Abstract
Background and Aims:
Ambrosia artemisiifolia is a ruderal weed introduced from North America to Europe. It produces large amount of achenes which are highly heterogeneous in size. Due to the preponderant role of propagules in invasive plant processes, the achene mass variability related to germination, dispersal strategy and life history traits of offspring were investigated within this species.
Methods:
The variability in achene mass was quantified among six populations sampled in different habitats. The effects of achene mass variation on germination were studied. The percentages of floating and non-floating achenes were evaluated in the studied populations. The consequences of floatability on the growth and traits of the offspring were studied.
Key Results:
Mean achene mass ranged from 1·72 to 3·60 mg, depending on the populations, and was highly variable. Variation among achenes within plants accounted for 63·9 % of the variance, whereas variances among plants within each population (22·2 %) and among populations (13·9 %) were lower. Achene masses were also positively correlated to the total germination percentage for four populations out of six. Two kinds of achenes were distinguished: floating and non-floating. The majority of floating achenes (90 %) sank 24 h after water immersion. Whatever the population, floating achenes were lighter, more dormant and germinated faster than non-floating achenes. Plants which issued from floating achenes had better growth than those from non-floating achenes.
Conclusions:
The capacity of A. artemisiifolia to be invasive in Europe appears to be high, possibly due to its huge plasticity in seed mass which may help it to cope with a wide range of conditions and to establish in disturbed habitats. Furthermore, the recent invasion of southern France by A. artemisiifolia could be partially explained by water dispersal of achenes through rivers and has pinpointed its colonization potential along French rivers.
Key words: Asteraceae, Ambrosia artemisiifolia, seed mass variation, achene, germination, growth, heteromorphism, hydrochory, invasive plant
INTRODUCTION
Seed size is an important trait for plant population dynamics, and an abundant literature has documented the evolutionary significance of interspecific seed mass variation (Stevens, 1957; Harper, 1977; Michaels et al., 1988; Guo et al., 2000). Harper (1977) suggested that variation in seed size is limited within most plant species. However, for the past 30 years, many studies have shown that seed mass can vary widely among populations or growing conditions, among plants within a population, within plants and even among seeds within a fruit (Harper et al., 1970; Pitelka et al., 1983; Thompson, 1984; Michaels et al., 1988; Obeso, 1993; Susko and Lovett-Doust, 2000). It is now well known that individual plants often produce seeds of quite variable size in such a way that the intra-individual variation can be higher than within, or among, populations (Venable, 1985; Michaels et al., 1988; Obeso, 1993).
Several hypotheses have been formulated to explain intraspecific variation in seed mass. Such variation may be due to trade-offs in resource allocation between seed mass and seed number (Venable, 1992), but other parameters such as plant size and population density have an effect on seed mass variation (Roach and Wulff, 1987; Matthies, 1990). The ovule position in the inflorescence on plant axes can also affect seed mass, and the pattern of flowering phenology may also contribute to individual variation (Harper et al., 1970; Obeso, 1993). Seed size may also vary in response to environmental heterogeneity (Janzen, 1977). Whereas phenotypic variation in seed size often results from environmental constraints (i.e. nutrients, temperature and humidity), selection can directly affect variation to promote seed size polymorphism. Such disruptive selection may enhance the ability to survive in a wider range of environmental conditions (Fenner and Thompson, 2005). The production of a ‘range’ of seed sizes is an effective evolutionary strategy that can minimize risk and increase the probabilities of reproducing in an unpredictable environment (Venable and Brown, 1988; Haig, 1996). This particularly concerns annual ruderal plant species that colonize disturbed habitats (Harper, 1977). Seed mass variation is directly connected to germination kinetics, dormancy, pattern of dispersal and rate of predation. However, it also affects seedling vigour and survival, and can even have consequences for the adult plant (Harper et al., 1970; Baskin and Baskin, 1998).
A particular case of seed polymorphism is the seed heteromorphism which is defined as the production by single individuals of seeds (or sometimes single-seeded fruit) of different forms or different behaviours (Venable, 1985). Seed heteromorphism is common in angiosperms (Imbert, 2002) and, from an evolutionary point of view, this heteromorphism is thought to represent a form of ‘bet-hedging’ in the face of temporal variation in environmental suitability (Harper, 1977; Silvertown, 1984). Some authors suggest that seed heteromorphism occurs because environmental differences are extreme and because intermediate adaptations have low fitness (Harper, 1977). However, seed heteromorphism can be cryptic. In such a case, a variable seed behaviour is not accompanied by a discriminant morphological variation (Imbert, 2002). Also supposed to be common in angiosperms, cryptic heteromorphism is probably underestimated (Venable, 1985) due to the difficulties in testing it (Silvertown, 1984).
Seed mass variation and seed heteromorphism could be a major feature explaining invasive plant success, as they can enhance colonization at both local and regional scales, but also facilitate the exploitation of spatial and temporal heterogeneous environments (Mandak and Pysek, 2001; Willis and Hulme, 2004). Furthermore, among seed dispersal mechanisms, hydrochory is frequently blamed for the very rapid spread of some invasive plants colonizing riparian habitats (Thébaud and Debussche, 1991; Pysek and Prach, 1993).
Ambrosia artemisiifolia (common ragweed) is an annual plant (Asteraceae), introduced from North America to Europe more than a century ago (Heckel, 1906). This invasive plant is currently widespread in numerous European countries (Clot et al., 2002; Török et al., 2003), including France (Chauvel et al., 2006). The species is both a weed colonizing spring crops and a ruderal plant developing in open disturbed habitats such as wastelands, roadsides or riverbanks (Basset and Crompton, 1975). The achenes of A. artemisiifolia (i.e. a hard coat involucre protecting a soft seed containing a unique embryo) have a central terminal beak surrounded by a ring of tiny spines (Basset and Crompton, 1975). The species can produce about 300–6000 achenes on average, with a maximum of 14 000 for the largest plants (Basset and Crompton, 1975).
