Abstract
Immunoglobulin domains are found in a wide variety of functionally diverse transmembrane proteins, and also in a smaller number of cytoplasmic proteins. Members of this latter group are usually associated with the actin cytoskeleton, and most of them bind directly to either actin or myosin, or both. Recently, studies of inherited human disorders have identified disease-causing mutations in five cytoplasmic Ig-domain proteins: myosin-binding protein C, titin, myotilin, palladin, and myopalladin. Together with results obtained from cultured cells and mouse models, these clinical studies have yielded novel insights into the unexpected roles of Ig domain proteins in mechanotransduction and signaling to the nucleus. An emerging theme in this field is that cytoskeleton-associated Ig domain proteins are more than structural elements of the cell, and may have evolved to fill different needs in different cellular compartments.
Keywords: titin, palladin, MyBP-C, myotilin, myopalladin
Introduction
The term immunoglobulin (Ig) superfamily is used to describe a large and diverse group of proteins that share a common domain, which is called the Ig domain or Ig fold. Ig domains consist of 70–100 amino acids, and they possess a characteristic “sandwich” structure, with between seven and nine β strands arranged into two sheets (Richardson et al., 1976; Williams and Barclay, 1988; Bork et al., 1994). The founding members of this superfamily were the immunoglobulins, and other family members include cell adhesion molecules such as NCAM and VCAM, integrin counter-receptors such as ICAM, and a variety of other trans-membrane proteins. Although most Ig domain proteins are targeted to the plasma membrane, a distinctive subset is found in the cytoplasm, and these typically interact with the actin cytoskeleton. The majority of these cytoskeleton-associated Ig-domain proteins are expressed in striated muscle, although a few are also expressed in non-muscle cell types. In recent years, studies of inherited human disorders have identified disease-causing mutations in many of the cytoplasmic Ig-domain proteins, suggesting that this group of molecules is critically involved in regulating actin-dependent cell functions. In this review, we will focus on five members of the group that have significant links to human disease: myosin binding protein C (MyBP-C), titin, myotilin, palladin and myopalladin.
The Overall Structure of the Ig Domain is Highly Conserved
Members of the Ig superfamily are found within groups of distantly related proteins, with diverse functions and tissue distributions, but are related by the presence of one or more small modules consisting of the Ig fold (Halaby et al., 1998). Although sequence similarity among Ig domains is low in many cases, the structure of the beta sandwich is conserved (Halaby et al., 1999). Whether Ig domains in unrelated proteins are homologous (generated from divergent evolution) or analogous (generated from convergent evolution) has been debated (Halaby et al., 1999), yet it is clear that the Ig fold has been utilized by nature in many ways, probably due to the stability of the fold and its utility in mediating protein-protein interactions (Bork et al., 1994, Williams and Barclay, 1988, Halaby et al., 1998).
By convention, the strands of Ig domains are sequentially named A, B, C, D, E, F, and G. All Ig domains share a common core composed of four beta strands (BCEF) and can be further subdivided based on the presence and position of an additional three to five beta strands (Bork et al., 1994). Most commonly, four types of Ig domains have been identified based on their structures, although alternate sub-classification has been proposed (Williams and Barclay, 1988; Hunkapiller and Hood, 1989; Harpaz and Chothia, 1994; Halaby et al., 1999). Originally, Ig domains were described as either V or C type, based on their similarities to the variable (V) and constant (C) domains of immunoglobulin, with the C type being further divided into C1 and C2 or H (Williams and Barclay, 1988; Hunkapiller and Hood, 1989). Members of the V-set are the largest; they contain nine beta strands. The core BCEF strands are present in V-set Ig domains, as well as the exterior A, D, G, C’, and C’’ strands. In V-set Ig domains, the A strand is discontinuous and the two parts of the strand are usually labeled A and A’. The A strand first hydrogen bonds to the B strand in an anti-parallel fashion, then crosses over to the other beta sheet where A’ hydrogen bonds to the G strand in a parallel fashion.
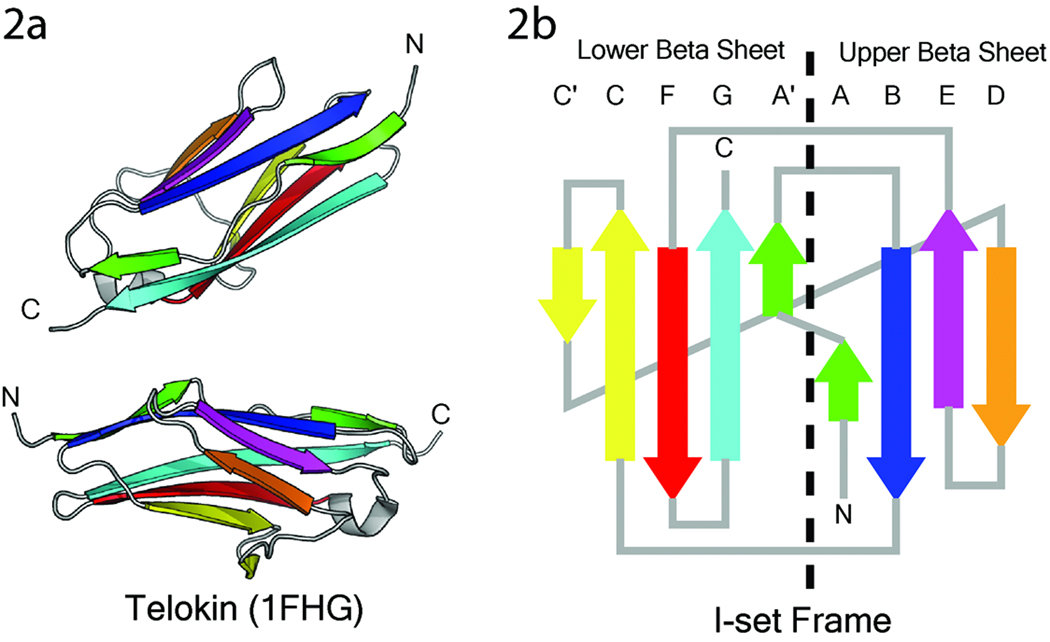
With the increase of available protein structures, it has become possible to subdivide the Ig superfamily further. The crystal structure of telokin (Holden et al, 1992) (Figure 1a), the C-terminal domain of myosin light chain kinase, was the first structure of an Ig domain from a muscle protein. The structure of telokin revealed a fold that contained features of both the V-set and C-set of Ig domains and prompted the identification of a new structural subgroup called the I-set (Harpaz and Chothia, 1994). Like the V-set Ig domains, members of the I-set have a discontinuous A strand that hydrogen bonds to the B strand in one of the beta sheets and then changes levels to hydrogen bond to the G strand of the second beta sheet (Figure 1b). Both a C’ and D strand are present in I-set Ig domain, making them larger than C-type and smaller than V-set Ig domains. Although the I-set was first identified in a muscle protein, it was subsequently recognized that many previously identified Ig domains were best classified as I-set Ig domains.
Figure 1. The crystal structure of telokin and the framework of an I-set Ig domain.
The crystal structure of telokin (pdb code 1FHG), the founding member of the I-set, is shown from both sides as a ribbon representation of the backbone. Members of the I-set of Ig domains share a common fold pattern consisting of two sandwiched beta sheets, depicted as a two dimensional template (2b). The upper beta sheet consists of beta strands A, B, D, and E; the lower beta sheet consists of beta strands A’, C, C’, F, and G. The coloring of the strands in the template is the same as the coloring of the beta strands in the structures of telokin.
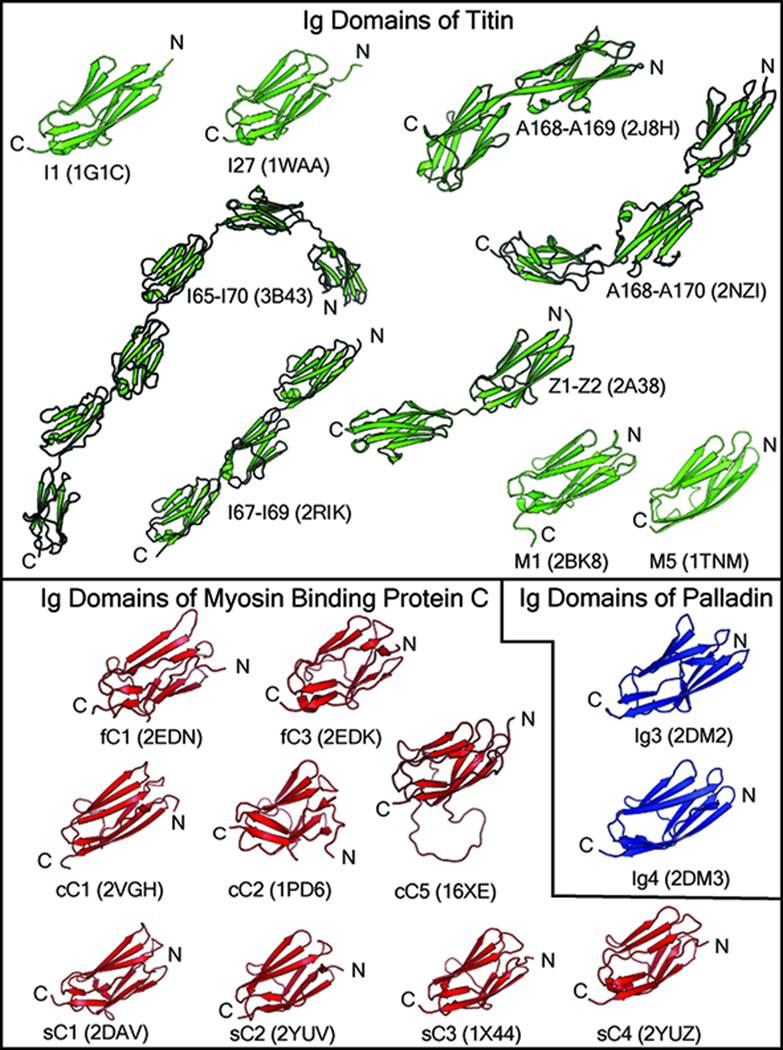
The Ig domains found in titin, MyBP-C, and palladin are members of the I-set. Structurally, these domains are very similar, though they appear to have evolved different functions (discussed in further detail below). In Figure 2, the currently available high-resolution structures of the Ig domains from each of these family members are shown. The Ig domains of titin are involved in the structural and regulatory functions of titin, conferring elasticity in the I-band and participating in binding interactions in the A-band, M-line, and Z-line that maintain the structural integrity of the sarcomere (Tskhovrebova and Trinick, 2004). The palladin Ig3 domain is an actin-binding domain (Dixon et al., 2008), and its Ig4 domain has been associated with actin bundling (Dixon et al., 2008) and, along with the Ig5 domain, the binding of ezrin (Mykkanen et al., 2001). The Ig domains of MyBP-C are involved in binding to titin, binding to actin, binding to myosin, and interdomain interactions (reviewed in Flashman et al., 2004).
Figure 2. Structures of Ig domains from titin, palladin, and myosin binding protein-C.
High-resolution structures of titin Ig domains have been solved for representative Ig domains from the proximal Ig segment (I1), the distal Ig segment (I27 and I65–I70), the P-zone of the A-band (A168–A169), the M-line (M1 and M5), and the Z-line (Z1–Z2). The A170 domain is a fibronectin type 3 (Fn3) domain that was solved in tandem with the A168 and A169 Ig domains (pdb code 2NZI). The palladin Ig3 and Ig4 domains are the only structures of Ig domains currently available from the palladin family of proteins. High-resolution structures are available for the fast-type isoform (fC1 and fC3), the cardiac isoform (cC1, cC2, and cC5), and the slow-type isoform (sC1, sC2, sC3, and sC4). The cC5 Ig domain contains an unstructured 28 amino acid insert between the C and D beta strands.
Myosin Binding Protein C: a Role in Sarcomere Function and Heart Disease
Myosin Binding Protein C (MyBP-C, also known as C-protein) was identified over thirty years ago as a 137 kD protein that contaminated preps of purified myosin isolated from vertebrate skeletal muscle (Offer et al., 1973). MyBP-C has a distinctive localization pattern in the sarcomere: it concentrates in vertical stripes, seven to nine in number depending on the muscle type (Winegrad 1999; Flashman et al., 2004). These are spaced approximately 43 nm apart, running parallel to the M-line (illustrated in Figure 3). There are three isoforms of MyBP-C encoded by different genes and classified as fast skeletal, slow skeletal, and cardiac. Both skeletal muscle isoforms contain 10 domains (C1–C10): seven copies of the Ig domain, and three copies of the fibronectin type III domain, in the order Ig-Ig-Ig-Ig-Ig-Fn-Fn-Ig-Fn-Ig (Einheber and Fischman,1990; Weber et al., 1993). The cardiac isoform (cMyBP-C) possesses an additional Ig domain (C0) added at the N-terminus, a LAGGGRRIS sequence within the MyBP-C motif between the C1 and C2 domains, and contains a 28 amino acid unstructured insertion in the central C5 domain (Yasuda et al., 1995). Between the C1 and C2 Ig domains there is a sequence of ~100 amino acids that has been named the MyBP-C motif. Small-angle X-ray scattering (SAXS) data has revealed that the MyBP-C motif has a compact structure with dimensions similar to an Ig fold, however a high-resolution structure of this sequence is not currently available and the sequence does not have significant sequence homology to the Ig or Fn3 domains of MyBP-C (Jeffries et al. 2008). Presently, the MyBP-C motif is classified as an ‘m-domain’ (Jeffries et al. 2008).
