Abstract
SGK3, which previously has been shown to play a key role in hair follicle development in mice, is a member of the AGC family of serine-threonine kinases. Mice lacking SGK3 have abnormal follicle cycling, which begins shortly after birth and ameliorates substantially with age. However, this developmental abnormality is not recapitulated in mice lacking closely related kinases Akt1, Akt2, or Akt3. To examine whether Akt2 interacts with SGK3 in postnatal hair development, we have generated and characterized Akt2/SGK3 double knockouts (DKOs). We find that the DKO mice have a defect in hair growth that is markedly worse than that of SGK3−/− mice and does not ameliorate with age. Morphologically, this defect is characterized by accelerated entry into catagen and through anagen, irregular hair follicle orientation, and increased expression of sebaceous glands. The defect is preceded by a profound failure to increase follicle matrix cell nuclear β-catenin accumulation and proliferation at the onset of morphogenesis. Furthermore, in cultured keratinocytes, transfected Akt2 and SGK3 both stimulate transcription of a β-catenin-LEF1-dependent reporter gene. Thus, SGK3 and Akt2 both appear to play important roles in postnatal hair follicle morphogenesis, likely because of their redundant regulation of β-catenin-dependent transcriptional processes, which control hair follicle cell proliferation.—Mauro, T. M., McCormick, J. A., Wang, J., Boini, K. M., Ray, L., Monks, B., Birnbaum, M. J., Lang, F., and Pearce, D. Akt2 and SGK3 are both determinants of postnatal hair follicle development.
Keywords: phosphatidylinositol-3-kinase, β-catenin, Lef1, proliferation, baldness
Signaling by growth factors (e.g., IGF-I, IL-3, and EGF) and insulin through the phosphatidylinositol-3-kinase (PI3K) pathway is mediated in substantial measure by two families of serine-threonine kinases, the Akts and SGKs (1). Each of these families comprises 3 isoforms, which share a high degree of sequence homology, phosphorylate common substrates, and are regulated by common upstream regulatory signaling pathways (2,3,4,5,6). There are notable differences between the Akts and SGKs, particularly within the amino-terminal regions, where the Akts bear a pleckstrin homology (PH) domain, which mediates interaction with phosphoinositides, primarily PtdIns(3,4,5)P3 (7); SGK3 has a phox homology (PX) domain, which mediates interaction with PtdIns(3)P (8, 9); SGK1 has a novel phosphoinositide interaction domain, with some features resembling that of the PX domain (10); and SGK2 has a short N terminus with no identifiable phosphoinositide interaction domain. Of the 6 Akt/SGK family members, all but SGK2 have been knocked out in mice (11,12,13,14,15,16,17). All 5 null strains have distinct, relatively mild phenotypes, indicating diverse physiological roles, including whole-body growth (Akt1) (11, 13), glucose and lipid homeostasis (Akt2 and SGK1) (12, 18,19,20), brain growth (Akt3) (15, 16), renal sodium reabsorption (SGK1) (14), intestinal transport (SGK1 and SGK3) (21, 22), potassium excretion (SGK1) (23), and hair growth (SGK3) (15). Notably, the phenotypes of the null mice were either only partially predicted or were even counter to predictions based on in vitro studies. Moreover, the mildness of the single-knockout (KO) phenotypes suggested that other factors, possibly other family members, might compensate. To date, 4 within-family compound KOs have been characterized. An Akt1/Akt2 double KO (DKO) displays a severe neonatal lethal phenotype, possibly due to respiratory failure (24); Akt1/Akt3-DKO mice die around embryonic day 12 (E12) with severe impairments in growth, cardiovascular development, and organization of the nervous system (25). Akt2/Akt3-DKO mice display a general growth defect in addition to reduced brain size, but glucose handling is no worse than that of Akt2 single-KO mice (26). Finally, SGK1/SGK3-DKO mice have a superposition of the two single null phenotypes (27), suggesting their functions are nonoverlapping. To date, no cross-family compound nulls have been characterized. Akt1 (26) as well as SGKs 1 and 3 (5, 28) are expressed in all tissues examined so far, whereas Akts 2 and 3 (12, 13, 29, 30) and SGK2 (5) show a more restricted tissue distribution. Given their partially overlapping expression patterns, it seemed possible that actions attributed to the Akts might be mediated by the SGKs or that they might have overlapping or redundant activities, in vivo. To begin to examine the possibility of functional overlap between these two kinase families in vivo, we have generated and characterized mice lacking both Akt2 and SGK3. These mice display a severe, unremitting alopecia, which begins at the onset of morphogenesis, and which is unusual in that no inflammation, scarring or cyst formation is seen. Instead, hair cycling is disturbed, associated with impaired β-catenin signaling. These findings suggest that specific Akt and SGK kinases control hair follicle cycling and growth.
MATERIALS AND METHODS
Generation of Akt2/Sgk3-DKO mice
All animal experiments were conducted following institutional Animal Research Committee approval. Targeted disruption of the Akt2 and SGK3 alleles has been described previously (12, 15). Akt2-KO mice were bred with SGK3-KO mice to generate compound heterozygote (Akt2+/−/Sgk3+/−) mice, which were then interbred to generate double-homozygous mice with a mixed B6;129 background.
PCR analysis of genotype
Genomic DNA was prepared from tail biopsies using the GenElute Mammalian Genomic DNA Minprep Kit (Sigma-Aldrich, St. Louis, MO, USA), and 250 ng DNA was used for genotyping. PCR analysis of the Akt2 allele has been described previously (12). For analysis of the SGK3 allele, the forward primer 5′GATTGCGAACTCTTCACTCATTTGC3′ and the reverse primer 5′GAACACAGCTGCTCATACAGACACAGG 3′ were used. Reactions were performed using LA Taq (TaKaRa; Otsu, Shiga, Japan) with the conditions 94°C for 1 min; 94°C for 30 s, 62°C for 45 s, 68°C for 7 min for 14 cycles, then increased extension by 15 s/cycle; final extension of 72°C for 15 min. PCR products were resolved on 2% agarose gels.
Histological studies
To analyze skin morphology, dorsal skin was biopsied and fixed overnight in 10% neutral buffered formalin (Fisher Scientific, Waltham, MA, USA). Samples were dehydrated, paraffin-embedded, and sectioned (6 μm). For basic morphology, sections were deparaffinized and stained with hematoxylin and eosin; samples were taken from Akt2−/−/ Sgk3+/− and Akt2−/−/Sgk3−/− littermates to obtain sufficient numbers.