The achenes of A. artemisiifolia possess no obvious morphological dispersal mechanism and are mostly dispersed by human activities through soil or seed transport (Basset and Crompton, 1975). However, some authors also reported the possibility of achenes being dispersed by birds or by flowing water, as a proportion of A. artemisiifolia achenes are able to float (Payne, 1962; Gebben, 1965). The occurrence of populations on newly formed sand and gravel bars clearly suggests that hydrochory is a dispersal mechanism of the species, although poorly documented. In France, hydrochory could explain the past and present spread of the species along the Loire and Rhône rivers (Heckel, 1906; Corillion, 1964) and its progression in new areas along the Dordogne river in the South of France (Felzines, 2004).
The aim of this study was (a) to quantify the degree of achene mass variation of A. artemisiifolia, among populations, among plants within populations and within a single plant; (b) to quantify the variability of related germination and dormancy of A. artemisiifolia growing in contrasting environments; (c) to quantify the floating ability of achenes of different A. artemisiifolia populations; and (d) to determine its consequences on seed dormancy, germination kinetics and seedling growth. This approach, based on life history traits, will potentially determine factors involved in the successful spread of A. artemisiifolia.
MATERIALS AND METHODS
Achene materials
Achenes of A. artemisiifolia L. were collected from 30 individual mother plants from six French populations at maturity in autumn 2003 (Table 1). Populations located from the centre to the periphery of invasive A. artemisiifolia areas in France were sampled in four different habitat types (Table 1). Achenes without an embryo were removed from the experiment by testing by hand for their resistance to pressure.
Table 1.
Location and habitat of natural populations of Ambrosia artemisiifolia sampled for seed material in France
| Populations | Location | Longitude (E) | Latitude (N) | Invasive location | Habitat |
|---|---|---|---|---|---|
| Co | Concoeur-Corboin | 4°57′44″ | 47°11′07″ | North | Field-crop |
| Lt | Labergement | 5°14′27″ | 47°14′34″ | North | Wasteland |
| Lu | Lux | 5°12′40″ | 47°27′22″ | North | Gravel pit |
| Ch | Chaponnay | 4°56′20″ | 45°37′41″ | Centre | Field-crop |
| Ra | Alex | 4°56′39″ | 44°44′26′ | South | Riverbank |
| Sp | St-Pierre-de-Chandieu | 5°00′52″ | 45°38′43″ | Centre | Field-crop |
Invasive location indicates the location of populations according to the invasive distribution area of A. artemisiifolia.
Achene characterization and life history traits of offspring
The variability of A. artemisiifolia achene mass was quantified among populations, among plants within populations and within plants. A set of 30 achenes per plant was randomly selected and individually weighed for 15 plants per population (n = 2699).
For all the plants (n = 30) collected per population, 100 achenes were randomly selected and were put in a 100 mL glass jar which was two-thirds full of tap water at room temperature. The water was agitated vigorously for 1 min with a magnetic agitator that forced the achenes to sink. The remaining floating achenes (F) were separated from sinking achenes, i.e. non-floating achenes (NF). The two sets of F and NF achenes were dried (35 °C with ventilation for 6 h) and weighed.
The sets of achenes were put on germination paper in Petri dishes and stratified for 3 weeks (wet, dark stratification at 4 °C) in order to break the primary dormancy. After this treatment, achenes were placed in optimum germination conditions (24 °C/11 h day and 15 °C/13 h night) and germinated achenes were checked daily for 2 weeks. Achenes that did not germinate after 2 weeks were crushed to check whether they had a living white embryo, and dormant achene proportions were deducted from non-germinated achenes.
One seedling (5 d old, cotyledon stage) from both the floating and non-floating sets was transplanted into individual small pots. Plants were grown in pots for 1 week in a greenhouse (25 °C, natural light) and were transplanted at the two-leaf stage in a garden experiment in Dijon in April 2005. Plants were spaced 0·5 m apart to overcome intraspecific competition. During the experiment, the soil was freed of other plants and A. artemisiifolia plants were watered daily. After 64 d of growth, the flowering A. artemisiifolia plants were harvested and their final individual height and shoot dry mass were recorded. Dry mass was determined after drying the plants at 80 °C for 48 h.
Floating ability of achenes
In order to evaluate the floating ability of A. artemisiifolia achenes, a sub-sample of 50 F achene sets per population was studied. As previously, the sets of achenes was put separately into agitated water, and the number of achenes which sank was recorded each hour (for the first 10 h), and then every 5 h until all achenes had sunk. The effect of achene density on floating ability was also studied using ten F and ten NF achenes per population. All achenes were weighed and their width (W) and length (L) measured. Achenes were then separately tested as before for their floating ability by putting them in water and measuring the time (in hours) it took them to sink.
Data analysis
The mean achene mass among populations and among plants was compared using a nested analysis of variance (ANOVA), with all effects treated as random to partition the variance among sources. The proportion of the total variance of achene mass that contributed to the variations among populations, among plants within populations and within plants (residual) was then calculated (Sokal and Rohlf, 1981). The amount of seed mass variation among populations was compared using the coefficient of variation (CV). The total germination percentage per plant and the rate of germination, i.e. time (in days) required for 50 % of achenes to germinate, were evaluated on sets of 100 achenes (including a mixture of F and NF achene types) and were compared among populations using one-way ANOVA, with populations as a fixed effect. Following the ANOVA, a multiple pairwise comparison with the Bonferroni method was used to test for pairwise population differences.
The correlations between total germination percentage and mean achene mass per plant (calculated on sets of 100 achenes) were calculated for each population using Pearson's correlation. A t-test analysis was used in order to ensure that the mean achene masses calculated for 100 achene sets in 30 plants per population were not different from those previously calculated with individual seeds in 15 plants.
The mean proportions of F and NF achenes and the mean number of heteromorphic plants (plants producing both F and NF achenes) among populations were compared using one-way ANOVA with populations as a fixed effect. Multiple pairwise comparisons between populations were carried out using the Bonferroni procedure. The achene mass, the total percentage germination, the rate of germination and the final height and shoot dry mass of offspring were compared among populations and achene types (F and NF), using two-way ANOVAs. The achene mass distribution of F and NF achene types within each population was compared using Kolmogorov–Smirnov two-sample tests.
