137 (4-1 BB). In all cases, the KO had similar values to the WT, by frequencies and by MFIs. PLRP2+/+: solid black line, PLRP2−/−: solid gray line. Unstained controls: shaded peak.
Rapid lysis was unchanged by the addition of r-PLRP2 to PLRP2−/−
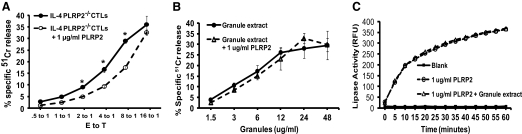
CTLs To determine whether restoration of PLRP2 could augment the deficiency in short-term cytotoxic function observed for IL-4-induced PLRP2−/− CTLs, we added recombinant human PLRP2 to 51Cr redirected lysis assays. The recombinant PLRP2 alone with the P815 target cells caused no cytotoxicity at 1 μg/ml, an amount of lipase that exceeds the triolein lipase activity released by IL-4 CTLs after TCR stimulation [27]. [The slightly greater lipase activities released by WT compared with PLRP2−/− CTLs were statistically insignificant (B. Alves, unpublished results).] Upon addition of 1 μg/ml recombinant PLRP2 to IL-4 -induced PLRP2−/− CTLs, the cytotoxic activity was actually reduced slightly, indicating that restoration of PLRP2 alone to the CTLs was insufficient to improve lysis (Fig. 6A). Lysis was unchanged when IL-2-induced PLRP2−/− CTLs received recombinant PLRP2 (data not illustrated). The addition of r-PLRP2 also failed to enhance perforin-dependent cytotoxicity medicated by cytotoxic granule extracts (Fig. 6B). We have established that the r-PLRP2 lipase activity is stable in the presence of the granzyme proteases that are exocytosed during killing (Fig. 6C). These results suggest that the function of PLRP2 appears to be independent of direct cytotoxic effects on cells attacked by CTLs.
Figure 6.
Exogenous rPLRP2 was unable to increase short-term cytolysis mediated by IL-4 induced CTLs or by cytotoxic granule extracts. (A) The cytotoxic activity of IL-4-induced PLRP2−/− CTLs was reduced, rather than increased, after rPLRP2 addition. The effects of recombinant PLRP2 addition to CTLs was tested using the 4 h 51Cr redirected lysis assay of P815 s. IL-4-induced PLRP2−/− CTLs were assayed in the presence or absence of 1 μg/ml rPLRP2. The lipase alone was nontoxic (not illustrated.) The cytotoxic activity was actually decreased by the addition of rPLRP2. (B) The cytotoxic activity of cytotoxic granule extracts was unchanged after rPLRP2 addition. The cytotoxic granule extracts, at several concentrations, were incubated with P815 cells alone or with the addition of 1 μg/ml r-PLRP2. (C) Stability of PLRP2 to granzyme proteases. We demonstrated that 1 μg/ml r-PLRP2 was stable in the presence of granzymes from 30 μg/ml cytotoxic granule extracts after preincubation for 10 min at room temperature and during measurement of lipase assay for 1 h at 37°C.
Perforin mRNA levels were unexpectedly different in IL-2-induced WT and PLRP2−/− CTLs
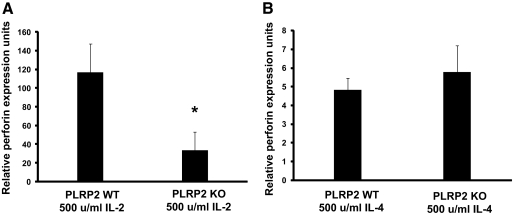
It is possible that ablation of the PLRP2 gene could unexpectedly affect perforin expression and thereby account for the differences in 51Cr-release short-term lysis. The best available method to monitor perforin at this time is to quantify the mRNA because the antibodies available to mouse perforin are unsuitable to detect perforin by flow cytometry and immunoblotting. We applied TaqMan RT-PCR to quantify mRNA to perforin and found that perforin mRNA levels were similar for IL-4-induced WT and KO CTLs (Fig. 7). This observation suggests that perforin differences are unlikely to account for PLRP2 KO effects in the IL-4-induced CTLs. The IL-2-induced WT CTLs had 20-fold more perforin mRNA than the IL-4 WT CTLs, consistent with the greater 51Cr lytic activity of IL-2 CTLs compared with IL-4 CTLs (for lysis, see WTs in Fig. 2, A and B). Unexpectedly, we observed that, in IL-2-treated CTLs (where PLRP2 mRNA is scarce), the perforin mRNA was fourfold greater in the WT than in the PLPR2 KO (P<0.05). As discussed below, PLRP2 and perforin genes are on different chromosomes, which makes the lower perforin mRNA in the PLRP2 gene-ablated cells hard to understand. The unexpected differences in perforin mRNA correlated with the unexpected differences in short-term lytic activity between the IL-2 WT and PLRP2−/− CTLs. In summary, we have two unexpected observations with IL-2 (cytotoxicity and perforin mRNA) to indicate that the PLRP2 gene ablation has unforeseen effects that could be independent of the lipase.
