Abstract
Background and Aims
Convolvulaceae is the most advanced plant family (asterid clade) that produces seeds with physical dormancy (water-impermeable seed coat). There are several different opinions about the nature of the specialized structure (‘water gap’) in the seed coat through which water initially enters seeds of Convolvulaceae, but none of them has been documented clearly. The primary aim of the study was to identify the water gap in seeds of Ipomoea lacunosa (Convolvulaceae) and to describe its morphology, anatomy and function.
Methods
Light microscopy, scanning electron microscopy, tissue-sectioning, dye-tracking and blocking experiments were used to describe the morphology, anatomy and function of the water gap in seeds of I. lacunosa.
Key Results
Dormancy-breaking treatments caused slits to form around the two bulges on the seed coat adjacent to the hilum, and dye entered the seed only via the disrupted bulges. Bulge anatomy differs from that of the rest of the seed coat. Sclereid cells of the bulges are more compacted and elongated than those in the hilum pad and in the rest of the seed coat away from the bulges.
Conclusions
The transition area between elongated and square-shaped sclereid cells is the place where the water gap opens. Morphology/anatomy of the water gap in Convolvulaceae differs from that of taxa in the other 11 angiosperm plant families that produce seeds with physical dormancy for which it has been described.
Key words: Convolvulaceae, Ipomoea, seed coat anatomy, seed dormancy, seed germination, palisade layer, physical dormancy, water gap, water-impermeable seed coat
INTRODUCTION
Physical dormancy (PY) in seeds is caused by a water impermeable seed (or fruit) coat (Baskin and Baskin, 1998, 2004), and it is known to occur in only 16 families of angiosperms (J. M. Baskin et al., 2000, 2006): one monocot family (Cannaceae) and 15 eudicot families. Within the eudicots, 14 of the families are in the rosids, and only one (Convolvulaceae) is in the highly evolutionary advanced asterid clade (Baskin and Baskin, 2000; J. M. Baskin et al., 2006). The phylogenetically isolated position of the Convolvulaceae and the fact that it is the most advanced family (APG, 2003) with PY (Baskin et al., 2000) make it an important family to study to advance our understanding of the evolutionary relationships of PY.
Impermeability of the seed (or fruit) coat to water develops during maturation drying of the seed (Van Staden et al., 1989; Baskin and Baskin, 1998) or fruit (Li et al., 1999). A palisade layer(s) in the seed (or fruit) coat is (are) responsible for the impermeability. The seed becomes permeable to water when an opening is formed via a specialized anatomical structure (‘water gap’) in the palisade layer(s), allowing water to enter the seed (Baskin et al., 2000). A water gap has been described in 12 of the 16 families known to have PY (Baskin et al., 2000). Further, several different kinds of water gaps occur in seeds of these 12 families, and they differ in origin, morphology and anatomy (Baskin et al., 2000).
Ipomoea lacunosa belongs to Ipomoeeae, the most advanced tribe in Convolvulaceae (Stefanovic et al., 2003). This species is a summer annual vine native to eastern USA (Abel and Austin, 1981; Gleason and Cronquist, 1991). Ipomoea lacunosa is resistant to glyphosate (Koger et al., 2004; Koger and Reddy, 2005) and thus difficult to control in crop fields. Therefore, it is a common noxious weed in agricultural crops, especially in corn (Anonymous, 1995), cotton, soybeans (Anonymous, 2000) and rice (Anonymous, 2001).
Seeds of I. lacunosa buried in soil can remain viable for at least 39 years (Toole and Brown, 1946). Gomes et al. (1978), Crowley and Buchanan (1980) and Oliveira and Norsworthy (2006) tested the effects of various environmental factors on germination of I. lacunosa seeds, but only Gomes et al. (1978) tested the effect of scarification on germination. They found that scarified seeds generally germinated to significantly higher percentages across a range of temperatures than non-scarified ones. This suggests, but does not prove, that seeds of I. lacunosa are water impermeable. They did not compare water uptake (imbibition) in scarified versus non-scarified seeds, and scarification can break dormancy in seeds with shallow physiological dormancy (see Baskin and Baskin, 2004) as well as in those with PY (see C. C. Baskin et al., 2006).
There are several claims of identification of the route of water entry into seeds of Convolvulaceae. However, none of these studies clearly documented a water gap. Koller and Cohen (1959) reported that a plug-like structure near the micropyle opens during the dormancy break, thereby allowing water to enter the seeds of Convolvulus lanatus, C. negevensis and C. secundus. However, they did not describe the structure in detail, nor did they clearly present evidence that led them to this conclusion. Callihan (1933) described the hilum slit as the route of water entry into ethanol-treated seeds of Convolvulus arvensis, which he observed to open at high RH. However, this was just a speculation, since he came to this conclusion after observing photomicrographs of the seeds and did not document water uptake by the seeds.
Hutchison and Ashton (1979) used the blocking method (lanolin–petroleum jelly) to investigate where water entered seeds of Cuscuta campestris that had been scarified with concentrated H2SO4. They concluded that the acid-scarified seeds took up water uniformly over the entire seed coat. Lyshede (1984) studied scanning electron micrographs of seeds of Cuscuta campestris made permeable by slight abrasion with sand paper and of those of C. pedicellata, which germinated without treatment, and she came to the same conclusion as that of Hutchison and Ashton. Lyshede suggested that papillae present on the seed coat are involved in water uptake. Thus, there is no clear description of the route of water entry into seeds of Convolvulaceae. The purpose of the present study was 3-fold: (1) to document whether seeds of I. lacunosa have PY, which has been shown to occur in I. purpurea (Brechu-Franco et al., 2000), I. hederacea (Thullen and Kelly, 1983), I. pandurata (Horak and Wax, 1991), I. crassicaulis [syn. I. carnea Jacquin ssp. fistulosa (Martius ex Choisy) D.F. Austin] (Misra, 1963), I. coccinea (Hardcastle, 1978), I. pes-caprae (Martinez et al., 2002) and various other Ipomoea species (Ogunwenmo, 2006); (2) to document the presence (or not) of a water gap in seeds of this species; and (3) to describe the water gap morphologically and anatomically.