The growth of A. artemisiifolia plants was analysed through the relative growth rate (RGR) in order to compare the relative performance of plants growing from the two achene types (F and NF) and from different populations. The RGR was calculated as:
where M is the plant dry mass (g) at final flowering (t2) and the achene mass before germination (t1) (in days).
The final height and the shoot dry mass of plants growing from both achene types were compared using two-way ANOVAs with populations and achene types (F and NF) as fixed effects.
The rate of achene sinking, i.e. the time required for 50 % of the achenes to sink (S50), was calculated to compare the floating ability of achenes in each population. The density of achenes was calculated using the volume mass formula of an ellipsoid:
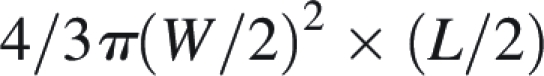 |
where W is the achene width and L the achene length. The achene density was compared among populations and achene types, using two-way ANOVA with all effects treated as fixed. The correlation between log (F achene density) and log (time of floatation) was calculated using Pearson's correlation.
All data were checked for normality and homogeneity of variances, and were log-transformed to correct deviations from these assumptions when necessary. Proportional data were arcsin-square root transformed before performing statistical analysis (Sokal and Rohlf, 1981). All statistical analyses were performed with SYSTAT 11® software for Windows (P < 0·05).
RESULTS
Variations in achene mass and germination
Mean (± s.e.) achene mass varied 2-fold among the six A. artemisiifolia populations, from 1·72 ± 0·04 to 3·60 ± 0·06 mg (Table 2). The achene masses were significantly different among populations and among plants within populations (Table 3). The nested ANOVA showed that 13·9 % of the total observed variation was due to variation among populations, 22·2 % was due to variation among plants within populations, and 63·9 % was due to variation within plants (Table 3). The mean achene mass of northern A. artemisiifolia populations [Concoeur-Corboin (Co), Labergement (Lt) and Lux (Lu)] was lower than those of the populations located in the centre or south [Chaponnay (Ch), Alex (Ra) and St-Pierre-de-Chandieu (Sp)]. The magnitude of achene mass variation within populations ranged from 16·5-fold for the Co population to 49-fold for the Lt population, and from 1·8-fold to 27-fold within individual plants. Among all the plants, the lightest achene weighed 0·2 mg whereas the heaviest weighed 9·8 mg. The amount of variation of achene mass among populations, expressed by the CV, ranged from 40·33 to 88·8 % (Table 2). CVs were 2-fold higher for northern populations than for central or southern ones. Finally, variations within populations ranged from 12·7 to 98·0 %.
Table 2.
Mean (± s.e.) achene mass per Ambrosia artemisiifolia population studied, with variations within populations
| Populations | Seed number | Achene mass (mg) | Range of achene mass (mg) | Magnitude of mass variation (heaviest ÷ lightest) | CV (%) | Range of CV (%) |
|---|---|---|---|---|---|---|
| Co | 450 | 1·72 (0·04) | 0·4–6·6 | 16·5 | 88·8 | 22·3–98·0 |
| Lu | 450 | 3·08 (0·08) | 0·2–7·6 | 38·0 | 75·77 | 22·6–68·5 |
| Lt | 450 | 3·07 (0·08) | 0·2–9·8 | 49·0 | 73·36 | 14·9–64·6 |
| Ch | 450 | 3·48 (0·07) | 0·3–8·0 | 26·7 | 43·77 | 22·5–50·4 |
| Ra | 450 | 3·60 (0·06) | 0·4–7·2 | 18·0 | 40·23 | 12·7–53·4 |
| Sp | 449 | 3·28 (0·08) | 0·4–7·7 | 19·3 | 45·16 | 14·1–77·0 |
The individual weight of 30 achenes for 15 plants within each population was used. CV, coefficient of variation; range of CV, minimum – maximum individual CV within a population.
Table 3.
Nested ANOVA of Ambrosia artemisiifolia achene mass analysed on log-transformed data
| Source of variation | d.f. | MS | F | P | Variance (%) |
|---|---|---|---|---|---|
| Populations | 5 | 5·964 | 108·95 | 0·001 | 13·9 |
| Plant (population) | 84 | 0·625 | 11·41 | 0·002 | 22·2 |
| Within plants | 2609 | 0·055 | 63·9 |
The proportion of the total variation that was due to the variation among populations, among plants within a population and within plants (error) is indicated.
The rate of germination (F = 14·90, P < 0·001) and total germination percentage (F = 34·89, P < 0·001) differed significantly among populations (Table 4). Patterns of variation among populations differed according to the germination parameter analysed. The mean number of days to reach 50 % germination (rate of germination) ranged from 3·6 ± 0·3 to 11·8 ± 0·7 d and the mean total germination percentage ranged from 15·6 ± 1·8 to 77·3 ± 2·0 % according to the population (Table 4).
Table 4.
Mean (± s.e.) rate of germination (number of days for 50 % germination) and total germination percentage of the six different Ambrosia artemisiifolia populations studied (30 plants each)
| Populations | Days to 50 % germination | Total germination (%) | r | P |
|---|---|---|---|---|
| Co | 11·8 (0·7)a | 15·6 (1·8)c | 0·56 | 0·001 |
| Lu | 6·9 (0·5)b | 56·2 (4·4)b | 0·61 | <0·001 |
| Lt | 3·6 (0·3)c | 64·3 (4·1)ab | 0·28 | 0·137 |
| Ch | 9·6 (0·6)a | 62·5 (2·9)b | 0·63 | <0·001 |
| Ra | 7·1 (0·6)b | 77·3 (2·0)a | 0·45 | 0·014 |
| Sp | 10·6 (0·8)a | 71·1 (2·3)ab | −0·15 | 0·419 |
Germination was calculated on sets of 100 achenes per plant. The results of Bonferroni's multiple comparison test (within columns) are indicated by different superscript letters where values differ significantly at P < 0·05. Pearson's correlations (r) were calculated between total germination and mean achene mass within each population.
