Abstract
Background and Aims
Bilabiate flowers have evolved in many lineages of the angiosperms, thus representing a convincing example of parallel evolution. Similar to keel blossoms, they have obviously evolved in order to protect pollen against pollen-collecting bees. Although many examples are known, a comprehensive survey on floral diversity and functional constraints of bilabiate flowers is lacking. Here, the concept is widened and described as a general pattern.
Methods
The present paper is a conceptional review including personal observations of the authors. To form a survey on the diversity of bilabiate blossoms, a search was made for examples across the angiosperms and these were combined with personal observations collected during the last 25 years, coupled with knowledge from the literature. New functional terms are introduced that are independent of morphological and taxonomic associations.
Key Results
Bilabiate constructions occur in at least 38 angiosperm families. They are characterized by dorsiventral organization and dorsal pollen transfer. They are most often realised on the level of a single flower, but may also be present in an inflorescence or as part of a so-called ‘walk-around flower’. Interestingly, in functional terms all nototribic blossoms represent bilabiate constructions. The great majority of specialized bee-flowers can thus be included under bilabiate and keel blossoms. The syndrome introduced here, however, also paves the way for the inclusion of larger animals such as birds and bats. The most important evolutionary trends appear to be in the saving of pollen and the precision of its transfer. With special reference to the Lamiales, selected examples of bilabiate flowers are presented and their functional significance is discussed.
Conclusions
Bilabiate blossoms protect their pollen against pollen-collecting bees and at the same time render their pollination more precisely. The huge diversity of realised forms indicate the high selection pressure towards the bilabiate syndrome. As bees are very inventive, however, bilabiate constructions will not represent the ultimate response to bees.
Key words: Bilabiate flowers, nototribic (dorsal) pollination, floral diversity, bee blossoms, functional morphology
INTRODUCTION
When we think of flowers, it is generally bee-flowers that are the first to come to mind, simply because of their sheer numbers. And when imagining bees and flowers, we usually think of dorsiventral blossoms. Why this overwhelming bias in favour of bees? And why do we usually encounter dorsiventral blossoms when searching for bee-flowers?
Flower symmetry has recently been discussed extensively (e.g. Donoghue et al., 1998; Neal et al., 1998; Endress, 1999, 2001; Giurfa et al., 1999; Rudall and Bateman, 2002, 2004), but the immediate interaction between animals and flowers and their consequences have hardly been considered. Thus, the subject is tackled in this paper. Taking into account what is already known, we widen the functional concept of bilabiate flowers, adding much new data and information from own observations and conclusions.
Dorsiventral flowers
Because of gravity and their earthbound, forward locomotion, animals have a dorsiventral bilateral organization. Their legs are directed towards the interface with the substrate, and thus are always in a ventral position. On the basis of the same reasoning, wings are inserted above the legs. This overall organization was already present when animals came into contact with flowers. For pollination to occur, a standardized and repeated contact of a definite animal surface with the reproductive organs of the flower is essential. The most secure point-to-point interaction will be reached when the forward movement of the visitor in relation to the flower is arrested. As pollen transfer (flower-to-visitor, visitor-to-flower), however, requires a minimal relative movement, it is no surprise that it occurs in the last moment before the visitor reaches a standstill or at the beginning of withdrawal in reverse gear. So, flower evolution has had to respect the basic organization of the animal, and the dorsiventral organization of eutropic (specialized; Loew, 1884/86, 1895) flowers is the consequence of the dorsiventral organization of the relevant animals, especially bees. The idea of relating dorsiventral flowers to the perceptive abilities of highly derived eusocial bees (Neal et al., 1998) is obviously a post hoc explanation.
Bee pollination
Certain groups of animals are lured to visit flowers by a wide variety of attractants, especially nectar. By far the most important group of pollinators are bees, a group of vegetarian wasps that uses pollen instead of animal flesh for larval food. To fill a brood cell with a sufficient mass of pollen to feed one offspring, a great number of flower visits is needed. Müller et al. (2006) report that between seven and 1100 flowers are needed, assuming that the bee has the entire mass of pollen at its disposal; as this rarely occurs in nature because of earlier visitors or pollen packaging, these authors suggest multiplying these figures by a factor of 2·5 in order to arrive at a more realistic number of flowers needed per offspring. This figure then has to be multiplied by the number of brood cells completed during a mother's lifetime (10–30, Müller et al., 2006; under extreme conditions up to 40, Westrich, 1989). As pollen is usually stored in a mixture with nectar or fatty oils, these fluids also have to be collected in great quantities, which again requires more flower visits. To allow for so many flower visits and all their associated activities, the bees have to replenish their energy and water supplies – resulting in an additional need to visit flowers. Compared with all other nectar-drinking animals that only have to refuel themselves from time to time, bees thus visit exponentially more blossoms during their lifetimes than any other group of flower visitors. Interestingly, the species with the lowest pollen per flower rate (and thus the highest revisitation necessity) in the study of Müller et al. (2006) were specialized bee-flowers.
Foraging for pollen is mechanically a much more complex activity than simply inserting mouthparts for drinking. It requires a certain handling skill, different from species to species. These skills are difficult to acquire and result, at least, in flower constancy (Darwin, 1876; Waser, 1986), as in polylectic species. To optimize foraging efficiency, bees generally specialize on a restricted number of flower species (oligolecty), to which they repeatedly return. Specialists produce more potential offspring per unit handling time because they manipulate flowers faster and collect more pollen per flower than do generalists (Lovell, 1912, 1913, 1914; Heinrich, 1976; Williams, 1977; Strickler, 1979).
When flower and bee phenologies depend on the same stimuli, and thus are similar year after year, bees encounter and visit the same flowers every season, identifying their host plant by inborn reactions to specific pollen scents (Dobson and Bergström, 2000). In this situation, even morphological specializations for removing pollen from certain flowers make sense (e.g. Peters, 1974; Thorp, 1979, 2000; Parker and Tepedino, 1982; Müller, 1995, 1996, 2006; Alves dos Santos and Wittmann, 2000; Fig. 1A). The fidelity, and thus the repeated return to the same species, not only have advantages for the bee, but are an inevitable prerequisite for reliable pollination to occur.
Fig. 1.

Bee flowers and bees. (A–F) Flower–bee interactions. (A) Rophites trispinosus with specialized hairs on the forehead to remove pollen directly from the anthers of certain lip flowers. (B) Pollen uptake is usually made using the mandibles and forelegs after landing directly at the stamens (Apis mellifera, Echium wildpretii). (C) Pollen in a safe position on the back of the bee (Xylocopa sp., Thunbergia grandiflora). (D) Xylocopa frontalis is too small for this lip blossom (Canavalia brasiliensis): the bee curves its body to finally reach and remove (steal) pollen from this flower. (E) Albuca maxima with Megachile sp. mounted in one meranthium to show the relative position of the organs. When leaving the flower, the cushion at the tip of the petal liberates the anther, which delivers pollen onto the back of the bee, while the gynoecium serves as a perch. (F) Apis mellifera hanging inverted on the upper lip of Salvia glutinosa, pulling out the hidden anthers to remove pollen. (G–J) Bilabiate flowers from different families: (G) Brillantaisia patula (Acanthaceae); (H) Galeopsis speciosum (Lamiaceae); (J) Melampyrum nemorosum (Scrophulariaceae).
Necessity of defence
Pollen, on the other hand, is an essential part of the plant reproductive process, serving as a transport container for male genetic information. Moreover, pollen is expensive and cannot be reproduced, like nectar drained by a previous visitor. As pollen grains have a finite number, every grain removed by a bee is lost for pollination. As plants and bees rely on the very same (generally) few pollen grains for their respective reproduction, a strong rivalry results (Westerkamp, 1997). To understand the options flowers have to defend at least part of their pollen for exclusive use in pollination, we have to look at the pollen foraging process (see also Westerkamp, 1987, 1996).
When considering pollen-collecting bees, it is usually common only to discuss the structures used in homeward transport, such as the scopae (brushes) or corbiculae situated on the hindlegs or the abdomen. Pollen located here is more-or-less safe against being stripped off by floral structures – including by the stigma. Moreover, this pollen is often mixed with liquids (regurgitated nectar and saliva) that improve sticking, but reduce germination. Much more important for the plant's reproductive success is the moment when pollen is taken up from the flower. The great majority of bees uses forelegs and mouthparts (especially mandibles) to actively brush and scrape off pollen (Fig. 1B; see also Grinfel'd, 1962; Michener et al., 1978). To gain prompt access to the pollen presenters (anthers or secondary sites; for an overview see Yeo, 1993), they alight directly on them. In this way, they can remove the grains in great quantity directly from the presenting structures. As the bee's reloading process towards the storage places for nestward transport includes all the legs, it usually occurs during flight. In a fixed pattern of rearward movement, pollen is passed from forelegs via midlegs to the hindlegs. The movements during the reloading process are more-or-less identical to those used in grooming (Michener et al., 1978). This cleaning process also brings into the load pollen that has (passively) contaminated other areas of the body surface. The only – but important – difference from the normal grooming process in other wasps is that the sweepings are not discarded immediately, but are stored temporarily in the transport container, from where they are removed only in the nest. Thus, grooming pollen was transformed into collecting pollen.
