Abstract
Background and Aims
The functional morphology of Salvia pratensis flowers was re-investigated, after new insights revealed that pollen dispensing is one of the main functions of the staminal lever. In particular, no detailed information was available regarding the process of pollen transfer and the forces arising between the pollen-bearing thecae and the pollinating bee's body. The assumption was made that these forces play a significant role in pollen dispensing.
Methods
The functional morphology of S. pratensis flowers and the interaction between flowers and bees (Apis mellifera) were studied by reconstructing stress and strains by using qualitative and semi-quantitative theoretical analysis. Flowers were manipulated to study the spatial arrangement of the filament and lever, and of the head and proboscis of the visiting bee inside the tube. Photographs and films of bee visits on flowers were used to analyse the interaction of pollinator and staminal lever.
Key Results
The spoon-shaped lower lever of S. pratensis has a small hole through which a bee introduces its proboscis into the corolla tube. Although mentioned for the first time by Kerner von Marilaun in 1891, presented here is the first drawing and the first photograph showing this interaction in detail. The analysis of the interaction of flower visitor and the lever mechanism revealed that the position of bees on different flowers is spatially very similar. Flower morphology constrains postures of legitimately nectar-probing bees within narrow bounds. A theoretical discussion on structural elements and force progression in the flower allows the principles of lightweight architecture in flower morphology to be recognized.
Conclusions
The staminal lever of S. pratensis is a pollen-dispensing device. It seems to influence the amount of pollen deposited on pollinators by determining the forces arising between the pollinator and the pollen. The relevant forces occur either during the first, dynamic phase or during the second, almost static phase of a flower visit.
Key words: Flower–pollinator interaction, bee, Apis mellifera, pollination, pollen uptake, see-saw mechanism, biomechanics, pollen dispensing
INTRODUCTION
The interaction of the staminal lever of Salvia pratensis and a flower-visiting bee has been described often in textbooks and articles [Sprengel, 1793; Hildebrand, 1865; Müller, 1873; Kerner von Marilaun, 1891; Knuth, 1898; Kirchner, 1911; Kugler, 1955; Werth, 1956; Faegri and Van der Pijl, 1979; Meeuse and Morris, 1984 (see Fig. 1); Huck, 1992; Kwak, 2002; Claßen-Bockhoff et al., 2003]. For example, Proctor and Yeo (1973, p. 208) describe a flower visit of a bee on S. pratensis as follows, ‘When a bee pushes its head into the flower, the connective plate is pushed upwards and backwards, and the fertile anther lobes swing downwards bringing their pollen into contact with the abdomen of the bee.’
Fig. 1.

Interaction of a bumblebee and a Salvia pratensis flower as depicted by Meeuse and Morris (1984). This figure shows the popular twentieth-century view on pollen uptake, transfer and pollinator–flower interaction; compare with the new findings in Figs 4 and 5.
The visit of a bee on a Salvia flower can be subdivided into three temporal phases: in the first phase the bee triggers the lever mechanism, and the lever moves. The second phase seems to be almost static, with the bee sucking nectar and the lever being triggered. During the third phase the bee leaves the flower and the lever swings back into position.
The law pertaining to a lever notes that, during equilibrium, the sum of all torques acting in the clockwise direction is equal to the sum of all torques acting in the anticlockwise direction (Young, 1989). Therefore, during the first phase of the flower visit the lever swings around its joint, as the force that the bee exerts on the lever is greater than the reset forces of the lever mechanism. In the third phase the reset force becomes greater than the forces exerted by the bee, and therefore the lever mechanism swings back to its original, untriggered position. The processes taking place during the second phase, however, have gone largely undescribed.
MATERIALS AND METHODS
Salvia pratensis L. flowers and flower visitors were investigated at the following locations: in wild native stands at Bötzingen (Kaiserstuhl), Freiburg (airport), Freiburg (Dreisam) and Oberrimsingen (Baden), and in cultivated plants originating from Burgenland (Austria) (leg. M. Reith) planted in the Botanical Garden, Freiburg.
