Introduction
Treatment of patients with cancer has radically changed over the last several years with the advent of “targeted therapeutics.” Whereas traditional chemotherapy was directed at all rapidly dividing cells, whether cancerous or not, today’s anti-cancer drugs are increasingly tailored to the specific genetics of each cancer. This targeted approach, predominantly via inhibition of tyrosine kinase activity, has markedly improved the management of cancers including chronic myeloid leukemia (CML), breast cancer, gastrointestinal stromal tumor (GIST), renal cell carcinoma (RCC), and colon carcinoma.1–5
Inhibitors of tyrosine kinases are of two classes – monoclonal antibodies (mAbs), typically targeting growth factor receptor tyrosine kinases, and small molecules, referred to as TKIs (tyrosine kinase inhibitors), targeting both receptor and non-receptor tyrosine kinases. The goal of targeted therapy is to improve anti-tumor activity with fewer toxic side-effects than traditional anti-cancer therapies; given the initial success of this approach, the number of targeted therapy drugs entering into development in the last five years has dramatically increased.6, 7 However, several recent studies have revealed unanticipated side-effects of targeted therapy, including cardiomyopathy and heart failure, the primary manifestations of cardiotoxicity we will be examining here.5, 8, 9
Herein, we will examine the potential risk of cardiomyopathy of targeted therapy, and the molecular mechanisms that underlie that risk. We will review the importance of tyrosine kinase signaling pathways both for oncogenesis and for the survival of normal cardiomyocytes. To understand basic mechanisms of cardiomyopathy of TKIs, it is critical to understand two general classes of toxicity. The first is “on-target” toxicity wherein the tyrosine kinase target regulating cancer cell survival and/or proliferation (and therefore is a good target in cancer therapy), also serves an important role in normal cardiomyocyte survival, and thus inhibition leads to myocardial dysfunction. “Off-target” toxicity occurs when a TKI leads to toxicity via inhibition of a kinase not intended to be a target of the drug. This type of toxicity is intrinsically related to two issues - 1) the inherent non-selectivity of TKIs and 2) a trend towards “multi-targeting” or purposefully designing drugs to inhibit a broad range of targets that include kinases regulating both tumorigenesis and tumor angiogenesis. Although multi-targeting may broaden efficacy of an anti-cancer agent, likelihood of toxicity would also increase.
With the growing number of FDA-approved agents, and scores more in development,6, 7 some of these will inhibit novel kinase targets for which little or no clinical data exist on risk of heart failure or cardiomyopathy. Therefore, we will also review basic science studies that raise concerns over potential risk of cardiomyopathy in patients treated with drugs that inhibit these kinases. Finally, we will discuss cardiovascular considerations for development of future targeted therapy that may maximize anti-tumor effects, while minimizing cardiac effects in patients being treated with these potentially life-saving medications.
Tyrosine Kinases in Signal Transduction
Response to extracellular and intracellular stimuli is vital for all complex living organisms. Activation of signal transduction cascades allows a relatively small stimulus to be amplified into a larger biologic response, such as the re-programming of gene expression.10 Tyrosine kinases, of which there are approximately 90 in the human genome,11 play central roles in transducing extracellular signals (i.e. growth factors and cytokines) into activation of signaling pathways that regulate cell growth, differentiation, metabolism, migration, and programmed cell death (apoptosis). Tyrosine kinases are families of enzymes that catalyze transfer of a phosphate residue from ATP to tyrosine residues in other proteins (substrates). Phosphorylation can change activity, subcellular location, stability, etc. of the phosphylorated substrate protein.
There are two major classes of tyrosine kinases. Receptor tyrosine kinases (RTKs) are embedded in the cell membrane with an extracellular ligand-binding domain and an intracellular kinase domain that signals to the interior of the cell. In contrast, non-receptor tyrosine kinases (NRTKs) are located within the cell. By their location, tyrosine kinases can mediate transduction of both extracellular and intracellular signals. Because of their critical role in normal cellular communication and maintenance of homeostasis, tyrosine kinase activity is tightly regulated.10 Tyrosine kinases are normally quiescent until activated by extracellular stimuli or ligands, such as growth factors (e.g. vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF)) or intracellular stimuli (such as oxidant stress, activating non-receptor tyrosine kinases). An exquisite balance between activity of tyrosine kinases and of tyrosine phosphatases which mediate dephosphorylation of tyrosine residues and therefore act in contra to kinases, controls the timing and duration of cell signaling.
Abnormal Tyrosine Kinase Activity and Cancer: Malignant transformation and tumor angiogenesis
Tyrosine kinase signaling is central to both the malignant transformation of cells and tumor angiogenesis.12 Malignant transformation often results from dysregulation of tyrosine kinase signaling. Constitutive activation (i.e. on-going, even in the absence of an activating signal) of tyrosine kinases has been implicated in ~70% of cancers (Table 1).12, 13 In leukemias and solid cancers, the gene encoding the causal (or contributory) kinase is either amplified or mutated; the former leads to overexpression of the kinase and the latter to a constitutively activated state. Both mechanisms drive proliferation of the cancerous clonal cells and/or prevent them from undergoing apoptosis.
Table 1.
Kinase Inhibitors in Cancer
| Agent | Class | Target(s) | Malignancies | Cardiovascular toxicity / (Rate) / Type |
|---|---|---|---|---|
| imatinib (Gleevec) |
TKI | ABL1/2, PDGFRα/β, KIT | CML, Ph+ B-ALL, CMML, HES, GIST |
Y / (low)*/ CHF |
| dasatinib (Sprycel) |
TKI | ABL1/2, PDGFRα/β, KIT, SRC family |
CML | Y / (low to mod)* / CHF, generalized edema |
| nilotinib (Tasigna) |
TKI | ABL1/2, PDGFRα/β, KIT | CML | Y / (low)* / QT prolongation, rare sudden death |
| sunitinib (Sutent) |
TKI | VEGFR1/2/3, KIT, PDGFRα/β, RET, CSF-1R, FLT3 |
RCC, GIST | Y / (mod) / CHF, HTN |
| lapatinib (Tykerb) |
TKI | EGFR (ERBB1), ERBB2 | HER2+ breast cancer | N |
| sorafenib (Nexavar) |
TKI S/TKI | Raf-1/B-Raf, VEGFR2/3, PDGFRα/β, KIT, FLT3 |
RCC, melanoma | Y / (low)* / ACS, HTN, CHF |
| gefitinib (Iressa) |
TKI | EGFR (ERBB1) | NSCLC | N * |
| erlotinib (Tarceva) |
TKI | EGFR (ERBB1) | NSCLC, pancreatic cancer, |
N * |
| temsirolimus (Torisel) |
novel | mTOR (indirect- binds to FKBP12 and complex inhibits mTOR) |
RCC | N * |
| trastuzumab (Herceptin) |
mAb | ERBB2 | HER2+ breast cancer | Y / (mod) / CHF |
| bevacizumab (Avastin) |
mAb | VEGF-A | Colorectal cancer, NSCLC |
Y / (low to mod)* / arterial thrombosis, HTN |
| cetuximab (Erbitux) |
mAb | EGFR (ERBB1) | Colorectal cancer, squamous cell carcinoma of head/neck |
N * |
| panitumumab (Vectibix) |
mAb | EGFR (ERBB1) | Colorectal | N * |
| rituximab (Rituxan) |
mAb | CD20 | B cell lymphoma | Unknown |
| alemtuzumab (Campath) |
mAb | CD52 | B-cell CLL; | Y (in patients with mycosis fungoides/Sezary syndrome13) / CHF |
| lestaurtinib# | TKI | JAK2/FLT3 | PCV, IMF | Unknown |
| pazopanib# | TKI | VEGFRs; PDGFRs; KIT | RCC | Unknown |
| vandetanib# | TKI | VEGFR/EGFR | NSCLC | Unknown |
| cediranib## | TKI | VEGFR | NSCLC | Unknown |
| alvocidib## | S/TKI | CDK | CLL | Unknown |
| enzastaurin## | S/TKI | PKCβ | B-cell lymphoma | Unknown |
effect on LV function has not been determined and therefore these represent best guesses
NDA expected 2008
NDA expected 2010.
Abbreviations: mAb, humanized monoclonal antibody; TKI, tyrosine kinase inhibitor; S/TKI, serine/threonine kinase inhibitor; ALL, acute lymphocytic leukemia; CMML, chronic myelomonocytic leukemia; HES, hypereosinophilic syndrome; GIST, gastrointestinal stromal tumor; RCC, renal cell carcinoma; NSCLC, non-small cell lung cancer; CLL, chronic lymphocytic leukemia; PCV, polycythemia vera; IMF, idiopathic myelofibrosis; mTOR, mammalian target of rapamycin; please see text for additional abbreviations. For agents not yet FDA approved, efficacy in malignancies is projected.
