Abstract
Background and Aims
Lobed leaves are considered selectively advantageous in conditions of high irradiance. However, most studies have involved woody species, with only a few considering the role of leaf lobation in herbaceous rosette species. In this study, it is hypothesized that, in addition to its adaptive value in high light, leaf lobation may add to the function of petioles as vertical spacers in herbaceous species in conditions of strong competition for light.
Methods
To test this hypothesis, leaf development was examined under seasonally changing natural light conditions and a field experiment was conducted in which light climate was manipulated in a wooded meadow population of Serratula tinctoria.
Key Results
No changes in leaf lobation were observed in response to experimental shading or different natural light conditions. However, in tall herbaceous vegetation, plants with highly lobed leaves achieved significantly greater vertical elongation than plants with less-lobed leaves. In contrast to herbaceous shade, tree shade had no effect on leaf elongation, suggesting differential responsiveness to competition from neighbouring herbs versus overhead shade. In shading treatments, imposed shade could only be responded to by the elongation of leaves that were produced late in development.
Conclusions
The results show that extensive leaf lobation can enable greater leaf elongation in response to shade from surrounding herbaceous vegetation. The different morphological responses displayed by Serratula tinctoria to different types of shade demonstrate the importance of critically assessing experimental designs when investigating phenotypic plasticity in response to shade.
Key words: Asteraceae, herbaceous shade, leaf lobation, phenotypic plasticity, Serratula tinctoria, shade avoidance, tree shade, leaf elongation
INTRODUCTION
Leaf shape has been shown to vary with plant ontogeny (Kerstetter and Poethig, 1998; Lynn and Waldren, 2001), light quality (red : far-red ratio) and quantity, air temperature and CO2 levels (Hanson, 1917; Talbert and Holch, 1957; Vogel, 1968; Sanchez and Cogliatti, 1975; Gurevitch, 1992; Jones, 1995; Thomas and Bazzaz, 1996; Zwieniecki et al., 2004). These patterns have led to the development of a variety of explanations for the adaptive value of leaf shape and its plasticity. Experiments conducted by Vogel (1968, 1970) demonstrated that the presence of lobes on a leaf increased heat dissipation per unit area and decreased the dependence of heat dissipation on leaf orientation. However, the effects of leaf shape were much smaller than the effects of leaf size (Vogel, 1970). It has also been shown that leaf lobation is not functionally advantageous to light interception; however, gradients of leaf-lobing along the length of shoots may be significant in terms of overall light interception (Niklas, 1989). Sisó et al. (2001) have suggested that lower hydraulic resistance in deeply lobed leaves may constitute a mechanism for improving water balance under dry atmospheric conditions. In addition, compound and lobed leaves are considered to be structures with low production costs that are adaptive in environments favouring the deciduous habit, and in light gaps or early successional vegetation where rapid upward growth and efficient competition for light are advantageous (Givnish, 1978).
It has been suggested that herbaceous plants may face the same ecological problems as early successional trees, in having to compromise between extensive branching and rapid vertical growth (Givnish, 1978). However, a considerable number of herbaceous species do not have vertically extending leafy stems and thus are not confronted with the choice between branching and not branching. We suggest that rosette-forming species experience different selective conditions from trees, and that the leaves of such herbaceous species have the additional function of acting as vertical spacers (i.e. structures that place photosynthesizing tissues higher in the canopy; Huber, 1996), a role that leaves of woody species do not have. Herbaceous canopies are characterized by a strong vertical light gradient, in which light availability increases towards the top of the canopy (e.g. Huber and Wiggerman, 1997). Therefore, the placement of photosynthesizing structures in higher layers of the herbaceous canopy by elongation will improve a plant's light capture. In herbaceous plants lacking vertical stems, petiole elongation may often represent the most important mechanism of shade avoidance (Huber, 1996; Huber and Wiggerman, 1997; Leeflang et al., 1998). In addition, herbaceous plants can experience shade from surrounding trees. Since there is little likelihood of most herbaceous species overtopping trees, reduced elongation responses to tree shade as compared with responses to herbaceous competitors should be favoured (Schmitt et al., 1999). This would, however, require plants to be able to discriminate between the two types of shade. Artificial shading in most experiments represents a third type of shade, which can have attributes of both natural herbaceous and tree shade and may not be easily interpreted in biological terms. These possibilities have to be considered when studying leaf elongation in response to shade.
We hypothesize that, in addition to its role as an adaptation to dry conditions and high irradiance, pinnatifid lobation in leaves (i.e. leaves deeply cut into pinnately arranged lobes, the lobes remaining connected by at least a narrow flange of leaf-blade bordering the midrib; Rose, 1981) may substitute for, or augment, the function of petioles in placing leaves higher in the canopy in response to herbaceous shade. In terms of biomass investment, it will be less expensive to elongate gaps between leaf lobes in response to shading than to occupy a similar vertical space with continuous leaf area. This hypothesis was tested using Serratula tinctoria, which is extremely variable in leaf shape (Fig. 1). The main objective was to relate patterns of variation in leaf shape, especially leaf lobation, to local light climate whilst discriminating between shade from trees and herbaceous species. Plant development was tracked under different natural light conditions and a field experiment was conducted in which light climate was altered in a wooded meadow population of S. tinctoria.
