Abstract
Ligands of peroxisome proliferator-activated receptor-γ (PPAR-γ) abrogate the stimulation of collagen gene transcription induced by transforming growth factor-beta (TGF-β). Here, we delineate the mechanisms underlying this important novel physiological function for PPAR-γ in connective tissue homeostasis. First, we demonstrated that antagonistic regulation of TGF-β activity by PPAR-γ ligands involves cellular PPAR-γ, since 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) failed to block TGF-β-induced responses in either primary cultures of PPAR-γ-null murine embryonic fibroblasts, or in normal human skin fibroblasts with RNAi-mediated knockdown of PPAR-γ. Next, we examined the molecular basis underlying the abrogation of TGF-β signaling by PPAR-γ in normal human fibroblasts in culture. The results demonstrated that Smad-dependent transcriptional responses were blocked by PPAR-γ without preventing Smad2/3 activation. In contrast, the interaction between activated Smad2/3 and the transcriptional coactivator and histone acetyltransferase p300 induced by TGF-β, and the accumulation of p300 on consensus Smad-binding DNA sequences and histone H4 hyperacetylation at the COL1A2 locus, were all prevented by PPAR-γ. Wild-type p300, but not a mutant form of p300 lacking functional histone acetyltransferase, was able to restore TGF-β-induced stimulation of COL1A2 in the presence of PPAR-γ ligands. Collectively, these results indicate that PPAR-γ blocked Smad-mediated transcriptional responses by preventing p300 recruitment and histone H4 hyperacetylation, resulting in the inhibition of TGF-β-induced collagen gene expression. Pharmacological activation of PPAR-γ thus may represent a novel therapeutic approach to target p300-dependent TGF-β profibrotic responses such as stimulation of collagen gene expression.—Ghosh, A. K., Bhattacharyya, S., Wei, J., Kim, S., Barak, Y., Mori, Y., and Varga, J. Peroxisome proliferator-activated receptor-γ abrogates Smad-dependent collagen stimulation by targeting the p300 transcriptional coactivator.
Keywords: fibrosis, 15d-PGJ2, type I collagen, fibroblast, acetyltransferase
The synthesis of collagen is normally tightly regulated, and excessive accumulation of collagen results in fibrosis (1). Multiple intracellular signaling pathways, transcription factors, and cofactors have been implicated as playing important roles in the regulation of collagen gene expression (2, 3). The multifunctional cytokine transforming growth factor-β (TGF-β) stimulates collagen synthesis and plays a key role in the pathogenesis of scleroderma and related fibrotic disorders (4). Cellular responses induced by TGF-β are mediated intracellularly primarily via the canonical Smad pathway. Upon phosphorylation by the activated type I TGF-β receptor (TβRI), cytoplasmic Smad2 and Smad3 heterodimerize with Smad4 and accumulate within the nucleus, where they recruit cofactors to Smad-binding element (SBE) DNA sequences and activate target gene transcription (5). We showed previously that stimulation of COL1A2 transcription by TGF-β required Smad3 (6, 7), as well as the interaction of activated Smad2/3 with the transcriptional coactivator and histone acetyltransferase p300 (8,9,10). Overexpression of E1A, an inhibitor of p300 function, prevented the stimulation of collagen gene expression by TGF-β (8). In addition to its critical role in mediating profibrotic TGF-β responses, p300 also integrates converging signaling pathways that positively or negatively modulate the expression of collagen genes (11).
The peroxisome proliferator-activated receptors such as PPAR-γ form a family of nuclear hormone receptors that play critical roles in adipogenesis and the regulation of glucose and lipid metabolism. Pathological alterations in PPAR-γ expression or function are implicated in diabetes, obesity, and the metabolic syndrome (12). Recent studies have begun to uncover additional important roles for PPAR-γ in the regulation of inflammation, connective tissue remodeling, and in pathological processes such as glomerulosclerosis, atherosclerosis, cancer, and arthritis (12,13,14,15,16,17,18,19). The biological activity of PPAR-γ is triggered upon its activation by natural ligands, such as 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), or by synthetic agonists such as rosiglitazone. Activated PPAR-γ acts as an inducible transcription factor that can stimulate, or in some cases, repress, transcription of PPAR-γ target genes (20). Transactivation-mediated by PPAR-γ is generally DNA-dependent, whereas transrepression is DNA independent (21). The full activity of PPAR-γ requires interaction with coactivators such as p300 (22, 23). Indeed, PPAR-γ-driven adipogenic differentiation is completely suppressed in progenitor cells lacking p300 (24).
We recently demonstrated that the PPAR-γ ligands 15d-PGJ2 and troglitazone, as well as transient ectopic expression of PPAR-γ in normal skin fibroblasts, abrogated TGF-β-induced stimulation of collagen synthesis (25). Furthermore, the inhibitory effect could be blocked by pretreatment of cells with a selective PPAR-γ antagonist (25). The molecular mechanisms underlying these important anti-TGF-β activities elicited by PPAR-γ ligands are currently not known. Accordingly, we have undertaken mechanistic studies in normal skin fibroblasts to investigate these mechanisms. We now report that either the transient expression of ectopic PPAR-γ, or ligand-induced activation of endogenous PPAR-γ, suppressed Smad3-dependent transcriptional responses without blocking Smad activation. We further show that TGF-β-induced interaction of p300 with Smad3, as well as recruitment of p300 to the DNA-bound transcriptional complex, and p300-mediated histone H4 hyperacetylation at the COL1A2 locus, were all abrogated. Moreover, forced expression of ectopic p300 rescued the stimulatory TGF-β response in the presence of PPAR-γ ligands. Together, these results delineate a novel mechanism for the anti-TGF-β activities of PPAR-γ, and identify p300 coactivator as an important molecular target.
MATERIALS AND METHODS
Reagents
Transforming growth factor-β2 (Genzyme, Farmingham, MA, USA), troglitazone and 15d-PGJ2 (both from Biomol, Plymouth Meeting, PA, USA), and SB431542 (Glaxo, King of Prussia, PA, USA) were used. Troglitazone and 15d-PGJ2 were used at a concentration of 10 μM unless otherwise indicated.
Cell cultures
Primary cultures of neonatal foreskin fibroblasts were established as described previously (6) and maintained in Eagle’s modified essential medium (EMEM; Biowhittaker, Walkersville, MD, USA) supplemented with 10% FBS, 1% vitamins, 1% penicillin/streptomycin, and 2 mM l-glutamine. Fibroblasts were studied between passages 3 and 7. Cell viability was determined by Trypan blue dye exclusion, and toxicity was measured using EZ4U kit (Alpco Diagnostic, Salem, NH, USA). Murine embryonic fibroblasts (MEFs) null for PPAR-γ (PPAR-γ−/−) and wild-type MEFs were established at embryonic day 13.5 from embryos homozygous for a loxP-flanked PPAR-γ allele (26) and carrying an epiblast-specific Sox2-Cre transgene (27), or matching wild-type controls, respectively. This allelic configuration drives >95% deletion of PPAR-γ in all embryonic tissues outside the placenta (unpublished data). This novel targeting strategy makes it possible to establish PPAR-γ-null MEF donors that survive the placental lethality of standard PPAR-γ-null embryos at E9.5 (28). Primary cultures of PPAR-γ null MEFs were maintained for up to 5 passages in vitro in Dulbecco’s modified Eagle’s medium (DMEM; Sigma Chemicals, St. Louis, MO, USA) supplemented with 10% FBS, 1% vitamins, 1% penicillin/ streptomycin, and 2 mM l-glutamine.