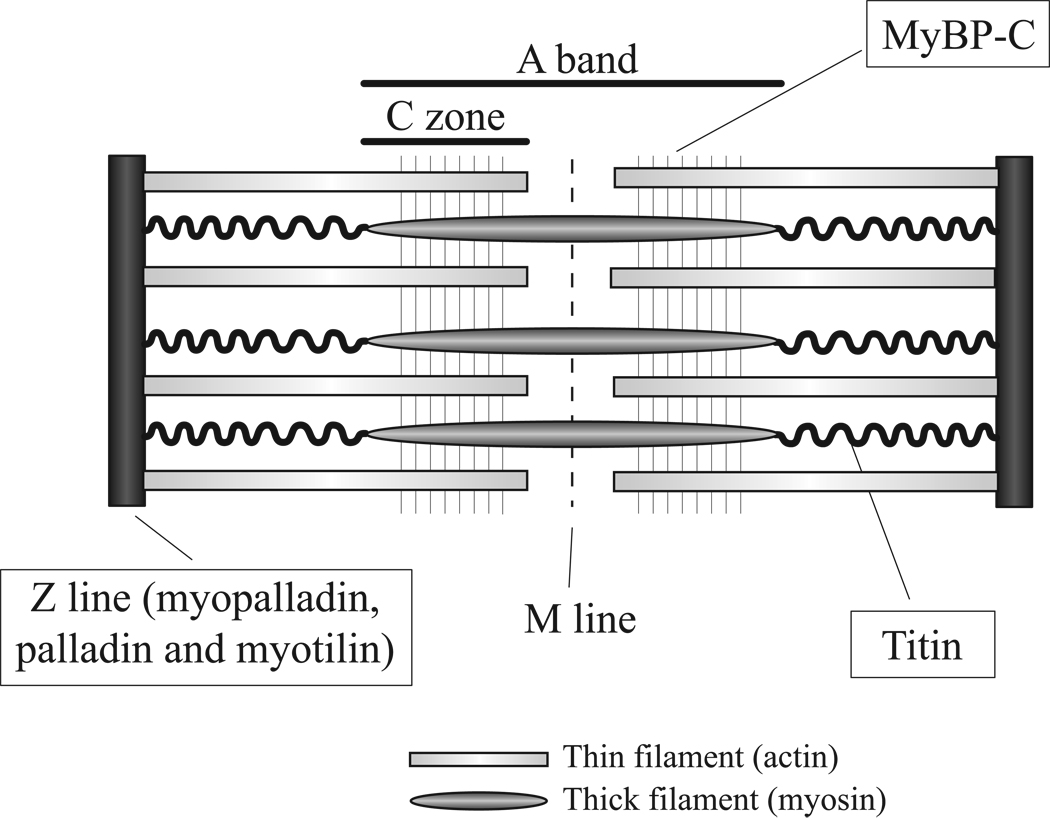
Figure 3. Location of Ig domains proteins within the sarcomere in striated muscle.
MyBP-C is found in transverse stripes in the C-Zone. Myopalladin, palladin and myotilin localize to the Z line. Titin spans from the Z-line to the M-line.
Interest in MyBP-C proteins has intensified in recent years because of research that links mutations in the gene encoding cardiac MyBP-C to two different types of familial heart disease: hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM). HCM is characterized by thickening of the walls of the left ventricle and an increased risk of heart failure, and it is a relatively common condition, occurring in 1 out of 500 individuals (reviewed in Tardiff, 2005). Although many patients with HCM live a normal lifespan, this disorder is believed to be a leading cause of sudden cardiac death in young athletes. The familial form of HCM exhibits an autosomal dominant pattern of inheritance, and 20–45% of the disease-causing mutations map to the gene encoding cMyBP-C (MYBPC3) (Watkins et al., 1995; Oakley et al., 2004; Tardiff, 2005). The majority of these are frame-shift mutations that result in the expression of truncated forms of cMyBP-C, but a number of point-mutations have also been identified. To date, disease-causing mutations have been found within seven of cMyBP-C’s eight Ig domains (Oakley et al., 2004). The cMyBP-C gene is also involved in familial DCM, a disorder characterized by dilatation and impaired contraction of either both ventricles or the left ventricle. In DCM families, three disease-causing mutations have been found in the cMyBP-C gene, including one in an Ig domain (reviewed in Chang and Potter, 2005). These results demonstrate that MyBP-C plays a critical role in normal cardiac physiology, and that its Ig domains are essential to that role.
MyBP-C binds with moderately high affinity to myosin, and more weakly to titin and actin (reviewed in Oakley et al., 2004). The biological function of these interactions continues to be a subject of intense research to determine if MyBP-C’s function in striated muscle is primarily structural or primarily regulatory, or both. To address this question, a knockout mouse was generated that lacks cMyBP-C expression in the heart (Harris et al., 2002). Characterization of this mouse line revealed that cMyBP-C is not essential for cardiac development or sarcomere assembly, as the knockout mice were viable; however, the mice displayed cardiac hypertrophy and contractility defects. Subsequent ultrastructural analysis of the knockout hearts demonstrated that gross sarcomeric structure is unperturbed in the absence of cMyBP-C, although subtle abnormalities were noted (Luther et al., 2008; Kensler and Harris, 2008). These results suggest that the role of MyBP-C may be primarily regulatory rather than structural.
The Ig domains of MyBP-C are all classified as I-set Ig domains and, with the exception of the unstructured 28 amino acid insertion of the C5 domain of the cardiac isoform of MyBP-C, the Ig domains of MyBP-C are similar in size and structure. The Ig domains of MyBP-C appear to be specialized for different binding interactions. MyBP-C possesses two myosin binding sites, one near the N-terminus and one near the C-terminus. The first has been mapped to a region called cC1C2, a fragment that contains the C1 and C2 Ig domains and the MyBP-C motif, the “m-domain”, that connects them. The cC1C2 fragment binds to myosin S2 and may play a role in regulating force generation (Gruen and Gautel, 1999; Flashman et al., 2004). Although the C2 domain is able to bind myosin in vitro (Ababou et al., 2007), the full fragment is considered the physiological binding fragment. The second myosin-binding site has been mapped to C8–C10, and this binds to the light meromyosin portion of the myosin rod (Moos et al., 1975; Okagaki et al., 1993; Alyonycheva et al., 1997). The titin binding site has been mapped to C9–C10 (Freiburg and Gautel, 1996). The C5 and C8 domains of MyBP-C are able to bind to each other, suggesting a model in which trimers of MyBP-C may be organized into a collar around the thick filament (Moolman-Smook et al., 2002).
Recently, NMR approaches were used in combination with site-directed mutagenesis of the MyBP-C N-terminal Ig domain to gain insight into MyBP-C’s interaction with myosin (Ababou et al., 2008; Govada et al., 2008). The results indicate that MyBP-C binds to myosin near the hinge that connects myosin’s S1 head to its neck region, adding support to the idea that MyBP-C plays a role in regulating the efficiency of muscle contraction. Biophysical and biochemical approaches have also generated new insights into the interactions between MyBP-C and actin (Kulikovskaya et al., 2003; Saber et al., 2008; Whitten et al., 2008). These results show that an N-terminal fragment of cMyBP-C decorates actin filaments in vitro, suggesting that cMyBP-C could displace tropomyosin, priming the thin filaments to interact with myosin. More recent co-sedimentation studies have demonstrated that multiple actin binding sites may be present within the cC1C2 fragment (Shaffer et al., 2009). Actin-binding sites were mapped to both the C1 and m domain and the combined fragment C1m was capable of cross-linking F-actin into tightly packed bundles (Shaffer et al., 2009). Thus, the current body of results indicates that MyBP-C proteins can influence the cardiac contractile cycle through interactions with both thin and thick filaments.
Titin: the Largest Ig-Domain Protein
In striated muscle, contractility is achieved when the thick filaments and thin filaments slide past each other. The sarcomere also contains a third filament type composed of the protein titin, which was originally named connectin (Maruyama, 1976; Maruyama, 1997). Titin is the largest protein known to date, with a molecular mass of 3–4 megadaltons, and a single titin molecule spans from the Z-disc to the M-band of the sarcomere (reviewed in Fukuda et al., 2008). Although titin is encoded by a single gene, alternative splicing gives rise to multiple titin isoforms that are specific to different types of muscle (Granzier et al., 2005; Fukuda et al., 2008). Titin serves at least three important functions in striated muscle: it contributes elasticity, it has a structural role in organizing the sarcomere, and it is linked to multiple signaling pathways, as described in detail below (reviewed in Tskhovrebova and Trinick, 2004; Granzier et al., 2005; Linke 2008). Titin’s multiple Ig domains, with more than 160 copies in some isoforms, contribute to each of these functions.
The elasticity of striated muscle is intrinsic to its physiological task, permitting the muscle to stretch and then return to its resting length with no disruption in sarcomeric organization. When titin was first sequenced in 1995, the protein’s multiple tandem Ig domains were immediately recognized as being a potential source of molecular elasticity, and recent structural studies have confirmed this idea (Labeit et al., 1990; Labeit and Kolmerer, 1995; Improta et al., 1998; Minajeva et al., 2001; von Castelmur et al., 2008). In addition to its role as an elastic element, titin also has an important function as a molecular scaffold. It binds to α-actinin, and may be responsible for anchoring α-actinin in the Z-disc (Young et al., 1998; Young and Gautel, 2000). Titin also binds to both actin and myosin, and cell culture experiments suggest that wild-type titin is required for normal assembly of thick filaments, Z-discs and myofibrils (Miller et al., 2003a).
In addition, titin participates in multiple signaling pathways. The Z-disc protein T-cap (also called telethonin) binds to titin’s N-terminus (Valle et al., 1997; Gregorio et al., 1998; Furukawa et al., 2001), and T-cap interacts with a variety of proteins, including a potassium channel subunit, a muscle growth factor, a muscle LIM domain protein that regulates cellular differentiation, and an ankyrin-repeat protein, suggesting that the titin/T-cap complex may participate in stretch-dependent signaling pathways (Faulkner et al., 2001; Knöll et al., 2002; Kojic et al., 2004). This idea is supported by the observation that mutations in titin’s molecular partner T-cap are associated with inherited cardiomyopathies (Olivé et al., 2008). Titin also binds to the RING finger protein MuRF-1, which appears to play a regulatory role in the degradation of muscle proteins (Witt et al., 2005). Titin’s Ig domains mediate binding to three different ankyrin-repeat proteins, CARP, Arpp and DARP (Miller et al., 2003b). The muscle ankyrin-repeat proteins are developmentally regulated and also upregulated in response to injury or stress, and their interactions with titin are thought to be part of a biomechanical sensing mechanism that may serve to link stretch-induced signals to changes in the levels of specific muscle proteins (Knöll et al., 2002; Ojima et al., 2005; Hoshijima 2006).
Structural Analysis of Titin’s Ig Domains
Titin, in itself, is a striking example of the evolution of Ig domains for diverse functions. In addition to Ig domains, titin contains multiple fibronectin type 3 domains, and these are confined to the A-band of titin. In contrast, Ig domains are distributed across the entire molecule and have been adapted for different functions in different regions of titin (reviewed in Tskhovrebova and Trinick, 2004). By sequence analysis, all of titin’s Ig domains were predicted to be I-set, because they all contain the key residues for the fold (Harpaz and Chothia, 1994; Pfuhl and Pastore, 1995). However, while sequence similarity between Ig domains from different regions of titin is low, consensus motifs are present for Ig domains from the different bands of titin, suggesting that solving the structures of only a few Ig domains from each of the regions of titin might be sufficient to generate homology models (Improta et al., 1996; Pfuhl and Pastore, 1995; Pfuhl et al., 1995). Representative high-resolution structures of individual Ig domains from the M-line (M1 and M5) and I-band (1I and I27), as well as tandem Ig domains from the I-band (I65–I70 and I67–I69) and A-band (A168–A169 and A168–A170), of titin have been solved by either NMR of X-ray crystallography (illustrated in Figure 2), and these have allowed for a better understanding of the structural and functional differences between the Ig domains. The structure of the M5 domain confirmed that the Ig domains of titin are members of the I-set (Pfuhl and Pastore, 1995). The M5 structure also revealed the absence of a C’ strand, so the C’ strand does not appear to be an essential feature of the I-set (Pfuhl and Pastore, 1995).
The basis for titin’s molecular elasticity in the I-band is still not understood completely; however, models for the role of Ig domains in elasticity have been proposed, based on structural and biophysical data obtained with individual and tandem Ig domains (Improta et al., 1996; Improta et al., 1998; von Castelmur et al., 2008). Unlike the Ig domains in the A-band and proximal to the M-line and Z-line, Ig domains from the I-band are arranged in tandem, without a linker sequence between them, so that the end of one Ig domain is contiguous with the start of its neighbor (Improta et al., 1996). The Ig27 domain was the first structure from this region of titin to be solved (Improta et al, 1996). The sequence of the I27 domain revealed the absence of otherwise conserved motifs in titin: a Pro-X-Pro in the BC loop and a Asn-X-X-Gly sequence near the C-terminus were both absent in I-band Ig domains, leading to the speculation that Ig domains in the I-band may be less stable than other Ig domains (Sebestyen et al., 1995). However, the stability studies indicated that Ig domains within the I-band were as stably folded as Ig domains from other regions of titin, and the structure of I27 confirmed this (Politou et al, 1994; Politou et al, 1995; Improta et al, 1996). The structure of I1 led to the observation that formation of a reversible disulfide bridge between the C and E strands could contribute to the elastic properties of the I-band and that this disulphide bond could be present in at least 40 of the Ig domains in the I-band (Mayans et al., 2001). More recently, the investigation of source of elasticity in the I-band has shifted from the properties of individual Ig domains to the interdomain orientations and interplay between the poly-Ig and PEVK sequences (von Castelmur et al., 2008). The structure of I65–I70 and small angle x-ray scattering revealed that the I-band contains segments of conformationally stiff tandem Ig domains with regularly spaced hinge regions that can be extended under mechanical stress (von Castelmur et al., 2008). Consequently, a new model of I-band elasticity has been proposed, called the “carpenter-ruler” model in which extension of the I-band is based on a few flexor points (von Castelmur et al., 2008).
