Abstract
Statins are widely used to treat hypercholesterolemia but can lead to a number of side effects in muscle, including rhabdomyolysis. Our recent findings implicated the induction of atrogin-1, a gene required for the development of muscle atrophy, in statin-induced muscle damage. Since statins inhibit many biochemical reactions besides cholesterol synthesis, we sought to define the statin-inhibited pathways responsible for atrogin-1 expression and muscle damage. We report here that lovastatin-induced atrogin-1 expression and muscle damage in cultured mouse myotubes and zebrafish can be prevented in the presence of geranylgeranol but not farnesol. Further, inhibitors of the transfer of geranylgeranyl isoprene units to protein targets cause statin muscle damage and atrogin-1 induction in cultured cells and in fish. These findings support the concept that dysfunction of small GTP-binding proteins lead to statin-induced muscle damage since these molecules require modification by geranylgeranyl moieties for their cellular localization and activity. Collectively, our animal and in vitro findings shed light on the molecular mechanism of statin-induced myopathy and suggest that atrogin-1 may be regulated by novel signaling pathways.—Cao, P., Hanai, J., Tanksale, P., Imamura, S., Sukhatme, V. P., Lecker, S. H. Statin-induced muscle damage and atrogin-1 induction is the result of a geranylgeranylation defect.
Keywords: atrophy, farnesylation
Statins (3-hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors, HMG Co A reductase inhibitors) are among the most commonly prescribed medications in developed countries (1). They impair cholesterol production by inhibiting the synthesis of mevalonate, the rate-limiting step in the cholesterol biosynthetic pathway. Statins are generally well tolerated but can produce a variety of skeletal muscle-associated, dose-dependent adverse reactions that range from muscle pain to muscle cell damage and severe rhabdomyolysis (2). Though the frequency of rhabdomyolysis is low, (1, 3, 4), symptomatic muscle weakness and pain are much more frequent. These less-severe side effects are difficult to quantitate, since objective measures of muscle damage such as elevation of creatine kinase in the serum of patients is often absent (5, 6). Estimates of prevalence of muscle complaints by statin users in typical clinical settings range as high a 20% and remain a major impediment that prevents patients and their physicians from complying with statin therapy guidelines (7,8,9).
Little is known about the molecular mechanisms by which HMG CoA reductase inhibitors produce skeletal muscle injury (2, 10,11,12). Recent experiments have suggested that some of the mechanisms that underlie the development of muscle atrophy as occurs in response to food deprivation and in many major disease states (e.g., cancer cachexia, diabetes, uremia, cardiac failure, sepsis), and with disuse (13, 14) are also activated in statin-induced muscle injury (15). As muscle atrophies, protein is rapidly mobilized through common cellular mechanisms involving similar biochemical and transcriptional adaptations (13, 16). Among the coordinately regulated genes induced is the ubiquitin-protein ligase or E3, atrogin-1/MAFbx (17, 18). Atrogin-1 is induced early during the atrophy process, and the rise in atrogin-1 expression precedes the loss of muscle weight (17). Animals lacking atrogin-1 are resistant to muscle atrophy following denervation (18). Atrogin-1 has a number of potential targets in muscle including myoD (19), calcineurin (20), the translation initiation factor, eIF3-f (21), and the transcription factor, FoxO1 (22). Which of these interactors leads to atrophy, and by what means, is still a mystery. We initially examined atrogin-1 mRNA levels in human quadriceps muscle biopsies and found that atrogin-1 expression was significantly higher in muscle samples obtained from symptomatic statin-treated patients (15). This is in contrast to a small recent study by Urso et. al (23), which found no increase in atrogin-1 expression in muscle biopsies following repeated leg muscle contractions in a statin-treated cohort. However, atrogin-1 was also strongly expressed in cultured muscle cells and in the muscles of whole zebrafish exposed to pharmacologic levels of statins (15).
Prior studies have shown that suppression of IGF-1/PI3K/AKT signaling, leading to dephosphorylation, nuclear translocation, and activation of FoxO3, is a key event in atrogin-1 induction (24). Our more recent studies suggest that as in conditions of muscle atrophy, statin-induced atrogin-1 transcription is mediated by FoxO dephosphorylation and activation. Additional experiments performed by others (25) also demonstrate suppression of IGF-1 signaling after statin treatment. This finding suggests a common mechanism for the induction of atrogin-1 following statin treatment and in muscle atrophy, where suppression of IGF-1 signaling leads to FoxO dephosphorylation, nuclear localization, and transcription of the atrogin-1 gene (24). Precisely how statin treatment leads to suppression of IGF-1 signaling is currently unclear.
Since mevalonate is an important precursor not only of cholesterol but also of ubiquinone, dolichols, and other isoprenoids (26), muscle toxicity could be mediated by many different intracellular pathways. Furthermore, recent experiments suggest that statins affect mitochondrial function (15, 27). Interestingly, ubiquinone (coenzyme Q10), a component of the inner mitochondrial membrane required for oxidative phosphorylation, is prenylated; thus its synthesis is inhibited by statins (28,29,30).
In the present study, we have dissected further the biosynthetic pathways dependent on mevalonate and thus inhibited by HMG CoA reductase inhibitors. Using specific inhibitors and rescue experiments in muscle cell culture and in zebrafish, we demonstrate that statin-inhibited protein geranylgeranylation promotes both atrogin-1 induction and muscle damage. We hypothesize that normal production and function of geranylgeranylated proteins such as small GTPases may be necessary to prevent atrogin-1 induction in normal muscle and may suggest novel ways to protect against the detrimental effects of statins.
MATERIALS AND METHODS
Plasmids, viral constructs, antibodies, and inhibitors
Polyclonal anti-atrogin-1 antibody was used as described previously (31). Anti-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and dynein antibodies were from Santa Cruz Biotechology (Santa Cruz, CA, USA). Farnesol, geranylgeranol, and perillyl alcohol were from Sigma (St. Louis, MO, USA). N-acetyl-S-farnesyl-l-cysteine (AFC) and N-acetyl-S-geranylgeranyl-l-cysteine (AGGC) were from Calbiochem (San Diego, CA, USA).