As bees have optimized pollen-foraging in order to produce more offspring, they are able entirely to remove pollen from a flower, leaving (next to) nothing for pollination. In the case of Campanula rapunculus, for example, Schlindwein et al. (2005) reported that more than 95 % of the total pollen was collected by its oligolectic pollinator, while less than 4 % contributed to pollination. As bees possess the great advantages of being mobile, dexterous and inventive animals whilst plants are sessile and immovable, flowers are forced to react in an evolutionary way in order to avoid total pollen loss.
There are two main techniques to protect pollen against bees, often encountered jointly: the offer of other incentives (especially nectar) to deviate the interest of the flower-visitor away from pollen, and the temporal hiding and portioning of pollen.
The inclusion of nectaries into flowers and the emphasis on nectar was certainly one of the most important novelties in the evolution of flowers; today, the great majority of flowers uses this attractant. On the other hand, this key innovation paved the way for the inclusion of a new suite of possible pollinators – lepidopterans, dipterans and vertebrates. Foraging actively for pollen and nectar are well separated in bees: in the latter case, the foragers may (and should be) contaminated with pollen, while in the former they have to refuel themselves with nectar; these two activities are never combined. Thus, the offer of a great quantity of nectar may in fact deviate the interest of a visitor from pollen to nectar, making this species a nectar-flower. The second option to save pollen against total removal is to hide it from direct access and to applicate a small portion of it insidiously out of the field of perception of the visitor. Perception of the contamination is thus impeded and so it is not immediately removed by the visitor, thereby allowing potential pollination of further flowers. Only after a suite of visits (and possible pollinations) is the contamination perceived and eventually removed (without interfering negatively with pollination). On the other hand, the small portion of pollen received by the bee necessitates many more visits (and eventual pollinations) before sufficient pollen is obtained for the production of one offspring.
Because of the dorsiventral organization of the bees, pollen may be hidden from them in two ways, above and below. In sternotribic blossoms, pollen is transferred from below to the ventral side of the pollinator. To save it from being collected, some flowers (e.g. Melastomataceae, Commelinaceae) hide their fertile anthers by cryptic colours and at the same time focus the bees' interest on attractive dummies. Much more effective are the so-called ‘keel blossoms’ (see Westerkamp, 1997), in which pollen is hidden within a keel. To get access to the nectar of these flowers, all six legs are needed in order to lower the hiding structures, thus no one of them is free for direct pollen uptake. As pollen still is within reach of the foraging legs, sophisticated structures are needed to keep all legs busy. At the same time, these structures have to ensure the automatic return of the pollen-presenting structures to within the keel as soon as only a single leg is lifted and aimed at direct pollen collection. Even in keel blossoms, there are flowers that are able to locate at least part of their pollen on the dorsal side of the visitor (e.g. Lathyrus latifolius, Westerkamp, 1993; Apios americana, Westerkamp and Paul, 1993; Cytisus scoparius, Westerkamp, 1997; all Fabaceae).
Mechanically, it is much more simple to avoid leg action at all, when all reproductive activity – including pollen placement – is located on the dorsal side of the bee in nototribic blossoms (Thorp, 2000), for example in the so-called ‘bilabiate’ flowers (Fig. 1G–J). It is hard to imagine how bees should have developed a ‘fondness’ for dorsal pollen, as suggested by Schremmer (1972) for Xylocopa bees. Applicating pollen, for example, above the insertions of the legs, where grooming is difficult, drastically reduces pollen loss to the bees. When even located above the wing insertions (Fig. 1C) pollen is safe also during flight, when reloading to the carrying containers usually occurs. Pollen is also secure below the upper lip during legitimate flower visits, when the bee searches for nectar at the flower base. In more advanced bilabiate flowers the upper lip is closed underneath in order to exclude pollen thieves. Pollen is invisible here and only becomes apparent when the bee's head has already passed by, being stuck to the insect insidiously from behind.
MATERIAL AND METHODS
Most of the observations summarized in the present paper have been collected over the past 25 years during field trips in Europe, to the tropics, South Africa and Australia, and by utilizing the collections of diverse Botanical Gardens, in particular at Berlin-Dahlem and Mainz University (both Germany). Some of our own observations are new and some of them have already been illustrated by other authors: nonetheless, to give a rough survey of the floral diversity of nototribic blossoms we combine both data sets in the Results and Discussion section below. Taxonomically we follow the Angiosperm Phylogeny Group (APG II, 2003). Methodologically, we proceed from a functional approach and for the first time describe the bilabiate syndrome independent of morphological and taxonomic levels. We introduce some new terms, which are generally applicable to all kinds of nototribic blossoms. We use the term ‘blossom’ (‘Blume’ in German) for any floral unit as the functional complement to the morphological terms ‘flower’ (‘Blüte’ in German) and ‘inflorescence’ (see Faegri and van der Pijl, 1979; Claßen-Bockhoff, 1991). Only when referring to a given organization (e.g. flower) and taxon (e.g. Lamiaceae) do we also use the familiar terms ‘corolla tube’, ‘upper lip’ and ‘lower lip’.
RESULTS AND DISCUSSION
Bilabiate blossoms
Bilabiate blossoms (including lip, gullet and throat flowers) are one-way constructions that in most cases offer nectar at their base (Fig. 2). Ventrally, they are confined by a ‘floor’, usually called the lower lip. Dorsally, they are covered by a ‘roof’, usually called the upper lip, with the reproductive surface (i.e. pollen and stigma) underneath. Floor and roof are at such a fixed distance that legitimate visitors inevitably contact the reproductive structures with their dorsal side. Bilabiate flowers thus are nototribic by definition.
Fig. 2.
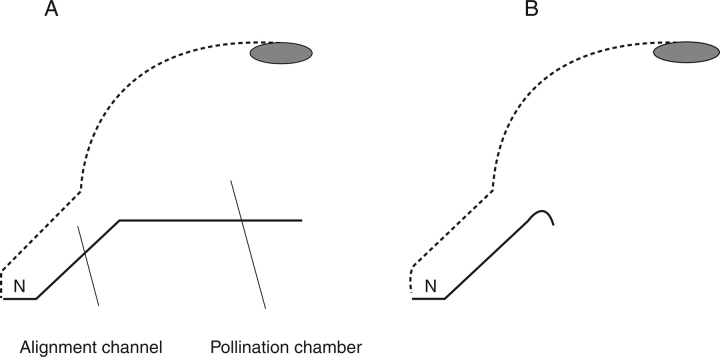
Schematic longitudinal sections of generalized bilabiate blossoms. (A) The bilabiate construction includes the roof (dotted line) with the reproductive surface (grey) on its lower side and the floor (continuous line) separated by the visitor path with (usually) nectar (N) at its proximal end; the interior of the flower is divided into the wide, outer pollination chamber and the narrow, inner alignment channel. (B) Blossom with a reduced (but still existing) floor.
In most cases, the visitor path is divided into two sections of different diameters, a wider distal element, that we call the ‘pollination chamber’, and a narrow proximal part, the ‘alignment channel’ (see Fig. 2).The length relationship between these two parts may vary over a wide range. Usually, the alignment channel is accessible to the mouthparts only (proboscis, beak, rostrum, snout, tongue) while the pollination chamber admits the entry of the head and even further parts of the visitor. Often the transition zone from the wide to the narrow part is of utmost importance for floral kinetics (see Reith et al., 2007). It is here that the forward movement of the visitor comes to an end, and thus it defines the fixed relationship between the pollination surfaces of the flower and the visitor. Often at this juncture a mechanical barrier is developed that may represent a nectar cover (e.g. hairs, staminodes, swellings; for example Thunbergia grandiflora, Acanthaceae, see Fig. 6F) and/or part of a lever mechanism (e.g. Salvia pratensis, Lamiaceae, see Fig. 6H).
Fig. 6.

Pollen protection and pollen transfer in bilabiate flowers. (A) Phlomis fruticosa, locked lip flower, opened lengthwise. (B) Iris reticulata, meranthium, cut lengthwise to show the upper lip formed by a stylar branch and the stamen and the lower lip formed by a tepal. (C) Camptandra sp., note the lever mechanism formed by the single curved and partly sterile anther. (D) Nemesia anisocarpa with locked entrance. (E) Leonotis leonurus (from below), note the outer part of the lower lip reduced and folded back. (F) Thunbergia grandiflora with locked nectar chamber and anther levers that open the thecae during a visit. (G, K) Columnea tessmannii: flower (G) with fused pollen sacs (K). (H) Salvia pratensis cut lengthwise to show the floral organs and the staminal lever mechanism (a, anther; n, nectar; s, style; sc, sterile connective arm; sl, staminal lever). (J) Thunbergia mysorensis with abbreviated lower lip. (L) representative of the Australian Westringieae with staminal levers coming from the adaxial stamens (upper lip removed). (M) Prunella grandiflora with four downward-orientated anthers. (N) Prunella vulgaris: note the filament formations placing the anthers towards the centre of the flower. (Photographs in G, K: Lars Nauheimer.)
