Abstract
Background and Aims
Based on molecular phylogenetic studies, the unigeneric family Eupteleaceae has a prominent phylogenetic position at or near the base of Ranunculales, which, in turn, appear at the base of eudicots. The aim of the present paper is to reveal developmental features of the flowers and to put the genus in a morphological context with other basal eudicots.
Methods
Flowers in all developmental stages of Euptelea pleiosperma were collected in the wild at intervals of 7–10 d in the critical stages and studied with a scanning electron microscope.
Key Results
Remnants of a perianth are lacking throughout flower development. Floral symmetry changes from monosymmetric to asymmetric to disymmetric during development. Asymmetry is expressed in that the sequence of stamen initiation is from the centre to both lateral sides on the adaxial side of the flower but starting from one lateral side and proceeding to the other on the abaxial side. Despite the pronounced floral disymmetry, a dimerous pattern of floral organs was not found. The carpel primordia arise between the already large stamens and alternate with them. Stamens and carpels each form a somewhat irregular whorl. The carpels are ascidiate from the beginning. The stigma differentiates as two crests along the ventral slit of the ovary. The few lateral ovules alternate with each other.
Conclusions
Although the flowers have some unusual autapomorphies (wind pollination, lack of a perianth, pronounced disymmetry of the floral base, long connective protrusion, long temporal gap between androecium and gynoecium initiation, small space for carpel initiation), they show some plesiomorphies at the level of basal eudicots (free carpels, basifixed anthers, whorled phyllotaxis), and thus fit well in Ranunculales.
Key words: Basal eudicots, Euptelea, Eupteleaceae, floral development, floral phyllotaxis, floral symmetry, Ranunculales, systematics
INTRODUCTION
Euptelea Sieb. et Zucc. is the only genus of Eupteleaceae, a family endemic to East Asia, with two species, E. pleiosperma Hook. f. & Thoms. in China and India, and E. polyandra Sieb. & Zucc. in Japan (Endress, 1993; Fu and Endress, 2001). Because of its structural features reminiscent of magnoliids and hamamelidids, earlier interpretations on its systematic position fluctuated between these two alliances but more recently concentrated on hamamelidids (Nast and Bailey, 1946; Takhtajan, 1980, 1997; Cronquist, 1981; Thorne, 1992). Pollen morphology and leaf architecture indicated a position in hamamelidids (Praglowski, 1975; Endress, 1986; Wolfe, 1989). Its simple, wind-pollinated flowers did not make the systematic interpretation of Euptelea easy (Endress, 1969, 1986).
Molecular systematic studies position Eupteleaceae in Ranunculales (APG, 1998, 2003). Initially Eupteleaceae appeared as the second clade in the grade of families (Chase et al., 1993; Qiu et al., 1993, 1999; Hoot and Crane, 1995; Soltis et al., 1997, 2000; Hoot et al., 1999; Magallón et al., 1999; Zanis et al., 2003). The same topology resulted from combined molecular and structural analyses (Doyle and Endress, 2000). Another topology is with Eupteleaceae as sister to all other Ranunculales, which appeared in some more recent molecular studies (Hilu et al., 2003; Soltis et al., 2003; Kim et al., 2004; Worberg et al., 2007), but also in the combined molecular and structural study by Nandi et al. (1998). A third variant is that Eupteleaceae plus Papaveraceae appear as sister to the remaining Ranunculales (Qiu et al., 2005). In all these recent studies Ranunculales are sister to all other eudicots. Thus the position of Eupteleaceae is invariably close to the base of eudicots.
Because of this prominent systematic position of Eupteleaceae and also the increasing focus of molecular developmental and evo-devo studies on flowers of Ranunculales (Kramer and Irish, 1999; Kramer et al., 2003, 2006; Becker et al., 2005; Di Stilio et al., 2005; Lee et al., 2005; Carlson et al., 2006; Cui et al., 2006; Howarth and Donoghue, 2006; Kölsch and Gleissberg, 2006; Kramer and Zimmer, 2006; Shan et al., 2006; Zahn et al., 2006) better knowledge of the floral development of Eupteleaceae becomes especially timely. Earlier studies in the family concentrated on Euptelea polyandra, in which floral structure and development, and embryology were studied with microtome section series (Endress, 1969). There is also a short study on the structure of the carpels by Leinfellner (1969), and Endress (1986) briefly described an early stage of floral development based on SEM studies. However, the present study is the first on the entire floral development of Euptelea investigated with the SEM, and also the first on the floral structure of Euptelea pleiosperma. As a number of other Ranunculales have been studied in the same way more recently, a fresh comparison within the order is now possible (Endress, 1989, 1995; Lehmann and Sattler, 1994; Feng et al., 1995; Erbar et al., 1998; Feng and Lu, 1998; Endress and Igersheim, 1999; Ren et al., 2004; Chang et al., 2005; Tucker and Hodges, 2005; Tian et al., 2006; Gu and Ren, 2007; Song et al., 2007; Zhang et al., 2007).
MATERIALS AND METHODS
Floral buds of Euptelea pleiosperma Hook. f. & Thoms. were collected in different developmental stages from more than 20 individuals on Mt Taibai (voucher: Bai Gen-lu136, 137, and 144, alt. 1200–1500 m, SANU) from 2002 to 2004 at intervals of 7–10 d. The material was fixed in FAA, dehydrated in ethanol and iso-amyl acetate series, treated with critical-point drying in CO2, vacuum evaporation, and observed with a Hitachi 800 SEM.