Plasmids
The 772COL1A2/CAT reporter construct contains the −772 to +58 bp fragment of the human COL1A2 gene in front of CAT gene (29). The expression vector pCMX-mPPAR-γ contains the full-length mouse PPAR-γ cDNA in pCMX vector (13). The pEGFP-Smad3 expression vector contains the full-length human Smad3 cDNA in pEGFPN1 vector (30). The pCI-p300-FLAG and pCI p300ΔHAT-FLAG expression vectors contain wild-type p300 and a HAT-deleted mutant p300 cDNA, respectively, in pCI vector (31). To reduce the cellular levels of PPAR-γ, dermal fibroblasts were transfected with short-hairpin RNA (shRNA) or scrambled control plasmids (SuperArray, Frederick, MD, USA) or lentiviruses (Sigma).
Transient transfection assays
Fibroblasts were transiently transfected with expression vectors for Smad3, PPAR-γ, p300, or p300ΔHAT, along with 772COL1A2/CAT reporter construct and internal standard pRLTK-luc using Superfect reagent (Qiagen, Valencia, CA, USA). Transfected fibroblasts were pretreated with 15d-PGJ2 for 60 min before the addition of TGF-β (12.5 ng/ml). Following further 48-h incubation in medium containing 10% FBS, cultures were harvested, and whole-cell lysates were prepared and assayed for their CAT and luciferase activities (6). Results were normalized for protein concentration in each sample, and transfection efficiency was monitored. To reduce endogenous PPAR-γ, fibroblasts were transfected or infected with PPAR-γ-shRNA plasmids (SuperArray) or lentiviruses (Sigma), or scrambled control oligonucleotides. Twenty four hours following transfection, fresh medium containing 15d-PGJ2 was added to the cultures for 60 min, followed by TGF-β. After an additional 24 h incubation, cultures were harvested and whole-cell lysates, nuclear extracts, or conditioned medium were prepared and subjected to Western blot analysis or electrophoretic mobility shift assays. Efficacy and specificity of suppression by shRNA were evaluated by analysis of protein and RNA levels.
Western and coupled immunoprecipitation-immunoblot analysis
Equal amounts of cellular proteins or aliquots of conditioned culture medium were resolved by electrophoresis in 4–20% Tris-glycine gradient gels (Bio-Rad, Hercules, CA, USA). In other experiments, whole-cell lysates (∼200 μg) were immunoprecipitated with indicated antibodies and subjected to immunoblot analysis (10). Membranes were probed with antibodies to human type I collagen (Southern Biotechnology, Birmingham, AL, USA), PPAR-γ (E-8), Smad7 (N-19), Smad4 (B8), Smad1/2/3 (H2), actin (C-2), or p300 (C-20) (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA), Smad3 (Zymed Lab, San Francisco, CA, USA), phospho-Smad2 or phospho-Smad3 (Cell Signaling, Beverly, MA, USA).
Electrophoretic mobility shift assays
Confluent fibroblasts were preincubated with troglitazone or 15d-PGJ2, followed by TGF-β for 60 min. At the end of the experiments, nuclear extracts were prepared and subjected to electrophoretic mobility shift assays with radiolabeled oligonucleotides harboring the SBE (32) as probes. Antibody supershift assays were performed by preincubating nuclear extracts with antibodies to Smad3 (I-20) (Santa Cruz Biotechnology) or IgG (9).
DNA affinity precipitation assays
Accumulation of DNA binding factors and cofactors on the consensus SBE DNA sequences was determined by DNA affinity precipitation assays (10). Briefly, equal amounts of nuclear proteins (∼200 μg) were incubated with biotin-labeled double-stranded deoxyoligonucleotides (3 μg) harboring the SBE sequences (forward: 5′GGAGTATGT CTAGACTGACAATGTAC-3′; reverse: 5′-GTACATTG TCAGTCTAGAC ATACTCC-3′). At the end of a 30-min incubation, a solution of streptavidin-agarose beads (4%) with 50% slurry (Sigma) was added, and mixtures were incubated at 4°C for 45 min on a rotator platform. The streptavidin-agarose beads were precipitated by centrifugation and pellets were washed three times with cold PBS. Bead-bound proteins were resuspended in SDS-loading buffer, boiled, separated in 4–20% denaturing gels, and processed for Western blot analysis.
Chromatin immunoprecipitation (ChIP) assays
In vivo interactions of nuclear factors with the COL1A2 promoter DNA were studied by ChIP assays using the EZ Magna ChIP Assay Kit (Upstate/Millipore, Billerica, MA, USA) following the manufacturer’s instructions. Briefly, fibroblasts were preincubated with 15d-PGJ2 for 60 min (or in selected experiments for 23 h), followed by TGF-β2 (12.5 ng/ml) for 60 min. At the end of the incubation period, 1% formaldehyde was added to cross-link DNA-protein in chromatin, cell lysates were sonicated, and equal aliquots were used for immunoprecipitation with monoclonal antibodies to human Smad1/2/3, p300, and acetylated H4 histone (Upstate/Millipore), or with mouse IgG (Upstate/Millipore). PCR amplification of the captured DNA sequences was performed using primers complementary to COL1A2 sequences flanking the SBE (33), or GAPDH-specific primers. Amplified PCR products were analyzed by electrophoresis in 2% agarose gels. Real-time quantitative PCR of antibody-captured DNA was performed with human COL1A2-specific primers (forward: 5′-AAATTCTGCCCATGTCGGG-3′; reverse: 5′-AAACTCTGGCTCGTTGTCTGC-3) on ABI-Prism 7300 sequence detection PCR machine with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), according to manufacturer’s protocol.
Confocal immunofluorescence microscopy
Fibroblasts were preincubated on coverslips in EMEM with troglitazone or 15d-PGJ2 followed by TGF-β. At the end of the indicated periods, cells were fixed with acetic acid and methanol mixture, and incubated with antibodies to Smad1/2/3 (Santa Cruz) for 60 min. Following washing in PBS, slides were incubated with fluorescein-conjugated anti-mouse IgG (Santa Cruz) for 30 min, and immunofluorescence was evaluated by laser scanning confocal microscopy (9).
Statistical analysis
The data are presented as the means ± sd of multiple determinations. The significance of differences between experimental and control groups was determined by analysis of variance (ANOVA), and the value of P < 0.05 by Student’s t test was considered statistically significant. Student’s t tests were performed using the GraphPad t test calculator (GraphPad, San Diego, CA, USA).