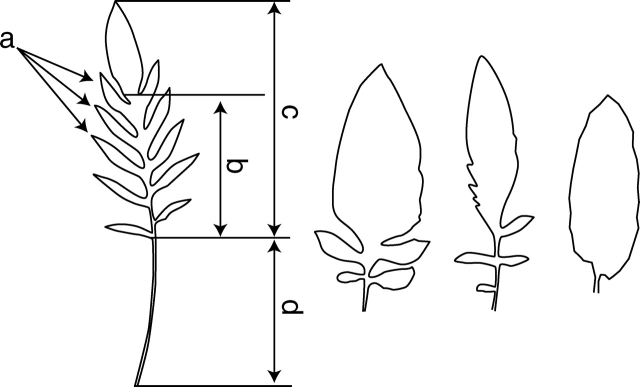
Fig. 1.
Leaf characteristics and examples of leaf shapes of Serratula tinctoria. a, Leaf lobes; b, dissected area of a leaf; c, leaf lamina; d, petiole. Numbers of lobes in leaves from left to right are ten, four, four and zero.
MATERIALS AND METHODS
Study species
Serratula tinctoria L. is a long-lived herbaceous perennial species of moist meadows, wooded meadows and open deciduous forests. The area of distribution of the species covers central and Atlantic Europe and the southern part of Scandinavia (Talts, 1978; Tutin et al., 1980). Leaves are finely toothed and vary in shape from undivided, lanceolate, to deeply pinnatifid with narrow lobes (Fig. 1).
Study site
The field study was carried out in a species-rich calcareous wooded meadow in Laelatu, western Estonia, on the eastern coast of the Baltic Sea (58°35′N, 23°34′E). The climate is seasonal temperate; mean temperature in July is 17·0 °C and in January, − 5·0 °C. The average annual precipitation is approx. 500 mm. The meadow has a scattered deciduous tree layer and it has been annually mown in July for hay for > 200 years. The majority of species in the herbaceous layer are perennials (98 %), 64 % of the species are forbs and 33 % are graminoids (Zobel and Liira, 1997). Plant species richness in the herbaceous layer is high (exceeding 60 species m−2 in some parts of the meadow; Kull and Zobel, 1991).
Experimental design
To minimize morphological variation between replicate plants due to differences in ontogenetic stages, adult vegetative individuals were selected for the experiment. Plants were selected at random across the meadow to ensure a gradient of natural light availability. Three shading treatments of different timing and duration, and an unshaded control treatment, were imposed between May and July 2004. The constant shading treatment lasted from 25 May to 26 July. Early shading was imposed by shading plants from 25 May to 26 June, and late shade was achieved by shading plants from 26 June to 26 July. Twenty-two replicate plants of S. tinctoria per treatment were examined. However, 18–22 replicates per treatment were used in the analysis as some plants started flowering and were therefore excluded from the analysis.
In the shading treatments, each replicate plant was shaded using two artificial plastic plants that mimicked both the shape and optical properties (Smith and Morgan, 1981) of approx. 28-cm-high forbs with four or five leaves, respectively (leaf width 8–11 cm, length 15–20 cm; Fig. 2). The artificial leaf material reduced the red : far-red ratio (measured using a two-channel sensor with 660-nm and 730-nm filters; SKR 110, Skye Instruments Ltd, Powys, UK) from 1·02 in daylight conditions to values around 0·32 under the artificial plants. Light availability under the artificial plants was reduced by approx. 48 % (measured using hemispherical photographs taken under the artificial plants and analysed by SCANOPY software, Regent Instruments Inc., Quebec, Canada). This was similar to the mean light availability beneath tree canopies in the study area (Table 1) and beneath the herbaceous layer at the beginning of summer (mean reduction in PAR beneath the canopy between 26 May and 26 June was 49 %). Gaps in the canopy of the artificial plants allowed the shaded plants to experience sunflecks during the day.
Fig. 2.
Artificial plants used in the experiment. The stems of the artificial plants were placed at equal distances on either side of a study plant (rooting point is shown with a black dot).
Table 1.
Mean values for natural light conditions and various leaf traits of Serratula tinctoria at five consecutive sampling dates (n = 60) during the summer of 2004
| Characteristic | Sampling date |
||||
|---|---|---|---|---|---|
| 25 May | 11 June | 26 June | 10 July | 26 July | |
| Height of herbaceous layer (cm) | 16 (0·3)a | 22 (0·4)b | 29 (0·6)c | 31 (0·6)d | 33 (0·6)e |
| ISF | 58·7 (1·6)a | 54·4 (1·8)b | –* | – | – |
| Leaf lamina length (cm) | 8·8 (0·20)a | 11·8 (0·27)b | 12·7 (0·33)c | 13·4 (0·38)cd | 13·5 (0·47)d |
| Petiole length (cm) | 6·6 (0·18)a | 8·3 (0·20)b | 8·9 (0·24)c | 9·3 (0·26)c | 9·8 (0·32)d |
| Density of lobation (lobes cm−1) | 2·4 (0·11)a | 1·7 (0·06)b | 1·5 (0·06)b | 1·5 (0·07)b | 1·5 (0·07)b |
| Number of lobes (lobes leaf−1) | 8·41(0·39)a | 9·05(0·39)b | 9·12(0·41)b | 9·32(0·41)b | 9·18(0·43)b |
Means with the same letter are not significantly different (P > 0·05, Tukey HSD test within repeated measures ANOVA). Standard errors are shown in parentheses.