Cell culture
Lovastatin (>98% purity) (mevinolin; Sigma) was prepared as a 50 mM stock solution in DMSO as reagent vehicle, further diluted in DMSO, and added into the medium. The final volume of DMSO in medium was not more than 0.125%, with no obvious cytotoxicity. Equal volumes of reagent vehicle were used for all experiments, and reagent vehicle alone served as control treatment. Each experiment was performed at least 3 times. Primary mouse myoblasts from atrogin-1-null mice (Regeneron, Tarrytown, NY, USA) were isolated as follows: Muscle was removed from the hind limbs of 2 wk old mice. After treatment with 0.1% collagenase D and Dispase II (Roche, Indianapolis, IN, USA), the isolated cells were plated on collagen (Type I, Roche)-coated dishes. Subsequently, myoblasts were enriched and cultured in F-10 nutrient medium with 20% fetal calf serum, 2.5 ng/ml bFGF (Invitrogen, Carlsbad, CA, USA). Myotubes were induced in differentiation medium. All media contained 1× Primocin (InvivoGen, San Diego, CA, USA). The cultures were maintained at 37°C, below 5 and 8% CO2 air-humidified atmosphere for myoblasts and myotubes, respectively. Cultures were ready to use in assays on d 2 in differentiation medium when the myotubes had formed and were contracting.
Myotube fiber size
Size was quantified by measuring a total of 200 tube diameters as described by Sandri et al. (24). Briefly, muscle fiber size from 4 random fields at ×100 was measured using Image software (Scion, Frederick, MD, USA). All data were expressed as means ± se.
Quantitative PCR
Atrogin-1 mRNA levels were determined by real-time PCR using the Applied Biosystems (Foster City, CA, USA) 7300 real-time PCR analyzer according to the method recently described by others (32, 33). Multiplexed amplification reactions were performed using 18S rRNA as an endogenous control (18S rRNA primers/VIC-labeled probe; Applied Biosystems 4310893E) using the TaqMan One Step PCR Master Mix reagents kit (4309169; Applied Biosystems). The following settings were used: stage 1 (reverse transcription), 48°C for 30 min; stage 2 (denaturation), 95°C for 10 min; and stage 3 (PCR), 95°C for 15 s and 60°C for 60 s for 40 cycles. The sequences of the forward, reverse, and double-labeled oligonucleotides for atrogin-1 were as follows: forward 5′-CTT TCA ACA GAC TGG ACT TCT CGA-3′, reverse 5′-CAG CTC CAA CAG CCT TAC TAC GT-3′; TaqMan probe sequence 5′-FAM-TGC CAT CCT GGA TTC CAG AAG ATT CAA C-TAMRA-3′. Fluorescence data were analyzed by SDS1.7 software (Applied Biosystems). The Ct (threshold cycle) values for each reaction were transferred to an Excel spreadsheet (Microsoft, Redmond, WA, USA), and calculation of relative gene expression was performed from this data according to published algorithms (TaqMan Cytokine Gene Expression Plate 1 protocol; Applied Biosystems). All RNA samples were analyzed in triplicate, with the mean value used in subsequent analyses.
Western blotting
Cultured cells after treatment were collected at specific times and solubilized in RIPA lysis buffer [50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1% Nonidet P-40; 0.5% sodium deoxycholate; 0.1% SDS (Boston Bioproducts, Boston, MA, USA); protease (Roche); and phosphatase (Sigma) inhibitor cocktail]. Proteins were separated by SDS-PAGE, transferred to PVDF membranes, and visualized by Western blotting using alkaline phosphatase-based CDP-star chemiluminescent detection according to the manufacturer’s protocol (Applied Biosystems). Zebrafish embryos were homogenized in SDS sample buffer (30 embryos/30 μl sample buffer) with a microfuge pestle until the lysate became uniform in consistency and no longer stringy inside. The lysate was boiled for 5 min, and centrifuged supernatant was processed for Western blotting (34).
Zebrafish lines and maintenance
Adult zebrafish (Danio rerio) were maintained as described under standard laboratory conditions at 28.5°C in a 14:10 h light-dark cycle (35) Developmental stages were determined by embryo morphology and hours postfertilization (hpf) (36). To examine the effects of statin, the embryos at 20–24 hpf were immersed in the embryonic water (500 μM NaCl, 170 μM KCl, 330 μM CaCl2, and 330 μM MgSO4) at a concentration of 0.005–10 μM of lovastatin (mevinolin; Sigma), including 0.003% 1-phenyl-2-thiourea (Sigma) to inhibit pigmentation in a 24-well plate. After 32 hpf, the embryos were fixed by 4% paraformaldehyde in PBS.
Whole-zebrafish antibody staining
Zebrafish embryos were fixed by 4% paraformaldehyde in PBS overnight. After fixation, the embryos were washed by PBS, stored for at least 1 h at −20°C in methanol, and permeabilized for 30 min at −20°C in acetone. Embryos were incubated with blocking buffer (1% BSA and 0.1% Tween-20 in PBS) and incubated with diluted primary antibody, anti-slow twitch myosin F59 [1:100; Developmental Studies Hybridoma Bank (DSHB), Department of Biological Sciences, University of Iowa, Iowa City, IA, USA] (37, 38), in blocking solution overnight at 4°C. Staining was detected by using Alexa Fluor 594 goat anti-mouse IgG (H+L) (1:200; Invitrogen) in blocking solution for 4 h at room temperature (39).
Antisense morpholino oligonucleotide (MO) sequence and injection
Antisense MOs were designed and synthesized by Gene Tools LLC (Philomath, OR, USA). MO used for knockdown of the z-GGTase I, β subunit: 5′-AAT CCA CCG ACT CAA AAT CCG CCA T-3′. Five-base mismatch control: 5′AAT CCA GCC AGT CAA AAT GCC CCA T-3′. MO used for knockdown of the z-GGTase II, β subunit: 5′-CTG ACT TCA GCC GTC ACA CAT ATA T-3′. Five-base mismatch control: 5′-CTC ACT TGA GCG GTC AGA CAT AAA T-3′. For injections, MOs were diluted to 500 or 1000 μM. MOs were injected at 8–16 ng/embryo into one-cell-stage embryos at the yolk and cytoplasm interface. All values are expressed as means ± se.