Meadow sage (S. pratensis) is a well-known European perennial. Flowers are usually bright blue to purple, but sometimes white or rose-coloured morphs can be found. Meadow sage is a representative of the genus with a derived staminal lever. The lower lever arm is spoon-shaped and blocks the flower entrance (Figs 2 and 3) (group B following Walker and Sytsma, 2007; type V according to Himmelbaur and Stibal, 1934). The internal structure and morphology of the flowers were studied in a number of longitudinal sections and cross-sections.
Fig. 2.

Longitudinal section of a Salvia pratensis flower. Lever mechanism halved (second stamen removed). (A) Overview, with lever in its original, untriggered position. (B) Detail, with lever in triggered position. Abbreviations: c, calyx; f, filament; fl, fold in the lower lip; fu, fold in the upper lip; h, hole in the spoon-shaped lever; j, position of the joint (joint hidden behind the lever); ll, lateral lobe of the lower lip; ml, middle lobe of the lower lip; nd, nectar disc (nectar glands); o, ovary; s, staminodium: sla, spoon-shaped lower lever arm; sti, stigma; sty, style; t, theca; ul, upper lip; ula, upper lever arm; vl, vascular bundle of the fertile stamen; vu, vascular bundle of the staminodium.
Fig. 3.
Spoon-shaped lower lever arm of Salvia pratensis. The arrow indicates the hole in its distal spoon-shaped region.
Generally, insect visits at S. pratensis flowers can be divided into two functional groups: first, illegitimate flower visits, for example nectar robbing or pollen theft in which the lever mechanism is usually not triggered; and, second, legitimate flower visits in which the lever is triggered. A remarkable diversity of bees and a few other insect visitors can be expected at S. pratensis flowers (see compilations in Knuth, 1898; Reith et al., 2006). This study concentrates on legitimate flower visits of honeybees (Apis mellifera). Honeybees are abundant pollinators of S. pratensis (see Claßen-Bockhoff et al., 2004a; Reith et al., 2005).
To permit a closer investigation into the corolla tube during flower visits, eight flowers were manipulated prior to flower visits. Flower tubes were cut open laterally (on one side) with surgical scissors in a way that keeps the flowers and the staminal mechanism functional, but allows observation of the spatial arrangement of floral structures and the bee's proboscis. Field observations were backed by photographs and video recordings (MotionScope® PCI, model 1000S 9400-0010, 250 frames s−1; Redlake MASD Inc., San Diego, CA, USA) that allow a close observation and thorough analyses of transient insect–flower interactions.
Categorical data were analysed by a chi-squared test of goodness of fit of the observed distribution to a random one. Random data were generated by distributing equal parts of the sum of observations (n) on all categories.
RESULTS
Flower morphology
The staminal levers of S. pratensis are attached to the two filaments. These filaments, together with a prominent fold in the lower lip, form a ring-like structure, which at its upper end is crossed by the lever arms. This ring-structure narrows the entrance to the flower tube of S. pratensis. The basic position of the spoon-shaped lower lever arm, consisting of the two lower connective parts, is between the filaments and just behind the fold in the lower lip (Fig. 2A). The ring-shaped distal end of the lower lever arm (Fig. 3) is therefore hidden behind this constriction of the flower (Fig. 2).
The distal end of the filaments that bears the joint of the lever mechanism is embedded in another fold of the flower tissue. As can be seen in Fig. 2, the staminodia are located just behind the distal end of the filaments.