MATERIALS AND METHODS
Seed collection
Seeds were collected from numerous plants of I. lacunosa (Gunn, 1969; Jones, 2005) in a corn field at Spindletop Farm, University of Kentucky, Lexington, KY, USA, in October 2005. They were pooled and stored in plastic bottles under ambient room conditions (22–24 °C, 50–60 % RH) until used. Experiments were carried out within 4 months of collection.
Documenting PY in seeds of I. lacunosa
Imbibition
The mass of each of 25 manually scarified (individually with a scalpel) and of 25 non-scarified seeds was determined at time 0 and at 1-h intervals until all scarified seeds had fully imbibed. Each seed was weighed initially and placed on moistened filter paper. At 1-h intervals, seeds were removed from the Petri dish, blotted dry with filter paper, weighed to the nearest 0·0001 g and returned to the wet filter paper in the dish. Imbibition experiments were carried out under ambient room conditions. Imbibition curves [increase in seed mass (fresh weight basis) over time] of scarified and of non-scarified seeds were constructed and compared to determine if scarification was required for seeds to imbibe (PY) or not (physiological dormancy or no dormancy).
Germination
Germination tests of manually scarified and of non-scarified (control) seeds were conducted in both light/dark (14/10 h) (approx. 40 µmol m–2 s–1, 400–700 nm, cool white fluorescent light) and constant darkness at (12/12 h) daily temperature regimes of 35/20, 30/15, 25/15, 20/10 and 15/6 °C. Darkness was provided by wrapping Petri dishes in aluminium foil. Three replicates of 25 seeds were used for each treatment. Seeds were incubated on moist sand in 5·5-cm-diameter Petri dishes, and the number of seeds germinated in light was counted every 2 d for 14 d. Dark-incubated seeds were checked for germination after 14 d. Petri dishes were allocated in a completely randomized design on incubator shelves. Germination percentages of non-treated and of manually scarified seeds were compared to determine if seeds have PY.
Breaking dormancy in intact seeds
The following treatments were applied to intact (not mechanically scarified) seeds in an attempt to break PY: seeds dipped in boiling water for 2, 4 and 6 s; seeds dry heated at 90 °C for 1, 5 and 10 min; seeds heated for 1, 2, 3 and 6 h at 35 °C on wet sand; and seeds heated for 1, 2, 3 and 6 h at 35 °C on dry sand. Treated seeds were tested for germination in the 25/15 °C light/dark regime described above. Three replicates of 25 seeds were used for each treatment. Germination percentages in each treatment were compared with those of control (non-scarified seeds incubated at 25/15 °C) to determine the effectiveness of the dormancy-breaking treatment.
Morphological changes during imbibition
Seeds made non-dormant by treating them for 3 h at 35 °C on wet sand were placed on moistened filter paper and photographed at 15 min intervals for 6 h using a Canon EOS 30 D camera with a Canon 95 mm macro lens. Photographs were compared to identify the water gap.
Dormant and non-dormant seeds were coated with gold–palladium in a Technics Hummer VI sputter coater. These coated samples were scanned with a Hitachi S-800 FE scanning electron microscope. Micrographs were compared to identify the water gap and changes on the seed surface after treatment.
Dye-tracking of imbibition pathway
Twenty non-dormant seeds were placed in a saturated solution (aqueous) of aniline blue, and two seeds were removed from the solution at 30-min intervals for 5 h. Transverse hand-cuts through the upper and lower hilum margins and longitudinal hand-cuts through the hilum and bulge were made and scanned using an EPSON Perfection #2400 scanner at 2400 DPI resolution or photographed using a Canon EOS 30 D camera with a Canon 95 mm macro lens, described above. The pathway of water into the seed was determined using aniline blue.
Blocking area of putative water gap
The hilum, bulge and hilum plus bulge in seeds made permeable by treating them at 35 °C for 3 h on wet sand were painted with Thompson's Water Seal (Thompson and Formby, Inc., Memphis, TN, USA) and tested for germination at the 25/15 °C regime. Three replicates of 25 seeds were used for each treatment. Germinated seeds were counted after 7 d. Radicle emergence was the criterion of germination.
Water gap anatomy
Hand and Vibratome (Vibratome 1500, St Louis, MO, USA) sections (25 µm) were made, and they were observed under an Olympus (Model BX50) light microscope. Photomicrographs were taken of the micropylar–hilum area. Photomicrographs and hand drawings (not shown) were used to describe the anatomy of the water gap.
Analysis of data
All germination data were analysed by ANOVA using GLM procedure in SAS software. Data were arcsine transformed before analysis to normalize them. Means were separated using Duncan's mean separation procedure and Dunnet's mean separation procedure (Scot et al., 1984).