Usually it is the narrow alignment channel that – as a result of the depth of the nectar and the lateral constraints – defines the exact positioning of the visitor, because of the stiff fixed spatial relationship between the mouthparts in their functional position and the remainder of the vistitor's body.
The alignment channel has previously been termed a ‘tube’ when discussing flowers of Lamiaceae s.s. only (e.g. Sprengel, 1793; Meeuse, 1992), but a sympetalous construction is not required, although the majority possess it. In fabaceous lip-flowers, for example, all petals are free; the calyx tube reinforces the fixed relationship between the parts.
In the great majority of cases, the floor of the pollination chamber serves as a perch for bees. As room inside the blossom is restricted, these animals cannot reach their dorsal side with their legs (to gain pollen from there), nor can they move sideways. Exit from the flower is only possible by a rearward movement.
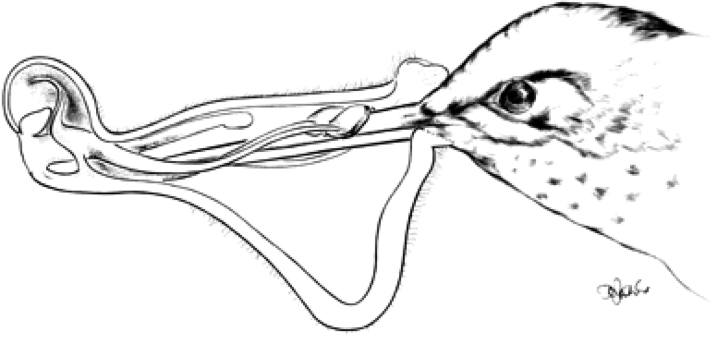
In our wide definition of bilabiate blossoms, there is no need of an incision between roof and floor, as is typical for the name-giving bilabiate flower in Lamiaceae. In this family, the incision largely affects the pollination chamber, while the alignment channel is the corolla tube. In fabaceous lip-flowers, roof and floor are entirely separated morphologically. In the hypocyrtoid flowers of some gesneriad examples (e.g. Alloplectus dodsonii; Fig. 3) an incision is totally absent. All transitional stages between these two extremes are observed in nature. As long as the functional unit is dorsiventral with the reproductive surface above the pollinator then it is a bilabiate blossom, independent of the morphological nature of the organs concerned and the overall appearance of the flower and inflorescence involved.
Fig. 3.
Alloplectus dodsonii (longitudinal section) pollinated by Aglaiocercus coelestris. Note the nototribic construction without free upper and lower lips (from Hübenthal et al., 2003).
Morphological diversity
The basic considerations given above apply independently of the organization level of the pollination unit (i.e. flower, partial flower, inflorescence). Faegri and van der Pijl (1979, their Fig. 2) have already used bilabiate (‘gullet’) blossoms to illustrate their definitions of meranthium, euanthium and pseudanthium (for the latter term see Claßen-Bockhoff, 1991).
The great majority of lip blossoms are formed by solitary flowers (Table 1).
Table 1.
Taxa including lip blossoms (systematic arrangement following APG II, 2003). For each family one genus is listed; if there are more genera with nototribic constructions this is indicated by ‘+’. Radial blossoms with nototribic subunits are indicated by*
| (A) Monocots | |
| Asparagales | |
| Alliaceae1 (Gilliesia) | |
| Hyacinthaceae (Albuca) | |
| Iridaceae (Iris +) | |
| Orchidaceae (Disa +) | |
| Commelinales | |
| Haemodoraceae (Anigozanthos) | |
| Zingiberales | |
| Bromeliaceae (Puya +) | |
| Cannaceae (Canna) | |
| Costaceae (Costus +) | |
| Lowiaceae2 (Orchidantha) | |
| Heliconiaceae (Heliconia) | |
| Zingiberaceae3 (Roscoea +) | |
| (B) Dicots | |
| Proteales | |
| Proteaceae (Mimetes +) | |
| Ranunculales | |
| Ranunculaceae*4 (Nigella +) | |
| Caryophyllales | |
| Cactaceae (Schlumbergera) | |
| Santalales | |
| Loranthaceae (Benthamia +) | |
| Geraniales | |
| Melianthaceae (Melianthus) | |
| Myrtales | |
| Myrtaceae (Calothamnus +) | |
| Fabales5 | |
| Caesalpiniaceae (Senna +) | |
| Fabaceae (Canavalia +) | |
| Malpighiales | |
| Chrysobalanaceae6 (Neocarya +) | |
| Passifloraceae* (Passiflora) | |
| Brassicales | |
| Moringaceae (Moringa) | |
| Malvales | |
| Malvaceae7 (Chiranthodendron +) | |
| Sapindales | |
| Sapindaceae (Hippocastanum) | |
| Ericales | |
| Balsaminaceae (Impatiens) | |
| Lecythidaceae (Couroupita +) | |
| Marcgraviaceae*8 (Marcgravia) | |
| Lamiales | |
| Acanthaceae (Brillantaisia +) | |
| Bignoniaceae (Kigelia +) | |
| Calceolariaceae (Calceolaria) | |
| Gesneriaceae9 (Columnea +) | |
| Lamiaceae (Salvia +) | |
| Lentibulariaceae (Utricularia +) | |
| Martyniaceae (Craniolaria +) | |
| Orobanchaceae (Orobanche) | |
| Paulowniaceae (Paulownia) | |
| Pedaliaceae (Harpagophyton +) | |
| Phrymaceae (Mimulus) | |
| Plantaginaceae (Linaria +) | |
| Schlegeliaceae (Schlegelia +) | |
| Scrophulariaceae (Nemesia) | |
| Stilbaceae (Halleria) | |
| Verbenaceae (Verbena +) | |
| Solanales | |
| Convolvulaceae (Mina +) | |
| Asterales | |
| Goodeniaceae (Goodenia +) | |
| Lobeliaceae (Lobelia) | |
| Stylidiaceae (Stylidium) | |
| Dipsacales | |
| Caprifoliaceae (Abelia) |
Sources for information or illustrations: 1Rudall et al., 2002; 2Sakai and Inoue, 1999; 3Troll, 1929; 4Weber, 1993; 5Lewis et al., 2005; 6Prance, 1985; Prance and White, 1988; 7Endress, 1999; Raju et al., 2004; 8Dressler and Tschapka, 2002; 9Hübenthal et al., 2003.
Meranthia are functional units, which morphologically represent only parts of a single flower. Thus, an overall radial flower may be composed of several bilabiate units as observed, for example, in Iris (Fig. 4A), Moraea and other Iridaceae, in which each flower has three lip blossoms, each being formed by a petaloid stigma arm and its opposed perigon lobe. Further examples are Albuca (Hyacinthaceae; C. Westerkamp and M. Kuhlmann, unpubl. res.), with bilabiate units formed by a petal with the stamen included and the opposed part of the gynoecium (Fig. 1E), as well as Nigella damascena (Ranunculaceae; Fig. 4B; Weber, 1993), and Passiflora spp. (Passifloraceae; e.g. P. foetida; fig. 12 in Gottsberger et al., 1988), in which the lip blossoms are formed by part of the stamens/styles (upper lip) and the perianth/nectar leaves (lower lip); in these latter cases, the bee comes to a standstill in front of the central column while sucking nectar, each unit thus representing a bilabiate blossom (Fig. 2A). Presumably, all nototribic ‘revolver flowers’ with several separate entrances towards nectar within a flower (usually termed ‘Umlaufblume’, a walk-around flower or a roundabout flower) belong within this category.
Fig. 4.
