Abstract
Objective
We and others showed that tyrosine kinase receptors (TKRs) such as the epidermal growth factor receptor stimulate G-protein coupled receptor (GPCR) kinase-interacting protein 1 (GIT1) phosphorylation via c-Src, which is required for phospholipase C-γ (PLCγ) activation, indicating that GIT1 participates in TKR signaling. VEGF is the most important TKR in endothelial cells (EC); essential for cell survival, migration and angiogenesis. Podosomes, actin-rich structures, were found to contribute to EC migration, tissue invasion and matrix remodeling suggesting a role for podosomes in angiogenesis. Since GIT1 is a substrate of c-Src, and podosome formation is c-Src dependent, we hypothesized that GIT1 plays an important role in VEGF-induced EC podosome formation and cell migration.
Methods and Results
Exposure of EC to VEGF for 30 minutes stimulated GIT1 co-localization with podosomes. Depletion of GIT1 by siRNA significantly decreased VEGF-induced podosome formation. A key role for PLCγ was suggested by several experiments. Double staining PLCγ and actin showed co-localization of PLCγ with podosomes. Podosome formation was dramatically reduced by PLCγ inhibitor U73122, Src inhibitor PP2, or expression of dominant negative small GTPases. Therefore, VEGF-induced EC podosome formation is dependent on Src, GIT1, PLCγ and small GTPases. In addition, matrix metalloprotease 2 (MMP2) and MT-MMP1 were detected at sites of VEGF-induced podosomes. Depletion of GIT1 by siRNA also significantly inhibited VEGF-induced MMP2 activation and extracellular matrix (ECM) degradation. Therefore, GIT1 mediates VEGF-induced MMP activation and ECM degradation by regulating podosome formation. Finally, depletion of GIT1 by siRNA significantly decreased VEGF-induced cell migration.
Conclusions
These data indicate that GIT1 is an essential mediator for VEGF-induced EC podosome formation and cell migration via PLCγ.
Keywords: GIT1, VEGF, PLCγ, podosomes, endothelial cells
Introduction
Angiogenesis, the formation of new blood vessels, is a tightly coordinated process. Extracellular matrix (ECM) degradation and migration of endothelial cells (EC) are prerequisites for angiogenesis, especially in wound healing. One of the key stimuli for angiogenesis is vascular endothelial growth factor (VEGF). VEGF binding to VEGF receptor-2 (VEGFR-2/Flk-1/KDR) stimulates several downstream signaling pathways including the tyrosine kinase c-Src.1-3 In addition, it has been shown that VEGF-induced recruitment and subsequent activation of phospholipase Cγ1 (PLCγ1) is essential for angiogenesis.4-6 Mice nullizygous for PLCγ1 experience embryonic lethality due to significantly impaired vasculogenesis and erythrogenesis.7, 8 Studies in zebrafish demonstrated that PLCγ1 is critically required for the function of VEGF and arterial development.9 These data suggest a critical physiological role for PLCγ1 in angiogenesis.
The G protein coupled receptor kinase (GRK)-2 interacting protein 1 (GIT1) was originally identified by its binding to GRK-2.10 GIT1 has 5 functional domains, including a zinc finger domain responsible for ARF-GAP activity, three ankyrin repeats, a Spa2 homology domain (SHD), a synaptic localization domain (SLD), and a conserved carboxyl-terminal region that interacts with paxillin (PBS).11 A major GIT1 function is regulation of cytoskeletal dynamics during cell spreading and migration by interacting with specific binding partners and targeting them spatially.12, 13 Our previous studies have demonstrated an important role for GIT1 in signal transduction mediated by tyrosine kinase receptors and angiotensin II (AngII), especially in activation of PLCγ, MEK1-ERK1/2 and FAK.12, 14 Specifically, we showed that GIT1 was a substrate for c-Src that undergoes tyrosine phosphorylation in response to AngII and EGF in vascular smooth muscle.12 GIT1 associates with PLCγ via the PLCγ Src homology 2 and 3 domains, and this interaction is required for PLCγ activation.14 To determine the physiological importance of GIT1 in vivo, our lab generated GIT1 traditional knockout mice. The GIT1-KO mouse phenocopies the VEGF120 mouse,15, 16 and resembles the PLCγ KO mouse which having a vascular phenotype,8 suggesting that GIT1 deficiency may abrogate VEGF-PLCγ signaling. Thus we propose that GIT1 is a novel regulator of PLCγ function that mediates PLCγ activation by c-Src in response to VEGF.
Podosomes are dynamic, actin-rich adhesion structures, shown to play a role in tissue invasion and cell migration by regulating MMP activity and ECM degradation.17-19 Podosome formation is regulated by several signaling pathways, including Rho family GTPases, actin regulatory pathways, protein tyrosine phosphorylation, and the microtubule system. Activation of c-Src is central to podosome formation.19 It has been shown that formation of podosomes increases polarization and motility of EC,18, 20, 21 suggesting a key role in angiogenesis. Because GIT1 is a c-Src substrate and plays an important role in EC adhesion and migration,12, 22 we hypothesized that GIT1 will mediate VEGF-induced EC migration by affecting EC podosome formation and ECM degradation.