ISF, Percentage of diffuse radiation passing tree canopies.
* Tree canopies were fully expanded by the third sampling date, and no further measurements were taken.
Measurements
The following data were recorded for each plant on 25 May, 11 June, 26 June, 10 July and 26 July: number of leaves on a plant; and leaf ordinal number, leaf angle (the number of degrees by which the leaf petiole deviated from horizontal), lamina length, petiole length, number of lobes and length of dissected area of each leaf (Fig. 1).
Availability of diffuse and direct radiation was estimated from hemispherical digital photographs taken at the rooting point of each plant measured on the first two sampling dates using WinSCANOPY software (Regent Instruments Inc., Quebec, Canada). The height of the hemispherical lens above the ground was 0·2 m. The direct and indirect site factors (DSF and ISF, respectively; proportion of direct and indirect radiation under the tree canopy relative to that above the tree canopy) were estimated from the photographs as an integral for a 3-month period (15 May to 15 August), using cosine correction (Machado and Reich, 1999). Since ISF and DSF estimates were highly correlated, only the one providing lower P-values (ISF) against plant morphological traits was used in the analyses. The height of the herbaceous layer was estimated on every sampling date as the average of several measurements taken within a 15-cm radius of each replicate.
Density of lobation was calculated as the number of lobes per unit length of dissected leaf portion (Fig. 1). This characteristic was used to describe changes in the density of lobes along the leaf midrib (the central vein of a leaf) due to leaf elongation. In contrast, the variation in leaf shape that is determined early during leaf formation was measured by differences in absolute numbers of lobes in a leaf. Leaf height was estimated as a function of leaf angle and the sum of lamina and petiole length: leaf height = (lamina length + petiole length) × sin (leaf angle). Above-ground parts of plants were harvested at the last sampling date, dried at 75 °C for 48 h, and weighed.
Statistical analysis
Temporal changes in mean values of plant traits and light conditions were analysed with a repeated measures ANOVA treating sampling time as a repeated measures factor. Two general linear models were used to examine the relationship between mean leaf height (dependent variable) and (a) the mean number of lobes per leaf and (b) mean density of lobation. In both models, sampling date was included as a repeated measures factor.
The examination of leaf trait as a function of sampling time implies the observation of leaves at different stages in their development. Differences between shading treatments can then be partially attributed to differences in plants' growth rates, i.e. resource-mediated responses. At some point in time, shaded leaves can be shorter on average than sun leaves, merely because of reduced growth rates rather than a developmental change (Wright and McConnaughay, 2002). Therefore only data on fully expanded leaves (i.e. leaves that did not increase in length at subsequent sampling dates) were analysed as a function of their ordinal number (i.e. the first leaf is the leaf that was produced earliest in the growing season), in order to exclude the confounding effects of sampling time, and to examine responses that are driven by shifts in ontogeny (‘ontogenetic plasticity’ in Wright and McConnaughay, 2002). Similarly, numerous studies have used the production of successive leaves as a reliable measure of the developmental stage of plants, which can be determined non-destructively (Collins and Jones, 1988; Huber and Stuefer, 1997). A general linear mixed model (Littell et al., 1996) was used to examine responses to light conditions, where leaf ordinal number (a fixed factor that indicates ontogenetic stage), shading treatment (a fixed factor with four levels: control, constant shade, early shade and late shade), indirect radiation availability (ISF, covariate), and height of surrounding herbaceous layer (covariate) were included to predict the following characteristics: lamina length, petiole length, number of lobes, density of lobation and leaf angle of fully expanded leaves. Plant identity, nested into treatment, was included in the model as a random factor. The definition of plant identity as a random factor takes into account the size of each plant and the correlation between single leaves within a plant. In the table of results, only the test of fixed effects is presented.
It is likely that the first leaves of the plants studied were produced before shading treatments were imposed, and that variation in traits of early leaves may therefore be independent of treatments. Any differences in leaf trait means caused by treatments should therefore appear later in development. Hence, interaction between leaf ordinal number and shading treatment was included in the model to describe the variation in effects of treatments during ontogeny. To test the hypothesis that leaf lobation is a trait enhancing leaf elongation and its plasticity in response to light conditions, leaf lobation and its interactions with light conditions were also included as predictors in the model examining plasticity in lamina and petiole length. For a clearer interpretation and graphical presentation of the interaction terms, leaf lobation was transformed into a categorical variable with three levels: 0–6 lobes, 7–12 lobes and > 12 lobes in a leaf. (A model with lobation as a continuous variable produced very similar output; results not shown.)