RESULTS
Documenting PY in seeds of I. lacunosa
Imbibition
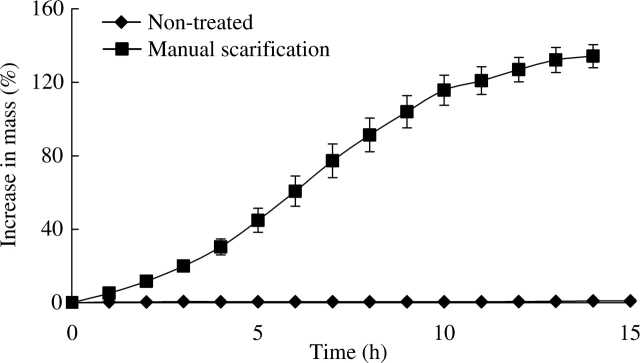
The mass of treated (manually scarified) seeds increased about 120 % in 15 h, at which time all of them were fully imbibed, whereas that of non-treated (non-scarified) seeds increased <1 % (Fig. 1).
Fig. 1.
Increase in mass of non-treated and of manually scarified I. lacunosa seeds on moist filter paper at ambient laboratory temperatures (22–24 °C).
Germination
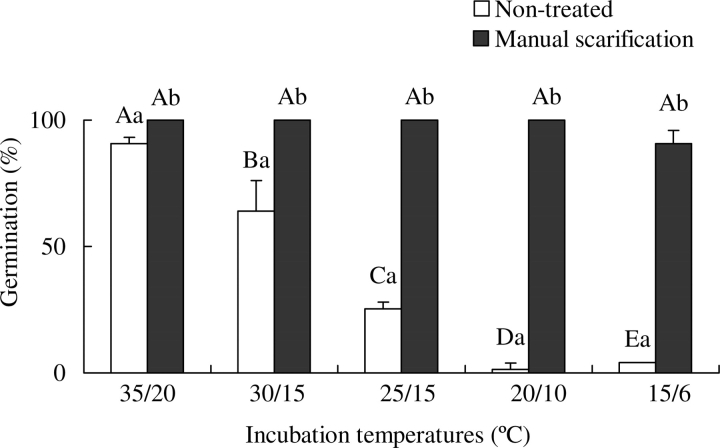
Germination percentages of non-treated seeds were very low at 25/15, 20/10 and 15/6 °C, moderately high at 30/15 °C and high at 35/20 °C, whereas manually scarified seeds germinated to high percentages in all five temperatures regimes in dark/light (Fig. 2). There was no difference between germination percentages of non-scarified and scarified seeds in dark and in dark/light (data not shown).
Fig. 2.
Germination of non-treated and of manually scarified I. lacunosa seeds in dark/light. Different upper-case letters within a treatment (non-scarified or scarified) indicate significant differences among all temperature regimes. Different lower-case letters indicate significant difference between treatments at same temperature regime.
Breaking dormancy in intact seeds
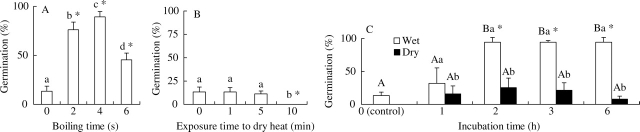
Seeds boiled for 2 s and 4 s germinated to high percentages, whereas those boiled for 6 s germinated to <50 % (Fig. 3A). Seeds dry heated at 90 °C for 1 min or 5 min germinated to <15 %, and no seeds heated for 10 min germinated (Fig. 3B). A high percentage of seeds kept on wet sand at 35 °C for 2, 3 or 6 h and then incubated at 25/15 °C germinated, whereas seeds kept on dry sand germinated to ≤20 % (Fig. 3C).
Fig. 3.
Effect of boiling (A), dry heat (B) and pretreatment incubation on wet and dry sand at 35 °C (C) on germination of I. lacunosa seeds at 25/15 °C. Different upper-case letters within a treatment (non-scarified or scarified) indicate significant differences among all temperature regimes. Different lower-case letters indicate significant differences among treatments at the same temperature regime. An asterisk indicates significant difference between treatments and control.
Morphological changes during imbibition
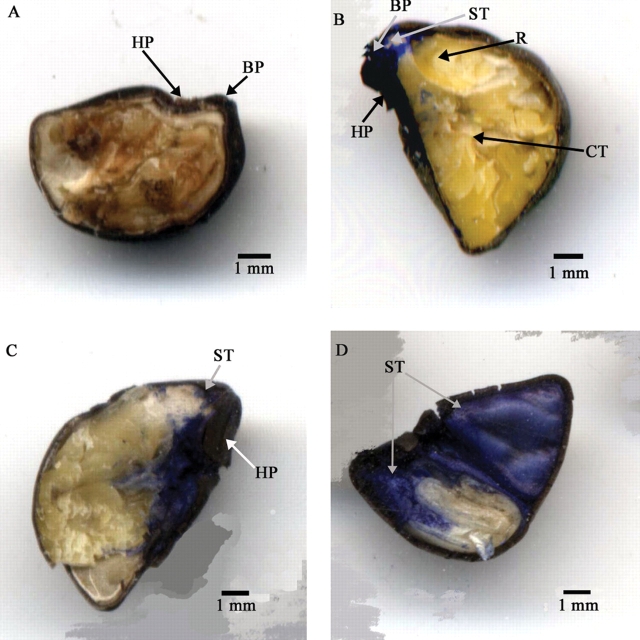
The hilar region of I. lacunosa is characterized by a large circular pad bordered by a hilar fissure located between the pad and hilum rim (Figs 4 and 5). There are two bulges adjacent to the hilar pad separated by the micropyle (Figs 4A and 5B). A ridge-like raphe extends below the hilum opposite the micropyle. Prior to initial imbibition, a slit forms on the hilar edge of one or both bulges, initiating water uptake and releasing seeds from physical dormancy (Figs 4B and 5B). Consequently, the bulges are raised and become detached from the seed coat between 30 min and 60 min from the start of imbibition (Figs 4D, E and 5D). The slits around the bulges extend through the entire seed coat, across both the palisade and sclereid layers, permitting free access to water uptake (Fig. 5D). By 2 h, the seed coat pulls away from the hilum pad along the hilar fissure and separates along the ridge of the raphe (Fig. 4D). Radicle emergence takes place above the micropyle between the two bulges.