RESULTS
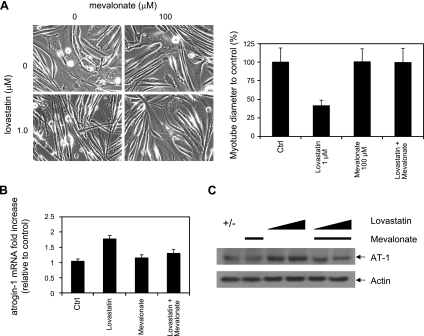
To dissect the intracellular pathways that mediate statin toxicity and atrogin-1 induction in muscle, we began by using myotubes derived from normal and atrogin-1-knockout mice. In wild-type cultures, administration of 0.5–1.0 μM lovastatin for 24 h led to a dose-dependent reduction in myotube diameter and concomitant induction of atrogin-1 mRNA and protein. Cells lacking atrogin-1 were resistant to this lovastatin-induced reduction in myotube width (Fig. 1). These results are in good agreement with our prior findings (15) and reflect serum lovastatin concentrations typically found in patients (40, 41). Statins inhibit HMG oA reductase, the first committed step in cholesterol synthesis that converts HMG CoA to mevalonate. To ensure that the effect of lovastatin on muscle cells is due to inhibition of this pathway rather than a nonspecific effect, we treated wild-type myotubes with lovastatin in combination with the product of HMG CoA reductase, mevalonate. Mevalonate (100 μM) had no effect on the muscle cultures alone but completely prevented the reduction of myotube diameter, atrogin-1 mRNA, and protein induction caused by treatment with lovastatin (Fig. 2).
Figure 1.
Lovastatin damage in cultured myotubes. Myoblasts derived from atrogin-1-knockout mice (−/−) and corresponding wild-type littermates (+/+) were differentiated into myotubes. Cultures were treated with varying concentrations of lovastatin for 24 h. A) Morphology (left), myotube diameter (right). Solid bars, +/+ cells; open bars, −/− cells. B) Atrogin-1 mRNA levels in +/+ cells assessed by real-time PCR. C) Atrogin-1 protein levels in +/+ cells assessed by immunoblot.
Figure 2.
Mevalonate rescues lovastatin-induced myotube damage. Primary myotubes from wild-type mice were treated with lovastatin or lovastatin and mevalonate for 24 h. A) Morphology (left), myotube diameter (right). B) Atrogin-1 mRNA. C) Atrogin-1 protein. Mevalonate concentration was 100 μM throughout. Lovastatin concentration was 1.0 μM (A, B); 0.5 and 1.0 μM (C).
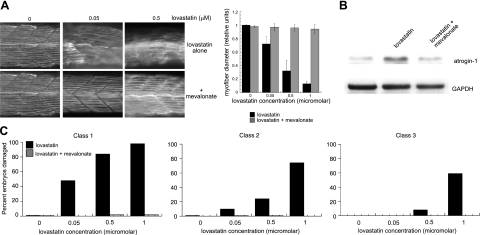
As in mammalian muscle cell culture, lovastatin leads to clear dose-dependent muscle phenotypes in a zebrafish in vivo model of statin-induced muscle damage, demonstrated by longitudinal muscle fiber staining with an antibody to myosin heavy chain (15) (Fig. 3). Muscle damage at low lovastatin concentration (0.025–0.05 μM) is evidenced by bowing, gap formation, and fiber disruption (class 1 changes). At higher lovastatin concentrations (0.05–0.5 μM), fiber damage is more severe. Fiber thinning and attenuation of staining with the MHC antibody is frequently seen (class 2 changes). At maximal lovastatin concentrations (1.0–5.0 μM), damage beyond the muscle is observed with the development of irregular somite boundaries (class 3 changes). Zebrafish also bear an atrogin-1 gene 75% homologous at the amino acid level to the human counterpart, and our prior studies have shown that knockdown of z-atrogin-1 prevents statin-induced muscle damage in the fish (15). As in mammalian myotube culture, 100 μM mevalonate completely prevented the development of zebrafish myofiber damage and zebrafish atrogin-1 induction (Fig. 3). Taken together, these data demonstrate that the effects of lovastatin on muscle morphology and atrogin-1 induction are by pathways dependent on HMG CoA reductase function.
Figure 3.
Mevalonate rescues lovastatin-induced myofiber damage in zebrafish embryos. A) Zebrafish embryos (20 hpf) were treated with concentrations of lovastatin ranging from 0.05 to 0.5 μM and 100 μM mevalonate for 12 h. Embryos were fixed and stained with anti-myosin heavy-chain antibody (F59) as described in Materials and Methods. Representative somite phenotypes are shown. All panels are side views, anterior, left. Muscle fiber diameter was measured following myosin heavy-chain staining as described in Materials and Methods. At least 500 fibers were measured at each concentration. Results were graphed as the ratio of mean ± se experimental fiber size/control fiber size. Control fiber size: 7.60 ± 0.19 μM. B) Immunoblot of zebrafish atrogin-1. C) Quantitation of muscle damage. Morphological phenotypes shown in panel A were grouped into three classes. Class 1 changes include bowing, gap formation, and blocked/disrupted fibers. Class 2 changes include irregular fibers and diffuse appearance. Class 3 changes are typified by irregular somite boundaries. Values are percentages of embryos displaying specific class defects as a function of lovastatin concentration; 100 embryos/group.