The three domains near the C-terminus, A168–A170, form a binding interface for MuRF-1 (Centner et al., 2001). MuRF-1 is a regulator of myofibril turnover and muscle atrophy (Bodine, et al., 2001). The structure of the tandem A168–A169 fragment revealed a 17 amino acid beta sheet connecting the two Ig domains, and forms an extended, rigid orientation of the two Ig domains (Müller et al., 2007). The two Ig domains and fibronectin type III domain (A170) exhibit long-range domain orientation that creates a binding interface for the recruitment of MuRF-1 in which all three domains are involved (Mrosek, et al., 2007). These results should provide the information needed for detailed studies of the molecular interaction between titin and MuRF-1, which may be central to understanding titin’s role as biomechanical sensor.
Role of Titin in Human Disorders
The enormous size of titin makes it challenging to investigate this protein’s function using common experimental tools, but much has been learned about titin’s physiological role from medical studies of human myopathies. In 2002, mutations in the titin gene were linked to a disorder called tibial muscular dystrophy, or TMD (Hackman et al., 2002). TMD is a late-onset myopathy that results in weakness and atrophy of the tibialis anterior muscle, located in the calf of the leg. Since the first titinopathy was described, additional mutations in the titin gene have been associated with a limb-girdle muscular dystrophy (LGMD2J), and with dilated cardiomyopathy (Gerull et al., 2002; Hein and Schaper, 2002; Gerull et al., 2006; LeWinter et al., 2007). These titinopathies are typically late-onset; for example, symptoms of TMD usually occur at age 35–45 years, or much later, even in the seventh decade of life. Recently, an unusual titinopathy was identified in two families of Moroccan and Sudanese origin. This disorder involves both the skeletal muscle and the heart, and the symptoms develop in childhood, resulting in a fatal form of dilated cardiomyopathy (Carmignac et al., 2007). Clearly, additional insights into the molecular function of titin will be needed in order to understand the precise molecular basis for these human disorders.
Myotilin: a Role in Organizing the Z-Disc
At 57 kD, myotilin is one of the smaller members of this protein family, containing only two Ig domains (Salmikangas et al., 1999). Like many cytoplasmic Ig-domain proteins, myotilin is expressed predominantly in striated muscle, where it localizes to the Z-disc (Figure 3). Myotilin was originally identified as a binding partner for α-actinin in a yeast two-hybrid screen, and it also binds directly to the Z-disc proteins FATZ-1 and FATZ-2 (Salmikangas et al., 1999; Gontier et al., 2005). In addition, myotilin binds to F-actin, and to the muscle isoform of filamin (filamin C or γ filamin), and both of these binding interactions map to a region that contains myotilin’s Ig-domains (van der Ven et al., 2000; Salmikangas et al., 2003; von Nandelstadh et al., 2005). Results obtained from yeast two-hybrid assays suggest that myotilin’s Ig domains also participate in the formation of anti-parallel homodimers (Salmikangas et al., 1999). Thus, myotilin’s two Ig domains have the potential to mediate binding to F-actin, filamin, and a second myotilin monomer.
The precise cellular function of myotilin is still under investigation, although the results of dominant negative studies suggest that it may play a role in sarcomere maturation (Salmikangas et al., 2003; van der Ven et al., 2000). In vitro, purified myotilin crosslinks actin filaments into bundles, and overexpression of myotilin in cultured cells results in the formation of actin-rich structures that resemble abnormally thick stress fibers (Salmikangas et al., 2003; von Nandelstadh et al., 2005). These arrays of hyper-bundled F-actin are believed to result from direct crosslinking of actin filaments via myotilin, and perhaps also from myotilin’s synergistic interactions with its molecular partner α-actinin.
Additional insights into myotilin function have arisen from clinical research, as mutations in the myotilin gene have been implicated in three distinct inherited muscle disorders (Selcen and Carpén, 2008). First, missense mutations in myotilin were discovered to cause limb girdle muscular dystrophy 1A (LGMD1A) (Hauser et al., 2000). This form of muscular dystrophy has a relatively late age of onset (mean 27 years), and is characterized by progressive muscle weakness and reduced tendon reflexes. Ultrastructural analysis of LGMD1A muscle revealed a striking disruption of the sarcomeric architecture, as the Z discs in these patients display a “streaming” morphology, with a smeared appearance in electron micrographs. These results motivated researchers to examine the myotilin gene in other inherited muscle disorders with similar phenotypes, which led to the discovery that myotilin is also mutated in patients with myofibrillar myopathy (MFM), a disorder characterized by progressive muscular dystrophy and sarcomeric disarray (Selcen and Engel, 2004). The third myotilinopathy is a rare condition called spheroid body myopathy (SBM). Affected individuals develop progressive muscle weakness in adulthood, usually in the fourth decade of life, and exhibit a distinctively nasal speech. In one well-studied kindred, all of the affected family members were found to have a point mutation in the myotilin gene (Foroud et al., 2005). Although the clinical symptoms of LCMD1A, MFM, and SBM are similar, the ultrastructural features of the disordered muscles are different, as biopsied samples from MFM and SBM patients display distinctive dense aggregates or inclusions that are not observed in LCMD1A patients. Interestingly, transgenic mice expressing a myotilin missense mutation (T571I) develop a progressive myofibrillar pathology that displays characteristics of all three human disorders, including Z-disc streaming and dense myotilin-rich aggregates (Garvey et al., 2006).
All of the known myotilinopathies result from mutations in the N-terminal region of myotilin rather than the C-terminal Ig domains, and the physiological functions of myotilin’s Ig domains are still under investigation. The known disease-causing mutations do not impact upon myotilin’s ability to bind or bundle actin (von Nandelstradh et al., 2005). Indeed, myotilin’s roles in normal mammalian development and physiology remain somewhat undefined. Since mutations in the human myotilin gene have been implicated in three different muscle disorders, it was logical to expect that expression of wild-type myotilin would be required for normal muscle development in mammals. Surprisingly, this was not the case, as a myotilin knockout mouse displayed no discernable muscle phenotype: a thorough analysis revealed no abnormalities in sarcomeric structure in either embryonic or adult mice, and neither muscle strength nor muscle performance were affected in the mice (Moza et al., 2007). Since myotilin is closely related to two other proteins, palladin and myopalladin (Figure 4), it is possible that these family members compensate for the absence of myotilin in the knockout mice. Thus, to understand myotilin’s role in muscle development, it may be necessary to develop a triple knockout mouse in which the genes encoding all three proteins are silenced.
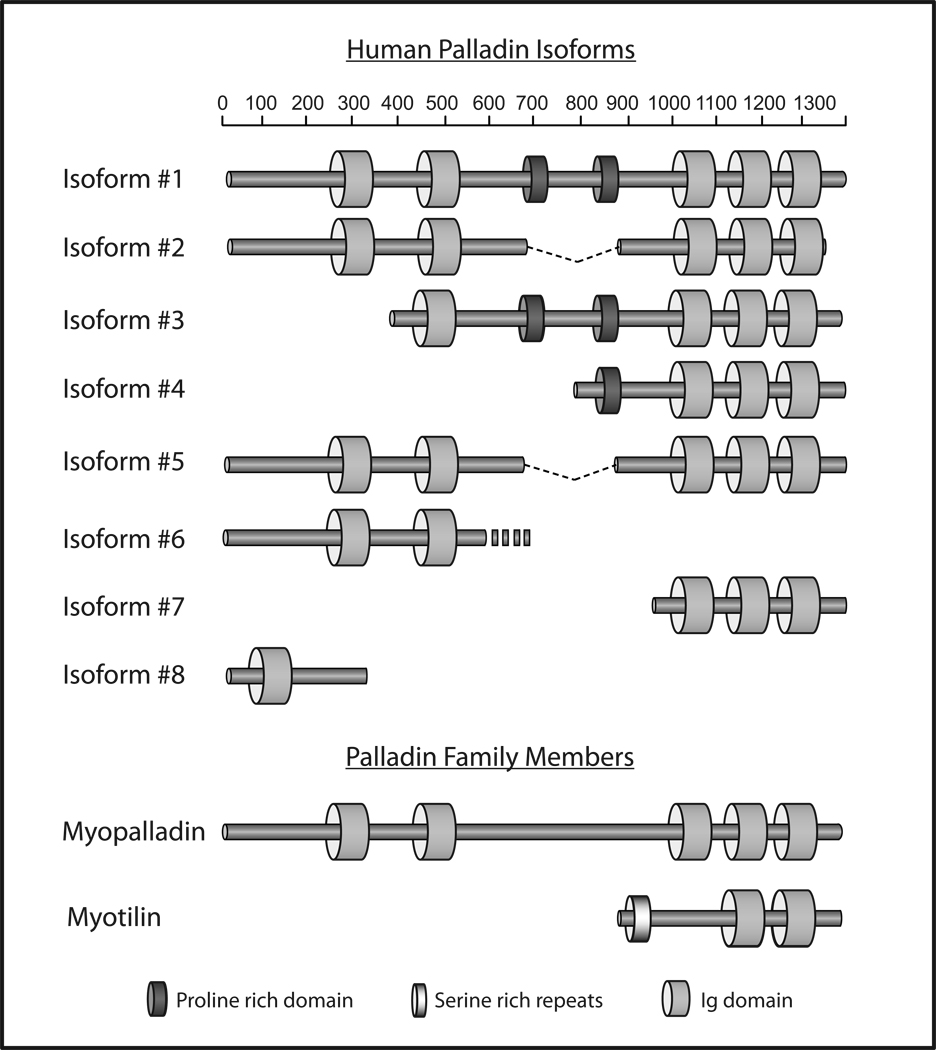
Figure 4. Myotilin-palladin-myopalladin family members.
Schematic representation of myotilin-palladin-myopalladin family members and palladin’s major isoforms, and their domain organization. PR, proline-rich region; Ig, immunoglobulin domain.
Palladin: the Ubiquitous Family Member
In contrast to most other cytoplasmic Ig domain proteins that are expressed predominantly in striated muscle, palladin is expressed in both muscle and non-muscle cell types, and is especially abundant in embryos and neonates (Parast and Otey, 2000; Rachlin and Otey, 2006; Wang and Moser, 2008). The palladin gene is highly conserved between vertebrate species, and it gives rise to multiple isoforms via alternative start sites and alternative splicing. Originally, three major isoforms (90–92, 140, and 200 kD) were described, and these are transcribed from different promoters (Parast and Otey, 2000; Rachlin and Otey, 2006). More recently, additional isoforms have been annotated by various transcriptome databases: the Universal Protein Database now lists seven human isoforms and an eighth isoform has been described experimentally (Rachlin and Otey, 2006) (Figure 4), while the NIH AceView Database lists fourteen potential isoforms, some of which have yet to be confirmed by immunoblot analysis. This high degree of isoform variability suggests that different palladin variants may be specialized for different functions, an idea that is supported by the observation that palladin isoforms are expressed in tissue-specific patterns. In mouse and chick, the 200 kD isoform is expressed predominantly in the heart, skeletal muscle, testis and bone, and is much less abundant in other tissues (Parast and Otey, 2000; Rachlin and Otey, 2006; Wang and Moser, 2008). The 140 kD isoform is widely expressed, although it is not detected in several major organs such as liver, muscle and skin (Parast and Otey, 2000; Rachlin and Otey, 2006; Wang and Moser, 2008). Originally, the 90–92 palladin doublet was reported to be the most widely-expressed isoform, as it was essentially ubiquitous in developing mouse organs (Parast and Otey, 2000). Recently, this observation was confirmed in a detailed study of palladin isoform expression in the mouse, which also reported that there are two alternatively spliced forms of 90–92 kD palladin (Wang and Moser, 2008).
Palladin’s Molecular Interactions
All of the palladin isoforms described to date contain at least one Ig domain, and as many as five (Figure 4). Two potential functions have been identified for palladin’s Ig domains. In a yeast two-hybrid assay, a palladin fragment containing Ig4 and Ig5 bound to ezrin, a member of the ezrin-radixin-moesin family of cytoskeleton-associated scaffold proteins (Mykkanen et al., 2001). More recently, purified palladin was found to function as an actin-crosslinking protein in vitro, and the Ig3 domain was identified as the smallest palladin fragment that binds directly to F-actin (Dixon et al., 2008). The functions of the N-terminal Ig domains (Ig1 and Ig2) remain to be determined.
