Abstract
Objective
To assess the effects of selective serotonin reuptake inhibitor (SSRI) use on risks of gestational hypertension (GHT) and preeclampsia.
Method
We used data from 5,731 women with non-malformed infants and no underlying hypertension who participated in the Slone Epidemiology Center Birth Defects Study in 1998-2007. GHT was defined as incident hypertension diagnosed after the 20th week of pregnancy with and without proteinuria (i.e., with and without preeclampsia). We compared the risks of GHT and preeclampsia between users and non-users of SSRIs during pregnancy. Relative risks (RR) and 95% confidence intervals (CI) were estimated by Cox proportional hazard models, adjusting for pre-pregnancy sociodemographic, reproductive, and medical factors.
Results
GHT was present in 9.0% of the 5,532 non-users and 19.1% of the 199 SSRI users; among users, GHT was present in 13.1% of the 107 women who used SSRIs only in the first trimester and in 26.1% of the 92 who continued SSRIs beyond the first trimester. For preeclampsia, the incidence was 2.4% among non-users, 3.7% among women who were exposed only in the first trimester and 15.2% among those who continued SSRIs beyond the first trimester. Compared with non-users, the adjusted RR of preeclampsia was 1.4 (95% CI: 0.5, 3.8) for women who discontinued and 4.9 (95% CI: 2.7, 8.8) for those who continued SSRIs.
Conclusion
Whether a causal factor or not, SSRI exposure late in pregnancy might identify women at increased risk for GHT and preeclampsia. Further investigation is required to separate the effects of SSRIs from those of underlying mood disorders.
Keywords: antidepressive agents, gestational hypertension, preeclampsia, selective serotonin reuptake inhibitors, epidemiology
Introduction
Studies suggest that 12-25% of all pregnant women display some signs of depression (1, 2) and 3-13% of pregnant women are treated with antidepressants (3, 4). The most commonly used antidepressants among pregnant women are the selective serotonin reuptake inhibitors (SSRIs) (3, 4). Antidepressants can control mood effectively and reduce the risks of serious consequences associated with untreated depression for both the mother and her offspring (5, 6). However, use of antidepressants during pregnancy could be potentially associated with adverse effects to the fetus. While findings have not been consistent for some outcomes (7-12), first trimester exposure to certain SSRIs has been associated with certain specific birth defects (13-16), and SSRI use late in pregnancy has been associated with pulmonary hypertension of the newborn (17), prematurity (18-21), low birth weight (20, 21), small for gestational age (22), and various other neonatal complications (18-20, 23).
Very few studies have focused on the potential medical and obstetrical adverse effects of antidepressants for the mothers themselves. A leading cause of morbidity and mortality in pregnancy is preeclampsia, clinically recognized by gestational hypertension (GHT) and proteinuria (24, 25). It has been suggested that psychological conditions such as stress (26) and anxiety and depression (27, 28) may trigger the pathogenic vascular processes that lead to this condition. Serotonin may play an important role in the etiology of preeclampsia through its vascular and hemostatic effects (29), and SSRIs have been shown to affect circulating serotonin levels (30). The few studies that suggested an elevated risk of preeclampsia among pregnant women with depression did not assess the potential independent effect of medications (27, 28). If this association is real, it would still be unclear whether it is due to biologic or behavioral factors intrinsic to women with mood disorders, to medications used to treat the disorder, or a combination of both.
Women treated with medications for depression who are pregnant or planning a pregnancy, and their doctors, often struggle with the decision about treatment options, and a critical clinical question is whether to continue or discontinue antidepressants during pregnancy. In this study, we investigated the risk of GHT and preeclampsia associated with continuing and discontinuing these antidepressants beyond the first trimester. To our knowledge, this is the first study to examine this question.
Methods
Study population
We used data from the Slone Epidemiology Center Birth Defects Study (BDS), a multi-center case-control surveillance program of birth defects in relation to environmental exposures (particularly medications) (13, 17). More than 35,000 mothers of babies with and without birth defects from the greater metropolitan areas of Philadelphia, San Diego, and Toronto, as well as selected regions in Iowa, Massachusetts and New York State, have been interviewed since 1976. Study subjects are identified through review of admissions and discharges at major birth hospitals and pediatric referral hospitals and clinics, logbooks in perinatal intensive care units, through weekly telephone contact with collaborators at newborn nurseries in community hospitals, and through collaborations with state birth defects registries. Since 1998, the study has also included a random sample of Massachusetts births. The mothers of non-malformed infants are recruited independently from any exposure and therefore provide an estimate of the distribution of exposures (including use of medications) in the study population. Institutional Review Board approval was obtained from each of the participating institutions and mothers provide informed consent before participation.
Inclusion and exclusion criteria
We restricted our analyses to a retrospective cohort of women who gave birth to non-malformed liveborns between 1998 and 2007 and were ascertained at either the hospital-based centers or through the Massachusetts birth registry (N=5,912). Women with elective terminations, miscarriages, or stillbirths were not included, because most are not at risk of the outcomes of interest, which occurred after 20 weeks of gestation.
Exposure ascertainment and definitions
Within six months of delivery, trained study nurses unaware of study hypotheses conduct a 45-60 minute telephone interview of the study mothers. The interview collects information on demographic, reproductive, and medical factors, as well as cigarette smoking, alcohol consumption, occupational exposures, and dietary intake. It also uses a series of increasingly detailed questions to collect information on medications (prescription, over-the-counter, vitamins/minerals, and herbal products) used anytime from two months prior to conception through the pregnancy (31). Standardized questions prompt with a list of indications and specific conditions (e.g., “depression”) and specific drugs (e.g., “Prozac (fluoxetine)”). When possible, reported medications are verified by asking the subject to read information from the medication container. Identification of timing of drug exposure is facilitated by use of a 12-month calendar covering periods before and after pregnancy; special dates (e.g., last menstrual period (LMP), holidays) are marked to help enhance recall. Data are collected on starting and stopping dates, duration, frequency, indication, form, and number of pills per day. The interview also elicits information on LMP, whether based on maternal recall or ultrasound exam; LMP allows estimation of the due date, approximate conception date, and gestational age at birth.