Fig. 4.
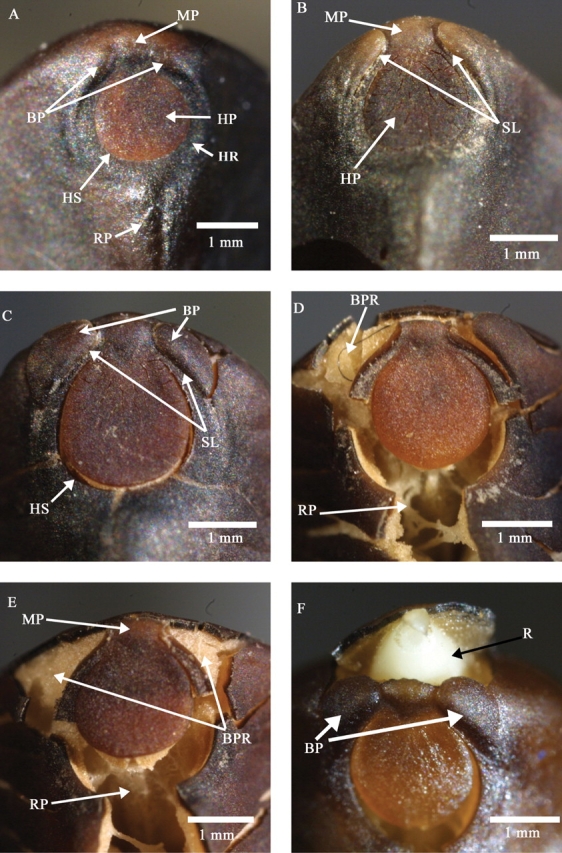
Morphology of the hilum area of a dormant seed (A), a non-dormant seed (B) and a seed after 1 h (C), 3 h (D), 6 h (E) and 14 h (F) of imbibition. BP, Bulge; BPR, bulge scar; HP, hilum pad; HR, hilar rim; HS, hilum fissure; MP, micropyle; R, radicle; RP, raphe; SL, slit around bulge. Bulges have fallen off seed in (E) but not those in (F).
Fig. 5.
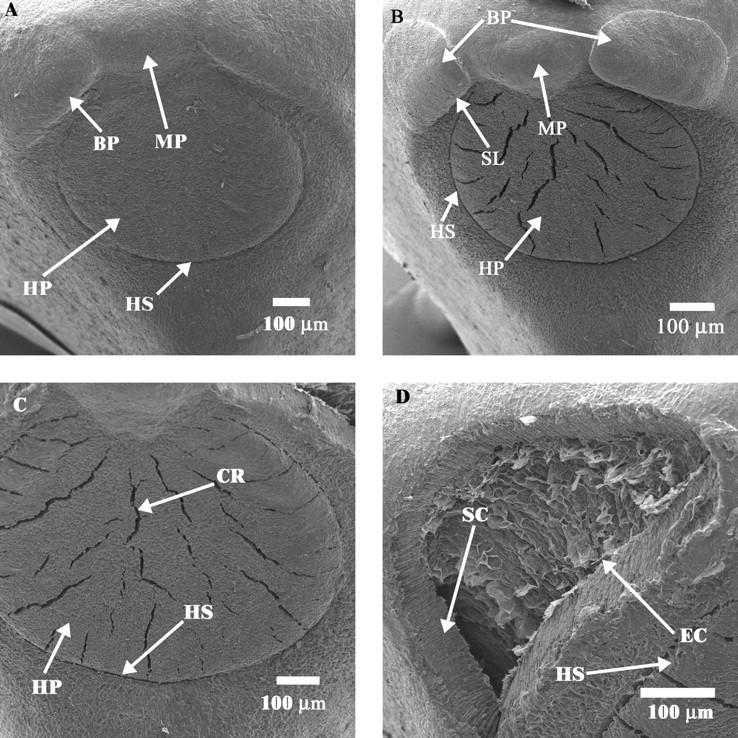
Electron micrographs of non-treated (dormant) and of treated (non-dormant) seeds: (A) hilum–microphyle area of a dormant seed; (B) hilum–micropyle area of a non-dormant seed; (C) hilum pad of a non-dormant seed; (D) opening where bulge has become detached from seed coat. BP, Bulge; CR, crack; EC, endodermal cells; HP, hilum pad; HS, hilar fissure; MP, micropyle; SC, seed coat showing palisade cells and sclereids; SL, slit through which water enters the seed.
