Abstract
Background and Aims
The European Chaerophyllum temulum and two North American Chaerophyllum species have a trans-Atlantic disjunct distribution. This work aimed to resolve requirements for dormancy break and germination of C. temulum seeds and to compare dormancy traits with those of the two North American congeners.
Methods
Phenology of germination and embryo growth was studied by regularly exhuming seeds sown in natural conditions. Temperature requirements for embryo growth, breaking of dormancy and germination were determined by incubating seeds under controlled laboratory conditions. Additionally the effect of GA3 on germination was tested to determine the specific dormancy type.
Key Results
In natural conditions, embryo growth starts in early winter. Seedlings emerge in late winter shortly after the embryos reached the critical ratio for embryo length to seed length (E : S) of approx. 0·95. Growth of the embryo only occurs during a prolonged incubation period at 5 °C. After stratification at 5 °C, which breaks physiological and morphological dormancy, seeds can germinate at a wide range of temperatures. GA3 did not substitute for cold stratification in seeds placed at 23 °C.
Conclusions
Chaerophyllum temulum has deep complex morphophysiological dormancy. This dormancy type differs considerably from that of the two North American congeners.
Key words: Chaerophyllum temulum, Apiaceae, trans-Atlantic, disjunct taxa, seed dormancy, gibberellic acid, morphophysiological dormancy, E : S ratio
INTRODUCTION
Representatives of many plant families typically have seeds containing an underdeveloped embryo at the time of dispersal (Martin, 1946). Nikolaeva (1977) defined these seeds as morphologically dormant (MD) in her classification of dormancy types, because the embryo must elongate to a critical length before the radicle emerges. Seeds are termed morphophysiologically dormant (MPD) if an additional physiological mechanism, inhibiting germination, has to be overcome. Eight types of MPD are currently being discerned, based on the ability of gibberellic acid (GA) to break dormancy, and on the temperature requirements for dormancy break and embryo growth (Baskin and Baskin, 1998).
Stasis and evolution of morphological traits have been extensively studied in plant species with an eastern North American–eastern Asian disjunct distribution (Wen, 1999; Milne and Abbott, 2002). Very often these disjunct plant taxa show a surprising similarity in morphology. In some disjunct species pairs, this morphological similarity has been attributed to morphological stasis (e.g. Hoey and Parks, 1991). Besides morphological resemblance, disjunct species pairs can also show stasis in ecophysiological traits such as dormancy and germination characteristics. Similar dormancy types have been found in eastern North American–eastern Asian disjunct species pairs of Jeffersonia, Panax (Baskin and Baskin, 1998) and Aristolochia (Adams et al., 2005). In Osmorhiza and Erythronium, however, eastern North American–eastern Asian disjunct species show major differences in dormancy characteristics. The Asian representatives of these genera require only a cold stratification for dormancy break, while the eastern North American species require an additional period at high temperatures before cold stratification is effective in breaking dormancy (Baskin et al., 1995; Walck et al., 2002; Wen et al., 2002). A similar pattern was found in three intercontinental disjunct Sambucus species. The Eurasian Sambucus racemosa requires only sufficient cold stratification to break dormancy, while the North American S. canadensis and S. pubens also require an additional period at high temperatures prior to cold stratification to break dormancy (Hidayati et al., 2000).
We are not aware of any studies comparing dormancy in North American–European disjunct taxa. Here we study dormancy in a representative of Chaerophyllum (Apiaceae), the largest genus in the subtribe Scandicinae, comprising > 30 species (Downie et al., 2000). Based on a DIVA analysis, Chung et al. (2005) considered Eurasia as the ancestral area of the Scandicinae. Except for C. procumbens and C. tainturieri, all Chaerophyllum species are native to Eurasia and North Africa. Spalik and Downie (2001) suggested four sections in Chaerophyllum based on ITS (internal transcribed spacer) and morphological data. In their classification, Chaerophyllum sect. Chaerophyllum encompasses C. temulum and two North American species C. procumbens and C. tainturieri. More recently the trans-Pacific disjunct alpine genus Oreomyrrhis was also nested within sect. Chaerophyllum based on ITS data (Chung et al., 2005). In this classification Oreomyrrhis is most closely related to C. procumbens and C. tainturieri. Spalik and Downie (2001) suggested that the North American Chaerophyllum species originated from a European ancestor by an incidental dispersal event, because no representatives of the clade are known to occur in Asia. This dispersal hypothesis is one of three possible migration scenarios obtained by a DIVA analysis which included Oreomyrrhis. Molecular dating methods indicate that divergence between C. temulum and the other species of sect. Chaerophyllum occurred during the late Miocene to early Pliocene (Chung et al., 2005).
The present study focuses on the determination of the temperature requirements for embryo growth, dormancy break and germination of C. temulum seeds. All test conditions were based on the phenology of embryo growth and seedling emergence in natural conditions. The effects of GA on germination were also investigated to define the dormancy type. The results are then compared with the germination characteristics of the two North American congeners of C. temulum, and with other related species. The aim was to verify whether stasis of dormancy traits has occurred, as is the case in some eastern Asian–eastern North American disjunct taxa.