We identified women who were receiving SSRIs two months before pregnancy and then categorized them into those who stopped their treatment before the end of the first trimester and those who continued to use their medications after the first trimester. Since very few women initiate SSRIs during pregnancy (N=36), assessing the effect of starting treatment during pregnancy would be less clinically relevant and statistically underpowered, and these women were not analyzed separately (no cases of preeclampsia were observed in these women). Use of non-SSRI antidepressants was classified in a similar manner.
Outcome ascertainment and definition
We specifically asked women if a health care provider had diagnosed “high blood pressure” or “toxemia or preeclampsia” during their pregnancy, the dates when the condition started and ended, and whether they had used medications to treat the conditions. Given the potential misclassification of pregnancy-induced hypertension with and without preeclampsia, we combined the two diagnoses in our main analyses. Thus, gestational hypertension (GHT) was defined as incident hypertension during pregnancy with and without proteinuria (i.e., with and without preeclampsia/toxemia). To exclude underlying hypertension as a potential source of both confounding and outcome misclassification bias, we restricted the definition of GHT to that first diagnosed after the 20th week of pregnancy; as a result, we excluded 145 women with an early diagnosis of hypertension from all the analyses. For each woman with GHT, the onset date was defined as the gestational month when GHT was first diagnosed.
Statistical Analysis
We considered our study a retrospective cohort design. Using as the reference group women with no SSRI use throughout pregnancy, we estimated the relative risk of GHT for those who stopped their SSRIs before the end of the first trimester and those who continued treatment beyond the first trimester. Characteristics of the latter two groups were compared by chi-square analyses and Fisher's exact tests.
Relative risks (RR) and 95 percent confidence intervals (95% CI) were estimated for each outcome using Cox proportional hazard models with time measured in days since the 20th week of gestation. We considered the following potential confounders: region, birth year, maternal age, race/ethnicity, education, family income, gravidity, number of fetuses, pre-pregnancy body mass index (BMI; kg/m2), age at menarche, diabetes mellitus, infertility treatment, cigarette smoking, coffee and alcohol intake, and use of illicit drugs or other psychotherapeutic medications during pregnancy (24, 32-36). We retained in the models those factors associated with the outcome in our population (even if the association was not statistically significant), as well as use of non-SSRI antidepressants. We repeated our analyses separately for preeclampsia and GHT without preeclampsia.
We conducted a number of secondary analyses. Because of the different heritability, clinical manifestations, and prognosis of early- and late-onset GHT, we distinguished these conditions according to timing of onset (33, 34, 37, 38). GHT was considered early-onset when it began between 20 and 32 weeks after the LMP and late-onset afterwards. We analyzed primigravidae separately from multigravidae as well as smokers separately from non-smokers, and restricted our analysis to singleton births (39). Effect measure modification on a multiplicative scale was formally tested by the Wald test comparing whether two stratum-specific RRs were equivalent. We verified the proportional hazards assumptions by including in the models an interaction term for time to event and SSRI use.
Results
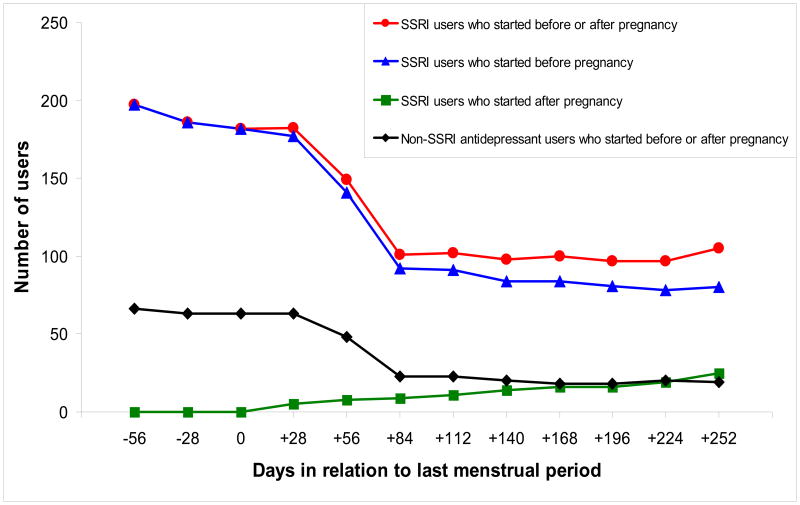
Of the 5,731 women without pre-gestational hypertension, 538 (9.4%) reported being diagnosed with GHT and among these 538 women, 153 (28.4%) developed preeclampsia (2.7% of all subjects). A total of 199 (3.5%) women were using SSRIs two months before conception (191 used these medications for mood disorders); 107 of them discontinued SSRIs before the first trimester (i.e., “discontinuers”), and 92 remained on the medications beyond the first trimester of pregnancy (i.e., “continuers”) (Figure 1). Most women maintained their exposure status during the later trimesters: 12 (11.0%) of the discontinuers restarted their treatment and 6 (6.5%) of the continuers stopped taking SSRIs.
Figure 1.