RESULTS
Abrogation of TGF-β stimulation of collagen synthesis by PPAR-γ ligands is PPAR-γ dependent
Brief pretreatment of dermal fibroblasts with 15d-PGJ2 abrogated the stimulation of collagen synthesis and of COL1A2 promoter activity induced by TGF-β (Fig. 1). Ligands of PPAR-γ can elicit cellular responses by binding to and inducing the transcriptional activity of endogenous PPAR-γ, as well as through PPAR-γ-independent signaling pathways (16). Two complementary strategies were pursued to determine whether the inhibitory effect of 15d-PGJ2 on the stimulation of collagen synthesis occurred via PPAR-γ. First, the regulation of collagen gene expression was examined in cells lacking endogenous PPAR-γ. Primary PPAR-γ-null fibroblasts were derived by a novel genetic targeting strategy using midgestation mouse embryos, in which loxP-flanked PPAR-γ was deleted by the epiblast-specific Sox2-Cre in all nonplacental tissues. The MEFs used here are primary fibroblasts, procured directly from mouse embryos in which they have been rendered PPAR-γ-null at the source by germ-line genetic manipulations, and used at early passage. Thus, comparisons to control wild-type MEFs harvested in parallel from wild-type littermates are not complicated by stochastic genetic differences other than deficiency for PPAR-γ. The results shown in Fig. 2A indicated that while 15d-PGJ2 reduced both basal and the TGF-β-induced stimulation of collagen synthesis and secretion in wild-type MEFs (compare lanes 1 and 3, and 2 and 4), PPAR-γ null MEFs were largely refractory to the inhibitory effects of 15d-PGJ2 (compare lanes 5 and 7, and 6 and 8). Levels of both cellular and secreted type I collagen are significantly higher in PPAR-γ-null MEFs compared to levels in wild-type MEFs (compare lanes 8 and 4).
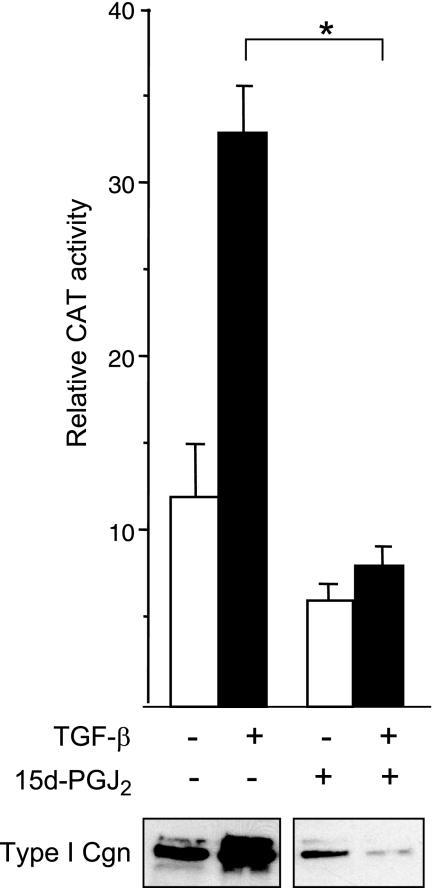
Figure 1.
15d-PGJ2 abrogates stimulation of collagen gene expression. Foreskin fibroblasts transiently transfected with 772COL1A2/CAT reporter construct were preincubated with 15d-PGJ2 (10 μM) for 60 min, followed by TGF-β2 (12.5 ng/ml) for a further 48 h. At the end of incubation, cell lysates were assayed for CAT and luciferase activities. Transfection efficiency was monitored by Renilla luciferase assays. Results shown represent means ± sd of triplicate determinations. Open bars denote untreated fibroblasts; solid bars denote TGF-β-treated fibroblasts. *P < 0.001. Bottom panel: culture supernatants were subjected to Western blot analysis using anti-type I collagen antibody.
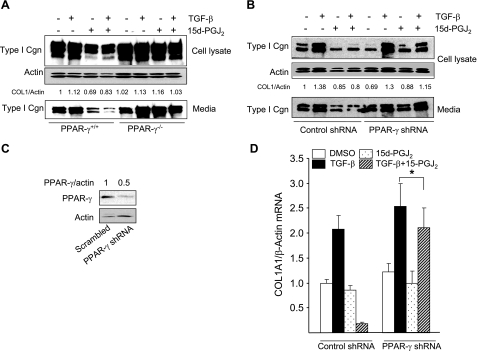
Figure 2.
15d-PGJ2 blocks stimulation of collagen gene expression via PPAR-γ. A) Primary cultures of wild-type (PPAR-γ+/+) and PPAR-γ null (PPAR-γ−/−) MEFs, generated as described in Materials and Methods, were pretreated with 15d-PGJ2 for 60 min, followed by TGF-β2 (12.5 ng/ml). Twenty-four hours later, cultures were harvested, and equal amounts of cell lysate proteins or aliquots of supernatants were subjected to Western blot analysis. B) Foreskin fibroblasts transfected with scrambled short-hairpin (sh) RNA or shRNA targeting mRNA sequences for PPAR-γ were incubated with TGF-β in the presence or absence of 15d-PGJ2 for 24 h. Cell lysates and conditioned medium were subjected to Western blot analysis. C) Foreskin fibroblasts were infected with scrambled shRNA or shRNA targeting PPAR-γ mRNA sequences and incubated for 24 h with TGF-β in the presence or absence of 15d-PGJ2. Whole-cell lysates were examined by Western blot analysis. Representative immunoblots are shown. D) Total RNA was subjected to qPCR analysis. β-Actin was used as an internal control. Results represent means ± sd of triplicate determinations. *P > 0.2.
The effect of 15d-PGJ2 on TGF-β stimulation of collagen synthesis was also examined in normal skin fibroblasts where endogenous PPAR-γ was suppressed by RNAi. To achieve PPAR-γ knockdown, confluent fibroblasts were transfected with shRNA constructs. A selective ∼50% reduction of PPAR-γ levels was consistently achieved (Fig. 2C and data not shown). In contrast to fibroblasts transfected with scrambled control shRNA (compare lanes 2 and 4), in fibroblasts transfected with PPAR-γ RNAi 15d-PGJ2 caused only a minimal suppression of collagen synthesis and secretion (Fig. 2B, compare lanes 6 and 8). Furthermore, 15d-PGJ2 similarly failed to block TGF-β-induced stimulation of COL1A1 mRNA expression in fibroblasts transfected with PPAR-γ shRNA (Fig. 2D). The effects of 15d-PGJ2 or troglitazone were not a reflection of altered fibroblast growth or viability (Table 1). Together, these results provide evidence that inhibition of TGF-β-stimulated increase in collagen gene expression by 15d-PGJ2 was due to bona fide ligand activation of endogenous PPAR-γ.
TABLE 1.