MATERIALS AND METHODS
Chaerophyllum temulum L. is a biennial or monocarpic perennial herb mainly growing in semi-shaded places such as open forests, forest edges, hedgerows and river banks (Hegi, 1975; Grime et al., 1988). It is distributed throughout Europe, the Caucasus and western North Africa (Hegi, 1975). Ripe seeds (mericarps) for this study were collected in August 2005 and 2006 in a moist open woodland near Diest, Belgium (50°48′N, 5°3′E). Unless otherwise mentioned, experiments started within 1 week after harvest. Seeds not used immediately in the experiments were stored dry at room temperature (about 20 °C). Preliminary experiments indicated that dry storage for several months did not affect seed viability or dormancy status.
Phenology of seedling emergence and embryo growth
Within 1 week after harvest in August 2005, three replicates of 50 seeds were sown in an experimental garden near Leuven, Belgium. Seeds were sown both at the soil surface and at a depth of 1 cm in plastic pots filled with potting soil, and buried at ground level. Emerged seedlings were counted and removed weekly during 1 year. A molluscicide was applied regularly to prevent seedling damage, and the pots were covered with a net to prevent disturbance by birds. Minimum and maximum soil temperatures at a depth of 1 cm were recorded daily and used to calculate mean weekly minimum and maximum temperatures.
Embryo growth phenology was monitored in seeds buried under natural conditions in August 2005. Twenty nylon bags were filled with a mixture of 30 seeds and 10 g of fine white sand, and buried to a depth of 5 cm in the experimental garden. Every 2 weeks a bag was exhumed and 20 seeds were selected randomly for embryo measurement. Seeds were cut open and embryo length and seed length were measured using a dissecting microscope with an ocular micrometer. The ratio of embryo length to seed length (E : S ratio) was calculated to correct for a positive correlation between seed length and embryo length. To determine the E:S ratio of seeds at time of germination, i.e. critical embryo length, the average E : S ratio of 20 seeds with split seed coats but no radicle protrusion (critical E : S ratio) was determined. In the study on embryo growth phenology, if a seed had germinated when it was removed from the bag, the critical E : S ratio was recorded.
Temperature requirements for embryo growth
All experiments in controlled laboratory conditions were performed with seeds collected in August 2006. The increase in embryo length during moist storage at three different temperature conditions was determined. Two-hundred and fifty seeds were stored continuously at 5 and 23 °C for 20 weeks, and 150 seeds were stored at 23 °C for 12 weeks and then transferred to 5 °C for another 12 weeks. Every 2 weeks 20 seeds were selected randomly from each temperature regime and the E : S ratio was determined as described above.
Temperature and light requirements for germination
In every test condition three replicates of 50 seeds were placed in Petri dishes on filter paper (Schleicher & Schuell No. 2282) moistened with distilled water. All germination experiments were performed in temperature-controlled incubators. For germination tests, seeds were placed in incubators at constant temperatures of 5, 10 and 23 °C and at daily 12/12 h alternating temperature regimes of 20/10 and 15/6 °C. Seeds were incubated either in complete darkness, in a wooden box, or in light (= 12 h daily photoperiod, PAR = 36 µmol m−2 s−1) provided by cool white fluorescent tubes (Philips TLD 80) during the 12 h high-temperature portion of the cycle. During storage in dark conditions, the seeds were never exposed to light until the experiment was terminated. The seeds were, however, moistened weekly with distilled water under a green safe lamp.
In a first experiment, fresh seeds were incubated in light at 5, 10, 23, 15/6 and 20/10 °C for 24 weeks, and at 2-week intervals germinated seeds were counted and discarded. Seeds dispersed in summer are exposed to a period of relatively high temperatures during late summer and autumn. Therefore, the effect of a high temperature pre-treatment on subsequent germination was tested. Seeds were placed on a moist filter paper in complete darkness at 23 °C for 0, 4, 8 and 12 weeks. During this period of warm stratification, the filter paper and seeds were regularly moistened under a green safe lamp. After this treatment, seeds were transferred to light at 5, 10, 15/6 and 20/10 °C for 16 weeks, and at 1-week intervals germinated seeds were counted and discarded. After 16 weeks the total percentage of germinated seeds was calculated.
At the beginning of spring, mean daily temperature rises and daily temperature fluctuations increase. To test the effect of these changing temperatures on germination of non-dormant seeds, seeds were stratified in darkness at 5 °C (to simulate winter conditions) for 0, 4, 8 and 12 weeks. After each stratification period, seeds were transferred to light and to darkness at several high temperatures regimes: 10, 23, 20/10 and 15/6 °C. Seeds were incubated for 2 weeks, after which the percentage of germinated seeds was determined for each test condition. The effect of pre-treatment and of incubation temperature on the final germination percentage was tested non-parametrically using the Scheirer–Ray–Hare extension of the Kruskal–Wallis test (Sokal and Rohlf, 1997).