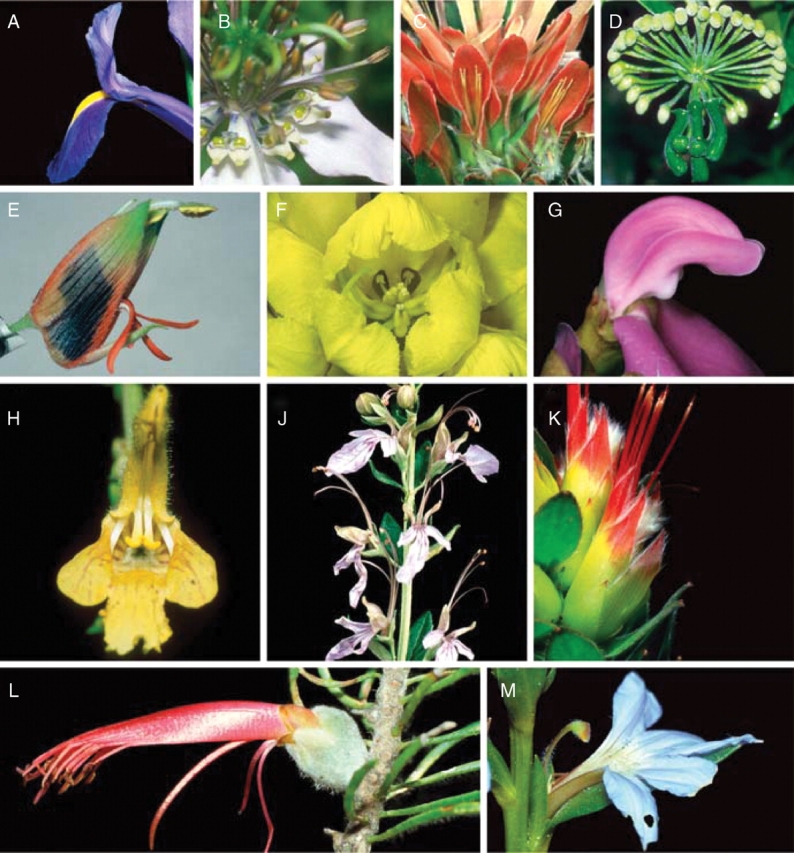
Floral diversity in bilabiate construction. (A, B) Meranthia: in Iris reticulata (A) the bilabiate construction is formed by a stigma lobe, a stamen and a tepal; in Nigella damascena (B) by stamens, styles and nectaries. (C, D) Inflorescences (pseudanthia): (C) Mimetes cf. cucullata with an upper lip formed by pollen presenters and subtending bracts of the next upper partial inflorescences; (D) Marcgravia umbellata with an upper lip formed by perfect flowers and a lower lip by nectar producing leaves. (E) Melianthus minor with abbreviated lower lip, exposed pollen and black nectar. (F) Senna alata, a pollen-only lip flower. (G) Canavalia brasiliensis, a fabaceous bilabiate flower; note the white outgrowths near the base of the roof (part of the wing petals) that take part in the opening of the closed pollen container. (H) Salvia glutinosa with pollen hidden in the roof and attractive sterile lever ends. (J) Teucrium fruticosum with an upper lip formed by the reproductive surface. (K) Mimetes hirtus with an upper lip primarily composed of the pollen presenters (styles) of the flowers clustered in this dense inflorescence blossom. (L) Calothamnus sanguineus (Myrtaceae) with an upper lip formed by a stamen bundle. (M) Scaevola sp. with the reproductive column forming the upper lip.
Pseudanthia sensu Faegri and van der Pijl (1979) are inflorescences in which several flowers form a single functional unit (see Claßen-Bockhoff, 1990). The first bilabiate example was described by Vogel (1954; see also Rourke, 1984) in ornithophilous Mimetes spp. (Proteaceae). Here, the styles of up to ten florets form the roof and their shrivelled corollas jointly form the floor (e.g. Mimetes hirtus, Fig. 4K). In M. cucullatus (Fig. 4C) the petaloid subtending bract of the partial inflorescence above is included into the upper lip of the blossom below (schematic representation in Claßen-Bockhoff, 1990). The hanging inflorescences of Marcgravia spp. (Marcgraviaceae) also belong here, representing a ‘roundabout’ construction on the inflorescence level (Fig. 4D). Each nectar-producing bract (‘pitcher’) of the sterile central flowers serves several surrounding flowers that do not possess these pitchers (see, for example, Wallace, 1889; Dressler and Tschapka, 2002). Each bilabiate unit is formed by one pitcher and part of the flowers.
Lip blossoms are not only produced on different organization levels, they also show an enormous diversity in the organs that participate in the formation of roof and floor. In most cases, the roof (‘upper lip’) is composed of corolla elements (single: Senna alata, Fig. 4F; Albuca maxima, Fig. 1E; multiple: Lamiales, many Fabaceae) and the androecium/gynoecium. But blossoms with a roof formed only by the androecium/gynoecium also exist, for example in Calothamnus (Myrtaceae: filament tube plus style, Fig. 4L), Iris (Iridaceae: stigmatic lobe plus anther, Fig. 6B) and several Lamiaceae (e.g. Teucrium: stamens and style, Fig. 4J). In Melianthus (Melianthaceae; Fig. 4E) and Acanthus (Acanthaceae), sepals assume the covering function of the corolla. In species with secondary pollen presentation on the style, the gynoecium alone may form the roof (e.g. Canna, Cannaceae; Scaevola, Goodeniaceae, Fig. 4M; many Proteaceae). In inflorescences (see above) subtending bracts and extrafloral nectaries take part in the formation of the roof.
The roof may even be composed of different materials along its length. In Impatiens (Balsaminaceae) the distal part is formed by the androecium and/or gynoecium, the proximal part by the roof of the sepal spur. In Orchidaceae, the column makes up the outer component and the roof of the labellum spur the inner one. In Linaria (Plantaginaceae) the upper petal and androecium/gynoecium jointly form the distal part and the roof of the lower petal spur the proximal part. And in Marcgravia (Marcgraviaceae) several entire flowers form the distal element and the roof of a nectar pitcher forms the proximal piece.
The lower lip in most cases consists of a single (vexillum in inverted Fabaceae, Fig. 4G) or multiple petals (e.g. Lamiales, Proteaceae). Where it is single, it may be formed by nectar leaves (Nigella damascena, Ranunculaceae, Fig. 4B), the androecium (e.g. Senna alata, Caesalpiniaceae, Fig. 4F) or even the gynoecium (Albuca maxima, Hyacinthaceae, Fig. 1E). Bracts participate in Marcgravia, Marcgraviaceae, and Mimetes (Proteaceae, see above).
The lateral constraints may also be formed by all of the afore-mentioned organs: gynoecium (Iris), androecium (Calothamnus, Senna), corolla, calyx (Melianthus) and even bracts (Mimetes, Marcgravia).
Systematic distribution
Bilabiate constructions are widely distributed within the angiosperms (Table 1). In the monocotyledons, we have found them in three orders and ten families, and in the dicotyledons in 16 orders and 38 families at least. Within the families, examples range from single incidents (e.g. Albuca) to nearly the whole taxon (e.g. Orchidaceae, Iridaceae, Lamiaceae, Proteaceae). Even within families, the distribution is uneven. In the Fabaceae (for a recent overview see Lewis et al., 2005), for example, bilabiate flowers (in contrast to the typical keel blossoms) evolved in at least nine (out of 28) tribes independently, with greater numbers occurring in the Phaseoleae, especially the Clitoriinae and Diocleinae. In the neighbouring Caesalpiniaceae, single cases of bilabiate flowers were observed in all four tribes.
Diversification
Bilabiate blossoms have not only evolved many times in parallel within the Angiosperms but may also represent key innovations promoting adaptive radiation (Givnish, 1997). The Lamiales, for instance, are by far the largest group of the Euasterids I (after APG II, 2003), including approximately 23600 species (Kadereit, 2004). They have thus nearly twice as many species as the Gentianales (about 14000 species) and nearly five times more species than the Solanales (about 4500 species), both forming together with the Lamiales the unresolved sister group of the Garryales (less than 20 species; Mabberley, 1997). Starting from the basic bilabiate construction, modifications of all single characters (except nototriby) result in a functional and structural diversity that will now be considered with emphasis on the Lamiaceae (Lamiales). The alterations can appear singly or in combinations, but it is important to emphasize that even the functional blossom form represents a syndrome or a suite of characters. This was demonstrated recently by Castellanos et al. (2004) who experimentally changed only a single character at a time in an attempt to determine its importance.
Locked entrance
Entrance to a blossom may be impeded in different ways, thus augmenting the efficiency of hiding the pollen. To give access to the interior of the flower, the blossom has to be opened forcefully. Thus the interest and the actions of the visitor are deviated from the pollen.
One option is to lower the end of the roof until it approaches the floor and thus nearly closes the entrance. This means that the roof has to be raised by the visitor, which requires not only an adequate settling of the feet but also a considerable physical force. In Phlomis fruticosa (Lamiaceae, Fig. 6A), for example, a bee contacts the lower lip while landing and first enters its proboscis into the open space between the two lips. Entry of the proboscis is not impeded and force only has to be applied when the head reaches the point of direct contact between the roof and perch; so the lifting of the upper lip only takes place when the head has already passed the region where the anthers are hidden. Thus, the bee is dusted with pollen only behind its eyes – out of its sight, insidiously from behind. In Iris (Iridaceae, Fig. 6B), the lowermost part of the roof is in the region of the stigmatic flap, which wipes over the forehead when the bee applies force whilst entering. The anther is higher up in the tunnel. So, there is free space to move forward in the direction of the nectar. Pollen is only applied when the tongue is in contact with the attractant. In Albuca maxima (Hyacinthaceae; M. Kuhlmann and C. Westerkamp, unpubl. res.) the entrance to the blossom is firmly closed by a cushion formed at an inward fold of the petal (Fig. 1E). The petal tip overlaps the end of the anther, a feature reflected in local names for this flower such as ‘soldier-in-the-box’ or ‘man-in-the-box’. When the distal cushion is lifted by an entering bee, the petal and stamen (that are basally fused) are elevated jointly. Pollen is only released when the bee is exiting the flower, at which time the tip of the petal unfolds and releases the anther to smear pollen on the back of the bee (Fig. 1E). When visiting a second flower pollen is combed off by the papillae of the cushion, from where pollen tubes grow along the transmitting tissue of the style after the formation of stigmatic slime at the end of anthesis (Kingston, 1998). In Phlomis and Iris, the floor is elongated in relation to the roof, thus offering a secure perch for the visitor and hence allowing it to apply the necessary forces. In Albuca, however, this auxiliary structure does not exist, making entrance more difficult and thus restricting access to a few highly specialized bees.