Interaction with honeybees
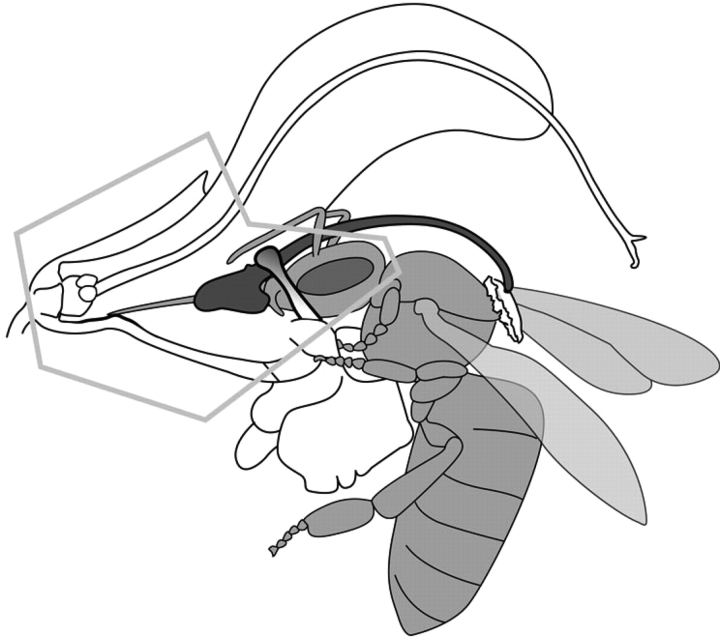
In S. pratensis honeybees land on the flower and use the lobes of the lower lip as a foothold (Table 1). While the bee is introducing its proboscis through the constriction into the flower tube, the lever starts to swing. The bee also introduces its mandibles into the flower tube. Most of the head does not pass the filaments, which narrow the flower tube, and the head remains clearly visible outside the flower tube (Table 2). The bee inserts its proboscis through the hole of the spoon-shaped part of the lever when introducing its proboscis into the flower. Although this last process was not visible in our photographs and video recordings, it can be inferred from the fact that in the subsequent phase of the flower visit, it is clearly visible that the proboscis was inserted through this hole (Figs 4 and 5).
Table 1.
Frequency of the position of the honeybee's legs, visiting different individual flowers of Salvia pratensis (n = 50)
| Position of legs | First leg | Middle leg | Hind leg | Random |
|---|---|---|---|---|
| Middle of the lower lip | 16 % | 16 % | – | 20 % |
| Lateral lobes of the lower lip | 84 % | 82 % | – | 20 % |
| Terminal lobe of the lower lip | – | 2 % | 90 % | 20 % |
| Calyx, stem or neighbouring flowers | – | – | 6 % | 20 % |
| Leg unattached | – | – | 4 % | 20 % |
| P (chi-squared test)* | 2·15×10−26 | 1·10×10−24 | 9·26×10−31 |
* Known distribution compared with random distribution (P < 0·01).
Table 2.
Frequency of the position of the honeybee's head, visiting different individual flowers of Salvia pratensis (n = 50)
| Position of a honeybee's head relative to the flower tube | Observed | Random |
|---|---|---|
| Head introduced into the flower tube: eyes covered by floral tissue (more than 25 %) | 0 % | 20 % |
| Head largely outside the flower tube: Eyes partly covered by floral tissue (less than 25 %) | 30 % | 20 % |
| Head outside the flower tube, eyes entirely visible, mouth parts covered | 28 % | 20 % |
| Head outside the flower tube, eyes entirely visible, mandibles partly visible | 42 % | 20 % |
| Head outside the flower tube, mandibles entirely visible: other mouthparts at least partly covered by floral tissue | 0 % | 20 % |
| P (chi-squared test)* | 1·64 × 10−6 |
* Known distribution compared with random distribution (P < 0·01).
Fig. 4.
Salvia pratensis flower, laterally cut open to show the interaction of a visiting bee's legs, head and proboscis (here honeybee) with a Salvia flower and the staminal lever mechanism (see Fig. 5 for a schematic drawing).
Fig. 5.
Schematic drawing of the interaction of a visiting bee (honeybee) with a Salvia pratensis flower and the staminal lever mechanism (see also Fig. 4).
During the next phase of the flower visit the bee often moves its head slightly deeper into the corolla tube. This results from the fact that it presses its head and proboscis towards the base of the flower where the nectaries and the nectar are located. A typical position of a bee on a flower is depicted in Figs 4 and 5. The angle between the abaxial side of the lower lever arm and the line that connects nectaries, proboscis and eyes of a bee, as measured on images of visits to manipulated flowers, is acute (10 ± 6°, n = 8; Table 3, Figs 4 and 6).
Table 3.
Average angle α between the lower lever arm and the line connecting nectaries–proboscis–eye of a honeybee visiting a manipulated (laterally cut open) flower (n = 8), and average force applied at 90° (from the abaxial direction) on the lever, in percentage of the total force applied (parallel to the line nectaries–proboscis–eye) by a visiting bee (n = 8)
Average angle  (n = 8) (n = 8) |
Average force applied at 90° on the lever  sin αi (n = 8) sin αi (n = 8) |
|---|---|
| 10±6° | 17±10 % |
Fig. 6.