Temperature range for germination
The range of constant temperatures at which seeds can germinate after cold stratification was determined in seeds that had been dry stored for 1 month. After 8 weeks of cold stratification in darkness at 5 °C, three replicates of 50 seeds were moved to water baths at different constant temperatures, ranging from 5 to 30 °C with an interval of 2·5 °C. Seeds were placed on a moist filter in Petri dishes that floated on the water surface. The water baths were placed in a growth chamber near a window providing seeds with natural light conditions (PAR between 2·6 and 6·5 µmol s−1 m−2). No additional light source was used and seeds were protected from direct sunlight. Seeds were incubated for 14 d, and germinated seeds were counted and discarded each day. For each species, the percentage of germinated seeds was determined, as was the time required to reach 50 % germination of the total amount of germinated seeds (T50).
Effect of GA3 on dormancy break
In some species, GA can substitute for a cold or warm stratification to break dormancy (Baskin and Baskin, 1998). To determine if GA3 can overcome dormancy in C. temulum, seeds were incubated in Petri dishes on filter paper moistened with GA3 solutions at concentrations of 10, 100 and 1000 mg L−1 or with distilled water (control) in light at 23, 10, 15/6 and 20/10 °C. At the beginning of the experiment, the seeds were moistened with 10 mL of a solution with the appropriate GA3 concentration or with distilled water. During the following weeks, filter papers were kept moist with distilled water. The experiment lasted for 12 weeks and germinated seeds were counted and discarded every 2 weeks. The effects of GA3 and incubation temperature on final germination percentage were analysed non-parametrically using the Scheirer–Ray–Hare extension of the Kruskal–Wallis test (Sokal and Rohlf, 1997).
RESULTS
Phenology of seedling emergence and embryo growth
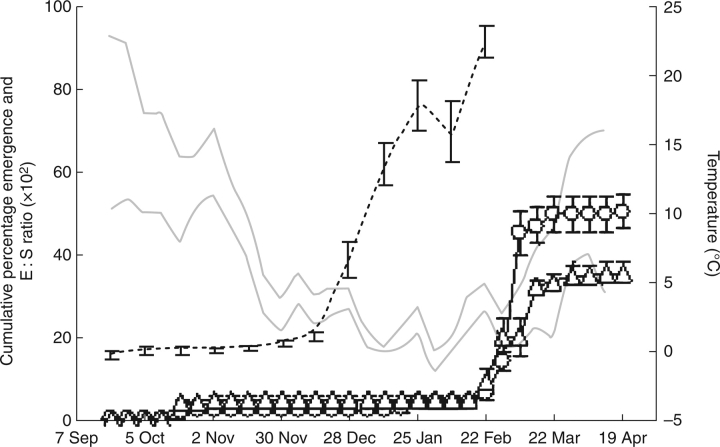
The average E:S ratio of freshly matured seeds was 0·16 ± 0·01 (mean ± s.e.). Significant growth of the embryo, in seeds buried in August 2005, started between 14 and 28 December 2005, when mean average maximum and minimum temperatures were between 0 and 5 °C (Fig. 1). Growth of the embryos continued until 25 January 2006, and by this time the embryo in most seeds had reached the critical E:S ratio (0·95 ± 0·01) required for germination. The final nylon bag for embryo measurements was dug up on 22 February 2006, when the mean maximum temperature was 4·9 °C. In this bag about 95 % of the seeds had already germinated.
Fig. 1.
Cumulative percentage of seedling emergence and phenology of embryo growth of C. temulum in natural conditions. Grey lines are mean weekly maximum and minimum temperature at 1 cm depth. Dotted line, embryo growth (n = 20); open circles, germination of seeds at the soil surface (n = 3); open triangles, germination of seeds buried at 1 cm depth (n = 3). Vertical bars represent the s.e. for embryo growth and the s.d. for seedling emergence.
About 5 % of the seeds sown at a depth of 1 cm and 2 % of those sown at the soil surface germinated in autumn, and the first seedlings were recorded on 19 October 2005. Mean maximum and minimum temperatures at the time of first emergence in autumn were 14·0 and 7·8 °C, respectively. Most seedlings, however, started emerging at the end of winter, from 22 February 2006 onwards, when mean daily maximum and minimum temperatures were 4·9 and 2·9 °C, respectively. Peak emergence of seedlings occurred when temperatures started rising in early March. By the end of March 2006, 49·3 % of the seeds sown at the soil surface and 35·3 % of those buried at 1 cm depth had produced seedlings. No additional seedlings emerged after this date.