Fibroblast viability in the presence and absence of PPAR-γ ligands
| Treatment | Medium (%) | TGF-β (%) |
|---|---|---|
| DMSO | 97 | 99 |
| 15d-PGJ2 | 98 | 98 |
| Troglitazone | 96 | 98 |
PPAR-γ attenuates Smad-dependent transcriptional activity
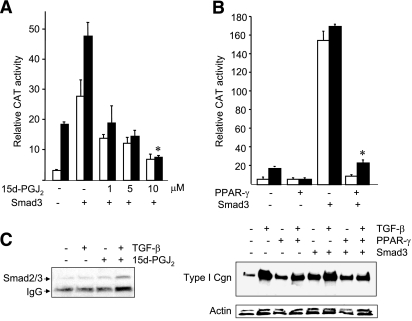
To explore the molecular mechanisms underlying the abrogation of TGF-β responses by 15d-PGJ2, we first focused on the canonical Smad signaling pathway. First, the regulation of Smad-dependent transcription by 15d-PGJ2 was examined in fibroblasts transfected with Smad3 expression vectors and 772COL1A2/ CAT. The results showed that while ectopic Smad3 induced an >6-fold increase in COL1A2 promoter activity, even in the absence of TGF-β, 15d-PGJ2 abrogated this response in a dose-dependent manner (Fig. 3A). Furthermore, enhanced Smad3 transactivation in the presence of TGF-β was also blocked by 15d-PGJ2. The high-affinity PPAR-γ ligand troglitazone exerted comparable inhibition of Smad3-mediated transactivation (data not shown). Expression of ectopic PPAR-γ in the fibroblasts was sufficient to mimic the effect of 15d-PGJ2 and abrogate the stimulation of COL1A2 promoter activity (Fig. 3B, top panel), and type I collagen synthesis (Fig. 3B, bottom panel) induced by Smad3. Taken together, these results indicate that PPAR-γ targets the transcriptional activity of Smad3, accounting for inhibition of TGF-β responses in fibroblasts. Coupled immunoprecipitation/immunoblot assays revealed a low level constitutive interaction of Smad3 with endogenous PPAR-γ in unstimulated fibroblasts that was modestly enhanced by TGF-β treatment, but unaffected by 15d-PGJ2 or by troglitazone (Fig. 3C and data not shown).
Figure 3.
Both 15d-PGJ2 and ectopic PPAR-γ abrogate Smad3-dependent transcription. A, B) Foreskin fibroblasts transiently transfected with Smad3 expression vector (A) or PPAR-γ expression vector (B) along with 772COL1A2/CAT reporter were preincubated with indicated concentrations of 15d-PGJ2 for 60 min, followed by TGF-β2 (12.5 ng/ml). After a further 48-h incubation, cell lysates were assayed for CAT and luciferase activities. Transfection efficiency was monitored by Renilla luciferase assays. Whole-cell lysates were subjected to Western blot analysis (B, bottom panel). C) Equal amounts of whole-cell lysates were immunoprecipitated with anti-PPAR-γ antibodies and subjected to Western blot analysis. Results represent means ± sd of triplicate determinations. Open bars denote untreated fibroblasts; solid bars denote TGF-β-treated fibroblasts.*P < 0.001 vs. Smad3 plus TGF-β.
Ligand-dependent Smad activation in the presence of PPAR-γ
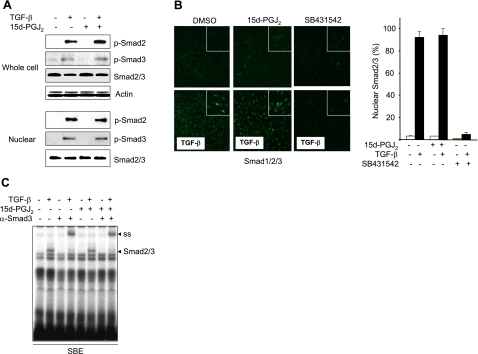
In some studies, the antagonistic modulation of TGF-β responses by PPAR-γ ligands was associated with interference with Smad2 or Smad3 phosphorylation in hepatic stellate cells and vascular smooth muscle cells (34,35,36,37,38). To investigate the effect of PPAR-γ ligands on Smad activation in fibroblasts, a series of experiments were performed. Western blot analysis indicated that TGF-β-induced rapid phosphorylation of Smad2/3 was unaffected by preincubation of fibroblasts with 15d-PGJ2 (Fig. 4A, top panel). Moreover, a comparable increase in nuclear accumulation of Smad2/3 and phospho-Smad2/3 was seen in the presence or absence of 15d-PGJ2 (Fig. 4A bottom panel, B) or troglitazone (data not shown). In contrast, the ALK5 inhibitor SB431542 completely blocked these TGF-β-induced responses, as expected (Fig. 4B).
Figure 4.
15d-PGJ2 fails to prevent Smad activation. Confluent fibroblasts pretreated with 15d-PGJ2 for 23 h (A) or for 60 min (B) were incubated with TGF-β2 for a further 60 min. A) Cultures were harvested and whole-cell lysates (top panel) or nuclear extracts (bottom panel) were subjected to Western blot analysis. Representative immunoblots are shown. B) Confocal microscopy. Cultures were preincubated with SB431542 or 15d-PGJ2 for 60 min, followed by incubation with TGF-β2 for 60 min. Representative images are shown. Original view: ×100. Right panel: proportion of fibroblasts showing predominantly nuclear Smad2/3 was quantified. Results represent means ± sd. C) Electrophoretic mobility shift assays. Nuclear extracts were incubated with radiolabeled oligonucleotides harboring consensus SBE sequences. TGF-β-induced DNA-protein complex was identified as Smad2/3 by antibody supershift assays (ss). Representative autoradiogram is shown.
To examine the effect of 15d-PGJ2 on Smad2/3 DNA binding, nuclear extracts were prepared from fibroblasts stimulated with TGF-β in the presence or absence of 15d-PGJ2 and incubated with radiolabeled oligonucleotide probes harboring SBE sequence, and subjected to electrophoretic mobility shift analysis. The results showed that TGF-β induced the formation of a protein-DNA complex that was identified as Smad3 by antibody supershift assays (Fig. 4C). Notably, the relative intensity of the Smad3-DNA complex was comparable in fibroblasts incubated in the presence or absence of 15d-PGJ2. Collectively, these results indicate that PPAR-γ did not interfere with TGF-β-induced activation of the Smad pathway, but rather abrogated Smad transcriptional activity.