Methods
Cell culture and transient transfection with siRNA
Human umbilical vein endothelial cells (HUVEC) were isolated as described previously and maintained in Medium 200 (Cascade Biologics) with low serum growth supplement. Cells were used at passages 2-4. HUVEC were seeded onto 35 mm dishes 24 hours prior to transfection, and transiently transfected with 100nM controlsiRNA or GIT1 siRNA per dish at 90% confluence with Lipofectamine 2000 reagent in OptiMEM medium. GIT1 siRNA was described previously and ordered from Ambion. Control siRNA was purchased from Qiagen. After 2 hours, 5% serum medium was added.
Cell lysate preparation
Cells were rinsed with ice-cold phosphate-buffered saline (PBS; 150mM NaCl, 20mM Na2PO4, pH 7.4) on ice and harvested in lysis buffer (150mM NaCl, 1mM EDTA, 1mM EGTA, 1% Triton X-100, 2.5mM sodium pyrophosphate, 5mM NaF, 1mM Na3VO4 plus 1:1000 protein inhibitor cocktail (PIC, Sigma) and clarified by centrifugation. The protein concentration was determined by the Bradford assay (Bio-Rad).
Western analysis
Total cell lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes, and the membranes were incubated with appropriate primary antibodies: GIT1 (Santa Cruz), Actin (Santa Cruz). After washing and incubating with secondary antibodies, immunoreactive proteins were visualized by the Odyssey infrared imaging system (LI-COR Biotechnology).
Immunofluorescence and podosome analysis
HUVEC were cultured in serum-free medium 200 for overnight and then stimulated with 50ng/ml VEGF. Cells were fixed with 4% formaldehyde for 10 min, permeabilized with 0.05% Triton for 5 min, and blocked with 10% normal goat serum for 1 h. Cells were incubated with GIT1 antibody (invitrogen) or PLCγ antibogy (BD) followed by Alexa Fluor 488 anti-rabbit IgG for green fluorescence and 2.5ug/ml TRITC-labeled phalloidin for red fluorescence. Podosomes were identified as big phalloidin positive ring. The number of cells containing podosomes were counted and analyzed by fluorescence microscope. Data were shown as percentage of podosome positive cells per 100 cells.
ECM degradation assay
FITC-gelatin was prepared as described previously.23 After transfection with control siRNA or GIT1 siRNA for 48 hours, cells were seeded on FITC-gelatin-coated coverslips. After treatment with VEGF, colocalization of dark areas and podosomes was visualised by merging FITC-gelatin (green) and F-Actin (red) images.
Matrix metalloproteinase (MMP) activity by zymography
HUVEC were plated in 60-mm-diameter culture dishes. MMP activity was detected in both supernatants and cell lysates. Gelatinolytic activity was assayed by SDS-PAGE, in 8% polyacrylamide gels containing 1 mg/ml gelatin as described.24 Supernatants were concentrated by using centrifugal filter device (Millipore). Cell lysates and concentrated supernatants were obtained by adding sample buffer (50 mM Tris-HCl, 2% SDS, 0.1% bromophenol blue, 10% glycerol, pH 6.8). After run at 25mA, gels were then incubated in 2.5% Triton X-100 for 60 minutes to remove SDS followed by overnight incubation in developing buffer (50 mM Tris-HCl, 0.2 M NaCl, 5 mM CaCl2, 0.02%Brij-35 pH 7.6). Then, gels were stained for 30 minutes in 0.5% Coomassie Blue G-250, 30% methanol, 10% glacial acetic acid, and destained for 30 minutes in 30% methanol, 10% glacial acetic acid.
Migration assay
A wound healing assay was performed.25 HUVEC were grown on 35mm dishes, the monolayer was scratched with a sterile disposable rubber policeman and the edge labeled with a traced line. After injury, the cells were gently washed with normal medium without serum. EC migration from the edge of the injured monolayer was quantified by measuring the area between the wound edges before and the recovered area after injury using light microscopy and the computer program ImageJ.
Statistical analysis
Data are shown as mean±SE. Differences between mean values were analyzed using the Student's t test. P < 0.05 was considered statistically significant.
Results
GIT1 co-localizes with VEGF-induced podosomes and is required for podosome formation
HUVEC cultured overnight in serum-free medium exhibited podosomes in 20±2% of cells. After treatment with VEGF for 30 min, there was a significant increase in podosomes to 35±1% of cells, consistent with other reports.26 To evaluate the role of GIT1 in VEGF-induced podosome formation, we used GIT1 siRNA to deplete GIT1 specifically. After transfection with GIT1 siRNA for 48 hours, GIT1 expression was reduced by 70%, whereas control siRNA had no effect (Figure 1A). Treatment with GIT1 siRNA and control siRNA did not alter cell viability and cell morphology (data not shown). After transfection with control siRNA or GIT1 siRNA, HUVEC were treated with VEGF for different times, and podosome formation was measured. Podosome formation was significantly increased by VEGF from 21±1% to 33±2% at 30 min in cells treated with control siRNA (Figure 1B). In contrast, podosome formation was dramatically reduced in GIT1 siRNA treated cells (Figure 1B, 19±1% in GIT1siRNA treated cells at 30min versus 33±2% in control siRNA treated cells). To test whether GIT1 co-localized with podosomes, we double stained HUVEC with antibodies to GIT1 and F-actin. In control conditions, there were few podosomes and minimal co-localization of GIT1 and F-actin (Figure 1C-E). In response to VEGF, podosomes rapidly appeared as assayed by F-actin, and GIT1 co-localized with F-actin in podosomes (Figure 1F-H). Higher magnification revealed that podosomes aggregated and formed large ring-like structures (Figure 1I-K). These results indicated that GIT1 co-localized with podosomes and was required for podosome formation.