Temperature requirements for embryo growth
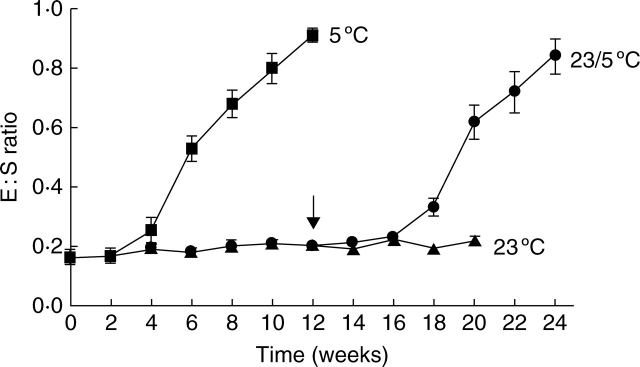
No increase in embryo length occurred in seeds continuously incubated on a moist substrate at 23 °C for 20 weeks (Fig. 2). In seeds placed immediately at 5 °C, the embryo started to grow in the second week, with the peak occurring between 4 and 6 weeks. After 12 weeks at 5 °C, the embryo had reached the critical length required for germination in all seeds. The small standard error indicates that growth of the embryo occurs at about the same rate in all seeds. In seeds given a 12 week pre-treatment at 23 °C before being transferred to 5 °C, the embryo only started to grow after transfer. The growth of the embryo after transfer from 23 °C occurred at approximately the same rate as that of the seeds placed immediately at 5 °C.
Fig. 2.
Embryo growth of C. temulum seeds at 5 °C, 23 °C and 12 weeks at 23 °C followed by 12 weeks at 5 °C. The arrow indicates transfer to 5 °C of the seeds in the latter condition. Vertical bars represent the s.e., n = 20.
Temperature and light requirements for germination
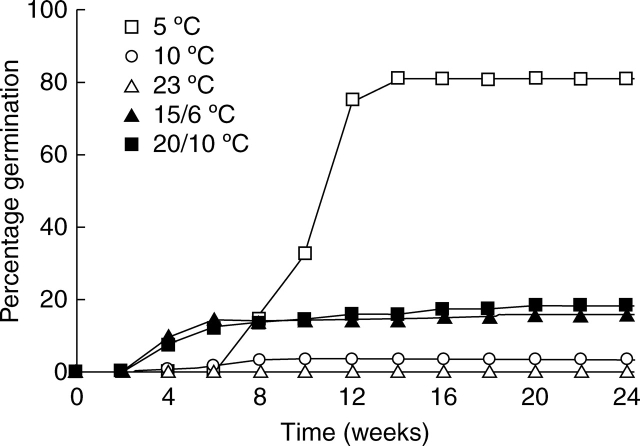
Fresh seeds germinated to 80 % after 24 weeks of incubation at 5 °C, with a peak of germination between the 10th and 12th week (Fig. 3). Germination of seeds incubated at 10 and 23 °C for 24 weeks was negligible. When seeds where incubated at 15/6 and 20/10 °C, about 15 % of the seeds had germinated after only 6 weeks, after which very little additional germination was recorded.
Fig. 3.
Cumulative percentage germination of freshly collected seeds of C. temulum during 24 weeks at constant temperatures of 5 °C, 10 °C and 23 °C, and daily fluctuating temperatures of 15/6 °C and 20/10 °C. n = 3.
Pre-treatment of seeds at 23 °C for 4, 8 or 12 weeks had no significant effect (P > 0·05) on the final percentage of seeds that germinated during 16 weeks of incubation at 5, 10, 15/6 and 20/10 °C (Table 1). The germination rate of seeds incubated at 5 °C was also similar for varying lengths of pre-treatment at 23 °C (results not shown). There was, however, a significant effect of incubation temperature (P < 0·001). Up to 80 % germination was recorded in seeds incubated at 5 °C, while < 20 % of the seeds germinated when incubated at 10, 15/6 or 20/10 °C. No germination at any test temperature was recorded within 2 weeks of incubation after transfer from 23 °C, while up to 7 % of the seeds had germinated within 4 weeks of incubation. A slight increase in final germination percentage with increasing duration of the pre-treatment at 23 °C was recorded in seeds incubated at 10 °C. The opposite was observed for seeds incubated at 15/6 and 20/10 °C, resulting in a significant interaction effect (P < 0·01).
Table 1.
Effects of a warm stratification (23 °C) of up to 12 weeks on the final germination percentage of C. temulum seeds incubated at different temperatures in light during 16 weeks
| Incubation temperature |
||||
|---|---|---|---|---|
| Weeks at 23 °C | 5 °C | 10 °C | 15/6 °C | 20/10 °C |
| 0 | 80·7 ± 2·4 | 3·3 ± 1·3 | 15·3 ± 3·3 | 17·3 ± 2·9 |
| 4 | 80·0 ± 2·3 | 9·3 ± 3·7 | 2·0 ± 1·2 | 10·0 ± 2·0 |
| 8 | 68·0 ± 2·0 | 13·3 ± 2·4 | 1·3 ± 0·7 | 7·3 ± 2·9 |
| 12 | 76·0 ± 3·1 | 17·3 ± 0·7 | 4·0 ± 4·0 | 10·7 ± 0·7 |
Values are means ± s.e.; n = 3.
An increase in the stratification period at 5 °C resulted in significantly higher germination percentages of seeds incubated at 10, 23, 15/6 and 20/10 °C in light and in darkness (P < 0·001). Seeds incubated in light germinated to much higher percentages than those incubated in darkness (Table 2). Increasing the length of stratification did not result in a higher germination percentage when seeds were incubated in darkness. No significant effect of incubation temperature was observed on germination in light and darkness of stratified seeds (P > 0·05). The interaction effect between duration of stratification and incubation temperature was also not significant (P > 0·05). In the 8 week cold stratification condition, 8·9 % of the seeds had already germinated during stratification, while 20·5 % had already germinated during 12 weeks of stratification.