In addition to producing cholesterol, mevalonate is a building block of the polyprenyl tails conjugated to many intracellular proteins. Some proteins (e.g., lamins, ras) are derivatized by farnesyl pyrophosphate, a 15-carbon adduct generated from 3 mevalonate moieties, while other proteins (e.g., rho, rac, rab, and rap1) are derivatized by geranylgeranyl pyrophosphate, a 20-carbon adduct generated from 4 mevalonate moieties. The fact that inhibition of squalene synthase and squalene epoxidase, distal enzymes specific only to cholesterol biosynthesis, do not cause toxicity in cultured muscle cells (42, 43) led us to test whether statin inhibition of farnesylation or geranylgeranylation might underlie atrogin-1 induction and muscle damage. The first approach taken to address this question was to explore whether the addition of farnesol or geranylgeranol, the cell-permeable alcohol precursors of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, respectively (44), could rescue the effects of lovastatin in mammalian muscle culture or in zebrafish embryos. Neither farnesol nor geranylgeranol themselves had any effect on fiber diameter or atrogin-1 level in myotube culture (Fig. 4) or in zebrafish embryos (Fig. 5) at concentrations similar to those used with lovavstatin. However, when administered in conjunction with lovastatin, 2.5 μM geranylgeranol but not 2.5 μM farnesol reduced the degree of atrogin-1 mRNA and protein induction as well as fiber damage in both models (Figs. 4and 5). These data strongly suggest that proteins that require geranylgeranylation for their activity mediate statin toxicity in muscle at least in part by causing atrogin-1 induction.
Figure 4.
Geranylgeranol but not farnesol rescues lovastatin-induced myotube damage in cultured myotubes. Primary myotubes from wild-type mice were treated with lovastatin (1.0 μM) or lovastatin (1.0 μM) and either farnesol (2.5 μM) or geranylgeranol (2.5 μM) for 24 h. A) Morphology (left), myotube diameter (right). B) Atrogin-1 mRNA. C) Atrogin-1 protein.
Figure 5.
Geranylgeranol but not farnesol rescues lovastatin-induced muscle damage in zebrafish embryos. A) Zebrafish embryos (20 hpf) were treated with concentrations of lovastatin ranging from 0.05 to 0.5 μM and 10 μM farnesol or geranylgeranol for 12 h. Embryos were fixed and stained as in Fig. 3. Muscle fiber diameter was measured as described in Fig. 3. B) Immunoblot of zebrafish atrogin-1. Lovastatin, 0.5 mM; geranylgeranol and farnesol, 10 mM. C) Quantitation of muscle damage; 100 embryos/group.
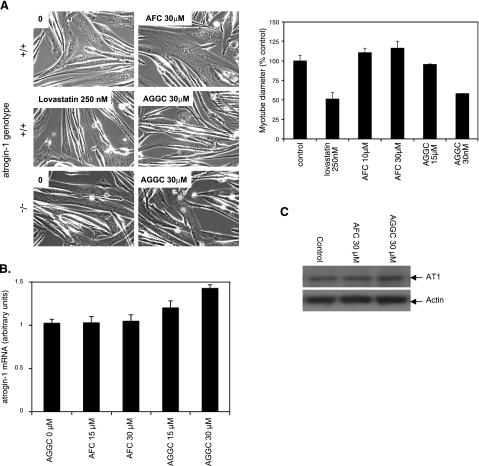
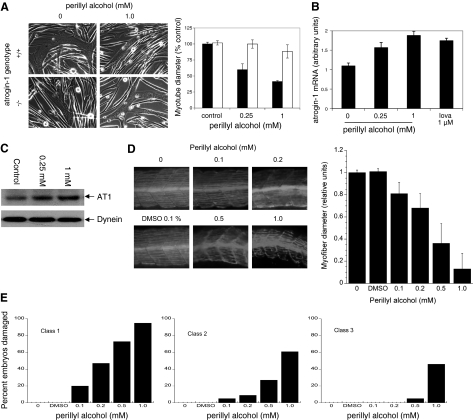
Further evidence that inhibition of geranylgeranylation is critical to the toxicity of statins in muscle comes from experiments in which we treated myotubes with AGGC, which inhibits processing of geranylgeranylated proteins by isoprenylcysteine carboxyl methyltransferase (ICMT). AGGC (30 μM) treatment caused a similar reduction of myotube diameter as 0.25 μM lovastatin and likewise was associated with atrogin-1 mRNA and protein increases in myotubes (Fig. 6). This effect was dependent on atrogin-1 since myotubes derived from atrogin-1-knockout mice were resistant to this reagent. In addition, 30 μM AFC, which inhibits processing of farnesylated proteins by ICMT, had no effect on myotube integrity or atrogin-1 levels (Fig. 6). Finally, perillyl alcohol treatment, which inhibits geranylgeranyl transferases (45), also led to marked damage of wild-type but not atrogin-1−/− myotubes as well as induction of atrogin-1 mRNA and protein at similar concentrations as lovastatin (Fig. 7A–C). Perillyl alcohol had similar effects in zebrafish embryos, where it led to dramatic thinning of muscle fibers and morphological damage (Fig. 7D–E). To confirm that the effect of perillyl alcohol on muscle fibers is due to inhibition of geranylgeranyl transferases and not a nonspecific effect, we knocked down the β subunit of GGTase I and GGTase II in zebrafish embryos using antisense MOs targeting the ATG region of the gene (ATG morpholino). Depletion of both z- geranylgeranyl transferase I and II demonstrated similar effects as perillyl alcohol and lovastatin treatment in zebrafish muscle fibers (Fig. 8). Altogether, these experiments provide strong evidence that geranylgeranylated proteins mediate lovastatin-induced atrogin-1 induction and muscle damage.
Figure 6.
AGGC but not AFC mimics lovastatin-induced myotube damage. Myotubes derived from atrogin-1-knockout mice (−/−) and corresponding wild-type littermates (+/+) were treated with lovastatin (250 nM) and AGGC (15–30 μM) or AFC (10–30 μM) for 24 h. A) Morphology (left); myotube diameter, +/+ cells only (right). B) Atrogin-1 mRNA levels in +/+ cells assessed by real-time PCR. C) Atrogin-1 protein levels in +/+ cells assessed by immunoblot.
Figure 7.