Schematic approximation of the forces acting on the lever mechanism. By measuring the angle between the abaxial side of the spoon-shaped lever and the direction of the forces (black arrows) exerted by the bee, the force acting at 90° (i.e. from the abaxial direction) on the lever can be calculated (see Table 3).
DISCUSSION
Theoretical discussion of force progression in the S. pratensis flower
A nectar-gathering bee that visits a flower will move its head towards the nectar source in order to reach it and gather the nectar effectively. In S. pratensis the nectar glands are located at the base of the flower tube and the flower tube is constricted at its entrance. As the data presented here show, movement of the head of the honeybee stops when the head is partly introduced into the flower tube. This indicates that the construction of the flower inhibits its further advancement. Several structures that narrowed the pathway of the bee's head and proboscis to the nectar were observed, such as the narrow flower entrance and the spoon-shaped lever. These structures have to withstand the forces exerted by the bee.
During the first phase of a flower visit, the proboscis of a bee triggering the lever mechanism of an S. pratensis flower is guided by the lateral lobes of the ‘spoon’ and the fold at the base of the lower lip towards the hole in the spoon-shaped lower lever arm. Thus, the proboscis is inserted through this hole. In the second phase of a flower visit a bee that is pressing its head deeper into the flower will increasingly exert forces on the corolla, the filament and the lower arm of the lever mechanism. The flower and the lever mechanism have to withstand these forces without being destroyed. There are four main ways in which these forces can be transferred.
First, head and mandibles of the bee are pressed against the filaments that are narrowing the flower entrance. As a consequence of this interaction, the narrow flower entrance is widened, as the filaments at the flower entrance are forced apart.
Second, as a result, stress occurs between the filament and the lower lip (Fig. 7), whereby the flower is strained between the filaments and the lateral lobes of the lower lip (Fig. 8A).
Fig. 7.
Forces exerted by a bee on a Salvia pratensis flower. The black arrows indicate the direction of the forces exerted by the visiting bee in relation to the flower and the staminal lever.
Fig. 8.
Strain in a Salvia pratensis flower as a result of the forces exerted by a visiting bee pressing its head and mouth parts into the flower. Direction of strain is indicated by black arrows.
Third, the mouthparts of the bee are pressed into the spoon-shaped lower lever arm, resulting in a force acting on the spoon-shaped lower lever (Fig. 7). This force is transferred via the joint from the lever to the filament and to the lateral lobes of the lower lip. As a consequence, the flower is strained between the lower lever arm and the lateral lobes of the lower lip (Fig. 8B).
Fourth, all these strains combine in the filaments (Fig. 9), which are fixed at their base to the corolla tube. The filament is either directly pushed by the bee's head, or pulled at its top via the joint to the spoon-shaped lever, or pushed and pulled at the same time. The corolla tube together with its particular morphological structures might thus help to stabilize the filament. Possible stabilizing structures are the folds of the corolla tube in which the filaments are embedded, the staminodia which are located near the upper end of each filament, and the rigid corolla tissue surrounding the staminode (see Fig. 2).
Fig. 9.
Schematic drawing showing the direction of forces acting on a filament of Salvia pratensis. The filament is fixed on the corolla (not shown) at its base. Bees pull at the upper part of the filament (via the spoon-shaped lever, which is attached by a joint to the filament) and push directly against the upright filament.
Rediscovery of the function of the spoon-shaped lever
Kerner von Marilaun (1891, p. 263) is, to our knowledge, the only author who has mentioned the hole in the spoon-shaped lever of S. pratensis as being used by bees through which to insert the proboscis: ‘Durch dieses Loch fahren die angeflogenen Insekten mit dem Rüssel ein und drücken dabei die Fallthür nach rückwärts und zugleich in die Höhe’ (‘landed insects thread the proboscis through this hole, thereby pushing the trap-door backwards and at the same time upwards’). The first detailed drawings and photographs documenting this interaction are presented here (Figs 4 and 5). Knowledge regarding this close flower–pollinator interaction seems to have been lost in the last century (compare Fig. 1), which is astonishing given that the morphology of S. pratensis is well known, and has often been cited as an example of adaptation of flowers to pollinators, and has also often been studied in the classroom.