A backwards stepwise linear regression was used to assess the significance of the mean number of lobes per leaf and plant above-ground biomass as predictors of leaf elongation. If the number of lobes and biomass are both significant predictors of leaf length, and have a positive effect on it in the regression, plants with extensively lobed leaves will produce longer leaf laminas than plants with less-lobed leaves, irrespective of their biomass.
Statistical analyses were performed using SAS 9·1 (SAS Institute, Cary, NC, USA). When necessary, dependent variables were ln-transformed to improve normality of residuals and homogeneity of variances.
RESULTS
Temporal changes in natural light conditions and plant growth
The largest changes in leaf morphology took place between the two first sampling dates, coinciding with the most pronounced changes in the height of the herbaceous layer (Table 1). There was a sharp increase in leaf lamina length and petiole length, as well as a decrease in the density of lobation, at the beginning of summer (25 May to 11 June), indicating rapid leaf elongation (Table 1). There was no correlation between the height of the herbaceous layer and the availability of overhead diffuse radiation (R2 at five measurements during the summer ranged from 0·0007 to 0·0174; P > 0·05).
Functional significance of leaf lobation
A significant positive correlation was observed between leaf lamina length and the number of lobes per leaf in all plant samples, independent of sampling date or leaf position (P < 0·006 for all correlations). Having a large number of lobes was directly associated with a higher vertical position of the rosette leaves, the dependence being apparently stronger at later sampling dates (Fig. 3; interaction between time and the number of lobes per leaf significant at P = 0·0144). Mean leaf height of the plants studied was independent of leaf lobation density at the beginning of the study period (r = 0·01, P = 0·9169) and was negatively correlated with it at subsequent measurements (r ranged from –0·41 to –0·63, P < 0·0012; interaction between time and density of lobation significant at P < 0·0001). This means that leaf vertical growth was achieved largely through extension of the lobed section of the leaf lamina. Mean petiole length, on the contrary, did not show any significant correlation with mean number of lobes.
Fig. 3.
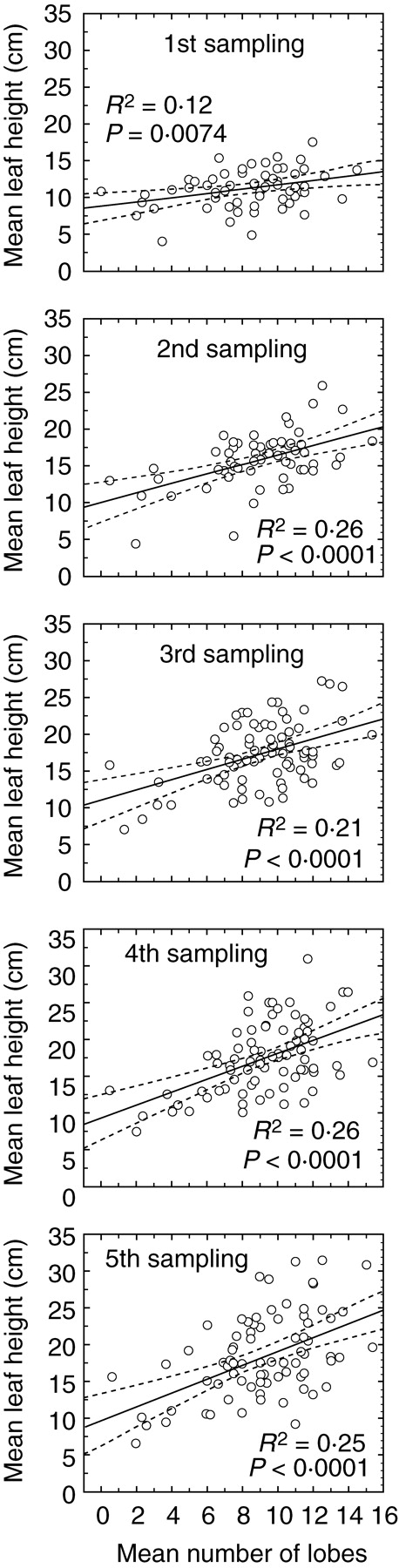
Dependence of mean height of rosette leaves on mean number of lobes per leaf in repeatedly measured plants of Serratula tinctoria at five consecutive samplings. Least-square regression lines and their 95 % confidence bands are shown. Coefficients of determination (R2) and P-values for relationships at each sampling time are given.
Mean number of lobes was also positively correlated with plant above-ground biomass at the end of the experiment (r = 0·31, P = 0·0051). In the backwards stepwise linear regression, both above-ground biomass and mean number of lobes were highly significant predictors of leaf length (both P < 0·0001), and a high tolerance value (0·90) suggests that a large number of lobes in a leaf increased leaf elongation irrespective of plant size.
Plasticity in response to natural conditions
Plants produced more upright leaves with longer petioles and leaf laminae, and arranged lobes more sparsely along the leaf midrib, in response to taller surrounding herbaceous vegetation, but not in response to tree shade (Table 2). No plasticity was observed in the number of lobes in response to either of the parameters of light availability measured (Table 2). However, plants with highly lobed leaves exhibited greater plasticity in leaf lamina length in response to the height of the herbaceous layer, as indicated by the significant interaction between leaf lobation and height of the herbaceous layer (Fig. 4 and Table 2; interaction term estimates of 0·27 and 0·19 for leaves with > 12 lobes and with 7–12 lobes per leaf, respectively, compared with the reference level of zero for leaves with fewer than seven lobes).