A significant positive correlation between germination percentages and mean mass of achenes per plants (measured on a set of 100 achenes per plant) was found for the populations Co, Lu, Ch and Ra, but no correlation was detected for the two populations Lt and Sp (Table 4).
Achene types, germination and seedlings
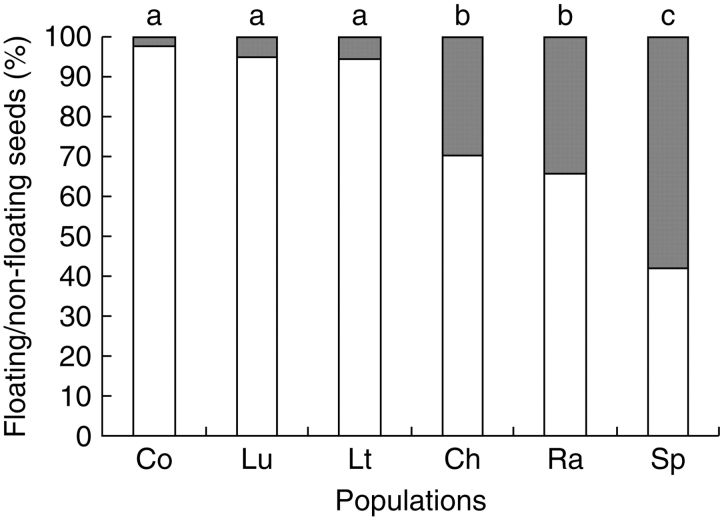
The proportion of F achenes was significantly different (Fig. 1) among populations (F = 59·40, P < 0·001). Two groups were distinguished, the first one composed of the three northern populations (Co, Lu and Lt) with 95–97 % of F achenes and the second one composed of the three central and southern populations (Ch, Ra and Sp) with 42–70 % of F achenes.
Fig. 1.
Mean proportion of floating (open bars) and non-floating (filled bars) achenes per Ambrosia artemisiifolia population. The proportions of floating and non-floating achenes were calculated on sets of 100 achenes per plant. The results of Bonferroni's multiple comparison test are indicated by different letters where values differ significantly at P < 0·05.
The populations were also significantly different (F = 17·53, P < 0·001), depending on the proportion of heteromorphic individuals (proportion of individuals with both F and NF achenes). The populations Co, Lu and Lt possessed 40, 40 and 67 %, respectively, of heteromorphic individuals, whereas the populations Ch, Ra and Sp, from the second group, possessed 100 % of heteromorphic individuals.
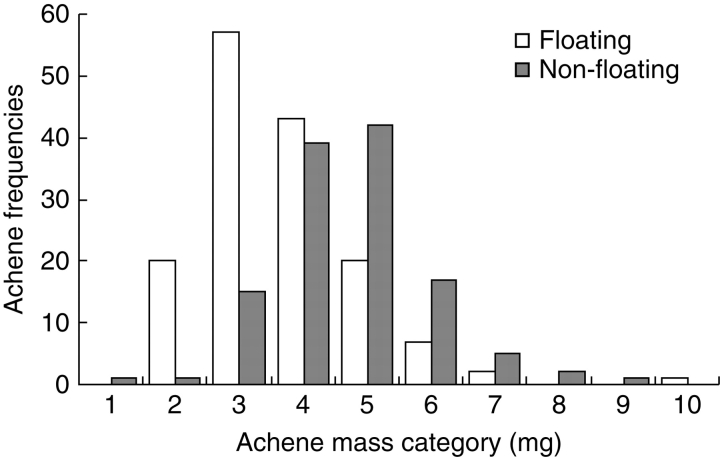
The distribution of achene mass between F and NF achenes across all populations pooled together is shown in Fig. 2. The distributions in each population of achene mass from F and NF types were significantly different (P < 0·05, Kolmogorov–Smirnov two-sample test) except for the population Lt (D = 0·317, P = 0·327). Achene mass and total germination percentages (Table 5) were significantly lower for F than for NF achenes (Table 6). Significant effects of populations and interaction (population × F/NF) were also detected.
Fig. 2.
Distribution of achene mass frequencies of floating and non-floating seeds within all pooled Ambrosia artemisiifolia populations tested. Data analyses were based on mean masses of 100 achenes (30 plants among six populations).
Table 5.
Mean (± s.e.) achene mass, number of days to reach 50 % germination, total percentage germination, plant dry mass and height of plants growing from the two achene types per population
| Populations | Achene type | Achene mass (mg) | Days to 50 % germination | Total germination (%) | Shoot dry mass (g) | Plant height (cm) |
|---|---|---|---|---|---|---|
| Co | F | 1·8 (0·1) | 11·6 (0·7) | 13·8 (1·6) | 35·6 (1·8) | 12·6 (1·4) |
| NF | 4·5 (0·4) | 16·5 (2·2) | 70·3 (11·6) | 37·1 (3·4) | 11·2 (2·0) | |
| Lu | F | 2·8 (0·1) | 6·8 (0·5) | 54·9 (4·4) | 39·2 (1·7) | 20·3 (2·1) |
| NF | 4·3 (0·2) | 9·5 (1·0) | 89·9 (4·0) | 38·4 (2·1) | 16·5 (2·1) | |
| Lt | F | 3·1 (0·2) | 3·6 (0·3) | 63·9 (4·1) | 46·3 (2·1) | 17·6 (1·4) |
| NF | 3·6 (0·3) | 5·5 (1·7) | 81·5 (8·7) | 44·7 (3·0) | 12·6 (2·4) | |
| Ch | F | 3·4 (0·3) | 9·2 (0·6) | 58·5 (3·2) | 40·2 (2·1) | 16·1 (1·3) |
| NF | 4·4 (0·2) | 11·4 (0·9) | 77·6 (3·0) | 31·4 (2·0) | 14·2 (1·1) | |
| Ra | F | 3·2 (0·2) | 6·9 (0·5) | 71·4 (2·7) | 39·2 (2·1) | 15·5 (1·6) |
| NF | 4·1 (0·2) | 8·0 (0·7) | 87·8 (2·5) | 34·9 (1·3) | 12·8 (1·0) | |
| Sp | F | 3·2 (0·3) | 9·1 (0·8) | 68·9 (2·6) | 42·0 (2·0) | 24·4 (2·7) |
| NF | 4·2 (0·3) | 11·4 (0·9) | 73·9 (2·9) | 40·0 (1·5) | 24·3 (2·1) |
F, floating; NF, non-floating.