Less often, the entrance is closed by the floor, which forms a palate, i.e. it is strongly curved upwards (Nemesia anisocarpa, Scrophulariaceae; Fig. 6D). In order to gain access, this impediment has to be removed. While working on this, the bee does not perceive that it is passing below the anthers and thus is dusted insidiously on its back. A lower lip that is curved up over part of its length, either transversally in the case of the palate or longitudinally in the form of rails (e.g. species of Jacaranda, Tabebuia, Thunbergia), offers an additional advantage: the visitor's body is elevated and thus its back is pressed against the reproductive surfaces. In Craterostigma plantaginea (Scrophulariaceae) two longitudinal bulges narrow the flower entrance. These are formed by the proximal parts of the two abaxial stamens, which are congenitally fused with the floor of the alignment channel and the lower lip (Magin et al., 1989). Roof and floor are included when both lips get closer to each other (e.g. Jacaranda, Tabebuia; Bignoniaceae). Here, the width of the entrance is increased at the cost of the height – the circumference being constant. A potential visitor has to apply considerable force to increase the height in order to be able to gain access. The advantages are as described above.
In many species, the roof is in a position high above the floor, and locked below enclosing the anthers. Thus, perching of a visitor is enabled, but the anthers are hidden (e.g. Salvia, Fig. 4H). Here, a novel mechanism had to be evolved to cause secondary release of the pollen either by lowering the pollen presenter or by removing the cover. An example of the first device is encountered in the well-studied case of Salvia and other taxa with lever mechanisms (see below); the second is demonstrated in Salvia verticillata (Hildebrand, 1865) and in Canavalia brasiliensis (Fabaceae; C. Westerkamp and L. P. A. Amaral Neto, unpubl. res.; Fig. 4G). In the latter, xylocopine bees land on the vexillum that serves as a perch in this inverted keel blossom and make their way towards the nectar, which is locked by combined structures of the roof (keel and wings). After inserting the proboscis, they open the upper lip with their forehead: cartilaginous outgrowths of the wing petals slide along the eyes and thus liberate the central column of the androecium and gynoecium. While the cover is pressed backwards, the free ends of the stamens and the style spread fanlike on the bee's head or thorax (Fig.1D), depending on the Xylocopa species. In Salvia verticillata, a sage without any lever mechanism, the pollen sacs are enclosed by the upper lip. The latter is mobile and can be pushed back by a bee searching for nectar. A diagonally running stiff ring of hairs forces the bee (e.g. Bombus spp., Apis mellifera) to insert its proboscis in such a way that it first touches the downward orientated stigma and then the upper lip, thus becoming loaded with pollen on its front (Claßen-Bockhoff et al., 2004a).
Reduced floor
There are remarkable cases of a reduced floor (Fig. 2B). These are particularly encountered in bird-pollinated species (Vogel, 1954; e.g. Anigozanthus, Haemodoraceae; Canna, Cannaceae; Heliconia, Heliconiaceae; Melianthus, Melianthaceae, Fig. 4E; Schlumbergera, Cactaceae; Isoplexis, Scrophulariaceae; Leonotis, Lamiaceae, Fig. 6E; for Salvia see Wester and Claßen-Bockhoff, 2007) and bat-pollinated species (e.g. Kigelia africana, Paliavana prasinata, Thunbergia mysorensis, Fig. 6J). This reduction, however, is restricted to the pollination chamber (Fig. 2); bilabiate blossoms can never dispense with the floor totally. The alignment channel part is not only essential to hold nectar in its position (Fig. 6F), but it is also indispensable and of utmost importance in necessitating an intimate contact between the visitor and the reproductive organs. Remnants of the pollination chamber floor are often encountered (Figs 2, 4E). They obviously still play a role during the insertion of the beak/snout into the flower (e.g. Temeles and Rankin, 2000).
When larger animals such as birds and bats are involved in pollinating bilabiate blossoms, it is the fixed distance between floor and roof that impedes access. With pollination functional (and thus the roof with pollen in a given position), it is only the perch that can be reduced to simplify approach from below. Because of their dimensions, these visitors alight outside (usually below; see Westerkamp, 1990) the individual flowers or forage whilst hovering. So they are not in need of an in-flower perch, which might ‘speed up’ the evolutionary reduction of the latter. Exceptions are found in ornithophilous legume lip flowers, with the floor (vexillum) even enlarged (e.g. Erythrina crista-galli, Strongylodon, Swainsonia). In the flowers of some Gesneriaceae (e.g. Alloplectus dodsonii, Fig. 3), the floor and roof are reduced into an extremely narrow orifice that guides the hummingbird's beak from its first contact onwards as it enters this hypocyrtoid flower (Hübenthal et al., 2003).
Alignment channel depth
A feature that has experienced much variation is the length of the alignment channel (Fig. 2). It is this element that houses the mouthparts of the visitor and that is responsible for the final guidance. Its length also defines the exact positioning of the visitor (e.g. Nilsson, 1988).
As nectar is hidden deeply in the alignment channel, the pollinators have to search for it. The immense number of coloured and tactile signals (e.g. spots, hairs, furrows, epidermal surfaces) found in nototribic blossoms are interpreted as aids for the pollinators (e.g. Vogel, 1978; Kevan and Lane, 1985; Lunau et al., 1996). These signals may at the same time direct the pollinator into a position that allows precise pollen transfer. This is, for instance, true for Torenia polygonoides and Craterostigma plantaginea (Scrophulariaceae) in which the two abaxial stamens form ‘filament knees’ placed on both sides of a yellow spot, or are themselves intensively yellow coloured (Magin et al., 1989).
With increasing distance to the nectar, the way was paved to include a new suite of long-tongued pollinators into bilabiate blossoms, such as long-tongued flies (e.g. Manning and Goldblatt, 1995; Johnson and Steiner, 1997), moths (e.g. Alexandersson and Johnson, 2002), and long-beaked birds (see Wester and Claßen-Bockhoff, 2007). With increasing length, the relative participation of the alignment channel grew as compared to the pollination chamber, reaching its extreme in the case of Angraecum sesquipedale (e.g. Wasserthal, 1997). On the other hand, the combination of the change from bees to other animal groups not interested in pollen together with the high precision of pollen placement and receipt has allowed for a reduction in the covering structures of the roof while the reproductive surfaces retain their position (see Wester and Claßen-Bockhoff, 2007).
Reproductive surface
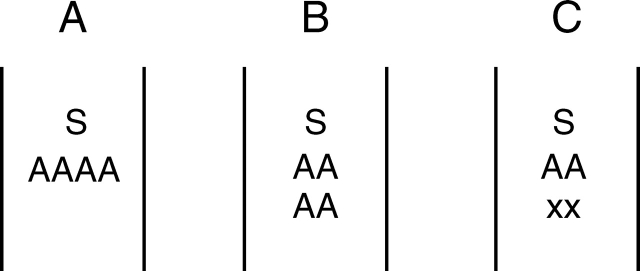
With the growing exactness of visitor positioning because of the narrow, elongated alignment channel, pollination success also grew – enabling the reduction of pollen production and even the number of anthers/thecae (Fig. 5). In Lamiales, the basic number of four anthers arranged at the same level below the upper lip still gives a lot of flexibility as regards the entering of a possible pollinator: a large area is dusted with pollen, so there is a good chance that some of it will be delivered to the single stigma of the next flower (usually made up of two carpels). Much pollen, however, is wasted in this four (anthers) to one (stigma) arrangement (Fig. 5A). With the growing exactness of entry of the pollinatior, there was no longer the need to dust so large an area; in addition, the outermost anthers no longer hit a region met by the stigma. Thus, the way was paved for the didynamous layout with two levels of two anthers each (Fig. 5B), typical for many Lamiales flowers. With increased precision, pollen production could be reduced to two anthers only (Fig. 5C; e.g. Brillantaisia, Acanthaceae, Fig. 1G; Calceolaria, Calceolariaceae). Even at this stage, further reductions to only two thecae per flower were possible, as in Salvia (see Claßen-Bockhoff et al., 2004b) or, on a totally different concept, in monandrous orchids. In Canna only a single theca remains of the originally six bithecate anthers of Monocots.
Fig. 5.
Schematic disposition of stigmas (S) and anthers (A) in lamiaceous flowers. (A) Four anthers at same height; (B) didynamous distribution; (C) reduction to two stamens (either the upper or the lower pair).