Perillyl alcohol mimics lovastatin-induced myotube damage. Myotubes derived from atrogin-1-knockout mice (−/−) and corresponding wild-type littermates (+/+) were treated with perillyl alcohol (0.25–1.0 mM) for 24 h. A) Morphology (left), myotube diameter. Solid bars, +/+ cells; open bars, −/− cells. B) Atrogin-1 mRNA levels in +/+ cells assessed by real-time PCR. C) Atrogin-1 protein levels in +/+ cells assessed by immunoblot. D) Zebrafish embryos (20 hpf) were treated with concentrations of perillyl alcohol ranging from 0.1 to 1.0 mM for 12 h. Embryos were fixed and stained as in Fig. 3. Representative myofiber morphology (top) and myofiber diameter (bottom). E) Quantitation of muscle damage. Embryos/group: 73 for 0.1% DMSO and 71, 93, 87, 81, and 85 for 0, 0.1, 0.2. 0.5, and 1.0 mM perillyl alcohol, respectively.
Figure 8.
Knockdown of geranylgeranyl transferase II in zebrafish mimics lovastatin-induced myofiber damage. A) Myosin heavy-chain staining of representative control morpholino-injected or z-GGTase I or II-injected zebrafish embryos (top), and myofiber diameter (bottom). B) Quantitation of muscle damage. Embryos/group: 100, 98, 102 for controls and 98, 96, 100 for z-GGT I knockdowns at 0, 8, 16 ng morpholino/embryo, respectively; 75, 91, 83 for controls and 75, 88, 95 for z-GGT II knockdowns at 0, 8, 16 ng morpholino/embryo, respectively.
DISCUSSION
Little is known about the mechanisms by which statins induce muscle damage. Our prior studies implicated the muscle-specific E3 ubiquitin ligase atrogin-1 in the development of statin myopathy, since its level dramatically rises in muscle cells exposed to lovastatin. Also, cells lacking or depleted in the gene product are resistant to the damaging effects of statins (15). That study suggested that important features of the atrophic response in muscle are also present in statin-induced injury. Furthermore, those experiments and others (25) demonstrated suppression of IGF-1 signaling after statin treatment, which suggests a common mechanism for the induction of atrogin-1 following statin treatment and in muscle atrophy, where suppression of IGF-1 signaling leads to FoxO dephosphorylation, nuclear localization, and transcription of the atrogin-1 gene (24). In this report, through a combination of complementary in vitro and animal experiments, we extend our initial findings by demonstrating that the lack of geranylgeranyl modifications resulting from statin inhibition of HMG CoA reductase accounts for at least part of this toxicity. We show that repletion of geranylgeranyl precursors in both muscle cell cultures and in zebrafish embryos exposed to lovastatin reduce expression of atrogin-1 and prevent muscle damage. Conversely, inhibition of the enzymes that couple geranylgeranyl precursors onto intracellular proteins mimics the effects of lovavstatin on both cell morphology and atrogin-1 induction. While others have implicated the geranylgeranylation pathway in statin muscle toxicity (46, 47), this is the first evidence that a defect in geranylgeranylation can lead to activation of atrogin-1 and that repletion of this intermediate can reduce atrogin-1 activation in response to lovastatin treatment. These results suggest that one of the ways in which statins exert their toxic effects in muscle is by inhibiting the function of a geranylgeranyl-conjugated protein that directly or indirectly leads to atrogin-1 expression.
Since HMG CoA reductase lies at the entry point to many biosynthetic pathways, statin inhibition can interfere with multiple processes besides cholesterol production. Statin-mediated muscle toxicity is likely related one or more of these noncholesterol pathways since in vitro studies show that blocking cholesterol production by inhibiting squalene synthesis does not cause muscle toxicity (42, 43). Likewise, statin-induced injury in rat skeletal muscle is not reversed with squalene supplementation (42), and enzymes of the distal cholesterol synthetic pathway are not associated with myopathy (11).
In contrast, isoprenyl modifications are necessary for several products important to skeletal muscle cell function. Most prominently, small GTPases require farnesylation or geranylgeranylation to target them to the membrane where they are active (48, 49), and statins have been shown to inhibit these modifications (50, 46). On the one hand, our results show that farnesol cannot rescue statin effects in mouse myotubes (in vitro) or in vivo zebrafish muscle fibers; ras, which is farnesylated, is unlikely to be the relevant GTPase. On the other hand, many other small GTPases, such as rac, rho, and rap1, are geranylgeranylated and could be at the basis of the effects of statins. One particular GTPase, rap1, is both geranylgeranylated (52) and known to potentiate IGF-1 signaling (50, 53,54,55). Others have shown that rap1 is present and active in muscle cells (56), and that it plays a role in muscle hypertrophy in the heart (57). Since statins both suppress IGF-1 signaling (15) and inhibit rap1 function, this signaling intermediate is an ideal candidate to couple the effects of statin with atrogin-1 induction.
Rheb is another small GTP-binding protein that requires isoprenyl modification for its membrane localization (58, 51). It typically undergoes farnesylation, although when that pathway is inhibited it can be modified by geranylgeranylation resulting in a functional but less active protein (59, 60). Rheb acts downstream of AKT in the IGF-1 signaling pathway, positively regulating mTOR and S6K. While suppression of AKT activity leads to atrogin-1 induction through FoxO dephosphorylation and nuclear translocation, it is not known whether suppression of IGF-1 signaling downstream of AKT, as statins would affect rheb, would also lead to atrogin-1 induction, and if so, through what mechanism. Of note, mice lacking S6K do not demonstrate increased cellular levels of atrogin-1 or other atrophy-specific E3s (61).