PPAR-γ blocks the recruitment of the p300 coactivator
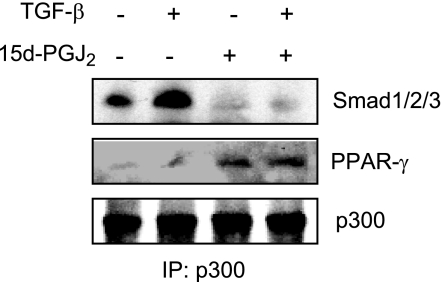
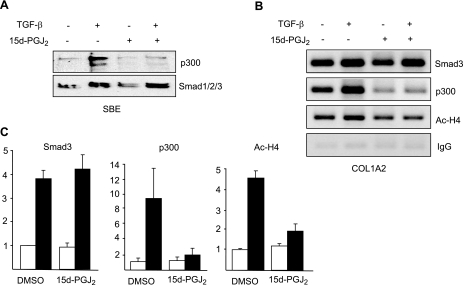
The transcriptional coactivator p300 is intimately involved in intracellular TGF-β signal transduction (11). Furthermore, by virtue of its intrinsic histone acetyltransferase activity, p300 plays a fundamental role in the induction of fibrotic responses (11). We had shown previously that Smad-dependent transactivation of COL1A2 in TGF-β-stimulated fibroblasts required the physical and functional interaction of p300 with activated Smad2/3 (9, 10). The transcriptional activities of PPAR-γ similarly require interaction with p300 (22). In light of the requirement for p300 as a shared cofactor that is indispensable for both Smad2/3-dependent and PPAR-γ-dependent transcription, we hypothesized that the antagonistic regulation of TGF-β signaling by PPAR-γ may be associated with disrupted p300 recruitment or function. This notion was examined by three complementary approaches. First, coupled immunoprecipitation-immunoblot assays revealed that while TGF-β stimulation enhanced the interaction between endogenous Smad3 and p300, as expected (Fig. 5), preincubation of the cultures with 15d-PGJ2 blocked this response, while at the same time enhancing the interaction of PPAR-γ with p300 (Fig. 5). Second, the effect of PPAR-γ on p300 recruitment was examined by DNA affinity precipitation assay using biotin-labeled oligonucleotides harboring SBE. The results demonstrated that while Smad2/3 binding to SBE sequences in TGF-β-treated fibroblasts was unaffected by 15d-PGJ2, recruitment of p300 was completely blocked (Fig. 6A). Similar results were obtained with troglitazone as the PPAR-γ ligand (data not shown). To extend these observations to the chromatin context in live cells, ChIP assays were performed. Fibroblasts were stimulated with TGF-β in the presence of absence of 15d-PGJ2, and formaldehyde crosslinked DNA-protein complexes were immunoprecipitated with antibodies to Smad2/3, p300 or acetylated histone H4. The results revealed that, whereas 15d-PGJ2 had no detectable effect on the increased levels of Smad2/3 accumulation on the COL1A2 promoter, in fibroblasts treated with TGF-β, both the increased recruitment of p300, as well as histone H4 hyperacetylation at the COL1A2 locus, were reduced to near-basal levels in the presence of 15d-PGJ2 (Figs. 6B, C).
Figure 5.
15d-PGJ2 abrogates induction of p300-Smad2/3 interaction. Following pretreatment with 15d-PGJ2 for 60 min, fibroblasts were incubated with TGF-β for 60 min. Whole-cell lysates were immunoprecipitated with antibodies to p300 and subjected to Western blot analysis. Representative immunoblots are shown.
Figure 6.
15d-PGJ2 abrogates p300 recruitment to SBE. A) DNA affinity precipitation assays. Nuclear extracts from fibroblasts treated with TGF-β in the presence and absence of 15d-PGJ2 were incubated with biotin-labeled oligonucleotide probes harboring consensus SBE sequences, and subjected to DNA affinity precipitation assay (DAPA), as described in Materials and Methods. Representative images are shown. B) ChIP assays. Formaldehyde cross-linked nuclear extracts were subjected to ChIP using indicated antibodies or IgG. Immunoprecipitated DNA was amplified using primers spanning for TGF-β response element of COL1A2 promoter. Representative images are shown. C) Immunoprecipitated DNA was subjected to real-time quantitative PCR using COL1A2 promoter-specific primers. Results are expressed as fold change in DNA precipitated by the indicated antibody from TGF-β-treated cultures (solid bars) compared to control cultures (open bars) in the presence and absence of 15d-PGJ2 and represent means ± sd of triplicate determinations.
Forced expression of p300 rescues collagen stimulation in the presence of PPAR-γ
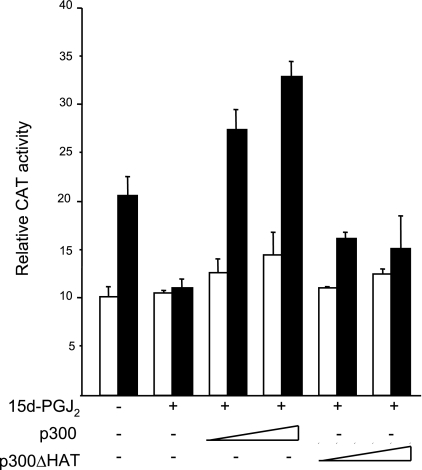
These findings led us to hypothesize that interference with the recruitment of p300 to the Smad2/3-containing transcriptional complex in 15d-PGJ2-treated fibroblasts resulted in loss of histone H4 hyperacetylation at target gene promoters, accounting for abrogation of Smad-dependent TGF-β responses. If the hypothesis is correct, then forced expression of p300 would be expected to rescue TGF-β stimulation in the presence of 15d-PGJ2 in a histone acetyltransferase-dependent manner. To test this notion, fibroblasts were cotransfected with expression vectors for wild-type p300 or a mutant form of p300 that lacks HAT domain (31). Transient transfection assays revealed that while 15d-PGJ2 almost completely prevented the stimulation of COL1A2 promoter activity in TGF-β-treated fibroblasts, ectopic p300 rescued the stimulatory response in a dose-dependent manner (Fig. 7). By itself, ectopic p300 caused a significant increase in basal, as well as TGF-β-stimulated, COL1A2 promoter activity (data not shown). In contrast, the HAT-deficient mutant p300 failed to reverse the suppression of TGF-β responses. These results indicate that p300 reversed the suppressive effects of 15d-PGJ2 on TGF-β responses, and the intrinsic acetyltransferase activity of the coactivator was required for this effect.
Figure 7.
Ectopic p300 rescues stimulation of COL1A2 activity in the presence of 15d-PGJ2. Confluent fibroblasts transiently cotransfected with increasing concentration of p300 or HAT-deleted mutant p300 (p300ΔHAT) expression vectors along with 772COL1A2/CAT were pretreated with 15d-PGJ2 for 60 min. Following further incubation with TGF-β2 (12.5 ng/ml) for 24 h, cell lysates were assayed for their CAT and luciferase activities. Transfection efficiency was monitored by Renilla luciferase assays. Results represent means ± sd of triplicate determinations. Open bars denote untreated fibroblasts; solid bars denote TGF-β-treated fibroblasts.
DISCUSSION
Aberrant TGF-β expression or activity of TGF-β is implicated in the pathogenesis of fibrosis in a broad range of conditions (1). In light of its critical role in fibrosis, there is substantial interest in understanding the physiological modulation of TGF-β signaling intensity, and in blocking the expression or function of TGF-β as potential therapeutic strategies (39). We previously demonstrated that ligands of PPAR-γ blocked the stimulation of collagen synthesis induced by TGF-β in dermal fibroblasts (25). Both natural and synthetic ligands exerted comparable inhibitory effects (25). Suppression of the stimulatory response occurred at the level of transcription. Other studies have reported similar effects of PPAR-γ in lung fibroblasts, mesangial cells and hepatic stellate cells (17, 40,41,42). In addition, PPAR-γ ligands have been shown to ameliorate fibrosis in vivo in rodent models of fibrosis of the kidney (43), liver (18), lung (44, 45), and dermis (46). Taken together, these observations point to a potentially important novel facet of PPAR-γ biology that is related to connective tissue homeostasis and the control of tissue remodeling. There is growing recognition of the potential roles of PPAR-γ in regulating physiological and pathological matrix remodeling (45). The molecular mechanisms underlying these effects are incompletely understood. The present studies demonstrate that the antagonistic regulation of profibrotic TGF-β signaling in normal fibroblasts by ligand agonists of PPAR-γ is a bona fide PPAR-γ response and involves suppression of Smad2/3 transcriptional activity. The inhibitory effect was not associated with disruption of Smad2/3 activation or nuclear accumulation or Smad-SBE interaction, but rather involved in suppression of the recruitment of p300, a coactivator and histone acetyltransferase that is indispensable for stimulation of collagen gene expression (11).