Table 2.
Effects of a cold stratification (5 °C) period on germination percentages of C. temulum seeds at different temperatures in light and darkness during 2 weeks.
| Incubation temperature in light |
Incubation temperature in darkness |
|||||||
|---|---|---|---|---|---|---|---|---|
| Weeks at 5 °C | 10 °C | 23 °C | 15/6 °C | 20/10 °C | 10 °C | 23 °C | 15/6 °C | 20/10 °C |
| 0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 | 0·0 ± 0·0 |
| 4 | 14·0 ± 1·2 | 9·3 ± 1·8 | 6·0 ± 2·0 | 6·0 ± 2·3 | 8·7 ± 2·4 | 0·0 ± 0·0 | 6·0 ± 3·1 | 4·0 ± 2·0 |
| 8 | 49·3 ± 0·7 | 40·0 ± 4·2 | 48·0 ± 8·3 | 52·0 ± 1·2 | 8·0 ± 1·2 | 4·0 ± 0·0 | 9·3 ± 4·1 | 12·7 ± 1·8 |
| 12* | 72·0 ± 6·0 | 68·0 ± 2·0 | 65·0 ± 1·0 | 59·0 ± 13·0 | 6·0 ± 0·0 | 2·0 ± 2·0 | 2·0 ± 0·0 | 1·0 ± 1·0 |
Values are means ± s.e. n = 3 or *n = 2.
Temperature range for germination
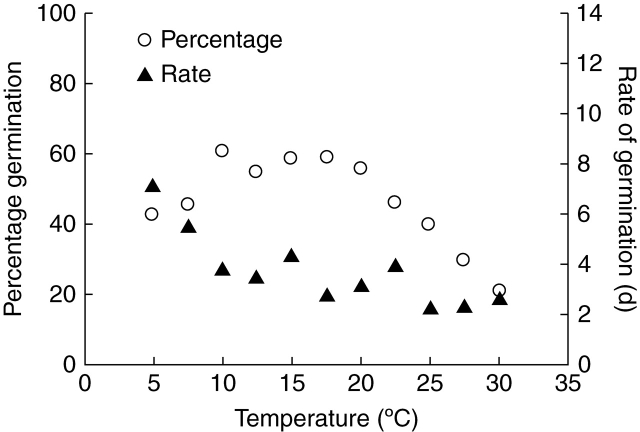
After 8 weeks of cold stratification, > 40 % of the seeds germinated during 2 weeks at all temperatures between 5 and 22·5 °C (Fig. 4). The percentage of germinated seeds decreased with an increase in temperatures above 22·5 °C. The T50 decreased with an increase in temperature up to about 10 °C (Fig. 4); at higher temperatures the T50 ranged from 2·5 to 4 d. These high germination rates indicate that a 14 d incubation period was sufficient in this experiment.
Fig. 4.
Germination percentage of C. temulum after 14 d at a range of temperatures following 8 weeks of cold stratification at 5 °C (open circles). Filled triangles represent the rate of germination, i.e. days until 50 % of all germinated seeds have germinated. n = 3.
Effect of GA3 on dormancy break
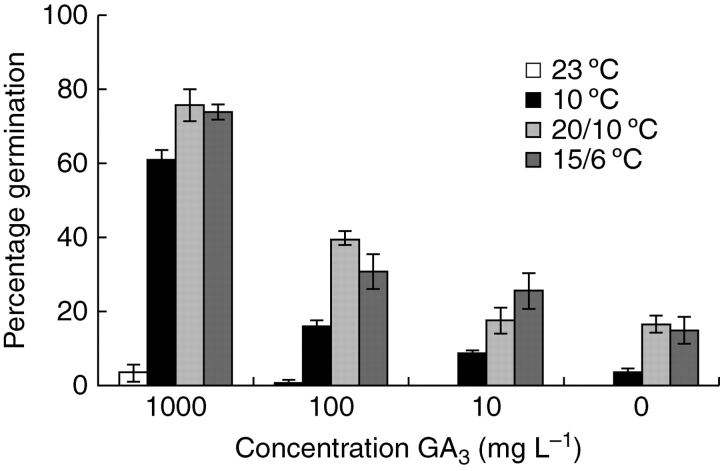
The concentration of GA3 and temperature condition had significant effects on the final germination percentage after 12 weeks of incubation (P < 0·001 and P < 0·01, respectively). The interaction effect was not significant (P > 0·05), meaning that germination was similar among temperatures within the different GA3 concentrations. In all temperature conditions, the final germination percentage increased with increasing concentration of GA3 (Fig. 5). The germination percentage was highest for seeds incubated at 15/6 and 20/10 °C, while seeds incubated at 23 °C had very low germinating percentages. The maximum final germination recorded at 23 °C was 3·4 % in 1000 mg L−1 GA3. In all conditions, very few seeds germinated after week 10, indicating that a 12 week incubation period was sufficient to test for effects of GA3 (results not shown).