Geranylgeranylation is also required for the synthesis of the isoprenyl tail of coenzyme Q10 (ubiquinone), an integral component of the mitochondrial electron transport apparatus required for oxidative phosphorylation. Several studies show that statins reduce circulating levels of CoQ10 (28,29,30), but it remains unclear whether this represents a true CoQ10 deficiency, because CoQ10 is transported in the circulation bound to LDL, the primary target of statin therapy (62). Studies examining the effects of statins on intramuscular CoQ10 levels have yielded inconsistent results (63), but recent experiments suggest that statins suppress mitochondrial function (27). Our prior experiments showed that augmentation of mitochondrial function and/or number could reverse induction of atrogin-1 by statins, but the involvement of CoQ10 in that effect is not known. Further studies will be required to ascertain whether statin inhibition of CoQ10 synthesis is at the basis of the induction of atrogin-1 expression and muscle damage caused by statins. However, since CoQ10 synthesis requires both geranylgeranyl as well as smaller isoprenyl precursors, geranylgeranol alone would not be predicted to fully restore its production, unless other tail-length CoQs can rescue CoQ10 deficiency. Finally, some N-linked glycosylated proteins, such as α-dystroglycan, a central protein of the skeletal muscle dystrophin–glycoprotein complex, requires dolichol-mediated modification. Dolichol is a polyprenylated alcohol also produced from mevanolate, and its synthesis is inhibited by statins (26). Despite these theoretical mechanisms of toxicity, no studies have been performed directly linking these molecules with statin-induced muscle injury.
Patients with statin-induced muscle injury represent a heterogenous group, ranging from those with overt muscle necrosis (rhabdomyolysis) to those with muscle pain and no serum creatine kinase elevation. They also present variably clinically, and the resolution of their symptoms after cessation of statin therapy can be variable in time course as well. Furthermore, atrogin-1 is induced in atrophying muscle in which frank muscle injury is rarely present. Currently, it is not known whether atrogin-1 expression correlates with particular signs or symptoms of statin-induced muscle injury; that will require larger biopsy studies of patients with statin-induced muscle damage and a more detailed biochemical understanding of atrogin-1 targets in muscle cells. It is conceivable that polymorphisms in the atrogin-1 gene or promoter might explain differences in susceptibility, clinical presentation, or course in patients with statin myopathy (64). Such studies should shed additional light on the molecular mechanisms of statin-induced myopathy and enable the stratification of patients who might be susceptible to such injury.
Acknowledgments
We thank members of the V.P.S. laboratory and the Alfred L. Goldberg laboratory (Harvard Medical School, Boston, MA, USA) for their useful discussions. We also thank Eriko Koshimizu and Nathaniel Doro for technical support. This work was supported by grants from the U.S. National Institutes of Health (DK062307) to S.H.L. and seed funds from Beth Israel Deaconess Medical Center to V.P.S.
References
- Staffa J A, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002;346:539–540. doi: 10.1056/NEJM200202143460721. [DOI] [PubMed] [Google Scholar]
- Thompson P D, Clarkson P, Karas R H. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- Ballantyne C M, Corsini A, Davidson M H, Holdaas H, Jacobson T A, Leitersdorf E, Marz W, Reckless J P, Stein E A. Risk for myopathy with statin therapy in high-risk patients. Arch Intern Med. 2003;163:553–564. doi: 10.1001/archinte.163.5.553. [DOI] [PubMed] [Google Scholar]
- Graham D J, Staffa J A, Shatin D, Andrade S E, Schech S D, La Grenade L, Gurwitz J H, Chan K A, Goodman M J, Platt R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–2590. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- Phillips P S, Haas R H, Bannykh S, Hathaway S, Gray N L, Kimura B J, Vladutiu G D, England J D. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–585. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- Antons K A, Williams C D, Baker S K, Phillips P S. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119:400–409. doi: 10.1016/j.amjmed.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Foley K A, Massing M W, Simpson R J, Jr, Alexander C M, Markson L E. Population implications of changes in lipid management in patients with coronary heart disease. Am J Cardiol. 2004;93:193–195. doi: 10.1016/j.amjcard.2003.09.035. [DOI] [PubMed] [Google Scholar]
- O'Meara J G, Kardia S L, Armon J J, Brown C A, Boerwinkle E, Turner S T. Ethnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med. 2004;164:1313–1318. doi: 10.1001/archinte.164.12.1313. [DOI] [PubMed] [Google Scholar]
- Buettner C, Davis R B, Leveille S G, Mittleman M A, Mukamal K J. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23:1182–1186. doi: 10.1007/s11606-008-0636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S K, Tarnopolsky M A. Statin myopathies: pathophysiologic and clinical perspectives. Clin Invest Med. 2001;24:258–272. [PubMed] [Google Scholar]
- Baker S K. Molecular clues into the pathogenesis of statin-mediated muscle toxicity. Muscle Nerve. 2005;31:572–580. doi: 10.1002/mus.20291. [DOI] [PubMed] [Google Scholar]
- Buettner C, Lecker S H. Molecular basis for statin-induced muscle toxicity: implications and possibilities. Pharmacogenomics. 2008;9:1133–1142. doi: 10.2217/14622416.9.8.1133. [DOI] [PubMed] [Google Scholar]
