Abstract
Virus particles morphologically similar to caliciviruses and rotaviruses were detected by electron microscopy (EM) in the intestinal contents of a 27-day-old diarrheic nursing pig. A third small spherical 23-nm virus-like particle was also observed. Calicivirus-like particles averaged 33 nm in diameter. Similar to rotaviruses, rotavirus-like particles were present as single-capsid 55-nm forms or double-capsid 70-nm particles. Most gnotobiotic pigs orally exposed to samples containing these three viruses developed diarrhea and villous atrophy of the small intestine, and all shed the three viruses in their intestinal contents. Attempts to propagate these viruses in cell culture were unsuccessful. The antigenic relationship of the rotavirus-like particles to known rotaviruses was explored by immune EM and immunofluorescent staining. By these techniques, the rotavirus-like particles did not cross-react with antisera to porcine, bovine, or human rotaviruses or to reovirus type 3. Antisera from gnotobiotic pigs exposed to all three viruses had enzyme-linked immunosorbent assay and virus neutralization titers of <4 against porcine rotavirus. Previous infection of gnotobiotic pigs with the mixture containing rotavirus-like particles failed to protect them against a subsequent challenge with porcine rotavirus. The antigenic relationship of the calicivirus-like particles to known caliciviruses was investigated by immune EM and virus neutralization. By these tests, the calicivirus-like particles did not react with antisera against feline calicivirus strain 255 or M-8. In a study conducted at Plum Island Animal Disease Center, antiserum against the three combined agents did not specifically neutralize any serotype of swine vesicular exanthema virus.
Full text
PDF


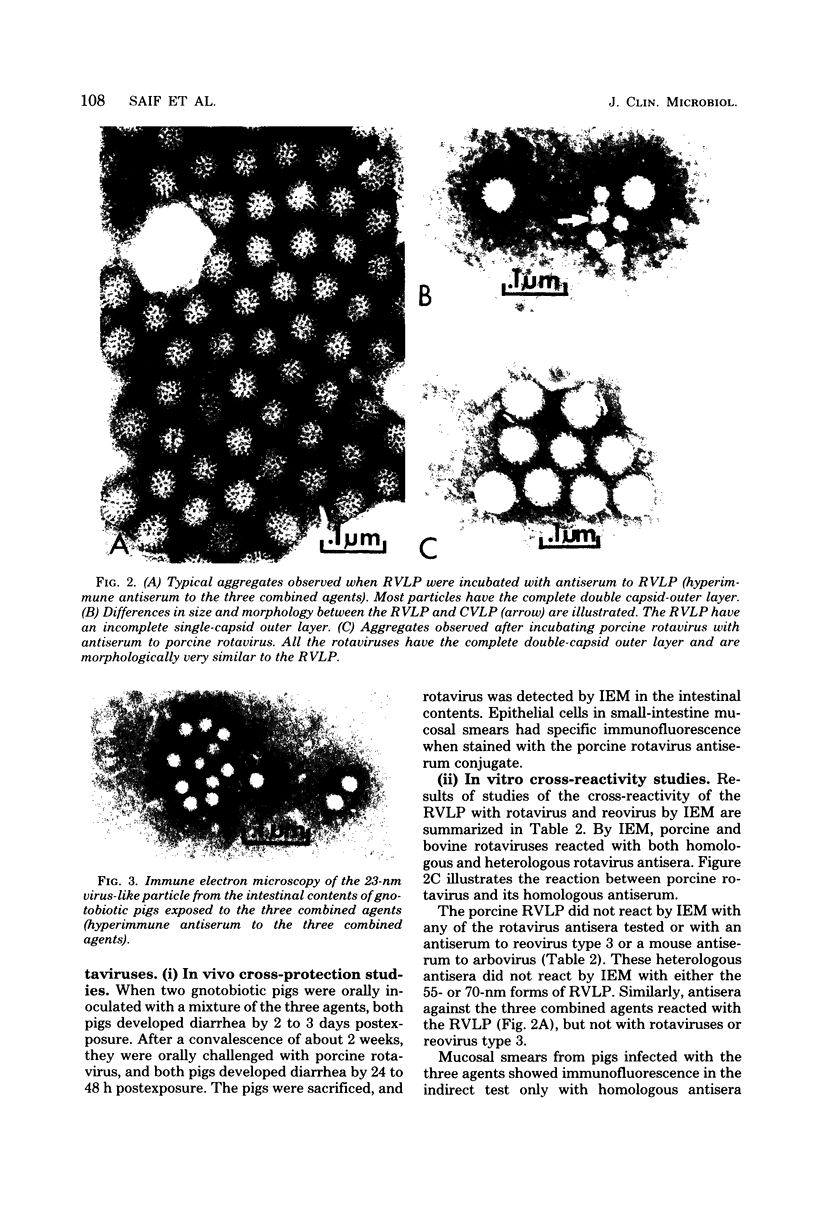
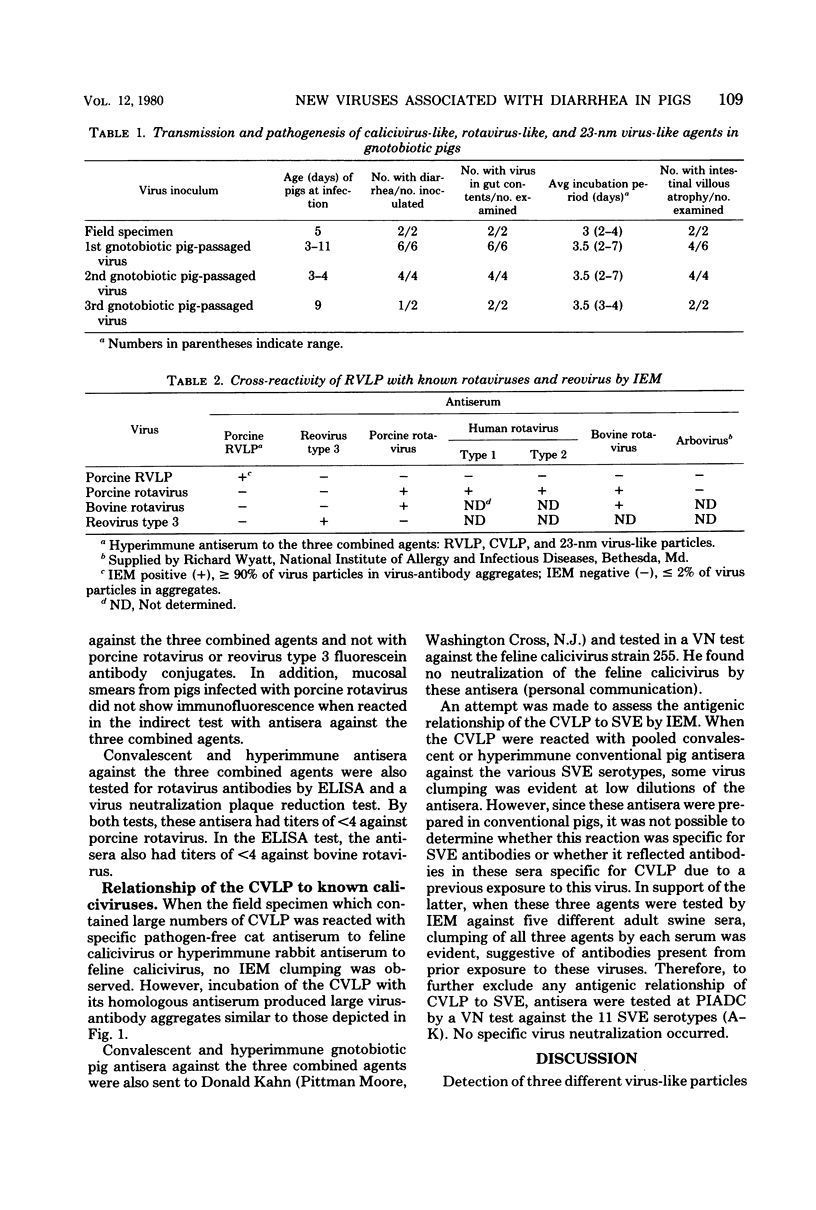


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D., Waterson A. P., Prydie J., Fletcher E. W. The structure of a feline picornavirus and its relevance to cubic viruses in general. Arch Gesamte Virusforsch. 1968;25(1):105–114. doi: 10.1007/BF01243095. [DOI] [PubMed] [Google Scholar]
- Bohl E. H., Kohler E. M., Saif L. J., Cross R. F., Agnes A. G., Theil K. W. Rotavirus as a cause of diarrhea in pigs. J Am Vet Med Assoc. 1978 Feb 15;172(4):458–463. [PubMed] [Google Scholar]
- Bridger J. C. Location of type-specific antigens in calf rotaviruses. J Clin Microbiol. 1978 Dec;8(6):625–628. doi: 10.1128/jcm.8.6.625-628.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D. J., Bishop R. F., Veenstra A. A., Barnes G. L., Holmes I. H., Ruck B. J. Pattern of shedding of two noncultivable viruses in stools of newborn babies. J Med Virol. 1978;2(1):7–13. doi: 10.1002/jmv.1890020103. [DOI] [PubMed] [Google Scholar]