Table 2.
Results of a general linear mixed model for five leaf traits of Serratula tinctoria
| Trait | n | Treatment | Leaf ordinal number | Leaf ordinal number × treatment | Height of herb layer | ISF | Lobation | Lobation × treatment | Lobation × height of herb layer | Lobation × ISF |
|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | 3 | 4 | 12 | 1 | 1 | 2 | 6 | 2 | 2 | |
| Lamina length | 315 | 0·0051 | <0·0001 | <0·0001 | 0·0001 (0·26) | 0·6492 (−0·06) | 0·5389 | 0·1990 | 0·0008 | 0·0799 |
| Petiole length | 315 | 0·1531 | <0·0001 | 0·0004 | <0·0001 (0·23) | 0·6909 (−0·05) | 0·8300 | 0·4646 | 0·3734 | 0·0540 |
| No. of lobes | 315 | 0·7544 | <0·0001 | 0·0509 | 0·1515 (0·06) | 0·2332 (0·03) | – | – | – | – |
| ln(density of lobation) | 298 | 0·3050 | <0·0001 | 0·0047 | 0·0001 (−0·02) | 0·9651 (0) | – | – | – | – |
| Leaf angle | 315 | 0·1400 | <0·0001 | 0·4400 | 0·0055 (0·51) | 0·0120 (0·20) | – | – | – | – |
Plant identity, nested into shading treatment, was included as a random factor (not shown in the table).
Leaves were observed at their maximum expansion (i.e. when they did not increase in their length at subsequent sampling dates).
The statistical significance of ontogenetic stage (indicated by leaf ordinal number), shading treatments (four levels: control, constant shade, shade for the first half of the experiment, and shade for the second half of the experiment) and natural light conditions (height of herbaceous layer and availability of diffuse radiation, i.e. ISF) are presented.
To examine the effect of leaf lobation on the plasticity of lamina length in response to light conditions, interactions between lobation (three categories: leaves with < 7 lobes, leaves with 7–12 lobes, and leaves with > 12 lobes) and light conditions were included in the model.
Numbers in bold indicate significant (P < 0·05) effects under a sequential Bonferroni criterion.
The estimates of the effects of natural light conditions are shown in parentheses.
Measurement units of leaf traits and light conditions are the same as in Table 1.
Leaf angle was measured as the deviation of the leaf petiole from horizontal (in degrees).
d.f., degrees of freedom; n, sample size.
Fig. 4.
The relationship between leaf lamina length and the height of the surrounding herbaceous layer. Data for fully expanded leaves is presented. Leaves were grouped into three categories of leaf lobation: leaves with < 7 lobes, leaves with 7–12 lobes, and leaves with > 12 lobes. See results of the linear model in Table 2.
Experimental shading
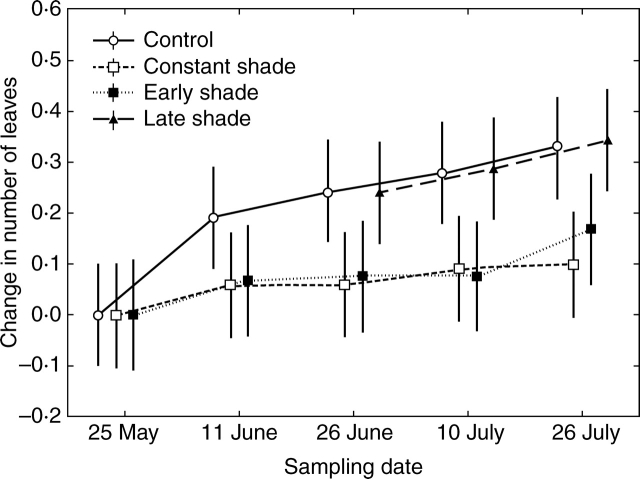
While control plants continued to produce new leaves until the end of the experiment, the formation of new leaves in shaded plants was retarded, and this restricted plants' elongation responses to leaves that were present before the shade was imposed (Fig. 5). Consequently, greater elongation of leaf laminae in shaded plants than in control plants was observed only as late as at the stage of the fourth leaf (Fig. 6A and Table 2). Elongation of leaf laminae was accompanied by a significant decrease in leaf lobation density (Fig. 6B and Table 2). Shading treatments imposed later in summer had almost no effect on leaf morphology (Figs 5 and 6). None of the treatments had a significant effect on the number of lobes per leaf (Table 2).
Fig. 5.
Changes in numbers of leaves in plants of Serratula tinctoria during the course of the experiment. Control plants, constantly shaded plants, plants shaded for the first half of the experiment, and plants shaded for the second half of the experiment, are distinguished. Original differences in mean numbers of leaves between treatments have been set to zero, and absolute changes from the first sampling are shown. For the treatment ‘late shade’, data were only recorded from the third sampling date, when shading was imposed, as plants were assumed to perform as controls prior to shading. The error bars denote 95 % confidence intervals for the mean.
Fig. 6.