Immunohistochemistry and immunofluorescence
Skin immunohistochemistry was performed on 5- or 6-μm longitudinal sections (paraffin-embedded) from Akt2+/+/Sgk3+/+, Akt2+/−/Sgk3+/+, Akt2−/−/Sgk3+/−, and Akt2−/−/Sgk3−/− mice. For Akt stains, wild-type mice were used at postnatal day 1 (P1) and P3, and Akt2 heterozygous (SGK3 wild type) mice were used at P5. Wild-type and Akt2-heterozygous animals were phenotypically indistinguishable at all ages examined and were labeled as normal. For Akt2 immunofluorescence, sections underwent deparaffinization, rehydration, and antigen-unmasking (Vector Antigen Unmasking Solution, Vector Laboratories, Burlingame, CA, USA). The sections then were incubated with 1× TBS/0.1% Tween-20 and washed with PBS, and endogenous peroxidase activity was blocked using 3% H2O2. Unspecific binding sites were then blocked using 1.5% goat serum in PBS, and sections were incubated with primary antibody (Cell Signaling, Danvers, MA, USA) at a dilution of 1:150 for 30 min. After several washes with PBS, samples were incubated with biotinylated secondary antibody (Elite Rabbit IgG Kit, Vector Laboratories) for 30 min, washed again with PBS, and incubated with ABC reagent (Elite Rabbit IgG Kit, Vector Laboratories) for 30 min. ABC reagent was removed, sections were washed in PBS, and DAB substrate (DAB peroxidase substrate kit, Vector Laboratories) was added to each section. Stain development was stopped by immersing slides in ddH2O. Sections were then dehydrated in a series of ethanol and xylene, and coverslips were mounted. Sections were counterstained with propidium iodide.
For PCNA and β-catenin, the M.O.M IHC kit and alkaline phosphatase detection system in conjunction with BCIP/NBT (Vector Laboratories) were used according to the manufacturer’s protocol, except with incubation times of 60 min for primary antibody, 20 min for secondary antibody, and 20 min for ABC. Dilutions of 1:100 of anti-mouse PCNA antibody (Novocastra Laboratories, Newcastle-On-Tyne, UK) and 1:50 of anti-mouse β-catenin antibody (BD Biosciences Transduction Laboratories, San Jose, CA, USA) were used. BCIP/NBT detection substrate was incubated for 20 to 80 min, to achieve optimal staining. For PCNA and β-catenin, sections were counterstained with Nuclear Fast Red, dehydrated, and permanently mounted. Staining of the differentiation markers cytokeratins 6, 10, and 14 was performed as a service by Dr. D. F. Kusewitt at Ohio State University (Columbus, OH, USA), using standard protocols.
Transient transfection assays
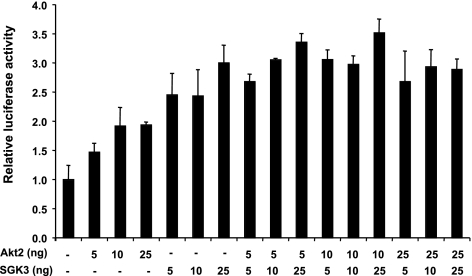
HaCaT cells (provided by Dr. Norbert E. Fusenig, German Cancer Research Center, Heidelberg, Germany) were maintained in DMEM supplemented with 10% FBS. The mouse Akt2 open reading frame was cloned from mouse kidney and subcloned into pCDNA3 and expression vector for constitutively active (Akt2-S474D/T309E, which substitutes acidic residues that mimic phosphorylation by PDK1; ref. 31) forms of Akt2 were generated by PCR-based mutagenesis. The mouse expression vector for constitutively active SGK3 (CA-SGK3) has been described previously (15). The Lef1-β-catenin reporter, consisting of 7 Lef1-response elements driving luciferase (p7 Lef-fos Luc), as well as Lef1 expression vector were provided by Dr. Rudolf Grosschedl (Max Planck Institute of Immunobiology, Freiburg, Germany). Transient transfections to assess the effects of Akt2 on Lef1-mediated gene transcription in HaCaT cells were performed, as described previously (15), using 100 ng of reporter, 100 ng of Lef1, and 5, 10, or 25 ng of CA-Akt2 and/or CA-SGK3. Twenty hours after transfection, complete growth medium was replaced for an additional 24 h, and then cells were harvested in 100 μl lysis buffer (50 mM Tris/Cl, pH 7.4; 150 mM NaCl; 1 mM EDTA; and 1% Triton X-100) at 4°C for 30 min, scraping and transferring the lysate to Eppendorf tubes, followed by 20 min centrifugation at 4°C. Luciferase activity of 5 μl of lysate was assayed using Promega luciferin reagent (Promega, Madison, WI, USA) and normalized to β-galactosidase activity, as described previously (32). Transfections were performed at least 3 times.
Image manipulation
The images in Figs. 4 and 6 were adjusted for brightness, contrast, and color balance when necessary to improve image quality. Images from both controls and experimental samples were subjected to identical manipulations to prevent generation of false differences.
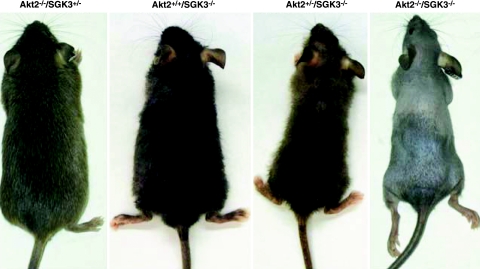
Figure 1.
Akt2/SGK3-DKO mice have a severe defect in hair growth. Photographs show typical appearance of the indicated genotypes at 12 wk of age for both sexes. Mice with disruption of both Akt2 and SGK3 (Akt2−/−/SGK3−/−; DKO) have a greatly exacerbated hair-growth defect compared with those lacking only SGK3 (Akt2+/+/SGK3−/−) or Akt2 (Akt2−/−/SGK3+/−). The latter is indistinguishable from Akt2−/−/SGK3+/+ or wild type (not shown). Akt2+/−/SGK3−/− mice display an intermediate phenotype.
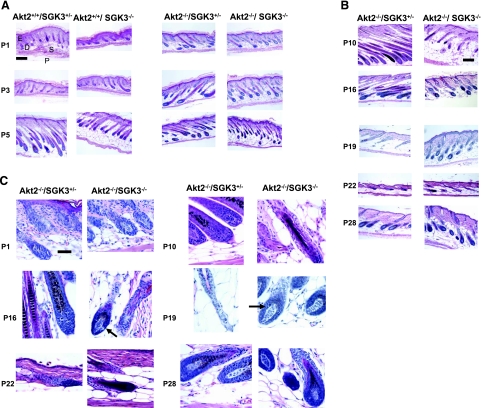
Figure 2.