In addition to the Ig domains, the two proline-rich (PR) domains of palladin play important roles in its molecular binding interactions. Palladin’s PR1 domain binds to Lasp-1, which is an actin-binding protein from the nebulin/nebulette family that localizes to focal adhesions, stress fibers and lamellipodia (Grunewald and Butt, 2008). Lasp-1 expression is required for normal cell motility, and misregulated Lasp-1 has been implicated in the pathological motility of ovarian cancer and breast cancer cells (Grunewald et al. 2007a, Grunewald et al. 2006, Lin et al. 2004). The three major palladin isoforms share a common PR2 domain that contains one binding site for VASP family members, and the 140 and 200 kD isoforms of palladin each possess two additional VASP binding sites in their PR1 domain (Boukhelifa et al., 2004; Rachlin and Otey, 2006). In cultured cells, palladin and VASP closely co-localize in puncta arrayed along stress fibers, and the two proteins interact in co-immunoprecipitation assays (Boukhelifa et al., 2004). VASP and its relatives (Mena, Ena, and EVL) play important roles in regulating actin filament growth and cell motility, and also function as actin-crosslinking proteins (Price and Brindle, 2000; Bear et al., 2002; Krause et al., 2003; Schirenbeck et al., 2006). Palladin’s PR2 domain also binds to the actin-regulating protein profilin, which forms complexes with VASP, suggesting that VASP and palladin may function together to recruit profilin to sites of actin polymerization (Krause et al., 2003; Witke 2004; Boukhelifa et al., 2005).
The PR2 domain of palladin also binds to Eps8, which is a substrate for the EGF receptor and many other receptor and non-receptor tyrosine kinases (Fazioli et al., 1993; Di Fiore and Scita, 2002; Goicoechea et al., 2006). Eps8 binds directly to actin and plays a critical role in regulating the length of actin filaments, and it has been implicated in the pathological motility of invasive cancer cells (Croce et al., 2004; Disanza et al., 2004; Goicoechea et al., 2006; Maa et al., 2007; Welsch et al., 2007). Palladin and Eps8 co-localize in the dorsal membrane ruffles that form transiently after cells are treated with certain growth factors, and in podosomes in vascular smooth muscle cells (Goicoechea et al., 2006). Palladin’s PR2 domain also binds to Src (Rönty et al., 2007), which is a non-receptor tyrosine kinase that, upon activation, reorganizes the cytoskeleton and interacts with many other cytoskeletal proteins.
Palladin’s PR2 domain binds to signaling intermediaries such as ArgBP-2 and SPIN-90 (Rönty et al. 2005, Rönty et al. 2007). Like its relatives myotilin and myopalladin, palladin binds to α-actinin, which is one of the classic actin crosslinking proteins (Rönty et al. 2004). In addition to binding F-actin, α-actinin also functions as a scaffolding molecule, and it interacts with multiple transmembrane proteins and signaling proteins (reviewed in Otey and Carpen, 2004). Palladin also binds to LPP, a relative of the cytoskeletal scaffolding protein zyxin that plays an important role in the physiology of vascular smooth muscle cells (Jin et al., 2007). In summary, then, palladin can be described as an actin-binding molecular scaffold that forms complexes with a wide variety of cytoskeletal regulators.
Palladin’s Cellular Function
In cultured cells, palladin localizes to diverse actin-based structures, and it has a critical role in the assembly of stress fibers, dorsal ruffles, cell adhesions, growth cones, and podosomes (Parast and Otey, 2000; Boukhelifa et al., 2001; Luo et al., 2005; Goicoechea et al., 2006; Liu et al., 2006; Goicoechea et al., 2009). The level of palladin expression appears to be tightly controlled by cells, and it can be dramatically altered during cellular differentiation or when cells make the transition from a stationary to a motile phenotype. For example, palladin is upregulated when monocytes differentiate into dendritic cells, neural crest cells begin to migrate, Rcho-1 stem cells differentiate into trophoblasts, tracheal epithelial cells undergo epithelial to mesenchymal transition, and human mesenchymal cells differentiate along an osteogenic lineage (Parast and Otey, 2000; Mykkanen et al., 2001; Gammill and Bronner-Fraser, 2002; Gultice et al., 2006; Wall et al., 2007; Alcorn et al., 2008). Each of these phenotypic or behavioral changes require large-scale reorganizations of the cytoskeleton.
Palladin is also involved in the tissue remodeling that occurs in response to injury. In a cell culture model, palladin is rapidly upregulated in astrocytes along a wound edge (Boukhelifa et al., 2003). Palladin upregulation has been observed in animal models of injury to the brain, aorta and skin, and in human wound granulation tissue (Boukhelifa et al., 2003; Rönty et al., 2006; Jin et al., 2007). In cultured vascular smooth muscle cells, which have a highly plastic cellular phenotype, palladin plays an important role in cell motility and contractility, and in the formation of dynamic membrane ruffles and podosomes (Goicoechea et al., 2006; Jin et al., 2007; Jin et al., 2009). The closing of wounds typically requires both increased cell motility (to fill the wound) and increased contractility (to bring the wound edges together), and the results acquired to date suggest that palladin could be an integral contributor to both of these processes.
The role of palladin in mammalian development has been explored in a knockout mouse, and the phenotype of the mouse was embryonic lethality at about embryonic day 15.5 (Luo et al., 2005). Palladin null embryos displayed multiple profound defects, including failures of the body wall to close both anteriorly (resulting in exencephaly) and dorsally (resulting in herniation of abdominal organs). Somewhat surprisingly, palladin null embryos were also anemic, and defects were noted in the formation of the erythroblastic islands that support differentiation of mature red blood cells (Liu et al., 2007). Erythroblastic islands depend upon the formation of specialized cell adhesions between the central macrophage and the developing erythrocytes, and also on the ability of the macrophage to phagocytose the erythroblasts’ extruded nuclei (Chasis and Mohandas, 2008). Both of these are actin-dependent functions that could involve palladin, and the precise role of palladin in erythroblastic islands remains to be determined.
All of the available evidence from knockdown, knockout, and overexpression studies supports the idea that palladin plays an important role in organizing the actin cytoskeleton, and thus participates in actin-dependent processes such as cell motility and contractility. However, recent observations suggest that palladin may have other functions that are distinct from its role in the cytoplasm. In cultured kidney podocytes, endogenous palladin was observed to accumulate in the nucleus when the cells were treated with a drug to block nuclear export (Endlich et al., 2009). When fragments of 90–92 kD palladin were expressed in the cells, the C-terminal half localized strongly to the nucleus, while the N-terminal half remained in the cytoplasm. It is not yet known if any of palladin’s three C-terminal Ig domains are required for nuclear import, yet these results suggest the intriguing possibility that by shuttling from the cytoplasm to the nucleus, palladin could serve an important regulatory or signaling function. In fact, studies with palladin-null embryoid bodies also implicate palladin in the regulation of gene expression, as the levels of expression of multiple proteins involved in contractility were found to be abnormally low in the absence of palladin expression (Jin et al., 2009). It is also important to note many of palladin’s numerous molecular partners have been detected in the nucleus: actinin-4, LASP-1, LPP and profilin have all been shown to shuttle between the cytoplasm and the nucleus (Honda et al., 1998; Skare et al., 2003; Petit et al., 2005; Guo et al., 2006; Grunewald et al., 2007b). Thus, it may be informative to determine if these proteins enter the nucleus in complexes, or have a shared function within the nuclear compartment.
Palladin’s Connection to Human Disease
Recently, a mutation within the human palladin gene was linked to a serious disease: familial pancreatic cancer. This discovery arose from the study of a large North American family with an exceptionally high incidence of pancreatic cancer, known as Family X. Through the use of linkage analysis, custom gene arrays and sequencing techniques, the palladin gene was found to be mutated in all of the afflicted members of Family X (Pogue-Geile et al., 2006). This mutation results in a single amino acid substitution from proline to serine at position 239, which is within the binding region for α-actinin, although it is not yet known if the P239S mutation alters palladin’s ability to bind α-actinin or its many other molecular partners. However, transfection of mutated palladin into HeLa cells resulted in significant changes in the organization of the actin cytoskeleton (Pogue-Geile et al., 2006). This P239S mutation appears to be specific to the single kindred in which it was identified (Slater et al., 2007; Zogopoulous et al., 2007), so that it may not be useful for large-scale genetic screening; nevertheless, a more thorough investigation of this mutation, and of the role of wild-type palladin in the pancreas, seems likely to yield new insights into the molecular pathways that underlie inherited pancreatic cancer. Palladin also has a connection to breast cancer, albeit through a different mechanism. In this disease, palladin levels correlated with increased invasiveness in multiple human tumor cell lines in culture, in human tumors grown within a mouse host, and in patient samples of primary and metastatic tumors (Wang et al., 2004; Goicoechea et al., 2009). In cultured breast cancer cells, knocking down palladin resulted in a 50% decrease in cell motility and invasiveness, suggesting that palladin may play an important role in organizing the actin-based structures that are utilized by tumor cells to invade across tissue boundaries (Goicoechea et al., 2009). These results suggest that it may be useful to explore palladin’s role in other types of invasive cancer.
Myopalladin: a Specialized Function in Cardiac Muscle
Myopalladin was first identified in a yeast two-hybrid screen as a novel binding partner for nebulin, which is a giant protein that plays a key role in sarcomere assembly (Bang et al., 2001). In its overall structure, myopalladin closely resembles palladin isoform #5, as both proteins contain a total of five Ig domains (two N-terminal and three C-terminal) and lack the proline-rich domains that are found in many other palladin isoforms. In addition to nebulin and its relative nebulette, myopalladin also binds to α-actinin and to the cardiac ankyrin repeat protein, CARP (Bang et al., 2001). CARP binds to an N-terminal region of myopalladin that includes two Ig domains, but precise mapping of the binding site within that region has not been performed to date. Similarly, α-actinin binds to the C-terminal region of myopalladin, but a specific role for the C-terminal Ig domains in this molecular interaction has not been demonstrated.
The observation that myopalladin binds to CARP was unexpected, since CARP localizes largely to the nucleus, and myopalladin was originally identified as a component of the sarcomere (Chu et al., 1995; Zou et al., 1997; Bang et al., 2001). In adult heart, CARP expression is downregulated, but it is re-expressed during the development of cardiac hypertrophy, suggesting that it may play a role in regulating the levels of various cardiac-specific proteins (Kuo et al., 1999; Aihara et al., 2000). To date, the precise function of the myopalladin/CARP interaction is not known, although in cultured cardiac myocytes, overexpression of myopalladin’s CARP-binding region resulted in gross disruptions of Z-line organization, suggesting an important role for myopalladin in sarcomeric architecture (Bang et al., 2001). Similar to palladin, myopalladin has also been detected in the nucleus, and it is not known if myopalladin shuttles into the nucleus alone or in complexes with CARP (Bang et al., 2001; Miller et al., 2003).
Overall, myopalladin’s diverse molecular interactions suggest that it may be a key intermediary involved in both the structural aspects of sarcomere assembly and in regulation of sarcomeric gene expression. This idea is supported by the recent discovery of four independent mutations in the myopalladin gene associated with dilated cardiomyopathy (DCM), two (R1088H and I83fsX105) in two families with idiopathic dilated cardiomyopathy, and two (V119M, P1112L) in individuals with sporadic (non-familial) forms of the disease (Duboscq-Bidot et al., 2008), suggesting that myopalladin plays a significant role in normal cardiac physiology. The three missense mutations linked with DCM are located in the C-terminal Ig domains of myopalladin. The R1088H mutation occurs in the fourth Ig domain in the loop between the A’ and B strand; this mutation is associated with a decreased localization of myopalladin to the Z-band area of left ventricular cardiac myofibrils (Duboscq-Bidot et al., 2008). The P1112L mutation is located in the fourth Ig domain of myopalladin at the end of the short C’ strand. The V1195M mutation is located in the B strand of the fifth Ig domain. Transfection of the P1112L and V1195M mutations into rat neonate cardiomyocytes led to sarcomere disorganization and premature cell death (Duboscq-Bidot et al., 2008). There are currently no structures available for the Ig domains of myopalladin, nor have any functions been assigned to the Ig domains, however, the correlation between DCM and mutations in the fourth and fifth Ig domains suggests that the Ig domains of myopalladin are critically involved in the organization of the sarcomere in cardiac muscle tissue.
Other cytoplasmic Ig-domain proteins
The focus of this article is on five Ig-domain proteins with diverse functions in the cytoskeleton, each with links to inherited disorders in humans; however, it is relevant to note that additional cytoskeleton-associated Ig-domain proteins serve important roles in normal physiology, especially in striated muscle. The Ig-domain proteins myomesin-1 (also known as skelemin), myomesin-2 (also known as M-protein) and myomesin-3 are major components of the M-band, a structure that contributes to the organization of thick filaments within the A-band of the sarcomere (Agarkova et al., 2003; Schoenauer et al., 2008). The precise cellular function of the myomesin proteins is not yet known, although it appears likely that they provide elasticity to the M-band and may participate in a stress-sensing mechanism (Agarkova et al., 2003; Agarkova and Perriard, 2005; Schoenauer et al., 2005). Similar to other Ig domain proteins such as palladin and myopalladin, myomesin-1 has also been observed inside the nucleus; in fact, in myocytes isolated from neonatal rat pups, myomesin-1 was detected almost exclusively in nuclear speckles (Reddy et al., 2008). There is preliminary evidence to suggest that nuclear myomesin-1 may play a role in the regulation of gene expression, although the molecular mechanism that underlies these effects has not been identified (Reddy et al., 2008).