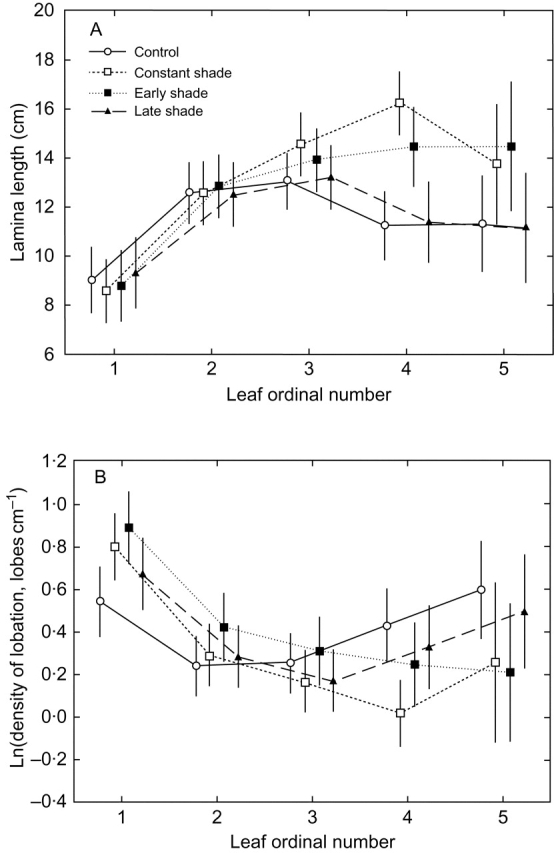
(A) Lamina length and (B) density of lobation of fully expanded leaves as a function of leaf ordinal number in plants of Serratula tinctoria grown in control conditions or exposed to three shading treatments. Early and late shade denote shading imposed for the first half and for the second half of the experiment, respectively. The error bars denote 95 % confidence intervals for the mean.
DISCUSSION
In all plants, a positive relationship between the number of lobes and leaf lamina length was observed. Two factors suggest that lobation causes greater leaf elongation rather than leaf elongation resulting in greater lobation. First, it has been shown that leaf shape, including the number of lobes, is determined at a very early stage of leaf development (Jones, 1995; Kerstetter and Poethig, 1998), i.e. lobes are not added as leaves elongate. Secondly, lamina length was highly responsive to herbaceous shade, whereas the number of lobes in a leaf was not influenced by light conditions. Comparison of the temporal changes in light conditions at the meadow and the growth pattern of S. tinctoria suggest how leaf lobation could contribute to plant competitive ability without the need for plasticity in this trait. In spring, plants had short leaves with densely packed lobes, and mean leaf height was only weakly related to the number of lobes per leaf (Fig. 3) and density of lobation. At the beginning of summer, as light conditions changed rapidly, plants elongated their leaves rapidly and leaf height became strongly correlated with the number of lobes per leaf (Fig. 3) and with the extension of the lobed section of a leaf (as shown by a strong negative correlation with density of lobation). To assure rapid vertical growth, plants require a strategy that involves minimal expense in terms of carbon (Reich, 2001; Westoby et al., 2002). This can be achieved by elongating lobed leaf portions along the vertical axis. A smaller biomass investment is needed to elongate gaps between leaf lobes than to produce a continuous leaf lamina along the extending leaf midrib. Thus, given the same amount of resource, lobed leaves can elongate more in response to shading than leaves with entire margins.
Furthermore, examination of phenotypic responses to natural light conditions revealed that extensive leaf lobation was associated with greater plasticity of leaf length in response to shading by surrounding herbaceous vegetation (Table 2). Within low vegetation, plants with highly lobed leaves produced laminas as short as plants with unlobed laminas, but within a tall herbaceous layer, where there was likely to be strong competition for light, only plants with highly lobed leaves were able to produce longer leaf blades and reach the top of the herbaceous canopy (Fig. 4).
Using the same argument, petiole elongation would be even less costly than elongation of lobed leaf lamina. The present data show that petiole length was indeed highly responsive to the herbaceous shade (Table 2). However, plants had similar rates of petiole elongation regardless of the number of lobes in a leaf. Thus, plants with few-lobed leaves were not able to increase petiole elongation to compensate for the inferior leaf lamina elongation. This finding suggests that there is a limit to petiole elongation, and that leaf lobation provides plants with an additional, biomass-efficient method of projecting leaves to a higher level in the vegetation profile.
In spring, before tree leaf canopies have expanded, and when the herbaceous layer is still low, plants can detect shading from herbaceous neighbours through changes in light quality, and alter their growth pattern to maintain their photosynthetic potential. The shading treatments in the present study (and in most such experimental studies) were imposed suddenly, precluding plants from escaping the shade. Primarily, reduced light availability retarded plants' growth rates so that they were not able to produce new leaves (Fig. 5). This fact alone considerably limited the ability of plants to respond to the rapid changes in light availability: perceived shade could only be responded to by the elongation of leaves that were produced late in development (i.e. leaves with ordinal number four or higher; Fig. 6). The delay between the perception of an environmental cue and the production of an altered phenotype (elongated leaves in this case) is considered to be one of the most important factors limiting the evolution of plasticity and limiting its value (DeWitt et al., 1998; Pigliucci, 2001). Also, virtually no response to shading treatments imposed in the middle of summer indicates that the preceding developmental and environmental histories affected the ability of plants to adapt to changing conditions (the phenomenon described in Novoplansky et al., 1994; Weinig and Delph, 2001).
