Abstract
It has been hypothesized that translational efficiency is determined by the amount of secondary structure in the 5′-untranslated region (5′-UTR) of mRNA. Here, we examined whether specific 5′-UTRs with excessive secondary structure selectively regulate translational efficiency in adult cardiocytes. Recombinant adenoviruses were generated to express reporter mRNAs consisting of the 5′-UTR derived from c-jun or ornithine decarboxylase (ODC) fused to β-galactosidase (βGal) coding sequence. Each adenovirus expressed GFP mRNA as a control for 5′-UTRs with minimal secondary structure. Subsequently, cardiocytes were electrically stimulated to contract at 1 Hz to accelerate protein synthesis as compared to quiescent controls. Translational efficiency was calculated by measuring protein expression as a function of mRNA levels. Translational efficiency of c-jun/βGal mRNA increased significantly by 3.7-fold in contracting vs. quiescent cardiocytes, but ODC/βGal mRNA was unchanged. Contraction increased c-jun/βGal mRNA levels in polyribosomes by 2.3-fold, which indicates that translational efficiency was enhanced by mobilization. A short, unstructured 5′-UTR was sufficient for efficient translation of βGal mRNA in quiescent and contracting cardiocytes. GFP mRNA produced similar results. These studies demonstrate that the 5′-UTR functions as a determinant of translational efficiency of specific mRNAs, such as c-jun, that regulate growth of the adult cardiocyte.—Spruill, L. S., McDermott, P. J. Role of the 5′-untranslated region in regulating translational efficiency of specific mRNAs in adult cardiocytes.
Keywords: cardiac hypertrophy, protein biosynthesis, polyribosomes, peptide chain initiation
Hypertrophic growth of the adult myocardium is produced by altering steady-state protein metabolism such that the rate of protein synthesis exceeds the rate of protein degradation (1). The resulting enlargement of cardiac muscle cells (cardiocytes) develops by proportionate increases in proteins that comprise the predominant organelles such as sarcomeres and mitochondria (2, 3). The rate of total protein synthesis is accelerated during hypertrophic growth by increasing the amount of ribosomes along with the activity of initiation factors and associated components of the translation machinery (4). These mechanisms generate more 43S preinitiation complexes, which are formed by binding of the ternary complex, eIF3, and other initiation factors to the 40S ribosomal subunit (5). As a result, the general (global) rate of peptide chain initiation increases because binding of 43S preinitiation complexes to mRNA is a rate-limiting step for translational initiation (6). Given that total mRNA is in excess relative to available 43S preinitiation complexes, individual mRNAs compete for binding in order to initiate translation. Thus, as demonstrated by measuring the synthesis of a highly abundant protein such as myosin, the rate of protein synthesis can be accelerated during pressure overload hypertrophy in vivo without a comparable increase in mRNA level (7, 8).
Hypertrophy also is characterized by selective changes in gene expression that regulate growth and modify the structural and functional properties of the myocardium (3). An array of transcriptional and post-transcriptional mechanisms produce these changes by regulating mRNA abundance, alternative splicing, and/or stability of specific mRNAs (9,10,11,12,13). Translational mechanisms provide an additional level at which expression of specific mRNAs can be regulated. For example, the 5′-UTR of individual mRNAs has distinct structural features and elements that affect the intrinsic rate of translational initiation (14, 15). Accordingly, these features are used to classify mRNAs as either weak or strong with respect to translational efficiency (16). Weak mRNAs have an excessive amount of stable, secondary structure in the 5′-UTR, which is based on a free energy estimate (ΔG) of −50 kcal/mol or less as predicted by mfold (17). Other distinctive features are a length exceeding 100 nucleotides (nt), upstream open reading frames (uORFs), and alternative AUG codons. A disproportionate number of weak mRNAs code for proteins such as transcription factors, proto-oncogenes, and growth factors. By comparison, the strong class constitute as much as 90% of cellular mRNAs and code for proteins such as myosin, actin, and tubulin. In general, these 5′-UTRs are relatively short, contain a minimal amount of stable secondary structure, and lack the types of distinct elements that impede initiation of weak mRNAs. Strong mRNAs are translated more efficiently because the intrinsic rate of initiation is higher than that of the weak class of mRNAs.
In classic studies, Lodish (6) developed a model of gene expression based on the principle that mRNAs differ in the rate at which they bind to 43S preinitiation complexes and initiate translation. Consequently, an increase in the general rate of peptide chain initiation exerts a selective effect on translational efficiency of weak vs. strong mRNAs. This model is particularly relevant to the regulation of cardiac protein synthesis. At steady state, the efficiency of total protein synthesis is high, as indicated by the fact that >85% of the ribosome pool is incorporated into polysomes and therefore active in translation (1). Strong mRNAs are translated preferentially over weak mRNAs because they have a higher initiation rate, which enables them to compete effectively for 43S preinitiation complexes (6). A hypertrophic stimulus can shift this imbalance by accelerating the rate of peptide chain initiation. The resulting increase in 43S preinitiation complexes could advance translation of weak mRNAs relative to the total mRNA pool because of limited potential to further increase translational efficiency of strong mRNAs (6, 18).