Antidepressant use in relation to the last menstrual period, Slone Epidemiology Center Birth Defects Study, 1998-2007 *
* SSRI: selective serotonin reuptake inhibitor. Non-SSRI antidepressants include tricyclic antidepressants, serotonin/norepinephrine reuptake inhibitors, and other antidepressant
Maternal characteristics by SSRI use status are shown in Table 1. SSRI discontinuers and continuers did not differ substantially in their baseline characteristics. The risk of GHT and/or preeclampsia was associated with region, younger maternal age, White race, lower family income, younger age at menarche, cigarette smoking, diabetes mellitus, higher pre-pregnancy BMI, multiple gestations (twins or more), primigravidae, and history of fertility treatment (data not shown). These variables were included in our final models.
Table 1.
Maternal characteristics by selective serotonin reuptake inhibitor (SSRI) use status, Slone Epidemiology Center Birth Defects Study, 1998-2007 *
| Characteristics | Non-users (N=5,532) † | Discontinuers (N=107)† | Continuers (N=92)† | p-value (discontinuers vs. continuers) ‡ |
|---|---|---|---|---|
| Maternal age (years) | ||||
| < 25 | 1,047 (18.9) | 23 (21.5) | 16 (17.4) | 0.56 |
| 25-29 | 1,284 (23.2) | 29 (27.1) | 18 (19.6) | |
| 30-34 | 1,979 (35.8) | 27 (25.2) | 26 (28.3) | |
| ≥ 35 | 1,196 (21.6) | 27 (25.2) | 31 (33.7) | |
| Maternal race/ethnicity | ||||
| Caucasian | 3,994 (72.2) | 86 (80.4) | 82 (89.1) | 0.13 |
| Hispanic | 739 (13.4) | 8 (7.5) | 6 (6.5) | |
| African American | 380 (6.9) | 8 (7.5) | 4 (4.4) | |
| Other | 417 (7.5) | 5 (4.7) | 0 (0.0) | |
| Maternal education (years) | ||||
| ≤ 12 | 1,470 (26.6) | 33 (30.9) | 25 (27.2) | 0.81 |
| 13-15 | 1,320 (23.9) | 29 (27.1) | 28 (30.4) | |
| > 15 | 2,739 (49.5) | 45 (42.1) | 39 (42.4) | |
| Married or living with baby's father | 4,969 (89.8) | 90 (84.1) | 83 (90.2) | 0.21 |
| Family income | ||||
| < 45,000 US dollars per year | 1,498 (27.1) | 34 (31.8) | 29 (31.5) | 0.84 |
| ≥ 45,000 US dollars per year | 3,577 (64.7) | 65 (60.8) | 58 (63.0) | |
| Diabetes mellitus | 262 (4.7) | 6 (5.6) | 5 (5.4) | 1.00 |
| Age at menarche | ||||
| < 12 | 983 (17.8) | 16 (15.0) | 20 (21.7) | 0.36 |
| ≥ 12 | 4,343 (78.5) | 89 (83.2) | 69 (75.0) | |
| Fertility treatment | 375 (6.8) | 5 (4.7) | 10 (10.9) | 0.11 |
| Number of fetuses | ||||
| Single | 5,376 (97.2) | 106 (99.1) | 87 (94.6) | 0.10 |
| Two or more | 156 (2.8) | 1 (1.0) | 5 (5.4) | |
| Gravidity | ||||
| Primigravidae | 1,718 (31.1) | 35 (32.7) | 24 (26.1) | 0.07 |
| Multigravidae, first liveborn | 449 (8.1) | 6 (5.6) | 15 (16.3) | |
| Multiparous, last pregnancy ≤ 3 years | 2,054 (37.1) | 30 (28.0) | 31 (33.7) | |
| Multiparous, last pregnancy > 3 years | 1,294 (23.4) | 35 (32.7) | 22 (23.9) | |
| Illicit dug use | 100 (1.8) | 3 (2.8) | 4 (4.4) | 0.71 |
| Non-SSRI antidepressant use | 53 (1.0) | 6 (5.6) | 9 (9.8) | 0.29 |
| Other psychotherapeutic drug use | 33 (0.6) | 7 (6.5) | 3 (3.3) | 0.35 |
| Pre-pregnancy body mass index§ | ||||
| < 18.5 | 260 (4.7) | 11 (10.3) | 5 (5.4) | 0.54 |
| 18.5 - 25.0 | 3,510 (63.5) | 60 (56.1) | 50 (54.4) | |
| 25.1 - 30.0 | 1,085 (19.6) | 19 (17.8) | 21 (22.8) | |
| > 30.0 | 583 (10.5) | 17 (15.9) | 16 (17.4) | |
| Smoking during pregnancy | ||||
| Never smoked | 3,297 (59.6) | 45 (42.1) | 43 (46.7) | 0.76 |
| Past smoker | 1,355 (24.5) | 27 (25.2) | 23 (25.0) | |
| Smoked < 10/day during pregnancy | 426 (7.7) | 17 (15.9) | 10 (10.9) | |
| Smoked ≥ 10/day during pregnancy | 454 (8.2) | 18 (16.8) | 16 (17.4) | |
| Alcohol intake during pregnancy | ||||
| Never drank | 2,535 (45.8) | 45 (42.1) | 38 (41.3) | 0.85 |
| Past drinker | 2,823 (51.0) | 57 (53.3) | 48 (52.2) | |
| Drank during pregnancy | 174 (3.2) | 5 (4.7) | 6 (6.5) | |
| Coffee drinking during pregnancy | ||||
| Never drank | 2,725 (49.3) | 50 (46.7) | 27 (29.4) | 0.04 |
| Past drinker | 2,050 (37.1) | 44 (41.1) | 49 (53.3) | |
| Drank during pregnancy | 757 (13.7) | 13 (12.2) | 16 (17.4) |
Numbers might not add up due to missing data. Numbers in parentheses represent proportions of all women in each category; independent categories were created for women with missing values
Non-users were women who were not exposed to SSRIs from two months before pregnancy through delivery; Discontinuers were women who used SSRIs before pregnancy, but stopped taking them after first trimester; Continuers were women who used SSRIs before pregnancy, and continued to use them after first trimester
Based on chi-square test, except for binary variables, which were based on Fisher's exact test (two-tailed)
Body mass index was calculated by weight/(height)2, kg/m2
Sixty-eight women were using non-SSRI antidepressants two months before pregnancy (16 used serotonin/norepinephrine reuptake inhibitors, SNRIs); 13 of them (19.1%; 3 used SNRIs) developed GHT and 4 (5.9%; 1 used SNRIs) developed preeclampsia. Compared with non-users, the adjusted RR for women who used non-SSRI antidepressants immediately before or during pregnancy was 1.58 (95% CI: 0.90-2.80) for GHT and 1.55 (95% CI: 0.55-4.38) for preeclampsia. Due to small numbers, non-SSRI antidepressant use was not analyzed further, except as a potential confounder.