- Mitch W E, Goldberg A L. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. New Engl J Med. 1996;335:1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- Attaix D, Mosoni L, Dardevet D, Combaret L, Mirand P P, Grizard J. Altered responses in skeletal muscle protein turnover during aging in anabolic and catabolic periods. Int J Biochem Cell Biol. 2005;37:1962–1973. doi: 10.1016/j.biocel.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Hanai J I, Cao P, Tanksale P, Imamura S, Koshimizu E, Zhao J, Kishi S, Yamashita M, Phillips P S, Sukhatme V P, Lecker S H. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117:3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker S H, Solomon V, Mitch W E, Goldberg A L. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129:227S–237S. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- Gomes M D, Lecker S H, Jagoe R T, Navon A, Goldberg A L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine S C, Latres E, Baumhueter S, Lai V K, Nunez L, Clarke B A, Poueymirou W T, Panaro F J, Na E, Dharmarajan K, Pan Z Q, Valenzuela D M, DeChiara T M, Stitt T N, Yancopoulos G D, Glass D J. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Tintignac L A, Lagirand J, Batonnet S, Sirri V, Leibovitch M P, Leibovitch S A. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem. 2005;280:2847–2856. doi: 10.1074/jbc.M411346200. [DOI] [PubMed] [Google Scholar]
- Li H H, Kedar V, Zhang C, McDonough H, Arya R, Wang D Z, Patterson C. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest. 2004;114:1058–1071. doi: 10.1172/JCI22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagirand-Cantaloube J, Offner N, Csibi A, Leibovitch M P, Batonnet-Pichon S, Tintignac L A, Segura C T, Leibovitch S A. The initiation factor eIF3-f is a major target for Atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J. 2008;27:1266–1276. doi: 10.1038/emboj.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H H, Willis M S, Lockyer P, Miller N, McDonough H, Glass D J, Patterson C. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J Clin Invest. 2007;117:3211–3223. doi: 10.1172/JCI31757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso M L, Clarkson P M, Hittel D, Hoffman E P, Thompson P D. Changes in ubiquitin proteasome pathway gene expression in skeletal muscle with exercise and statins. Arterioscler Thromb Vasc Biol. 2005;25:2560–2566. doi: 10.1161/01.ATV.0000190608.28704.71. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker S H, Goldberg A L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddals K W, Marshman E, Westwood M, Gibson J M. Abrogation of insulin-like growth factor-I (IGF-I) and insulin action by mevalonic acid depletion: synergy between protein prenylation and receptor glycosylation pathways. J Biol Chem. 2004;279:38353–38359. doi: 10.1074/jbc.M404838200. [DOI] [PubMed] [Google Scholar]
- Liao J K. Isoprenoids as mediators of the biological effects of statins. J Clin Invest. 2002;110:285–288. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann P, Torok M, Zahno A, Waldhauser K M, Brecht K, Krahenbuhl S. Toxicity of statins on rat skeletal muscle mitochondria. Cell Mol Life Sci. 2006;63:2415–2425. doi: 10.1007/s00018-006-6235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambudiri A M, Ranganathan S, Rudney H. The role of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in the regulation of ubiquinone synthesis in human fibroblasts. J Biol Chem. 1980;255:5894–5899. [PubMed] [Google Scholar]
- Berthold H K, Naini A, Di Mauro S, Hallikainen M, Gylling H, Krone W, Gouni-Berthold I. Effect of ezetimibe and/or simvastatin on coenzyme Q10 levels in plasma: a randomised trial. Drug Saf. 2006;29:703–712. doi: 10.2165/00002018-200629080-00007. [DOI] [PubMed] [Google Scholar]
- Lamperti C, Naini A B, Lucchini V, Prelle A, Bresolin N, Moggio M, Sciacco M, Kaufmann P, DiMauro S. Muscle coenzyme Q10 level in statin-related myopathy. Arch Neurol. 2005;62:1709–1712. doi: 10.1001/archneur.62.11.1709. [DOI] [PubMed] [Google Scholar]
- Bdolah Y, Segal A, Tanksale P, Karumanchi S A, Lecker S H. Atrophy-related ubiquitin ligases atrogin-1 and MuRF-1 are associated with uterine smooth muscle involution in the postpartum period. Am J Physiol. 2007;292:R971–R976. doi: 10.1152/ajpregu.00617.2006. [DOI] [PubMed] [Google Scholar]
- Okuno Y, Huettner C S, Radomska H S, Petkova V, Iwasaki H, Akashi K, Tenen D G. Distal elements are critical for human CD34 expression in vivo. Blood. 2002;100:4420–4426. doi: 10.1182/blood-2002-03-0788. [DOI] [PubMed] [Google Scholar]
- Yang H, Menconi M J, Wei W, Petkova V, Hasselgren P O. Dexamethasone upregulates the expression of the nuclear cofactor p300 and its interaction with C/EBPbeta in cultured myotubes. J Cell Biochem. 2005;94:1058–1067. doi: 10.1002/jcb.20371. [DOI] [PubMed] [Google Scholar]
- Hanai J, Gloy J, Karumanchi S A, Kale S, Tang J, Hu G, Chan B, Ramchandran R, Jha V, Sukhatme V P, Sokol S. Endostatin is a potential inhibitor of Wnt signaling. J Cell Biol. 2002;158:529–539. doi: 10.1083/jcb.200203064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. University of Oregon, Eugene, OR, USA: Institute of Neuroscience; The Zebrafish BookA Guide for the Laboratory Use of Zebrafish Danio (Brachydanio) rerio. 1993 [Google Scholar]
- Kimmel C B, Ballard W W, Kimmel S R, Ullmann B, Schilling T F. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Crow M T, Stockdale F E. The developmental program of fast myosin heavy chain expression in avian skeletal muscles. Dev Biol. 1986;118:333–342. doi: 10.1016/0012-1606(86)90002-3. [DOI] [PubMed] [Google Scholar]
- Devoto S H, Melancon E, Eisen J S, Westerfield M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 1996;122:3371–3380. doi: 10.1242/dev.122.11.3371. [DOI] [PubMed] [Google Scholar]
- Birely J, Schneider V A, Santana E, Dosch R, Wagner D S, Mullins M C, Granato M. Genetic screens for genes controlling motor nerve-muscle development and interactions. Dev Biol. 2005;280:162–176. doi: 10.1016/j.ydbio.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Pan H Y, DeVault A R, Wang-Iverson D, Ivashkiv E, Swanson B N, Sugerman A A. Comparative pharmacokinetics and pharmacodynamics of pravastatin and lovastatin. J Clin Pharmacol. 1990;30:1128–1135. doi: 10.1002/j.1552-4604.1990.tb01856.x. [DOI] [PubMed] [Google Scholar]