In previous studies, translational efficiency of a luciferase reporter mRNA was reduced in adult cardiocytes by inserting G-C rich repeats into the 5′-UTR to increase the amount of secondary structure (19). Here, we tested whether 5′-UTRs derived from endogenous mRNAs selectively regulate translational efficiency in adult cardiocytes. Recombinant adenoviruses were generated that express reporter mRNAs consisting of the 5′-UTR of either c-jun mRNA or ornithine decarboxylase (ODC) mRNA fused to the β-galactosidase (βGal) coding sequence. Both of these 5′-UTRs contain an excessive amount of secondary structure, which is considered a hallmark of mRNAs that are weak with respect to translational efficiency (14). For comparison, a βGal reporter mRNA with minimal secondary structure in the 5′-UTR was generated as a control for strong mRNAs. Electrically stimulated contraction of adult cardiocytes in vitro was used to accelerate the rate of total protein synthesis, which is caused by an increase in the general rate of peptide chain initiation as compared to quiescent (noncontracting) cardiocytes (20,21,22,23). Using this approach, changes in translational efficiency were measured directly by quantifying expression of reporter protein as a function of increasing reporter mRNA levels. These studies demonstrate that an increase in the general rate of peptide chain initiation as produced in contracting cardiocytes exerts a selective effect on translational efficiency that is determined by the 5′-UTR of the reporter mRNA.
MATERIALS AND METHODS
Expression of reporter mRNAs by adenoviral gene transfer
Recombinant adenoviruses were generated in order to express reporter mRNAs consisting of the 5′-UTR of c-jun mRNA or ODC mRNA fused to the βGal coding sequence. For comparison, a control adenovirus was produced to express βGal mRNA with a short, unstructured 5′-UTR. Transcription of the 5′-UTR/βGal reporter gene was controlled by the minimal sodium-calcium exchanger (NCX) promoter of the feline gene (24). In addition, each adenovirus expressed GFP under control of the cytomegalovirus promoter. The procedures used to generate these adenoviruses are described in Supplemental Data.
Preparation of adult feline cardiocytes for primary culture
Cardiocytes were isolated from the left ventricle of adult feline myocardium as described before (20). The investigation conforms to the Guide for the Care and Use of Laboratory Animals, published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996). The procedure was approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina. The cardiocytes were plated onto 4-well, laminin-coated culture trays (Nalge Nunc International, Rochester, NY, USA) at an initial plating density of 3 × 105 cardiocytes/well, and maintained in serum-free medium (25). The next day, cardiocytes were infected for 4 h with reporter adenovirus at increasing multiplicity of infection (m.o.i.), which was calculated by dividing the number of plaque-forming units by the cardiocyte plating density. After an 18-h incubation, cardiocytes were stimulated to contract at a frequency of 1 Hz by delivering 5-ms electrical pulses of alternating polarity through the culture medium (25). Companion dishes of nonstimulated cardiocytes were quiescent and used as controls.
Measurement of translational efficiency of reporter mRNAs
After infection with adenovirus, cardiocytes were electrically stimulated to contract for 48 h or maintained as nonstimulated (quiescent) controls. At each m.o.i., 2 wells/culture tray were used to measure levels of βGal and GFP mRNA, normalized to GAPDH mRNA. The other wells were used to assay expression of βGal protein by a chemiluminescent reporter assay and expression of GFP protein by measuring fluorescence. Reporter protein activities were normalized to cardiocyte protein (μg) in each assay. Translational efficiency (protein/mRNA) is equivalent to the slope as calculated by the following formula:
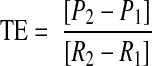 |
where TE is translational efficiency, P is the activity of reporter protein measured at each m.o.i., and R is the relative increase in reporter mRNA levels as calculated by setting R1 in each experiment to a value of 1.
Total RNA was extracted from cardiocytes by the Trizol method (Invitrogen, Carlsbad, CA, USA). The levels of each RNA were measured by real-time RT-PCR as described before (26). The primer sets are shown in Supplemental Table 1. The size and purity of the PCR products were verified using agarose gels. Standard curves were generated by making serial dilutions of a stock sample of RNA derived from cardiocytes infected with βGal or GFP adenovirus. The levels of βGal mRNA and GFP mRNA in each sample were normalized to endogenous levels of GAPDH mRNA.
To measure expression of βGal and GFP reporter proteins, cardiocytes at each m.o.i. were rinsed in phosphate-buffered saline, scraped in lysis buffer containing 100 mM K2CO3-KHCO3 (pH 7.8) and 0.2% Triton X-100, and frozen immediately in liquid N2. The lysates were thawed on ice and centrifuged at 12,000 g for 10 min. βGal activity in the supernatant was measured using the Galacto-Light Plus System (Applied Biosystems, Bedford, MA, USA). Lysates derived from noninfected cardiocytes were used to measure background luminescence. GFP activity was measured in the same lysates by fluorometry. Total protein concentration was measured by the BCA protein assay (Pierce Biotechnology, Inc., Rockford, IL, USA).
Distribution of reporter mRNAs in monosomes and polysomes
The localization of reporter mRNAs in monosomes (mRNPs and ribosome subunits) and polysomes was determined by fractionation of cardiocyte homogenates on 15 to 50% (w/v) linear sucrose gradients as described before (22). RNA was extracted from the gradient fractions using the Trizol method and prepared for analysis by real-time RT-PCR as described above. βGal mRNA and GFP mRNA levels in each fraction were normalized to the corresponding levels of 18S rRNA, which were measured using the primer set in Supplemental Table 1.
RESULTS
Adenoviral-mediated expression of reporter mRNAs
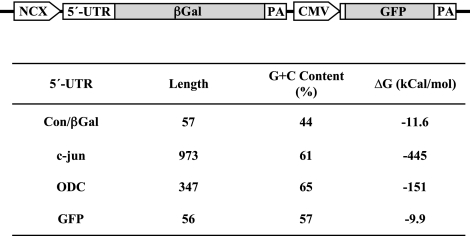
As illustrated in Fig. 1, recombinant adenoviruses were generated that express reporter mRNAs consisting of the 5′-UTR fused to the βGal coding sequence. The 5′-UTRs of c-jun and ODC are relatively long and contain an excessive amount of secondary structure, features that are typical of weak mRNAs. In contrast, the 5′-UTR of Con/βGal is representative of strong mRNAs, which comprise a large percentage of the total mRNA pool. Expression of βGal reporter mRNAs was regulated by the minimal, cardiac-specific NCX promoter (24). This promoter has constitutive activity in adult cardiocytes, but it is not activated by electrically stimulated contraction. Figure 1 shows further that each adenovirus expressed GFP mRNA, which has a 5′-UTR characteristic of strong mRNAs. In each experiment, real-time RT-PCR data established that expression of βGal reporter mRNAs increased as a function of m.o.i. Northern blotting also was done to verify that the 5′-UTR of either c-jun or ODC did not alter the stability of βGal reporter mRNA. Each adenovirus produced a single βGal mRNA transcript consistent with the predicted sizes of 4.1 and 3.5 kb, respectively (Supplemental Data).