Figure 1. GIT1 mediates VEGF-induced podosomes.
(A) EC were transfected with 100 nM control siRNA or GIT1 siRNA using Lipofectamine 2000 for 48 hours, and cell lysates were immunoblotted with GIT1 antibody. Actin blot shows equal loading. (B) HUVEC were transfected with 100 nM siRNA as in (A), then treated with VEGF for varying times. Podosomes were identified as large phalloidin positive rings. Percentage of podosome positive cells (%) was determined. (*, P<0.05 vs control siRNA at time 0 min; #, P<0.05 vs control siRNA at 15 and 30 min) (C-K) HUVEC were seeded in 2% gelatin coated dishes. After cells were treated with 50 ng/ml VEGF for 30 min, cells were stained for GIT1 (Invitrogen, green) and F-actin (Sigma, red). Arrow shows podosomes. Bar represents 10 um.
PLCγ colocalizes and mediates podosome formation induced by VEGF
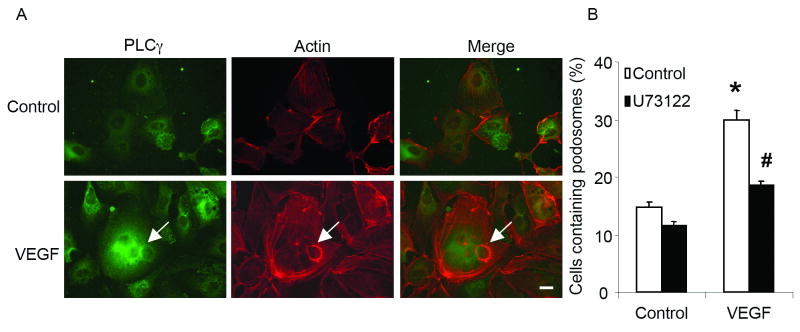
We have shown that interaction between GIT1 and PLCγ is required for EGF-induced PLCγ activation.14 Recently, a key role for PLCγ in cell spreading was reported, which required PLCγ association with a βPIX/GIT1 complex and activation of small GTPases.27 Therefore, we hypothesized that GIT1 mediated VEGF-induced podosome formation and requires PLCγ activation. As shown in figure 2A, PLCγ co-localized with F-actin in podosomes in response to VEGF (50 ng/ml for 30 min). To examine whether PLCγ plays a critical role in podosome formation, HUVEC were pre-treated with the PLCγ inhibitor U73122. As shown in figure 2B, VEGF-stimulated podosome formation was dramatically reduced by U73122 (from 30±2% to 18±1%). These data suggested that VEGF-induced podosome formation requires GIT1-PLCγ co-localization and activation.
Figure 2. PLCγ mediates VEGF-induced podosome formation.
(A) Co-localization of PLCγ with podosomes. HUVEC were treated with 50 ng/ml VEGF for 30 min. Then cells were stained with PLCγ antibody (BD, green) and F-actin (Sigma, red). Arrow shows podosomes. Bar represents 10 um. (B) VEGF-induced podosome formation is dependent on PLCγ. HUVEC were pretreated with 5 μM U73122 for 30 min, then stimulated with 50 ng/ml VEGF. Percentage of podosome positive cells (%) was determined. (*, P<0.05 vs no VEGF treatment control; #, P<0.05 vs no U73312 treatment control).
VEGF-induced podosome formation is c-Src and small GTPase dependent
We next hypothesized that VEGF-induced podosome formation is dependent on c-Src and small GTPases based on three reports: 1) TGFβ-induced podosome formation is dependent on Src and small GTPases,28 2) Src is required for N-WASP-induced podosome formation in HUVEC,26 and 3) Src and small GTPases are essential for PMA-induced podosome formation in HUVEC.29 To test our hypothesis, we pretreated HUVEC with Src inhibitor PP2 or expressed dominant negative small GTPases (DN-GTPase), and then stimulated the cells with VEGF. Both PP2 (Figure 3A) and DN-small GTPases (Figure 3B) dramatically decreased VEGF-induced podosome formation. Among the DN-GTPases, the greatest inhibition occurred with DN-Rac consistent with previous reports.26 Therefore, VEGF-induced podosome formation is also dependent on c-Src and small GTPases.
Figure 3. VEGF-induced podosomes are Src and small GTPases dependent.