Figure 7.
mRNA encoding perforin was unaffected by PLRP2 KO in IL-4-induced CTLs. Perforin and cyclophilin mRNAs production were measured using TaqMan quantitative RT-PCR and then the ratios were calculated. These ratios were then compared with determine the relative levels of perforin among the different cells. The ratio of perforin to cyclophilin mRNAs in the cells used to standardize the assay was arbitrarily set to a perforin expression unit of 1 (even though there were ∼500 fold more cyclophilin mRNAs than perforin mRNAs within these standard cells.) (A) Perforin mRNA expression was (unexpectedly) reduced in PLRP2−/− IL-2 s induced CTLs, compared with the mRNA expressed in the WT CTLs. The P value for the difference was <0.05. (B) Similar perforin mRNA expression was found in PLRP2+/+ and PLRP2−/− CTLs induced with IL-4. Note that the ordinate scale of A is 0–160, while that of B is 0–8 for the ratios of Pfn to cyclophilin mRNAs. As expected, on the basis of a previous report [44], perforin mRNA in the presence of IL-4 was depressed.
Analyses for collateral genetic effects as a result of PLRL2 gene ablation
Gene ablation can have unanticipated effects on the expression of neighboring genes [28,29,30]. First, we considered proteins known to be important to CTL killing to see whether they were on mouse chromosome 19 with PLRP2. Using NCBI Gene (http://www.ncbi.nlm.nih.gov/sites/entrez), we found that, while mouse PLRP2 is on chromosome 19, granzymes B-H and N are on chromosome 14; granzymes A and K on chromosome 13; granzyme M, perforin and CD18 (part of LFA-1) on chromosome 10; the other heterodimeric protein chain of the adhesion molecule LFA-1 (CD11a) is on chromosome 7, and the TCR coreceptor CD8 is on chromosome 2, indicating that these proteins important for cytotoxicity are very unlikely to be perturbed by damage at the PLRP2 locus. Activation of the IL-4 receptor of CD8 T cells induces a wide spectrum of intracellular signaling through two cascades [31] and will undoubtedly affect many genes other than the ones considered above. We also examined the genes flanking the PLRP2 site using NCBI Mapviewer (http://www.ncbi.nlm.nih.gov/mapview) for candidate genes within a 3,000 kB site. The 12 genes upstream and the 17 genes downstream of PLRP2 appear unrelated to cytotoxicity, although 10 of these 29 genes have currently unidentified functions.
DISCUSSION
Since the discovery of PLRP2 in cytotoxic T cells by Grusby et al. [8] and the later findings that PLRP2−/− T cells have reduced cytotoxic activity compared with WT T cells [10], it has been hypothesized that PLRP2 lipase helps destroy cells targeted for death by T cells [8,9,10, 32, 33]. Although this idea has been accepted, we demonstrated here for the first time that freshly isolated T cells upon activation and exposure to IL-4 express both PLRP2 mRNA and protein. PLRP2 is expressed in only CD8+ T cells after induction with IL-4, even under these culture conditions the CD4+ T cells also contained cytotoxic granules with granzyme B and mediated cytotoxicity (not illustrated). Since CD8 T cells are the main mediators of killing by T cells, these data strengthen the idea that PLRP2 plays a role in cytotoxicity, although control of cytotoxic granule release would appear to be unlikely. However, on the basis of the data presented here, this previous hypothesis has come into question. The critical data that raise questions are 1) lack of PLRP2 expression by IL-2 induced CTLs, 2) the greater activity of WT vs. PLRP2−/− CTLs under the IL-2 conditions (in which PLRP2 may be completely un-expressed), 3) the higher amount of perforin mRNA in the WT vs. PLRP2−/− IL-2 CTLs, and 4) the inability of r-PLRP2 to increase the cytotoxicity of PLRP2−/− IL-4-cultured CTLs.