Figure 1.
Schematic of reporter adenoviruses. The 5′-UTR was fused to the coding sequence for βGal to generate Con/βGal, c-jun/βGal and ODC/βGal, respectively. Expression of βGal mRNA was controlled by the minimal NCX promoter. Each adenovirus expressed GFP under control of the CMV promoter. PA, polyadenylation sequence. The predicted amount of secondary structure was determined by mfold (17).
Relation between expression of reporter mRNAs and reporter proteins
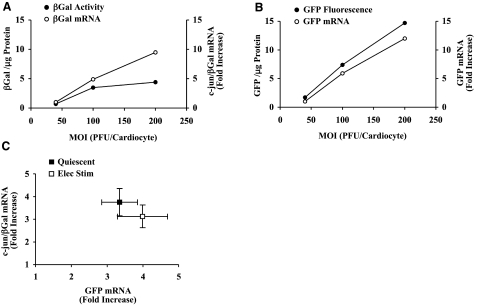
Translational efficiency was measured by infecting cardiocytes with adenovirus at increasing m.o.i., thereby raising the levels of reporter mRNA incrementally. Subsequently, the accumulation of protein product was divided by the relative increase in the corresponding pool of reporter mRNA. Figure 2A shows that βGal protein levels rose as a function of m.o.i., but expression reached a plateau when reporter mRNA levels were increased between ∼5- and 10-fold. In contrast, GFP protein activity increased in proportion with GFP mRNA levels over an extended range of m.o.i., which proves that translational efficiency was not affected adversely by relatively high levels of adenoviral-mediated gene expression (Fig. 2B). βGal expression reached a plateau at higher m.o.i. because the CMV promoter driving GFP expression was much stronger than the NCX promoter. As a result, GFP mRNA levels in the cardiocyte were 50- to 70-fold higher than βGal mRNA. Assuming that ribosome binding to GFP mRNA was more efficient than βGal mRNA, fewer ribosomes would be available for translational initiation on βGal mRNA as the m.o.i. was raised progressively.
Figure 2.
Effects of m.o.i. on reporter mRNA and protein levels. A) Cardiocytes were infected with increasing m.o.i. of c-jun/βGal adenovirus and electrically stimulated to contract at 1 Hz for 48 h. c-jun/βGal mRNA levels are expressed as the fold increase, which was calculated by setting the βGal RNA level measured at the lowest m.o.i. to a value of 1. B) Corresponding measurements of GFP mRNA and GFP fluorescence. C) Summary data showing relation between GFP mRNA and c-jun/βGal mRNA levels. Values are means ± se, n = 8 experiments.
The type of curve shown in Fig. 2A, B was generated for each experiment in order to quantify changes in reporter mRNA levels. In Fig. 2C, summary data show the increase in c-jun/βGal mRNA plotted as a function of the corresponding increase in GFP mRNA. These data demonstrate that adenoviral-mediated delivery produced comparable increases in c-jun/βGal mRNA and GFP mRNA levels in quiescent and contracting cardiocytes. Furthermore, the magnitude of these increases ensured that translational efficiency of c-jun/βGal mRNA was not measured when βGal protein expression had reached the plateau phase (compare Fig. 2C with A, B).
Translational efficiency of reporter mRNAs in quiescent and contracting cardiocytes
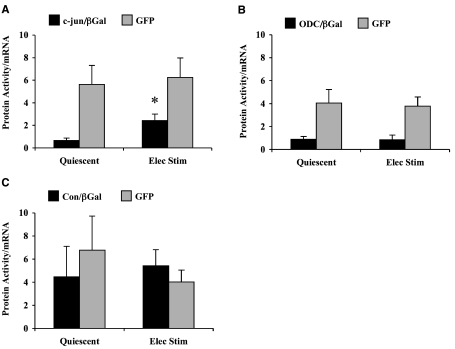
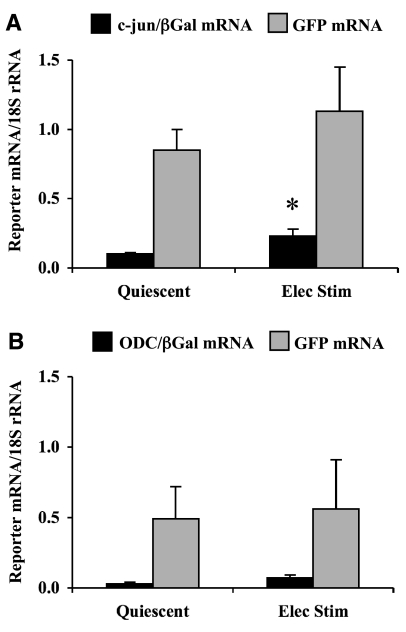
To examine the role of the 5′-UTR in regulating gene expression, direct measurements of translational efficiencies of reporter mRNAs were compared in quiescent and contracting cardiocytes (Fig. 3). Translational efficiency of c-jun/βGal mRNA was low in quiescent cardiocytes, but increased significantly by electrically stimulated contraction (Fig. 3A). Thus, a contraction-induced increase in the general rate of initiation was sufficient to enhance translation of the c-jun/βGal mRNA reporter. By comparison, translational efficiency of GFP mRNA was higher than c-jun/βGal mRNA in quiescent cardiocytes, and no further increase occurred in contracting cardiocytes. These results confirmed that GFP mRNA functions as a strong reporter, thus, translational efficiency was near its maximum in quiescent cardiocytes.