Like the myomesins, the giant Ig-domain protein obscurin may also be involved in stress-sensing. Obscurin is linked to both Ca2+-mediated and Rho-GTPase signaling pathways, and appears to be concentrated primarily at the periphery of the M-band, where it interacts with the sarcoplasmic reticulum (Bang et al., 2001; Young et al., 2001; Kontrogianni-Konstantopoulos and Bloch, 2005; Carlsson et al., 2008). Knockdown of obscurin in cultured myocytes has revealed a critical role for this protein in sarcomere assembly (Kontrogianni-Konstantopoulos et al., 2006), and current efforts are focused on understanding how molecular interactions between titin, obscurin and myomesins contribute to the assembly and maintenance of the M-band (Bowman et al., 2008; Fukuzawa et al., 2008). In this context, it is especially noteworthy that several disease-causing mutations in titin have been shown to weaken the molecular interactions between titin and obscurin, suggesting that the molecular interaction between titin and obscurin may play a central role in preserving sarcomere integrity (Fukuzawa et al., 2008).
MyBP-H is related to MyBP-C, and it contains two Ig domains and one fibronectin domain (Vaughan et al., 1993). The precise role of MyBP-H in striated muscle has not been defined, but it is interesting to note that MyBP-H is dramatically underexpressed in pelvic muscles from patients with pelvic organ prolapse, a condition that results from weakened muscles in the pelvic floor (Visco and Yuan, 2003; Hundley et al., 2006). These results suggest the possibility that downregulation of MyBP-H may be associated with a loss of myofibril structure or function within the levator ani muscles.
In contrast to the muscle-specific proteins described above, the actin-binding protein filamin is widely expressed. In humans, each filamin monomer contains an actin-binding domain and 24 Ig domains, and the monomers dimerize to form a classical F-actin crosslinking protein (Fucini et al., 1997). Filamin binds to a variety of molecular partners, suggesting that it may participate in signal transduction pathways, in addition to its structural role (Popowicz et al., 2006). In humans, there are three genes encoding filamins A, B, and C, and mutations in different filamin genes have been implicated in disorders of the nervous system, musculoskeletal system, and cardiovascular system (Fox et al., 1998; Sheen et al., 2001; Robertson et al., 2003; Stefanova et al., 2005; Vorgerd et al., 2005; Zhou et al., 2007). It is interesting to note that a specific C-terminal fragment of filamin A can translocate into the nucleus (Ozanne et al., 2000; Loy et al., 2003). Cytoplasmic filamin A interacts with a pair of transcription factors, suggesting that it may be involved in regulating gene expression (Sasaki et al., 2001; Watanabe et al., 2005; Yoshida et al., 2005), and filamin A also appears to participate in a DNA-repair mechanism in the nucleus (Meng et al., 2004), suggesting that the cellular functions of this Ig-domain protein extend far beyond its structural role in organizing actin arrays.
Concluding Remarks and Future Directions
Overall, a comparison of the proteins discussed here suggests that Ig domains have evolved to occupy multiple functional niches. Certain Ig domains serve as sites for protein-protein interactions; for example, both MyBP-C and titin utilize Ig domains to bind both myosin and F-actin (Oakley et al., 2004). In addition, myotilin and palladin bind to F-actin through Ig domains, as does another member of this group that is expressed in some invertebrate species, kettin (van Straaten et al., 1999; Bullard et al., 2006; Ono et al., 2006). This suggests that Ig domains should be considered as another form of actin-binding motif. But the question remains: what are the specific structural features that define an actin-binding type of Ig domain, as opposed to an Ig domain that binds to myosin, or to signaling intermediaries or nuclear components? In the future, as the structures of more Ig domains are solved, this question may be answered.
From studies focused on titin, we have learned that Ig domains have distinctive mechanical properties, and that their “sandwich” structure confers elasticity, especially to proteins that contain multiple Ig domains in sequence. In the case of titin, the biological role of molecular elasticity is easily understood within the context of striated muscle. In proteins such as myotilin, palladin, and filamin, which participate in actin-crosslinking, the importance of molecular elasticity is less clear. This distinctive mechanical feature may be related to another unexpected property of the Ig domain proteins, which is their ability to influence events in the nucleus. Certain Ig domain proteins (for example, titin) bind to signaling intermediaries that regulate the levels of multiple proteins, and other Ig domain proteins (such as palladin, myopalladin, myomesin-I, and filamin) appear to shuttle between the nucleus and the cytoplasm and thus may influence cellular differentiation directly. In the future, it will be important to determine if these cytoskeleton-associated Ig domain proteins are part of a mechanism that allows the cell to integrate mechanical signals such as stretch or contractility with changes in gene expression.
Acknowledgements
The authors are grateful to Drs. Sharon Campbell, Moriah Beck and Daniel Arneman for helpful discussions and Dr. Rafael García-Mata for assistance with figures. This work was partially supported by grants from the NIH, the Elsa U. Pardee foundation, the American Heart Association, the North Carolina Biotechnology Center, and the UNC Center for Gastrointestinal Biology and Disease.
References cited
- Ababou A, Rostkova E, Mistry S, Le Masurier C, Gautel M, Pfuhl M. Myosin binding protein C positioned to play a key role in regulation of muscle contraction: structure and interactions of domain C1. J Mol Biol. 2008;384:615–630. doi: 10.1016/j.jmb.2008.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarkova I, Ehler E, Lange S, Schoenauer R, Perriard JC. M-band: a safeguard for sarcomere stability? J Muscle Res Cell Motil. 2003;24:191–203. doi: 10.1023/a:1026094924677. [DOI] [PubMed] [Google Scholar]
- Agarkova I, Perriard JC. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. doi: 10.1016/j.tcb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Aihara Y, Kurabayashi M, Saito Y, Ohyama Y, Tanaka T, Takeda S, Tomaru K, Sekiguchi K, Arai M, Nakamura T, Nagai R. Cardiac ankyrin repeat protein is a novel marker of cardiac hypertrophy: role of M-CAT element within the promoter. Hypertension. 2000;36:48–53. doi: 10.1161/01.hyp.36.1.48. [DOI] [PubMed] [Google Scholar]
- Alyonycheva TN, Mikawa T, Reinach FC, Fischman DA. Isoform-specific interaction of the myosin-binding proteins (MyBPs) with skeletal and cardiac myosin is a property of the C-terminal immunoglobulin domain. J Biol Chem. 1997;272:20866–20872. doi: 10.1074/jbc.272.33.20866. [DOI] [PubMed] [Google Scholar]
- Alcorn JF, Guala AS, van der Velden J, McElhinney B, Irvin CG, Davis RJ, Janssen-Heininger YM. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J Cell Sci. 2008;121:1036–1045. doi: 10.1242/jcs.019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to Iband linking system. Circ Res. 2001;89:1065–1072. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- Bang ML, Mudry RE, McElhinny AS, Trombitas K, Geach AJ, Yamasaki R, Sorimachi H, Granzier H, Gregorio CC, Labeit S. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol. 2001;153:413–427. doi: 10.1083/jcb.153.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Bork P, Holm L, Sander C. The immunoglobulin fold: Structural classification, sequence patterns and common core. J Mol Biol. 1994;242:309–320. doi: 10.1006/jmbi.1994.1582. [DOI] [PubMed] [Google Scholar]
- Boukhelifa M, Parast MM, Valtschanoff JG, LaMantia AS, Meeker RB, Otey CA. A role for the cytoskeleton-associated protein palladin in neurite outgrowth. Mol Biol Cell. 2001;12:2721–2729. doi: 10.1091/mbc.12.9.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhelifa M, Hwang S-J, Valtschanoff JG, Meeker R, Rustioni A, Otey C. A critical role for palladin in astrocyte morphology and response to injury. Molec. Cell. Neurosci. 2003;23:661–668. doi: 10.1016/s1044-7431(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Boukhelifa M, Parast MM, Bear J, Gertler F, Otey CA. Palladin is a novel binding partner for Ena/VASP proteins. Cell Motil Cytoskel. 2004;58:17–29. doi: 10.1002/cm.10173. [DOI] [PubMed] [Google Scholar]
- Boukhelifa M, Rachlin A, Parast MM, Moza M, Johansson T, Carpen O, Karlsson R, Otey CA. The proline-rich protein palladin binds directly to profilin. FEBS J. 2005;273:26–33. doi: 10.1111/j.1742-4658.2005.05036.x. [DOI] [PubMed] [Google Scholar]
- Bowman AL, Catino DH, Strong JC, Randall WR, Kontrogianni-Konstantopoulos A, Bloch RJ. The rho-guanine nucleotide exchange factor domain of obscurin regulates assembly of titin at the Z-disk through interactions with Ran binding protein 9. Mol Biol Cell. 2008;19:3782–3792. doi: 10.1091/mbc.E08-03-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard B, Garcia T, Benes V, Leake MC, Linke WA, Oberhauser AF. The molecular elasticity of the insect flight muscle proteins projectin and kettin. Proc Natl Acad Sci U S A. 2006;103:4451–4456. doi: 10.1073/pnas.0509016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson L, Yu JG, Thornell LE. New aspects of obscurin in human striated muscles. Histochem Cell Biol. 2008;130:91–103. doi: 10.1007/s00418-008-0413-z. [DOI] [PubMed] [Google Scholar]
- Carmignac V, Salih MA, Quijano-Roy S, Marchand S, Al Rayess MM, Mukhtar MM, Urtizberea JA, Labeit S, Guicheney P, Leturcq F, Gautel M, Fardeau M, Campbell KP, Richard I, Estournet B, Ferreiro A. C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Ann Neurol. 2007;61:340–351. doi: 10.1002/ana.21089. [DOI] [PubMed] [Google Scholar]
- Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol. 2001;306:717–726. doi: 10.1006/jmbi.2001.4448. [DOI] [PubMed] [Google Scholar]
- Chang AN, Potter JD. Sarcomeric protein mutations in dilated cardiomyopathy. Heart Fail Rev. 2005;10:225–235. doi: 10.1007/s10741-005-5252-6. [DOI] [PubMed] [Google Scholar]
- Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112:470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W, Burns DK, Swerlick RA, Presky DH. Identification and characterization of a novel cytokine-inducible nuclear protein from human endothelial cells. J Biol Chem. 1995;270:10236–10245. doi: 10.1074/jbc.270.17.10236. [DOI] [PubMed] [Google Scholar]
- Croce A, Cassata G, Disanza A, Gagliani MC, Tacchetti C, Malabarba MG, Carlier MF, Scita G, Baumeister R, Di Fiore PP. A novel actin barbed-end-capping activity in EPS-8 regulates apical morphogenesis in intestinal cells of Caenorhabditis elegans. Nat Cell Biol. 2004;6:1173–1179. doi: 10.1038/ncb1198. [DOI] [PubMed] [Google Scholar]
- Di Fiore PP, Scita G. Eps8 in the midst of GTPases. Int J Biochem Cell Biol. 2002;34:1178–1183. doi: 10.1016/s1357-2725(02)00064-x. [DOI] [PubMed] [Google Scholar]
- Disanza A, Carlier MF, Stradal TE, Didry D, Frittoli E, Confalonieri S, Croce A, Wehland J, Di Fiore PP, Scita G. Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat Cell Biol. 2004;6:1180–1188. doi: 10.1038/ncb1199. [DOI] [PubMed] [Google Scholar]
- Dixon RDS, Arneman DK, Rachlin AS, Sundaresan N, Costello JM, Campbell SL, Otey CA. Palladin is an actin crosslinking protein that uses immunoglobulin-like domains to bind filamentous actin. J Biol Chem. 2008;283:6222–6231. doi: 10.1074/jbc.M707694200. [DOI] [PubMed] [Google Scholar]
- Duboscq-Bidot L, Xu P, Charron P, Neyroud N, Dilanian G, Millaire A, Bors V, Komajda M, Villard E. Mutations in the Z-band protein myopalladin gene and idiopathic dilated cardiomyopathy. Cardiovasc Res. 2008;77:118–125. doi: 10.1093/cvr/cvm015. [DOI] [PubMed] [Google Scholar]
- Einheber S, Fischman DA. Isolation and characterization of a cDNA clone encoding avian skeletal muscle C-protein: an intracellular member of the immunoglobulin superfamily. Proc Natl Acad Sci U S A. 1990;87:2157–2161. doi: 10.1073/pnas.87.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endlich N, Schordan E, Cohen CD, Kretzler M, Lewko B, Welsch T, Kriz W, Otey CA, Endlich K. Palladin is a dynamic actin-associated protein in podocytes. Kidney International. 2009;75:214–226. doi: 10.1038/ki.2008.486. [DOI] [PubMed] [Google Scholar]