The same kind of interaction was also documented in S. amplexicaulis Lam., S. sclarea L., S. transsylvanica (Schur ex Griseb.) Schur, S. verbenaca L., S. virgata Jacq. and their pollinators in the Freiburg Botanical Garden (M. Reith, unpubl. data). (Claßen-Bockhoff et al. 2004b), citing Hildebrand (1865), postulate a similar interaction between the spoon-shaped lever of S. argentea and its pollinators. All these sages share the special spoon-shaped lever that seems to be typical for a monophyletic group within the genus Salvia (group B after Walker and Sytsma, 2007). We hypothesize that the holes in the levers of Salvia species of this group are generally used by pollinators through which to insert the proboscis (Kerner von Marilaun, 1891).
In detail, the particular lever construction in this group of sages defines two constrictions in the flower through which legitimate visitors insert the proboscis. Therefore, the minimal functional proboscis length of legitimate visitors of this Salvia group is expected to be determined by the distance between the nectar droplet near the nectar disc (Fig. 2B: nd) and either the very narrow flower entrance (Fig. 2B: f; for example in S. sclarea) or the triggered spoon-shaped lever (Fig. 2B: sla), when the flower entrance is somewhat wider (for example in S. pratensis). The spoon-shaped lever therefore defines a functional flower tube length, which plays an important role in specialization of Salvia species on pollinators (Reith et al., 2006).
The staminal lever: a pollen-dispensing device
Plants can distribute pollen among pollinators by either packaging or dispensing mechanisms (Harder and Thomson, 1989). Packaging means the total amount of pollen is divided in separate units (packages) that sequentially become available to pollinators. The total pollen production of a Salvia plant is packaged into several flowers. But the pollen of a single Salvia flower is not further packaged. Repeated triggering of the staminal lever in a Salvia flower has been observed by numerous scientists since Sprengel (1793). According to Claßen-Bockhoff et al. (2004a), the pollen of an S. pratensis flower is distributed by the lever mechanism during 12–17 consecutive visits. Therefore, the staminal lever in Salvia meets the definition of a pollen-dispensing mechanism (sensu Lloyed in Harder and Thomson, 1989).
Most pollen-dispensing devices limit the access to pollen and release pollen over a protracted period, as in the pollen squeezers in Asteraceae and Fabaceae (Yeo, 1993), the gradual opening anthers in Erythronium (Harder and Thomson, 1989), or the poricidal anthers in Ericaceae (Buchmann and Hurley, 1978). Others are known to influence the forces involved in pollen uptake, for example the forces necessary for detachment of the pollen from the flower, or the forces necessary for attachment of pollen to pollinators. In Wahlenbergia (Campanulaceae) the pollen of a flower is freely accessible but firmly attached to the site of pollen presentation by means of hairs (Yeo, 1993). These hairs gradually shrink, and therefore the forces required to detach pollen are diminished over time (Lloyed and Yates, 1982). This means that pollen dispensing in Wahlenbergia is achieved by modifying the forces necessary for pollen detachment. Curiously, until now only a small number of dispensing mechanisms are known that influence or regulate the forces that a pollinator exerts on the pollen (but see, for example, the adhesives that are smeared on the pollinators and help to attach pollen to them in some Polygalaceae and Apocynaceae; Yeo, 1993). Nonetheless, the forces acting between pollinator and pollen might play an important role in pollen uptake.
The data presented here show that the position of a bee's head and legs is determined by the floral morphology of S. pratensis flowers (cf. Tables 1 and 2). During the second phase of a flower visit the lever is fixed at its lower arm by the position of the proboscis sticking through it. The lower lever arm can therefore be regarded as fixed in relation to the position of the bee's head. As the lever is additionally attached on its two joints, the whole floral mechanism is fixed on at least three different sites to the flower–pollinator system. Furthermore, the position of the bee's legs is always very similar. Therefore, the posture a bee adopts on a flower is not random but constrained by floral morphology.