In Salvia the flower entrance is often locked by staminal structures (for details and illustration see Claßen-Bockhoff et al., 2003, 2004a; Wester and Claßen-Bockhoff, 2007). Here, an extreme growth of the connective results in a lever mechanism, with one of the thecae of both fertile stamens usually placed below the roof while the other arm, with a plate-like widening, closes the entrance to different degrees. After inserting the proboscis below the combined plates of the two posterior arms, the forehead usually pushes against these plates in bee-pollinated species. Thus, each of the connectives is rotated around its pivot at the end of the filament. Finally, the pollen load of the fertile thecae is pressed onto the back of the visitor by its own force. When the bee moves out of the tube, the parts of the connective return to their original positions (see Reith et al., 2007).
Nototribic lever mechanisms are also known in other Lamiaceae such as Hemigenia and Microcorys (Australian Prostantherioideae–Westringieae; see Guerin, 2005), in which the lever is likewise formed by the widened connective (Fig. 6L). The lever arm in Calceolaria (Calceolariaceae) may likewise also have connective or thecal origins (Sérsic, 2004). In the Zingiberaceae (e.g. Curcuma, Roscoea, Camptandra; Fig. 6C; Troll, 1929, 1951; Holttum, 1950) basal appendages on the pollen sacs form the lever (Endress, 1994). This is also true in the case of Thunbergia grandiflora (Acanthaceae, Fig. 6F; Burkill, 1906; C. Westerkamp, unpubl. res.) where the lever, however, serves to open the theca, thus liberating pollen on demand.
To guarantee dorsal pollination (nototriby) the anthers have to be placed below the upper lip. This can either be achieved during early bud development by relocating the entire abaxial stamens in an adaxial position, or at a later stage by setting merely the pollen sacs in an upright position. In the case of introrse anthers, the abaxial pollen sacs positioned under the upper lip have to turn around in order to guarantee dorsal dusting (for Salvia see Claßen-Bockhoff et al., 2004b).
The precision of pollen deposition can be increased by various further mechanisms. In the Gesneriaceae, all pollen sacs are in such close contact that they post-genitally fuse and form a single functional unit (Fig. 6G, K). In Prunella grandiflora (Lamiaceae) the abaxial filaments are forced into close contact by corolla invaginations at their bases (R. Claßen-Bockhoff, unpubl. res.). All anthers are orientated downward and inward by an asymmetric bending at their base (Fig. 6M). In P. vulgaris (Fig. 6N), they are stabilised in their position by prominent thorn-like outgrowths developing from the filament below the bent anthers (Loew, 1886).
The dislocation of the abaxial anthers can be combined with additional functions of the filament and/or with a sterilization of the abaxial stamens and even pollen sacs. The first case is observed in the Scrophulariaceae. In many flowers the anthers of the lower (abaxial) stamen pair are dislodged under the upper lip by their long filaments, the flower entrance remains open and is marked by a yellow signal on the lower lip. In Craterostigma plantaginea the filament is fused to the lower lip. Its backwards bending starts within the fused zone, resulting in a common bulge, which is conspicuously yellow-coloured and narrows the entrance. In Torenia polygonoides the bending of the filament only starts above the fused zone resulting in free ‘filament knees’, which guide the visitors. The yellow signal again is on the lower lip. In Lindernia the abaxial stamen pair is sterile and completely modified into coloured guiding structures (R. Claßen-Bockhoff, unpubl. res.).
The second case is found in Salvia, in which the adaxial pair of stamens is always sterile, forming small-to-minute staminodes, while the abaxial pair is modified into lever-like structures. The lever arms are formed by the widened connective, which thereby separates the two thecae from each other. Dependent on the distance between the two thecae, the stamens tend to become monothecate. While, for instance, in S. officinalis, S. scabra or S. roemeriana both thecae still produce pollen, the posterior connective arms are modified into sterile plates of high morphological and functional diversity in the majority of the sages (see Claßen-Bockhoff et al., 2004a, b; Walker and Sytsma, 2007; Wester and Claßen-Bockhoff, 2007). Lever-like stamens on the adaxial side of the flower are observed in Australian Lamiaceae (Fig. 6L), only in a few species compared with the immense number of sages (Guerin, 2005). Further studies on the biomechanics of these adaxial levers are needed to show whether it is the dorsal attachment that makes the lever mechanism less precise compared to abaxial levers, which to different degrees support themselves by their filaments (Fig. 6H).
Pollinators
The great majority of bilabiate blossoms are melittophilous, i.e. pollinated by bees. As noted above, bats and especially birds form further important pollinator guilds. Obviously, upward movements of the bird's beak are less brusque and thus less injuring than those in the opposite direction, thus favouring lip blossoms. Moreover, ornithophilous keel blossoms are next to impossible (with the exception of Strelitzia; Westerkamp, 1997) as the flowers rarely reach the size necessary for a bird to perch on the wing-keel complex. When perching outside the flower, the curvature of the bird's beak and that of the keel and the column contained within it mean that it is unable to open the keel and thus liberate stigma and pollen. When inserting the beak from the opposite (vexillar) side, however, keel, column and beak have the same curvature and thus a liberation of stigma and pollen is possible. Thus, melittophilous keel blossoms changed into ornithophilous lip blossoms in the Fabaceae.
Moths and long-tongued flies were able to enter after a considerable relative lengthening of the alignment channel. Other flies (for example in Guazuma ulmifolia, Malvaceae; Westerkamp et al., 2006) and beetles (in Orchidantha, Lowiaceae; Sakai and Inoue, 1999) to our knowledge only rarely participate in bilabiate flower pollination.
CONCLUSIONS
In our wide definition, every specialized nototribic construction is a bilabiate blossom. As the overwhelming number of lip flowers with melittophilous specialization demonstrates, the latter are obviously a perfect response to the biology and morphology of bees. New radiations became possible with the utilization of bees as pollinators, as demonstrated by the success of lip flowers in, for example, Lamiales, Zingiberales and Orchidaceae. The nototribic pollen deposition favoured not only the pollination by pollen-interested bees, but also by birds that usually are only concerned with nectar.
But evolution goes on – perhaps with the exception of highly eusocial bees largely independent of their environment (Westerkamp, 1987, 1991). Thus, even good fittings may lose their significance. A striking example is the reduction of the staminal lever mechanism in Salvia due to the shift from bee to bird pollination (see Wester and Claßen-Bockhoff, 2007). On the one hand, bees (and honey wasps, Celonites abbreviatus, Masaridae; Schremmer, 1959, Müller, 1996) may evolve specialized stiff hairs with different inclinations (Ebmer and Schwammberger, 1986) on the forehead (clypeus and/or frons; Müller, 1996; Fig. 1A: Rophites trispinosus, Halictidae) with which they scrape pollen directly from the anthers above the visitor; Müller (1996), Thorp (2000) and Gonzalez and Chavez (2004) mention examples from Halicidae, Andrenidae, Megachilidae and Anthophoridae. Thorp (2000) refers to the genus Pectinapis (Eucerini) with a comb of flattened setae (the pectin) on the head. The behaviour of these bees may differ: while some species make nodding or rubbing movements, others press their head against the anthers and sonicate at the same time; Rophites is even said to combine these behaviours (Müller, 1996). On the other hand, bees are inventive, especially when there is a need to find more pollen. This behaviour is not restricted to eusocial bees (Fig. 1B, see Westerkamp, 1987), but is also observed in other groups. In Salvia glutinosa, Megachile circumcincta (Megachilidae) lands on top of the upper lip, then moves to a hanging position below it, grips the fertile ends of the levers, removes them from their cover and brushes pollen from the thecae with the hindlegs (Schremmer, 1941). In the same flower species, Anthidium manicatum (Megachilidae) lands on the lower lip and enters the flower so far as to liberate the fertile ends of the lever, and then begins brushing pollen from the anthers with the upper side of the abdomen (Westerkamp, 1987). In the lip flower of Canavalia brasiliensis (Fabaceae, see above, Fig. 1D), adapted to large Xylocopa species, the small X. frontalis strongly curves its body after freeing the anthers from their cover in order to use the length of its abdomen to give support for brushing pollen from the anthers, which are otherwise inaccessible to it (Fig. 1D).
So clearly the construction of bilabiate flowers is a good response to bees, but certainly not the ultimate one; because, as always, the better is the enemy of the good.
ACKNOWLEDGEMENTS
We thank Petra Wester for critically reading the manuscript, Lars Nauheimer for providing us with two photographs (Fig. 6G, K) and Linda Klöckner (both Mainz) for working over our illustrations. We are grateful to two anonymous reviewers for their remarks, which helped to improve the text.