RESULTS
Floral morphology
Each of the 5–8 bisexual flowers is in the axil of a bract at the base of a leafy shoot (Fig. 1A, B). The pedicel is about 6–12 mm long. A perianth is absent. The 6–14 stamens are 0·8–1·9 cm long, free, and have filiform to slightly flattened filaments and red, narrowly oblong anthers. The anther is longer than the filament and has a prominent connective appendage 0·7–2 mm long. The 5–13 carpels are 1–3 mm long, free, stalked, and have an obliquely decurrent stigma along the ovary. The carpels have 1–4 ovules.
Fig. 1.
Flowering of Euptelea pleiosperma: (A) flowering shoot; (B) flower. C, Carpel; F, flower; L, leaf; P, pedicle; S, stamen; Sc, scale). Scale bars = 0·5 mm.
Inflorescence and shape of floral primordia
From base to apex the flowering shoot consists of 5–6 scales, followed by 5–8 bracts, each with a flower in the axil, and 4–6 foliage leaves. Flowers at the primordial stage are larger in the middle part of the inflorescence than basally and distally (Fig. 2A).
Fig. 2.
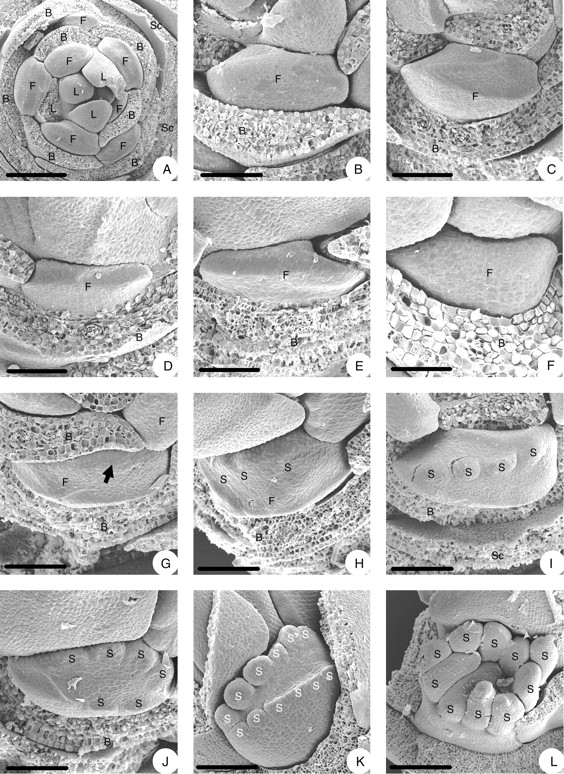
Floral morphogenesis of Euptelea pleiosperma: (A) young inflorescence bud (scales and floral-subtending bracts removed); (B) floral primordium in the axil of a bract; (C–F) floral primordia with irregular shapes due to pressure by neighbouring bracts; (G–K) initiation of stamens (G, adaxial side of floral becoming higher, indicated by an arrow; H, first stamen primordia appear on adaxial side; I, stamen primordia more distinct; J, some stamen primordia appear on abaxial side; K, number of stamen primordia increased on both sides of flower – no other floral organ found outside the stamens); (L) stamens enlarge. B, Floral-subtending bract; F, flower; L, leaf; S, stamen; Sc, scale. Scale bars: A = 250 µm; B, C, H–J = 100 µm; D, G = 86 µm; E = 75 µm; F = 50 µm; K = 120 µm; L = 500 µm.
The young flower is tangentially elongate and of somewhat asymmetrical shape owing to various degrees of lateral (or radial) expansion of neighbouring bracts (Fig. 2B–F).
Stamen development
Before stamen primordia become apparent, the middle part of the adaxial side of the flower becomes higher than the abaxial. Thus the floral apex becomes somewhat oblique (Fig. 2G, arrow). The first stamen primordia appear in the middle part of the adaxial side in collateral arrangement (Fig. 2H). Then additional ones arise on both sides of the first ones (Fig. 2I), and later in the middle part of the abaxial (lower) side of the flower (Fig. 2J) and finally on both sides of those. The complete set of stamens forms two rows, one on the adaxial and one on the abaxial side of the flower (Fig. 2K), joining into a flat ring (Fig. 2L). The stamens are finger-like at this stage. As they enlarge, they differentiate into a very short filament and a long anther with a connective appendage (Fig. 3A). The anther and the connective appendage become more prominent after carpel initiation (Fig. 3B, C). In later bud stage the connective appendage may attain the shape of an arrowhead (Fig. 3D, E). In the fully differentiated but undehisced anther, each theca has a longitudinal stomium that bifurcates at both ends so that the prospective dehiscence line is I-shaped (Fig. 3E–G). Thus anther dehiscence is valvate. The filament has become about as long as the anther.
Fig. 3.