Interestingly, plants exhibited enhanced leaf elongation in response to increasing height of herbaceous vegetation, but not in response to the development of tree shade (Table 2). Comparative studies have shown that species/populations of open habitats were more responsive to simulated vegetation shade than those originating from closed-canopy wooded habitats (Corré, 1983; Dudley and Schmitt, 1995; van Hinsberg and van Tienderen, 1997; von Wettberg and Schmitt, 2005). The possibility of differential responsiveness to herbaceous (mostly lateral) and overhead shade (i.e. from a tree canopy) in plants has been hypothesized by Schmitt et al. (1999), but to the best of our knowledge no population experiencing both tree and herb shade has been examined so far to test this hypothesis. The present results show that plants of S. tinctoria responded differently to shading caused by neighbouring herbs and trees. Tree canopies, being more distant from herbaceous species, impose rather different environmental conditions than adjacent herbs (e.g. light direction and distribution, air humidity, wind speed, plant volatile concentrations; Niklas, 1994; Hirose and Werger, 1995; Leeflang et al., 1998; Pierik et al., 2004), and plants may be able to use these cues to discriminate between shade from these different sources.
In conclusion, the present results show that S. tinctoria, a rosette-forming herbaceous species, can benefit from having an extensively lobed leaf form as this allows vigorous vertical growth in conditions of limited light availability caused by herbaceous competitors. The absence of plasticity in leaf lobation (i.e. number of lobes in a leaf) in the species studied implies that it can do equally well with the same numbers of lobes per leaf in both shade and sunlight, or that there are constraints on plasticity in that trait. The present study also suggests that plants may have different responses to herbaceous and tree shade, and have limited responsiveness to the abrupt imposition of artificial shade.
ACKNOWLEDGEMENTS
We thank M. Lepik for assistance with data collection, J. Liira for help with the statistics, and Prof. M. J. Hutchings for insightful discussion and comments on an earlier version of the manuscript. The work was supported by grant 5535 from Estonian Science Foundation and by University of Tartu (2540).
LITERATURE CITED
- Collins RP, Jones MB. The effects of temperature on leaf growth in Cyperus longus, a temperate C4 species. Annals of Botany. 1988;61:355–362. [Google Scholar]
- Corré WJ. Growth and morphogenesis of sun and shade plants. II. The influence of light quality. Acta Botanica Neerlandica. 1983;32:185–202. [Google Scholar]
- DeWitt TJ, Sih A, Wilson DS. Costs and limits of phenotypic plasticity. Trends in Ecology and Evolution. 1998;13:77–81. doi: 10.1016/s0169-5347(97)01274-3. [DOI] [PubMed] [Google Scholar]
- Dudley SA, Schmitt J. Genetic differentiation in morphological responses to simulated foliage shade between populations of Impatiens capensis from open and woodland sites. Functional Ecology. 1995;9:655–666. [Google Scholar]
- Givnish TJ. On the adaptive significance of compound leaves, with particular reference to tropical trees. In: Tomlinson PB, Zimmerman MH, editors. Tropical trees as living systems. Cambidge: Cambridge University Press; 1978. pp. 351–380. [Google Scholar]
- Gurevitch J. Sources of variation in leaf shape among two populations of Achillea lanulosa. Genetics. 1992;130:383–394. doi: 10.1093/genetics/130.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson HC. Leaf-structure as related to environment. American Journal of Botany. 1917;4:533–560. [Google Scholar]
- van Hinsberg A, van Tienderen P. Variation in growth form in relation to spectral light quality (red/far-red ratio) in Plantago lanceolata L. in sun and shade populations. Oecologia. 1997;111:452–459. doi: 10.1007/s004420050258. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA. Canopy structure and photon flux partitioning among species in a herbaceous plant community. Ecology. 1995;76:466–474. [Google Scholar]
- Huber H. Plasticity of internodes and petioles in prostrate and erect Potentilla species. Functional Ecology. 1996;10:401–409. [Google Scholar]
- Huber H, Stuefer JF. Shade-induced changes in the branching pattern of a stoloniferous herb: functional response or allometric effect? Oecologia. 1997;110:478–486. doi: 10.1007/s004420050183. [DOI] [PubMed] [Google Scholar]
- Huber H, Wiggerman L. Shade avoidance in the clonal herb Trifolium fragiferum: a field study with experimentally manipulated vegetation height. Plant Ecology. 1997;130:53–62. [Google Scholar]
- Jones CS. Does shade prolong juvenile development? A morphological analysis of leaf shape changes in Cucurbita argyrosperma subsp. sororia (Cucurbitaceae) American Journal of Botany. 1995;82:346–359. [Google Scholar]