LITERATURE CITED
- Alexandersson R, Johnson SD. Pollinator-mediated selection of flower-tube length in a hawkmoth-pollinated Gladiolus (Iridaceae). Proceedings of the Royal Society of London, Series B., Biological Sciences; 2002. pp. 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves dos Santos I, Wittmann D. The proboscis of the long-tongued Ancyloscelis bees (Anthophoridae/Apoidea), with remarks on flower visits and pollen collecting with the mouthparts. Journal of the Kansas Entomological Society. 2000;72:277–288. [Google Scholar]
- Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- Burkill IH. Notes on the pollination of flowers in India. Note No. 1. The pollination of Thunbergia grandiflora, Roxb., in Calcutta. Journal and Proceedings of the Asiatic Society of Bengal, N.S. 1906;2:511–515. [Google Scholar]
- Castellanos MC, Wilson P, Thomson JD. ‘Anti-bee’ and ‘pro-bird’ changes during the evolution of hummingbird pollination in Penstemon flowers. Journal of Evolutionary Biology. 2004;17:876–885. doi: 10.1111/j.1420-9101.2004.00729.x. [DOI] [PubMed] [Google Scholar]
- Claßen-Bockhoff R. Pattern analysis in pseudanthia. Plant Systematics and Evolution. 1990;171:57–88. [Google Scholar]
- Claßen-Bockhoff R. Anthodien, Pseudanthien und Infloreszenzblumen. Beiträge zur Biologie der Pflanzen. 1991;66:221–240. [Google Scholar]
- Claßen-Bockhoff R, Wester P, Tweraser E. The staminal lever mechanism in Salvia L. (Lamiaceae) – a review. Plant Biology. 2003;5:33–41. [Google Scholar]
- Claßen-Bockhoff R, Speck T, Tweraser E, Wester P, Thimm S, Reith M. The staminal lever mechanism in Salvia L. (Lamiaceae): a key innovation for adaptive radiation? Organisms, Diversity and Evolution. 2004;4:189–205. [Google Scholar]
- Claßen-Bockhoff R, Crone M, Baikova E. Stamen development in Salvia L.: Homology reinvestigated. International Journal of Plant Science. 2004;165:475–498. [Google Scholar]
- Darwin C. On the effects of cross and self fertilization in the vegetable kingdom. London: Murray; 1876. [Google Scholar]
- Dobson HEM, Bergström G. The ecology and evolution of pollen odors. Plant Systematics and Evolution. 2000;222:63–87. [Google Scholar]
- Donoghue MJ, Ree RH, Baum DA. Phylogeny and the evolution of flower symmetry in the Asteridae. Trends in Plant Sciences. 1998;3:311–317. [Google Scholar]
- Dressler S, Tschapka M. Bird versus bat pollination in the genus Marcgravia and the description of a new species. Curtis's Botanical Magazine (series 6) 2002;19:104–114. [Google Scholar]
- Ebmer AW, Schwammberger KH. Die Bienengattung Rophites SPINOLA 1808 (Insecta: Hymenoptera: Apoidea: Halictidae: Dufoureinae). Illustrierte Bestimmungstabellen. Senckenbergiana biologica. 1986;66:271–304. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Endress PK. Symmetry in flowers: diversity and evolution. International Journal of Plant Science. 1999;160(Suppl.):S3–S23. doi: 10.1086/314211. [DOI] [PubMed] [Google Scholar]
- Endress PK. Evolution of floral symmetry. Current Opinion in Plant Biology. 2001;4:86–91. doi: 10.1016/s1369-5266(00)00140-0. [DOI] [PubMed] [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. 3rd edn. Oxford: Pergamon Press; 1979. [Google Scholar]
- Giurfa M, Dafni A, Neal PR. Floral symmetry and its role in plant-pollinator systems. International Journal of Plant Science. 1999;160(suppl.):S41–S50. doi: 10.1086/314214. [DOI] [PubMed] [Google Scholar]
- Givnish TJ. Adaptive radiation and molecular systematics: issues and approaches. In: Givnish TJ, Sytsma KJ, editors. Molecular evolution and adaptive radiation. Cambridge: University Press; 1997. pp. 1–54. [Google Scholar]
- Gonzalez VH, Chavez FC. Nesting biology of a new high Andean bee, Anthophora walteri Gonzalez (Hymenoptera: Apidae: Anthophorinni) Journal of the Kansas Entomological Society. 2004;77:584–592. [Google Scholar]
- Gottsberger G, Camargo JMF, Silberbauer-Gottsberger I. A bee-pollinated tropical community: the beach dune vegetation of Ilha de Sao Luís, Maranhão, Brazil. Botanische Jahrbücher. 1988;109:469–500. [Google Scholar]
- Grinfel'd EK. Origin and development of the apparatus for pollen collection in bees (Hymenoptera: Apoidea) Entomological Review. 1962;41:37–42. [Google Scholar]
- Guerin G. Floral biology of Hemigenia and Microcorys (Lamiaceae) Australian Journal of Botany. 2005;53:147–162. [Google Scholar]
- Heinrich B. Foraging specializations of individual bumble bees. Ecological Monographs. 1976;46:105–128. [Google Scholar]
- Hildebrand F. Über die Befruchtung der Salviaarten mit Hilfe der Insekten. Jahrbuch für wissenschaftliche Botanik. 1865;4:451–476. [Google Scholar]
- Holttum RE. The Zingiberaceae of the Malay Peninsula. Garden Bulletin Singapore. 1950;13:1–250. [Google Scholar]
- Hübenthal JA, Kraemer M, Claßen-Bockhoff R. Mechanical isolation among sympatric hummingbird isolated Alloplectus species (Gesneriaceae) in the Ecuadorian cloud forest. Palmarum Hortus Francofurtensis. 2003;7:170. [Google Scholar]
- Johnson SD, Steiner KE. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae) Evolution. 1997;51:45–53. doi: 10.1111/j.1558-5646.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Kadereit JW. Lamiales: Introduction and Conspectus. In: Kadereit JW, editor. The families and genera of vascular plants. Vol. VII, Flowering plants, dicotyledons, Lamiales (except Acanthaceae including Avicenniaceae). Berlin, Heidelberg: Springer; 2004. pp. 1–8. [Google Scholar]
- Kevan PG, Lane MA. Flower petal microtexture is a tactile cue for bees. Proceedings of the National Academy of Science; 1985. pp. 4750–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston BL. Papillate tepal hoods and delayed pollen germination in Albuca L. (Liliaceae) Annals of Botany. 1998;82:263–266. [Google Scholar]
- Lewis G, Schrire B, Mackinder B, Lock M. Legumes of the World. Kew, London: Royal Botanic Gardens; 2005. [Google Scholar]
- Loew E. Beobachtungen über den Blumenbesuch von Insekten an Freilandpflanzen des Botanischen Gartens zu Berlin. Jahrbuch Königlich Botanischer Garten zu Berlin. 1884/6;1884:69–118. 253–296, 1886 93 178. [Google Scholar]
- Loew E. Einführung in die Blütenbiologie auf historischer Grundlage. Berlin: Dümmler; 1895. [Google Scholar]
- Lovell JH. Bees which visit only one species of flower. Population Science Monthly. 1912;81:197–203. [Google Scholar]
- Lovell JH. The origin of the oligotropic habit among bees. Entomological News. 1913;24:104–112. [Google Scholar]
- Lovell JH. The origin of oligotropism. Entomological News. 1914;25:314–321. [Google Scholar]
- Lunau K, Wacht S, Chittka L. Colour choices of naive bumble bees and their implications for colour perception. Journal of Comparative Physiology A. 1996;178:477–489. [Google Scholar]
- Mabberley DJ. The plant book. A portable dictionary of the vascular plants. 2nd edn. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Magin N, Claßen R, Gack C. The morphology of false anthers in Craterostigma plantagineum and Torenia polygonoides (Scrophulariaceae) Canadian Journal of Botany. 1989;67:1931–1937. [Google Scholar]
- Manning JC, Goldblatt P. The Prosoeca peringueiyi (Diptera: Nemestrinidae) pollination guild in southern Africa: long-tongued flies and their tubular flowers. Annals of the Missouri Botanical Garden. 1995;82:517–534. [Google Scholar]
- Meeuse ADJ. Anthecology of the Labiatae: an armchair approach. In: Harley RM, Reynolds T, editors. Advances in labiate science. Kew, London: Royal Botanic Gardens; 1992. pp. 183–191. [Google Scholar]
- Michener CD, Winston ML, Jander R. Pollen manipulation and related activities and structures in bees of the family Apidae. University of Kansas Science Bulletin. 1978;51:575–601. [Google Scholar]
- Müller A. Morphological specializations in the Central European bees for the uptake of pollen from flowers with anthers hidden in narrow corolla tubes (Hymenoptera: Apoidea) Entomologia Generalis. 1995;20:43–57. [Google Scholar]
- Müller A. Convergent evolution of morphological specializations in Central European bee and honey wasp species as an adaptation to the uptake of pollen from nototribic flowers (Hymenoptera, Apoidea and Masaridae) Biological Journal of the Linnean Society. 1996;57:235–252. [Google Scholar]