Figure 3.
Translational efficiency of reporter mRNAs in quiescent and contracting cardiocytes. A) Translational efficiencies of c-jun/βGal mRNA and GFP mRNA. Values are means ± se, n = 8 experiments. *P < 0.05 vs. quiescent cardiocytes; Student’s t test. B) Translational efficiencies of ODC/βGal mRNA and GFP mRNA. Values are means ± se, n = 5 experiments. C) Translational efficiencies of Con/βGal mRNA and GFP mRNA. Values are means ± se, n = 4 experiments.
Figure 3B shows the results derived from the ODC/βGal adenovirus. In these experiments, the mean increases in ODC/βGal mRNA levels were 3.8 ± 0.6 and 6.0 ± 1.2 in quiescent and contracting cardiocytes, respectively (mean±se, n=5). Similar to c-jun/βGal mRNA, translational efficiency of ODC/βGal mRNA was low in quiescent cardiocytes. However, contraction had no effect on translational efficiency of ODC/βGal mRNA. Translational efficiency of GFP mRNA remained strong and was not significantly different in quiescent vs. contracting cardiocytes. These data show that the 5′-UTR of ODC mRNA was not sufficient to enhance translation in contracting cardiocytes.
In Fig. 3C, the Con/βGal mRNA was used as a control for strong mRNAs that have a minimal amount of secondary structure in the 5′-UTR. As compared to c-jun/βGal and ODC/βGal, translational efficiency was higher in both quiescent and contracting cardiocytes. These results were not due to differences in expression by the adenovirus because Con/βGal mRNA levels increased by 2.9 ± 0.7 and 3.5 ± 0.9 in quiescent and contracting cardiocytes, respectively (mean±se, n=4). The strength of Con/βGal mRNA is supported further by the fact that translational efficiency was comparable to GFP mRNA in both quiescent and contracting cardiocytes.
It has been demonstrated that the α1-adrenergic agonist phenylephrine (PE) directly stimulates growth of adult rat cardiocytes in vitro (27). To determine whether an alternative growth stimulus affected translational efficiency of reporter mRNAs, quiescent cardiocytes were infected with increasing titers of adenovirus and then maintained in the presence or absence of 10 μM PE for 48 h. The cardiocytes did not contract in response to PE treatment. PE produced small increases in translational efficiency of each reporter mRNA relative to nontreated controls, but these changes did not achieve statistical significance (Supplemental Data). Thus, in contrast to the mechanical stimulus of contraction, translational efficiency of c-jun mRNA reporter was not enhanced by PE relative to strong mRNAs such as Con/βGal or GFP.
Functional distribution of reporter mRNAs
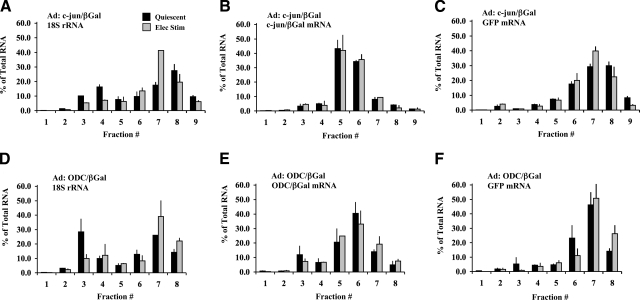
Translational efficiency can be enhanced by two basic mechanisms: increasing the rate of initiation on mRNAs that are already active in translation, and mobilizing more mRNA from the inactive (free) pool and incorporating it into polysomes. To determine the effect on initiation rate, the distribution of reporter mRNAs was measured in individual monosome (free ribosomes and mRNPs) and polysome fractions isolated on linear sucrose gradients (Supplemental Data). In Fig. 4, the distribution of βGal mRNA, GFP mRNA, and 18S rRNA is plotted for cardiocytes infected with either c-jun/βGal (Fig. 4A–C) or ODC/βGal (Fig. 4D–F). A shift of mRNA into larger polysome fractions is indicative of a higher rate of translational initiation, that is, a greater number of ribosomes loaded onto the reporter mRNA.
Figure 4.
Functional distribution of 18S rRNA and reporter mRNAs in quiescent and contracting cardiocytes. Cardiocytes were infected with c-jun/βGal (A–C) or ODC/βGal adenovirus (D–F). Cardiocytes were electrically stimulated to contract at 1 Hz for 24 h or maintained as quiescent controls. Amount of 18S rRNA, βGal mRNA, and GFP mRNA in individual monosome and polysome fractions was quantified by real-time RT-PCR and expressed as percentage of total amount of each RNA on the gradient. Values are averages of 2 experiments each. Bars show range of the two experiments.
Figure 4A, D compares the distribution of ribosomes by plotting the percentage of 18S rRNA in each gradient fraction. Approximately 60–70% of 18S rRNA in quiescent cardiocytes was recovered in polysome fractions, which is indicative of 40S ribosomes active in translation. The general rate of translational initiation increased in contracting cardiocytes, as demonstrated by a shift of 18S rRNA from monosomes into polysomes (80–90%). The paucity of free 40S ribosomes in contracting cardiocytes indicates that efficiency of total protein synthesis was close to maximum.
The distribution of c-jun/βGal and ODC/βGal reporter mRNAs is compared in Fig. 4B, E. The highest percentages of both mRNAs were located in the smaller polysome fractions (fractions 5 and 6), which contain mRNAs with <5 ribosomes. Given that the ORF for βGal mRNA is 3.1 kb in length, the ribosome density was relatively low. These data further demonstrate that electrically stimulated contraction did not shift βGal mRNA into larger polysomes. Thus, the contraction-induced increase in translational efficiency of c-jun/βGal mRNA was not caused by an increase in the rate of initiation.