Ligands of PPAR-γ are known to exert their effect on target gene expression in both PPAR-γ-dependent and PPAR-γ-independent manner (47). We demonstrate here that PPAR-γ ligands blocked TGF-β-induced stimulation of collagen synthesis via PPAR-γ. That endogenous PPAR-γ was a bona fide mediator of the inhibitory effect of PPAR-γ ligands on TGF-β responses was established by several lines of observation (25, 48). First, we showed that 15d-PGJ2 was unable to abrogate TGF-β-induced transcription in MEFs lacking endogenous PPAR-γ. Second, the inhibitory effect was abrogated by pretreatment of normal fibroblasts with a selective and irreversible PPAR-γ antagonist. A potential mechanism for the antifibrotic effects of PPAR-γ is the ability to antagonize Smad-mediated profibrotic responses elicited by TGF-β. Blockade of TGF-β signaling by PPAR-γ has been shown to involve disruption of Smad nuclear translocation in NRK fibroblasts (37) or phosphorylation in hepatic stellate cells (35). However, in other studies with vascular smooth muscle cells, PPAR-γ ligands enhanced, or had no effect on, Smad phosphorylation and nuclear translocation (34, 37, 38). The present results indicate that PPAR-γ targeted the transcriptional activity of Smads in skin fibroblasts. We found that 15d-PGJ2 blocked Smad-mediated transcriptional responses without disrupting TGF-β-induced Smad2/3 activation. The effects of PPAR-γ on Smad signal transduction may therefore depend on the cell type. The antifibrotic activities of PPAR-γ may be mediated via the induction of endogenous repressor molecules. For instance, in mesangial cells, PPAR-γ ligands stimulated the expression of hepatocyte growth factor, which, in turn, induced the transcriptional corepressor TGIF (37). Recent studies implicate induction of the tumor suppressor PTEN as responsible for the attenuation of bleomycin-induced pulmonary fibrosis by PPAR-γ (49,50,51).
The dual-function coactivator and histone acetyltransferase p300 is essential for Smad-dependent TGF-β responses. Full transcriptional stimulation of TGF-β target genes requires recruitment of p300 to DNA and the accompanying locus-specific histone hyperacetylation and chromatin remodeling (52). Ligand-dependent Smad2/3 interaction with p300 is implicated in TGF-β stimulation of collagen synthesis (10, 53). As a promiscuous cofactor, p300 interacts with and is required for the transcriptional activity of multiple DNA-binding factors (54). Furthermore, p300, as well as its orthologue CBP, also serves as an essential cofactor for nuclear receptors, including PPAR-γ (22). Because p300 is available in cells in limiting amounts, it has been suggested that PPAR-γ competes in a cell-type-specific manner with ligand-inducible transcription factors for binding to limiting p300, a mechanism called squelching (55). We believe that this may represent a generalized mechanism for regulation of other genes. For example, a strong correlation between PPAR-γ interaction with p300 and repression of inflammation induced-iNOS promoter activity has been found (56). A similar mechanism underlies PPAR-γ suppression of TGF-β-induced CTGF gene expression (57). Squelching is also implicated as a mechanism for PPAR-γ abrogation of inflammatory responses such as Cox-2 production (58). Our results identify a similar mechanism in TGF-β-stimulated fibroblasts treated with PPAR-γ ligands. Competition by activated PPAR-γ for limiting amounts of p300 in activated fibroblasts could interfere with the recruitment of p300 to the COL1A2 promoter, thereby reducing local histone modification and inhibiting collagen transcription. In the present studies, we found that treatment with 15d-PGJ2 reduced p300 accumulation and H4 histone hyperacetylation at the COL1A2 locus in TGF-β-treated fibroblasts. Ligand activation of cellular PPAR-γ disrupted the interaction of Smad2/3 with p300 induced by TGF-β. Taken together, these observations implicate competition for limiting amounts of p300 as a potential mechanism accounting for the antifibrotic effects of PPAR-γ ligands, and point to mechanistic parallels between the anti-inflammatory and the antifibrotic effects of PPAR-γ, both of which appear to involve squelching as the mechanism for transrepression (59). Alternate mechanisms to explain the inhibitory activities of PPAR-γ have also been identified. For instance, an inducible interaction of PPAR-γ with an inhibitory transcriptional complex containing CIITA and RFX-5 in IMR-90 lung fibroblasts has been implicated in repression of collagen synthesis by interferon-γ (60).
The present results now allow us to propose a novel mechanistic model to explain how PPAR-γ causes suppression of Smad-dependent transcriptional responses in the context of fibrogenesis. In normal fibroblasts, TGF-β stimulated the recruitment of p300 histone acetyltransferase to the COL1A2 promoter, which was associated with hyperacetylation of histone H4 at the COL1A2 locus. Recruitment of p300 to the Smad complex on the COL1A2 promoter SBE sequences is required for maximal transactivation induced by TGF-β. PPAR-γ inhibited the recruitment of p300 to the promoter in TGF-β-treated fibroblasts, and prevented histone H4 hyperacetylation. Inhibition of p300 recruitment is likely to account for the suppression of histone acetylation. We, therefore, propose that PPAR-γ-mediated abrogation of the stimulatory TGF-β response was due, at least in part, to decreased histone H4 acetylation at the COL1A2 promoter, possibly due to the reduced recruitment of p300. This observation may be relevant for the development of targeted antifibrotic therapies. Scleroderma fibroblasts are characterized by elevated p300 expression, constitutive Smad2/3 activation and constitutive interaction of Smad2/3 with p300 (53, 61, 62). By disrupting recruitment and function of p300, PPAR-γ might normalize altered fibroblast function in scleroderma and control TGF-β-dependent fibrotic processes.
Acknowledgments
We are grateful to Christopher K. Glass (University of California, San Diego, CA, USA) and Joan Boyes (Institute of Cancer Research, London, UK) for PPAR-γ and p300 expression vectors. This work was supported by grants from the Scleroderma Foundation to A.K.G. and the National Institutes of Health (AR-49025) to J.V.