If the posture of a bee can be assumed as being highly constrained or even fixed in relation to the flower (Fig. 10), then the forces that act between the thecae and the bee's body largely are determined by flower morphology. Namely, if the curved upper lever arm is flexible and deforms during a flower visit due to the forces acting on it, then this deformation is constant in different visits to the same flower (if it is assumed that bees are of comparable size). This means that the forces between the thecae and the bee's body depend largely on the length, curvature and mechanical properties of the upper lever arm. This becomes obvious in a thought experiment in which an S. pratensis flower with a hypothetical straight lever is visited by a bee (Fig. 11). As the thecae of the hypothetical straight lever do not touch the bee, the forces between the thecae and the bee's body are zero. When the curvature of the upper lever arm is increased, the forces remain zero until the lever is so strongly curved that it touches the bee's body. The forces between the thecae and the bee's body then increase with increasing curvature of the lever and increasing lever arm stiffness. These results raise the possibility that the lever mechanism in Salvia achieves pollen dispensing by regulating the forces that a pollinator exerts on the pollen-dispensing thecae during the second almost static phase of the visit on a flower.
Fig. 10.
Honeybee visiting a flower of Salvia pratensis. The position of the bee's head can be regarded as fixed in relation to the filaments, lower lever arm and corolla tube.
Fig. 11.
Honeybee visiting a hypothetical Salvia pratensis flower containing a straight upper lever arm. As the lever arm is straight, the thecae do not touch the bee. In real flowers the forces between the thecae and the bee might depend largely on the morphology and elasticity of the upper lever arm.
On the other hand, the lever mechanism could also be seen as a reversible explosion mechanism, shedding the pollen on the pollinator during the dynamic first phase of its visit, similar to clapping a chalk-eraser against a blackboard. However, explosive mechanisms in flowers usually do not function as dispensing devices. Most explosive flowers release their pollen at once (Yeo, 1993; Aluri and Reddy, 1995). Only a few explosive flowers are known to distribute pollen on several pollinators. This is, for example, the case in Kalmia, where each flower has several stamens that can be released separately (Harder and Wilson, 1994). Therefore, it seems unclear how a reversible explosion mechanism could retain pollen during the first triggering and dispense it on consecutive visitors. It is possible that in Salvia the forces and energies during the first ‘clapping’ might be smaller than in other explosive mechanisms and that therefore at least some of the pollen remains in the thecae. The terminal velocity of the thecae in Salvia clearly depends on the relative length of the lever arms (if it is assumed that every bee inserts its proboscis at the same speed and pushes the lever at the same point). This raises the possibility that the lever mechanism in Salvia may achieve pollen dispensing by regulating the forces that occur between pollen-dispensing thecae and pollinator during the first dynamic phase of the visit on a flower.
Until now it has been unclear when the forces important in pollen uptake by pollinators of S. pratensis arise, i.e. whether in the first, dynamic phase of a visit or in the second, static phase. A comparative measurement of the forces during the dynamic and the static phase of an insect's visit on a flower is technically very difficult. Furthermore, a manipulation of the flowers will influence the interaction of lever mechanism and pollinator during both phases. Therefore, it appears to be necessary to model these two scenarios, in order to analyse in which phase – the dynamic, the static, or in both phases – forces arise that are sufficient to detach pollen from the thecae and attach it to pollinators. These models will enable us to understand staminal levers in Salvia and other plant genera and explosion mechanisms in more detail and are the subject of our forthcoming work.
CONCLUSIONS
A spoon-shaped lever enables a very tight fit between flower and its pollinator. It might therefore improve, in an evolutionary sense, the control of the plant on the posture of a flower-visiting insect. Postures of pollinators are often constrained by floral morphology (e.g. Wester and Claßen-Bockhoff, 2006) and are also known to influence pollen dispensing (Hurlbert et al., 1996; Temeles and Rankin, 2000; Fetscher et al., 2002). The time a flower-visiting animal spends at a flower or touches the thecae is often used to account for differences in pollen uptake (e.g. Hurlbert et al., 1996; Temeles and Rankin, 2000; Fetscher et al., 2002). We consider that the speed of rubbing of the hairy or feathery body and the (frictional) forces that arise between the flower visitor's body and the thecae are two other very important – but owing to restricted technical equipment, largely uninvestigated – factors influencing pollen uptake by flower visitors (Harder and Barrett, 1993; Hurlbert et al., 1996). The levers in S. pratensis flowers are an example of a pollen-dispensing device that controls pollen deposition on pollinators by determining the forces arising between the pollinator and the pollen-dispensing thecae, either during the first, dynamic phase or the second, almost static phase of a flower visit.