GFP mRNA was measured as a reporter for a strong mRNA expressed in high abundance. Figure 4C shows that the larger polysome fractions (fractions 7 and 8) contained the highest percentages of GFP mRNA. Essentially the same results were obtained for GFP mRNA expressed by the ODC/βGal adenovirus (Fig. 4F). The large polysome fractions contain mRNAs with >5 ribosomes. Given that the coding sequence of GFP mRNA is 0.7 kb in length, the average ribosome density was substantially higher than βGal mRNA. These differences in ribosome density indicate that the intrinsic rate of initiation of GFP mRNA was higher than βGal mRNAs and therefore more efficient with respect to translation. Electrically stimulated contraction did not shift GFP mRNA into larger polysomes compared to quiescent cardiocytes, which demonstrates that the initiation rate of GFP mRNA remained constant.
Mobilization of reporter mRNA into polysomes
Given that contraction did not affect the initiation rate of c-jun/βGal mRNA, we examined whether translational efficiency was increased by mobilizing more c-jun/βGal mRNA into polysomes. Mobilization was assayed by pooling polysome fractions and measuring the levels of βGal mRNA and GFP mRNA normalized to 18S rRNA. Figure 5 shows that contraction significantly increased c-jun/βGal mRNA in polysomes by 2.3-fold over quiescent cardiocytes. The mobilization of c-jun/βGal mRNA was comparable to the increase in translational efficiency (compare to Fig. 3A). In contrast, the amount of GFP mRNA in polysomes was higher than c-jun/βGal mRNA, and this difference correlated with the higher levels of GFP mRNA expression produced by the adenovirus. Contraction did not mobilize more GFP mRNA into polysomes, a result that further supports the conclusion that GFP mRNA was strong with respect to translational efficiency.
Figure 5.
Comparative effects of 5′-UTR on mobilization of reporter mRNA into polysomes. Cardiocytes were infected with c-jun/βGal (A) or ODC/βGal adenovirus (B). Cardiocytes were stimulated electrically to contract at 1 Hz for 24 h or maintained as quiescent controls. Polysome fractions were pooled, and amount of 18S rRNA, βGal mRNA, and GFP mRNA was quantified by real-time RT-PCR. Values are means ± se, n = 5 experiments. *P < 0.05 vs. quiescent cardiocytes; Student’s t test.
The mobilization of reporter mRNAs derived from ODC/βGal adenovirus is compared in Fig. 5B. These data demonstrate that ODC/βGal mRNA was low in polysomes derived from quiescent cardiocytes and that electrically stimulated contraction did not increase mobilization. This result was expected given that contraction did not have a significant effect on translational efficiency of ODC/βGal mRNA (compare to Fig. 3B). GFP mRNA in polysomes was higher than ODC/βGal mRNA, but no difference was found between quiescent and contracting cardiocytes. These data confirm that the 5′-UTR of ODC mRNA was not sufficient to increase mobilization of the reporter mRNA into polysomes.
DISCUSSION
As in other cell types, a large proportion of mRNAs in the terminally differentiated adult cardiocyte can be classified as strong because a high intrinsic rate of initiation enables them to compete effectively for 43S preinitiation complexes (18). Thus, total protein synthesis is exceedingly efficient in the cardiocyte, both at steady state and during periods of hypertrophic growth (1). The structural basis of strong mRNAs is a relatively short, unstructured 5′-UTR that is hypothesized to optimize binding of the ribosome to the 5′-end. In the cardiocyte, highly abundant mRNAs that code for myofibrillar proteins such as β-myosin heavy chain and α-cardiac actin have a 5′-UTR that fits the profile of a strong mRNA. Consequently, synthesis of the corresponding proteins are accelerated during cardiac growth by increasing the general rate of initiation (8, 22). Selective changes in gene expression of strong mRNAs are regulated largely through mechanisms that alter relative abundance, for example, transcriptional control of α and β myosin heavy chain isoform expression during cardiac growth and development (28). GFP was taken to represent a strong mRNA that maintains efficient translation constitutively, as such, GFP expression rose as a function of mRNA levels in both quiescent and contracting cardiocytes. Accordingly, GFP mRNA was localized mainly in larger polysome fractions, which confirms a high rate of translational initiation (29).
By directly measuring translational efficiency of reporter mRNAs, these studies demonstrate that the 5′-UTR of c-jun mRNA is involved in selective regulation of gene expression in the adult cardiocyte. This finding supports the hypothesis that an increase in the general rate of peptide chain initiation, which is responsible for accelerating the rate of total protein synthesis in contracting cardiocytes, exerts a selective effect on translational efficiency of weak mRNAs (6, 18). The bases for selectivity are the intrinsic initiation rates of weak vs. strong mRNAs and the corresponding ability to compete for 43S preinitiation complexes. Thus, translational efficiency of c-jun/βGal mRNA was enhanced in response to contraction because greater availability of 43S preinitiation complexes facilitates mobilization of more c-jun/βGal mRNA into polysomes. In the case of strong mRNAs such as Con/βGal and GFP, the pool of available 43S preinitiation complexes was sufficient for efficient translation in quiescent and contracting cardiocytes.