(A) HUVEC were pretreated with 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo(3,4-d) pyrimidine (PP2) for 30 minutes, then stimulated with 50 ng/ml VEGF. Percentage of podosome positive cells (%) was determined. (*, P<0.05 vs no VEGF treatment control; #, P<0.05 vs no PP2 treatment control) (B) HUVEC were infected with Ad.DN small GTPases (Rho, RhoA, Cdc42) before treatment with VEGF, then cells were stained for podosomes. Percentage of podosome positive cells (%) was determined. (*, P<0.05 vs LacZ no VEGF treatment control; #, P<0.05 vs LacZ VEGF treatment control) (C) At the same time, cell lysates were used for western blotting to confirm the expression of adenovirus. Actin blots show equal loading.
Role of GIT1 in VEGF-induced MMP2 activation
A major function of podosomes is to regulate MMP activity and ECM degradation. By dual immunofluorescence, we found that MMP2 co-localized with F-actin in VEGF-induced podosomes (Figure 4D-F). Because MT1-MMP, which can cleave pro-MMP2 into active MMP2, has been shown to co-localize with podosomes in EC, we also analyzed the presence of MT-MMP1 in VEGF-induced podosomes, and observed significant co-localization (Figure 4J-L).
Figure 4. Co-localization of MMP2 and MT1-MMP with podosomes.
After treatment with 50 ng/ml VEGF for 30 minutes, cells were fixed and stained with MMP-2(A-F) or MT1-MMP (G-L) antibody (green), and TRITC-phalloidin (red). Arrows show podosomes. Bar represents 10 μm.
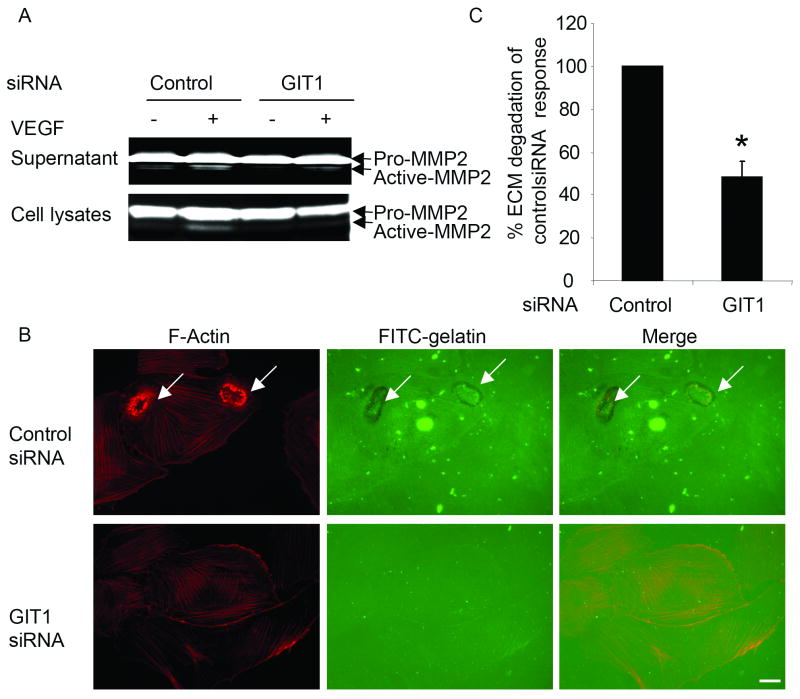
To explore the role of GIT1 in MMP2 activation, we analyzed the effect of GIT1 depletion on VEGF-induced gelatinase acitivity. Both culture supernatants and cell lysates were run on gelatin-containing polyacrylamide gels. After treatment with VEGF, active MMP2 was detected in both supernatants and cell lysates (Figure 5A). GIT1 siRNA significantly decreased MMP2 activation induced by VEGF (Figure 5A). Because GIT1 siRNA strongly inhibited VEGF-induced podosome formation (Figure 1B), we propose that GIT1 mediates MMP activation by regulating podosome formation.
Figure 5. Role of GIT1 in MMP activity and ECM degradation.
(A) HUVEC were transfected with 100 nM control siRNA or GIT1 siRNA, followed by treatment with 50ng/ml VEGF. Both concentrated supernatants and cell lysates were were analysed by 1% gelatin zymography. The active form of MMP-2 (lower molecular weight) indicates cleavage of pro-MMP2. (B) HUVEC were transfected with 100 nM control siRNA or GIT1 siRNA for 48 hours. Then, cells were seeded on FITC-gelatin-coated coverslips followed by treatment with 50 ng/ml VEGF. Cells were fixed and stained for F-Actin. Arrows show areas of degraded gelatin that overlap with podosomes. (C) Quantitative data were presented as percentages of control siRNA response obtained in the presence of VEGF. (*, P<0.05 vs control siRNA response)
Role of GIT1 in VEGF-induced ECM degradation
Proteolytic activity is associated with podosomes in v-Src-transformed fibroblasts, tumor cells, osteoclasts and EC.30, 31 To determine whether VEGF-induced podosomes in EC are able to locally degrade ECM, we performed an in vitro ECM degradation assay as described previously.24 As shown in figure 5B, after treatment with VEGF, cells displayed podosomes assayed by F-actin. ECM degradation, visualized as dark areas in the fluorescent ECM (FITC-gelatin), co-localized with podosomes (red fluorescence). To explore the role of GIT1 in ECM degradation, we performed the same experiment after cells were treated with GIT1 siRNA. As shown in figure 5C, GIT1 siRNA dramatically decreased ECM degradation by 48±6%. These results suggest GIT1 is required for VEGF-induced ECM degradation by regulating podosome formation.