Some of the critical data were unexpected, but data presented in previous reports were prescient, although at the time, their significance was unrecognized. IL-2 cultured long-term CD8 T cell lines lacked PLRP2, which was central to the concept of PLRP2 being an IL-4-induced gene [8]. However, in a separate report [10], it appeared that IL-2-cultured WT splenic CTLs had greater short-term cytotoxic activity than PLRP2−/− CTLs, but this report had no trouble attributing the effect to PLRP2 because it was unrecognized that the WT control CTLs probably lacked PLRP2. The most compelling data that challenge the hypothesis are the greater perforin mRNA levels that we observed in WT compared with PLRP2−/− CTLs induced with IL-2. Essentially, the differences in perforin mRNA levels are sufficient to explain the difference in lysis and without PLRP2 mRNA expression in the IL-2 WT controls, one needs some other difference. (Curiously, the perforin mRNA levels were similar for WT and PLRP2−/− CTLs induced with IL-4, and in some replicas, the perforin mRNA levels were greater for the PLRP2−/− CTLs. So in the absence of information about the IL-2 cells, one would have been tempted to attribute the difference in IL-4-supported lytic activity to PLRP2. Now the IL-4 differences are simply unexplained.) The second part of the critical data that challenge the original hypothesis is the inability of r-PLRP2 to increase the cytotoxicity of PLRP2−/− CTLs. This challenge is of lesser impact, as restoration of lysis with rPLRP2 would have been interpretable as evidence for a PLRP2 function in lysis, while the negative result, failure to restore lysis, is inconclusive because of the issues of site-specific delivery of lipase by CTLs, etc. Thus, the data, past and present, form a consistent picture that weakens the hypothesis.
Another of our findings warrants discussion. The short-term, perforin-dependent, assays were affected by PLRP2 gene ablation, while the long-term assays were unaffected. The long-term assays can function without perforin, indicating that there is a perforin-independent secondary mechanism that can terminate target cells if perforin fails to do so. The simplest interpretation of our results is that a perforin-dependent mechanism is affected by the PLRP2 gene ablation; although rigorous logic leaves open the possibility that there may be a new, unknown and rapid, perforin-independent mechanism, induced by IL-4 that is affected by PLRP2 gene ablation.
We are left with the question: What is this perforin-dependent mechanism that is affected by the PLRP2 gene ablation? We considered the possibility of upstream or downstream attenuation of other genes but were unable to identify candidate genes involved in short-term T-cell cytotoxicity. If the effect is really due to loss of PLRP2 rather than extraneous chromosomal effects, it is possible that cytotoxic activity could be developmentally affected by the malnutrition and delayed growth that the PLRP2−/− mice experience during suckling.
The effects of early malnutrition are worth discussion. In fact, dietary triglycerides provide ∼90% of the energy during the first 6 mo of human development after birth [34, 35]. To use the energy stored in these triglycerides, lipases must hydrolyze the lipids into fatty acids. PLRP2 is the main lipase responsible for the breakdown of triglycerides in neonates [36,37,38]. The suckling PLRP2−/− mice had a lower weight gain of 0.33 g/day when compared with the WT suckling mice that gained weight at 0.44 g/day [10]. The body mass of the PLRP2−/− mice was decreased by ∼20% by 23 days of age. With the onset of PTL lipase expression at the time of weaning, the PLRP2−/− mice exhibited catch-up growth, and their adult weights became indistinguishable from wild-type littermates. The PLRP2−/− mice were malnourished at just the time of thymic development. It is also relevant that the thymus involutes at puberty and would be unavailable later in adults to compensate when body mass was restored. Nutritional deficiencies have been associated with impairment of immune function. Malnourishment during human development results in premature atrophy of the thymus and prolonged impairment of cell-mediated immunity [39,40,41]. A reduction in the number T lymphocytes and their ability to respond to mitogens has been found in both human and animal models of malnourishment [39, 40, 42]. Although many effects of malnourishment are reversible in adults with proper nutrition, temporarily malnourished infants who gained weight later continued to exhibit impaired cellular immunity. The effects included higher risk of upper and lower respiratory infections for years after nutrition was restored [43], infections that require CTLs for control. Thus, it is possible that the effects of PLRP2 ablation on CTLs occurred well before the CTLs were activated.