- Müller A. Unusual host plant of Hoplitis pici, a bee with hooked bristles on its mouthparts (Hymenoptera: Megachilidae: Osmiini) European Journal of Entomology. 2006;103:497–500. [Google Scholar]
- Müller A, Diener S, Schnyder S, Stutz K, Sedivy C, Dorn S. Quantitative pollen requirements of solitary bees: Implications for bee conservation and the evolution of bee-flower relationships. Biological Conservation. 2006;130:604–615. [Google Scholar]
- Neal PR, Dafni A, Giurfa M. Floral symmetry and its role in plant-pollinator systems: terminology, distribution, and hypotheses. Annual Reviews of Ecolological Systematics. 1998;29:345–373. [Google Scholar]
- Nilsson LA. The evolution of flowers with deep corolla tubes. Nature. 1988;334:147–149. [Google Scholar]
- Parker FD, Tepedino VJ. A nest and pollen-collection records of Osmia scullenii Sandhouse, a bee with hooked hairs on the mouthparts (Hymenoptera: Megachilidae) Journal of the Kansas Entomological Society. 1982;55:329–334. [Google Scholar]
- Peters DS. Über die Untergattung Haetosmia Popov 1952. Senckenbergiana Biologica. 1974;55:293–309. [Google Scholar]
- Prance GT. The pollination of Amazonian plants In. In: Prance GT, Lovejoy TE, editors. Key environments: Amazonia. Oxford: Pergamon Press; 1985. pp. 166–191. [Google Scholar]
- Prance GT, White F. The genera of the Chrysobalanaceae: a study in practical and theoretical taxonomy and its relevance to evolutionary biology. Philosphical Transactions of the Royal Society of London, B. 1988;320:1–184. [Google Scholar]
- Raju AJS, Rao SP, Ezdaranam V. Bird pollination in Sterculia colorata Roxb. (Sterculiaceae), a rare tree species in the Eastern Ghats of Visakhapatanam and East Godavari Districts of Andhra Pradesh. Current Science. 2004;87:28–31. [Google Scholar]
- Reith M, Baumann G, Claßen-Bockhoff R, Speck T. New insights into the functional morphology of the lever mechanism of Salvia pratensis (Lamiaceae) Annals of Botany. 2007;100:393–400. doi: 10.1093/aob/mcm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke JP. A revision of the genus Mimetes Salisb. (Proteaceae) Journal of South African Botany. 1984;50:171–236. [Google Scholar]
- Rudall PJ, Bateman RM. Roles of synorganization, zygomorphy and heterotopy in floral evolution: the Gynostemium and labellum of orchids and other lilioid monocots. Biological Reviews. 2002;77:403–441. doi: 10.1017/s1464793102005936. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Bateman RM. Evolution of zygomorphy in monocot flowers: iterative patterns and developmental constraints. New Phytologist. 2004;162:25–44. [Google Scholar]
- Rudall PJ, Bateman RM, Fay MF, Eastman A. Floral anatomy and systematics of Alliaceae with special reference to Gilliesia, a presumed insect mimic with strongly zygomorphic flowers. American Journal of Botany. 2002;89:1867–1883. doi: 10.3732/ajb.89.12.1867. [DOI] [PubMed] [Google Scholar]
- Sakai S, Inoue T. A new pollination system: dung-beetle pollination discovered in Orchidantha inouei (Lowiaceae, Zingiberales) in Sarawak, Malaysia. American Journal of Botany. 1999;86:56–61. [PubMed] [Google Scholar]
- Schlindwein C, Wittmann D, Martins CF, Hamm A, Siqueira JA, Schiffler D, Machado IC. Pollination of Campanula rapunculus L. (Campanulaceae): how much pollen flows into pollination and intro reproduction of oligolectic pollinators? Plant Systematics and Evolution. 2005;250:147–156. [Google Scholar]
- Schremmer F. Eine Bauchsammelbiene (Megachile circumcincta K.) als Zerstörer der Blüten von Salvia glutinosa. Zoologischer Anzeiger. 1941;133:230–232. [Google Scholar]
- Schremmer F. Der bisher unbekannte Pollensammelapparat der Honigwespe Celonites abbreviatus Vill. (Vespidae, Masarinae) Zeitschrift für Morphologie und Ökologie der Tiere. 1959;48:424–438. [Google Scholar]
- Schremmer F. Der Stechsaugrüssel, der Nektarraub, das Pollensammeln und der Blütenbesuch der Holzbienen (Xylocopa) (Hymenoptera, Apidae) Zeitschrift für Morphologie und Ökologie der Tiere. 1972;72:263–294. [Google Scholar]
- Sérsic AN. Pollination biology in the genus Calceolaria L. (Calceolariaceae) Stapfia. 2004;82:1–121. [Google Scholar]
- Sprengel CK. Das entdeckte Geheimniss der Natur im Bau und in der Befruchtung der Blumen. Berlin: Vieweg; 1793. (Reprint 1972: Cramer) [Google Scholar]
- Strickler K. Specialization and foraging efficiency of solitary bees. Ecology. 1979;60:998–1009. [Google Scholar]
- Temeles EJ, Rankin AG. The effect of the lower lip of Monarda didyma on pollen removal by hummingbirds. Canadian Journal of Botany. 2000;78:1164–1168. [Google Scholar]
- Thorp RW. Structural, behavioral, and physiological adaptations of bees (Apoidea) for collecting pollen. Annals of the Missouri Botanical Garden. 1979;66:788–812. [Google Scholar]
- Thorp RW. The collection of pollen by bees. Plant Systematics and Evolution. 2000;222:211–223. [Google Scholar]
- Troll W. Roscoea purpurea Sm., eine Zingiberacee mit Hebelmechanismus in den Blüten. Planta. 1929;7:1–28. Mit Bemerkungen über die Entfaltungen der fertilen Staubblätter von Salvia. [Google Scholar]
- Troll W. Botanische Notizen II. Abhandlungen der Akademie der Wissenschaften und der Literatur Mainz, Mathematisch-Naturwissenschaftliche Klasse. 1951;1951:25–80. [Google Scholar]
- Vogel S. Blütenbiologische Typen als Elemente der Sippengliederung, dargestellt anhand der Flora Südafrikas. Botanische Studien. 1954;1:1–338. [Google Scholar]
- Vogel S. Evolutionary shifts from reward to deception in pollen flowers. Linnean Society Symposium Series. 1978;6:89–96. [Google Scholar]
- Walker JB, Sytsma KJ. Staminal evolution in the genus Salvia (Lamiaceae): molecular phylogenetic evidence for multiple origins of the staminal lever. Annals of Botany. 2007;100:375–391. doi: 10.1093/aob/mcl176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AR. Darwinism – an exposition of the theory of natural selection with some of its applications. London: MacMillan; 1889. [Google Scholar]
- Waser NM. Flower constancy: definition, cause, and measure. American Naturalist. 1986;127:593–603. [Google Scholar]
- Wasserthal LT. The pollinators of Malagasy star orchids Angraecum sesquipedale, A. sororium and A. compactum and the evolution of extremely long spurs by pollinator shift. Botanica Acta. 1997;110:1–17. [Google Scholar]
- Weber A. Struktur, Antheseverlauf und Bestäubung der Blüte von Nigella arvensis (Ranunculaceae) Verhandlungen Zoologisch-Botanische Gesellschaft Österreich. 1993;130:99–125. [Google Scholar]
- Wester P, Claßen-Bockhoff R. Floral diversity and pollen transfer in bird-pollinated Salvia species. Annals of Botany. 2007;100:401–421. doi: 10.1093/aob/mcm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerkamp C. Das Pollensammelverhalten der sozialen Bienen in Bezug auf die Anpassungen der Blüten. Germany: University of Mainz; 1987. Doctoral Thesis. [Google Scholar]
- Westerkamp C. Bird-flowers: hovering versus perching exploitation. Botanica Acta. 1990;103:366–371. [Google Scholar]
- Westerkamp C. Honeybees are poor pollinators – why? Plant Systematics and Evolution. 1991;177:71–75. [Google Scholar]
- Westerkamp C. The co-operation between the asymmetric flower of Lathyrus latifolius (Fabaceae–Vicieae) and its flower visitors. Phyton. 1993;33:121–137. [Google Scholar]
- Westerkamp C. Pollen in bee-flower relations. Some considerations on melittophily. Botanica Acta. 1996;109:325–332. [Google Scholar]
- Westerkamp C. Keel blossoms: bee flowers with adaptations against bees. Flora. 1997;192:125–132. [Google Scholar]
- Westerkamp C, Paul H. Apios americana, a fly-pollinated papilionaceous flower? Plant Systematics and Evolution. 1993;187:135–144. [Google Scholar]
- Westerkamp C, Soares AA, Amaral Neto LPA. Guazuma ulmifolia (Malvaceae–Byttnerioideae): female and male booths with separate entrances. Flora. 2006;201:389–395. [Google Scholar]
- Westrich P. Die Wildbienen Baden-Württembergs. Stuttgart: Ulmer; 1989. [Google Scholar]
- Williams IH. Behaviour of insects foraging on pigeon pea (Cajanus cajan (L.) Millsp.) in India. Tropical Agriculture. 1977;54:353–363. [Google Scholar]
- Yeo PF. Secondary pollen presentation. Form, function and evolution. Plant Systematics and Evolution. 1993;6:1–268. (Suppl.) [Google Scholar]