GIT1 mediates VEGF-induced EC migration by affecting podosome formation
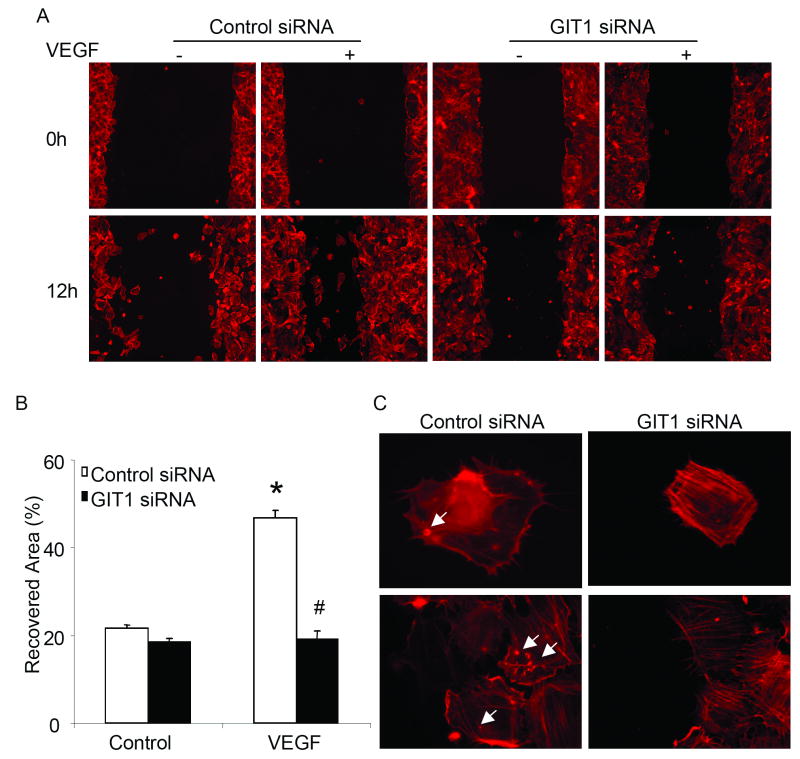
We previously showed that GIT1 was required for cell migration of VSMC, HEK293 cells and HeLa cells.12 Recent studies of podosomes in EC suggested a key role for podosomes in migration, tissue invasion, and ECMdegradation.26, 28 To study the role of GIT1 in EC migration, we used a scratch would healing assay and depleted GIT1 with siRNA. We found that VEGF induced a significant increase in the recovered area from 22±1% to 47±2% in control siRNA transfected HUVEC (Figure 6B). Transfection with GIT1 siRNA completely inhibited cell migration with a recovered area of only 20±3% (Figure 6B). Immunostaining for F-actin showed that the migrating cells in the control siRNA group were podosome positive. In contrast, the cells in the GIT1 siRNA group were podosome negative (Figure 6C). These results suggest that GIT1 mediates EC migration in response to VEGF by enhancing podosome formation.
Figure 6. GIT1 mediates VEGF-induced endothelial cell migration by affecting podosome formation.
(A) After HUVEC were transfected with 100 nM control siRNA or GIT1 siRNA, scratch wound injury was performed with or without VEGF. (B) Migration rate was analyzed with NIH-Image software (*, p < 0.05 versus control siRNA at time 0hr, #, p< 0.05 versus control siRNA at time 12 hour). (C) Cells were fixed and stained with TRITC-phalloidin. Arrows show podosomes. Bar represents 10 μm.
Discussion
The major finding of this study is that GIT1 is a key mediator of VEGF stimulated podosome formation in endothelial cells. Specifically, we define a novel role for GIT1 to mediate EC migration and ECM degradation by activating PLCγ, and regulating podosome formation. GIT1 has been previously shown to regulate migration of SMC and HEK293 cells.32-34 We propose that VEGF stimulates GIT1-PLCγ translocation to the site where podosomes will form, and then recruits Src, which phosphorylates and activates PLCγ. Functionally, GIT1 mediates cell invasion and migration by increasing podosome formation and ECM degradation. Evidence to support this concept includes: 1) GIT1 knockdown inhibits EC podosome formation induced by VEGF (Figure 1); 2) GIT1 colocalizes with podosomes induced by VEGF (Figure 1); 3) PLCγ colocalizes with podosomes induced by VEGF (Figure 2); 4) Inhibiting PLCγ with U73122 blocks podosome formation induced by VEGF (Figure 2); 5) VEGF-induced podosome formation is also dependent on Src and small GTPase (Figure 3); 6) Downregulation of GIT1 by siRNA significantly inhibited VEGF-induced MMP activation and ECM degradation (Figures 4 and 5); and 7) GIT1 mediated VEGF-induced cell migration correlated with podosome formation (Figure 6).