- Holstein S A, Knapp H R, Clamon G H, Murry D J, Hohl R J. Pharmacodynamic effects of high dose lovastatin in subjects with advanced malignancies. Cancer Chemother Pharmacol. 2006;57:155–164. doi: 10.1007/s00280-005-0013-8. [DOI] [PubMed] [Google Scholar]
- Flint O P, Masters B A, Gregg R E, Durham S K. Inhibition of cholesterol synthesis by squalene synthase inhibitors does not induce myotoxicity in vitro. Toxicol Appl Pharmacol. 1997;145:91–98. doi: 10.1006/taap.1997.8131. [DOI] [PubMed] [Google Scholar]
- Matzno S, Yamauchi T, Gohda M, Ishida N, Katsuura K, Hanasaki Y, Tokunaga T, Itoh H, Nakamura N. Inhibition of cholesterol biosynthesis by squalene epoxidase inhibitor avoids apoptotic cell death in L6 myoblasts. J Lipid Res. 1997;38:1639–1648. [PubMed] [Google Scholar]
- Crick D C, Andres D A, Waechter C J. Novel salvage pathway utilizing farnesol and geranylgeraniol for protein isoprenylation. Biochem Biophys Res Commun. 1997;237:483–487. doi: 10.1006/bbrc.1997.7145. [DOI] [PubMed] [Google Scholar]
- Ren Z, Elson C E, Gould M N. Inhibition of type I and type II geranylgeranyl-protein transferases by the monoterpene perillyl alcohol in NIH3T3 cells. Biochem Pharmacol. 1997;54:113–120. doi: 10.1016/s0006-2952(97)00151-2. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Honda T, Yokoya S, Waguri S, Kimura J. Rab-small GTPases are involved in fluvastatin and pravastatin-induced vacuolation in rat skeletal myofibers. FASEB J. 2007;21:4087–4094. doi: 10.1096/fj.07-8713com. [DOI] [PubMed] [Google Scholar]
- Baba T T, Nemoto T K, Miyazaki T, Oida S. Simvastatin suppresses the differentiation of C2C12 myoblast cells via a Rac pathway. J Muscle Res Cell Motil. 2008;29:127–134. doi: 10.1007/s10974-008-9146-9. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Ivorra C, Kubicek M, Gonzalez J M, Sanz-Gonzalez S M, Alvarez-Barrientos A, O'Connor J E, Burke B, Andres V. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev. 2006;20:307–320. doi: 10.1101/gad.349506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu F, Adamo M L. Cyclic AMP inhibits extracellular signal-regulated kinase and phosphatidylinositol 3-kinase/Akt pathways by inhibiting Rap1. J Biol Chem. 2001;276:37242–37249. doi: 10.1074/jbc.M105089200. [DOI] [PubMed] [Google Scholar]
- Buerger C, DeVries B, Stambolic V. Localization of Rheb to the endomembrane is critical for its signaling function. Biochem Biophys Res Commun. 2006;344:869–880. doi: 10.1016/j.bbrc.2006.03.220. [DOI] [PubMed] [Google Scholar]
- Hattori M, Minato N. Rap1 GTPase: functions, regulation, and malignancy. J Biochem. 2003;134:479–484. doi: 10.1093/jb/mvg180. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Sanchez-Franco F, Palacios N, Sanchez I, Cacicedo L. IGF-I and vasoactive intestinal peptide (VIP) regulate cAMP-response element-binding protein (CREB)-dependent transcription via the mitogen-activated protein kinase (MAPK) pathway in pituitary cells: requirement of Rap1. J Mol Endocrinol. 2005;34:699–712. doi: 10.1677/jme.1.01703. [DOI] [PubMed] [Google Scholar]
- Romano D, Pertuit M, Rasolonjanahary R, Barnier J V, Magalon K, Enjalbert A, Gerard C. Regulation of the RAP1/RAF-1/extracellularly regulated kinase-1/2 cascade and prolactin release by the phosphoinositide 3-kinase/AKT pathway in pituitary cells. Endocrinology. 2006;147:6036–6045. doi: 10.1210/en.2006-0325. [DOI] [PubMed] [Google Scholar]
- Van Kolen K, Gilany K, Moens L, Esmans E L, Slegers H. P2Y12 receptor signalling towards PKB proceeds through IGF-I receptor cross-talk and requires activation of Src, Pyk2 and Rap1. Cell Signal. 2006;18:1169–1181. doi: 10.1016/j.cellsig.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Pizon V, Baldacci G. Rap1A protein interferes with various MAP kinase activating pathways in skeletal myogenic cells. Oncogene. 2000;19:6074–6081. doi: 10.1038/sj.onc.1203984. [DOI] [PubMed] [Google Scholar]
- Ulucan C, Wang X, Baljinnyam E, Bai Y, Okumura S, Sato M, Minamisawa S, Hirotani S, Ishikawa Y. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H1662–H1672. doi: 10.1152/ajpheart.00159.2007. [DOI] [PubMed] [Google Scholar]
- Castro A F, Rebhun J F, Clark G J, Quilliam L A. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J Biol Chem. 2003;278:32493–32496. doi: 10.1074/jbc.C300226200. [DOI] [PubMed] [Google Scholar]
- Maurer-Stroh S, Koranda M, Benetka W, Schneider G, Sirota F L, Eisenhaber F. Towards complete sets of farnesylated and geranylgeranylated proteins. PLoS Comput Biol. 2007;3:e66. doi: 10.1371/journal.pcbi.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Inoki K, Guan K L. Biochemical and functional characterizations of small GTPase Rheb and TSC2 GAP activity. Mol Cell Biol. 2004;24:7965–7975. doi: 10.1128/MCB.24.18.7965-7975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieulet V, Roceri M, Espeillac C, Sotiropoulos A, Ohanna M, Oorschot V, Klumperman J, Sandri M, Pende M. S6 kinase inactivation impairs growth and translational target phosphorylation in muscle cells maintaining proper regulation of protein turnover. Am J Physiol Cell Physiol. 2007;293:C712–C722. doi: 10.1152/ajpcell.00499.2006. [DOI] [PubMed] [Google Scholar]
- Tomasetti M, Alleva R, Solenghi M D, Littarru G P. Distribution of antioxidants among blood components and lipoproteins: significance of lipids/CoQ10 ratio as a possible marker of increased risk for atherosclerosis. Biofactors. 1999;9:231–240. doi: 10.1002/biof.5520090218. [DOI] [PubMed] [Google Scholar]
- Marcoff L, Thompson P D. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–2237. doi: 10.1016/j.jacc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]