The pollen-dispensing schedule of plants strongly influences male reproductive success (Harder and Wilson, 1994). Therefore, the biomechanics and functional morphology of the staminal lever and flowers in Salvia might be subject to significant sexual selection. The triple role of the staminal lever, being subject to sexual selection and at the same time achieving mechanical (Grant and Grant, 1964; Ramamoorthy and Elliot, 1993; but see Wester and Claßen-Bockhoff, 2006) and ethological isolation (at least in the spoon-shaped lever group; but see Reith et al., 2006, for a general view), might explain the immense functional and morphological diversity of lever mechanisms in the genus Salvia (Himmelbaur and Stibal, 1934; Claßen-Bockhoff et al., 2003, 2004a).
ACKNOWLEDGEMENTS
We thank C. Bräuchler, H. Meimberg, J. B. Walker and other participants of the Lamiaceae section in Vienna for helpful and interesting discussions, Claudia Gack for support with photographic equipment and REM, the team of the Freiburg Botanical Garden and Walter Wolf for providing plant material. Additional thanks go to Randy Cassada for improving the English, Linda Klöckner (Mainz) for work on the illustrations, and two anonymous reviewers for their helpful comments. The Deutsche Forschungsgemeinschaft (SPP 1127) is gratefully acknowledged for financial support (Cl 81/9-1, Sp 534 5-1 & 5-2).
LITERATURE CITED
- Aluri RJS, Reddi CS. Explosive pollen release and pollination in flowering plants. Proceedings of the Indian National Science Academy Part B Biological Sciences. 1995;61:323–332. [Google Scholar]
- Buchmann SL, Hurley JP. A biophysical model for buzz pollination in angiosperms. Journal of Theoretical Biology. 1978;72:639–657. doi: 10.1016/0022-5193(78)90277-1. [DOI] [PubMed] [Google Scholar]
- Claßen-Bockhoff R, Wester P, Tweraser E. The staminal lever mechanism in Salvia L. (Lamiaceae): a review. Plant Biology (Stuttgart) 2003;5:33–41. [Google Scholar]
- Claßen-Bockhoff R, Speck T, Tweraser E, Wester P, Thimm S, Reith M. The staminal lever mechanism in Salvia L. (Lamiaceae): a key innovation for adaptive radiation? Organisms, Diversity and Evolution. 2004a;4:189–205. [Google Scholar]
- Claßen-Bockhoff R, Crone M, Baikova E. Stamen development in Salvia L.: homology reinvestigated. International Journal of Plant Science. 2004b;165:475–498. [Google Scholar]
- Faegri K, Van der Pijl L. The principles of pollination ecology. Oxford: Pergamon Press; 1979. [Google Scholar]
- Fetscher EA, Rupert SM, Kohn JR. Hummingbird foraging position is altered by the touch-sensitive stigma of bush monkeyflower. Oecologia. 2002;133:551–558. doi: 10.1007/s00442-002-1079-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Grant V. Mechanical isolation of Salvia apiana and Salvia mellifera. Evolution. 1964;18:196–212. [Google Scholar]
- Harder LD, Barrett SCH. Pollen removal from tristylous Pontederia cordata: effects of anther position and pollinator specialisation. Ecology. 1993;74:1059–1072. [Google Scholar]
- Harder LD, Thomson JD. Evolutionary options for maximizing pollen dispersal of animal-pollinated plants. American Naturalist. 1989;133:323–344. [Google Scholar]
- Harder LD, Wilson WG. Floral evolution and male reproductive success: optimal dispensing schedules for pollen dispersal by animal-pollinated plants. Evolutionary Ecology. 1994;8:542–559. [Google Scholar]
- Hildebrand F. Ueber die Befruchtung der Salviaarten mit Hilfe von Insekten. Jahrbücher für wissenschaftliche Botanik. 1865;4:451–476. [Google Scholar]
- Himmelbaur W, Stibal E. Entwicklungsrichtungen in der Blütenregion der Gattung Salvia L. II. Biologia generalis. 1934;9:129–150. [Google Scholar]