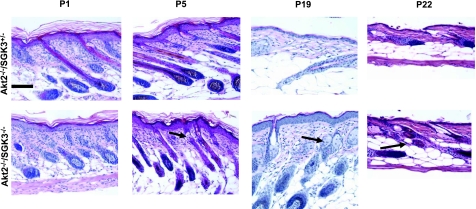
Hair morphology in Akt2/SGK3 mutant mice. Dorsal skin was harvested from mice of specified genotypes at postnatal days shown. Paraffin-embedded skin sections (6 μm) were prepared and stained with hematoxylin and eosin, and examined by light microscopy. A) Akt2+/+/SGK3−/+, Akt+/+/SGK3−/−, Akt−/−/SGK3−/+, and Akt2−/−/SGK3−/− (DKO) mice at P1, P3, and P5. D, dermis; E, epidermis; S, subcutis; P, panniculus carnosus. B) Akt−/−/SGK3−/+ and DKO mice at P10, P16, P19, P22, and P28. C) High-power views of Akt−/−/SGK3−/+ and DKO mice at P1, P10, P16, P19, P22, and P28. DKO follicles enter anagen (arrows) by P16, continue in anagen at P19, are in catagen at P22, and begin a second anagen at P28. This is in contrast to Akt2−/−/SGK3+/−mice, which are indistinguishable from wild type (not shown) from P10 on. Scale bars = 200 μm (A, B); 50 μm (C).
Figure 3.
Hair follicle morphology in DKO mice. Although hair follicle morphology is normal at P1, it becomes distorted and irregular by P10 (arrows). These abnormalities do not ameliorate with age. Samples were prepared as in Fig. 2. Scale bar = 50 μm.
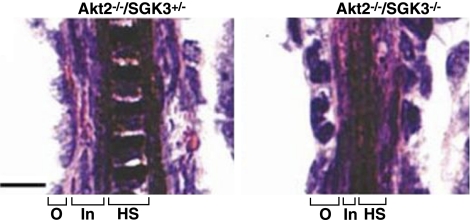
Figure 4.
Differentiation defects in DKO hair follicles. P5 Akt2−/−/SGK3−/− hair follicles show differentiation defects as reflected by an expanded outer root sheath (O) and reduced inner root sheath (In) and hair shaft (HS). Samples were prepared as in Fig. 2. Scale bar = 10 μm.
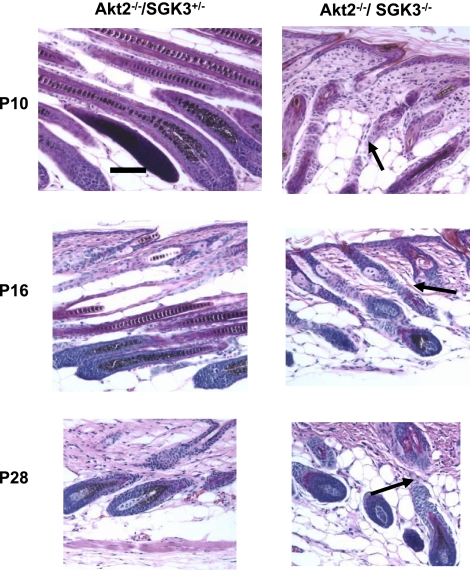
Figure 5.
Increased sebaceous gland number and size in DKO mice. Few sebaceous glands are noted at P1 in either Akt−/−/SGK3−/+ or Akt−/−/SGK3−/− mice. By P5, sebaceous glands are seen in both genotypes; however, those of DKO are larger and more abundant (arrow). This difference is particularly evident at P19 and P22. Scale bar = 100 μm.
Figure 6.
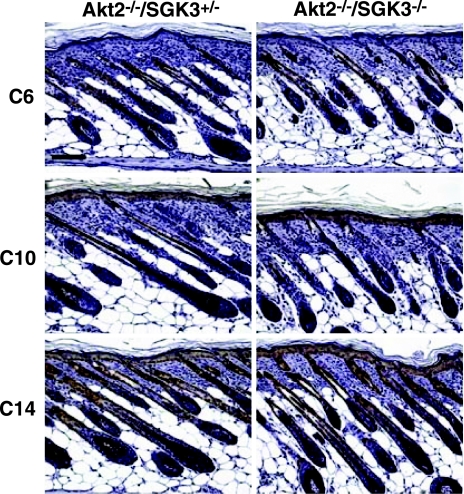
Normal expression of differentiation markers in Akt2−/−/SGK3−/− skin. Immunoflourescence was performed on 6-μm longitudinal sections of dorsal skin from P5 mice as described for differentiation-specific markers. Spinous layers were stained with anti-cytokeratin 10 (C10), the basal layer anti-cytokeratin 14 (C14), and the ORS of the hair follicle with anti-cytokeratin 6 (C6). Brown staining represents expression of each marker; sections were counterstained with hematoxylin and eosin. Scale bar = 200 μm.
Statistical analyses
All data were analyzed using Microsoft Excel (Microsoft, Redmond, WA, USA). Statistical significance was determined by Student’s unpaired t test, with P < 0.05 taken as statistically significant.
RESULTS
Mice lacking both Akt2 and SGK3 have a severe hair-growth defect
Akt2/SGK3-DKO mice were generated by interbreeding Akt2+/−/Sgk3+/− mice, and were born at a normal Mendelian frequency and at a normal sex ratio (data not shown). Similar to SGK3-KO (15, 33) and Akt2-KO (12, 18) mice, Akt2/SGK3-DKO mice appeared normal at birth. By P5, gross examination revealed a clear defect in hair follicle morphogenesis, consisting of sparse growth of curly hair and curly vibrissae, initially resembling the hair-growth pattern of SGK3-KO mice. Histological analysis of multiple organs and blood analysis revealed no other defects in adult Akt2/SGK3-DKO mice, except pancreas, which showed islet expansion similar to that of the Akt2 single-KO mice (data not shown).
As the animals grew, it became apparent from gross inspection that in contrast to SGK3-KO mice, Akt2/SGK3-DKO mice displayed persistent severely defective hair growth without later compensation (Fig. 1 and ref. 15). Akt2+/−/Sgk3−/− mice appeared to display an intermediate phenotype, but more closely resembled SGK3-KO mice, demonstrating substantial improvement in hair growth with age. Interestingly, Akt2−/−/Sgk3+/− mice showed normal hair growth on gross inspection at all ages, suggesting that a single copy of the SGK3 allele is sufficient to confer normal hair growth in Akt2-KO mice, as it is in mice that are wild type at the Akt2 locus.
Hair growth in humans and rodents is a cyclical process that proceeds through proliferative (anagen), regressive (catagen), and quiescent (telogen) phases (34). In mice, hair follicle morphogenesis begins at E14.5 with placode formation, followed by progressive hair follicle growth and hair shaft production until the induction of catagen at P16 (34). The catagen stage, which is characterized by hair bulb involution and a reduction in hair follicle length, ends with the resting telogen phase at about P22 [the subcutis thins, and follicles reside entirely in the dermis and lack an inner root sheath (IRS)]. The second anagen phase begins at approximately P26: the dermal papilla enlarges, the hair bulb and IRS reform, and a new hair shaft begins to develop (34).