- Cubitt W. D., McSwiggan D. A., Moore W. Winter vomiting disease caused by calicivirus. J Clin Pathol. 1979 Aug;32(8):786–793. doi: 10.1136/jcp.32.8.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewett T. H., Bryden A. S., Davies H., Woode G. N., Bridger J. C., Derrick J. M. Relation between viruses from acute gastroenteritis of children and newborn calves. Lancet. 1974 Jul 13;2(7872):61–63. doi: 10.1016/s0140-6736(74)91631-6. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantile enteritis viruses: morphogenesis and morphology. J Virol. 1975 Oct;16(4):937–943. doi: 10.1128/jvi.16.4.937-943.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Rodriguez W. J., Ross S., Cline W. L., Parrott R. H., Chanock R. M. Reoviruslike agent in stools: association with infantile diarrhea and development of serologic tests. Science. 1974 Sep 20;185(4156):1049–1053. doi: 10.1126/science.185.4156.1049. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Dolin R., Thornhill T. S., Kalica A. R., Chanock R. M. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972 Nov;10(5):1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsberg E. Small spherical viruses in faeces from gastroenteritis patients. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):351–354. doi: 10.1111/j.1699-0463.1977.tb01988.x. [DOI] [PubMed] [Google Scholar]
- Kurtz J. B., Lee T. W., Pickering D. Astrovirus associated gastroenteritis in a children's ward. J Clin Pathol. 1977 Oct;30(10):948–952. doi: 10.1136/jcp.30.10.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecce J. G., King M. W., Mock R. Reovirus-like agent associated with fatal diarrhea in neonatal pigs. Infect Immun. 1976 Sep;14(3):816–825. doi: 10.1128/iai.14.3.816-825.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeley C. R. Comparison of the features of astroviruses and caliciviruses seen in samples of feces by electron microscopy. J Infect Dis. 1979 May;139(5):519–523. doi: 10.1093/infdis/139.5.519. [DOI] [PubMed] [Google Scholar]
- Madeley C. R., Cosgrove B. P. Letter: 28 nm particles in faeces in infantile gastroenteritis. Lancet. 1975 Sep 6;2(7932):451–452. doi: 10.1016/s0140-6736(75)90858-2. [DOI] [PubMed] [Google Scholar]
- Madeley C. R., Cosgrove B. P. Letter: Caliciviruses in man. Lancet. 1976 Jan 24;1(7952):199–200. doi: 10.1016/s0140-6736(76)91309-x. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Inouye S., Kono R. Plaque assay of neonatal calf diarrhea virus and the neutralizing antibody in human sera. J Clin Microbiol. 1977 Jan;5(1):1–4. doi: 10.1128/jcm.5.1.1-4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E. L., Martin M. L., Hatch M. H., Gary G. W., Jr Biophysical properties of a non-cultivable 29-nm enteric virus. J Gen Virol. 1979 Sep;44(3):833–837. doi: 10.1099/0022-1317-44-3-833. [DOI] [PubMed] [Google Scholar]