At this time, our evaluation of the hypothesis that PLRP2 lipase functions in T-cell cytotoxicity leaves the hypothesis in doubt. Our data provide warnings for investigators who might pursue the hypothesis further. Our findings raise a potential new issue as to effects of animal development on cytotoxic T cells.
Supplementary Material
Acknowledgments
The authors thank Drs. William J. Murphy (University of California, Davis), Alexandra M. Livingstone, (University of Rochester, NY) and Jennifer V. Welser (Scripps Research Institute) for their critical comments, as well as Dr. Frederick Carriere, (Centre National de la Recherche Scientifique, UPR9025 Laboratoire d’Enzymologie Interfaciale et de Physiologie de la Lipolyse, Marseille, France) for the generous gift of r-PLRP2. This study was supported in part by National Institutes of Health (NIH) R01CA38942-20 (D. H.), R01HD33060,and DK053100 (M. L.) and T32 09563 (B. N. A. and D. T.), a University of Nevada Undergraduate Fellowship (J. L.), a GSA graduate student grant (B. N. A.), and the NIH Nevada INBRE P20RR016463 for flow cytometry.
Footnotes
Abbreviations: CD=cluster of differentiation, ConA=concanavalin A, CTLs=cytotoxic T lymphocytes, E:T=effector lymphocyte to tumor target ratio, Grz=granzyme, Ig=immunoglobulin, IL=interleukin, P815=a mastocytoma tumor cell line used as a target for cell-mediated cytotoxicity, FITC=fluorescein isothiocyanate, PE=phycoerythrin, Pfn, perforin, PLA2=phospholipase A2, PLRP1=pancreatic lipase-related protein, PLRP2=pancreatic lipase-related protein 2, PMA=phorbol myristic acid, PTL=pancreatic triglyceride lipase, r=recombinant
References
- Thirstrup K, Verger R, Carriere F. Evidence for a pancreatic lipase subfamily with new kinetic properties. Biochemistry. 1994;33:2748–2756. doi: 10.1021/bi00176a002. [DOI] [PubMed] [Google Scholar]
- Sias B, Ferrato F, Grandval P, Lafont D, Boullanger P, De Caro A, Leboeuf B, Verger R, Carriere F. Human pancreatic lipase-related protein 2 is a galactolipase. Biochemistry. 2004;43:10138–10148. doi: 10.1021/bi049818d. [DOI] [PubMed] [Google Scholar]
- Roussel A, Yang Y, Ferrato F, Verger R, Cambillau C, Lowe M. Structure and activity of rat pancreatic lipase-related protein 2. J Biol Chem. 1998;273:32121–32128. doi: 10.1074/jbc.273.48.32121. [DOI] [PubMed] [Google Scholar]
- Jennens M L, Lowe M E. Rat GP-3 is a pancreatic lipase with kinetic properties that differ from colipase-dependent pancreatic lipase. J Lipid Res. 1995;36:2374–2382. [PubMed] [Google Scholar]
- Eydoux C, De Caro J, Ferrato F, Boullanger P, Lafont D, Laugier R, Carriere F, De Caro A. Further biochemical characterization of human pancreatic lipase-related protein 2 expressed in yeast cells. J Lipid Res. 2007;48:1539–1549. doi: 10.1194/jlr.M600486-JLR200. [DOI] [PubMed] [Google Scholar]
- Giller T, Buchwald P, Blum-Kaelin D, Hunziker W. Two novel human pancreatic lipase-related proteins, hPLRP1 and hPLRP2. Differences in colipase dependence and in lipase activity. J Biol Chem. 1992;267:16509–16516. [PubMed] [Google Scholar]
- Andersson L, Carriere F, Lowe M E, Nilsson A, Verger R. Pancreatic lipase-related protein 2 but not classical pancreatic lipase hydrolyzes galactolipids. Biochim Biophys Acta. 1996;1302:236–240. doi: 10.1016/0005-2760(96)00068-9. [DOI] [PubMed] [Google Scholar]
- Grusby M J, Nabavi N, Wong H, Dick R F, Bluestone J A, Schotz M C, Glimcher L H. Cloning of an interleukin-4 inducible gene from cytotoxic T lymphocytes and its identification as a lipase. Cell. 1990;60:451–459. doi: 10.1016/0092-8674(90)90596-7. [DOI] [PubMed] [Google Scholar]