To determine whether the more severe alopecia seen in Akt2/SGK3-DKO mice was caused by differences in hair follicle morphogenesis during the initial hair cycle, dorsal skin was harvested at various time points from P1 to P28 from DKO mice and Akt2−/−/SGK3+/− littermates, sectioned, and examined by light microscopy (Fig. 2). Skin samples from Akt2+/+/SKG3−/+ and Akt2+/+/SGK3−/− mice also were included for comparison at P1, P3, and P5 (Fig. 2A). At all time points examined, the hair follicles of Akt2+/+/SGK3+/− mice underwent appropriately timed development and were indistinguishable from those of wild-type mice (Fig. 2A and data not shown). Likewise, the SGK3−/− single-KO mice displayed the previously reported premature follicle regression at P5, and premature entry into anagen at P19 (15, 33) (Fig. 2A and data not shown). Although grossly normal, Akt2−/−/SGK3+/− mice displayed histological evidence of accelerated morphogenesis at P1 and P3. However, the follicular cycling in these mutants normalized in the later stages of the first cycle, and resembled that of the control Akt2+/+/SGK3+/− mice, suggesting that a single functional allele of SGK3 is sufficient to enable near-normal hair cycling. Like Akt2 single-KO mice, DKO mice displayed accelerated morphogenesis at P1 and P3. However, in contrast to all other genotypes, the DKO follicles markedly regressed at P5 and P10. Notably, the hair bulbs failed to enlarge and did not migrate fully into the subcutis (Fig. 2A, B). Although it initially was suggested that SGK3-null mice undergo a delayed first anagen (15), it is now clear that they undergo an accelerated first hair cycle, with follicles failing to complete a normal first anagen, and enter catagen as early as P9, the majority entering the next anagen by P15 (33). Histological findings in DKO mice were similar to those observed in SGK3-KO mice: At P10, we observed catagen hair follicles, and by P16, follicles in DKO mice had reentered anagen (Fig. 2B, C). Akt2/SGK3-DKO mice then progressed to a second catagen by d 22, a stage by which the Akt2−/− single KO was in telogen, similar to control animals (34), and reentered a second anagen by P28 (Fig. 2B, C), thus roughly doubling the number of hair cycles seen in normal postnatal hair cycling.
Morphologically, the developing hair follicles in Akt2/SGK3-DKO mice were highly disorganized, and this was especially striking from P10 onward (Fig. 3). At P5, as in SGK3-KO mice, the cells of the outer root sheath (ORS) were disorganized (Fig. 4) (although normally differentiated; see below), and the layers of the IRS were reduced, as was the hair shaft. These defects are the most likely cause of the wavy hair growth. Through telogen, Akt2/SGK3-DKO mice displayed enlarged and more numerous sebaceous glands (Fig. 5), though this defect was no longer present at P28 when the follicles reentered anagen (data not shown).
As with the SGK3-null mice, histological abnormalities were confined to the follicles themselves: interfollicular epidermis appeared normal, and the progressive thickening of the dermis and subcutaneous fat that accompanies anagen in wild-type and Akt2−/−/Sgk3+/− mice proceeded normally in DKO mice (Fig. 2A). Analysis of differentiation-specific markers indicated that epidermal differentiation is normal in Akt2/SGK3-DKO mice. Specifically, the spinous layers (stained with anti-cytokeratin 10), the basal layer (stained with anti-cytokeratin 14), and the ORS (stained with anti-cytokeratin 6) all appeared normal (Fig. 6). No epidermal cysts, inflammation, or scarring were seen at any time.
Akt2 is highly expressed in hair follicles
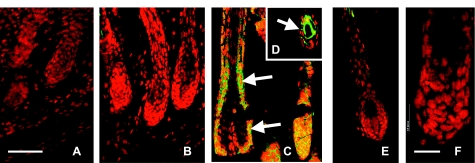
SGK3 mRNA is expressed at low levels in the early postnatal hair follicle but reaches high levels by P5 (15, 35) in root sheath, with lower-level expression seen in matrix cells of the hair bulb. To determine whether Akt2 shows the same pattern of expression in the postnatal follicular epithelium, Akt2 expression also was localized by immunofluorescence in dorsal skin sections from normal P5 mice (Fig. 7). Like SGK3, Akt2 was expressed at low levels at P1 and P3 but showed robust expression at P5. Akt2 expression was high in the hair follicle root sheath (both outer and inner layers), and also within the hair bulb matrix cells (which give rise to the IRS and precursors of the hair shaft) (Fig. 7), where it showed substantial overlap with that of SGK3. This expression pattern is consistent with the idea that Akt2 can compensate for a lack of SGK3. In addition, Akt2 was widely expressed in all skin layers (24, 36), consistent with the finding that Akt1/Akt2-DKO mice display proliferation defects in all layers (24).
Figure 7.
Akt2 expression in normal follicular epithelium increases with onset of morphogenesis. Immunofluorescence was performed on 5-μm longitudinal sections of dorsal skin from normal control mice at P1 (A), P3 (B), and P5 (C), using Akt2 primary antibody at a dilution of 1:150, and propidium iodide as a counterstain, as described in Materials and Methods. Akt2 was expressed at high levels throughout the follicular epithelium in P5 hair follicles (green staining in C, D; arrows) but was not expressed or expressed only at low levels at P1 or P3. Akt2 staining was not seen above background levels in DKO follicular epithelium (E) or in negative control hair follicles (F), in which the primary antibody had been omitted. Scale bars = 50 μm (A–E); 20 μm (F).
Akt2/SGK3-DKO mice display a severe impairment in hair follicle matrix cell proliferation and β-catenin nuclear accumulation at P5
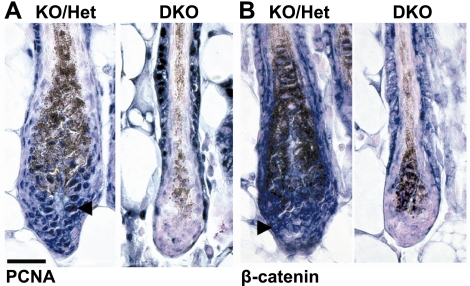
In hair bulbs lacking SGK3, there is a profound reduction in keratinocyte proliferation at P4 relative to normal hair bulbs; while still significant, this defect is diminished by P5 (15). To determine whether Akt2/SGK3-DKO hair bulbs had a similar proliferation defect, PCNA immunolocalization was performed on P5 skin sections to assess cell proliferation. Matrix cells of Akt2/SGK3-DKO mice displayed a strikingly low rate of proliferation at P4, but unlike the single KO, this defect persisted into P5 (compare with matrix cells of Akt2−/−/ Sgk3+/− hair bulbs, which behave as wild type) (Fig. 8A). Furthermore, there was intense PCNA staining in the ORS of Akt2/SGK3-DKO follicles relative to Akt2−/−/Sgk3+/− (Fig. 8A). In both genotypes, interfollicular epidermis resembled that of wild type (data not shown), displaying appropriate proliferation for age and developmental stage. Thus, the proliferation defect in Akt2/SGK3-DKO mice appears to persist at P5, and there is an apparent “shift” in proliferation from the hair bulb to the ORS, possibly due to an impairment of cell migration down through the ORS to the hair bulb (37). These observations are in contrast to the SGK3 single KO, in which the proliferation defect was partially ameliorated by P5.