Fig. 5.
Germination of C. temulum after 12 weeks of incubation at different GA3 concentrations. Seeds are incubated at 23 °C, 10 °C, 20/10 °C and 15/6 °C. Vertical bars represent the s.e.; n = 3.
DISCUSSION
Up to 80 % of the seeds of C. temulum germinated during 16 weeks of stratification at 5 °C (Table 1). Significantly lower germination percentages were recorded when fresh seeds were incubated at higher temperature conditions. This implies that a prolonged period at low temperatures was required to break physiological dormancy (PD) in most seeds of C. temulum. Similar results were obtained by Janiesch (1971), who studied dormancy in seeds of C. temulum collected in northern Germany and recorded approx. 75 % germination during 100 d of incubation at 5 °C. Grime et al. (1981) recorded 100 % germination during 3 months of incubation at 5 °C. In addition to PD, seeds of C. temulum, with an average E:S ratio of 0·16 at the moment of dispersal also have morphological dormancy. Before the radicle can emerge, the embryo must elongate to a critical length (critical E : S ratio 0·95). Embryo growth in most seeds occurred gradually during incubation at 5 °C (Fig. 2). This means that breaking of PD and MD occurs simultaneously in most seeds of C. temulum. A pre-treatment at high temperature (23 °C) did not affect the growth rate of embryos at 5 °C, nor did it increase germination percentages of seeds stratified at 5 °C (Table 1). Very few seeds germinated when incubated at 10 and 23 °C, but when seeds were incubated at daily fluctuating temperatures, 15 % germinated within 6 weeks (Fig. 3). Seeds not germinating within 30 d are considered to be dormant (Baskin and Baskin, 1998). Following this definition, a small fraction of the C. temulum seeds are either only MD or they are MD with a low level of PD.
The low temperature requirement for embryo growth and germination in most seeds, and the lack of a stimulating effect of a high temperature pre-treatment indicate that most seeds of C. temulum have an intermediate complex or deep complex type of MPD (Baskin and Baskin, 1998). Deep complex MPD has previously been observed in a number of other Apiaceae species (Baskin et al., 1995; Walck and Hidayati, 2004). No report was found of Apiaceae species with seeds that have intermediate complex MPD. However, in some species (e.g. Heracleum sphondylium), which require only cold stratification to break dormancy, authors have not tested whether GA could substitute for cold stratification (Stokes, 1952). Because GA substitutes for cold stratification in seeds with intermediate complex MPD, it is not clear whether seeds of these species have intermediate or deep complex MPD. In seeds of C. temulum, GA3 did not substitute for cold stratification when seeds were incubated at 23 °C for 12 weeks (Fig. 5). Therefore, it can be concluded that most seeds of C. temulum have deep complex MPD. In some species, e.g. Aegopodium podagraria, GA3 can induce growth of the embryo without subsequent germination (unpublished results). Test were not conducted to determine if GA3 promoted embryo growth in seeds of C. temulum, but it seems that GA3 does not substitute for cold stratification as suggested by Nikolaeva (1977).
Seedlings of European Apiaceae very often emerge in spring after seed dormancy is broken during winter (Roberts, 1979, 1986; Thompson and Baster, 1992). Chaerophyllum temulum seems to be no exception to this pattern, since most seedlings emerge at the beginning of the growing season in late February and early March in the first year after sowing (Fig. 1). By emerging in spring, vulnerable seedlings avoid severe winter conditions and competition with other plant species (Masuda and Washitani, 1990). Once MPD in C. temulum seeds is broken by stratification, seeds germinate at a wide range of temperatures (Table 2). Moreover, the rate of germination also increases when seeds are incubated at higher temperatures (Fig. 4). This allows seeds to germinate rapidly, if a period of increased temperatures occurs in late winter or early spring. After 1 year, 35·3 % of the seeds buried at 1 cm depth and 50·0 % of seeds sown at the soil surface were recruited into seedlings. Roberts (1986) found many more seedlings emerging (approx. 82 %). Roberts regularly disturbed the seedbed in his experiment, however, very probably resulting in higher germination percentages.
Chaerophyllum bulbosum is the only other Eurasian Chaerophyllum representative whose germination characteristics have been studied extensively. Although it has a more eastern distribution than C. temulum (Hegi, 1975), the germination and dormancy breaking requirements of these two species are very similar (Janiesch, 1971; Augé et al., 1989). Embryo growth and germination of C. bulbosum seeds only occurs during incubation at low temperatures (Janiesch, 1971). Addition of GA, however, did not promote germination at 22 °C (Augé et al., 1989). Therefore, these seeds have deep complex MPD. This type of dormancy is also very common among other temperate European and North American Apiaceae that germinate in late winter or spring.