- Lowe M E. Properties and function of pancreatic lipase related protein 2. Biochimie. 2000;82:997–1004. doi: 10.1016/s0300-9084(00)01184-6. [DOI] [PubMed] [Google Scholar]
- Lowe M E, Kaplan M H, Jackson-Grusby L, D'Agostino D, Grusby M J. Decreased neonatal dietary fat absorption and T cell cytotoxicity in pancreatic lipase-related protein 2-deficient mice. J Biol Chem. 1998;273:31215–31221. doi: 10.1074/jbc.273.47.31215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth M J, Thia K Y, Cretney E, Kelly J M, Snook M B, Forbes C A, Scalzo A A. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999;162:6658–6662. [PubMed] [Google Scholar]
- Kienzle N, Olver S, Buttigieg K, Kelso A. The fluorolysis assay, a highly sensitive method for measuring the cytolytic activity of T cells at very low numbers. J Immunol Methods. 2002;267:99–108. doi: 10.1016/s0022-1759(02)00150-3. [DOI] [PubMed] [Google Scholar]
- Hu-Li J, Ohara J, Watson C, Tsang W, Paul W E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S) J Immunol. 1989;142:800–807. [PubMed] [Google Scholar]
- Shah D S, Russell R R. A novel glucan-binding protein with lipase activity from the oral pathogen Streptococcus mutans. Microbiology. 2004;150:1947–1956. doi: 10.1099/mic.0.26955-0. [DOI] [PubMed] [Google Scholar]
- Bevan M J, Langman R E, Cohn M. H-2 antigen-specific cytotoxic T cells induced by concanavalin A: estimation of their relative frequency. Eur J Immunol. 1976;6:150–156. doi: 10.1002/eji.1830060303. [DOI] [PubMed] [Google Scholar]
- Bryant J, Day R, Whiteside T L, Herberman R B. Calculation of lytic units for the expression of cell-mediated cytotoxicity. J Immunol Methods. 1992;146:91–103. doi: 10.1016/0022-1759(92)90052-u. [DOI] [PubMed] [Google Scholar]
- Teixeira M M, Talvani A, Tafuri W L, Lukacs N W, Hellewell P G. Eosinophil recruitment into sites of delayed-type hypersensitivity reactions in mice. J Leukoc Biol. 2001;69:353–360. [PubMed] [Google Scholar]
- Fernandez-Botran R, Sanders V M, Mosmann T R, Vitetta E S. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med. 1988;168:543–558. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T F, Fitch F W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245–4252. [PubMed] [Google Scholar]
- Gajewski T F, Joyce J, Fitch F W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989;143:15–22. [PubMed] [Google Scholar]
- Gajewski T F, Schell S R, Nau G, Fitch F W. Regulation of T-cell activation: differences among T-cell subsets. Immunol Rev. 1989;111:79–110. doi: 10.1111/j.1600-065x.1989.tb00543.x. 79-110. [DOI] [PubMed] [Google Scholar]
- Li L, Sad S, Kagi D, Mosmann T R. CD8Tc1 and Tc2 cells secrete distinct cytokine patterns in vitro and in vivo but induce similar inflammatory reactions. J Immunol. 1997;158:4152–4161. [PubMed] [Google Scholar]
- Mosmann T R, Li L, Sad S. Functions of CD8 T-cell subsets secreting different cytokine patterns. Semin Immunol. 1997;9:87–92. doi: 10.1006/smim.1997.0065. [DOI] [PubMed] [Google Scholar]
- Sad S, Marcotte R, Mosmann T R. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- Pollok K E, Kim S H, Kwon B S. Regulation of 4-1BB expression by cell-cell interactions and the cytokines, interleukin-2 and interleukin-4. Eur J Immunol. 1995;25:488–494. doi: 10.1002/eji.1830250227. [DOI] [PubMed] [Google Scholar]
- Vinay D S, Kwon B S. Differential expression and costimulatory effect of 4-1BB (CD137) and CD28 molecules on cytokine-induced murine CD8(+) Tc1 and Tc2 cells. Cell Immunol. 1999;192:63–71. doi: 10.1006/cimm.1998.1433. [DOI] [PubMed] [Google Scholar]