Table 6.
Two-way ANOVA of achene mass and germination characteristics of Ambrosia artemisiifolia according to the population and achene types
| Achene mass |
Days to 50 % germination |
Total germination |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source of variation | d.f. | MS | F | P | MS | F | P | MS | F | P |
| Population | 5 | 0·066 | 3·462 | 0·005 | 344·139 | 21·365 | <0·001 | 0·931 | 13·983 | <0·001 |
| F/NF | 1 | 2·166 | 113·066 | <0·001 | 410·605 | 25·492 | <0·001 | 8·534 | 128·138 | <0·001 |
| Population × F/NF | 5 | 0·127 | 6·640 | <0·001 | 13·743 | 0·853 | 0·513 | 0·613 | 9·197 | <0·001 |
| Error | 302 | 0·019 | 16·107 | 0·067 | ||||||
F, floating; NF, non-floating.
Within populations, the F achenes germinated significantly faster than NF achenes, as shown by the percentage germination (Tables 5 and 6). A significant effect of populations was also detected, whereas no interaction (population × F/NF) was found. The total germination percentage (Table 5) was significantly lower for F than for NF achene types (Table 6). As previously, significant differences among populations, but also significant interaction (population × F/NF), were observed.
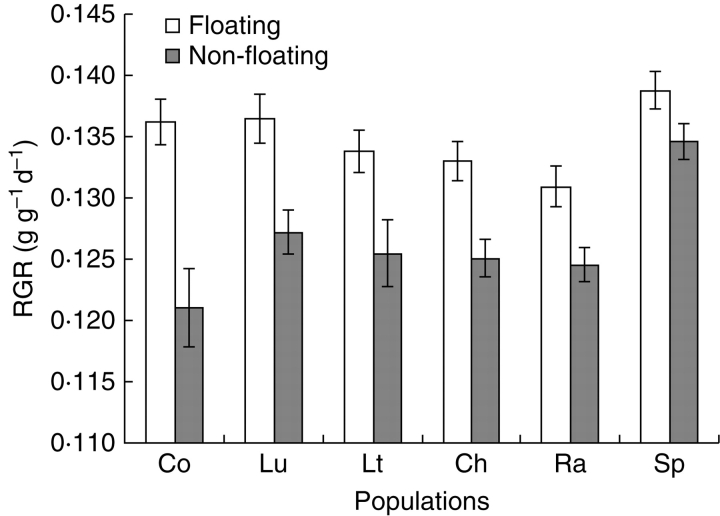
The values of final shoot dry mass and height of A. artemisiifolia plants growing from F achenes were significantly higher than for plants from NF achenes (Tables 5 and 6). A significant effect of populations was found, but no (population × F/NF) interaction was detected. Moreover, the RGR of A. artemisiifolia plants (Fig. 3) growing from F achenes was also significantly higher (Table 7) than that of those from NF achenes. A significant difference in RGR was also detected among populations, whereas no significant (population × F/NF) interaction was found.
Fig. 3.
Mean (± s.e.) of relative growth rate (RGR) of Ambrosia artemisiifolia plants per population for plants growing from floating and non-floating achenes.
Table 7.
Two-way ANOVA of shoot dry mass, height and relative growth rate (RGR) of Ambrosia artemisiifolia plants growing from different populations and achene types
| Source of variation | Shoot dry mass |
Plant height |
RGR |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | MS | F | P | MS | F | P | MS | F | P | |
| Population | 5 | 948·402 | 11·164 | <0·001 | 529·867 | 5·086 | <0·001 | 0·001 | 7·568 | <0·001 |
| F/NF | 1 | 390·886 | 4·601 | 0·033 | 454·093 | 4·359 | 0·038 | 0·005 | 57·667 | <0·001 |
| Population × F/NF | 5 | 33·280 | 0·392 | 0·854 | 147·336 | 1·414 | 0·219 | <0·001 | 1·475 | 0·198 |
| Error | 293 | 84·955 | 104·180 | <0·001 | ||||||
F, floating; NF, non-floating.
Floating ability
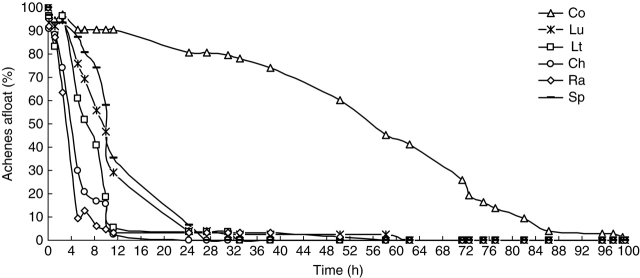
The floating capacity of A. artemisiifolia achenes over time was different among populations (Fig. 4). For the majority of the populations (except for Co), 50 % of achenes sank between 3·5 and 10·5 h after being put into water (S50: Ra = 3·5 h, Sp = 10·5 h, Lt = 7·2 h, Lu = 9·5 h, Ch = 4·2 h) and > 90 % by around 24 h. For the population Co, the S50 was 56·2 h, and 90 % of the achenes sank by 80 h after being put in water. The maximal time for 100 % achenes to sink was 60 h for all the populations, except for the population Co (100 h). The A. artemisiifolia population growing in the river habitat (Ra) was the quickest to sink.
Fig. 4.
Buoyancy pattern of A. artemisiifolia seeds over time according to different populations in the laboratory experiment.
The mean density of F achenes (0·86) was significantly lower (F = 15·753, P < 0·001) than that of NF achenes (0·94). A significant effect of population (F = 8·994, P < 0·001) and interaction (population × F/NF) (F = 15·033, P < 0·001) was also detected. A negative significant correlation was found (r = –0·74, P < 0·001) between the log (F achene density) and the log (hours of floatation), indicating that the highly dense achenes sank faster than the others (data not shown).