Figure 2 shows the cumulative incidence of GHT and preeclampsia by SSRI exposure status. SSRI users had a higher risk of GHT, compared with women not exposed to SSRIs; the risk was greater for continuers than for discontinuers (Table 2). The increased risk observed among continuers appeared to be largely attributable to preeclampsia, although there was also a suggestion of elevated risk for GHT without preeclampsia. The results were similar when we compared discontinuers who did not restart their treatment with continuers who did not stop using SSRIs during later trimesters. RRs did not vary greatly by gravidity or smoking status; restricting our analyses to singleton births, or using women exposed to SSRIs only (and not to non-SSRI antidepressants concomitantly) did not materially change the results (data not shown). We did not have the power to evaluate specific SSRIs.
Figure 2.
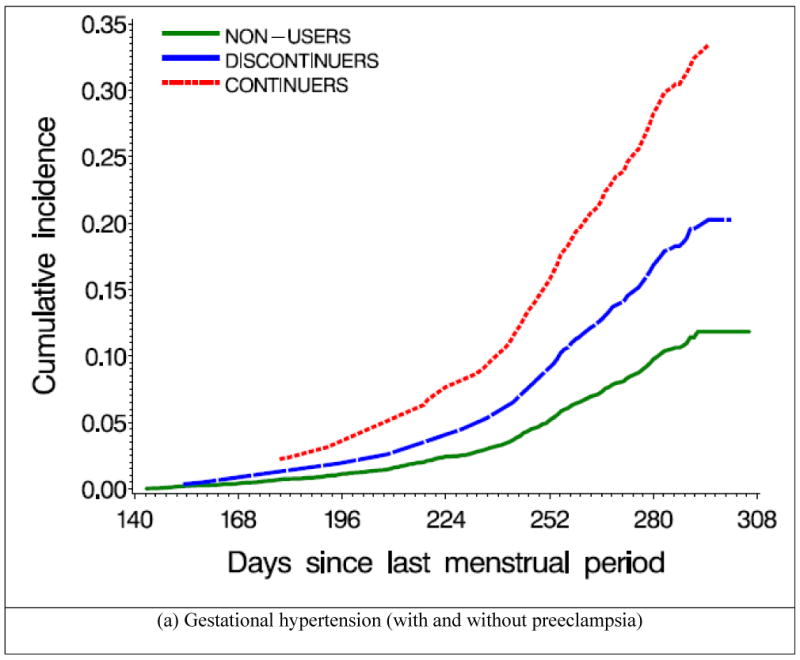
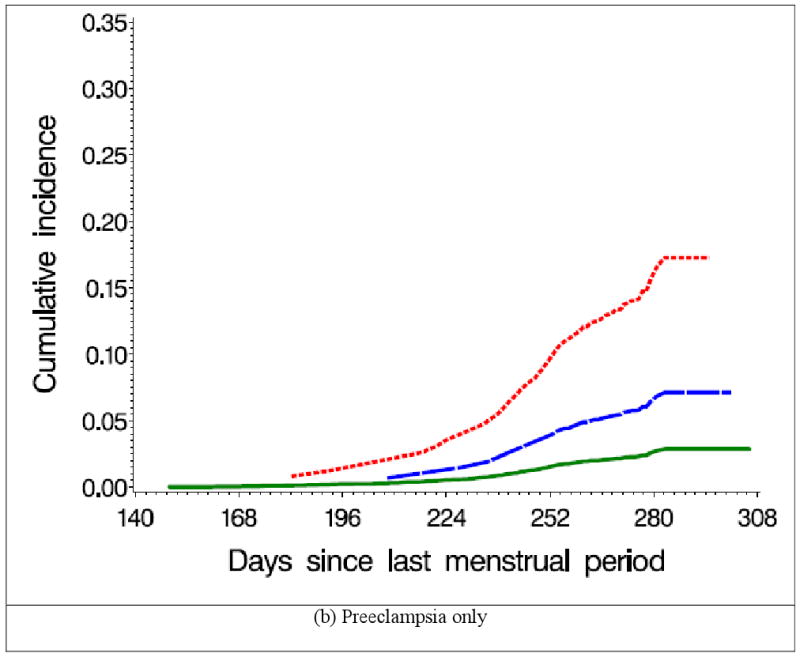
Cumulative incidence of gestational hypertension and preeclampsia by selective serotonin reuptake inhibitor (SSRI) use, Slone Epidemiology Center Birth Defects Study, 1998-2007 *
* Non-users (N=5,532) were women who were not exposed to SSRIs from two months before pregnancy through delivery; Discontinuers (N=107) were women who used SSRIs two months before pregnancy, but stopped taking them by the end of the first trimester; Continuers (N=92) were women who used SSRIs two months before pregnancy, and continued to use them after the first trimester
Table 2.