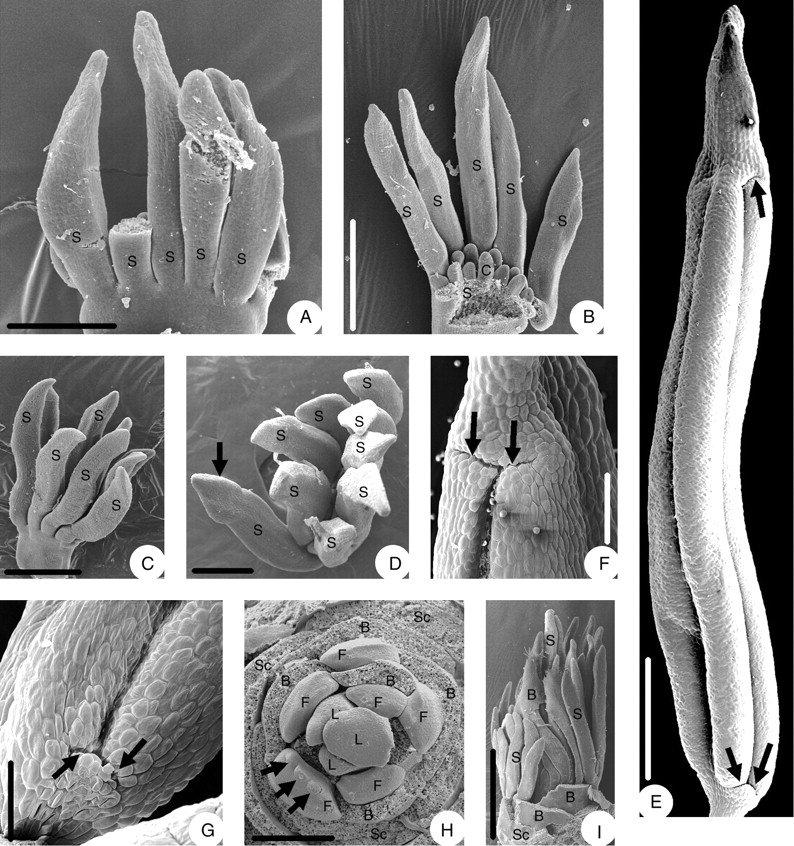
Floral morphogenesis of Euptelea pleiosperma: (A) young stamens differentiated into anther with connective appendage, filament still very short; (B, C) stamen filaments more distinct after carpel formation; (D) young flower from above, connective appendages arrowhead-shaped; (E) mature stamen before dehiscence, showing longitudinal and horizontal extension of dehiscence lines (arrows), from side; (F) close-up of dehiscence line with horizontal extensions (arrows); (G) close-up of lower part of dehiscence line with horizontal extensions (arrows); (H) stamen primordia in the second flower from the bottom of an inflorescence appear first (arrows); (I) stamens in upper flowers are longer than those of lower flowers. B, Floral-subtending bract; C, carpel; F, flower; L, leaf; S, stamen; Sc, scale. Scale bars: A, D, H = 250 µm; B = 0·5 mm; C = 0·6 mm; E = 0·86 mm; F = 231 µm; G = 205 µm; I = 1 mm.
No other organs were observed outside of the stamens in the young flowers (Fig. 2K, L).
The mentioned early retardation of flower development at the base of an inflorescence is also expressed at stamen initiation, as stamens in the second flower appear earlier than those in the basalmost flower (Fig. 3H). Later this basal retardation is even more pronounced, as stamens in the upper flowers of an inflorescence become longer than those of the lower (Fig. 3I).
Carpel development
There is little space left in the centre of the flower after initiation of all stamens. There are small triangular areas alternating with the stamens (Fig. 4A). Each of them enlarges and gives rise to a carpel (Fig. 4B). The carpels are slightly triangular in the beginning (Fig. 4C) but then become round (Fig. 4D, E). The carpels form the second ring (whorl) of floral organs (Fig. 4E, F) and are of different sizes, without a regular pattern; however, the transversal ones tend to be smaller than the more central ones (Figs 3B and 4E, F). The carpels elongate and differentiate into a narrower basal part and an ovoid upper part. At the base of the ovoid part, a longitudinal concavity appears (Fig. 5A), the incipient ovary locule and the first manifestation of the ascidiate zone. As the carpels enlarge, the concavity becomes deeper, resulting in a chair-like shape (Fig. 5B). In the upper part, the carpel becomes plicate as the flanks come together and form a ventral slit (Fig. 5C, D). The carpels are still of different sizes and their stalks become obvious at this stage. With further enlargement of the carpels the ventral slit becomes more pronounced (Fig. 5E). The area flanking the ventral slit becomes more massive (Fig. 5F) and differentiates into two papillate stigmatic crests (Fig. 5G, H). In the mature carpel, the stigmatic region encompasses almost the entire length of the ovary, the carpel stalk is relatively long and slender (Fig. 5I). The two stigmatic crests have long, unicellular papillae (Fig. 5J, K). The few lateral, alternating ovules are immature at anthesis (Fig. 5L).
Fig. 4.
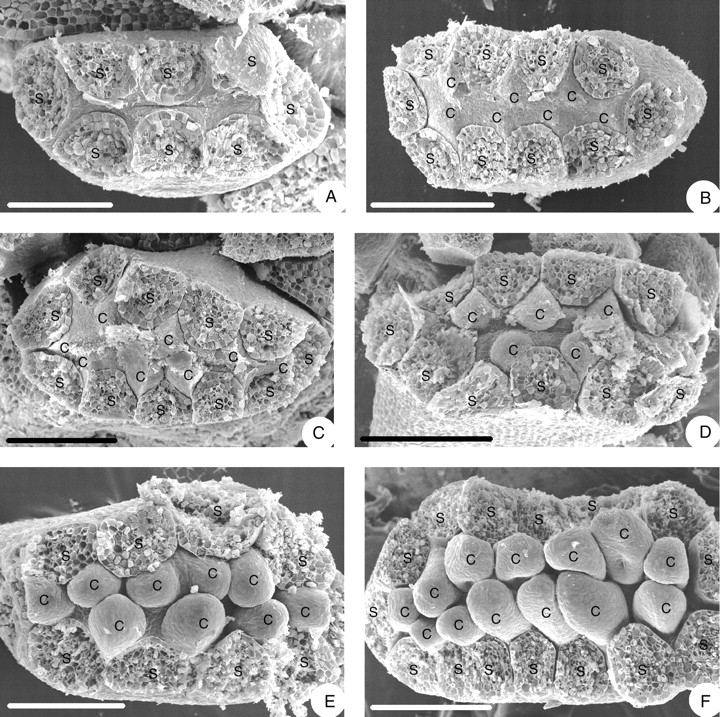
Floral morphogenesis of Euptelea pleiosperma showing young flowers from above (stamens removed): (A) before carpel initiation; (B) carpels initiated, alternating with stamens; (C) carpel primordia enlarged, irregular in shape; (D) carpel primordia more regular in shape; (E) young carpels rounded; (F) young carpels in chair-like stage. C, Carpel; S, stamen. Scale bars: A = 100 µm; B = 136 µm; C–E = 120 µm; F = 150 µm.