- Paver W. K., Ashley C. R., Caul E. O., Clarke S. K. A small virus in human faeces. Lancet. 1973 Feb 3;1(7797):237–240. doi: 10.1016/s0140-6736(73)90072-x. [DOI] [PubMed] [Google Scholar]
- Povey R. C. Serological relationships among feline caliciviruses. Infect Immun. 1974 Dec;10(6):1307–1314. doi: 10.1128/iai.10.6.1307-1314.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L. J., Bohl E. H., Kohler E. M., Hughes J. H. Immune electron microscopy of transmissible gastroenteritis virus and rotavirus (reovirus-like agent) of swine. Am J Vet Res. 1977 Jan;38(1):13–20. [PubMed] [Google Scholar]
- Smith A. W., Prato C., Skilling D. E. Caliciviruses infecting monkeys and possibly man. Am J Vet Res. 1978 Feb;39(2):287–289. [PubMed] [Google Scholar]
- Smith A. W., Skilling D. E., Ritchie A. E. Immunoelectron microscopic comparisons of caliciviruses. Am J Vet Res. 1978 Sep;39(9):1531–1533. [PubMed] [Google Scholar]
- Studdert M. J. Caliciviruses. Brief review. Arch Virol. 1978;58(3):157–191. doi: 10.1007/BF01317600. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Virus-like particle, 35 to 40 nm, associated with an institutional outbreak of acute gastroenteritis in adults. J Clin Microbiol. 1979 Nov;10(5):730–736. doi: 10.1128/jcm.10.5.730-736.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C. Isolation of small viruses resembling astroviruses and caliciviruses from acute enteritis of calves. J Med Microbiol. 1978 Nov;11(4):441–452. doi: 10.1099/00222615-11-4-441. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C., Jones J. M., Flewett T. H., Davies H. A., Davis H. A., White G. B. Morphological and antigenic relationships between viruses (rotaviruses) from acute gastroenteritis of children, calves, piglets, mice, and foals. Infect Immun. 1976 Sep;14(3):804–810. doi: 10.1128/iai.14.3.804-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bridger J., Hall G. A., Jones J. M., Jackson G. The isolation of reovirus-like agents (rota-viruses) from acute gastroenteritis of piglets. J Med Microbiol. 1976 May;9(2):203–209. doi: 10.1099/00222615-9-2-203. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Barbour B., Wyatt R. G., Kalica A. R., Kapikian A. Z., Chanock R. M. Enzyme-linked immunosorbent assay for identification of rotaviruses from different animal species. Science. 1978 Jul 21;201(4352):259–262. doi: 10.1126/science.208150. [DOI] [PubMed] [Google Scholar]