References
- Wynn T A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A K. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp Biol Med. 2002;227:301–314. doi: 10.1177/153537020222700502. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Tanaka S, Bou-Gharios G. Transcriptional regulation of the human α2(I) collagen gene (COL1A2) an informative model system to study fibrotic diseases. Matrix Biol. 2006;25:365–372. doi: 10.1016/j.matbio.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Chen S J, Yuan W, Mori Y, Levenson A, Trojanowska M, Varga J. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-β: involvement of Smad 3. J Invest Dermatol. 1999;112:49–57. doi: 10.1046/j.1523-1747.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- Mori Y, Chen S J, Varga J. Modulation of endogenous Smad expression in normal skin fibroblasts by transforming growth factor-β. Exp Cell Res. 2000;258:374–383. doi: 10.1006/excr.2000.4930. [DOI] [PubMed] [Google Scholar]
- Ghosh A K, Yuan W, Mori Y, Varga J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-β involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene. 2000;19:3546–3555. doi: 10.1038/sj.onc.1203693. [DOI] [PubMed] [Google Scholar]
- Ghosh A K, Yuan W, Mori Y, Chen S J, Varga J. Antagonistic regulation of type I collagen gene expression by interferon-γ and transforming growth factor-β. Integration at the level of p300/CBP transcriptional coactivators. J Biol Chem. 2001;276:11041–11048. doi: 10.1074/jbc.M004709200. [DOI] [PubMed] [Google Scholar]
- Ghosh A K, Bhattacharyya S, Varga J. The tumor suppressor p53 abrogates Smad-dependent collagen gene induction in mesenchymal cells. J Biol Chem. 2004;279:47455–47463. doi: 10.1074/jbc.M403477200. [DOI] [PubMed] [Google Scholar]
- Ghosh A K, Varga J. The transcriptional coactivator and acetyltransferase p300 in fibroblast biology and fibrosis. J Cell Physiol. 2007;213:663–671. doi: 10.1002/jcp.21162. [DOI] [PubMed] [Google Scholar]
- Lehrke M, Lazar M A. The many faces of PPARγ. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li A C, Willson T M, Kelly C J, Glass C K. The peroxisome proliferators-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- McCarthy K J, Routh R E, Shaw W, Walsh K, Welbourne T C, Johnson J H. Troglitazone halts diabetic glomerulosclerosis by blockade of mesangial expansion. Kidney Int. 2000;58:2341–2350. doi: 10.1046/j.1523-1755.2000.00418.x. [DOI] [PubMed] [Google Scholar]
- Ma L J, Marcantoni C, Linton M F, Fazio S, Fogo A B. Peroxisome proliferator-activated receptor-γ agonist troglitazone protects against nondiabetic glomerulosclerosis in rats. Kidney Int. 2001;59:1899–1910. doi: 10.1046/j.1523-1755.2001.0590051899.x. [DOI] [PubMed] [Google Scholar]
- Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans R M. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- Galli A, Crabb D W, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, Ridolfi F, Trozzi L, Surrenti C, Casini A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924–1940. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- Kon K, Ikejima K, Hiros M, Yoshikawa M, Enomoto N, Kitamura T, Takei Y, Sato N. Pioglitazone prevents early-phase hepatic fibrogenesis caused by carbon tetrachloride. Biochem Biophys Res Commun. 2002;291:55–61. doi: 10.1006/bbrc.2002.6385. [DOI] [PubMed] [Google Scholar]
- Sertznig P, Seifert M, Tilgen W, Reichrath J. Present concepts and future outlook: Function of peroxisome proliferator-activated receptors (PPARs) for pathogenesis, progression, and therapy of cancer. J Cell Physiol. 2007;212:1–12. doi: 10.1002/jcp.20998. [DOI] [PubMed] [Google Scholar]
- Straus D S, Glass C K. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med Res Rev. 2001;21:185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- Berger J, Moller D E. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Gelman L, Zhou G, Fajas L, Raspe E, Fruchart J C, Auwerx J. p300 interacts with the N- and C-terminal part of PPARγ2 in a ligand-independent and -dependent manner, respectively. J Biol Chem. 1999;274:7681–7688. doi: 10.1074/jbc.274.12.7681. [DOI] [PubMed] [Google Scholar]
- Kodera Y, Takeyama K, Murayama A, Suzawa M, Masuhiro Y, Kato S. Ligand type-specific interactions of peroxisome proliferator-activated receptor γ with transcriptional coactivators. J Biol Chem. 2000;275:33201–33204. doi: 10.1074/jbc.C000517200. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kavada T, Yamamoto T, Goto T, Taimatsu A, Aoki N, Kawasaki H, Taira K, Yokoyama K K, Kamei Y, Fushiki T. Overexpression and ribozyme-mediated targeting of transcriptional coactivators CREB-binding protein and p300 revealed their indispensable roles in adipocyte differentiation through the regulation of peroxisome proliferators-activated receptor-γ. J Biol Chem. 2002;277:16906–16912. doi: 10.1074/jbc.M200585200. [DOI] [PubMed] [Google Scholar]
- Ghosh A K, Bhattacharyya S, Lakos G, Mori Y, Chen S-J, Varga J. Peroxisome proliferator-activated receptor-γ signaling and profibrotic responses in normal skin disrupts TGF-β fibroblasts. Arthritis Rheum. 2004;50:1305–1318. doi: 10.1002/art.20104. [DOI] [PubMed] [Google Scholar]
- He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky J M, Evans R M. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon A P. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119:S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Barak Y, Nelson M C, Ong E S, Jones Y Z, Ruiz-Lozano P, Chien K R, Koder A, Evans R M. PPARγ is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–495. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Ihn H, Ohnishi K, Tamaki T, LeRoy E C, Trojanowska M. Transcriptional regulation of human α2(I) collagen gene: combined action of upstream stimulatory and inhibitory cis-acting elements. J Biol Chem. 1996;271:26717–26723. doi: 10.1074/jbc.271.43.26717. [DOI] [PubMed] [Google Scholar]
- Liu X, Sun Y, Constantinescu S N, Karam E, Weinberg R A, Lodish H F. Transforming growth factor beta-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc Natl Acad Sci U S A. 1997;94:10669–10674. doi: 10.1073/pnas.94.20.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- Zawel L, Dai J L, Buckhaults P, Zhou S, Kinzler K W, Vogelstein B, Kern S E. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell. 1998;1:611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- Ghosh A K, Bhattacharyya S, Mori Y, Varga J. Inhibition of collagen gene expression by interferon-γ: novel role of the CCAAT/enhancer binding protein β (C/EBPβ) J Cell Physiol. 2006;207:251–260. doi: 10.1002/jcp.20559. [DOI] [PubMed] [Google Scholar]
- Redondo S, Ruiz E, Santos-Gallego C G, Padilla E, Tejerina T. Pioglitazone induces vascular smooth muscle cell apoptosis through a peroxisome proliferator-activated receptor-gamma, transforming growth factor-β1, and a Smad2-dependent mechanism. Diabetes. 2005;54:811–817. doi: 10.2337/diabetes.54.3.811. [DOI] [PubMed] [Google Scholar]