PLCγ has been reported to associate with GIT1-βPIX complex (p21-activated kinase interacting exchange factor), which requires both GIT1 and β-PIX.35 Tyrosine phosphorylation of the βPIX/GIT1 complex is essential for the interaction with PLCγ, the subsequent activation of PLCγ, and the progression to an elongated cell morphology.35 Depletion of βPIX, GIT1 or PLCγ shows that all three components of the complex are necessary to promote cell spreading, and overexpression of individual components is not sufficient to replace deficiencies in the other components of the PLCγ/GIT1/βPIX complex.35 However, constitutively active forms of Cdc42 or Rac1 were able to rescue the elongation of these cells, suggesting that PLCγ, with complexes containing GIT1 and βPIX, is essential for cell spreading and motility by activating Cdc42 and Rac1.35 Data from several labs, including ours, demonstrate important roles for at least three small GTPases in podosome formation (Figure 3). The apparent redundancy in effects of inhibiting a single GTPase (Figure 3) limits our ability to comment on the specific and hierarchical nature of their roles in podosome formation. We can only suggest that there are likely interdependent pathways for the small GTPases. In contrast, we show that both GIT1 and PLCγ localize in podosomes (Figure 1 and 2) and are required for VEGF-induced podosome formation (Figure 1 and 2). Therefore we propose that PLCγ is a critical component of a PLCγ-GIT1-PIX complex that mediates activation of small GTPases and is required for podosome formation.35 The present study demonstrates that VEGF increases PLCγ activation, which is GIT1 dependent. We previously showed that GIT1 mediated activation of PLCγ by angiotensin II and epidermal growth factor in SMC, which is dependent on GIT1 tyrosine phosphorylation via c-Src.14 Therefore, we propose that VEGF stimulated, Src-dependent, tyrosine phosphorylation of GIT1 creates a scaffold to mediate localization and activation of PLCγ, regulation of podosome formation, and cell migration by stimulating small GTPases.
Several small GTPases have been reported to mediate podosome formation in different cell types.19, 28, 36, 37 Our data show that Rac1, RhoA and CDC42 are involved in EC podosome formation in response to VEGF (Figure 3), but Rac1 had the greatest effect (Figure 3). It is possible that the prominent role for Rac1 depends on PLCγ-mediated calcium dependent events, because PLCγ has been reported to regulate cell spreading by increasing intracellular calcium.38 Specifically, calcium-activated proteases, calpains, associate with βPIX, and cleavage of βPIX by calpains is an early event required for Rac activation in some cellular systems.39 Therefore, GIT1 may act as a scaffold to link PLCγ with βPIX, which is small GTPase GEF. The role of PLCγ in the complex could be to increase local calcium concentration which causes calpain activation and subsequent Rac1 stimulation by the GEF function of βPIX.40
PIX has been shown to play a central role in podosome formation, dependent in part, on its GEF activity.41 A PIX-PAK complex was shown to function both upstream and downstream of small GTPases and generate localized feedback loops that could regulate podosome formation.41-43 Our lab previously showed that GIT1 interacted with MEK1 directly, and acted as a scaffold to mediate ERK1/2 activation in focal adhesions.13 Recently, a MEK1 - ERK1/2 - caldesmon signaling cascade was shown to regulate PKC-mediated podosome dynamics in A7r5 cells.44 Based on these data we suggest that GIT1 serves as a scaffold to facilitate the localization and activation of PLCγ, small GTPases, PIX and ERK1/2, thereby promoting podosome formation.
Besides podosomes, filopodia and lamellipodia are also important structures for endothelial cell migration and angiogenesis.45 Recently, bone morphogenetic protein-6 was shown to be a potent stimulator of angiogenesis by regulating filopodial assembly in endothelial tip cells.46 Specifically these authors found that myosin-X translocated into filopodia, stimulated filopodial motility, and increased EC migration and angiogenesis.46 GIT1 may also regulate lamellipodia and filopodia formation by mediating Rac activation due to the Arf-GAP activity of GIT1.47 Our data demonstrate that GIT1 is essential for podosome formation by mediating PLCγ activation. These data suggest that GIT1 may be involved in functions of several macromolecular structures that regulate EC migration and angiogenesis.
In summary, we showed that a c-Src - GIT1 - PLCγ signaling pathway is required for VEGF-mediated podosome formation and cell migration. Because podosomes play an important role in cell invasion and migration, these findings suggest a novel function for GIT1 in EC as a mediator of angiogenesis and tissue remodeling.
Acknowledgments
The authors thank Dr. Kegi Fujiwara, Dr. Joseph Miano and Dr. Michael Zuscik for helpful suggestions and thoughtful discussion of data.
Sources of Funding: This work was supported by National Institutes of Health grant HL 63462 to Dr. Bradford C. Berk and HL 77789 to Dr. Chen Yan and American Heart Association Scientist Development Grant 740021N to Dr. Chen Yan.
Footnotes
Disclosures: None.