Figure 8.
Proliferation and β-catenin nuclear accumulation defects in Akt2−/−/SGK3−/− mice. A) Increased numbers of PCNA-positive keratinocytes were observed in Akt2−/−/SGK3+/− (KO/Het) but not in Akt2−/−/SGK3−/− (DKO) hair bulbs. Arrowhead indicates example of PCNA-positive cell. Note that melanin granules stain dark brown. B) Large numbers of keratinocytes with cytoplasmic and nuclear β-catenin are observed in Akt2−/−/SGK3+/− (KO/Het) hair bulbs at P5, similar to wild-type mice (not shown). Akt2−/−/SGK3−/− (DKO) hair bulbs display little expression of cytoplasmic or nuclear β-catenin. Arrowhead indicates example of normal cell in Akt2−/−/SGK3+/− displaying nuclear β-catenin. Scale bar = 50 μm.
The role of the Wnt/β-catenin pathway in hair follicle development and cycling has been extensively examined using mouse genetic models (38,39,40,41); increased signaling in this pathway has been found to increase follicular proliferation (40), while its disruption blocks hair follicle development or cycling (41). Transgenic mice carrying a β-catenin/Lef1-dependent reporter (TOPGAL) demonstrate reporter expression during hair follicle development (42) that overlaps with expression of Sgk3 (15) and Akt2 (Fig. 7A). We previously reported that at P5 SGK3-KO hair bulbs display substantially fewer β-catenin-positive nuclei in the lower root sheath and matrix cells (15). A recent study describing the YPC mutant mouse strain, which bears an SGK3 nonsense mutation, also suggests SGK3 regulates β-catenin-dependent pathways (35). With these prior observations in mind, we performed immunohistochemistry using an anti-β-catenin antibody on skin sections from P5-DKO mice; Akt2−/−/SGK3+/− mice (which have normal skin and fur) are shown for reference. In interfollicular epidermis, β-catenin was associated with the plasma membrane, and there was no difference between Akt2−/−/SGK3+/− and DKO animals at any time point (data not shown). In the follicular keratinocytes of Akt2−/−/SGK3+/−, there was plasma membrane β-catenin staining in both the IRS and ORS, and in the matrix cells of the hair bulb (Fig. 8B). In the matrix cells, there was also extensive, intense cytoplasmic, and nuclear β-catenin staining (Fig. 8B). This pattern of β-catenin staining matched that observed in wild-type skin and hair follicles (15). In Akt2/SGK3-DKO follicles, ORS β-catenin staining resembled that of Akt2−/−/SGK3+/− follicles (Fig. 8B). In contrast, there was virtually no β-catenin staining in the IRS and virtually no matrix cells displaying cytoplasmic and nuclear β-catenin accumulation (Fig. 8B). Overall, this pattern of β-catenin staining was similar to that observed in SGK3-KO hair follicles (15) and YPC mice (35), although the defect is more severe in Akt2/SGK3-DKO follicles, with little nuclear β-catenin detected, even in heavily stained sections.
Akt2 and SGK3 have additive effects on Lef1-mediated gene transcription in cultured keratinocytes
We previously showed that SGK3 strongly stimulates Lef1/β-catenin-mediated gene transcription in cultured keratinocytes, likely by inhibiting GSK3-β (15). To determine whether Akt2 also stimulates Lef1-mediated gene transcription, we transfected keratinocyte-derived HaCaT cells (43) with a constitutively active Akt2 mutant (Akt2-S474D/T309E; CA-Akt2) and a β-catenin/Lef1-driven reporter gene (p7 Lef-fos Luc) (44), with and without SGK3 (Fig. 9). HaCat cells have very low levels of total SGK3 or Akt, and undetectable levels of phosphorylated Akt (data not shown) and thus provide a low level of endogenous baseline activity against which to test the effects of the transfected kinases. In these cells, Akt2 stimulated reporter activity significantly, although less potently than SGK3 (Fig. 9), and the effects of the two kinases were additive, not synergistic. These results are consistent with the idea that SGK3 and Akt2 have overlapping functions in the control of Lef1-β-catenin-mediated transcription, with SGK3 playing the more prominent role. However, it remains possible that SGK3 and Akt2 act in distinct keratinocyte subtypes (see Discussion).
Figure 9.
Akt2 and SGK3 have additive effects on β-catenin/Lef1-mediated gene transcription in cultured keratinocytes. Immortalized keratinocytes (HaCaT cells) were transfected with p7 Lef-fos Luc (containing 7 Lef1 binding sites driving luciferase), Lef1 expression vector, and expression vectors for Akt2 and SGK3, as shown. Luciferase activity is represented as mean ± sd activity relative to basal reporter activity in the absence of either Akt2 or SGK3. *P ≤ 0.01; Student’s t test. There was no significant difference between effects of Akt2 and SGK3 together and the sum of their separate activities, at any given level of expression.
Together with the marked reduction in nuclear β-catenin and the low rate of follicle cell proliferation in DKO mice (Fig. 8A), these observations support the idea that Akt2 and SGK3 both stimulate β-catenin/Lef1-mediated gene transcription in hair follicle cells, and that a severe defect in this pathway underlies the failure of normal proliferation and therefore of follicle development in DKO mice. While up-regulating β-catenin leads to development of follicular-based skin tumors (45), no DKO mice developed skin tumors, even when followed at ages up to 1 yr (data not shown).