Risks of gestational hypertension with and without pre-eclampsia according to selective serotonin reuptake inhibitor (SSRI) exposure status, Slone Epidemiology Center Birth Defects Study, 1998-2007 *
| Perinatal outcomes | Number of cases (%) |
Crude relative risk (95% confidence interval) |
Adjusted relative risk † (95% confidence interval) |
|---|---|---|---|
| Any gestational hypertension | |||
| Pre-pregnancy SSRI use | |||
| Non-users | 500 (9.0) | Reference | Reference |
| Users | 38 (19.1) | 2.29 (1.64, 3.18) | 1.90 (1.35, 2.67) |
| Discontinuers | 14 (13.1) | 1.44 (0.85, 2.45) | 1.33 (0.78, 2.27) |
| Continuers | 24 (26.1) | 3.47 (2.30, 5.23) | 2.49 (1.62, 3.83) |
| Preeclampsia | |||
| Pre-pregnancy SSRI use | |||
| Non-users | 135 (2.4) | Reference | Reference |
| Users | 18 (9.0) | 3.91 (2.39, 6.39) | 3.16 (1.89, 5.29) |
| Discontinuers | 4 (3.7) | 1.51 (0.56, 4.08) | 1.37 (0.50, 3.76) |
| Continuers | 14 (15.2) | 7.16 (4.13, 12.43) | 4.86 (2.70, 8.76) |
| Gestational hypertension without preeclampsia | |||
| Pre-pregnancy SSRI use | |||
| Non-users | 365 (6.6) | Reference | Reference |
| Users | 20 (10.0) | 1.61 (1.03, 2.53) | 1.36 (0.85, 2.15) |
| Discontinuers | 10 (9.4) | 1.41 (0.75, 2.64) | 1.30 (0.69, 2.46) |
| Continuers | 10 (10.9) | 1.88 (1.00, 3.53) | 1.41 (0.74, 2.69) |
Non-users (N=5,532) were women who were not exposed to SSRIs from two months before pregnancy through delivery; Discontinuers (N=107) were women who used SSRIs two months before pregnancy, but stopped taking them by the end of the first trimester; Continuers (N=92) were women who used SSRIs two months before pregnancy, and continued to use them after the first trimester
Adjusted for region, maternal age, race/ethnicity, marital status, family income, age at menarche, diabetes mellitus, cigarette smoking, pre-pregnancy body mass index, use of non-SSRI antidepressants, number of fetuses, gravidity, and history of fertility treatment. See Table 1 for all potential confounders considered
There were 146 and 35 early-onset GHT and preeclampsia cases, respectively. The RRs for continuers and discontinuers were similar for early- and late-onset GHT compared with non-users. However, comparing continuers with non-users, the RR of early-onset preeclampsia was 16.4 (95% CI: 6.2, 43.7; based on 6 cases among continuers), and the RR of late-onset preeclampsia was 3.5 (95% CI: 1.6, 7.5; 8 cases among continuers). There was no indication of violation of the proportional hazards assumption in any analyses.
Discussion
We found that peri-conceptional SSRI use was associated with a higher risk of GHT, and particularly preeclampsia. Further, the risk of preeclampsia was greater among women who continued treatment after the first trimester (15.2%), compared with both non-users (2.4%) and those who discontinued SSRIs (3.7%). Our findings may have at least four interpretations: 1) SSRIs cause these outcomes; 2) underlying mood disorders are associated with an increased risk for these outcomes; 3) methodological limitations create a spurious association between SSRIs and these outcomes; and 4) a combination of the above.
Causal effect of SSRIs
In our study, SSRI use exclusively in early pregnancy, when placentation occurs, was not associated with a higher risk of preeclampsia. Thus, if real, the effect of SSRIs on preeclampsia might not be mediated via placentation but rather through hypertension or vascular effects later in pregnancy. Serotonin may play a role in the pathophysiology of preeclampsia through its effects on hemostasis and vascular tone in uteroplacental tissues (29, 40, 41). In addition, SSRIs inhibit synthesis of nitric oxide, a vasodilator that appears to play a role in vascular tone and reactivity both in utero and during postnatal life (42-44). The more recently introduced SNRIs can cause elevations of diastolic blood pressure, probably due to their noradrenergic effects (45), but we did not have sufficient numbers of exposed women to examine the outcomes associated with these medications.
Confounding by depression or other mood disorders
The association between SSRIs and preeclampsia might stem from the underlying mood disorders (e.g., depression, anxiety), which could reflect both the indication for treatment and the ultimate cause of preeclampsia, or it could represent a marker for a higher risk (e.g. linked genetic predisposition for the two conditions). For example, depression or anxiety could induce vasoconstriction and uterine artery resistance through an altered excretion of vasoactive hormones and other neuroendocrine transmitters (5). In a Finnish study, anxiety and depression during pregnancy were associated with a three-fold increased risk for preeclampsia (27), and a similar association was found in a recent case-control study (28). However, these studies did not examine the effects of these conditions independent of treatment. In our study, the greater incidence of GHT and preeclampsia found among users of non-SSRI antidepressants compared to non-users suggests that women who need antidepressive agents might be at an increased risk for these outcomes, and that the increased risk might not be related to the SSRIs themselves.
Unfortunately, we were not able to compare SSRI users with non-users among women with depression since we did not identify mood disorders in study participants who did not report drug treatment. The small number of outcomes among women receiving non-SSRI antidepressants (e.g., SNRIs, tricyclic antidepressants) limited our ability to examine the effect of SSRIs compared to these medications. However, while it might appear that such comparisons would be ideal to adjust for confounding by the underlying condition, depression severity and other potential risk factors for preeclampsia among women with depression may differ between users and non-users of specific antidepressants. For example, it has been observed that among pregnant women with a diagnosis of depression, those receiving SSRIs have clinical indicators suggesting a more severe depression than non-users (22).