References
- 1.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. Faseb J. 1999;13:9–22. [PubMed] [Google Scholar]
- 2.Werdich XQ, Penn JS. Src, Fyn and Yes play differential roles in VEGF-mediated endothelial cell events. Angiogenesis. 2005;8:315–326. doi: 10.1007/s10456-005-9021-x. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Ghazaleh R, Kabir J, Jia H, Lobo M, Zachary I. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem J. 2001;360:255–264. doi: 10.1042/0264-6021:3600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A- dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. Embo J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer RD, Latz C, Rahimi N. Recruitment and activation of phospholipase Cgamma1 by vascular endothelial growth factor receptor-2 are required for tubulogenesis and differentiation of endothelial cells. J Biol Chem. 2003;278:16347–16355. doi: 10.1074/jbc.M300259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc Natl Acad Sci U S A. 2005;102:1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji QS, Winnier GE, Niswender KD, Horstman D, Wisdom R, Magnuson MA, Carpenter G. Essential role of the tyrosine kinase substrate phospholipase C-gamma1 in mammalian growth and development. Proc Natl Acad Sci U S A. 1997;94:2999–3003. doi: 10.1073/pnas.94.7.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao HJ, Kume T, McKay C, Xu MJ, Ihle JN, Carpenter G. Absence of erythrogenesis and vasculogenesis in Plcg1-deficient mice. J Biol Chem. 2002;277:9335–9341. doi: 10.1074/jbc.M109955200. [DOI] [PubMed] [Google Scholar]
- 9.Lawson ND, Mugford JW, Diamond BA, Weinstein BM. phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 2003;17:1346–1351. doi: 10.1101/gad.1072203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Premont RT, Claing A, Vitale N, Freeman JL, Pitcher JA, Patton WA, Moss J, Vaughan M, Lefkowitz RJ. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc Natl Acad Sci U S A. 1998;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natarajan K, Yin G, Berk BC. Scaffolds direct Src-specific signaling in response to angiotensin II: new roles for Cas and GIT1. Mol Pharmacol. 2004;65:822–825. doi: 10.1124/mol.65.4.822. [DOI] [PubMed] [Google Scholar]
- 12.Yin G, Haendeler J, Yan C, Berk BC. GIT1 functions as a scaffold for MEK1-extracellular signal-regulated kinase 1 and 2 activation by angiotensin II and epidermal growth factor. Mol Cell Biol. 2004;24:875–885. doi: 10.1128/MCB.24.2.875-885.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin G, Zheng Q, Yan C, Berk BC. GIT1 Is a Scaffold for ERK1/2 Activation in Focal Adhesions. J Biol Chem. 2005;280:27705–27712. doi: 10.1074/jbc.M502271200. [DOI] [PubMed] [Google Scholar]
- 14.Haendeler J, Yin G, Hojo Y, Saito Y, Melaragno M, Yan C, Sharma VK, Heller M, Aebersold R, Berk BC. GIT1 mediates Src-dependent activation of phospholipase Cgamma by angiotensin II and epidermal growth factor. J Biol Chem. 2003;278:49936–49944. doi: 10.1074/jbc.M307317200. [DOI] [PubMed] [Google Scholar]
- 15.Galambos C, deMello DE. Molecular mechanisms of pulmonary vascular development. Pediatr Dev Pathol. 2007;10:1–17. doi: 10.2350/06-06-0122.1. [DOI] [PubMed] [Google Scholar]
- 16.Galambos C, Ng YS, Ali A, Noguchi A, Lovejoy S, D'Amore PA, DeMello DE. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol. 2002;27:194–203. doi: 10.1165/ajrcmb.27.2.4703. [DOI] [PubMed] [Google Scholar]
- 17.McNiven MA, Baldassarre M, Buccione R. The role of dynamin in the assembly and function of podosomes and invadopodia. Front Biosci. 2004;9:1944–1953. doi: 10.2741/1348. [DOI] [PubMed] [Google Scholar]
- 18.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 20.Moreau V, Tatin F, Varon C, Genot E. Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Mol Cell Biol. 2003;23:6809–6822. doi: 10.1128/MCB.23.19.6809-6822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreau V, Tatin F, Varon C, Anies G, Savona-Baron C, Genot E. Cdc42-driven podosome formation in endothelial cells. Eur J Cell Biol. 2006;85:319–325. doi: 10.1016/j.ejcb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Yin G, Yan C, Berk BC. Angiotensin II signaling pathways mediated by tyrosine kinases. Int J Biochem Cell Biol. 2003;35:780–783. doi: 10.1016/s1357-2725(02)00300-x. [DOI] [PubMed] [Google Scholar]
- 23.Bowden ET, Coopman PJ, Mueller SC. Invadopodia: unique methods for measurement of extracellular matrix degradation in vitro. Methods Cell Biol. 2001;63:613–627. doi: 10.1016/s0091-679x(01)63033-4. [DOI] [PubMed] [Google Scholar]
- 24.Stetler-Stevenson M, Mansoor A, Lim M, Fukushima P, Kehrl J, Marti G, Ptaszynski K, Wang J, Stetler-Stevenson WG. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in reactive and neoplastic lymphoid cells. Blood. 1997;89:1708–1715. [PubMed] [Google Scholar]