ODC controls the initial step in polyamine biosynthesis by catalyzing formation of putrescine from ornithine. Although polyamines perform a wide range of functions in regulating normal and neoplastic growth, our primary rationale for examining ODC mRNA in cardiocytes is the 5′-UTR contains several elements that control its translational efficiency (30). In the proximal half of ODC mRNA, the 5′-UTR is folded optimally into stable hairpin loops with a uORF positioned behind it. These elements function by inhibiting cap-dependent translation (31). The ability to enhance ODC mRNA translation probably involves mechanisms activated by the cell cycle, which in the terminally differentiated adult cardiocyte may not be utilized in response to a mechanical stimulus such as contraction. Thus, it is not surprising that translational efficiency of ODC/βGal mRNA remained low in both quiescent and contracting cardiocytes. It has been demonstrated that the 5′-UTR of ODC mRNA has an internal ribosome entry site (IRES) located in close proximity to the AUG start codon. The functional relevance of the IRES in the adult cardiocyte is questionable, because cap-independent translation of ODC mRNA has been demonstrated only in dividing cells.
Contraction was sufficient to enhance translation of c-jun mRNA despite the fact that its 5′-UTR is longer than ODC, and the amount of secondary structure is much greater. Optimal folding of the c-jun 5′-UTR indicates that base pairing is most stable in the region between nt 300 and 700. Consequently, c-jun/βGal mRNA could compete more effectively for ribosome binding than ODC/βGal mRNA, as the latter contains several hairpins near the 5′-end. Once the preinitiation complex is assembled, neither length nor overall amount of secondary structure would necessarily prevent linear scanning along the c-jun 5′-UTR (15).
Translational efficiency of c-jun/βGal mRNA was enhanced in contracting cardiocytes by a mechanism that increased mobilization into polysomes. The intrinsic rate of initiation was not affected, because polysome distribution of c-jun/βGal mRNA was the same in quiescent and contracting cardiocytes. Similar results were obtained following induction of endogenous c-jun mRNA expression in adult cardiocytes by treatment with phorbol ester; that is, substantial flux of c-jun mRNA occurred from the monosome pool into polysomes without a shift in polysome distribution (26). In contrast, contraction had no effect on the mobilization of ODC/βGal mRNA into polysomes or its rate of initiation, as revealed by shifts in polysome distribution. Studies in other cell types have shown that increased activity of eIF4E facilitates translation of ODC mRNA, probably by alleviating the inhibitory effects of secondary structure in the 5′-UTR (18, 30). Given that eIF4E phosphorylation and eIF4E expression are increased in contracting cardiocytes, these data suggest that changes in eIF4E activity were not sufficient to regulate translation of ODC/βGal mRNA (21, 32). Nevertheless, we cannot rule out the possibility that mobilization of ODC/βGal mRNA into polysomes was impaired in contracting cardiocytes by the absence of a key sequence element that is required for regulating initiation of ODC mRNA. An alternative possibility is that an increase in rate of peptide chain initiation produced in contracting cardiocytes was not of sufficient magnitude to facilitate mobilization of ODC/βGal mRNA into polysomes.
Many signaling pathways involved in cardiac growth target effectors that regulate the rate of peptide chain initiation, particularly eIF2/eIF2B and the eIF4F complex (33, 34). Phosphorylation of the ε subunit of eIF2B by glycogen synthase kinase 3β (GSK-3β) inhibits guanine nucleotide exchange activity, which is required for reactivation of the ternary complex [eIF2·Met-tRNAi·GTP]. Cardiac-specific overexpression of GSK-3β inhibits physiological growth in mice, while overexpression of a dominant-negative GSK-3β mutant is sufficient to induce compensatory hypertrophy (35, 36). The effects of GSK-3β have been attributed directly to changes in the rate of peptide chain initiation caused by modifying eIF2B activity, although GSK-3β activates many other effectors, including transcription factors (33). Assuming that loss of GSK-3β function increased the general rate of peptide chain initiation, the rate of total protein synthesis would be accelerated because the total mRNA pool consists mainly of strong mRNAs. Furthermore, loss in GSK-3β activity could lead also to selective increases in translational efficiency of weak mRNAs that code for proteins integral to cardiac growth.
It is hypothesized that increasing the activity of eIF4F facilitates translational initiation of mRNAs that have excessive secondary structure in their corresponding 5′-UTRs (18, 37). eIF4F activity is regulated by increasing the amount of eIF4E that is available for assembly into active eIF4F complexes or by changing the cap binding affinity of eIF4E through phosphorylation (5). In previous work, we demonstrated that eIF4F complex formation and eIF4E phosphorylation were increased in response to contraction (38, 39). However, the following results from our more recent studies suggest that eIF4F may not be responsible for the contraction-induced increase in translational efficiency of the c-jun/βGal reporter. First, overexpression of eIF4E had no effect on the flux of endogenous c-jun mRNA into polysomes. Second, translation of endogenous c-jun mRNA in cardiocytes was not affected by overexpression of either the eIF4E kinase Mnk1 to increase eIF4E phosphorylation or an eIF4E mutant in which phosphorylation of Ser-209 was blocked (26).
Supplemental Table 2 underscores that a substantial number of transcription factors required for cardiac hypertrophy are translated from mRNAs that contain a 5′-UTR characteristic of weak mRNAs. Translational efficiency of weak mRNAs is constitutively lower than that of the mRNA population as a whole; consequently, synthesis of the corresponding proteins is tightly controlled and limited to periods of growth. During pressure overload hypertrophy, the general rate of peptide initiation is accelerated by mechanisms that increase both the activity and amount of translation machinery (4). Contraction produces similar effects on the translation machinery in the adult cardiocyte model (20,21,22,23). As a result, translational efficiency of weak mRNAs can be enhanced in contracting cardiocytes by mobilization into polysomes. A parallel can be drawn with translational regulation of maternal mRNAs that occurs during early embryogenesis in Drosophila (40). In this model system, distinct functional classes of mRNA are recruited into polysomes via increased mobilization as measured by ribosome occupancy, rather than an increased initiation rate as reflected by ribosome density of individual mRNAs. Unique mechanisms also exist to regulate translation of specific mRNAs in the cardiocyte. For example, miRNAs have been shown to regulate cardiac gene expression by binding to specific sequence elements that can be located in any region along the mRNA molecule (41). It has been demonstrated that miRNAs can inhibit translation by blocking the initiation step of translation (42, 43). This raises the intriguing possibility that miRNAs target specific mRNAs for translational repression by lowering the intrinsic rate of initiation, thereby coupling expression to changes in the rate of myocardial protein synthesis. Regardless of the determinants of intrinsic initiation rates, these studies indicate that changes in the general rate of peptide chain initiation can underlie selective translation of specific mRNAs in the cardiocyte.