Therefore, in addition to comparing antidepressant users with non-users, we compared those who discontinued to those who continued SSRI use after the first trimester. Although the two groups did not differ in most baseline variables (Table 1), these women might differ in other unmeasured characteristics that could potentially explain our findings. In particular, we did not have a measure of depression severity for women who discontinued or continued SSRI use. Those continuing SSRIs might have better control of their symptoms than those who discontinued their treatment, or they might have a more severe condition that requires pharmacologic treatment. Thus, the higher risk of preeclampsia associated with continuation of treatment after the first trimester might simply reflect a more severe mood disorder. In the absence of treatment randomization, comparing these two groups might reduce, but not eliminate, the possibility of confounding by depression severity.
Confounding by other factors
Characteristics such as high pre-pregnancy BMI are positively associated with both SSRI use and preeclampsia (46). Failure to adjust for these confounders could overestimate the risks associated with these drugs; in our analyses, estimates adjusted for these confounders did not differ substantially from the unadjusted estimates. Other important socioeconomic and lifestyle factors, such as family income and maternal education level, were also considered, but did not appear to be major confounders in our study population. For an unmeasured confounder to have an appreciable impact, it would have to be reasonably prevalent and must be strongly associated with both SSRI use during pregnancy and risk of GHT.
Information bias
A potential weakness of this study lies in the outcome information, which was based on self-report because we did not have routine access to obstetric notes in mothers' medical records. Thus, under- and over-reporting of events, misclassification of the exact date of onset, and cross-classification of preeclampsia and transient hypertension in pregnancy, are possible. Under-diagnosis is unlikely since over 99% of the women in our population had had prenatal care, where screening for GHT and proteinuria is standard practice. Information errors are likely to have been minimized by use of a carefully designed questionnaire, with specific questions on hypertension onset and preeclampsia as diagnosed by a health care provider, and administered within six months of delivery by trained nurses.
Further, the data offer evidence of the general validity of the outcome classification: First, the reported incidence of GHT (12.6%) among primiparous women, and the overall incidence of preeclampsia (2.7%) in our population are similar to those in population-based studies (47, 48). Second, the frequency and effect of other known risk factors (e.g., gravidity, number of fetuses, and maternal weight) are similar to those consistently reported in the literature (24, 32-36). Other previously suggested risk factors, such as advanced maternal age, were not associated with a higher frequency of GHT in our study, most likely because we excluded women with pre-gestational hypertension (i.e., these factors were associated with hypertension diagnosed before 20 weeks of gestation). Our failure to find the previously suggested protective effect of smoking (49) might be due to various factors, including differences among characteristics of smokers over time and methodological factors.
In addition, if reported outcomes were misclassified similarly for users and non-users of SSRIs, the effect would be to bias our results towards a null effect of SSRIs. Of greater concern would be differential misclassification of outcome among SSRI users and non-users: For example, if women on chronic treatments such as antidepressant therapy were diagnosed with GHT or preeclampsia more readily, this situation would indeed tend to bias the association between SSRIs and GHT/preeclampsia towards an increased risk among users, and for continuers vs. discontinuers. However, while more intensive monitoring and diagnosis would likely produce stronger associations for GHT, an event that may be variably detected, they would be less likely to do so for preeclampsia, which is a clinically significant event that is difficult to miss.
Studies using medication prescribing or dispensing data might not capture actual use and time of use during pregnancy, leading to potential misclassification of exposure (50). Our study collected information on medications actually taken by the mother, and timing of use was systematically collected. Although any data based on maternal recall might be subject to misclassification, we do not believe recall bias was a major concern in our study since SSRIs are used on a regular basis and for nontrivial reasons and the study interviewers specifically probed for use of antidepressants and were unaware of the study hypothesis. While underreporting of antidepressant use is possible since women might be reluctant to disclose such information, it is reassuring that the prevalence of SSRI use in our study was consistent with that reported by prospective studies (4, 22). Further, if women with the study outcomes were less likely to report their SSRI use, such underreporting would lead to an underestimation of the true risk.
SSRI, preeclampsia, and prematurity/fetal growth restriction
Several (18-22), but not all (8-12) studies have suggested that SSRIs may be associated with a greater risk of prematurity and/or fetal growth restriction. Since preeclampsia is a known risk factor for these outcomes (51), it is possible that the suggested associations between SSRIs and prematurity or fetal growth restriction are mediated through an elevated risk of preeclampsia among SSRI users.
Conclusion
The benefits of treating depression in pregnancy (52) and the risk of relapse associated with treatment discontinuation (6) are well described, and this study can only inform the risk component of the risk-benefit assessment. Our data suggest that SSRI exposure in pregnancy might identify women at increased risk for GHT and preeclampsia. Continuation of SSRI treatment beyond the first trimester might be associated with a higher risk than discontinuation of SSRIs soon after pregnancy awareness. However, we were unable to disentangle whether the risk is attributable to the drugs or to the underlying mood disorder, or a combination of both. In addition, we were limited by potential differential misclassification of the outcomes. Thus, future efforts, including well-designed and powered prospective cohort studies, are needed to confirm or refute these findings. Independent of a causal link or mechanism, if these findings are confirmed, women exposed to SSRIs during pregnancy should be carefully monitored for the occurrence of GHT and preeclampsia.
Acknowledgments
We thank Dawn Jacobs, RN, MPH, Fiona Rice, MPH, Rita Krolak, RN, Kathleen Sheehan, RN, Karen Bennett Mark, RN, Clare Coughlin, RN, Nastia Dynkin, Nancy Rodriguez-Sheridan, and Meghan Malone-Moses, MPH for their assistance in data collection and computer programming; and the staff of the Massachusetts Department of Public Health Center for Birth Defects Research and Prevention; we also thank all the mothers who participated in the study.