- 25.Pi X, Garin G, Xie L, Zheng Q, Wei H, Abe J, Yan C, Berk BC. BMK1/ERK5 is a novel regulator of angiogenesis by destabilizing hypoxia inducible factor 1alpha. Circ Res. 2005;96:1145–1151. doi: 10.1161/01.RES.0000168802.43528.e1. [DOI] [PubMed] [Google Scholar]
- 26.Osiak AE, Zenner G, Linder S. Subconfluent endothelial cells form podosomes downstream of cytokine and RhoGTPase signaling. Exp Cell Res. 2005;307:342–353. doi: 10.1016/j.yexcr.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 27.Choi JH, Yang YR, Lee SK, Kim IS, Ha SH, Kim EK, Bae YS, Ryu SH, Suh PG. Phospholipase C-gamma1 potentiates integrin-dependent cell spreading and migration through Pyk2/paxillin activation. Cell Signal. 2007;19:1784–1796. doi: 10.1016/j.cellsig.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Varon C, Tatin F, Moreau V, Van Obberghen-Schilling E, Fernandez-Sauze S, Reuzeau E, Kramer I, Genot E. Transforming growth factor beta induces rosettes of podosomes in primary aortic endothelial cells. Mol Cell Biol. 2006;26:3582–3594. doi: 10.1128/MCB.26.9.3582-3594.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatin F, Varon C, Genot E, Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci. 2006;119:769–781. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- 30.Mizutani K, Miki H, He H, Maruta H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002;62:669–674. [PubMed] [Google Scholar]
- 31.Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–185. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- 32.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 33.Webb DJ, Kovalenko M, Whitmore L, Horwitz AF. Phosphorylation of serine 709 in GIT1 regulates protrusive activity in cells. Biochem Biophys Res Commun. 2006;346:1284–1288. doi: 10.1016/j.bbrc.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 34.Manabe R, Kovalenko M, Webb DJ, Horwitz AR. GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J Cell Sci. 2002;115:1497–1510. doi: 10.1242/jcs.115.7.1497. [DOI] [PubMed] [Google Scholar]
- 35.Jones NP, Katan M. Role of phospholipase Cgamma1 in cell spreading requires association with a beta-Pix/GIT1-containing complex, leading to activation of Cdc42 and Rac1. Mol Cell Biol. 2007;27:5790–5805. doi: 10.1128/MCB.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hai CM, Hahne P, Harrington EO, Gimona M. Conventional protein kinase C mediates phorbol-dibutyrate-induced cytoskeletal remodeling in a7r5 smooth muscle cells. Exp Cell Res. 2002;280:64–74. doi: 10.1006/excr.2002.5592. [DOI] [PubMed] [Google Scholar]
- 37.Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, Schlessinger J. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2/mice. J Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satish L, Blair HC, Glading A, Wells A. Interferon-inducible protein 9 (CXCL11)-induced cell motility in keratinocytes requires calcium flux-dependent activation of mu-calpain. Mol Cell Biol. 2005;25:1922–1941. doi: 10.1128/MCB.25.5.1922-1941.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulkarni S, Saido TC, Suzuki K, Fox JE. Calpain mediates integrin-induced signaling at a point upstream of Rho family members. J Biol Chem. 1999;274:21265–21275. doi: 10.1074/jbc.274.30.21265. [DOI] [PubMed] [Google Scholar]
- 40.Jones NP, Peak J, Brader S, Eccles SA, Katan M. PLCgamma1 is essential for early events in integrin signalling required for cell motility. J Cell Sci. 2005;118:2695–2706. doi: 10.1242/jcs.02374. [DOI] [PubMed] [Google Scholar]
- 41.Gringel A, Walz D, Rosenberger G, Minden A, Kutsche K, Kopp P, Linder S. PAK4 and alphaPIX determine podosome size and number in macrophages through localized actin regulation. J Cell Physiol. 2006;209:568–579. doi: 10.1002/jcp.20777. [DOI] [PubMed] [Google Scholar]
- 42.Obermeier A, Ahmed S, Manser E, Yen SC, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. Embo J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baird D, Feng Q, Cerione RA. The Cool-2/alpha-Pix protein mediates a Cdc42-Rac signaling cascade. Curr Biol. 2005;15:1–10. doi: 10.1016/j.cub.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 44.Gu Z, Kordowska J, Williams GL, Wang CL, Hai CM. Erk1/2 MAPK and caldesmon differentially regulate podosome dynamics in A7r5 vascular smooth muscle cells. Exp Cell Res. 2007;313:849–866. doi: 10.1016/j.yexcr.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pi X, Ren R, Kelley R, Zhang C, Moser M, Bohil AB, Divito M, Cheney RE, Patterson C. Sequential roles for myosin-X in BMP6-dependent filopodial extension, migration, and activation of BMP receptors. J Cell Biol. 2007;179:1569–1582. doi: 10.1083/jcb.200704010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishiya N, Kiosses WB, Han J, Ginsberg MH. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat Cell Biol. 2005;7:343–352. doi: 10.1038/ncb1234. [DOI] [PubMed] [Google Scholar]