- Fazioli F, Minichiello L, Matoska V, Castagnino P, Miki T, Wong WT, Di Fiore PP. Eps8, a substrate for the epidermal growth factor receptor kinase, enhances EGF-dependent mitogenic signals. EMBO J. 1993;12:3799–3808. doi: 10.1002/j.1460-2075.1993.tb06058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner G, Lanfranchi G, Valle G. Telethonin and other new proteins of the Z-disc of skeletal muscle. IUBMB Life. 2001;51:275–282. doi: 10.1080/152165401317190761. [DOI] [PubMed] [Google Scholar]
- Flashman E, Redwood C, Moolman-Smook J, Watkins H. Cardiac myosin binding protein C: its role in physiology and disease. Circ Res. 2004;94:1279–1289. doi: 10.1161/01.RES.0000127175.21818.C2. [DOI] [PubMed] [Google Scholar]
- Foroud T, Pankratz N, Batchman AP, Pauciulo MW, Vidal R, Miravalle L, Goebel HH, Cushman LJ, Azzarelli B, Horak H, Farlow M, Nichols WC. A mutation in myotilin causes spheroid body myopathy. Neurology. 2005;65:1936–1940. doi: 10.1212/01.wnl.0000188872.28149.9a. [DOI] [PubMed] [Google Scholar]
- Fox JW, Lamperti ED, Ekşioğlu YZ, Hong SE, Feng Y, Graham DA, Scheffer IE, Dobyns WB, Hirsch BA, Radtke RA, Berkovic SF, Huttenlocher PR, Walsh CA. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron. 1998;21:1315–1325. doi: 10.1016/s0896-6273(00)80651-0. [DOI] [PubMed] [Google Scholar]
- Freiburg A, Gautel M. A molecular map of the interactions between titin and myosin-binding protein C. Implications for sarcomeric assembly in familial hypertrophic cardiomyopathy. Eur J Biochem. 1996;235:317–323. doi: 10.1111/j.1432-1033.1996.00317.x. [DOI] [PubMed] [Google Scholar]
- Fucini P, Renner C, Herberhold C, Noegel AA, Holak TA. The repeating segments of the F-actin cross-linking gelation factor (ABP-120) have an immunoglobulin-like fold. Nat Struct Biol. 1997;4:223–230. doi: 10.1038/nsb0397-223. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Granzier HL, Ishiwata S, Kurihara S. Physiological functions of the giant elastic protein titin in mammalian striated muscle. J Physiol Sci. 2008;58:151–159. doi: 10.2170/physiolsci.RV005408. [DOI] [PubMed] [Google Scholar]
- Fukuzawa A, Lange S, Holt M, Vihola A, Carmignac V, Ferreiro A, Udd B, Gautel M. Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band: implications for hereditary myopathies. J Cell Sci. 2008;121:1841–1851. doi: 10.1242/jcs.028019. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Ono Y, Tsuchiya H, Katayama Y, Bang ML, Labeit D, Labeit S, Inagaki N, Gregorio CC. Specific interaction of the potassium channel beta-subunit minK with the sarcomeric protein T-cap suggests a T-tubule-myofibril linking system. J Mol Biol. 2001;313:775–784. doi: 10.1006/jmbi.2001.5053. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Bronner-Fraser M. Genomic analysis of neural crest induction. Development. 2002;129:5731–5741. doi: 10.1242/dev.00175. [DOI] [PubMed] [Google Scholar]
- Garvey SM, Miller SE, Claflin DR, Faulkner JA, Hauser MA. Transgenic mice expressing the myotilin T57I mutation unite the pathology associated with LGMD1A and MFM. Hum Mol Genet. 2006;15:2348–2362. doi: 10.1093/hmg/ddl160. [DOI] [PubMed] [Google Scholar]
- Gerull B, Gramlich M, Atherton J, McNabb M, Trombitas K, Sasse-Klaassen S, Seidman JG, Seidman C, Granzier H, Labeit S, Frenneaux M, Thierfelder L. Mutations of TTN, encoding the giant muscle filament titin, cause familial dilated cardiomyopathy. Nat Genet. 2002;30:201–204. doi: 10.1038/ng815. [DOI] [PubMed] [Google Scholar]
- Gerull B, Atherton J, Geupel A, Sasse-Klaassen S, Heuser A, Frenneaux M, McNabb M, Granzier H, Labeit S, Thierfelder L. Identification of a novel frameshift mutation in the giant muscle filament titin in a large Australian family with dilated cardiomyopathy. J Mol Med. 2006;84:478–483. doi: 10.1007/s00109-006-0060-6. [DOI] [PubMed] [Google Scholar]
- Goicoechea S, Disanza A, Arneman D, Scita G, Otey CA. Palladin binds to Eps8 and participates in dorsal ruffle and podosome formation. J. Cell Sci. 2006;119:3316–3324. doi: 10.1242/jcs.03076. [DOI] [PubMed] [Google Scholar]
- Goicoechea SM, Bednarski B, García-Mata R, Prentice-Dunn H, Kim HJ, Otey CA. Palladin contributes to invasive motility in human breast cancer cells. Oncogene. 2009;28:587–598. doi: 10.1038/onc.2008.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontier Y, Taivainen A, Fontao L, Sonnenberg A, van der Flier A, Carpen O, Faulkner G, Borradori L. The Z-disc proteins myotilin and FATZ-1 interact with each other and are connected to the sarcolemma via muscle-specific filamins. J Cell Sci. 2005;118:3739–3749. doi: 10.1242/jcs.02484. [DOI] [PubMed] [Google Scholar]
- Govada L, Carpenter L, da Fonseca PC, Helliwell JR, Rizkallah P, Flashman E, Chayen NE, Redwood C, Squire JM. Crystal structure of the C1 domain of cardiac myosin binding protein-C: implications for hypertrophic cardiomyopathy. J Mol Biol. 2008;378:387–397. doi: 10.1016/j.jmb.2008.02.044. [DOI] [PubMed] [Google Scholar]
- Granzier H, Wu Y, Labeit S, LeWinter M. Titin: physiological function and role in cardiomyopathy and failure. Heart Fail Rev. 2005;10:211–223. doi: 10.1007/s10741-005-5251-7. [DOI] [PubMed] [Google Scholar]
- Gregorio CC, Trombitás K, Centner T, Kolmerer B, Stier G, Kunke K, Suzuki K, Obermayr F, Herrmann B, Granzier H, Sorimachi H, Labeit S. The NH2 terminus of titin spans the Z-disc: its interaction with a novel 19-kD ligand (T-cap) is required for sarcomeric integrity. J Cell Biol. 1998;143:1013–1027. doi: 10.1083/jcb.143.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen M, Gautel M. Mutations in beta-myosin S2 that cause familial hypertrophic cardiomyopathy (FHC) abolish the interaction with the regulatory domain of myosin-binding protein-C. J Mol Biol. 1999;286:9339–9349. doi: 10.1006/jmbi.1998.2522. [DOI] [PubMed] [Google Scholar]
- Grunewald TG, Kammerer U, Schulze E, Schindler D, Honig A, Zimmer M, Butt E. Silencing of LASP-1 influences zyxin localization, inhibits proliferation and reduces migration in breast cancer cells. Exp Cell Res. 2006;312:974–982. doi: 10.1016/j.yexcr.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Grunewald TG, Kammerer U, Winkler C, Schindler D, Sickmann A, Honig A, Butt E. Overexpression of LASP-1 mediates migration and proliferation of human ovarian cancer cells and influences zyxin localisation. Br J Cancer. 2007a;96:296–305. doi: 10.1038/sj.bjc.6603545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald TG, Kammerer U, Kapp M, Eck M, Dietl J, Butt E, Honig A. Nuclear localization and cytosolic overexpression of LASP-1 correlates with tumor size and nodal-positivity of human breast carcinoma. BMC Cancer. 2007b;7:198. doi: 10.1186/1471-2407-7-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald TG, Butt E. The LIM and SH3 domain protein family: structural proteins or signal transducers or both? Mol Cancer. 2008;7:31. doi: 10.1186/1476-4598-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gultice AD, Selesniemi KL, Brown TL. Hypoxia inhibits differentiation of lineage-specific Rcho-1 trophoblast giant cells. Biol Reprod. 2006;74:1041–1050. doi: 10.1095/biolreprod.105.047845. [DOI] [PubMed] [Google Scholar]
- Guo B, Sallis RE, Greenall A, Petit MM, Jansen E, Young L, Van de Ven WJ, Sharrocks AD. The LIM domain protein LPP is a coactivator for the ETS domain transcription factor PEA3. Mol Cell Biol. 2006;26:4529–4538. doi: 10.1128/MCB.01667-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman P, Vihola A, Haravuori H, Marchand S, Sarparanta J, De Seze J, Labeit S, Witt C, Peltonen L, Richard I, Udd B. Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin. Am J Hum Genet. 2002;71:492–500. doi: 10.1086/342380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaby DM, Poupon A, Mornon J. The immunoglobulin fold family: sequence analysis and 3D structure comparisons. Protein Eng. 1999;12:563–571. doi: 10.1093/protein/12.7.563. [DOI] [PubMed] [Google Scholar]
- Halaby DM, Mornon JP. The immunoglobulin superfamily: an insight on its tissular, species, and functional diversity. J Mol Evol. 1998;46:389–400. doi: 10.1007/pl00006318. [DOI] [PubMed] [Google Scholar]
- Harpaz Y, Chothia C. Many of the immunoglobulin superfamily domains in cell adhesion molecules and surface receptors belong to a new structural set which is close to that containing variable domains. J Mol Biol. 1994;238:528–539. doi: 10.1006/jmbi.1994.1312. [DOI] [PubMed] [Google Scholar]
- Harris SP, Bartley CR, Hacker TA, McDonald KS, Douglas PS, Greaser ML, Powers PA, Moss RL. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res. 2002;90:594–601. doi: 10.1161/01.res.0000012222.70819.64. [DOI] [PubMed] [Google Scholar]
- Hauser MA, Horrigan SK, Salmikangas P, Torian UM, Viles KD, Dancel R, Tim RW, Taivainen A, Bartoloni L, Gilchrist JM, Stajich JM, Gaskell PC, Gilbert JR, Vance JM, Pericak-Vance MA, Carpen O, Westbrook CA, Speer MC. Myotilin is mutated in limb girdle muscular dystrophy 1A. Hum Mol Genet. 2000;9:2141–2147. doi: 10.1093/hmg/9.14.2141. [DOI] [PubMed] [Google Scholar]
- Hein S, Schaper J. Weakness of a giant: mutations of the sarcomeric protein titin. Trends Mol Med. 2002;8:311–313. doi: 10.1016/s1471-4914(02)02372-9. [DOI] [PubMed] [Google Scholar]
- Holden HM, Ito M, Hartshorne DJ, Rayment I. X-ray structure determination of telokin, the C-terminal domain of myosin light chain kinase, at 2.8 A resolution. J Mol Biol. 1992;227:840–851. doi: 10.1016/0022-2836(92)90226-a. [DOI] [PubMed] [Google Scholar]
- Honda K, Yamada T, Endo R, Ino Y, Gotoh M, Tsuda H, Yamada Y, Chiba H, Hirohashi S. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol. 1998;140:1383–1393. doi: 10.1083/jcb.140.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290:H1313–H1325. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley AF, Yuan L, Visco AG. Skeletal muscle heavy-chain polypeptide 3 and myosin binding protein H in the pubococcygeus muscle in patients with and without pelvic organ prolapse. Am J Obstet Gynecol. 2006;194:1404–1410. doi: 10.1016/j.ajog.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Hunkapiller T, Hood L. Diversity of the immunoglobulin gene superfamily. Adv Immunol. 1989;44:1–63. doi: 10.1016/s0065-2776(08)60639-2. [DOI] [PubMed] [Google Scholar]
- Improta S, Politou AS, Pastore A. Immunoglobulin-like modules from titin I-band:extensible components of muscle elasticity. Structure. 1996;4:323–337. doi: 10.1016/s0969-2126(96)00036-6. [DOI] [PubMed] [Google Scholar]
- Improta S, Krueger JK, Gautel M, Atkinson RA, Lefèvre JF, Moulton S, Trewhella J, Pastore A. The assembly of immunoglobulin-like modules in titin: implications for muscle elasticity. J Mol Biol. 1998;284:761–777. doi: 10.1006/jmbi.1998.2028. [DOI] [PubMed] [Google Scholar]
- Jeffries CM, Whitten AE, Harris SP, Trewhella J. Small-angle X-ray scattering reveals the N-terminal domain organization of cardiac myosin binding protein C. J Mol Biol. 2008;377:1186–1199. doi: 10.1016/j.jmb.2008.01.080. [DOI] [PubMed] [Google Scholar]
- Jin L, Kern MJ, Otey CA, Somlyo AV. Angiotensin II, FAK and PRX1 enhance smooth muscle expression of LPP and a newly identified LPP binding partner palladin to promote cell migration. Circ Research. 2007;100:817–825. doi: 10.1161/01.RES.0000261351.54147.de. [DOI] [PubMed] [Google Scholar]
- Jin L, Yoshida T, Ho R, Owens GK, Somlyo AV. The actin-associated protein Palladin is required for development of normal contractile properties of smooth muscle cells derived from embryoid bodies. J Biol Chem. 2009;284:2121–2130. doi: 10.1074/jbc.M806095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler RW, Harris SP. The structure of isolated cardiac Myosin thick filaments from cardiac Myosin binding protein-C knockout mice. Biophys J. 2008;94:1707–1718. doi: 10.1529/biophysj.107.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, Hayashi T, Shiga N, Yasukawa H, Schaper W, McKenna W, Yokoyama M, Schork NJ, Omens JH, McCulloch AD, Kimura A, Gregorio CC, Poller W, Schaper J, Schultheiss HP, Chien KR. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111:943–955. doi: 10.1016/s0092-8674(02)01226-6. [DOI] [PubMed] [Google Scholar]