Supplementary Material
Acknowledgments
We thank Daisy Dominick, Shaun Wahl, Harinath Kasiganesan, and Gina Keller for their excellent technical assistance. This work was supported by the National Institutes of Health (grant PO1 HL-48788) and by the Research and Development Service, Department of Veterans Affairs (Merit Review Award).
References
- Morgan H E, Beinlich C J. Contributions of increased efficiency and capacity of protein synthesis to rapid cardiac growth. Mol Cell Biochem. 1997;176:145–151. [PubMed] [Google Scholar]
- Lindsey M L, Goshorn D K, Comte-Walters S, Hendrick J W, Hapke E, Zile M R, Schey K. A multidimensional proteomic approach to identify hypertrophy-associated proteins. Proteomics. 2006;6:2225–2235. doi: 10.1002/pmic.200500013. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Phenotypic plasticity of adult myocardium: molecular mechanisms. J Exp Biol. 2006;209:2320–2327. doi: 10.1242/jeb.02084. [DOI] [PubMed] [Google Scholar]
- Hannan R D, Jenkins A, Jenkins A K, Brandenburger Y. Cardiac hypertrophy: a matter of translation. Clin Exp Pharmacol Physiol. 2003;30:517–527. doi: 10.1046/j.1440-1681.2003.03873.x. [DOI] [PubMed] [Google Scholar]
- Gebauer F, Hentze M W. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol. 2004;5:827–835. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Wiesner R J, Ehmke H, Faulhaber J, Zak R, Ruegg J C. Dissociation of left ventricular hypertrophy, beta-myosin heavy chain gene expression, and myosin isoform switch in rats after ascending aortic stenosis. Circulation. 1997;95:1253–1259. doi: 10.1161/01.cir.95.5.1253. [DOI] [PubMed] [Google Scholar]
- Nagatomo Y, Carabello B A, Hamawaki M, Nemoto S, Matsuo T, McDermott P J. Translational mechanisms accelerate the rate of protein synthesis during canine pressure-overload hypertrophy. Am J Physiol. 1999;277:H2176–2184. doi: 10.1152/ajpheart.1999.277.6.H2176. [DOI] [PubMed] [Google Scholar]
- Heineke J, Molkentin J D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- Colomer J M, Mao L, Rockman H A, Means A R. Pressure overload selectively up-regulates Ca2+/calmodulin-dependent protein kinase II in vivo. Mol Endocrinol. 2003;17:183–192. doi: 10.1210/me.2002-0350. [DOI] [PubMed] [Google Scholar]
- Frey N, Olson E N. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- Xu X, Yang D, Ding J H, Wang W, Chu P H, Dalton N D, Wang H Y, Bermingham J R, Jr, Ye Z, Liu F, Rosenfeld M G, Manley J L, Ross J, Jr, Chen J, Xiao R P, Cheng H, Fu X D. ASF/SF2-regulated CaMKIIdelta alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell. 2005;120:59–72. doi: 10.1016/j.cell.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Misquitta C M, Iyer V R, Werstiuk E S, Grover A K. The role of 3′-untranslated region (3′-UTR) mediated mRNA stability in cardiovascular pathophysiology. Mol Cell Biochem. 2001;224:53–67. doi: 10.1023/a:1011982932645. [DOI] [PubMed] [Google Scholar]
- Pesole G, Mignone F, Gissi C, Grillo G, Licciulli F, Liuni S. Structural and functional features of eukaryotic mRNA untranslated regions. Gene. 2001;276:73–81. doi: 10.1016/s0378-1119(01)00674-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. Regulation of translation via mRNA structure in prokaryotes and eukaryotes. Gene. 2005;361:13–37. doi: 10.1016/j.gene.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Davuluri R V, Suzuki Y, Sugano S, Zhang M Q. CART classification of human 5′ UTR sequences. Genome Res. 2000;10:1807–1816. doi: 10.1101/gr.gr-1460r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucl Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A, Graff J R. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–3199. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- Tuxworth W J, Jr, Saghir A N, Spruill L S, Menick D R, McDermott P J. Regulation of protein synthesis by eIF4E phosphorylation in adult cardiocytes: the consequence of secondary structure in the 5′-untranslated region of mRNA. Biochem J. 2004;378:73–82. doi: 10.1042/BJ20031027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivester C T, Kent R L, Tagawa H, Tsutsui H, Imamura T, Cooper G, I V, McDermott P J. Electrically stimulated contraction accelerates protein synthesis rates in adult feline cardiocytes. Am J Physiol. 1993;265:H666–674. doi: 10.1152/ajpheart.1993.265.2.H666. [DOI] [PubMed] [Google Scholar]
- Wada H, Zile M R, Ivester C T, Cooper G, I V, McDermott P J. Comparative effects of contraction and angiotensin II on growth of adult feline cardiocytes in primary culture. Am J Physiol. 1996;271:H29–37. doi: 10.1152/ajpheart.1996.271.1.H29. [DOI] [PubMed] [Google Scholar]
- Ivester C T, Tuxworth W J, Cooper G, I V, McDermott P J. Contraction accelerates myosin heavy chain synthesis rates in adult cardiocytes by an increase in the rate of translational initiation. J Biol Chem. 1995;270:21950–21957. doi: 10.1074/jbc.270.37.21950. [DOI] [PubMed] [Google Scholar]