Fig. 5.
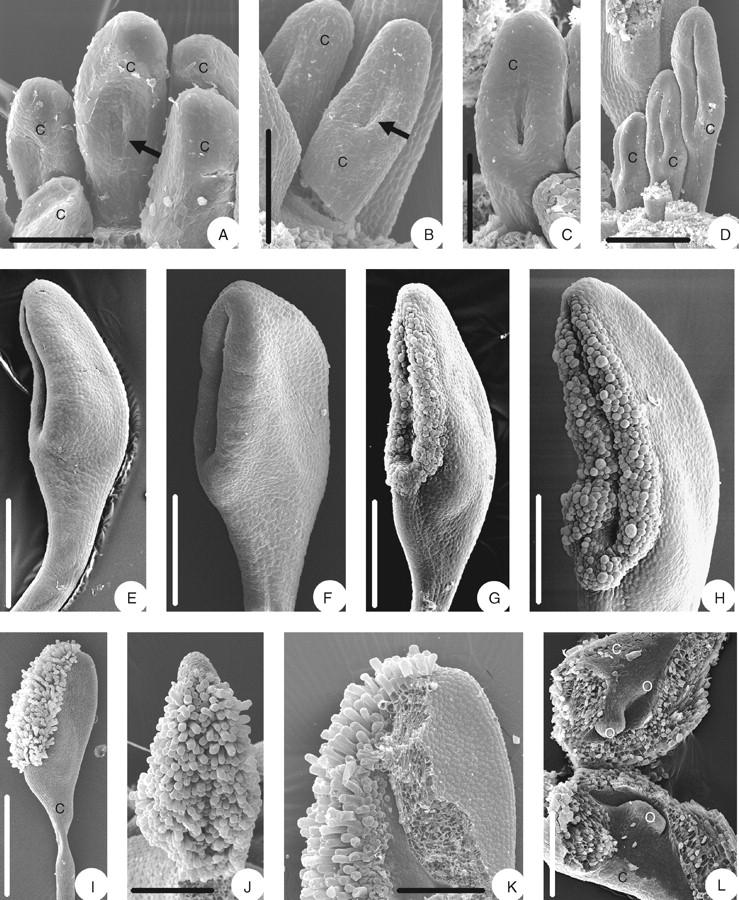
Floral morphogenesis of Euptelea pleiosperma: (A) carpel in the centre has become concave (arrow) in the middle part; (B) carpels with concave part (arrow) deeper and extending upwards; (C) carpel flanks beginning to close; (D) carpels more or less closed and ventral slit formed, carpel stalk formed; (E) more or less closed carpel from the side; (F) carpel with stigmatic crests formed; (G) carpel with stigmatic crests becoming papillate; (H) almost mature carpel with stigmatic crests; (I) mature carpel with oblique stigma and long stalk; (J) mature stigma, from ventral; (K) upper part of carpel, with one flank removed, showing stigmatic tissue extending deep into ventral slit; (L) opened ovary, showing three young ovules. Scale bars: A, C = 60 µm; B = 100 µm; D, F, J, K = 200 µm; E, G = 270 µm; H = 231 µm; I = 0·3 mm; L = 150 µm.
DISCUSSION
Floral symmetry, phyllotaxis and merism
At the time the stamens are initiated the flowers of E. pleiosperma appear transversely extended and somewhat irregular in shape, caused by pressure of neighbouring floral subtending bracts. This is also the case in E. polyandra (Endress, 1986), and an irregular floral shape occurs in general in a number of angiosperms that lack a perianth, which otherwise shapes the initial floral form (Endress, 1990).
The mature flowers of E. pleiosperma are still somewhat transversely extended, which roughly results in a disymmetric shape (for E. polyandra; Endress, 1986). However, in earlier stages the shape appears more monosymmetric because the adaxial side of the young flower is higher than the abaxial side (or even asymmetric because of the mentioned irregular shape). Asymmetry also occurs in the initiation sequence of the stamens, which is from the centre to both sides on the adaxial side of the flower but from one side to another on the abaxial side. The monosymmetric component of floral shape is no longer apparent in later stages. This phenomenon of transient early monosymmetry in otherwise polysymmetric flowers is not uncommon in angiosperm flowers (Endress, 2006).
The irregular size of the young carpels appears to be caused by the somewhat irregular spaces between the stamens, which tend to be larger between the central stamens than between the transverse ones (e.g. Fig. 4C).
Floral phyllotaxis appears to be irregularly whorled, with one approximate whorl of stamens and a somewhat irregularly alternating whorl of carpels. Because the flowers are transversely extended, these whorls are not circular but form narrow ellipses. There is a general trend for floral phyllotaxis to be irregular in flowers that lack a perianth. It appears that the perianth with its broad organs initiates the regular phyllotaxis of the following floral organs. In contrast, if the stamens, which are much narrower than the floral apex, are the first organs to be formed, a regular arrangement is more difficult to achieve (Endress, 1990). Another example of irregular floral phyllotaxis and lacking perianth in Ranunculales is Achlys (Berberidaceae) (Endress, 1989).
The flowers are often dimerous or trimerous in basal eudicots (Drinnan et al., 1994). Hoot et al. (1999) speculated that the floral disymmetry of Euptelea as described by Endress (1986) could indicate a basically dimerous groundplan. Although this is a possible scenario, floral development does not show any traits of dimery.