- Kojic S, Medeot E, Guccione E, Krmac H, Zara I, Martinelli V, Valle G, Faulkner G. The Ankrd2 protein, a link between the sarcomere and the nucleus in skeletal muscle. J Mol Biol. 2004;339:313–325. doi: 10.1016/j.jmb.2004.03.071. [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A, Bloch RJ. Obscurin: a multitasking muscle giant. J Muscle Res Cell Motil. 2005;26:419–426. doi: 10.1007/s10974-005-9024-7. [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A, Catino DH, Strong JC, Sutter S, Borisov AB, Pumplin DW, Russell MW, Bloch RJ. Obscurin modulates the assembly and organization of sarcomeres and the sarcoplasmic reticulum. FASEB J. 2006;20:2102–2111. doi: 10.1096/fj.06-5761com. [DOI] [PubMed] [Google Scholar]
- Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- Kulikovskaya I, McClellan G, Flavigny J, Carrier L, Winegrad S. Effect of MyBP-C binding to actin on contractility in heart muscle. J Gen Physiol. 2003;122:761–774. doi: 10.1085/jgp.200308941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H, Chen J, Ruiz-Lozano P, Zou Y, Nemer M, Chien KR. Control of segmental expression of the cardiac-restricted ankyrin repeat protein gene by distinct regulatory pathways in murine cardiogenesis. Development. 1999;126:4223–4234. doi: 10.1242/dev.126.19.4223. [DOI] [PubMed] [Google Scholar]
- Labeitx S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270:293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- Labeit S, Barlow DP, Gautel M, Gibson T, Holt J, Hsieh CL. A regular pattern of two types of 100-residue motif in the sequence of titin. Nature. 1990;345:273–276. doi: 10.1038/345273a0. [DOI] [PubMed] [Google Scholar]
- LeWinter MM, Wu Y, Labeit S, Granzier H. Cardiac titin: structure, functions and role in disease. Clin Chim Acta. 2007;375:1–9. doi: 10.1016/j.cca.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Lin YH, Park ZY, Lin D, Brahmbhatt AA, Rio MC, Yates JR, 3rd, Klemke RL. Regulation of cell migration and survival by focal adhesion targeting of Lasp-1. J Cell Biol. 2004;165:421–432. doi: 10.1083/jcb.200311045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke WA. Stretching molecular springs: elasticity of titin filaments in vertebrate striated muscle. Histol Histopathol. 2000;15:799–811. doi: 10.14670/HH-15.799. [DOI] [PubMed] [Google Scholar]
- Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res. 2008;77:637–648. doi: 10.1016/j.cardiores.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Liu XS, Luo HJ, Yang H, Wang L, Kong H, Jin YE, Wang F, Gu MM, Chen Z, Lu ZY, Wang ZG. Palladin regulates cell and extracellular matrix interaction through maintaining normal actin cytoskeleton architecture and stabilizing Beta1-integrin. J Cell Biochem. 2006;100:1288–1300. doi: 10.1002/jcb.21126. [DOI] [PubMed] [Google Scholar]
- Liu XS, Li XH, Wang Y, Shu RZ, Wang L, Lu SY, Kong H, Jin YE, Zhang LJ, Fei J, Chen SJ, Chen Z, Gu MM, Lu ZY, Wang ZG. Disruption of palladin leads to defects in definitive erythropoiesis by interfering with erythroblastic island formation in mouse fetal liver. Blood. 2007;110:870–876. doi: 10.1182/blood-2007-01-068528. [DOI] [PubMed] [Google Scholar]
- Loy CJ, Sim KS, Yong EL. Filamin-A fragment localizes to the nucleus to regulate androgen receptor and coactivator functions. Proc Natl Acad Sci U S A. 2003;100:4562–4567. doi: 10.1073/pnas.0736237100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther PK, Bennett PM, Knupp C, Craig R, Padrón R, Harris SP, Patel J, Moss RL. Understanding the organization and role of myosin binding protein C in normal striated muscle. J Mol Biol. 2008;384:60–72. doi: 10.1016/j.jmb.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Liu X, Wang F, Huang Q, Shen S, Wang L, Xu G, Sun X, Kong H, Gu M, Chen S, Chen Z, Wang Z. Disruption of palladin results in neural tube closure defects in mice. Mol Cell Neurosci. 2005;29:507–515. doi: 10.1016/j.mcn.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Maa MC, Lee JC, Chen YJ, Chen YJ, Lee YC, Wang ST, Huang CC, Chow NH, Leu TH. Eps8 facilitates cellular growth and motility of colon cancer cells by increasing the expression and activity of focal adhesion kinase. J Biol Chem. 2007;282:19399–19409. doi: 10.1074/jbc.M610280200. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Connectin, an elastic protein from myofibrils. J Biochem. 1976;80:405–407. doi: 10.1093/oxfordjournals.jbchem.a131291. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Connectin/titin, giant elastic protein of muscle. FASEB J. 1997;11:341–345. doi: 10.1096/fasebj.11.5.9141500. [DOI] [PubMed] [Google Scholar]
- Mayans O, Wuerges J, Canela S, Gautel M, Wilmanns M. Structural evidence for a possible role of reversible disulphide bridge formation in the elasticity of the muscle protein titin. Structure. 2001;9:331–340. doi: 10.1016/s0969-2126(01)00591-3. [DOI] [PubMed] [Google Scholar]
- Meng X, Yuan Y, Maestas A, Shen Z. Recovery from DNA damage-induced G2 arrest requires actin-binding protein filamin-A/actin-binding protein 280. J Biol Chem. 2004;279:6098–6105. doi: 10.1074/jbc.M306794200. [DOI] [PubMed] [Google Scholar]
- Miller G, Musa H, Gautel M, Peckham M. A targeted deletion of the C-terminal end of titin, including the titin kinase domain, impairs myofibrillogenesis. J Cell Sci. 2003a;116:4811–4819. doi: 10.1242/jcs.00768. [DOI] [PubMed] [Google Scholar]
- Miller MK, Bang ML, Witt CC, Labeit D, Trombitas C, Watanabe K, Granzier H, McElhinny AS, Gregorio CC, Labeit S. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol. 2003b;333:951–964. doi: 10.1016/j.jmb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Minajeva A, Kulke M, Fernandez JM, Linke WA. Unfolding of titin domains explains the viscoelastic behavior of skeletal myofibrils. Biophys J. 2001;80:1442–1451. doi: 10.1016/S0006-3495(01)76116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolman-Smook J, Flashman E, de Lange W, Li Z, Corfield V, Redwood C, Watkins H. Identification of novel interactions between domains of Myosin binding protein-C that are modulated by hypertrophic cardiomyopathy missense mutations. Circ Res. 2002;91:704–711. doi: 10.1161/01.res.0000036750.81083.83. [DOI] [PubMed] [Google Scholar]
- Moos C, Offer G, Starr R, Bennett P. Interaction of C-protein with myosin, myosin rod and light meromyosin. J Mol Biol. 1975;97:1–9. doi: 10.1016/s0022-2836(75)80017-9. [DOI] [PubMed] [Google Scholar]
- Moza M, Mologni L, Trokovic R, Faulkner G, Partanen J, Carpén O. Targeted deletion of the muscular dystrophy gene myotilin does not perturb muscle structure or function in mice. Mol Cell Biol. 2007;27:244–252. doi: 10.1128/MCB.00561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrosek M, Labeit D, Witt S, Heerklotz H, von Castelmur E, Labeit S, Mayans O. Molecular determinants for the recruitment of the ubiquitin-ligase MuRF-1 onto M-line titin. FASEB J. 2007;21:1383–1392. doi: 10.1096/fj.06-7644com. [DOI] [PubMed] [Google Scholar]
- Müller S, Lange S, Gautel M, Wilmanns M. Rigid conformation of an immunoglobulin domain tandem repeat in the A-band of the elastic muscle protein titin. J Mol Biol. 2007;371:469–480. doi: 10.1016/j.jmb.2007.05.055. [DOI] [PubMed] [Google Scholar]
- Mykkanen OM, Gronholm M, Ronty M, Lalowski M, Salmikangas P, Suila H. Characterization of human palladin, a microfilament-associated protein. Mol Biol Cell. 2001;12:3060–3073. doi: 10.1091/mbc.12.10.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley CE, Chamoun J, Brown LJ, Hambly BD. Myosin binding protein-C: enigmatic regulator of cardiac contraction. Int J Biochem Cell Biol. 2007;39:2161–2166. doi: 10.1016/j.biocel.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Offer G, Moos C, Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973;74:653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- Okagaki T, Weber FE, Fischman DA, Vaughan KT, Mikawa T, Reinach FC. The major myosin-binding domain of skeletal muscle MyBP-C (C protein) resides in the COOH-terminal, immunoglobulin C2 motif. J Cell Biol. 1993;123:619–626. doi: 10.1083/jcb.123.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivé M, Shatunov A, Gonzalez L, Carmona O, Moreno D, Quereda LG, Martinez-Matos JA, Goldfarb LG, Ferrer I. Transcription-terminating mutation in telethonin causing autosomal recessive muscular dystrophy type 2G in a European patient. Neuromuscul Disord. 2008;18:929–933. doi: 10.1016/j.nmd.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojima K, Ono Y, Hata S, Koyama S, Doi N, Sorimachi H. Possible functions of p94 in connectin-mediated signaling pathways in skeletal muscle cells. J Muscle Res Cell Motil. 2005;26:409–417. doi: 10.1007/s10974-005-9023-8. [DOI] [PubMed] [Google Scholar]
- Ono K, Yu R, Mohri K, Ono S. Caenorhabditis elegans Kettin, a Large Immunoglobulin-like Repeat Protein, Binds to Filamentous Actin and Provides Mechanical Stability to the Contractile Apparatuses in Body Wall Muscle. Mol Biol Cell. 2006;17:2722–2734. doi: 10.1091/mbc.E06-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey CA, Carpen O. α-Actinin Re-visited: a fresh look at an old player. Cell Motil. Cytoskelel. 2004;58:104–111. doi: 10.1002/cm.20007. [DOI] [PubMed] [Google Scholar]
- Ozanne DM, Brady ME, Cook S, Gaughan L, Neal DE, Robson CN. Androgen receptor nuclear translocation is facilitated by the f-actin cross-linking protein filamin. Mol Endocrinol. 2000;14:1618–1626. doi: 10.1210/mend.14.10.0541. [DOI] [PubMed] [Google Scholar]
- Parast MM, Otey CA. Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J Cell Biol. 2000;150:643–656. doi: 10.1083/jcb.150.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit MM, Meulemans SM, Alen P, Ayoubi TA, Jansen E, Van de Ven WJ. The tumor suppressor Scrib interacts with the zyxin-related protein LPP, which shuttles between cell adhesion sites and the nucleus. BMC Cell Biol. 2005;6:1. doi: 10.1186/1471-2121-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfuhl M, Pastore A. Tertiary structure of an immunoglobulin-like domain from the giant muscle protein titin: a new member of the I set. Structure. 1995;3:391–401. doi: 10.1016/s0969-2126(01)00170-8. [DOI] [PubMed] [Google Scholar]
- Pfuhl M, Gautel M, Politou AS, Joseph C, Pastore A. Secondary structure determination by NMR spectroscopy of an immunoglobulin-like domain from the giant muscle protein titin. J Biomol NMR. 1995;6:48–58. doi: 10.1007/BF00417491. [DOI] [PubMed] [Google Scholar]
- Pogue-Geile K, Chen R, Bronner MP, Crnogorac-Jurcevic T, Moyes KW, Dowen S, Otey CA, Crispin DA, George RD, Whitcomb DC, Brentnall TA. Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism. PloS Medicine. 2006;3:2216–2227. doi: 10.1371/journal.pmed.0030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politou AS, Gautel M, Pfuhl M, Labeit S, Pastore A. Immunoglobulin-type domains of titin: same fold, different stability? Biochemistry. 1994;33:4730–4737. doi: 10.1021/bi00181a604. [DOI] [PubMed] [Google Scholar]
- Politou AS, Thomas DJ, Pastore A. The folding and stability of titin immunoglobulin-like modules, with implications for the mechanism of elasticity. Biophys J. 1995;69:2601–2610. doi: 10.1016/S0006-3495(95)80131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popowicz GM, Schleicher M, Noegel AA, Holak TA. Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem Sci. 2006;31:411–419. doi: 10.1016/j.tibs.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Price CJ, Brindle NP. Vasodilator-stimulated phosphoprotein is involved in stress-fiber and membrane ruffle formation in endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:2051–2056. doi: 10.1161/01.atv.20.9.2051. [DOI] [PubMed] [Google Scholar]
- Rachlin A, Otey CA. Identification of palladin Isoforms and characterization of an isoform-specific Interaction between Lasp-1 and palladin. J Cell Sci. 2006;119:995–1004. doi: 10.1242/jcs.02825. [DOI] [PubMed] [Google Scholar]
- Reddy KB, Fox JE, Price MG, Kulkarni S, Gupta S, Das B, Smith DM. Nuclear localization of Myomesin-1: possible functions. J Muscle Res Cell Motil. 2008;29:1–8. doi: 10.1007/s10974-008-9137-x. [DOI] [PubMed] [Google Scholar]
- Richardson JS, Richardson DC, Thomas KA, Silverton EW, Davies DR. Similarity of three-dimensional structure between the immunoglobulin domain and the copper, zinc superoxide dismutase subunit. J Mol Biol. 1976;102:221–235. doi: 10.1016/s0022-2836(76)80050-2. [DOI] [PubMed] [Google Scholar]