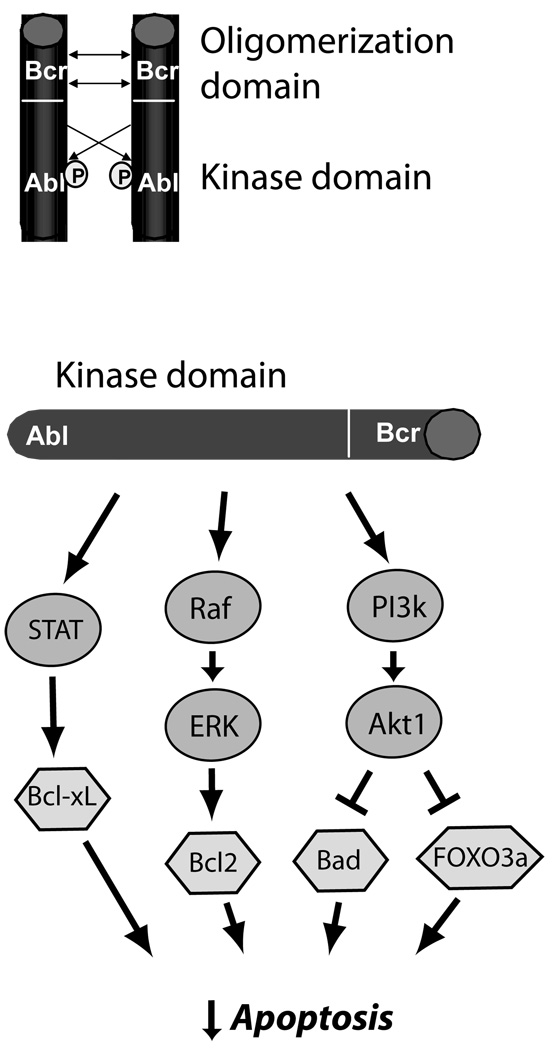
Chronic myeloid leukemia (CML) is a classical example of a cancer that results from a genetic mutation that creates a constitutively active tyrosine kinase. Four decades ago, Peter Nowell first described the association of CML with the Philadelphia chromosome, which is created by a balanced translocation in myeloid precursors. The Philadelphia chromosome encodes a fusion protein of the Bcr (breakpoint cluster region) protein kinase and the NRTK Abl (named after Herbert Abelson who first identified v-Abl, a viral oncogene in mice encoding the kinase).12 The Bcr-Abl fusion protein spontaneously forms homodimers consisting of two Bcr-Abl proteins that interact via the Bcr domains (Figure 1). This leads to constitutive activation of the Abl kinase portion of Bcr-Abl. Dimers of Bcr-Abl then activate multiple downstream signaling pathways that result primarily in inhibition of apoptosis in CML cells (Figure 1).12, 14
Figure 1.
Mechanisms of carcinogenesis of Bcr-Abl in CML. Oligomerization and cross-phosphorylation (P) of Bcr-Abl fusion proteins (top) leads to constitutive activation of the Abl kinase domain. This leads (bottom) to activation of three key pro-survival pathways, STAT5 (signal transducer and activator of transcription 5), which leads to increased expression of anti-apoptotic Bcl-xL, the Raf → ERK (extracellular signal-regulated kinase, or MAP kinase) pathway, which increases expression of anti-apoptotic Bcl2, and the phosphoinositide-3 kinase → Akt pathway, a major anti-apoptotic pathway in cancer cells and cardiomyocytes, that inhibits pro-apoptotic factors FOXO3a and Bad. This culminates in potent inhibition of apoptosis in CML cells.12
In addition to driving tumorigenesis, tyrosine kinase signaling also plays a central role in mediating tumor angiogenesis. The vital role of tumor angiogenesis was proposed more than 3 decades ago by the pioneer Judah Folkman.15 Cancers more than a few millimeters in size are dependent on formation of new microvessels for their continued growth and ability to metastasize.15 Almost all the pro-angiogenic growth factors that were subsequently identified (i.e. VEGF, Placental Growth Factor (PlGF), PDGF, TGFα, and FGF), are ligands of RTKs.
Inhibitors of tyrosine kinases as anti-cancer agents
The dependency of certain cancers on one or just a few genes for maintenance of the malignant phenotype, termed “oncogene addiction”, provides a rationale for molecular targeting in cancer therapy.16 Inhibition of aberrant tyrosine kinase activity has become a central and exciting focus of anti-cancer therapy. Two classes of targeted tyrosine kinase therapeutics have been developed-- humanized monoclonal antibodies (mAb) and small molecule inhibitors of kinase activity (TKIs)17 (Table 1; Figure 2). Monoclonal antibodies are antibodies produced from a single parent clonal cell. For anti-cancer therapy, mAbs are designed to bind the cancer cell-specific antigens, commonly to the extracellular portion of RTKs, thereby inhibiting tyrosine kinase activation (Figure 2). The binding of mAbs to the extracellular domain of the RTKs can block ligand-binding to the receptor, inhibit subsequent dimerization and activation of the tyrosine kinase domain, and/or induce downregulation of expression of the receptor.17 Furthermore, mAbs also may induce an immune response against the targeted tumor cell. An examples of a mAb that binds to receptors is trastuzumab (Herceptin; Genentech), which binds to the ERBB2 receptor (also known as HER2; Table 1). Other mAbs do not bind to the kinase receptors themselves, but instead bind the growth factor ligands that activate the receptors. For example, bevacizumab (Avastin; Genentech) targets VEGF-A, thereby preventing it from interacting with the VEGF receptor, and leading to inhibition of tumor angiogenesis.
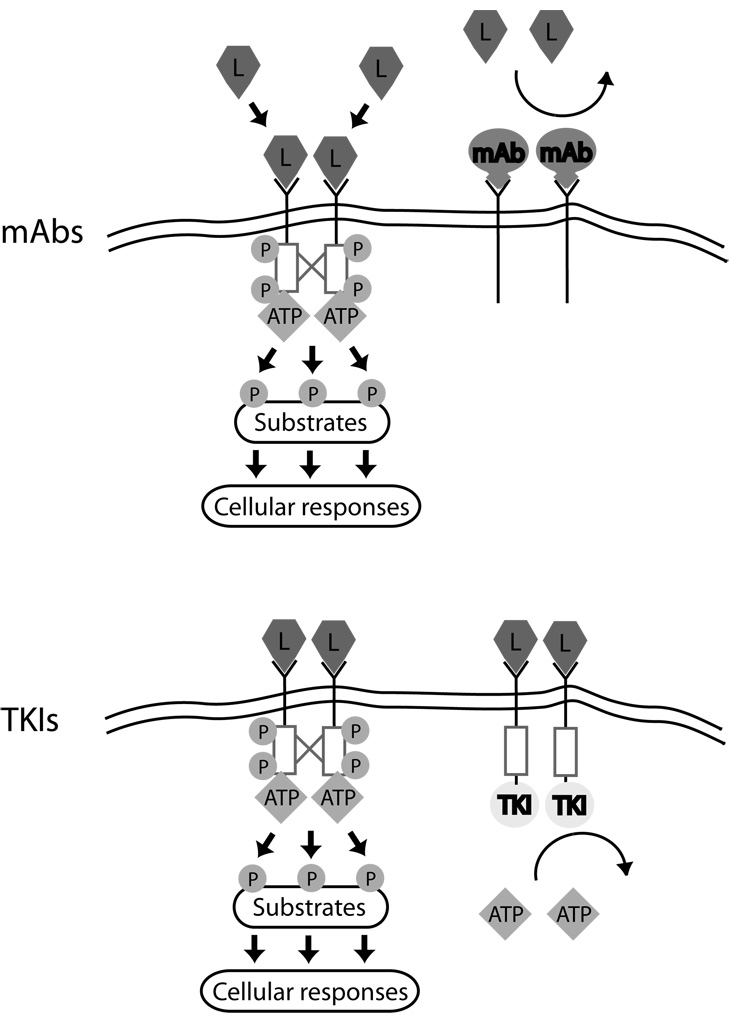
Figure 2.
Mechanisms of action of monoclonal antibodies vs. small molecule tyrosine kinase inhibitors (TKIs). Ligand (L) binding to RTKs leads to receptor dimerization, cross phosphorylation (red lines and P), and activation of the intracellular tyrosine kinase domain (red boxes). Substrates are then phosphorylated, leading to cellular responses. Monoclonal antibodies (mAbs, Top) interfere with ligand binding to receptor and/or receptor dimerization and cross-phosphorylation, blocking activation of the RTKs.17 TKIs (Bottom) do not prevent ligand binding or dimerization, but by preventing ATP from binding to the kinase domain, they block cross-phosphorylation of receptors and phosphorylation of substrates.