Comparison with other Ranunculales
In view of the systematic position of Eupteleaceae in the basal grade of Ranunculales, either as the basal branch, or the second basal branch after Papaveraceae, or in a basal branch together with Papaveraceae, as explained in the Introduction, separate comparisons of the floral structure with Papaveraceae and with the remainder of Ranunculales (Berberidaceae, Circaeasteraceae, Lardizabalaceae, Menispermaceae and Ranunculaceae) are of interest. For convenience the clade consisting of all Ranunculales families minus Eupteleaceae and Papaveraceae is here called ‘core Ranunculales’.
At the outset it should be emphasized that Euptelea has an unusual floral biology among Ranunculales. The flowers have a wind-pollination syndrome, including lack of perianth, elongate anthers concomitant with a long connective protrusion, and carpels with a relatively large stigma with long papillae (Endress, 1969, 1986). The flowers are difficult to compare with those of other Ranunculales, because a wind-pollination syndrome is otherwise very rare in Ranunculales. Relatively similar, wind-pollinated flowers are only known from Macleaya and Bocconia (Papaveraceae) and some Thalictrum species (Ranunculaceae) (see fig. 6 in Endress, 2002). However, both groups, Macleaya/Bocconia and Thalictrum are not related to Euptelea, since they are both deeply nested in their respective families. Their similarity is to be interpreted as the result of convergent evolution based on a similar bauplan.
Comparison with Papaveraceae
Flowers of Papaveraceae are different from those of Eupteleaceae in having basically dimerous flowers (Karrer, 1991). Except for some derived genera the gynoecium has two carpels, and these are always completely united. A perianth is always present in Papaveraceae, although in the wind-pollinated Bocconia and Macleaya petals have been lost. Floral phyllotaxis is whorled. Only in derived groups with an increased number of stamens may stamen phyllotaxis be slightly irregular. If disymmetry in flowers of Papaveraceae is pronounced, this is caused by the formation of two spurs (Fumarioideae) or the strong dorsal development of the two carpels, such as in Macleaya (Papaveroideae s.l.), in which the fruit has two wings, whereas in Euptelea the pronounced disymmetry is not related to the gynoecium. Winged fruits as in Euptelea are only present in Macleaya (but not based on single carpels). Early floral monosymmetry or asymmetry is not known from Papaveraceae (monosymmetry in Fumarioideae is not median, and thus different from the early median monosymmetry in Euptelea). The presence of a long temporal gap between the initiation of the androecium and gynoecium in Euptelea is unusual and not present in Papaveraceae (Karrer, 1991). The presence of an I-shaped stomium in the thecae and a long connective appendage as in Euptelea is not known from Papaveraceae.
Comparison with core Ranunculales
As the floral structure in the core Ranunculales families is more diverse, a comparison with Euptelea is more difficult. The flowers are dimerous and trimerous (Drinnan et al., 1994), with a trend to pentamery in Ranunculaceae. Phyllotaxis of dimerous and trimerous flowers is whorled (Schöffel, 1932; Hiepko, 1965; Endress, 1995), as present in part of all families, except Circaeasteraceae. Spiral floral phyllotaxis occurs in some Ranunculaceae (Schöffel, 1932; Hiepko, 1965), some Menispermaceae (Endress, 1995) and in Circaeasteraceae (Ren et al., 2004; Tian et al., 2006). Dimerous flowers may be disymmetric but this symmetry is never pronounced (e.g. Thalictrum; Schöffel, 1932; and perhaps also the basal genera Glaucidium and Hydrastis; all three genera in Ranunculaceae; or Epimedium, Berberidaceae). A perianth is always present in core Ranunculales, except for the derived genus Achlys (Berberidaceae) (Endress, 1989), which does not appear to be wind-pollinated but has brush flowers. A conspicuous temporal gap between androecium and gynoecium initiation has not been recorded in the core Ranunculales. A long connective appendage is unusual. An I-shaped stomium and thus valvate anther opening is rare but not completely absent; it is known from some Ranunculaceae (Endress and Hufford, 1989; Weber, 1993). Valvate anther opening, but of a different type, is common in Berberidaceae (Endress and Hufford, 1989). The stipitate carpels, which turn into one-seeded, winged fruitlets in Euptelea have a counterpart in those of Thalictrum (Ranunculaceae).
Such sporadic similarities with some core Ranunculales may not signify a closer relationship, but may just be an artefact due to the greater diversity of core Ranunculales than Papaveraceae, especially as the genera in question are not phylogenetically basal in the respective families. Euptelea shares with core Ranunculales some plesiomorphic characteristics such as basifixed anthers and free carpels. Carpel form is diverse in Ranunculales, varying between completely plicate and completely ascidiate. However, pronouncedly ascidiate carpels as in Euptelea are relatively widespread in core Ranunculales, such as in some Ranunculaceae (van Heel, 1981, 1983, 1984; Endress and Igersheim, 1999; Chang et al., 2005), in Berberidaceae (Endress, 1995; Endress and Igersheim, 1999; Brückner, 2000) and Circaeasteraceae (Ren et al., 2004; Tian et al., 2006). In contrast, in Papaveraceae the (completely united) carpels are plicate, and ascidiate carpels are only known from the derived Romneya (Karrer, 1991; Endress and Igersheim, 1999; Brückner, 2000). In general, in the core Ranunculales, carpels that are one- or few-seeded and are indehiscent in fruit tend to be more pronouncedly ascidiate than those that have many seeds and open longitudinally along the ventral side.