Dye-tracking of imbibition pathway
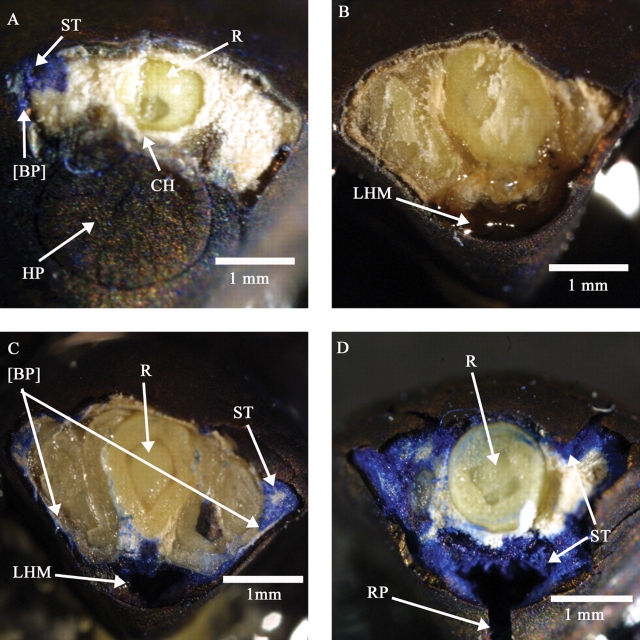
There was no staining of tissue in dormant seeds (Fig. 6A), showing that dye did not penetrate the seed coat. In non-dormant seeds, blue-staining occurred first under the bulge after 15 min (Figs 6B and 7A). At this time, there was no stain in the hilum area (Fig. 7B). After 2–3 h of imbibition, cracks had formed over all of the seed coat, and dye also had entered the seed through them. Therefore, the whole seed had become stained (Figs 6D and 7D).
Fig. 6.
Longitudinal cuts (hand sections) showing dye uptake by non-dormant I. lacunosa seeds: (A) dormant seed after 6 h in dye; (B) non-dormant seed after 15 min imbibition; (C) non-dormant seed after 4 h imbibition; (D) non-dormant seed after 6 h imbibition. BP, Bulge; CT, cotyledon; HP, hilar pad; R, radicle; ST, stain.
Fig. 7.
Transverse cuts (and sections) showing dye uptake by non-dormant I. lacunosa seeds: (A) cut in upper hilum margin after 15 min imbibition; (B) cut in lower hilum margin after 15 min imbibition; (C) cut through lower hilum margin after 4 h imbibition; (D) cut through lower hilum margin after 6 h imbibition. [BP], Area where bulge has been removed; CH, cells under hilum; HP, hilum pad; LHM, lower hilum margin; R, radicle; RP, slit in raphe; ST, stain.
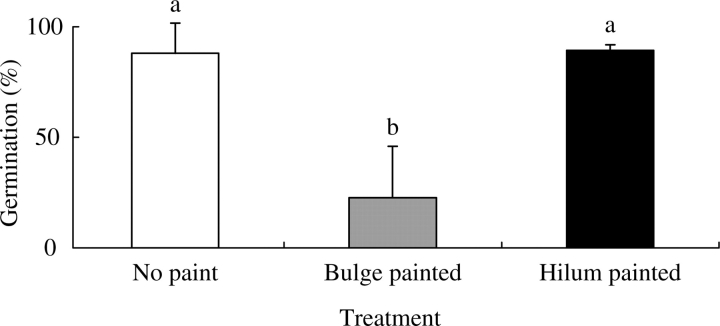
Blocking area of putative water gap
Non-painted seeds and seeds with the hilum painted germinated to 88 % and 89 %, respectively, whereas only 22 % of those with the bulges painted imbibed and germinated (Fig. 8).
Fig. 8.
Effect of painting bulge and hilum on germination of I. lacunosa seeds. Seeds were kept on wet sand for 3 h to break dormancy, after which bulges or hilum was painted. No paint was applied to any part of control seeds. Different letters indicate significant differences between treatments.
Water gap anatomy
The seed coat away from the hilar and bulge area of I. lacunosa has a palisade layer with a light line (linea lucida) and several layers of sclereid cells below it. A single-cell epidermal layer is present above the palisade layer (Fig. 9A). In the hilum pad, seed coat anatomy differs only a little from that of the seed coat away from the hilar and bulge area, except that there is a counter palisade layer without a light line (Fig. 9B, D). Several layers of sclereid cells can be seen under the palisade layer. A second palisade layer with a light line occurs below this sclereid layer. A second sclereid area that is several cell layers thick is located below the second palisade cell layer (Fig. 9B). The micropylar area has the same anatomy as that of the seed coat away from the hilar and bulge area. There is no trace of the micropyle in the mature seed coat (Fig. 9C). The fissure between the hilar pad and the seed coat adjacent to the hilar pad extends down into the subpalisade layers of the seed coat (Fig. 9D). However, this fissure is closed tightly in mature seeds. A slit is not formed in the micropyle, and the hilum fissure does not open during the dormancy break (Fig. 9E, F).
Fig. 9.