The vast majority of small molecule inhibitors used in cancer therapy are directed at tyrosine kinases, although some target serine/threonine kinases, the other superfamily of kinases involved in intracellular signaling (see below). Small molecule inhibitors have been designed to target both classes of tyrosine kinases, RTKs and NRTKs. Inhibitors of RTKs block activity of the intracellular kinase domain. Normally, ligand binding to a RTK initiates dimerization and cross-phosphorylation of one kinase domain by the other, thereby activating the kinase (Figure 2). The activated kinase dimer then phosphorylates downstream substrates in a signaling cascade that ultimately results in altered gene expression, cell proliferation, etc. TKIs can directly inhibit the cross-phosphyloration of the kinase domains and also inhibit phosphorylation of downstream substrates, thereby terminating the signaling cascade. TKIs that block signaling by NRTKs (e.g. Abl) target intracellular kinases and work in a similar fashion as those that target RTKs.
TKIs can block substrate phosphorylation in three ways (18 and references therein). Substrate phosphorylation is dependent on the binding of both ATP and the substrate to an activated kinase. Type I inhibitors (e.g. sunitinib) compete with ATP for binding to the ATP-pocket of a fully activated kinase, and are by far the dominant type in use today. However, they generally lack selectivity due to the highly conserved structure of the ATP pocket across the >500 kinases in the human genome and thus typically inhibit several kinases. Type II inhibitors (e.g. imatinib and nilotinib) bind two different regions on the kinase- the ATP pocket and an adjacent region that is accessible only when the kinase is inactive. Type II inhibitors thus bind and lock kinases in an inactive state. Type II TKIs generally are more potent and more selective than Type I. However, Type II agents still typically inhibit ≥ three kinases (Table 1). Type III inhibitors (e.g. the archetypal ERK pathway inhibitors, PD98059 and U0126) bind to sites remote from the ATP pocket, such as the substrate recognition region (blocking binding of substrate to kinase), or other regions of kinases that are much more divergent across the genome. Consequently, Type III inhibitors promise to be the most selective. Despite their potential for greater selectivity, however, Type III inhibitors represent a small minority of TKIs in development, because they are more difficult to design and construct and not as predictably effective. Overall, TKIs are inherently less selective than mAbs and typically inhibit several kinases, some known and others not.
One particular subgroup of TKIs is the so-called multi-targeted agents. Theoretically agents that inhibit growth factors or their receptors involved in angiogenesis, as well as kinases involved in tumor cell proliferation, could have very broad anti-cancer activity arising from this dual pharmacological effect.19 This concept led to the development of the multi-targeted agents sorafenib (Nexavar, Onyx-Bayer) and sunitinib (Sutent, Pfizer). This strategy, and its real and potential problems, will be discussed in detail, below.
Success with targeted therapeutics
Monoclonal antibodies and small molecule TKIs have validated the “oncogene addiction theory” and greatly improved the management of certain cancers. Trastuzumab was the first FDA-approved targeted anti-cancer agent, and it was approved for the treatment of women with metastatic ERBB2-positive breast cancer. Overexpression of the ERBB2 receptor was associated with more poorly-differentiated tumors, higher rates of metastases and poorer patient survival.20 Trastuzumab, a recombinant, humanized, IgG mAb that binds to the extracellular domain of ERBB2, causes growth inhibition and apoptosis of tumor cells expressing ERBB2.12 In women with metastatic breast cancer, addition of trastuzumab to standard chemotherapy resulted in a 20% decrease in risk of death among patients at one year.5 Furthermore, in five Phase III randomized trials of women with early stage ERBB2-positive breast cancer, trastuzumab used in the adjuvant setting after standard chemotherapy, reduced the risk of disease recurrence at 3 years.20
At about the same time, imatinib (Gleevec, Novartis), a drug that inhibits Bcr-Abl, the causal factor in 90% of the cases of CML, dramatically improved the survival of patients with this disease,4 leading to its approval by the FDA in 2001. Ninety percent of patients with CML treated with imatinib are alive 5 years after diagnosis of a disease that was uniformly fatal prior to this targeted therapy.4
Based on the success of trastuzumab and imatinib, the development of targeted therapeutics has exploded in the last few years. Currently, there are 29 FDA-approved agents that inhibit kinase activity, 21 mAbs and 8 TKIs (Table 1).6, 7 Three New Drug Application (NDA) filings for TKIs are expected in 2008 and an additional three in 2010 (Table 1). However, this number is truly the tip of the iceberg since there are ~175 mAbs and 150 TKIs in clinical trials with many more in pre-clinical development.6, 7 Currently there are approximately 600 agents somewhere between discovery and market, with 80% being developed as anti-cancer agents. Although sales of TKIs were only ~$4 billion in 2005–2006, with imatinib accounting for $2.5 billion, sales of TKIs are projected to grow substantially in the next few years.6
Cardiac Side-effects
Targeted anti-cancer drugs were initially thought to affect tumors but not normal tissue in which kinases were not constitutively active. Thus the hope of targeted therapy was high efficacy with minimal side-effects. As with many drugs, clinical trials have revealed unanticipated side-effects of targeted therapies involving the heart and other organs.21 Cardiovascular side-effects have, in general, been able to be managed medically and typically have not prevented their use. That said, the need to use TKIs on a long-term basis emphasizes the importance of knowing which agents are associated with cardiac effects and understanding mechanisms that underlie that toxicity.
Cardiovascular side-effects of TKIs are varied and have included heart failure, cardiomyopathy, conduction abnormalities, QT prolongation, acute coronary syndromes, myocardial injury, arterial thromboses and hypertension8, 9, 14, 22 (and please see Yeh23 for additional toxicities with cancer therapeutics in general). Overall, systolic dysfunction or cardiomyopathy with resultant heart failure is one of the most common important side-effects. This often occurs because pathways that induce the pathologic survival and abnormal proliferation of cancer cells may also regulate the survival of normal cells including cardiomyocytes. Targeting these pathways in cancer cells may inherently lead to “on-target” cardiotoxicity, manifest as cardiomyopathy, due to inhibition of these same pro-survival kinases in normal cardiomyocytes. We will introduce examples of on-target cardiotoxicity of the widely used drugs, trastuzumab and imatinib, and probable off-target toxicity of another popular agent, sunitinib, to illustrate molecular mechanisms of cardiotoxicity.
Trastuzumab
The classical example of on-target cardiotoxicity of tyrosine kinase inhibition may be the cardiac effects of trastuzumab. As noted above, trastuzumab is directed at the ERBB2 RTK.5, 24 Activation of ERBB2 leads to activation of intracellular signaling pathways in breast cancer cells similar to those seen with Bcr-Abl including ERK, PI3K/Akt, and STAT5 that drive tumor cell growth and prevent apoptosis (Figure 3).12, 14 Trastuzumab is very effective in blocking activation of these signaling pathways in ERBB2+ breast cancers cells.
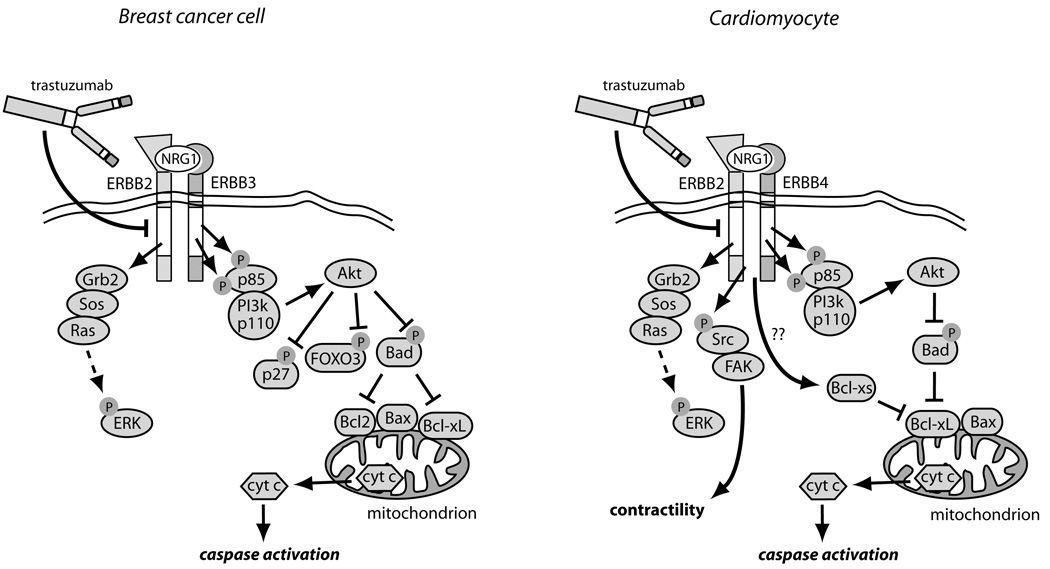
Figure 3.