Comparison with other basal eudicots
Euptelea shares some unusual floral features with Tetracentron, another, though unrelated, basal eudicot [although they were sometimes classified together in the order Trochodendrales (e.g. Endress, 1986) or Trochodendranae (Takhtajan, 1997)]: (a) an unusually long temporal gap between the initiation of androecium and gynoecium (Chen et al., 2007; this study); (b) only minimal increase of the floral apex after androecium formation so that the carpel primordia are squeezed in between the already large stamens (Chen et al., 2007; this study); and (c) an I-shaped dehiscence line and valvate opening of the thecae (Endress, 1986; Hufford and Endress, 1989; Chen et al., 2007; this study). In view of the phylogenetic relationships discussed above, these shared characters are either plesiomorphies or, more probably, autapomorphies.
Summarizing, the flowers of Eupteleaceae show several plesiomorphies at the level of basal eudicots (free carpels, basifixed anthers, whorled floral phyllotaxis; Ronse De Craene et al., 2003; Ronse De Craene et al., 2003; Endress and Doyle, 2007) and as such fit well in Ranunculales. Their unusual traits (wind pollination, lack of a perianth, pronounced disymmetry of the floral base, long connective protrusion, long temporal gap between androecium and gynoecium initiation, small space for carpel initiation) are likely autapomorphies.
ACKNOWLEDGEMENTS
This project was supported by the National Nature Science Foundation of China (No. 30640013).
LITERATURE CITED
- APG. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden. 1998;85:531–553. [Google Scholar]
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- Becker A, Gleissberg S, Smyth DR. Floral and vegetative morphogenesis in California poppy (Eschscholzia californica Cham.) International Journal of Plant Sciences. 2005;166:537–555. [Google Scholar]
- Brückner C. Clarification of the carpel number in Papaverales, Capparales, and Berberidaceae. Botanical Review. 2000;66:155–307. [Google Scholar]
- Carlson JE, Leebens-Mack JH, Wall PK, Zahn LM, Mueller LA, Landherr LL, et al. EST database for early flower development in California poppy (Eschscholzia californica Cham., Papaveraceae) tags over 6000 genes from a basal eudicot. Plant Molecular Biology. 2006;62:351–369. doi: 10.1007/s11103-006-9025-y. [DOI] [PubMed] [Google Scholar]
- Chang H-L, Ren Y, Lu A-M. Floral morphogenesis of Anemone rivularis Buch.-Ham. ex DC. var. flore-minore Maxim. (Ranunculaceae) with special emphasis on androecium development sequence. Journal of Integrative Plant Biology. 2005;47:257–263. [Google Scholar]
- Chase MW, Soltis DE, Olmstead RG, Morgan D, Les DH, Mishler BD, et al. Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden. 1993;80:528–580. [Google Scholar]
- Chen L, Ren Y, Endress PK, Tian X-H, Zhang X-H. Floral organogenesis in Tetracentron sinense (Trochodendraceae) and its systematic significance. Plant Systematics and Evolution. 2007;264:183–193. [Google Scholar]
- Cronquist A. An integrated system of classification of flowering plants. New York, NY: Columbia University Press; 1981. [Google Scholar]
- Cui L, Wall PK, Leebens-Mack JH, Lindsay BG, Soltis DE, Doyle JJ, et al. Widespread genome duplications throughout the history of flowering plants. Genome Research. 2006;16:738–749. doi: 10.1101/gr.4825606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stilio VS, Kramer EM, Baum DA. Floral MADS box genes and homeotic gender dimorphism in Thalictrum dioicum (Ranunculaceae) – a new model for the study of dioecy. The Plant Journal. 2005;41:755–766. doi: 10.1111/j.1365-313X.2005.02336.x. [DOI] [PubMed] [Google Scholar]
- Doyle JA, Endress PK. Morphological phylogenetic analysis of basal angiosperms: comparison and combination with molecular data. International Journal of Plant Sciences. 2000;161(Suppl. 6):S121–S153. [Google Scholar]
- Drinnan AN, Crane PR, Hoot SB. Patterns of floral evolution in the early diversification of non-magnoliid dicotyledons (eudicots) Plant Systematics and Evolution, Supplement. 1994;8:93–122. [Google Scholar]
- Endress PK. Gesichtspunkte zur systematischen Stellung der Eupteleaceen (Magnoliales). Untersuchungen über Bau und Entwicklung der generativen Region bei Euptelea polyandra Sieb. et Zucc. Berichte der Schweizerischen Botanischen Gesellschaft. 1969;79:229–278. [Google Scholar]
- Endress PK. Floral structure, systematics and phylogeny of Trochodendrales. Annals of the Missouri Botanical Garden. 1986;73:297–324. [Google Scholar]
- Endress PK. Chaotic floral phyllotaxis and reduced perianth in Achlys (Berberidaceae) Botanica Acta. 1989;102:159–163. [Google Scholar]
- Endress PK. Patterns of floral construction in ontogeny and phylogeny. Biological Journal of the Linnean Society. 1990;39:153–175. [Google Scholar]
- Endress PK. Eupteleaceae. In: Kubitzki K, Rohwer JG, Bittrich V, editors. The families and genera of vascular plants. Vol. 2. Berlin: Springer; 1993. pp. 299–300. [Google Scholar]
- Endress PK. Floral structure and evolution in Ranunculanae. Plant Systematics and Evolution. Supplement. 1995;9:47–61. [Google Scholar]