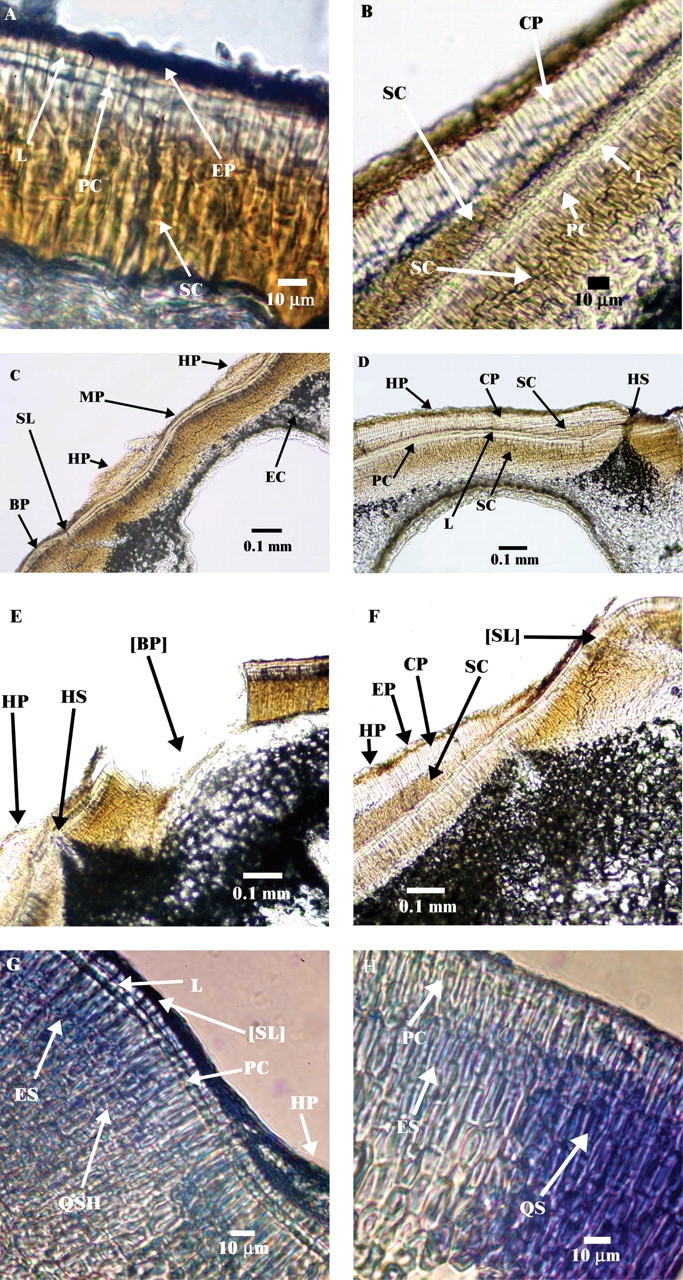
Vibrotome sections of I. lacunosa seeds showing seed coat away from hilar and bulge area (A); seed coat under hilum (B); bulge and micropyle area (C); hilum fissure (D); bulges detached (E); hilum–bulge area (F); bulge–hilum margin (G); and bulge–adjacent seed coat margin (H). BP, Bulge; [BP], detached bulge; CP, counter palisade; EC, endodermal cells; EP, epidermal cells; ES, elongated and compacted sclereid cells under bulge; HP, hilum pad; HS, hilar fissure; L, light line; MP, micropylar region; PC, palisade cells; QS, square-shaped, less compacted sclereids in seed coat away from the hilar and bulge area; QSH, square-shaped, less compacted sclereids in hilum pad; SC, sclereid cells; SL, slit; [SL], place where slit occurs.
The anatomy of the bulge is very similar to that of the seed coat away from the hilar and bulge area, having a palisade layer with a light line and several layers of sclereid cells. However, the shape of the sclereid cells in the bulge differs from that of those in the seed coat away from the hilar and bulge area and of those in the hilum pad. In the bulge, sclereid cells are elongated and compacted, whereas in the hilum pad and in the seed coat away from hilar and bulge area they are square (Fig. 9G, H). The transition from a square to an elongated shape of the sclereid cells is at the place where the slit through which water uptake occurs is formed (Fig. 9G). The palisade cell layer and the sclereid cell layers also undergo a change in orientation in this transition area.
DISCUSSION
Manually scarified seeds of I. lacunosa took up water rapidly at ambient laboratory temperatures (22–24 °C) (Fig. 1), and they germinated to high percentages over a wide range of temperatures within a few days (Fig. 2). In contrast, non-scarified (control) seeds did not take up water at ambient laboratory temperatures (Fig. 1), and they germinated to >25 % only at 30/15 and 35/20 ºC (Fig. 2). Further, I. lacunosa seeds incubated at 35 °C on wet sand for 2 h or longer and those dipped in boiling water for 4 s imbibed water as readily as manually scarified seeds (data not shown), and they germinated to a high percentage at 25/15 °C (the only temperature tested) (Fig. 3A–C). Thus, the results confirm the assumption of Crowley and Buchanan (1980) and of Oliveira and Norsworthy (2006) and the results of Gomes et al. (1978) that seeds of I. lacunosa have physical dormancy.
When I. lacunosa seeds are made permeable by boiling for 4 s or by incubating them at 35 °C for 3 h on wet sand, a slit develops around each of the two bulges (Figs 4B and 5B). In some seeds, a slit may develop around only one bulge. Cracks also developed in the hilum pad of some seeds after they were made permeable (Figs 4B and 5B, C). These slits around the bulges and the cracks in the hilum pad appeared to be possible routes of water entry into the seed. However, dye-tracking experiments showed that the initial route of water entry into the seed is via slits developed around the bulges and not via cracks in the hilum pad. That is, cells below the sclereid layer in the subpalisade under the bulge were stained, whereas those under the hilum pad were not (Figs 6A and 7A). Thus, the slits around the bulges extend through the whole seed coat (Fig. 5D), while cracks formed in the hilum pad do not extend though the impermeable layer(s) of the seed coat. After imbibition for 30 min to 1 h, the bulges become detached from most seeds. These results are supported by those of water exclusion experiments. When the bulge area of permeable seeds was painted, the seeds did not imbibe water and thus did not germinate. On the other hand, when the hilum was painted permeable seeds imbibed and germinated as readily as non-painted permeable seeds (Fig. 8).