DISCUSSION
The mean achene mass in A. artemisiifolia species found in the present study was relatively high compared with other weeds and/or ruderal plant species belonging to the Asteraceae family (Stevens, 1957; Mazer, 1989). The variability in individual seed masses of A. artemisiifolia grown in natural conditions was mostly detected within plants (63·9 % of total variance), while the variability among plants within populations (22·2 %) and then among populations (13·9 %) was lower. Similarly, Obeso (1993) found, for Asphodelus albus Mill., that the variation in seed mass was higher within plants (56·2 %) than among plants within populations (30·9 %) or among populations (12·9 %). In a study of 39 species, Michaels et al. (1988) found that the variation of seed mass within plants was the larger component of the total variance (62 % of the total variance on average) for 29 of the 39 species.
Although the individual achene mass of A. artemisiifolia varied 2-fold among populations, it varied from 16·5- to 49-fold within populations. Such a variation was also observed for another congeneric alien species, Ambrosia trifida (Washitani and Nishiyama, 1992); however, such considerable variation within populations is not common in plant species. For example, achene mass in Aster acuminatus varied 2·5-fold among populations and 2·3-fold within populations (Pitelka et al., 1983), and varied 5-fold among populations and 4-fold within populations in A. albus (Obeso, 1993). However, Thompson (1984) reported a 15·8-fold variation within Lomatium grayi populations grown under similar conditions. A very high achene mass intra-plant variation, from 1·8- to 27-fold, was also observed in this study. In general, within-plant variation in other species is lower, such as 2- to 3-fold for Alliaria petiolata (Susko and Lovett-Doust, 2000) or 2·6- to 8·1-fold for L. grayi (Thompson, 1984). The average coefficient of variation among the mean seed masses of all A. artemisiifolia plants (57·9 %) was higher than what is commonly observed in other species (Pitelka et al., 1983; Thompson, 1984; Michaels et al., 1988) or even in invasive plants (Willis and Hulme, 2004). An explanation could be that such variability may be favoured by selection in a variable environment (Pitelka et al., 1983; Thompson, 1984; Fenner and Thompson, 2005).
The high seed polymorphism observed within introduced populations of A. artemisiifolia could be connected to the high genetic diversity found within populations. In a recent study, Genton et al. (2005) demonstrated that in the introduced area, the genetic diversity of populations was higher than in the native plants, suggesting a link between diversity and invasibility. The increase of variability in neutral polymorphism could also be observed in polygenic traits such as achene traits and then could affect the invasibility potential of the species.
Even if most of the highest variability of seed mass was found first within plants and secondly within populations, a geographical variation in mean population seed mass was detected. The mean seed masses of populations from northern latitudes were lower than those found for central or southern latitudes. Some studies have previously highlighted clinal patterns of seed mass variation with respect to latitude (McWilliams et al., 1968) or species range distribution (Susko and Lovett-Doust, 2000).
As previously described for A. artemisiifolia (Washitani and Nishiyama, 1992), germination and dormancy were variable among and within populations. According to Baskin and Baskin (1998) and Fenner and Thompson (2005), the germination response to plant size depended on the species investigated and could increase, decrease or remain unaffected by differences in seed size. In the present study, a positive correlation between the mean achene mass per plant and the rate of germination/dormancy was detected. The northern populations exhibited a lower germination percentage but a higher proportion of dormant achenes, which could be interpreted as a strategy for temporal colonization in unpredictable and disturbed environments. According to Harper (1977), larger seeds can have a competitive advantage that may last until maturity and have effects on germination or dormancy, but, conversely, smaller seeds may be dispersed more efficiently.
Two types of achenes, differing in their floating ability, were observed in A. artemisiifolia. Payne (1962) previously indicated that the achenes of A. artemisiifolia were able to float. However, no particular achene morphology was associated with floating ability, although differences in achene type proportion were detected between northern and southern populations.
Staniforth and Cavers (1976) showed that achenes of Polygonum spp. were able to float for 3·5 d in turbulent conditions and, with the calculated flow rate, deduced that achene dispersal could be over considerable distances. As seen for Cakile edentula (Payne and Maun, 1981), achenes of A. artemisiifolia are well suited for long-distance dispersal because they have several essential characteristics, such as the ability to float, the inhibition of germination during floatation and the retention of germination after floatation. For all A. artemisiifolia populations, 50 % of F achenes were able to float from 3·5 to 10·5 h according to the population, but 90 % of such achenes sank within 24 h.
Among the angiosperms, the Asteraceae family is the major group sharing seed heteromorphism characters (Mandak, 1997; Imbert 2002). Whereas many species produce heteromorphic seeds with divergent morphology [e.g. Spergularia marina (Telenius and Torstensson, 1989) or the genus Crepis (Imbert, 2002)], some cases of cryptic seed heteromorphism have been reported, where ecological differentiation was observed in the absence of apparent morphological differences (Imbert, 2002). An example of such cryptic heteromorphism was provided by Burke (1995) for Geigeria alata (an Asteraceae species) which had two kinds of dispersal ability according to the position of the achenes on the plant, although no morphological characteristics differentiated the seeds. In the case of A. artemisiifolia, floating seeds could be regarded as cryptic heteromorphism. According to Fenner and Thompson (2005), water dispersal ability is often difficult to recognize from an examination of the diaspore alone. Although many species possess recognizable buoyancy aids, such as the winged seeds of S. marina (Telenius and Torstensson, 1989), others can float without any obvious morphological adaptations (Staniforth and Cavers, 1976). As in the present study, Mandak and Pysek (2001) observed three types of seeds in Atriplex sagittata, with two of them differing only in size and weight and showing different water dispersal ability.