Comparison of ERBB2 signaling and its inhibition by trastuzumab in breast cancer cells vs. cardiomyocytes.14 In breast cancer cells over-expressing ERBB2, ERBB2 homodimers or ERBB2/ERBB3 heterodimers form, leading to constitutive activation of the ERK, PI3K/Akt, and STAT3 pathways (latter not shown). Akt blocks apoptosis by phosphorylating and inhibiting two key pro-apoptotic factors, Bad and FOXO3A, and also inactivates the cyclin-dependent kinase inhibitor, p27, thereby enhancing cell proliferation. Trastuzumab blocks all downstream signaling, but particularly important may be reversing the inhibition of Bad, leading to activation of Bax, cyctochrome c release and apoptosis. In cardiomyocytes exposed to Nrg1, ERBB2/ERBB4 heterodimers form, again activating ERK and Akt. Trastuzumab blocks this activation and, via multiple mechanisms including alterations in levels of Bcl-X family members48, leads to mitochondrial dysfunction, energy compromise, and cytochrome c release. Trastuzumab also blocks Nrg1-mediated activation of Src and Fak, and this appears to worsen LV dysfunction.49
In a pivotal Phase III clinical trial of trastuzumab efficacy, the addition of trastuzumab to anthracycline improved survival in women with metastatic breast cancer.5 However, cardiomyopathy was an unanticipated finding with 27% of patients treated with the regimen of anthracycline, cyclophosphoamide, and trastuzumab developing heart failure. Several large studies subsequently confirmed the importance of trastuzumab in increasing disease-free survival from cancer, but also confirmed the association with heart failure(20, 25 and references therein). When anthracyclines were not administered concurrently with trastuzumab, the incidence of cardiomyopathy decreased to 13% in patients previously treated with anthracycline and then treated with paclitaxel and trastuzumab. In the adjuvant trials, 1.7 – 4.1% of trastuzumab-treated patients developed CHF.25 The incidence of cardiotoxicity of trastuzumab in non-trial settings is beginning to be examined. Recently McArthur et al reported a 20% risk of left ventricular dysfunction in patients being treated with trastuzumab after anthracycline therapy when treated off-trial.26
The finding of trastuzumab-induced cardiomyopathy in breast cancer patients led to a search for the molecular mechanisms of this effect. There was abundant evidence in mice that ERBB2, and its activating ligand, neuregulin-1 (Nrg1), play important roles during cardiac development. Germline deletion of ERBB227 or Nrg128 in mice is lethal in mid-gestation with failure of the ventricles to form properly, suggesting that ERBB2 signaling is required for cardiomyocyte proliferation during development. Mice with cardiac-specific deletion of ERBB2, after cardiac development was complete, were viable. However, these mice developed dilated cardiomyopathy as they aged and had decreased survival when subjected to pressure overload induced by aortic banding (Table 2).29, 30 Cardiomyocytes from these mice also exhibited enhanced sensitivity to anthracyclines, explaining in part the enhanced toxicity of the combination in patients.29
Table 2.
Evidence from experimental models suggesting cardiotoxicity of TKIs by TK target.
| TK target(s) |
TKIs | Model | Cardiac phenotype of model | References |
|---|---|---|---|---|
| ERBB2 | trastuzumab lapatinib |
ERBB2 KO: ±TAC | Spontaneous dilated CMP; worsened heart failure with pressure load; enhanced anthracycline sensitivity. |
29, 30 |
| VEGF VEGFRs |
sunitinib sorafenib bevacizumab |
WT: VEGF Trap + TAC |
Pathologic remodeling in response to pressure load. |
31–33 |
| KIT | imatinib/D/N sunitinib sorafenib |
1) W/WV mouse (KIT-deficient) + MI 2) WT: Arterial injury + imatinib |
1) Adverse remodeling post MI due to reduced homing of bone marrow stem cells to sites of injury; 2) Reduced stenosis post arterial injury. |
34–36 |
| Raf-1/B- Raf |
sorafenib | Raf-1 KO and dominant negative + TAC |
LV dilatation and CHF with pressure load. | 37, 38 |
| PDGFRs | imatinib/D/N sunitinib sorafenib |
WT: MI + Administration of PDGF |
Reduced injury (ischemic protection). | 39–41 |
| JAK2 | lestaurtinib | STAT3 KO: MI; aging; anthracycline administration; pregnancy |
Increased ischemic injury; reduced capillary density with aging; increased anthracylcine toxicity; peri-partum cardiomyopathy. |
42, 43 |
| Abl/Arg | imatinib/D/N | WT: imatinib | Decline in LV function; induction of ER stress. | 9, 44 |
| Met (HGF receptor) |
N/A | WT: MI or CMP models + administration of HGF |
Reduced fibrosis in MI and CMP models. Neoangiogenesis with HGF. |
45 and refs. therein |
| FGFR1/3 | N/A | Cell culture models: Administration of FGF |
Enhanced proliferation of cardiomyocytes and cardiac-resident stem cells. |
46 and refs. therein |
Abbreviations: WT, wild type; KO, knockout- gene deleted; D/N, dasatinib, nilotinib; ER, endoplasmic reticulum; CMP, cardiomyopathy
HGF, hepatocyte growth factor (ligand for Met); FGFR fibroblast growth factor receptor; MI, myocardial infarction.
See text for other abbreviations.
As in cancer cells, Nrg1-induced activation of ERBB2 in cardiomyocytes activates the ERK and PI3K/Akt pathways that promote cardiomyocyte proliferation during development and cardiomyocyte survival during adulthood (Figure 3).47 The Src/focal adhesion kinase (Fak) pathway, which enhances cardiac contractility is also activated by Nrg1.49 Expression of the anti-apoptotic protein Bcl-XL in hearts of newborn mice by adenoviral gene transfer partially prevented the heart chamber dilation and the impaired contractility seen in the adult.29 Thus inhibition of ERBB2 signaling appears to lead to dysfunction and death both in breast cancer cells over-expressing the ERBB2 receptor as well as in normal cardiomyocytes (i.e. “on-target” toxicity).
Surprisingly, unlike trastuzumab, lapatinib (Tykerb, GlaxoSmithKline), the small molecule dual inhibitor of ERBB2 and EGFR (also known as ERBB1; Table 1), shows limited depression of cardiac function.50, 51 Whereas future trials will be important to confirm this apparent absence of cardiac dysfunction, inherent differences in mechanism of action between mAbs and TKIs may also contribute to the different cardiotoxicity profiles reported between trastuzumab and lapatinib. Monoclonal antibodies, as opposed to small molecule inhibitors, initiate antibody-dependent cell cytotoxicity and complement-dependent cytotoxicity that could augment cardiotoxicity.17 Furthermore, differential inhibition/activation by lapatinib vs. trastuzumab of downstream signaling pathways may also contribute to the difference in observed rates of heart failure and cardiomyopathy. For example, lapatinib activates the cytoprotective AMP-activated protein kinase (AMPK) in cardiomyocytes whereas trastuzumab does not.52 It is obviously critical to identify mechanisms of the apparent difference in cardiotoxicity with the two agents since it could significantly impact approaches to treatment of patients with ERBB2-positive breast cancer.
Imatinib
Imatinib is indicated for the treatment of Philadelphia chromosome-positive CML and ALL, GIST, driven by activating mutations in either c-Kit (the receptor for stem cell factor) or PDGFRα, and for chronic myelomonocytic or eosinophilic leukemias due to PDGFR mutations (Table 1).12, 14 Although not common,53 imatinib-associated heart failure does occur.9, 54, 55 In mice, imatinib causes modest, but consistent, declines in LV function, but more striking was a loss of myocardial mass, consistent with cell loss. In cardiomyocytes in culture, imatinib led to cell death with features of both apoptosis and necrosis.9
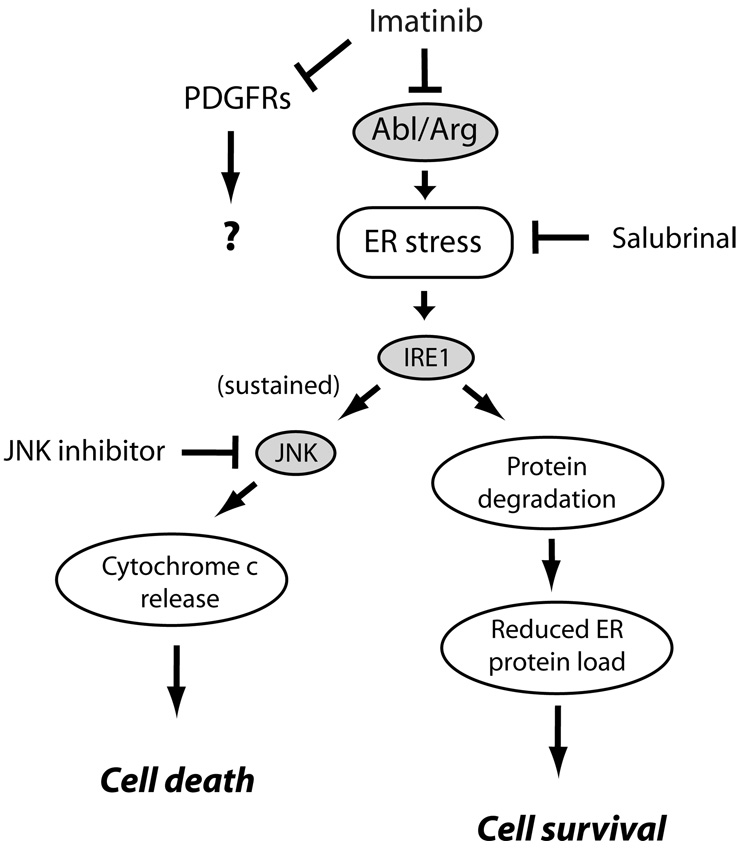
The pathway mediating cell death appeared to be induction of the endoplasmic reticulum (ER) stress response56, in which a buildup of misfolded proteins in the ER induces cellular apoptosis (Figure 4).9, 57 Imatinib also induces ER stress in CML cells and in GIST cells, and this is believed to play a role in inducing their death.58 The c-Jun N-terminal kinase (JNK) family of stress-activated MAP kinases functions as one key downstream pathway activated by the ER stress response that ultimately mediates cell death (Figure 4). Of note, imatinib-induced cell death was prevented by both salubrinal, a compound that protects cells from ER stress57 and JNK inhibition.9
Figure 4.