DISCUSSION
The data presented here provide the first evidence that members of the Akt and SGK families share overlapping functions in vivo. In particular, we have found that mice lacking both Akt2 and SGK3 have abnormalities in hair follicle development, which cannot be explained by simple superposition of the two single-KO phenotypes. Initial characterization of SGK3-null mice revealed an abnormality in hair follicle development, and further characterization identified additional roles (22, 46). In contrast, Akt2-null mice have abnormalities in insulin-regulated glucose metabolism and normal fur (Fig. 1, ref. 12, and unpublished results); a role for Akt2 was only unmasked in the context of the double-null animals (Figs. 12345). Like the SGK3 single-KO mice, the DKO mice display abnormally fast hair cycling. Unlike the SGK3 single-KO mice, these defects do not improve; in fact, the defects in proliferation and β-catenin nuclear localization are more severe from P5 onward. By P10, the follicular disorganization has become clearly more pronounced and, in contrast to the SGK3 single-KO mice, does not improve with subsequent hair cycles; the DKO mice remain severely compromised, with limited body hair growth at least until 1 yr of age. The robust expression of Akt2 from P5 onward (Fig. 7) provides a clear basis for the compensation that is seen in SGK3-KO mice. However, the physiological and mechanistic basis for induction of Akt2 expression at this point in postnatal development remains unclear at present.
Although many structural and signaling proteins control hair growth and cycling, relatively few mouse models demonstrate premature catagen entry. Mice in which nerve growth factor is overexpressed accelerate catagen, suggesting that p75 neurotrophin receptor controls hair entry into catagen (47). In contrast, inhibiting BCL-2 blocks entry into catagen (48). More recently, bone morphogenic protein (BMP) signaling has been shown to control entry into catagen. Mice that overexpress noggin, which inhibits BMP, display premature entry into catagen, complete loss of nontylotrich follicles, and ectopic sebocyte differentiation (49). BMP also controls hair follicle pigmentation (50). BMP signaling seems to balance WNT/catenin signaling in the hair cycle induction (51). The additional deletion of Akt in the DKO mice may worsen the SGK3-induced alopecia via Akt interaction with BMP receptors (52). Finally, defects in β-catenin/Lef1 signaling also have been found in vitamin D receptor-deficient mice (53). These mice also display a profound alopecia after the first postnatal hair cycle and increased sebaceous activity (53, 54). However, accelerated hair cycling is not seen in the vitamin D receptor-null mice, while dermal cyst formation is prominent, as opposed to the Akt2/SGK3-DKO mice we present in this report. Taken together, these reports suggest that the β-catenin/Lef1/wnt signaling pathway control important aspects of hair cycling and hair follicle epithelium proliferation and differentiation.
The signaling of growth factor and insulin receptors through Akt and SGK family members is well established, including their phosphorylation and inhibition of GSK3-β. However, there has been conflicting evidence regarding the regulation of β-catenin stability, and hence of Lef1/β-catenin-mediated gene transcription by this pathway (55,56,57,58,59). Notably, although expression of the Lef1/β-catenin-dependent reporter TOPGAL was not reduced in SGK3-KO mouse keratinocytes (33), this may not reflect a lack of regulation by SGK3, but rather a lack of uniform reporter response to nuclear Lef1/β-catenin. Although SGK3 is expressed in areas of TOPGAL activity in the IRS and the progenitor cells, it is also expressed in the matrix cells of the hair bulb, which do not express TOPGAL in wild-type mice. It is the latter that manifest the most striking defect in nuclear accumulation of endogenous β-catenin in both SGK3-KO (15) and Akt2/SGK3-DKO mice; a similar defect was also observed in YPC mice (35). We propose that Akt2 and SGK3 modulate β-catenin-mediated proliferative effects rather than differentiation, and this is supported by normal expression patterns of several keratins in both SGK3-KO (33) and Akt2/SGK3-DKO mice (Fig. 3). Furthermore, we found that Akt2 stimulates β-catenin/Lef1-mediated gene transcription in cultured keratinocytes, but less potently than SGK3 (Fig. 8 and unpublished results). The effects of the two kinases were additive, consistent with overlapping functions, since their effects should be synergistic if they act on distinct steps in convergent pathways (60). These effects of Akt2 and SGK3 are consistent with most—but not all—of the in vivo mouse data. We propose that in the absence of the more potent SGK3, β-catenin-Lef1 activity drops below a critical level required for coordinated hair cycling, and the abnormal fur phenotype emerges. The abnormality is blunted by Akt2, and hence, in the absence of both, the severe phenotype emerges. However, not all of our data are consistent with this interpretation: DKO mice demonstrate a marked increase in proliferation in the ORS, where β-catenin expression is relatively low in the nucleus and high in the plasma membrane. This observation is consistent with the possibility that Akt2 and SGK3 stimulate migration of ORS cells into the hair bulb and do not influence follicle cell proliferation per se. Further studies will be necessary to address this possibility.
SGK3 and Akt2 are both expressed in hair follicles (15), intestine (22, 61), and pancreatic β cells (62, 63). The observation that DKO mice have markedly worse hair growth than either of the single-KO mice in the face of similar mechanisms of molecular action suggests that these kinases are partially redundant. However, it remains possible that the DKO phenotype results from distinct roles of each kinase in distinct tissues or in distinct signaling steps in the same cell type. Additional studies in single and compound KOs and in tissue-selective KOs will be needed to address the various mechanistic possibilities.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) training grant T32DK007219 (to J.A.M.), NIH grant AR051930 (to T.M.), NIH grant DK-56886 (to M.B.), Deutsche Forschungsgemeinschaft (La 315/4-6) (to F.L.), and NIH grant DK56695 (to D.P.). We thank D. Kusewitt (Ohio State University Mouse Phenotyping Service, Columbus, OH, USA) for multiple-tissue histological analysis, blood chemistry and cytokeratin immunostaining; Z. Huang and Melanie Hupe for technical assistance; L. Prentice for skin section preparation; and Louella Lee for assistance with preparation of the manuscript. We gratefully thank Rudy Grosschedl (Max Planck Institute of Immunobiology, Freiburg, Germany) for providing Lef1 reporter and expression vector.