- Alves B N, Marxhall K, Tamang D L, Leong J, Redelman D, Elliott V, Lowe M E, Hudig D. Lipid-dependent cytotoxicity by the lipase PLRP2 and by PLRP2-positive cytotoxic T lymphocytes (CTLs) Cell Biochem Funct. 2009 doi: 10.1002/cbf.1573. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham C T, MacIvor D M, Hug B A, Heusel J W, Ley T J. Long-range disruption of gene expression by a selectable marker cassette. Proc Natl Acad Sci USA. 1996;93:13090–13095. doi: 10.1073/pnas.93.23.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E N, Arnold H H, Rigby P W, Wold B J. Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell. 1996;85:1–4. doi: 10.1016/s0092-8674(00)81073-9. [DOI] [PubMed] [Google Scholar]
- Rijli F M, Dolle P, Fraulob V, LeMeur M, Chambon P. Insertion of a targeting construct in a Hoxd-10 allele can influence the control of Hoxd-9 expression. Dev Dyn. 1994;201:366–377. doi: 10.1002/aja.1002010408. [DOI] [PubMed] [Google Scholar]
- Acacia de Sa Pinheiro A, Morrot A, Chakravarty S, Overstreet M, Bream J H, Irusta P M, Zavala F. IL-4 induces a wide-spectrum intracellular signaling cascade in CD8+ T cells. J Leukoc Biol. 2007;81:1102–1110. doi: 10.1189/jlb.0906583. [DOI] [PubMed] [Google Scholar]
- Lowe M E. The triglyceride lipases of the pancreas. J Lipid Res. 2002;43:2007–2016. doi: 10.1194/jlr.r200012-jlr200. [DOI] [PubMed] [Google Scholar]
- Thomson A W, Lotze M T. London: Academic Press; The Cytokine Handbook. 2003 [Google Scholar]
- Fomon S J, Ziegler E E, Thomas L N, Jensen R L, Filer L J., Jr Excretion of fat by normal full-term infants fed various milks and formulas. Am J Clin Nutr. 1970;23:1299–1313. doi: 10.1093/ajcn/23.10.1299. [DOI] [PubMed] [Google Scholar]
- Koletzko B, Bremer H J. Fat content and fatty acid composition of infant formulas. Acta Paediatr Scand. 1989;78:513–521. doi: 10.1111/j.1651-2227.1989.tb17929.x. [DOI] [PubMed] [Google Scholar]
- Ghishan F K, Moran J R, Durie P R, Greene H L. Isolated congenital lipase-colipase deficiency. Gastroenterology. 1984;86:1580–1582. [PubMed] [Google Scholar]
- D'Agostino D, Cordle R A, Kullman J, Erlanson-Albertsson C, Muglia L J, Lowe M E. Decreased postnatal survival and altered body weight regulation in procolipase-deficient mice. J Biol Chem. 2002;277:7170–7177. doi: 10.1074/jbc.M108328200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figarella C, De Caro A, Leupold D, Poley J R. Congenital pancreatic lipase deficiency. J Pediatr. 1980;96:412–416. doi: 10.1016/s0022-3476(80)80683-4. [DOI] [PubMed] [Google Scholar]
- Chandra R K. Nutrition and the immune system: an introduction. Am J Clin Nutr. 1997;66:460S–463S. doi: 10.1093/ajcn/66.2.460S. [DOI] [PubMed] [Google Scholar]
- Chandra R K. Numerical and functional deficiency in T helper cells in protein energy malnutrition. Clin Exp Immunol. 1983;51:126–132. [PMC free article] [PubMed] [Google Scholar]
- Chandra R K. Protein-energy malnutrition and immunological responses. J Nutr. 1992;122:597–600. doi: 10.1093/jn/122.suppl_3.597. [DOI] [PubMed] [Google Scholar]
- Moscatelli P, Bricarelli F D, Piccinini A, Tomatis C, Dufour M A. Defective immunocompetence in foetal undernutrition. Helv Paediatr Acta. 1976;31:241–247. [PubMed] [Google Scholar]
- Chandra RK. Nutrition and the immune system from birth to old age. Eur J Clin Nutr. 2002;56:S73–6. doi: 10.1038/sj.ejcn.1601492. S73–S76. [DOI] [PubMed] [Google Scholar]
- Kienzle N, Buttigieg K, Groves P, Kawula T, Kelso A. A clonal culture system demonstrates that IL-4 induces a subpopulation of noncytolytic T cells with low CD8, perforin, and granzyme expression. J Immunol. 2002;168:1672–1681. doi: 10.4049/jimmunol.168.4.1672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.