- Kerstetter RA, Poethig RS. The specification of leaf identity during shoot development. Annual Review of Cell and Developmental Biology. 1998;14:373–398. doi: 10.1146/annurev.cellbio.14.1.373. [DOI] [PubMed] [Google Scholar]
- Kull K, Zobel K. High species richness in an Estonian wooded meadow. Journal of Vegetation Science. 1991;2:711–714. [Google Scholar]
- Leeflang L, During HJ, Werger MJA. The role of petioles in light acquisition by Hydrocotyle vulgaris L. in a vertical light gradient. Oecologia. 1998;117:235–238. doi: 10.1007/s004420050653. [DOI] [PubMed] [Google Scholar]
- Littel RC, Milliken GA, Stroup WW, Wolfinger RD. SAS ystem for mixed models. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- Lynn DE, Waldren S. Morphological variation in populations of Ranunculus repens from the temporary limestone lakes (turloughs) in the west of Ireland. Annals of Botany. 2001;87:9–17. doi: 10.1093/aob/mcf125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado J-L, Reich PB. Evaluation of several measures of canopy openness as predictors of photosynthetic photon flux density in deeply shaded conifer-dominated forest understory. Canadian Journal of Forest Research. 1999;29:1438–1444. [Google Scholar]
- Niklas KJ. The effect of leaf-lobing on the interception of direct solar radiation. Oecologia. 1989;80:59–64. doi: 10.1007/BF00789932. [DOI] [PubMed] [Google Scholar]
- Niklas KJ. Plant allometry. Chicago, IL: University of Chicago Press; 1994. [Google Scholar]
- Novoplansky A, Cohen D, Sachs T. Responses of an annual plant to temporal changes in light environment: an interplay between plasticity and determination. Oikos. 1994;69:437–446. [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LACJ, de Kroon H, Visser EJW. Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signalling. The Plant Journal. 2004;38:310–319. doi: 10.1111/j.1365-313X.2004.02044.x. [DOI] [PubMed] [Google Scholar]
- Pigliucci M. Phenotypic plasticity: beyond nature and nurture. Baltimore, MD: John Hopkins University Press; 2001. [Google Scholar]
- Reich PB. Body size, geometry, longevity and metabolism: do plant leaves behave like animal bodies? Trends in Ecology and Evolution. 2001;16:674–680. [Google Scholar]
- Rose F. The wild flower key. London: Frederick Warne (Publishers) Ltd; 1981. [Google Scholar]
- Sanchez RA, Cogliatti D. The interaction between phytochrome and white light irradiance in the control of leaf shape in Taraxacum officinale. Botanical Gazette. 1975;136:281–285. [Google Scholar]
- Schmitt J, Dudley SA, Pigliucci M. Manipulative approaches to testing adaptive plasticity: phytochrome-mediated shade-avoidance responses in plants. American Naturalist. 1999;154(Supplement):S43–S54. doi: 10.1086/303282. [DOI] [PubMed] [Google Scholar]
- Sisó S, Camarero JJ, Gil-Pelegrín E. Relationship between hydraulic resistance and leaf morphology in broadleaf Quercus species: a new interpretation of leaf lobation. Trees. 2001;15:341–345. [Google Scholar]
- Smith H, Morgan DC. The spectral characteristics of the visible radiation incident upon the surface of the earth. In: Smith H, editor. Plants and the daylight spectrum. London: Academic Press; 1981. pp. 3–20. [Google Scholar]
- Talbert CM, Holch AE. A study of the lobing of sun and shade leaves. Ecology. 1957;38:655–658. [Google Scholar]
- Talts S. Eesti NSV Floora VI. Tallinn: Valgus; 1978. [Google Scholar]
- Thomas SC, Bazzaz FA. Elevated CO2 and leaf shape: are dandelions getting toothier? American Journal of Botany. 1996;83:106–111. [Google Scholar]
- Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, et al. Flora Europaea. Cambridge: Cambridge University Press; 1980. [Google Scholar]
- Vogel S. ‘Sun leaves’ and ‘shade leaves’: differences in convective heat dissipation. Ecology. 1968;49:1203–1204. [Google Scholar]
- Vogel S. Convective cooling at low airspeeds and the shapes of broad leaves. Journal of Experimental Botany. 1970;21:91–101. [Google Scholar]
- Weinig C, Delph LF. Phenotypic plasticity early in life constrains developmental responses later. Evolution. 2001;55:930–936. doi: 10.1554/0014-3820(2001)055[0930:ppeilc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics. 2002;33:125–159. [Google Scholar]
- von Wettberg EJ, Schmitt J. Physiological mechanism of population differentiation in shade-avoidance responses between woodland and clearing genotypes of Impatiens capensis. American Journal of Botany. 2005;92:868–874. doi: 10.3732/ajb.92.5.868. [DOI] [PubMed] [Google Scholar]
- Wright SD, McConnaughay KDM. Interpreting phenotypic plasticity: the importance of ontogeny. Plant Species Biology. 2002;17:119–131. [Google Scholar]
- Zobel K, Liira J. A scale-independent approach to the richness vs. biomass relationship in ground-layer plant communities. Oikos. 1997;80:325–332. [Google Scholar]
- Zwieniecki MA, Boyce CK, Holbrook NM. Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves. Plant, Cell and Environment. 2004;27:357–365. [Google Scholar]