Pathways of imatinib-induced cardiomyocyte toxicity. Imatinib, via inhibition of Abl/Arg, leads via unclear mechanisms to induction of ER stress. This activates protein kinase IRE1 which upregulates factors involved in degradation of mis-folded proteins in the ER, thereby attempting to restore homeostasis.56 If ER stress is sustained, however, JNKs are activated leading to activation of the intrinsic apoptosis program and cell death. Both salubrinal, an inhibitor of ER stress57, and JNK inhibition by a peptide antagonist, protected from imatinib cardiomyocyte toxicity. The role, if any, of inhibition of PDGFRs in imatinib-induced cardiotoxicity is unknown at this time.
TKIs often inhibit several TKs and it is important to identify the key target whose inhibition induces cell death. Imatinib targets Abl, ARG (Abl-related gene), PDGFRα/β and c-Kit, but since c-Kit is not expressed in adult cardiomyocytes, the toxicity was presumably due to inhibition of either Abl/ARG, PDGFRs, or an unknown kinase. However, since lentivirus-mediated expression of an imatinib-resistant mutant of Abl blocked imatinib toxicity in cardiomyocytes, inhibition of Abl appeared to be the mechanism of imatinib cardiotoxicity.9 This information led Fernandez and co-workers44 to engineer a variant of imatinib, WBZ4, that no longer inhibited Abl but retained activity against c-Kit. Therefore, WBZ4 retained efficacy comparable to imatinib in treating mouse models of GIST (driven by c-Kit), but did not have associated cardiac dysfunction, presumably because Abl was not inhibited.44 This type of approach could prove instrumental for future design of kinase inhibitors; however, the caveat is that re-design will be impossible in situations in which the kinase driving cancer progression is also critical for cardiomyocyte function or survival. In these situations, cardiotoxicity may be unavoidable, though hopefully treatable, if physicians are aware of the potential problem.
Sunitinib: a multi-targeted TKI
Sunitinib targets VEGFR1-3, PDGFR α and β, c-Kit, FMS-like tyrosine kinase-3, colony-stimulating factor-1 receptor, and the product of the human RET gene (RET, mutated in medullary thyroid carcinomas/multiple endocrine neoplasias).19, 59 Cardiac events (myocardial infarction, heart failure or cardiovascular death) were observed in 11% of patients with imatinib-resistant GIST treated with sunitinib for a median of 30.5 weeks (range 10.7 to 84.9), highlighting that cardiotoxicity with TKIs can take months to develop.8 Heart failure was recorded in 8% of patients. Forty-seven percent of individuals developed hypertension (>150/100 mm Hg) while on-study. Endomyocardial biopsy of the two index patients with heart failure showed cardiomyocyte hypertrophy and mitochondrial abnormalities, but no cardiomyocyte necrosis, and no fibrosis or inflammation. Importantly, the majority of patients were able to resume sunitinib after withholding therapy and institution of standard heart failure management. Studies in vitro and in mouse models showed significant mitochondrial dysfunction, and cardiomyocyte apoptosis was present when sunitinib-treated animals were also hypertensive.8
Given the planned multi-targeting of sunitinib, and the lack of selectivity of Type I TKIs in general, the cardiomyopathy may reflect on- and/or off-target effects. The mechanisms of sunitinib-associated cardiomyopathy are not known at this time. However, it is important to identify these mechanisms given the efficacy of sunitinib in GIST and RCC.
How to assess new agents in development
One obvious question is whether cardiomyopathy associated with TKIs is a class effect, analogous to that which is observed with anthracyclines. However, it is probably misleading to refer to the TKIs as a class since, unlike β-blockers or angiotensin receptor antagonists, the specific targets and, thus, the biological effects, vary widely across the members of the TKI “class.” Thus each agent must be understood on a case-by-case basis, focusing on the specific kinases inhibited. With that understood, it is probably not surprising that cardiomyopathy does not seem to be common to all (or probably even most) TKIs, as illustrated by the apparent lack of cardiotoxicity with EGFR inhibitors (Table 1). In addition, many TKI targets are not known to be expressed in the heart (e.g. RET, FLT3, CSF-1R) and, therefore, cardiotoxicity is unlikely with agents targeting these kinases. However, there are a large number of TKIs on the horizon with targets that are expressed in the heart, for which we do not have (and may never have) good prospective data concerning cardiac effects. What strategies can identify agents that might induce cardiac dysfunction vs. those not likely to? We believe the best approach is to take a target-focused approach to examine what is known from the basic science literature that might raise concerns about cardiotoxicity of TKIs directed at popular kinase targets in cancer (see Table 2). With the understanding that the phenotypes of mouse models deleted for a specific kinase may not necessarily correlate with the phenotype caused by the incomplete inhibition of kinase activity with a drug, we believe findings in the mouse models should be instructive. Herein we will briefly review several important tyrosine kinase targets in cancer that are current focuses of drug development, and discuss the phenotypes resulting from manipulation of these kinases in mouse models. We will also discuss any available clinical data on the cardiac effects of TKIs inhibiting these same targets.
Receptor tyrosine kinases
1. VEGF/VEGFR inhibition
VEGF is a central regulator of angiogenesis, acting on its cognate tyrosine kinase receptor, VEGFR2. It has now been estimated that of the ~200 different types of human cancers, ~60% overexpress VEGF, resulting in tumor progression and metastasis.60 Proof of principle of the effectiveness of anti-VEGF therapy in cancer came when Hurwitz et al. reported improved overall survival and progression-free survival in metastatic colon cancer patients treated with chemotherapy plus bevacizumab (the mAb that sequesters VEGF) vs. chemotherapy alone.3 Many solid tumors also express additional angiogenic factors including PlGF (a homolog of VEGF that is the ligand for VEGFR1), FGF, PDGF, TGFα and HGF, all of which act in part by upregulating expression of VEGF.61, 62 Multi-targeted TKIs such as sunitinib and sorafenib inhibit PDGFRs in addition to VEGFRs.14
All of the VEGF/VEGFR-targeted therapeutics lead to blockade of endothelial cell (EC) proliferation (thereby blocking angiogenesis) and to EC apoptosis (causing regression of vessels).62 Hypertension has emerged as an important side-effect of all VEGF/VEGFR inhibitors, with the incidence of hypertension ranging from 16 – 47%.8, 63 Since VEGFR signaling is an important regulator of nitric oxide production by ECs, it is likely that impaired nitric oxide production leading to endothelial dysfunction is one factor driving the hypertension seen in patients.64 Regardless of mechanism, the trials clearly demonstrate that a certain baseline level of VEGF/VEGFR signaling is necessary for maintaining normal function of ECs and the vasculature.62 Furthermore, large vessel thrombosis, particularly in the elderly, has been noted with bevacizumab, suggesting platelet/EC interactions are also altered when VEGF/VEGFR signaling is inhibited.65
In patients with poorly controlled hypertension, inhibition of VEGF/VEGFR signaling may be even more serious. Studies in mice demonstrate that angiogenesis is a key component of a normal adaptive response to a pressure load (Figure 5).31–33 Two studies employing a ‘VEGF-trap” strategy similar in concept to bevacizumab, demonstrated that pressure overload resulted in reduction of myocardial capillary density, global contractile dysfunction, cardiac fibrosis, and eventually decompensated heart failure.31, 33 Therefore, special attention to blood pressure management seems prudent in patients treated with VEGF/VEGFR inhibitors.