Dr. Hernández-Díaz was partially supported by grant R01 HD046595 from the National Institute of Child and Human Development (NICHD). Dr. Mitchell serves (unpaid) on the GlaxoSmithKline lamotrigine pregnancy registry. The Slone Epidemiology Center and the Pharmacoepidemiology program at Harvard School of Public Health have received research and/or training grants or contracts from pharmaceutical companies, some of which manufacture drugs discussed in this manuscript (GlaxoSmithKline, Pfizer, Eli Lilly, Wyeth, Novartis, and AstraZeneca).
Footnotes
Disclosures: The current analysis and manuscript were not directly financed by any organization and the authors declare no other competing interest.
References
- 1.Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323(7307):257–60. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotlib IH, Whiffen VE, Mount JH, Milne K, Cordy NI. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269–74. doi: 10.1037//0022-006x.57.2.269. [DOI] [PubMed] [Google Scholar]
- 3.Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544 e1–5. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, Rolnick SJ, Roblin D, Smith DH, Willy ME, Staffa JA, Platt R. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194 e1–5. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 5.Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–35. doi: 10.1177/070674370404901103. [DOI] [PubMed] [Google Scholar]
- 6.Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrick V, Reminick AM, Loughead A, Vitonis AF, Stowe ZN. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- 7.Hallberg P, Sjoblom V. The use of selective serotonin reuptake inhibitors during pregnancy and breast-feeding: a review and clinical aspects. J Clin Psychopharmacol. 2005;25(1):59–73. doi: 10.1097/01.jcp.0000150228.61501.e4. [DOI] [PubMed] [Google Scholar]
- 8.Malm H, Klaukka T, Neuvonen PJ. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol. 2005;106(6):1289–96. doi: 10.1097/01.AOG.0000187302.61812.53. [DOI] [PubMed] [Google Scholar]
- 9.Pastuszak A, Schick-Boschetto B, Zuber C, Feldkamp M, Pinelli M, Sihn S, Donnenfeld A, McCormack M, Leen-Mitchell M, Woodland C, et al. Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac) JAMA. 1993;269(17):2246–8. [PubMed] [Google Scholar]
- 10.Nulman I, Rovet J, Stewart DE, Wolpin J, Gardner HA, Theis JG, Kulin N, Koren G. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336(4):258–62. doi: 10.1056/NEJM199701233360404. [DOI] [PubMed] [Google Scholar]
- 11.Kulin NA, Pastuszak A, Sage SR, Schick-Boschetto B, Spivey G, Feldkamp M, Ormond K, Matsui D, Stein-Schechman AK, Cook L, Brochu J, Rieder M, Koren G. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study. JAMA. 1998;279(8):609–10. doi: 10.1001/jama.279.8.609. [DOI] [PubMed] [Google Scholar]
- 12.Suri R, Altshuler L, Hendrick V, Rasgon N, Lee E, Mintz J. The impact of depression and fluoxetine treatment on obstetrical outcome. Arch Womens Ment Health. 2004;7(3):193–200. doi: 10.1007/s00737-004-0057-5. [DOI] [PubMed] [Google Scholar]
- 13.Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356(26):2675–83. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- 14.Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684–92. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- 15.Cole JA, Ephross SA, Cosmatos IS, Walker AM. Paroxetine in the first trimester and the prevalence of congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16(10):1075–85. doi: 10.1002/pds.1463. [DOI] [PubMed] [Google Scholar]
- 16.Wogelius P, Norgaard M, Gislum M, Pedersen L, Munk E, Mortensen PB, Lipworth L, Sorensen HT. Maternal use of selective serotonin reuptake inhibitors and risk of congenital malformations. Epidemiology. 2006;17(6):701–4. doi: 10.1097/01.ede.0000239581.76793.ae. [DOI] [PubMed] [Google Scholar]
- 17.Chambers CD, Hernández-Díaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchell AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–87. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- 18.Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335(14):1010–5. doi: 10.1056/NEJM199610033351402. [DOI] [PubMed] [Google Scholar]
- 19.Davis RL, Rubanowice D, McPhillips H, Raebel MA, Andrade SE, Smith D, Yood MU, Platt R. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug Saf. 2007;16(10):1086–94. doi: 10.1002/pds.1462. [DOI] [PubMed] [Google Scholar]
- 20.Kallen B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med. 2004;158(4):312–6. doi: 10.1001/archpedi.158.4.312. [DOI] [PubMed] [Google Scholar]
- 21.Wen SW, Yang Q, Garner P, Fraser W, Olatunbosun O, Nimrod C, Walker M. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol. 2006;194(4):961–6. doi: 10.1016/j.ajog.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63(8):898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 23.Costei AM, Kozer E, Ho T, Ito S, Koren G. Perinatal outcome following third trimester exposure to paroxetine. Arch Pediatr Adolesc Med. 2002;156(11):1129–32. doi: 10.1001/archpedi.156.11.1129. [DOI] [PubMed] [Google Scholar]
- 24.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 25.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97(4):533–8. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 26.Klonoff-Cohen HS, Cross JL, Pieper CF. Job stress and preeclampsia. Epidemiology. 1996;7(3):245–9. doi: 10.1097/00001648-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95(4):487–90. doi: 10.1016/s0029-7844(99)00602-x. [DOI] [PubMed] [Google Scholar]