Koller and Cohen (1959) described a plug in the micropyle of three species of Convolvulvus that grow in the Negev Desert, Israel, and they concluded that dislodgment of this plug allowed water to enter the seeds. However, they did not demonstrate that water entered the seeds via the micropyle. The present results clearly show that the route of water entry into seeds of I. lacunosa is via a specialized morpho-anatomical area of the seed coat located adjacent to the hilum and micropyle and not through the micropyle itself. Although the water gap is described only in Ipomoea lacunosa here, the water gap in Convolvulvus arvensis and in several other species of Convolvulaceae is morphologically and functionally similar to the one in I. lacunosa (K. Jayasuriya et al., unpubl. res.). Callihan (1933) observed an open hilum fissure in ethanol-treated Convolvulus arvensis seeds stored at high humidity (>50 % RH), and thus he speculated that the hilum is the route of water entry into non-dormant seeds of this species. However, he did not support his speculation with data. Callihan merely suggested this in an attempt to explain the high germination percentage of seeds of C. arvensis after treating them with ethanol. The present study clearly shows that water enters the seed initially through the slits around the bulges, but not through the hilar fissure or through cracks in the hilum.
Hutchison and Ashton (1979) used concentrated H2SO4 to make seeds of Cuscuta compestris permeable to water. They blocked the hilum area of the seeds with lanolin-petroleum jelly and compared water uptake with that of permeable seeds to which no petroleum jelly was applied. There was no difference between painted and non-painted seeds. They also compared electron micrographs of dormant and acid-treated (permeable) seeds. In these micrographs, openings caused by damage to palisade cells can be seen everywhere on the seed coat. They stated that the upper part of the palisade layer was disrupted during the H2SO4 treatment. However, H2SO4 scarification is not a natural way for Cuscuta seeds to become water permeable. Lyshede (1984) compared electron micrographs of non-dormant (made permeable by slightly abrading them with sand paper) and dormant seeds of C. campestris and of non-dormant (apparently not water impermeable) seeds of C. pedicellata. He speculated that the bulging-papillae on the seed coat of non-dormant seeds of C. campestris and of C. pedicellata served as routes of water entry into the seed. Bulging of papillae did not occur on dormant seeds of C. campestris.
Several kinds of water gaps occur in the 11 families of angiosperms (excluding Convolvulaceae) for which they have been documented (Baskin et al., 2000). However, the morphology, anatomy and origin of the water gap within a family or within a group of closely related families (e.g. the bixoid chalazal plug in Cistaceae and related Malvanae; Nandi, 1998) usually are quite similar, but they may differ considerably between families or higher taxa (Baskin et al., 2000). The present study on I. lacunosa indicates that the water gap in Convolvulaceae differs from that of the other 11 families studied to date and is the only one described thus far in which two openings are formed. In Fabaceae, there are anatomical differences in the palisade layer between the water gap (lens) and the seed coat away from the lens (see Dell, 1980; Hanna, 1984; Serrato-Valenti et al., 1995), but differences do not occur in the palisade layer between these two regions in the seed coat in I. lacunosa (Fig. 9G and H). In several families of Malvales, plug removal is responsible for imbibition, whereas in I. lacunose formation of the slit around the bulge, and not bulge removal, is responsible for imbibition. The water gap in Malvales (i.e. bixoid chalazal plug) is considerably more complex anatomically (Chopra and Kaur, 1965; Nandi, 1998) than the one in I. lacunosa.
Water gaps serve as environmental signal detectors for dormancy break (Baskin and Baskin, 2000) that allow the seeds to germinate when conditions are suitable for subsequent establishment of the plant. In I. lacunosa, high temperature combined with high humidity (or free water) causes the water gap to open in summer, when climate is suitable for the seed to germinate and for subsequent establishment and growth of the plant to maturity.
LITERATURE CITED
- Abel WE, Austin DF. Introgressive hybridization between Ipomoea trichocarpa and I. lacunosa. Bulletin of the Torrey Botanical Club. 1981;108:231–239. [Google Scholar]
- Anonymous. Weed survey – Southern states, broad leaf crops subsection. Proceedings, Southern Weed Science Society. 1995;48:290–305. [Google Scholar]
- Anonymous. Weed survey – Southern states, grass crop subsection. Proceedings, Southern Weed Science Society. 2000;53:247–274. [Google Scholar]
- Anonymous. Weed survey – Southern states, broadleaf crops subsection. Proceedings, Southern Weed Science Society. 2001;54:244–259. [Google Scholar]
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Baskin CC, Thompson K, Baskin JM. Mistakes in germination ecology and how to avoid them. Seed Science Research. 2006;16:165–168. [Google Scholar]
- Baskin JM, Baskin CC. Evolutionary consideration of claims of physical dormancy-break by microbial action and abrasion by soil particles. Seed Science Research. 2000;10:409–413. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Baskin JM, Baskin CC, Li X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology. 2000;15:139–152. [Google Scholar]