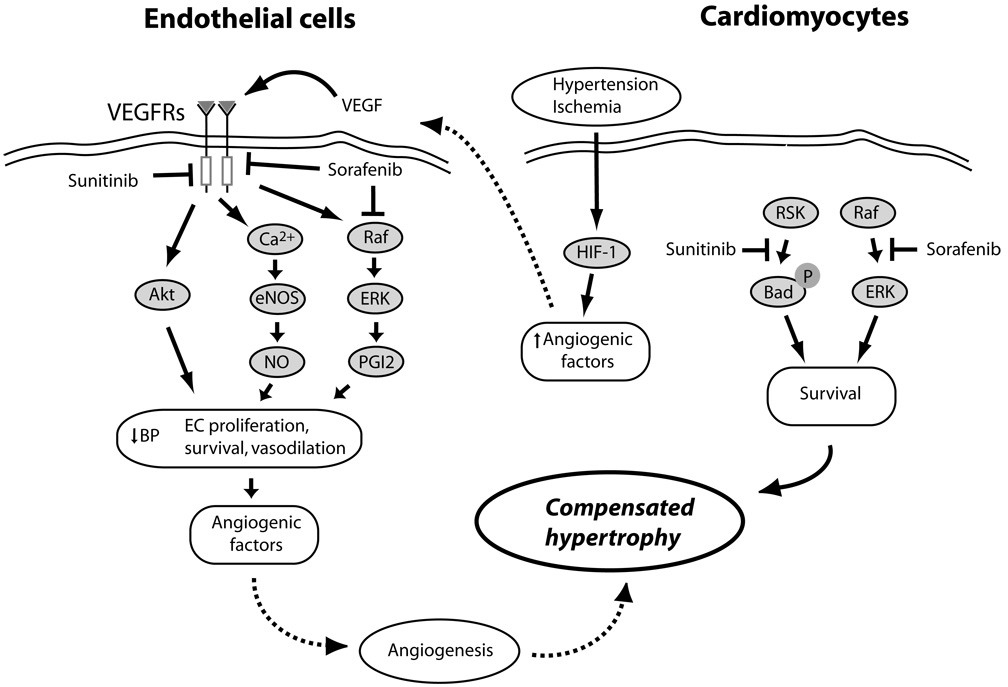
Figure 5.
Mechanisms of VEGF/VEGFR-mediated protection of cardiomyocytes from pressure stress and ischemia, and potential interactions of sunitinib and sorafenib. Hypertensive stress in the heart and the resulting relative ischemia/hypoxia of cardiomyocytes activate HIF-1, leading to the release of angiogenic factors including VEGF.32 VEGF activates VEGFRs on the surface of endothelial cells (EC), activating a number of pathways that induce vasodilation (NO and PGI2), EC proliferation and survival (Akt and ERKs), and the further release of proangiogenic factors, all of which lead to angiogenesis in the heart, allowing compensated hypertrophy to occur.31, 33 Sunitinib and sorafenib inhibit VEGFRs in ECs, potentially blocking angiogenesis and leading to decompensated hypertrophy. Sunitinib, via an off-target effect, may also inhibit various cytoprotective pathways in the heart (e.g. RSK, which would otherwise inhibit pro-apoptotic Bad). Sorafenib, by inhibiting the Raf/ERK pathway, could also induce apoptosis in ECs and cardiomyocytes.
2. PDGFR inhibition
PDGFRs are ubiquitously expressed including on cardiomyocytes and endothelial cells. Mutations and over-expression of PDGFRs, play key roles in a number of cancers including chronic myelomonocytic leukemia, GIST, glioblastoma, and osteosarcoma.12 Thus far it is unclear whether there are cardiac effects with inhibition of PDGF signaling. Data in mice suggest that exogenous delivery of PDGF to the heart may be protective in the ischemic heart (Table 2).39–41 However, it is unknown whether inhibition of endogenous PDGFR signaling by TKIs in patients will be detrimental or not. Ongoing clinical trials involving agents that inhibit PDGFRs should provide clues to the role of PDGFRs in cardiomyocytes and suggest whether there is toxicity with its inhibition and what form the toxicity might take.
3. EGFR inhibition
EGFR (or ERBB1) is mutated and/or over-expressed in many different types of tumors including gliomas, and carcinoma of the breast, ovary, and lung.17 More than 80% of non-small cell lung cancers (the leading cause of cancer death worldwide) harbor gene amplifications or mutations of the EGFR.66 To date we are aware of no reported cases clearly implicating mAbs or TKIs targeting EGFR (Table 1) in inducing CHF or cardiomyopathy in patients. There is also relatively little in the basic scienc literature that raises concerns. That said, Rockman and co-workers showed that treatment of mice with the EGFR TKI, erlotinib, an agent approved for the treatment of lung and pancreatic cancer (Table 1), enhanced myocardial injury induced by isoproteronol infusion.67 They concluded that EGFR signaling may be protective in settings of catecholamine excess. Furthermore, vigilance for potential cardiotoxicity may be prudent with the TKI canertinib which is now in clinical trials. This combination ERBB inhibitor causes irreversible inhibition of EGFR and, more importantly, ERBB2 and ERBB4, by a unique mechanism-- forming covalent bonds with cysteine residues in the active site of the kinases.
4. c-Kit inhibition
c-Kit is the receptor tyrosine kinase for stem cell factor. Point mutations, and overexpression or deletions of portions of this receptor are seen in a wide variety of cancers, which include AML, GIST, small cell lung cancer, and sarcomas (Table 1).12 Not surprisingly, a large number of trials are ongoing with Kit inhibitors.
Kit is normally expressed on hemangioblasts, which are the precursors for both hematopoietic stem cells and endothelial progenitor cells. Studies in the c-KitW/W-v mouse (in which one c-Kit allele is deleted and the other encodes a protein with reduced kinase activity) demonstrated that c-Kit is necessary for proper homing of bone marrow-derived pro-angiogenic stem/progenitor cells to regions of infarction (Table 2). This homing, in turn, is necessary to prevent adverse remodeling following myocardial infarction.34, 35 These data raise potential concerns about the use of c-Kit inhibitors in cancer patients with a history of coronary artery disease. Possibly balancing that concern is the finding that imatinib reduced intimal hyperplasia following vascular injury in the femoral artery of mice.36 The finding was thought to be due to reduced homing of bone marrow cells, which are vascular smooth muscle cell progenitors, to the site of injury. The cardiovascular effects of c-Kit inhibition in patients remain to be elucidated.
Non-receptor kinases
1. Abl inhibition
In addition to imatinib, dasatinib (Sprycel, Bristol-Myers Squibb) and nilotinib (Tasigna, Novartis) also target Abl/Arg, PDGFRs, and c-Kit. Both are effective in treating Philadelphia chromosome-positive imatinib-resistant CML. Nilotinib prolongs the QT interval and rarely sudden deaths have been reported (www.fda.gov/cder/Offices/OODP/whatsnew/nilotinib.htm). Dasatinib, which also targets the Src family of non-receptor tyrosine kinases, is associated with fluid retention including pericardial and pleural effusions. However, the mechanism has yet to be clarified. Dasatinib has also been implicated in the development of heart failure with up to 4% of patients in some series developing CHF (dasatinib prescribing information; https://www.sprycell.com/pdf/pi.pdf). Although this could certainly be due to inhibition of Abl,9, 44 dasatinib also inhibits Src and was recently reported to inhibit a number of other kinases,68 and any of these could play a role as well.
2. RAF family inhibition
Raf (standing for rapidly accelerated fibrosarcoma) is a family of cytoplasmic serine/threonine (as opposed to tyrosine) kinases consisting of Raf-1, A-Raf, and B-Raf.69 Activating mutations of B-Raf occur in 66% of melanomas and to a lesser degree in a variety of solid tumors including the thyroid, colon and ovary. Raf-1 is rarely mutated in cancers but is over-expressed in some including squamous cell cancer of the head and neck. The Raf kinases, in particular B-Raf, are upstream of the pro-survival ERKs.70 Many RTK oncogenic mutants signal via Raf-1/ERKs to induce cell cycle entry and proliferation, and to block apoptosis. Thus, the Raf family is a very popular target of small molecule inhibitors.
Raf-1 plays a protective role in the heart, particularly in settings of pressure overload stress (Table 2). In mice, deletion of Raf-1 in the heart led to a dilated, hypocontractile heart with enhanced cardiomyocyte apoptosis and fibrosis38. Furthermore, when mice with a mutation which blocked Raf signaling (via cardiac-specific expression of a dominant inhibitory Raf-1) were subjected to pressure overload, there was cardiomyocyte apoptosis and significant mortality.37 The roles of B-Raf and A-Raf in the heart are not clear.