- Zhao C, Chen W, Yang L, Chen L, Stimpson S A, Diehl A M. PPARγ agonists prevent TGFβ1/Smad3-signaling in human hepatic stellate cells. Biochem Biophys Res Commun. 2006;350:385–391. doi: 10.1016/j.bbrc.2006.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D F, Niu X L, Hao G H, Peng N, Wei J, Ning N, Wang N P. Rosiglitazone inhibits angiotensin II-induced CTGF expression in vascular smooth muscle cells - role of PPAR-γ in vascular fibrosis. Biochem Pharmacol. 2007;73:185–197. doi: 10.1016/j.bcp.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Li Y, Wen X, Spataro B C, Hu K, Dai C, Liu Y. Hepatocyte growth factor is a downstream effector that mediates the antifibrotic action of peroxisome proliferator-activated receptor-gamma agonists. J Am Soc Nephrol. 2006;17:54–65. doi: 10.1681/ASN.2005030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz E, Redondo S, Gordillo-Moscoso A, Tejerina T. Pioglitazone induces apoptosis in human vascular smooth muscle cells from diabetic patients involving the transforming growth factor-beta/activin receptor-like kinase-4/5/7/Smad2 signaling pathway. J Pharmacol Exp Ther. 2007;321:431–438. doi: 10.1124/jpet.106.114934. [DOI] [PubMed] [Google Scholar]
- Pasche B, Varga J. Antitransforming growth factor-beta therapy in fibrosis: recent progress and implications for systemic sclerosis. Curr Opin Rheumatol. 2008;20:720–728. doi: 10.1097/BOR.0b013e32830e48e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Fornoni A, Elliot S J, Guan Y, Breyer M D, Striker L J, Striker G E. Upregulation of type I collagen by TGF-β in mesangial cells is blocked by PPARγ activation. Am J Physiol Renal Physiol. 2002;282:F639–F648. doi: 10.1152/ajprenal.00189.2001. [DOI] [PubMed] [Google Scholar]
- Burgess H A, Daugherty L E, Thatcher T H, Lakatos H F, Ray D M, Redonnet M, Phipps R P, Sime P J. PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1146–L1153. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- Yavrom S, Chen L, Xiong S, Wang J, Rippe R A, Tsukamoto H. Peroxisome proliferator-activated receptor gamma suppresses proximal α1(I) collagen promoter via inhibition of p300-facilitated NF-I binding to DNA in hepatic stellate cells. J Biol Chem. 2005;280:40650–40659. doi: 10.1074/jbc.M510094200. [DOI] [PubMed] [Google Scholar]
- Panchapakesan U, Sumual S, Pollock C A, Chen X. PPARγ agonists exert antifibrotic effects in renal tubular cells exposed to high glucose. Am J Physiol Renal Physiol. 2005;289:F1153–F1158. doi: 10.1152/ajprenal.00097.2005. [DOI] [PubMed] [Google Scholar]
- Genovese T, Cuzzocrea S, Di Paola R, Mazzon E, Mastruzzo C, Catalano P, Sortino M, Crimi N, Caputi A P, Thiemermann C, Vancheri C. Effect of rosiglitazone and 15-×Delta12,14-prostaglandin J2 on bleomycin-induced lung injury. Eur Respir J. 2005;25:225–234. doi: 10.1183/09031936.05.00049704. [DOI] [PubMed] [Google Scholar]
- Lakatos H F, Thatcher T H, Kottmann R M, Garcia T M, Phipps R P, Sime P J. The roles of PPARs in lung fibrosis. [Online] PPAR Res. 2007;2007:71323. doi: 10.1155/2007/71323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M H, Melichian D, Chang E, Warner-Blankenship M, Ghosh A K, Varga J. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. Am J Pathol. 2009;174:519–533. doi: 10.2353/ajpath.2009.080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia R, Yi J H, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-γ agonists. Front Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A K, Wei J, Wu M, Varga J. Constitutive Smad signaling and Smad-dependent collagen gene expression in mouse embryonic fibroblasts lacking peroxisome proliferators-activated receptor-γ. Bichem Biophys Res Commun. 2008;374:231–236. doi: 10.1016/j.bbrc.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teresi R E, Shaiu C W, Chen C S, Chatterjee V K, Waite K A, Eng C. Increased PTEN expression due to transcriptional activation of PPARγ by lovastatin and rosiglitazone. Int J Cancer. 2006;118:2390–2398. doi: 10.1002/ijc.21799. [DOI] [PubMed] [Google Scholar]
- White E S, Atrasz R G, Hu B, Phan S H, Stambolic V, Mak T W, Hogaboam C M, Flaherty K R, Martinez F J, Kontos C D, Toews G B. Negative regulation of myofibroblast differentiation by PTEN (phosphatase and tensin homolog deleted on chromosome. 10. Am J Respir Crit Care Med. 2006;173:112–121. doi: 10.1164/rccm.200507-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Cheung E, Petrakis T G, Howell M, Kraus W L, Hill C S. Smads orchestrate specific histone modifications and chromatin remodeling to activate transcription. EMBO J. 2006;25:4490–4502. doi: 10.1038/sj.emboj.7601332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Ghosh A K, Pannu J, Mori Y, Takagawa S, Chen G, Trojanowska M, Gilliam A C, Varga J. Fibroblast expression of the coactivator p300 governs the intensity of profibrotic response to TGF-β. Arthritis Rheum. 2005;52:1248–1258. doi: 10.1002/art.20996. [DOI] [PubMed] [Google Scholar]
- Vo N, Goodman R H. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Li M, Pascual G, Glass C K. Peroxisome proliferator-activated receptor γ-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol. 2000;20:4699–4707. doi: 10.1128/mcb.20.13.4699-4707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner O A, Lahme B, Rehbein K, Siluschek M, Weiskirchen R, Gressner A M. Pharmacological application of caffeine inhibits TGF-β-stimulated connective tissue growth factor expression in hepatocytes via PPARγ and SMAD2/3-dependent pathways. J Hepatol. 2008;49:758–767. doi: 10.1016/j.jhep.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Farrajota K, Cheng S, Martel-Pelletier J, Afif H, Pelletier J P, Li X, Rangerm P, Fahmi H. Inhibition of interleukin-1γ-induced cyclooxygenase 2 expression in human synovial fibroblasts by 15-×Δ12,14-prostaglandin J2 through a histone deacetylase-independent mechanism. Arthritis Rheum. 2005;52:94–104. doi: 10.1002/art.20714. [DOI] [PubMed] [Google Scholar]
- Ricote M, Glass C K. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Farmer S R, Smith B D. Peroxisome proliferator-activated receptor g interacts with CIITA. RFX5 complex to repress type I collagen gene expression. J Biol Chem. 2007;282:26046–26056. doi: 10.1074/jbc.M703652200. [DOI] [PubMed] [Google Scholar]
- Mori Y, Chen S J, Varga J. Expression and regulation of intracellular SMAD signaling in scleroderma skin fibroblasts. Arthritis Rheum. 2003;48:1964–1978. doi: 10.1002/art.11157. [DOI] [PubMed] [Google Scholar]
- Ihn H, Yamane K, Asano Y, Jinnin M, Tamaki K. Constitutively phosphorylated Smad3 interacts with Sp1 and p300 in scleroderma fibroblasts. Rheumatology (Oxf) 2006;45:157–165. doi: 10.1093/rheumatology/kei124. [DOI] [PubMed] [Google Scholar]