The only two representatives of Chaerophyllum in North America, C. procumbens and C. tainturieri, are phylogenetically closely related to C. temulum (Downie et al., 2000). The life cycle strategy and dormancy characteristics of these two North American species differ considerably, however, from those of C. temulum. Chaerophyllum procumbens and C. tainturieri are both winter annuals with non-deep simple MPD. They germinate in autumn after dormancy is broken by high summer temperatures (Baskin and Baskin, 1990; Baskin et al., 2004). Seeds of C. procumbens and C. tainturieri both require light to germinate, enabling them to form a persistent seed bank. The present experiments indicate that seeds of C. temulum also require light to germinate after a cold stratification enabling them to form a persistent seed bank. However, all other evidence seems to rule against this hypothesis. In the present experiments, 95 % of the seeds buried 5 cm deep for embryo measurements had germinated at the end of the first winter after sowing. Roberts (1986) recorded that 82 % of the seedlings had emerged in the first spring after sowing, and few additional seedlings emerged in the following years.
Spalik and Downie (2001) suggested that C. procumbens and C. tainturieri are both descendants of a common Eurasian ancestor that reached North America by incidental bird dispersal, because no representatives of sect. Chaerophyllum occur in Asia. Moreover the North American species lack male flowers and their corollas are almost completely reduced. These characters are typical for self-pollinating species and they are also found in the related Oreomyrrhis species, but not in the European C. temulum. Molecular dating techniques suggest that C. temulum diverged from the other sect. Chaerophyllum species about 5–11 million years ago (Chung et al., 2005). Besides the Bering land bridge, the North Atlantic land bridge (NALB) formed an alternative route through which Eurasian species could migrate to North America during the early Tertiary. Geological evidence suggests that the NALB was discontinuous during the larger part of the later Tertiary (Tiffney, 1985). Consequently, since the Middle Eocene, any exchange of species between North America and Europe via the Atlantic probably occurred via long-distance dispersal or dispersal via island hopping (Tiffney and Manchester, 2001). Long-distance dispersal and especially dispersal through island hopping excludes stasis in morphological and physiological traits because of founder effects occurring in newly colonizing organisms (Milne and Abbott, 2002). Therefore, this dispersal scenario via the NALB supports the fact that major changes in life cycle strategy and dormancy characteristics have occurred in the North American representatives of sect. Chaerophyllum.
Any conclusions drawn on the evolution of dormancy in species from Chaerophyllum sect. Chaerophyllum are highly speculative, because data on dormancy types of related Apiaceae species are scarce. Nonetheless some arguments can be put forward for deep complex MPD to be the plesiomorphic dormancy type. The most important argument is that this dormancy type occurs in C. temulum and C. bulbosum, two species occurring in the centre of distribution of Chaerophyllum. Furthermore, deep complex dormancy also occurs in many other species of the subtribe Scandicinae. It is the plesiomorphic dormancy state in Osmorhiza species (Walck and Hidayati, 2004) and it is present in Anthriscus sylvestris and Myrrhis odorata, two Apiaceae with a mainly European distribution (Lhotska, 1977; Baskin et al., 2000). This implies that non-deep simple MPD is a derived dormancy type that was developed in a common ancestor of C. procumbens and C. tainturieri. This dormancy type was maintained in the two sister species, despite the fact that they grow in different habitats. Chaerophyllum tainturieri mainly occurs in fields and waste places, while C. procumbens typically grows in mesic deciduous forests (Baskin and Baskin, 1990; Baskin et al., 2004). Additional research on dormancy of Oreomyrrhis species and other Chaerophyllum species could further elucidate evolutionary pathways of dormancy in this group.
LITERATURE CITED
- Adams CA, Baskin JM, Baskin CC. Trait stasis versus adaptation in disjunct relict species: evolutionary changes in seed dormancy breaking and germination requirements in a subclade of Aristolochia subgenus Siphisia (Piperales) Seed Science Research. 2005;15:161–173. [Google Scholar]
- Augé R, Bourgeais P, Péron JY. Etude des conditions de la germination des semences de cerfeuil tubéreux (Chaerophyllum bulbosum L.) Acta Horticulturae. 1989;242:239–247. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Baskin CC, Meyer SE, Baskin JM. Two types of morphophysiological dormancy in seeds of two genera (Osmorhiza and Erythronium) with an arcto-tertiary distribution pattern. American Journal of Botany. 1995;82:293–298. [Google Scholar]
- Baskin CC, Milberg P, Andersson L, Baskin JM. Deep complex morphophysiological dormancy in seeds of Anthriscus sylvestris (Apiaceae) Flora. 2000;3:245–251. [Google Scholar]