References
- Lang F, Cohen P. Regulation and physiological roles of serum- and glucocorticoid-induced protein kinase isoforms. Sci STKE. 2001;2001:RE17. doi: 10.1126/stke.2001.108.re17. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Brunet A, Park J, Tran H, Hu L S, Hemmings B A, Greenberg M E. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai F, Yu L, He H, Chen Y, Yu J, Yang Y, Xu Y, Ling W, Zhao S. Human serum and glucocorticoid-inducible kinase-like kinase (SGKL) phosphorylates glycogen syntheses kinase 3 beta (GSK-3beta) at serine-9 through direct interaction. Biochem Biophys Res Commun. 2002;293:1191–1196. doi: 10.1016/S0006-291X(02)00349-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344:189–197. [PMC free article] [PubMed] [Google Scholar]
- Liu D, Yang X, Songyang Z. Identification of CISK, a new member of the SGK kinase family that promotes IL-3-dependent survival. Curr Biol. 2000;10:1233–1236. doi: 10.1016/s0960-9822(00)00733-8. [DOI] [PubMed] [Google Scholar]
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- Virbasius J V, Song X, Pomerleau D P, Zhan Y, Zhou G W, Czech M P. Activation of the Akt-related cytokine-independent survival kinase requires interaction of its phox domain with endosomal phosphatidylinositol 3-phosphate. Proc Natl Acad Sci U S A. 2001;98:12908–12913. doi: 10.1073/pnas.221352898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liu D, Gill G, Songyang Z. Regulation of cytokine-independent survival kinase (CISK) by the Phox homology domain and phosphoinositides. J Cell Biol. 2001;154:699–705. doi: 10.1083/jcb.200105089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao A C, McCormick J A, Li H, Siu J, Govaerts C, Bhalla V, Soundararajan R, Pearce D. NH2 terminus of serum and glucocorticoid-regulated kinase 1 binds to phosphoinositides and is essential for isoform-specific physiological functions. Am J Physiol Renal Physiol. 2007;292:F1741–F1750. doi: 10.1152/ajprenal.00027.2007. [DOI] [PubMed] [Google Scholar]
- Chen W S, Xu P Z, Gottlob K, Chen M L, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim J K, Thorvaldsen J L, Chu Q, Crenshaw E B, 3rd, Kaestner K H, Bartolomei M S, Shulman G I, Birnbaum M J. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen J L, Chu Q, Feng F, Birnbaum M J. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Wulff P, Vallon V, Huang D Y, Volkl H, Yu F, Richter K, Jansen M, Schlunz M, Klingel K, Loffing J, Kauselmann G, Bosl M R, Lang F, Kuhl D. Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Invest. 2002;110:1263–1268. doi: 10.1172/JCI15696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J A, Feng Y, Dawson K, Behne M J, Yu B, Wang J, Wyatt A W, Henke G, Grahammer F, Mauro T M, Lang F, Pearce D. Targeted disruption of the protein kinase SGK3/CISK impairs postnatal hair follicle development. Mol Biol Cell. 2004;15:4278–4288. doi: 10.1091/mbc.E04-01-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton R M, Cho H, Roovers K, Shineman D W, Mizrahi M, Forman M S, Lee V M, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum M J. Role for Akt3/protein kinase Bγ in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp O, Yang Z Z, Brodbeck D, Dummler B A, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings B A. Essential role of protein kinase B γ (PKB γ/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- Garofalo R S, Orena S J, Rafidi K, Torchia A J, Stock J L, Hildebrandt A L, Coskran T, Black S C, Brees D J, Wicks J R, McNeish J D, Coleman K G. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boini K M, Hennige A M, Huang D Y, Friedrich B, Palmada M, Boehmer C, Grahammer F, Artunc F, Ullrich S, Avram D, Osswald H, Wulff P, Kuhl D, Vallon V, Haring H U, Lang F. Serum- and glucocorticoid-inducible kinase 1 mediates salt sensitivity of glucose tolerance. Diabetes. 2006;55:2059–2066. doi: 10.2337/db05-1038. [DOI] [PubMed] [Google Scholar]
- Ullrich S, Berchtold S, Ranta F, Seebohm G, Henke G, Lupescu A, Mack A F, Chao C M, Su J, Nitschke R, Alexander D, Friedrich B, Wulff P, Kuhl D, Lang F. Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates glucocorticoid-induced inhibition of insulin secretion. Diabetes. 2005;54:1090–1099. doi: 10.2337/diabetes.54.4.1090. [DOI] [PubMed] [Google Scholar]
- Grahammer F, Henke G, Sandu C, Rexhepaj R, Hussain A, Friedrich B, Risler T, Metzger M, Just L, Skutella T, Wulff P, Kuhl D, Lang F. Intestinal function of gene-targeted mice lacking serum- and glucocorticoid-inducible kinase 1. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1114–G1123. doi: 10.1152/ajpgi.00231.2005. [DOI] [PubMed] [Google Scholar]
- Sandu C, Rexhepaj R, Grahammer F, McCormick J A, Henke G, Palmada M, Nammi S, Lang U, Metzger M, Just L, Skutella T, Dawson K, Wang J, Pearce D, Lang F. Decreased intestinal glucose transport in the sgk3-knockout mouse. Pflügers Arch. 2005;451:437–444. doi: 10.1007/s00424-005-1474-7. [DOI] [PubMed] [Google Scholar]
- Huang D Y, Wulff P, Volkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V. Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol. 2004;15:885–891. doi: 10.1097/01.asn.0000120368.59693.a8. [DOI] [PubMed] [Google Scholar]
- Peng X D, Xu P Z, Chen M L, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen W S, Crawford S E, Coleman K G, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z Z, Tschopp O, Di-Poi N, Bruder E, Baudry A, Dummler B, Wahli W, Hemmings B A. Dosage-dependent effects of Akt1/protein kinase Bα (PKBα) and Akt3/PKBγ on thymus, skin, and cardiovascular and nervous system development in mice. Mol Cell Biol. 2005;25:10407–10418. doi: 10.1128/MCB.25.23.10407-10418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummler B, Tschopp O, Hynx D, Yang Z Z, Dirnhofer S, Hemmings B A. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol. 2006;26:8042–8051. doi: 10.1128/MCB.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahammer F, Artunc F, Sandulache D, Rexhepaj R, Friedrich B, Risler T, McCormick J A, Dawson K, Wang J, Pearce D, Wulff P, Kuhl D, Lang F. Renal function of gene-targeted mice lacking both SGK1 and SGK3. Am J Physiol Regul Integr Comp Physiol. 2006;290:R945–R950. doi: 10.1152/ajpregu.00484.2005. [DOI] [PubMed] [Google Scholar]
- Dai F, Yu L, He H, Zhao Y, Yang J, Zhang X, Zhao S. Cloning and mapping of a novel human serum/glucocorticoid regulated kinase-like gene, SGKL, to chromosome 8q12.3-q13.1. Genomics. 1999;62:95–97. doi: 10.1006/geno.1999.5969. [DOI] [PubMed] [Google Scholar]
- Yang Z Z, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings B A. Protein kinase B α/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- Altomare D A, Lyons G E, Mitsuuchi Y, Cheng J Q, Testa J R. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene. 1998;16:2407–2411. doi: 10.1038/sj.onc.1201750. [DOI] [PubMed] [Google Scholar]