The differences between F and NF achenes were also related to other life history traits of their offspring. In this study, the F achenes were lighter, less dense with a lower rate of germination and germination percentage, and higher dormancy percentage than NF achenes. According to Fenner and Thompson (2005), small seeds are more likely to be buried and are more dormant, which contributes to temporal escape through a more persistent seed bank. This germination trend was previously observed in dimorphic seeds of C. edulenta where smaller seed types germinate earlier than larger ones (Zhang, 1993). The advantage of fast germination is the ability to occupy available space and use limited resources in competitive environments (Ross and Harper, 1972). Easily dispersed seeds usually germinate better and are less dormant, although the probability of seedling survival is low. However, seeds with lower dispersal ability are often dormant but produce seedlings with increased probability of survival.
Much evidence showed that differences in seed morphs could result in different dispersal, dormancy and germination patterns (Zhang, 1993; Imbert, 1999). However, few studies have demonstrated that the divergent growth and the variation of life history traits resulted from different seed morphs (Zhang, 1993; Mandak and Pysek, 2005). In A. artemisiifolia, the plants developing from F achenes grew faster and had a significant higher shoot dry mass and plant height than those resulting from NF achenes. In their study, Grotkopp et al. (2002) demonstrated that these invasive plants had on average a higher RGR and lighter seeds in comparison with native species. A higher RGR can provide a competitive advantage in the case of annual weed species growing in disturbed habitats. However, as plant biomass is positively correlated with the number of seeds produced in A. artemisiifolia (Fumanal et al., 2005), the results of the present study suggest that the plants from F achenes may have a higher fitness than NF achenes. Such effects depend on achene types and may be related to initial germination stages and correlated with fast growth.
From all the results of the present study, such a pattern of cryptic heteromorphism is certainly not only a simple continuous achene polymorphism, but also a selected strategy. Functional differences among achene types are necessary for the existence of temporal or spatial variation in their relative success. According to Mandak and Pysek (2005), current explanations of seed heteromorphism are largely adaptationistic.
The high variation in achene characteristics (mass, germination and dormancy) combined with the observed cryptic heteromorphism could certainly favour its spread. As for the European invasive species Impatiens glandulifera (Willis and Hulme, 2004), the success of A. artemisiifolia in colonizing a heterogeneous environment could be partially explained by this important variability in achenes. In order to understand and explain the seed mass variation in this troublesome annual weed, future research should be directed towards the role of parental growing environment, achene position within plants and dispersal phenology. Hydrochory could play a major role in long-distance dispersal of A. artemisiifolia and could also explain local spread along roadsides owing to water run-off (Weed, 1910). Therefore, hydrochory of A. artemisiifolia achenes should be taken into account to prevent its spread in new areas all over France through the major rivers such as the Rhône, Loire and Dordogne, and their tributaries.
ACKNOWLEDGEMENTS
We thank Jean-Luc Demizieux for his helpful comments on the manuscript. This work was partly supported by the Regional Council of Rhône-Alpes and the Regional Council of Burgundy.
LITERATURE CITED
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Bassett IJ, Crompton CW. The biology of Canadian weeds: 11 – Ambrosia artemisiifolia L. and A. psilostachya DC. Canadian Journal of Plant Science. 1975;55:463–476. [Google Scholar]
- Burke A. Geigeria alata in the Namib desert: seed heteromorphism in an extremely arid environment. Journal of Vegetation Science. 1995;6:473–478. [Google Scholar]
- Chauvel B, Dessaint F, Cardinal-Legrand C, Bretagnolle F. The historical spread of Ambrosia artemisiifolia L. in France from herbarium records. Journal of Biogeography. 2006;33:665–673. [Google Scholar]
- Clot B, Schneiter D, Tercier P, Gehrig R, Annie G, Thibaudon M. Ambrosia pollen in Switzerland: local production or transport? Allergie et Immunologie. 2002;34:126–128. [PubMed] [Google Scholar]
- Corillion R. Ambrosia artemisiifolia L. (Composées, Ambrosianées) adventice en extension dans le Val de Loire. Bulletin de Mayenne-Sciences. 1964:47–49. [Google Scholar]
- Felzines JC. Introduction et naturalisation d'espèces dans les groupements végétaux aquatiques et alluviaux de la Dordogne quercynoise: situation actuelle et modifications au cours du XXème siècle. Le Monde des Plantes. 2004;484:21–24. [Google Scholar]
- Fenner M, Thompson K. The ecology of seeds. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Fumanal B, Chauvel B, Bretagnolle F. Demography of an allergenic European invasive plant: Ambrosia artemisiifolia. In: Alford DV, Backhaus GF, editors. Plant protection and plant health in Europe: introduction and spread of invasive species. Alton: British Crop Production Council; 2005. pp. 225–226. [Google Scholar]
- Gebben AI. The ecology of common ragweed Ambrosia artemisiifolia L.) in southeastern Michigan. USA: University of Michigan; 1965. PhD Thesis. [Google Scholar]
- Genton BJ, Shykoff JA, Giraud T. High genetic diversity in French invasive populations of common ragweed, Ambrosia artemisiifolia, as a result of multiple sources of introduction. Molecular Ecology. 2005;14:4275–4285. doi: 10.1111/j.1365-294X.2005.02750.x. [DOI] [PubMed] [Google Scholar]
- Grotkopp E, Rejmanek M, Rost TL. Toward a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. American Naturalist. 2002;159:396–419. doi: 10.1086/338995. [DOI] [PubMed] [Google Scholar]
- Guo QF, Brown JH, Valone TJ, Kachman SD. Constraints of seed size on plant distribution and abundance. Ecology. 2000;81:2149–2155. [Google Scholar]