- Robertson SP, Twigg SR, Sutherland-Smith AJ, Biancalana V, Gorlin RJ, Horn D, Kenwrick SJ, Kim CA, Morava E, Newbury-Ecob R, Orstavik KH, Quarrell OW, Schwartz CE, Shears DJ, Suri M, Kendrick-Jones J, Wilkie AO. Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans. Nat Genet. 2003;33:487–491. doi: 10.1038/ng1119. [DOI] [PubMed] [Google Scholar]
- Rönty M, Taivainene A, Moza M, Otey CA, Carpen O. Molecular analysis of the interaction between palladin and α-actinin. FEBS Lett. 2004;566:30–34. doi: 10.1016/j.febslet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Rönty M, Taivainen A, Moza M, Kruh GD, Ehler E, Carpen O. Involvement of paladin and alpha-actinin in targeting of the Abl/Arg kinase adaptor ArgBP2 to the actin cytoskeleton. Exp Cell Res. 2005;310:88–98. doi: 10.1016/j.yexcr.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Rönty M, Leivonen SK, Rachlin A, Otey C, Carpén O. Isoform-specific regulation of the actin-organizing protein palladin during TGF-β1-induced myofibroblast differentiation. J. Investigative Dermatology. 2006;126:2387–2396. doi: 10.1038/sj.jid.5700427. [DOI] [PubMed] [Google Scholar]
- Rönty M, Taivainen A, Heiska L, Otey C, Ehler E, Song WK, Carpen O. Palladin interacts with SH3 domains of SPIN90 and Src and is required for Src-induced cytoskeletal remodeling. Exp Cell Res. 2007;313:2575–2585. doi: 10.1016/j.yexcr.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber W, Begin KJ, Warshaw DM, VanBuren P. Cardiac myosin binding protein-C modulates actomyosin binding and kinetics in the in vitro motility assay. J Mol Cell Cardiol. 2008;44:1053–1061. doi: 10.1016/j.yjmcc.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmikangas P, Mykkanen OM, Gronholm M, Heiska L, Kere J, Carpen O. Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy. Hum Mol Genet. 1999;8:1329–1336. doi: 10.1093/hmg/8.7.1329. [DOI] [PubMed] [Google Scholar]
- Salmikangas P, van der Ven PF, Lalowski M, Taivainen A, Zhao F, Suila H, Schroder R, Lappalainen P, Furst DO, Carpen O. Myotilin, the limb-girdle muscular dystrophy 1A (LGMD1A) protein, cross-links actin filaments and controls sarcomere assembly. Hum Mol Genet. 2003;12:189–203. doi: 10.1093/hmg/ddg020. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Masuda Y, Ohta Y, Ikeda K, Watanabe K. Filamin associates with Smads and regulates transforming growth factor-beta signaling. J Biol Chem. 2001;276:17871–17877. doi: 10.1074/jbc.M008422200. [DOI] [PubMed] [Google Scholar]
- Schirenbeck A, Arasada R, Bretschneider T, Stradal TE, Schleicher M, Faix J. The bundling activity of vasodilator-stimulated phosphoprotein is required for filopodium formation. Proc Natl Acad Sci U S A. 2006;103:7694–7699. doi: 10.1073/pnas.0511243103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenauer R, Bertoncini P, Machaidze G, Aebi U, Perriard JC, Hegner M, Agarkova I. Myomesin is a molecular spring with adaptable elasticity. J Mol Biol. 2005;349:367–379. doi: 10.1016/j.jmb.2005.03.055. [DOI] [PubMed] [Google Scholar]
- Schoenauer R, Lange S, Hirschy A, Ehler E, Perriard JC, Agarkova I. Myomesin 3, a novel structural component of the M-band in striated muscle. J Mol Biol. 2008;376:338–351. doi: 10.1016/j.jmb.2007.11.048. [DOI] [PubMed] [Google Scholar]
- Sebestyén MG, Wolff JA, Greaser ML. Characterization of a 5.4 kb cDNA fragment from the Z-line region of rabbit cardiac titin reveals phosphorylation sites for proline-directed kinases. J Cell Sci. 1995;108:3029–3037. doi: 10.1242/jcs.108.9.3029. [DOI] [PubMed] [Google Scholar]
- Selcen D, Carpén O. The Z-disk diseases. Adv Exp Med Biol. 2008;642:116–130. doi: 10.1007/978-0-387-84847-1_10. [DOI] [PubMed] [Google Scholar]
- Selcen D, Engel AG. Mutations in myotilin cause myofibrillar myopathy. Neurology. 2004;62:1363–1371. doi: 10.1212/01.wnl.0000123576.74801.75. [DOI] [PubMed] [Google Scholar]
- Shaffer JF, Kensler RW, Harris SP. The myosin binding protein C motif binds F-actin in a phosphorylation sensitive manner. J Biol Chem. 2009 doi: 10.1074/jbc.M808850200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen VL, Dixon PH, Fox JW, Hong SE, Kinton L, Sisodiya SM, Duncan JS, Dubeau F, Scheffer IE, Schachter SC, Wilner A, Henchy R, Crino P, Kamuro K, DiMario F, Berg M, Kuzniecky R, Cole AJ, Bromfield E, Biber M, Schomer D, Wheless J, Silver K, Mochida GH, Berkovic SF, Andermann F, Andermann E, Dobyns WB, Wood NW, Walsh CA. Mutations in the X-linked filamin 1 gene cause periventricular nodular heterotopia in males as well as in females. Hum Mol Genet. 2001;10:1775–1783. doi: 10.1093/hmg/10.17.1775. [DOI] [PubMed] [Google Scholar]
- Skare P, Kreivi JP, Bergström A, Karlsson R. Profilin I colocalizes with speckles and Cajal bodies: a possible role in pre-mRNA splicing. Exp Cell Res. 2003;286:12–21. doi: 10.1016/s0014-4827(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Slater E, Amrillaeva V, Fendrich V, Bartsch D, Earl J, Vitone LJ, Neoptolemos JP, Greenhalf W. Palladin mutation causes familial pancreatic cancer: absence in European families. PLoS Med. 2007;4:e164. doi: 10.1371/journal.pmed.0040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova M, Meinecke P, Gal A, Bolz H. A novel 9 bp deletion in the filamin a gene causes an otopalatodigital-spectrum disorder with a variable, intermediate phenotype. Am J Med Genet A. 2005;132:386–390. doi: 10.1002/ajmg.a.30484. [DOI] [PubMed] [Google Scholar]
- Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev. 2005;10:237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- Tskhovrebova L, Trinick J. Properties of titin immunoglobulin and fibronectin-3 domains. J Biol Chem. 2004;279:46351–46354. doi: 10.1074/jbc.R400023200. [DOI] [PubMed] [Google Scholar]
- Valle G, Faulkner G, De Antoni A, Pacchioni B, Pallavicini A, Pandolfo D, Tiso N, Toppo S, Trevisan S, Lanfranchi G. Telethonin, a novel sarcomeric protein of heart and skeletal muscle. FEBS Lett. 1997;415:163–168. doi: 10.1016/s0014-5793(97)01108-3. [DOI] [PubMed] [Google Scholar]
- van der Ven PF, Bartsch JW, Gautel M, Jockusch H, Fürst DO. A functional knock-out of titin results in defective myofibril assembly. J Cell Sci. 2000;113:1405–1414. doi: 10.1242/jcs.113.8.1405. [DOI] [PubMed] [Google Scholar]
- van Straaten M, Goulding D, Kolmerer B, Labeit S, Clayton J, Leonard K, Bullard B. Association of kettin with actin in the Z-disc of insect flight muscle. J Mol Biol. 1999;285:1549–1562. doi: 10.1006/jmbi.1998.2386. [DOI] [PubMed] [Google Scholar]
- Vaughan KT, Weber FE, Einheber S, Fischman DA. Molecular cloning of chicken myosin-binding protein (MyBP) H (86-kDa protein) reveals extensive homology with MyBP-C (C-protein) with conserved immunoglobulin C2 and fibronectin type III motifs. J Biol Chem. 1993;268:3670–3676. [PubMed] [Google Scholar]
- Visco AG, Yuan L. Differential gene expression in pubococcygeus muscle from patients with pelvic organ prolapse. Am J Obstet Gynecol. 2003;189:102–112. doi: 10.1067/mob.2003.372. [DOI] [PubMed] [Google Scholar]
- von Castelmur E, Marino M, Svergun DI, Kreplak L, Ucurum-Fotiadis Z, Konarev PV, Urzhumtsev A, Labeit D, Labeit S, Mayans O. A regular pattern of Ig super-motifs defines segmental flexibility as the elastic mechanism of the titin chain. Proc Natl Acad Sci U S A. 2008;105:1186–1191. doi: 10.1073/pnas.0707163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Nandelstadh P, Gronholm M, Moza M, Lamberg A, Savilahti H, Carpen O. Actin-organising properties of the muscular dystrophy protein myotilin. Exp Cell Res. 2005;310:131–139. doi: 10.1016/j.yexcr.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Vorgerd M, van der Ven PF, Bruchertseifer V, Löwe T, Kley RA, Schröder R, Lochmüller H, Himmel M, Koehler K, Fürst DO, Huebner A. A mutation in the dimerization domain of filamin c causes a novel type of autosomal dominant myofibrillar myopathy. Am J Hum Genet. 2005;77:297–304. doi: 10.1086/431959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall ME, Rachlin A, Otey CA, Loboa EG. Human adipose-derived adult stem cells upregulate palladin during osteogenesis and in response to cyclic tensile strain. Am J Physiol Cell Physiol. 2007;293:C1532–C1538. doi: 10.1152/ajpcell.00065.2007. [DOI] [PubMed] [Google Scholar]
- Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- Wang HV, Moser M. Comparative expression analysis of the murine palladin isoforms. Dev Dyn. 2008;237:3342–33451. doi: 10.1002/dvdy.21755. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yoshida N, Satake M. Biological implications of filamin A-bound PEBP2beta/CBFbeta retention in the cytoplasm. Crit Rev Eukaryot Gene Expr. 2005;15:197–206. doi: 10.1615/critreveukargeneexpr.v15.i3.20. [DOI] [PubMed] [Google Scholar]
- Watkins H, Conner D, Thierfelder L, Jarcho JA, MacRae C, McKenna WJ, Maron BJ, Seidman JG, Seidman CE. Mutations in the cardiac myosin binding protein-C gene on chromosome 11 cause familial hypertrophic cardiomyopathy. Nat Genet. 1995;11:434–437. doi: 10.1038/ng1295-434. [DOI] [PubMed] [Google Scholar]
- Weber FE, Vaughan KT, Reinach FC, Fischman DA. Complete sequence of human fast-type and slow-type muscle myosin-binding-protein C (MyBP-C). Differential expression, conserved domain structure and chromosome assignment. Eur J Biochem. 1993;216:661–669. doi: 10.1111/j.1432-1033.1993.tb18186.x. [DOI] [PubMed] [Google Scholar]
- Welsch T, Endlich K, Giese T, Büchler MW, Schmidt J. Eps8 is increased in pancreatic cancer and required for dynamic actin-based cell protrusions and intercellular cytoskeletal organization. Cancer Lett. 2007;255:205–218. doi: 10.1016/j.canlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Whitten AE, Jeffries CM, Harris SP, Trewhella J. Cardiac myosin-binding protein C decorates F-actin: Implications for cardiac function. Proc Natl Acad Sci U S A. 2008;105:18360–18365. doi: 10.1073/pnas.0808903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AF, Barclay AN. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Winegrad S. Cardiac myosin binding protein C. Circ Res. 1999;84:1117–1126. doi: 10.1161/01.res.84.10.1117. [DOI] [PubMed] [Google Scholar]
- Witke W. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 2004;14:461–469. doi: 10.1016/j.tcb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Witt SH, Granzier H, Witt CC, Labeit S. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol. 2005;350:713–722. doi: 10.1016/j.jmb.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Koshida S, Sato N, Obinata T. Complete primary structure of chicken cardiac C-protein (MyBP-C) and its expression in developing striated muscles. J Mol Cell Cardiol. 1995;27:2275–2286. doi: 10.1016/s0022-2828(95)91731-4. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Ogata T, Tanabe K, Li S, Nakazato M, Kohu K, Takafuta T, Shapiro S, Ohta Y, Satake M, Watanabe T. Filamin A-bound PEBP2beta/CBFbeta is retained in the cytoplasm and prevented from functioning as a partner of the Runx1 transcription factor. Mol Cell Biol. 2005;25:1003–1012. doi: 10.1128/MCB.25.3.1003-1012.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Ferguson C, Bañuelos S, Gautel M. Molecular structure of the sarcomeric Z-disk: two types of titin interactions lead to an asymmetrical sorting of alpha-actinin. EMBO J. 1998;17:1614–1624. doi: 10.1093/emboj/17.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Gautel M. The interaction of titin and alpha-actinin is controlled by a phospholipid-regulated intramolecular pseudoligand mechanism. EMBO J. 2000;19:6331–6340. doi: 10.1093/emboj/19.23.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Ehler E, Gautel M. Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly. J Cell Biol. 2001;154:123–136. doi: 10.1083/jcb.200102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Borén J, Akyürek LM. Filamins in cardiovascular development. Trends Cardiovasc Med. 2007;17:222–229. doi: 10.1016/j.tcm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Zogopoulous G, Rothenmund H, Eppel A, Ash C, Akbari MR, Hedley D, Narod SA, Gallinger S. The P239S palladin variant does not account for a significant fraction of hereditary or early onset pancreas cancer. Hum Genet. 2007;121:635–637. doi: 10.1007/s00439-007-0361-z. [DOI] [PubMed] [Google Scholar]
- Zou Y, Evans S, Chen J, Kuo HC, Harvey RP, Chien KR. CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2-5 homeobox gene pathway. Development. 1997;124:793–804. doi: 10.1242/dev.124.4.793. [DOI] [PubMed] [Google Scholar]