- Endress PK. Morphology and angiosperm systematics in the molecular era. Botanical Review. 2002;68:545–570. [Google Scholar]
- Endress PK. Angiosperm floral evolution: morphological developmental framework. Advances in Botanical Research. 2006;44:1–61. [Google Scholar]
- Endress PK, Doyle JA. Floral phyllotaxis in basal angiosperms: development and evolution. Current Opinion in Plant Biology. 2007;10:52–57. doi: 10.1016/j.pbi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Endress PK, Hufford LD. The diversity of stamens structures and dehiscence patterns among Magnoliidae. Botanical Journal of the Linnean Society. 1989;100:45–85. [Google Scholar]
- Endress PK, Igersheim A. Gynoecium diversity and systematics of the basal eudicots. Botanical Journal of the Linnean Society. 1999;130:305–393. [Google Scholar]
- Erbar C, Kusma S, Leins P. Development and interpretation of nectary organs in Ranunculaceae. Flora. 1998;194:317–332. [Google Scholar]
- Feng M, Lu A-M. Floral organogenesis and its systematic significance of the genus Nandina (Berberidaceae) Acta Botanica Sinica. 1998;40:102–108. [Google Scholar]
- Feng M, Fu D-Z, Liang H-X, Lu A-M. Floral morphogenesis of Aquilegia L. (Ranunculaceae) Acta Botanica Sinica. 1995;37:791–794. [Google Scholar]
- Fu D-Z, Endress PK. Eupteleaceae. In: Wu Z, Raven PH, Hong D, editors. Flora of China. Vol. 6. Beijing: Science Press; 2001. p. 123. [Google Scholar]
- Gu T-Q, Ren Y. Floral morphogenesis of Coptis (Ranunculaceae) Chinese Bulletin of Botany. 2007;24:80–86. [Google Scholar]
- van Heel WA. A SEM-investigation on the development of free carpels. Blumea. 1981;27:499–522. [Google Scholar]
- van Heel WA. The ascidiform early development of free carpels, a SEM-investigation. Blumea. 1983;28:231–270. [Google Scholar]
- van Heel WA. Variation in the development of ascidiform carpels, an SEM-investigation. Blumea. 1984;29:443–452. [Google Scholar]
- Hiepko P. Vergleichend-morphologische und entwicklungsgeschichtliche Untersuchungen über das Perianth bei den Polycarpicae. Botanische Jahrbücher für Systematik. 1965;84:359–508. [Google Scholar]
- Hilu KW, Borsch T, Müller K, Soltis DE, Soltis PS, Savolainen V, et al. Angiosperm phylogeny based on matK sequence information. American Journal of Botany. 2003;90:1758–1776. doi: 10.3732/ajb.90.12.1758. [DOI] [PubMed] [Google Scholar]
- Hoot SB, Crane PR. Inter-familial relationships in the Ranunculidae based on molecular systematics. Plant Systematics and Evolution, Supplement. 1995;9:119–131. [Google Scholar]
- Hoot SB, Magallón S, Crane PR. Phylogeny of basal eudicots based on three molecular datasets: atpB, rbcL, and 18S nuclear ribosomal DNA sequences. Annals of the Missouri Botanical Garden. 1999;86:1–32. [Google Scholar]
- Howarth DG, Donoghue MJ. Phylogenetic analysis of the ‘ECE’ (CYC/TB1) clade reveals duplications predating the core eudicots. Proceedings of the National Academy of Sciences of the USA. 2006;103:9101–9106. doi: 10.1073/pnas.0602827103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufford LD, Endress PK. The diversity of anther structures and dehiscence patterns among Hamamelididae. Botanical Journal of the Linnean Society. 1989;99:301–346. [Google Scholar]
- Karrer A. Doctoral dissertation. ADAG, Zurich: University of Zurich; 1991. Blütenentwicklung und systematische Stellung der Papaveraceae und Capparaceae. [Google Scholar]
- Kim S, Soltis DE, Soltis PS, Zanis MJ, Suh Y. Phylogenetic relationships among early-diverging eudicots based on four genes: were the eudicots ancestrally woody? Molecular Phylogenetics and Evolution. 2004;31:16–30. doi: 10.1016/j.ympev.2003.07.017. [DOI] [PubMed] [Google Scholar]
- Kölsch A, Gleissberg S. Diversification of CYCLOIDEA-like TCP genes in the basal eudicot families Fumariaceae and Papaveraceae s.str. Plant Biology. 2006;8:680–687. doi: 10.1055/s-2006-924286. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Irish VF. Evolution of genetic mechanisms controlling petal development. Nature. 1999;399:144–148. doi: 10.1038/20172. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Zimmer EA. Gene duplication and floral developmental genetics of basal eudicots. Advances in Botanical Research. 2006;44:353–384. [Google Scholar]
- Kramer EM, Di Stilio VS, Schluter P. Complex patterns of gene duplication in the APETALA3 and PISTILLATA lineages of the Ranunculaceae. International Journal of Plant Sciences. 2003;164:1–11. [Google Scholar]
- Kramer EM, Su H-J, Wu C-C, Hu J-M. A simplified explanation for the frameshift mutation that created a novel C-terminal motif in the APETALA3 gene lineage. BMC Evolutionary Biology. 2006;6:30. doi: 10.1186/1471-2148-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-Y, Baum SF, Oh S-H, Jiang C-Z, Chen J-C, Bowman JL. Recruitment of CRABS CLAW to promote nectary development within the eudicot clade. Development. 2005;132:5021–5032. doi: 10.1242/dev.02067. [DOI] [PubMed] [Google Scholar]
- Lehmann NL, Sattler R. Floral development and homeosis in Actaea rubra (Ranunculaceae) International Journal of Plant Sciences. 1994;155:658–671. [Google Scholar]
- Leinfellner W. Über die Karpelle verschiedener Magnoliales. VII. Euptelea (Eupteleaceae) Österreichische Botanische Zeitschrift. 1969;116:159–166. [Google Scholar]