- Chen X, Al-Hasani H, Olausson T, Wenthzel A M, Smith U, Cushman S W. Activity, phosphorylation state and subcellular distribution of GLUT4-targeted Akt2 in rat adipose cells. J Cell Sci. 2003;116:3511–3518. doi: 10.1242/jcs.00675. [DOI] [PubMed] [Google Scholar]
- Chen S-Y, Wang J, Yu G-Q, Liu W-H, Pearce D. Androgen and glucocorticoid receptor heterodimer formation. A possible mechanism for mutual inhibition of transcriptional activity. J Biol Chem. 1997;272:14087–14092. doi: 10.1074/jbc.272.22.14087. [DOI] [PubMed] [Google Scholar]
- Alonso L, Okada H, Pasolli H A, Wakeham A, You-Ten A I, Mak T W, Fuchs E. Sgk3 links growth factor signaling to maintenance of progenitor cells in the hair follicle. J Cell Biol. 2005;170:559–570. doi: 10.1083/jcb.200504131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay I A, Stenn K S, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Okada T, Ishii Y, Masujin K, Yasoshima A, Matsuda J, Ogura A, Nakayama H, Kunieda T, Doi K. The critical roles of serum/glucocorticoid-regulated kinase 3 (SGK3) in the hair follicle morphogenesis and homeostasis: the allelic difference provides novel insights into hair follicle biology. Am J Pathol. 2006;168:1119–1133. doi: 10.2353/ajpath.2006.050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shaughnessy R F, Akgul B, Storey A, Pfister H, Harwood C A, Byrne C. Cutaneous human papillomaviruses down-regulate AKT1, whereas AKT2 up-regulation and activation associates with tumors. Cancer Res. 2007;67:8207–8215. doi: 10.1158/0008-5472.CAN-07-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy S T, Gaddapara T, Millar S E. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Merrill B J, Jamora C, DasGupta R. At the roots of a never-ending cycle. Dev Cell. 2001;1:13–25. doi: 10.1016/s1534-5807(01)00022-3. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska R T, Breitkreutz D, Hornung J, Markham A, Fusenig N E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese K, Grosschedl R. LEF-1 contains an activation domain that stimulates transcription only in a specific context of factor-binding sites. EMBO J. 1993;12:4667–4676. doi: 10.1002/j.1460-2075.1993.tb06155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E F, Gat U, McNiff J M, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- Strutz-Seebohm N, Seebohm G, Mack A F, Wagner H J, Just L, Skutella T, Lang U E, Henke G, Striegel M, Hollmann M, Rouach N, Nicoll R A, McCormick J A, Wang J, Pearce D, Lang F. Regulation of GluR1 abundance in murine hippocampal neurones by serum- and glucocorticoid-inducible kinase 3. J Physiol. 2005;565:381–390. doi: 10.1113/jphysiol.2004.079582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev V A, Botchkareva N V, Albers K M, Chen L H, Welker P, Paus R. A role for p75 neurotrophin receptor in the control of apoptosis-driven hair follicle regression. FASEB J. 2000;14:1931–1942. doi: 10.1096/fj.99-0930com. [DOI] [PubMed] [Google Scholar]
- Botchkarev V A, Komarova E A, Siebenhaar F, Botchkareva N V, Sharov A A, Komarov P G, Maurer M, Gudkov A V, Gilchrest B A. p53 Involvement in the control of murine hair follicle regression. Am J Pathol. 2001;158:1913–1919. doi: 10.1016/S0002-9440(10)64659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha U, Mecklenburg L, Cowin P, Kan L, O'Guin W M, D'Vizio D, Pestell R G, Paus R, Kessler J A. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729–740. doi: 10.1016/S0002-9440(10)63336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov A A, Fessing M, Atoyan R, Sharova T Y, Haskell-Luevano C, Weiner L, Funa K, Brissette J L, Gilchrest B A, Botchkarev V A. Bone morphogenetic protein (BMP) signaling controls hair pigmentation by means of cross-talk with the melanocortin receptor-1 pathway. Proc Natl Acad Sci U S A. 2005;102:93–98. doi: 10.1073/pnas.0408455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus M V, Mayer J A, de la Cruz D, Baker R E, Maini P K, Maxson R, Chuong C M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianferotti L, Cox M, Skorija K, Demay M B. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci U S A. 2007;104:9428–9433. doi: 10.1073/pnas.0702884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D D, Elalieh H, Chang S, Xie Z, Sundberg J P. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340–353. doi: 10.1002/jcp.20578. [DOI] [PubMed] [Google Scholar]
- Edlund S, Lee S Y, Grimsby S, Zhang S, Aspenstrom P, Heldin C H, Landstrom M. Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis. Mol Cell Biol. 2005;25:1475–1488. doi: 10.1128/MCB.25.4.1475-1488.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Hsieh C M, Maemura K, Layne M D, Yet S F, Lee K H, Matsui T, Rosenzweig A, Taylor W G, Rubin J S, Perrella M A, Lee M E. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- Maupas-Schwalm F, Robinet C, Auge N, Thiers J C, Garcia V, Cambus J P, Salvayre R, Negre-Salvayre A. Activation of the β-catenin/T-cell-specific transcription factor/lymphoid enhancer factor-1 pathway by plasminogen activators in ECV304 carcinoma cells. Cancer Res. 2005;65:526–532. [PubMed] [Google Scholar]
- Tian Q, Feetham M C, Tao W A, He X C, Li L, Aebersold R, Hood L. Proteomic analysis identifies that 14–3-3zeta interacts with beta-catenin and facilitates its activation by Akt. Proc Natl Acad Sci U S A. 2004;101:15370–15375. doi: 10.1073/pnas.0406499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, He X C, Hood L, Li L. Bridging the BMP and Wnt pathways by PI3 kinase/Akt and 14–3-3zeta. Cell Cycle. 2005;4:215–216. [PubMed] [Google Scholar]
- Wang J, Ellwood K, Lehman A, Carey M F, She Z S. A mathematical model for synergistic eukaryotic gene activation. J Mol Biol. 1999;286:315–325. doi: 10.1006/jmbi.1998.2489. [DOI] [PubMed] [Google Scholar]
- Li X, Leu S, Cheong A, Zhang H, Baibakov B, Shih C, Birnbaum M J, Donowitz M. Akt2, phosphatidylinositol 3-kinase, and PTEN are in lipid rafts of intestinal cells: role in absorption and differentiation. Gastroenterology. 2004;126:122–135. doi: 10.1053/j.gastro.2003.10.061. [DOI] [PubMed] [Google Scholar]
- Muller D, Huang G C, Amiel S, Jones P M, Persaud S J. Identification of insulin signaling elements in human beta-cells: autocrine regulation of insulin gene expression. Diabetes. 2006;55:2835–2842. doi: 10.2337/db06-0532. [DOI] [PubMed] [Google Scholar]
- Muller D, Huang G C, Amiel S, Jones P M, Persaud S J. Gene expression heterogeneity in human islet endocrine cells in vitro: the insulin signalling cascade. Diabetologia. 2007;50:1239–1242. doi: 10.1007/s00125-007-0671-7. [DOI] [PubMed] [Google Scholar]