- Baskin CC, Hawkins TS, Baskin JM. Ecological life cycle of Chaerophyllum procumbens variety shortii (Apiaceae), a winter annual of the North American eastern deciduous forest. Journal of the Torrey Botanical Society. 2004;131:126–139. [Google Scholar]
- Baskin JM, Baskin CC. Germination ecophysiology of seeds of the winter annual Chaerophyllum tainturieri – a new type of morphophysiological dormancy. Journal of Ecology. 1990;78:993–1004. [Google Scholar]
- Chung KF, Peng CI, Downie SR, Spalik K, Schaal BA. Molecular systematics of the Trans-Pacific alpine genus Oreomyrrhis (Apiaceae): phylogenetic affinities and biogeographic implications. American Journal of Botany. 2005;92:2054–2071. doi: 10.3732/ajb.92.12.2054. [DOI] [PubMed] [Google Scholar]
- Downie SR, Katz-Downie DS, Spalik K. A phylogeny of Apiaceae tribe Scandiceae: evidence from nuclear ribosomal DNA internal transcribed spacer sequences. American Journal of Botany. 2000;87:76–95. [PubMed] [Google Scholar]
- Grime JP, Mason G, Curtis AV, Rodman J, Band SR, Mowforth MAG, Neal AM, Shaw S. A comparative study of germination characteristics in a local flora. Journal of Ecology. 1981;69:1017–1059. [Google Scholar]
- Grime JP, Hodgson JG, Hunt R. Comparative plant ecology: a functional approach to common British species. London: Unwin-Hyman; 1988. [Google Scholar]
- Hegi G. Illustrierte Flora von Mittel-Europa. V. 2. Dicotyledons. München: JF Lehmanns; 1975. [Google Scholar]
- Hidayati SN, Baskin JM, Baskin CC. Morphophysiological dormancy in seeds of two North American and one Eurasian species of Sambucus (Caprifoliaceae) with underdeveloped spatulate embryos. American Journal of Botany. 2000;87:1669–1678. [PubMed] [Google Scholar]
- Hoey MT, Parks CR. Isozyme divergence between eastern Asian, North American and Turkish species of Liquidambar (Hamamelidaceae) American Journal of Botany. 1991;78:938–947. [Google Scholar]
- Janiesch P. Zur Physiologie der Nachreife von Umbelliferen nitrophiler Säume. Flora. 1971;160:518–525. [Google Scholar]
- Lhotska M. Notes on the ecology of germination in Myrrhis odorata. Folia Geobotanica and Phyto-taxonomica. 1977;12:209–213. [Google Scholar]
- Martin AC. The comparative internal morphology of seeds. American Midland Naturalist. 1946;36:513–660. [Google Scholar]
- Masuda M, Washitani I. A comparative ecology of the seasonal schedules for reproduction by seeds in a moist tall grassland community. Functional Ecology. 1990;4:169–182. [Google Scholar]
- Milne RI, Abbott RJ. The origin and evolution of Tertiary relict floras. Advances in Botanical Research. 2002;38:281–314. [Google Scholar]
- Nikolaeva MG. Factors controlling the seed dormancy pattern. In: Kahn AA,, editor. The physiology and biochemistry of seed dormancy and germination. Amsterdam: North-Holland; 1977. pp. 51–74. [Google Scholar]
- Roberts HA. Periodicity of seedling emergence and seed survival in some Umbelliferae. Journal of Applied Ecology. 1979;16:195–201. [Google Scholar]
- Roberts HA. Seed persistence in soil and seasonal emergence in plant species from different habitats. Journal of Applied Ecology. 1986;23:639–656. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd edn. New York: WH Freeman and Co; 1997. [Google Scholar]
- Spalik K, Downie SR. The utility of morphological characters for inferring phylogeny in Scandiceae subtribe Scandicinae (Apiaceae) Annals of the Missouri Botanical Garden. 2001;88:270–301. [Google Scholar]
- Stokes P. A physiological study of embryo development in Heracleum sphondylium L. I. The effect of temperature on embryo development. Annals of Botany. 1952;16:441–447. [Google Scholar]
- Thompson K, Baster K. Establishment from seed of selected Umbelliferae in unmanaged grassland. Functional Ecology. 1992;6:346–352. [Google Scholar]
- Tiffney BH. The Eocene North Atlantic Land Bridge – its importance in Tertiary and modern phytogeography of the northern hemisphere. Journal of the Arnold Arboretum. 1985;66:243–273. [Google Scholar]
- Tiffney BH, Manchester SR. The use of geological and paleontological evidence in evaluating plant phylogeographic hypotheses in the northern hemisphere Tertiary. International Journal of Plant Sciences. 2001;162 supplement:S3–S17. [Google Scholar]
- Walck JL, Hidayati SN. Germination ecophysiology of the western North American species Osmorhiza depauperata (Apiaceae): implications of pre-adaptation and phylogenetic niche conservatism in seed dormancy evolution. Seed Science Research. 2004;14:387–394. [Google Scholar]
- Walck JL, Hidayati SN, Okagami N. Seed germination ecophysiology of the Asian species Osmorhiza aristata (Apiaceae): comparison with its North American congeners and implications for evolution of types of dormancy. American Journal of Botany. 2002;89:829–835. doi: 10.3732/ajb.89.5.829. [DOI] [PubMed] [Google Scholar]
- Wen J. Evolution of eastern Asian and eastern North American disjunct distributions in flowering plants. Annual Review of Ecology and Systematics. 1999;30:421–455. [Google Scholar]
- Wen J, Lowry PP, Walck JL, Yoo KO. Phylogenetic and biogeographic diversification in Osmorhiza (Apiaceae) Annals of the Missouri Botanical Garden. 2002;89:414–428. [Google Scholar]