- Magallón S, Crane PR, Herendeen PS. Phylogenetic pattern, diversity, and diversification of eudicots. Annals of the Missouri Botanical Garden. 1999;86:297–372. [Google Scholar]
- Nandi OI, Chase MW, Endress PK. A combined cladistic analysis of angiosperms using rbcL and non-molecular data sets. Annals of the Missouri Botanical Garden. 1998;85:137–212. [Google Scholar]
- Nast CG, Bailey IW. Morphology of Euptelea and comparison with Trochodendron. Journal of the Arnold Arboretum. 1946;27:186–192. [Google Scholar]
- Praglowski J. The pollen morphology of the Trochodendraceae, Tetracentraceae, Cercidiphyllaceae, and Eupteleaceae, with reference to taxonomy. Pollen et Spores. 1975;16:449–467. [Google Scholar]
- Qiu Y-L, Chase MW, Les DH, Parks CR. Molecular phylogenetics of the Magnoliidae: cladistic analyses of nucleotide sequences of the plastid gene rbcL. Annals of the Missouri Botanical Garden. 1993;80:587–606. [Google Scholar]
- Qiu Y-L, Lee J, Bernasconi-Quadroni F, Soltis DE, Soltis PS, Zanis MJ, et al. The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature. 1999;402:404–407. doi: 10.1038/46536. [DOI] [PubMed] [Google Scholar]
- Qiu Y-L, Dombrowska O, Lee J, Li L, Whitlock BA, Bernasconi-Quadroni F, et al. Phylogenetic analyses of basal angiosperms based on nine plastid, mitochondrial, and nuclear genes. International Journal of Plant Sciences. 2005;166:815–842. [Google Scholar]
- Ren Y, Li Z-J, Chang H-L, Lei Y-J, Lu A-M. Floral development of Kingdonia (Ranunculaceae s.l., Ranunculales) Plant Systematics and Evolution. 2004;247:145–153. [Google Scholar]
- Ronse De Craene LP, Soltis PS, Soltis DE. Evolution of floral structures in basal angiosperms. International Journal of Plant Sciences. 2003;164(Suppl. 5):S329–S363. [Google Scholar]
- Schöffel K. Untersuchungen über den Blütenbau der Ranunculaceen. Flora. 1932;17:315–371. [Google Scholar]
- Shan H, Su K, Lu W, Kong H, Chen Z, Meng Z. Conservation and divergence of candidate class B genes in Akebia trifoliata (Lardizabalaceae) Development, Genes and Evolution. 2006;216:785–795. doi: 10.1007/s00427-006-0107-2. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS, Nickrent DL, Johnson LA, Hahn WJ, Hoot SB, et al. Angiosperm phylogeny inferred from 18S ribosomal DNA sequences. Annals of the Missouri Botanical Garden. 1997;84:1–49. [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis M, et al. Angiosperm phylogeny inferred from 18S rDNA, rbcL and atpB sequences. Botanical Journal of the Linnean Society. 2000;133:381–461. [Google Scholar]
- Soltis DE, Senters A, Zanis M, Kim S, Thompson JD, Soltis PS, et al. Gunnerales are sister to other core eudicots: implications for the evolution of pentamery. American Journal of Botany. 2003;90:461–470. doi: 10.3732/ajb.90.3.461. [DOI] [PubMed] [Google Scholar]
- Song P, Tian X-H, Ren Y. Floral morphogenesis of Caltha and Trollius (Ranunculaceae) and the systematic significance. Acta Phytotaxonomica Sinica. 2007 (in press) [Google Scholar]
- Takhtajan AL. Outline of the classification of flowering plants (Magnoliophyta) Botanical Review. 1980;46:263–350. [Google Scholar]
- Takhtajan AL. Diversity and classification of flowering plants. New York, NY: Columbia University Press; 1997. [Google Scholar]
- Thorne RF. An updated phylogenetic classification of the flowering plants. Aliso. 1992;13:365–389. [Google Scholar]
- Tian X-H, Zhang L, Ren Y. Morphogenesis of flowers and inflorescences of Circaeaster (Circaeasteraceae, Ranunculales) Plant Systematics and Evolution. 2006;256:89–96. [Google Scholar]
- Tucker SC, Hodges SC. Floral ontogeny of Aquilegia, Semiaquilegia, and Enemion (Ranunculaceae) International Journal of Plant Sciences. 2005;166:557–574. [Google Scholar]
- Weber A. Struktur, Antheseverlauf und Bestäubung der Blüte von Nigella arvensis. Verhandlungen der Zoologisch-Botanischen Gesellschaft in Österreich. 1993;130:99–125. [Google Scholar]
- Wolfe JA. Leaf-architectural analysis of the Hamamelidae. In: Crane PR, Blackmore S, editors. Evolution, systematics, and fossil history of the Hamamelidae 1. Introduction and ‘lower’ Hamamelidae. Vol. 1. Oxford: Clarendon Press; 1989. pp. 75–104. [Google Scholar]
- Worberg A, Quandt D, Barniske A-M, Löhne C, Hilu KW, Borsch T. Phylogeny of basal eudicots: insights from non-coding and rapidly evolving DNA. Organisms, Diversity and Evolution. 2007;7:55–77. [Google Scholar]
- Zahn LM, Leebens-Mack J, Arringtonas JM, Hu Y, Landherr L, dePamphilis CW, et al. Conservation and divergence in the AGAMOUS subfamily of MADS-box genes: evidence of independent sub- and neofunctionalization events. Evolution and Development. 2006;8:30–45. doi: 10.1111/j.1525-142X.2006.05073.x. [DOI] [PubMed] [Google Scholar]
- Zanis MJ, Soltis PS, Qiu Y-L, Zimmer E, Soltis DE. Phylogenetic analyses and perianth evolution in basal angiosperms. Annals of the Missouri Botanical Garden. 2003;90:129–150. [Google Scholar]
- Zhang X-H, Ren Y, Tian X-H. Floral morphogenesis of the genus Sinofranchetia (Lardizabalaceae) and its systematic significance. Botanical Journal of the Linnean Society. 2007 (in press) [Google Scholar]