The Raf family is a target of sorafenib (Nexavar, Bayer) and a number of other drugs in development71. Approved for both metastatic RCC and hepatocellular carcinoma,63 sorafenib also inhibits VEGFR-2/3, FLT-3, c-Kit, and PDGFRs (Table 1). In clinical trials, sorafenib was associated with acute coronary syndromes, including myocardial infarction, in 2.9% of patients vs. 0.4% in placebo-treated patients (sorafenib prescribing information; http://www.univgraph.com/bayer/inserts/nexavar.pdf). As with other anti-VEGF/VEGFR agents, hypertension is a significant problem. Overall, the basic science findings noted above suggest vigilance for cardiovascular side-effects with sorafenib, particularly in patients with poorly controlled hypertension. Interestingly, gain-of-function mutations in Raf-1 and other components of the Raf pathway are causal in some cases of hypertrophic cardiomyopathy (Noonan and LEOPARD syndromes),72 raising the prospect that inhibitors targeting Raf-1 might be useful in these rare Mendelian syndromes.
3. JAK/STAT inhibition
The Janus kinase (JAK) family of non-receptor tyrosine kinases takes part in the regulation of cellular responses to numerous growth factors and cytokines including members of the interferon family and interleukins. Jaks are also activated by Ang II in cardiomyocytes. There are four Jaks, and they re-program gene expression by phosphorylating and activating STATs, of which there are seven.73
Mutated RTKs and NRTKs (e.g. Abl) often utilize Jak/STAT signaling to drive tumor growth and in some cases tumor angiogenesis. Furthermore, an activating point mutation (V617F) in Jak2 is present in nearly every patient with the myeloproliferative disorder polycythemia vera and in about half of patients with essential thrombocythemia and chronic idiopathic myelofibrosis12. The mutant JAK2 appears to act, at least in part, by activating Stat3 and Stat5, in leukemic cells.
In the heart, it is generally believed that Jak/STAT signaling is protective (Table 2).42, 43, 74 Mice lacking cardiac STAT3 had enhanced susceptibility to myocardial ischemia, doxorubicin-induced cardiotoxicity and age-related heart failure. STAT3 appears to be critical for maintaining cardiac capillary density via multiple mechanisms.42 These findings suggest potential for cardiotoxicity of Jak inhibitors, particularly if they have activity against multiple Jak family kinases.
The most advanced Jak inhibitor in clinical development is lestaurtinib (CEP-701), which targets Jak2 and Flt3 (mutated in ~30% of cases of AML), but also inhibits others including neurotrophin receptors. It is primarily being tested in patients with AML.75 A NDA is expected to be filed in 2008.6 Phase I/II trials of this agent have thus far not raised major concerns over cardiotoxicity, however, to out knowledge, these studies do not include monitoring of cardiac function. Given lestaurtinib’s apparent lack of selectivity, off-target and on-target effects are a concern.
Conclusions and future directions
Much still needs to be learned about the cardiac effects of agents that inhibit tyrosine kinase activity. With the number of drugs in development and on the market, a collaborative interdisciplinary approach between oncologists and cardiologists, would greatly further our understanding of these agents. As with any developing field, careful clinical observations and investigation of symptom constellations suggestive of CHF will provide early recognition of possible cardiotoxicity. These vital clinical observations help guide and inform basic scientists in the search for mechanisms of cardiotoxicity.
One important area for future research is the refinement of approaches to improve the selectivity of drugs in development. Methods now exist to screen TKIs for inhibitory activity against very large numbers of kinases (>200).68 The majority of TKIs studied have been found to inhibit many more kinases than intended. SInce many of these play no role in tumorigenesis, risk of toxicity is increased with no apparent gain in therapeutic efficacy. However, as knowledge of cancer and cardiomyocyte biology improves, supported by the striking advances in kinase crystal structure determination, we should be better able to tailor treatment to very specific cancer targets, reducing the risk of off-target effects. When it is impossible to “design around” a critical target of tumorigenesis, knowledge of the role of that kinase in the heart and the effects of its inhibition will serve to alert clinicians to potential problems.
One final unresolved issue is the question of reversibility of the cardiotoxicity associated with tyrosine kinase inhibition, as opposed to anthracycline cardiotoxicity, and the basic mechanisms underlying these differences. Some degree of reversibility has been seen with both trastuzumab and sunitinib,8, 76 and as noted above, a number of patients have been able to return to treatment after temporary cessation of therapy and/or institution of standard heart failure management. However, a dissenting report has recently surfaced regarding trastuzumab.77 At this time, we simply do not have adequate long-term follow-up of patients treated with sunitinib to be able to assess the degree and duration of reversibility. This is obviously a critical issue to address since if true, patients may be able to be maintained on these potentially life-saving therapies, albeit with very careful follow-up. Ultimately, this will require a close partnership between cardiologists and oncologists.
Acknowledgments
We thank Tammy Chu and Christopher Walsh for reviewing the manuscript.
Funding Sources:
Supported by the Translational Research Fund for Cancer and Cardiology, and the Department of Cardiology, Children’s Hospital Boston (MHC); Finnish Heart Foundation and Finnish Cultural Foundation (RK); and The National Heart, Lung, and Blood Institute (HL61688 and HL67371)(TF).
Footnotes
All authors have read and agree with the manuscript as written.
Conflict of Interest Disclosures:
None
References
- 1.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Sherbenou DW, Druker BJ. Applying the discovery of the Philadelphia chromosome. J. Clin. Invest. 2007;117:2067–2074. doi: 10.1172/JCI31988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Norman P. Kinase therapeutic pipelines: An assessment of targets and agents in development. Insight Pharma Reports. 2007 www.insightpharmareports.com/reports/2007/90_Kinase_Inhibitors/overview.asp.
- 7.Via MC. Monoclonal antibodies: Pipeline analysis and competitive assessment. Insight Pharma Reports. 2007 www.insightpharmareports.com/reports/88_Monoclonal_Antibodies/overview.asp.
- 8.Chu T, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan JA, Harris DM, Ismail NS, Chen J-H, Schoen FJ, Demetri GD, Force T, Chen MH. Cardiotoxicity associated with the tyrosine kinase inhibitor sunitinib. Lancet. 2007;270:2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat. Med. 2006;12:908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 10.Paul MK, Mukhopadhyay AK. Tyrosine kinase - Role and significance in Cancer. Int. J. Med. Sci. 2004;1:101–115. doi: 10.7150/ijms.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 12.Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N. Engl. Jour. Med. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 13.Lenihan DJ, Alencar AJ, Yang D, Kurzrock R, Keating MJ, Duvic M. Cardiac toxicity of alemtuzumab in patients with mycosis fungoides/Sezary syndrome. Blood. 2004;104:655–658. doi: 10.1182/blood-2003-07-2345. [DOI] [PubMed] [Google Scholar]
- 14.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat. Rev. Cancer. 2007;7:332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 15.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein IB, Joe AK. Mechanisms of disease: Oncogene addiction--a rationale for molecular targeting in cancer therapy. Nat. Clin. Pract. Oncol. 2006;3:448–457. doi: 10.1038/ncponc0558. [DOI] [PubMed] [Google Scholar]
- 17.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer. 2006;6:714–727. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- 18.Okram B, Nagle A, Adrian FJ, Lee C, Ren P, Wang X, Sim T, Xie Y, Wang X, Xia G, Spraggon G, Warmuth M, Liu Y, Gray NS. A general strategy for creating "inactive-conformation" abl inhibitors. Chemistry & biology. 2006;13:779–786. doi: 10.1016/j.chembiol.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 2007;6:734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 20.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N. Engl. J. Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 21.Maitland ML, Ratain MJ. Terminal ballistics of kinase inhibitors: There are no magic bullets. Ann. Intern. Med. 2006;145:702–703. doi: 10.7326/0003-4819-145-9-200611070-00015. [DOI] [PubMed] [Google Scholar]
- 22.Khakoo AY, Kassiotis CM, Tannir N, Plana JC, Halushka M, Bickford C, Trent J, 2nd, Champion JC, Durand JB, Lenihan DJ. Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer. 2008 doi: 10.1002/cncr.23460. In press. [DOI] [PubMed] [Google Scholar]
- 23.Yeh ET. Cardiotoxicity induced by chemotherapy and antibody therapy. Annu. Rev. Med. 2006;57:485–498. doi: 10.1146/annurev.med.57.121304.131240. [DOI] [PubMed] [Google Scholar]
- 24.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 25.Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008;14:14–24. doi: 10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
- 26.McArthur HL, Chia S. Cardiotoxicity of trastuzumab in clinical practice. N. Engl. J. Med. 2007;357:94–95. doi: 10.1056/NEJMc070065. [DOI] [PubMed] [Google Scholar]