- 28.Qiu C, Sanchez SE, Lam N, Garcia P, Williams MA. Associations of depression and depressive symptoms with preeclampsia: Results from a Peruvian case-control study. BMC Womens Health. 2007;7(1):15. doi: 10.1186/1472-6874-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolte AC, van Geijn HP, Dekker GA. Pathophysiology of preeclampsia and the role of serotonin. Eur J Obstet Gynecol Reprod Biol. 2001;95(1):12–21. doi: 10.1016/s0301-2115(00)00367-5. [DOI] [PubMed] [Google Scholar]
- 30.Skop BP, Brown TM. Potential vascular and bleeding complications of treatment with selective serotonin reuptake inhibitors. Psychosomatics. 1996;37(1):12–6. doi: 10.1016/S0033-3182(96)71592-X. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell AA, Cottler LB, Shapiro S. Effect of questionnaire design on recall of drug exposure in pregnancy. Am J Epidemiol. 1986;123(4):670–6. doi: 10.1093/oxfordjournals.aje.a114286. [DOI] [PubMed] [Google Scholar]
- 32.Walker JJ. Pre-eclampsia. Lancet. 2000;356(9237):1260–5. doi: 10.1016/S0140-6736(00)02800-2. [DOI] [PubMed] [Google Scholar]
- 33.Myatt L, Miodovnik M. Prediction of preeclampsia. Semin Perinatol. 1999;23(1):45–57. doi: 10.1016/s0146-0005(99)80059-7. [DOI] [PubMed] [Google Scholar]
- 34.Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Risk factors and clinical manifestations of pre-eclampsia. BJOG. 2000;107(11):1410–6. doi: 10.1111/j.1471-0528.2000.tb11657.x. [DOI] [PubMed] [Google Scholar]
- 35.Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175(5):1365–70. doi: 10.1016/s0002-9378(96)70056-x. [DOI] [PubMed] [Google Scholar]
- 36.Mills JL, DerSimonian R, Raymond E, Morrow JD, Roberts LJ, 2nd, Clemens JD, Hauth JC, Catalano P, Sibai B, Curet LB, Levine RJ. Prostacyclin and thromboxane changes predating clinical onset of preeclampsia: a multicenter prospective study. JAMA. 1999;282(4):356–62. doi: 10.1001/jama.282.4.356. [DOI] [PubMed] [Google Scholar]
- 37.CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. Lancet. 1994;343(8898):619–29. [PubMed] [Google Scholar]
- 38.Sibai BM, Mercer B, Sarinoglu C. Severe preeclampsia in the second trimester: recurrence risk and long-term prognosis. Am J Obstet Gynecol. 1991;165(5 Pt 1):1408–12. doi: 10.1016/0002-9378(91)90379-6. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JR, de Swiet M. Blood-pressure measurement and classification in pregnancy. Lancet. 2001;357(9250):131–5. doi: 10.1016/S0140-6736(00)03552-2. [DOI] [PubMed] [Google Scholar]
- 40.Bjoro K, Stray-Pedersen S. In vitro perfusion studies on human umbilical arteries. I. Vasoactive effects of serotonin, PGF2 alpha and PGE2. Acta Obstet Gynecol Scand. 1986;65(4):351–5. doi: 10.3109/00016348609157359. [DOI] [PubMed] [Google Scholar]
- 41.Haugen G, Bjoro K, Stray-Pedersen S. Vasoactive effects of intra- and extravascular serotonin, PGE2 and PGF2 alpha in human umbilical arteries. Gynecol Obstet Invest. 1991;31(4):208–12. doi: 10.1159/000293160. [DOI] [PubMed] [Google Scholar]
- 42.Abman SH. New developments in the pathogenesis and treatment of neonatal pulmonary hypertension. Pediatr Pulmonol Suppl. 1999;18:201–4. [PubMed] [Google Scholar]
- 43.Yaron I, Shirazi I, Judovich R, Levartovsky D, Caspi D, Yaron M. Fluoxetine and amitriptyline inhibit nitric oxide, prostaglandin E2, and hyaluronic acid production in human synovial cells and synovial tissue cultures. Arthritis Rheum. 1999;42(12):2561–8. doi: 10.1002/1529-0131(199912)42:12<2561::AID-ANR8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 44.Finkel MS, Laghrissi-Thode F, Pollock BG, Rong J. Paroxetine is a novel nitric oxide synthase inhibitor. Psychopharmacol Bull. 1996;32(4):653–8. [PubMed] [Google Scholar]
- 45.Thase ME. Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry. 1998;59(10):502–8. doi: 10.4088/jcp.v59n1002. [DOI] [PubMed] [Google Scholar]
- 46.Hernandez-Diaz S, Werler MM, Mitchell AA. Gestational hypertension in pregnancies supported by infertility treatments: role of infertility, treatments, and multiple gestations. Fertil Steril. 2007;88(2):438–45. doi: 10.1016/j.fertnstert.2006.11.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens. 2008;21(5):521–6. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 48.Vollset SE, Refsum H, Irgens LM, Emblem BM, Tverdal A, Gjessing HK, Monsen AL, Ueland PM. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. Am J Clin Nutr. 2000;71(4):962–8. doi: 10.1093/ajcn/71.4.962. [DOI] [PubMed] [Google Scholar]
- 49.Conde-Agudelo A, Althabe F, Belizan JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol. 1999;181(4):1026–35. doi: 10.1016/s0002-9378(99)70341-8. [DOI] [PubMed] [Google Scholar]
- 50.Toh S, Mitchell AA, Werler MM, Hernandez-Diaz S. Sensitivity and specificity of computerized algorithms to classify gestational periods in the absence of information on date of conception. Am J Epidemiol. 2008;167(6):633–40. doi: 10.1093/aje/kwm367. [DOI] [PubMed] [Google Scholar]
- 51.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 52.Cohen LS, Rosenbaum JF. Psychotropic drug use during pregnancy: weighing the risks. J Clin Psychiatry. 1998;59 2:18–28. [PubMed] [Google Scholar]