- Haig D. The pea and the coconut: seed size in safe sites. Trends in Ecology and Evolution. 1996;11:1–2. doi: 10.1016/0169-5347(96)81052-4. [DOI] [PubMed] [Google Scholar]
- Harper JL. Population biology of plants. London: Academic Press; 1977. [Google Scholar]
- Harper JL, Lovell PH, Moore KG. The shapes and sizes of seeds. Annual Review of Ecology and Systematics. 1970;1:327–356. [Google Scholar]
- Heckel E. Sur l'Ambrosia artemisiifolia L. et sa naturalisation en France. Bulletin de la Société Botanique de France. 1906;53:600–620. [Google Scholar]
- Imbert E. The effects of achene dimorphism on the dispersal in time and space in Crepis sancta (Asteraceae) Canadian Journal of Botany. 1999;77:508–513. [Google Scholar]
- Imbert E. Ecological consequences and ontogeny of seed heteromorphism. Perspectives in Plant Ecology, Evolution and Systematics. 2002;5:13–36. [Google Scholar]
- Janzen DH. Variation in seed size within a crop of a Costa Rican Mucuna andreana (Leguminosae) American Journal of Botany. 1977;64:347–349. [Google Scholar]
- Mandak B. Seed heteromorphism and the life cycle of plants: a literature review. Preslia. 1997;69:129–159. [Google Scholar]
- Mandak B, Pysek P. Fruit dispersal and seed banks in Atriplex sagittata: the role of heterocarpy. Journal of Ecology. 2001;89:159–165. [Google Scholar]
- Mandak B, Pysek P. How does seed heteromorphism influence the life history stages of Atriplex sagittata (Chenopodiaceae)? Flora. 2005;200:516–526. [Google Scholar]
- Matthies D. Plasticity of reproductive components at different stages of development in the annual plant Thlaspi arvense. Oecologia. 1990;83:105–116. doi: 10.1007/BF00324641. [DOI] [PubMed] [Google Scholar]
- Mazer SJ. Ecological, taxonomic and life history correlates of seed mass among Indiana dune angiosperms. Ecological Monographs. 1989;59:153–175.. [Google Scholar]
- McWilliams EL, Landers RQ, Mahlstede JP. Variation in seed weight and germination in populations of Amaranthus retroflexus L. Ecology. 1968;49:290–296.. [Google Scholar]
- Michaels HJ, Benner B, Hartgerink AP, Lee TD, Rice S. Seed size variation: magnitude, distribution, and ecological correlates. Evolutionary Ecology. 1988;2:157–166. [Google Scholar]
- Obeso JR. Seed mass variation in the perennial herb Asphodelus albus: sources of variation and position effect. Oecologia. 1993;93:571–575. doi: 10.1007/BF00328967. [DOI] [PubMed] [Google Scholar]
- Payne AM, Maun MA. Dispersal and floating ability of dimorphic fruit segments of Cakile edentula var. lacustris. Canadian Journal of Botany. 1981;59:2595–2602. [Google Scholar]
- Payne WW. Biosystematic studies of four widespread weeding species of ragweeds (Ambrosia: Compositae) USA: University of Michigan; 1962. PhD thesis. [Google Scholar]
- Pitelka LF, Thayer ME, Hansen SB. Variation in achene weight in Aster acuminatus. Canadian Journal of Botany. 1983;61:1415–1420. [Google Scholar]
- Pysek P, Prach K. Plant invasions and the role of riparian habitats: a comparison of four species alien to central Europe. Journal of Biogeography. 1993;20:413–420. [Google Scholar]
- Roach DA, Wulff RD. Maternal effects in plants. Annual Review of Ecology and Systematics. 1987;18:209–235. [Google Scholar]
- Ross MA, Harper JL. Occupation of biological space during seedling establishment. Journal of Ecology. 1972;60:77–88. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 2nd edn. New York: W. H. Freeman and Co; 1981. [Google Scholar]
- Silvertown JW. Phenotypic variety in seed germination behavior the ontogeny and evolution of somatic polymorphism in seeds. American Naturalist. 1984;124:1–16.. [Google Scholar]
- Staniforth RJ, Cavers PB. An experimental study of water dispersal in Polygonum spp. Canadian Journal of Botany. 1976;54:2587–2596. [Google Scholar]
- Stevens OA. Weights of seeds and numbers per plant. Weeds. 1957;5:46–55. [Google Scholar]
- Susko DJ, Lovett-Doust L. Patterns of seed mass variation and their effects on seedling traits in Alliaria petiolata (Brassicaceae) American Journal of Botany. 2000;87:56–66. [PubMed] [Google Scholar]
- Telenius A, Torstensson P. The seed dimorphism of Spergularia marina in relation to dispersal by wind and water. Oecologia. 1989;80:206–210. doi: 10.1007/BF00380152. [DOI] [PubMed] [Google Scholar]
- Thébaud C, Debussche M. Rapid invasion of Fraxinus ornus L. along the Herault River system in southern France: the importance of seed dispersal by water. Journal of Biogeography. 1991;18:7–12. [Google Scholar]
- Thompson JN. Variation among individual seed masses in Lomatium grayi (Umbelliferae) under controlled conditions: magnitude and partitioning of the variance. Ecology. 1984;65:626–631. [Google Scholar]
- Török K, Botta-Dukat Z, Dancza I, Németh I, Kiss J, Mihaly B, Magyar D. Invasion gateways and corridors in the Carpathian basin: biological invasions in Hungary. Biological Invasions. 2003;5:349–356. [Google Scholar]
- Venable DL. The evolutionary ecology of seed heteromorphism. American Naturalist. 1985;126:577–595. [Google Scholar]
- Venable DL. Size–number trade-offs and the variation of seed size with plant resource status. American Naturalist. 1992;140:287–304. [Google Scholar]
- Venable DL, Brown JS. The selective interactions of dispersal, dormancy and seed size as adaptations for reducing risk in variable environments. American Naturalist. 1988;131:360–384. [Google Scholar]
- Washitani I, Nishiyama S. Effects of seed size and seedling emergence time on the fitness components of Ambrosia trifida and A. artemisiaefolia var. elatior in competition with grass perennials. Plant Species Biology. 1992;7:11–19. [Google Scholar]
- Weed CM. Farm friends and farm foes: a textbook of agricultural science. Boston: Heat DC and Co; 1910. [Google Scholar]
- Willis SG, Hulme PE. Environmental severity and variation in the reproductive traits of Impatiens glandulifera. Functional Ecology. 2004;18:887–898. [Google Scholar]
- Zhang J. Seed dimorphism in relation to germination and growth of Cakile edentula. Canadian Journal of Botany. 1993;71:1231–1235. [Google Scholar]