- Huck RB. Overview of pollination biology in the Lamiaceae. In: Harley RM, Reynolds T, editors. Advances in Labiatae science. Kew: Royal Botanic Garden; 1992. pp. 167–181. [Google Scholar]
- Hurlbert AH, Hosoi SA, Temeles EJ, Ewald PW. Mobility of Impatiens capensis flowers: effect on pollen deposition and hummingbird foraging. Oecologia. 1996;105:243–246. doi: 10.1007/BF00328553. [DOI] [PubMed] [Google Scholar]
- Kerner von Marilaun A. Pflanzenleben. Bd. 2. Leipzig and Vienna: bibliographisches Institut; 1891. [Google Scholar]
- Kirchner Ov. Blumen und Insekten. Leipzig: B.G.Teubner; 1911. [Google Scholar]
- Knuth P. Handbuch der Bluetenbiologie. Leipzig: Engelmann; 1898. [Google Scholar]
- Kugler H. Einführung in die Blütenökologie. Stuttgart: Fischer; 1955. [Google Scholar]
- Kwak MM. Entomologische Berichten. Vol. 62. Amsterdam: 2002. Bumblebees as flower visitors: pollinators and profiteers; pp. 73–81. [Google Scholar]
- Lloyed DG, Yates JMA. Intrasexual selection and the segregation of pollen and stigmas in hermaphrodite plants, exemplified by Wahlenbergia albomarginata (Campanulaceae) Evolution. 1982;36:903–913. doi: 10.1111/j.1558-5646.1982.tb05462.x. [DOI] [PubMed] [Google Scholar]
- Meeuse B, Morris S. The sex life of flowers. Cologne: DuMont; 1984. [Google Scholar]
- Müller H. Befruchtung der Blumen durch Insekten und die gegenseitigen Anpassungen beider. Leipzig: W. Engelmann; 1873. [Google Scholar]
- Proctor M, Yeo P. The pollination of flowers. London: Collins; 1973. [Google Scholar]
- Ramamoorthy TP, Elliot M. Mexican Labiatae: diversity, distribution, endemism and evolution. In: Ramamoorthy TP, Bye R, Lot A, Fa J, editors. Biological diversity of Mexico: origins and distribution. New York: Oxford University Press; 1993. pp. 513–539. [Google Scholar]
- Reith M, Baumann G, Claßen-Bockhoff R, Speck T. Sharing without mixing? Quantitative analyses of pollen placement on Apis mellifera as a pollinator of Salvia pratensis and Salvia nemorosa. XVII International Botanical Congress – Abstracts; 2005. IBC Organizing Committee. [Google Scholar]
- Reith M, Claßen-Bockhoff R, Speck T. Biomechanics in Salvia flowers, the role of lever and flower tube in specialization on pollinators. In: Herrel A, Speck T, Rowe N, editors. Ecology and biomechanics: a mechanical approach to the ecology of animals and plants. Boca Raton: CRC Press; 2006. pp. 123–146. (FL). [Google Scholar]
- Sprengel CK. Das ent deckte Geheimnis der Natur im Bau und in der Befruchtung der Blumen. Berlin: Vieweg; 1793. [Google Scholar]
- Temeles EJ, Rankin AG. Effect of the lower lip of Monarda didyma on pollen removal by hummingbirds. Canadian Journal of Botany. 2000;78:1164–1168. [Google Scholar]
- Walker J, Sytsma K. Staminal evolution in the genus Salvia (Lamiaceae): molecular phylogenetic evidence for multiple origins of the staminal lever. Annals of Botany. 2007;100:375–391. doi: 10.1093/aob/mcl176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth E. Zur Kenntnis des Androeceums der Gattung Salvia und seiner stammesgeschichtlichen Wandlung. Berichte der deutschen botanischen Gesellschaft. 1956;69:381–386. [Google Scholar]
- Wester P, Claßen-Bockhoff R. Hummingbird pollination in Salvia haenkei Benth. (Lamiaceae) lacking the “typical” lever mechanism. Plant Systematics and Evolution. 2006;257:133–146. [Google Scholar]
- Young WC. Roark's formulas for stress & strain. 6th edn. New York: McGraw-Hill; 1989. [Google Scholar]
- Yeo PF. Secondary pollen presentation. Form, function and evolution. New York: Springer; 1993. [Google Scholar]