- Saghir A N, Tuxworth W J, Jr, Hagedorn C H, McDermott P J. Modifications of eukaryotic initiation factor 4F (eIF4F) in adult cardiocytes by adenoviral gene transfer: differential effects on eIF4F activity and total protein synthesis rates. Biochem J. 2001;356:557–566. doi: 10.1042/0264-6021:3560557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Hagen T P, Dawson M L, Barnes K V, Menick D R. The role of GATA, CArG, E-box, and a novel element in the regulation of cardiac expression of the Na+-Ca2+ exchanger gene. J Biol Chem. 1999;274:12819–12826. doi: 10.1074/jbc.274.18.12819. [DOI] [PubMed] [Google Scholar]
- Kato S, Ivester C T, Cooper G, I V, Zile M R, McDermott P J. Growth effects of electrically stimulated contraction on adult feline cardiocytes in primary culture. Am J Physiol. 1995;268:H2495–2504. doi: 10.1152/ajpheart.1995.268.6.H2495. [DOI] [PubMed] [Google Scholar]
- Spruill L S, McDermott P J. Regulation of c-jun mRNA expression in adult cardiocytes by MAP kinase interacting kinase-1 (MNK1) FASEB J. 2006;20:E1465–E1475. doi: 10.1096/fj.06-6245fje. [DOI] [PubMed] [Google Scholar]
- Schluter K D, Schafer M, Balser C, Taimor G, Piper H M. Influence of pHi and creatine phosphate on alpha-adrenoceptor-mediated cardiac hypertrophy. J Mol Cell Cardiol. 1998;30:763–771. doi: 10.1006/jmcc.1998.0640. [DOI] [PubMed] [Google Scholar]
- Gupta M P. Factors controlling cardiac myosin-isoform shift during hypertrophy and heart failure. J Mol Cell Cardiol. 2007;43:388–403. doi: 10.1016/j.yjmcc.2007.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M B, Sonenberg N, Hershey J W B. Origins and principles of translational control. Sonenberg N, Hershey J W B, Mathews M B, editors. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; Translational Control of Gene Expression. 2000:1–32. [Google Scholar]
- Pegg A E. Regulation of ornithine decarboxylase. J Biol Chem. 2006;281:14529–14532. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- Pyronnet S, Pradayrol L, Sonenberg N. Alternative splicing facilitates internal ribosome entry on the ornithine decarboxylase mRNA. Cell Mol Life Sci. 2005;62:1267–1274. doi: 10.1007/s00018-005-5020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhlouf A A, McDermott P J. Increased expression of eukaryotic initiation factor 4E during growth of neonatal rat cardiocytes in vitro. Am J Physiol. 1998;274:H2133–2142. doi: 10.1152/ajpheart.1998.274.6.H2133. [DOI] [PubMed] [Google Scholar]
- Hardt S E, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90:1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- Ruggero D, Sonenberg N. The Akt of translational control. Oncogene. 2005;24:7426–7434. doi: 10.1038/sj.onc.1209098. [DOI] [PubMed] [Google Scholar]
- Michael A, Haq S, Chen X, Hsich E, Cui L, Walters B, Shao Z, Bhattacharya K, Kilter H, Huggins G, Andreucci M, Periasamy M, Solomon R N, Liao R, Patten R, Molkentin J D, Force T. Glycogen synthase kinase-3beta regulates growth, calcium homeostasis, and diastolic function in the heart. J Biol Chem. 2004;279:21383–21393. doi: 10.1074/jbc.M401413200. [DOI] [PubMed] [Google Scholar]
- Hirotani S, Zhai P, Tomita H, Galeotti J, Marquez J P, Gao S, Hong C, Yatani A, Avila J, Sadoshima J. Inhibition of glycogen synthase kinase 3beta during heart failure is protective. Circ Res. 2007;101:1164–1174. doi: 10.1161/CIRCRESAHA.107.160614. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, Martineau Y, Sato T A, Larsson O, Rajasekhar V K, Sonenberg N. Epigenetic activation of a subset of mRNAs by eIF4E explains its effects on cell proliferation. PLoS ONE. 2007;2:e242. doi: 10.1371/journal.pone.0000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxworth W J, Jr, Wada H, Ishibashi Y, McDermott P J. Role of load in regulating eIF-4F complex formation in adult feline cardiocytes. Am J Physiol. 1999;277:H1273–1282. doi: 10.1152/ajpheart.1999.277.4.H1273. [DOI] [PubMed] [Google Scholar]
- Wada H, Ivester C T, Carabello B A, Cooper G, I V, McDermott P J. Translational initiation factor eIF-4E. A link between cardiac load and protein synthesis. J Biol Chem. 1996;271:8359–8364. doi: 10.1074/jbc.271.14.8359. [DOI] [PubMed] [Google Scholar]
- Qin X, Ahn S, Speed T P, Rubin G M. Global analyses of mRNA translational control during early Drosophila embryogenesis. Genome Biol. 2007;8:R63. doi: 10.1186/gb-2007-8-4-r63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooij E, Olson E N. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–2376. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathonnet G, Fabian M R, Svitkin Y V, Parsyan A, Huck L, Murata T, Biffo S, Merrick W C, Darzynkiewicz E, Pillai R S, Filipowicz W, Duchaine T F, Sonenberg N. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science. 2007;317:1764–1767. doi: 10.1126/science.1146067. [DOI] [PubMed] [Google Scholar]
- Standart N, Jackson R J. MicroRNAs repress translation of m7Gppp-capped target mRNAs in vitro by inhibiting initiation and promoting deadenylation. Genes Dev. 2007;21:1975–1982. doi: 10.1101/gad.1591507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.