- Baskin JM, Baskin CC, Dixon KW. Physical dormancy in the endemic Australian genus Stylobasium, a first report for the family Surianaceae (Fabales) Seed Science Research. 2006;16:229–232. [Google Scholar]
- Brechu-Franco AE, Ponce-Salazer RM, Laguna-Herandez G, Marques-Guzman J. Effect of thermal storage on seed coat dormancy and germination of Ipomoea purpurea (L.) Roth (Convolvulaceae) seeds. Journal of Experimental Botany. 2000;67:187–194. [Google Scholar]
- Callihan RH. Corvallis: Oregon State College; 1933. The hard seed mechanism in Convolvulus arvensis L. and influence of environmental variables upon germination. [Google Scholar]
- Chopra RN, Kaur H. Embryology of Bixa orellana Linn. Pytomorphology. 1965;15:211–214. [Google Scholar]
- Crowley RH, Buchanan GA. Response of Ipomoea spp. and smallflower morningglory (Jacquemontia tamnifolia) to temperature and osmotic stresses. Weed Science. 1980;28:76–82. [Google Scholar]
- Dell B. Structure and function of the strophiolar plug in seeds of Albizia lophantha. American Journal of Botany. 1980;67:556–563. [Google Scholar]
- Gleason HA, Cronquist A. Manual of vascular plants of northeastern United States and adjacent Canada. 2nd edn. New York Botanic Garden: Bronx, NY; 1991. [Google Scholar]
- Gomes LF, Chandler JM, Vaughan CE. Aspects of emergence and seed production of three Ipomoea taxa. Weed Science. 1978;26:245–248. [Google Scholar]
- Gunn CR. Seeds of the United States noxious and common weeds in the Convolvulaceae, excluding the genus Cuscuta. Proceedings of Association of Official Seed Analysts of North America. 1969;59:101–115. [Google Scholar]
- Hanna PJ. Anatomical features of the seed coat of Acacia kempeana (Mueller) which relate to increased germination rate induced by heat treatment. New Phytologist. 1984;96:23–29. [Google Scholar]
- Hardcastle WS. Influence of temperature and acid scarification duration on scarlet morningglory (Ipomoea coccinea) seed germination. Weed Science. 1978;26:261–263. [Google Scholar]
- Horak MJ, Wax LM. Germination and seedling development of bigroot morningglory (Ipomoea pandurata) Weed Science. 1991;39:390–396. [Google Scholar]
- Hutchison JM, Ashton FM. Effect of desiccation and scarification on the permeability and structure of the seed coat of Cuscuta campestris. American Journal of Botany. 1979;66:40–46. [Google Scholar]
- Jones RL. Plant life of Kentucky: an illustrated guide to the vascular flora. Lexington, KY: University Press of Kentucky; 2005. [Google Scholar]
- Koger CH, Reddy KN. Glyphosate efficacy, absorption, and translocation in pitted morningglory (Ipomoea lacunosa) Weed Science. 2005;53:277–283. [Google Scholar]
- Koger CH, Poston DH, Reddy KN. Effect of spray coverage on control of pitted morningglory (Ipomoea lacunosa) Weed Technology. 2004;18:124–130. [Google Scholar]
- Koller D, Cohen D. Germination-regulating mechanisms in some desert seeds. VI. Convolvulus lanatus Vahl, Convolvulvus negevensis Zoh. and Convolvulus secundus Desr. Bulletin of the Research Council of Israel. 1959;7D:180–195. [Google Scholar]
- Li X, Baskin JM, Baskin CC. Anatomy of two mechanisms of breaking physical dormancy by experimental treatments in seeds of two North American Rhus species (Anacardiaceae) American Journal of Botany. 1999;86:1505–1511. [PubMed] [Google Scholar]
- Lyshede OB. Seed structure and germination in Cuscuta pedicellata with some notes on C. campestris. Nordic Journal of Botany. 1984;4:669–674. [Google Scholar]
- Martinez ML, Vazquez G, White DA, Thivet G, Brengues M. Effect of burial by sand and induction by fresh and seawater on seed germination of five tropical beach species. Canadian Journal of Botany. 2002;80:416–424. [Google Scholar]
- Misra BN. Germination of seeds of Ipomoea crassicaulis (Benth.) Robinson. Journal of the Indian Botanical Society. 1963;42:358–366. [Google Scholar]
- Nandi OI. Ovule and seed anatomy of Cistaceae and related Malvanae. Plant Systematics and Evolution. 1998;209:239–264. [Google Scholar]
- Ogunwenmo KO. Variation in fruit and seed morphology, germination and seedling behavior of some taxa of Ipomoea L. (Convolvulaceae) Feddes Repertorium. 2006;117:207–216. [Google Scholar]
- Oliveira MJ, Norsworthy JK. Pitted morningglory (Ipomoea lacunosa) germination and emergence as affected by environmental factors and seeding depth. Weed Science. 2006;54:910–916. [Google Scholar]
- Scot SJ, Jones RA, Williams WA. Review of data analysis methods for seed germination. Crop Science. 1984;24:1192–1199. [Google Scholar]
- Serrato-Valenti G, De Vries M, Cornara L. The hilar region in Leucaena leucocephala Lam. (De Wit) seed: structure, histochemistry and the role of the lens in germination. Annals of Botany. 1995;75:569–574. [Google Scholar]
- Stefanovic S, Austin DF, Olmstead G. Classification of Convolvulaceae: a phylogenetic approach. Systematic Botany. 2003;28:791–806. [Google Scholar]
- Thullen RJ, Keeley PE. Germination, growth and seed production of Ipomoea hederacea when planted at monthly intervals. Weed Science. 1983;31:837–840. [Google Scholar]
- Toole EH, Brown E. Final results of the Duvel buried seed experiment. Journal of Agricultural Research. 1946;72:201–210. [Google Scholar]
- Van Staden J, Manning JC, Kelly KM. Legume seeds: the structure:function equation. In: Stirton CH, Zarucchi JL, editors. Advances in legume biology. Vol. 29. St Louis, MO: Missouri Botanical Garden; 1989. pp. 417–450. Monographs in Systematic Botany. [Google Scholar]