- 27.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 28.Meyer D, Birchmeier C. Muliple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 29.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 30.Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc. Nat. Acad. Sci. 2002;99:8880–8885. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–893. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007 Mar 22;446(7134):444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 33.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and antiogenesis contributes to the trasntion fo heart failure. J. Clin. Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, Higuchi K, Siatskas C, Lee P, Lim H, Zhang J, Cukerman E, Stanford WL, Medin JA, Liu PP. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc. Nat. Acad. Sci. 2006;103:2304–2309. doi: 10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fazel S, Cimini M, Chen LS, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li R-K. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J. Clin. Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CH, Anderson N, Li SH, Szmitko PE, Cherng WJ, Fedak PW, Fazel S, Li RK, Yau TM, Weisel RD, Stanford WL, Verma S. Stem cell factor deficiency is vasculoprotective: unraveling a new therapeutic potential of imatinib mesylate. Circ. Res. 2006;99:617–625. doi: 10.1161/01.RES.0000243210.79654.fd. [DOI] [PubMed] [Google Scholar]
- 37.Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi O, Watanabe T, Nishida K, Kashiwase K, Higuchi Y, Takeda T, Hikoso S, Hirotani S, Asahi M, Taniike M, Nakai A, Tsujimoto I, Matsumura Y, Miyazaki J, Chien KR, Matsuzawa A, Sadamitsu C, Ichijo H, Baccarani M, Hori M, Otsu K. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J. Clin. Invest. 2004;114:937–943. doi: 10.1172/JCI20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edelberg JM, Lee SH, Kaur M, Tang L, Feirt NM, McCabe S, Bramwell O, Wong SC, Hong MK. Platelet-derived growth factor-AB limits the extent of myocardial infarction in a rat model: feasibility of restoring impaired angiogenic capacity in the aging heart. Circulation. 2002;105:608–613. doi: 10.1161/hc0502.103672. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J. Clin. Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh PC, MacGillvray C, Gannon J, Cruz FU, Lee RT. Local controlled intramyocardial delivery of platelet-derived growth factor improves postinfarction ventricular function without pulmonary toxicity. Circulation. 2006;114:637–644. doi: 10.1161/CIRCULATIONAHA.106.639831. [DOI] [PubMed] [Google Scholar]
- 42.Hilfiker-Kleiner D, Limbourg A, Drexler H. STAT3-mediated activation of myocardial capillary growth. Trends Cardiovasc. Med. 2005;15:152–157. doi: 10.1016/j.tcm.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Yamada S, Okabe M, Kishimoto T, Yamauchi-Takihara K. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc. Natl. Acad. Sci. 2000;97:315–319. doi: 10.1073/pnas.97.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernandez A, Sanguino A, Peng Z, Ozturk E, Chen J, Crespo A, Wulf S, Shavrin A, Qin C, Ma J, Trent J, Lin Y, Han H-D, Mangala LS, Bankson JA, Gelovani J, Samarel A, Bornmann W, A.K. S, Lopez-Bernstein G. An anticancer c-Kit kinase inhibitor is reengineered to make it more active and less cardiotoxic. J Clin Invest. 2007;117:4044–4054. doi: 10.1172/JCI32373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson CD, Heydarkhan-Hagvall S, Schenke-Layland K, Yang JQ, Jordan MC, Kim JK, Brown DA, Zuk PA, Laks H, Roos KP, Maclellan WR, Beygui RE. The Role of Cytoprotective Cytokines in Cardiac Ischemia/Reperfusion Injury. J. Surg. Res. 2007 doi: 10.1016/j.jss.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc. Natl. Acad. Sci. U S A. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. J. Biol. Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 48.Grazette L, Boecker W, Matsui T, Semigran M, Force T, Hajjar R, Rosenzweig A. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J. Am. Coll. Cardiol. 2004;44:2231–2238. doi: 10.1016/j.jacc.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 49.Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J. Mol. Cell. Cardiol. 2006;41:228–235. doi: 10.1016/j.yjmcc.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bilancia D, Rosati G, Dinota A, Germano D, Romano R, Manzione L. Lapatinib in breast cancer. Ann Oncol. 2007;18 Suppl 6:vi26–vi30. doi: 10.1093/annonc/mdm220. [DOI] [PubMed] [Google Scholar]
- 51.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 52.Spector NL, Yarden Y, Smith B, Lyass L, Trusk P, Pry K, Hill JE, Xia W, Seger R, Bacus SS. Activation of AMP-activated protein kinase by human EGF receptor 2/EGF receptor tyrosine kinase inhibitor protects cardiac cells. Proc. Natl. Acad. Sci. USA. 2007;104:10607–10612. doi: 10.1073/pnas.0701286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atallah E, Durand JB, Kantarjian H, Cortes J. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood. 2007;110:1233–1237. doi: 10.1182/blood-2007-01-070144. [DOI] [PubMed] [Google Scholar]
- 54.Park YH, Park HJ, Kim BS, Ha E, Jung KH, Yoon SH, Yim SV, Chung JH. BNP as a marker of the heart failure in the treatment of imatinib mesylate. Cancer Lett. 2006;243:16–22. doi: 10.1016/j.canlet.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 55.Sohn SK, Kim JG, Kim DH, Lee KB. Cardiac morbidity in advanced chronic myelogenous leukaemia patients treated by successive allogeneic stem cell transplantation with busulphan/cyclophosphamide conditioning after imatinib mesylate administration. Br. J. Haematol. 2003;121:469–472. doi: 10.1046/j.1365-2141.2003.04288.x. [DOI] [PubMed] [Google Scholar]
- 56.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Molec Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 57.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2a dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 58.Pattacini L, Mancini M, Mazzacurati L, Brusa G, Benvenuti M, Martinelli G, Baccarani M, Santucci MA. Endoplasmic reticulum stress initiates apoptotic death induced by STI571 inhibition of p210 bcr-abl tyrosine kinase. Leukemia Research. 2004;28:191–202. doi: 10.1016/s0145-2126(03)00218-2. [DOI] [PubMed] [Google Scholar]
- 59.Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J. Clin. Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 60.Folkman J. Angiogenesis. Annu. Rev. Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 61.Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J. Am. Coll. Cardiol. 2007;49:1015–1026. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 62.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 63.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 64.Veronese ML, Mosenkis A, Flaherty KT, Gallagher M, Stevenson JP, Townsend RR, O'Dwyer PJ. Mechanisms of hypertension associated with BAY 43–9006. J Clin Oncol. 2006;24:1363–1369. doi: 10.1200/JCO.2005.02.0503. [DOI] [PubMed] [Google Scholar]
- 65.Verheul HMW, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat. Rev. Cancer. 2007;7:475–485. doi: 10.1038/nrc2152. [DOI] [PubMed] [Google Scholar]
- 66.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 67.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J. Clin. Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bantscheff M, Eberhard D, Abraham Y, Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau C, Reader V, Sweetman G, Bauer A, Bouwmeester T, Hopf C, Kruse U, Neubauer G, Ramsden N, Rick J, Kuster B, Drewes G. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat. Biotechnol. 2007;25:1035–1044. doi: 10.1038/nbt1328. [DOI] [PubMed] [Google Scholar]
- 69.Zebisch A, Czernilofsky AP, Keri G, Smigelskaite J, Sill H, Troppmair J. Signaling through RAS-RAF-MEK-ERK: from basics to bedside. Curr. Med. Chem. 2007;14:601–623. doi: 10.2174/092986707780059670. [DOI] [PubMed] [Google Scholar]
- 70.Kyriakis JM, Force TL, Rapp UR, Bonventre JV, Avruch J. Mitogen regulation of c-Raf-1 protein kinase activity toward mitogen-activated protein kinase kinase. J. Biol. Chem. 1993;268:16009–16019. [PubMed] [Google Scholar]
- 71.Gollob JA, Wilhelm S, Carter C, Kelley SL. Role of Raf kinase in cancer: therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin. Oncol. 2006;33:392–406. doi: 10.1053/j.seminoncol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Pogna EA, Schackwitz W, Ustaszewska A, Landstrom A, Bos JM, Ommen SR, Esposito G, Lepri F, Faul C, Mundel P, Lopez Siguero JP, Tenconi R, Selicorni A, Rossi C, Mazzanti L, Torrente I, Marino B, Digilio MC, Zampino G, Ackerman MJ, Dallapiccola B, Tartaglia M, Gelb BD. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nature genetics. 2007;39:1007–1012. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 73.Schindler CW. Series introduction. JAK-STAT signaling in human disease. J. Clin. Invest. 2002;109:1133–1137. doi: 10.1172/JCI15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barry SP, Townsend PA, Latchman DS, Stephanou A. Role of the JAK-STAT pathway in myocardial injury. Trends Mol. Med. 2007;13:82–89. doi: 10.1016/j.molmed.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, Murphy KM, Dauses T, Allebach J, Small D. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 76.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J. Clin. Oncol. 2005;23:2900–2902. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 77.Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J. Clin. Oncol. 2007;25:3525–3533. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]