Abstract
The integrity of lung alveoli is maintained by proper circulating levels of α-1 antitrypsin (A1AT). Next to cigarette smoking, A1AT deficiency is a major risk factor for lung emphysema development. We recently reported that in addition to neutralizing neutrophil elastases in the extracellular compartment, A1AT is internalized by lung endothelial cells and inhibits apoptosis. We hypothesized that the intracellular uptake of A1AT by endothelial cells may be required for its protective function; therefore, we studied the mechanisms of A1AT internalization by primary rat lung microvascular endothelial cells and the effect of cigarette smoke on this process both in vitro and in vivo (in mice). Purified A1AT was taken up intracellularly by endothelial cells in a time-dependent, dose-dependent, and conformer-specific manner and was detected in the cytoplasm of endothelial cells of nondiseased human lung sections. Despite a critical role for caveoli in endothelial cell endocytosis in general, specific inhibition of clathrin-mediated, but not caveoli-mediated, endocytosis profoundly decreased A1AT internalization and reversed the A1AT’s antiapoptotic action. Further more, A1AT associated with clathrin heavy chains, but not with caveolin-1 in the plasma membrane fraction of endothelial cells. Interestingly, cigarette smoke exposure significantly inhibited A1AT uptake both in endothelial cells and in the mouse lung and altered the intracellular distribution of clathrin heavy chains. Our results suggest that clathrin-mediated endocytosis regulates A1AT intracellular function in the lung endothelium and may be an important determinant of the serpin’s protection against developing cigarette smoke-induced emphysema. Sohrab, S., Petrusca, D. N., Lockett, A. D., Schweitzer, K. S., Rush, N. I., Gu, Y., Kamocki, K., Garrison, J., Petrache, I. Mechanism of α-1 antitrypsin endocytosis by lung endothelium.
Keywords: vascular, serpin, cigarette smoke, caveolin, COPD, apoptosis
α-1 Antitrypsin is the archetype of the serine protease inhibitor or serpin superfamily, which protects the lung by inhibiting serine proteases, including neutrophil elastase, cathepsin G, and proteinase-3 (1). This inhibitory function occurs primarily extracellularly after the protein has been secreted from its principal source, the liver. We recently demonstrated a novel antiapoptotic role for A1AT in the lung both in vivo and in vitro in microvascular endothelial cells (2, 3). The antiapoptotic effect on lung endothelium was associated with intracellular presence of A1AT (2). Because lung endothelial cells do not produce A1AT and because the intracellular A1AT activity in the microvascular endothelium may be of critical importance to the maintenance of lung alveolar integrity and function, we investigated the mechanism responsible for A1AT uptake by lung endothelium.
To take up nutrients or to scavenge unwanted molecules from their environment, cells utilize several endocytosis mechanisms, including clathrin-, caveoli-, and nonclathrin, noncaveoli-mediated endocytosis, as well as macropinocytosis (4). Endothelial cells primarily utilize caveoli-mediated endocytosis to internalize molecules, including proteins such as albumin, whereas clathrin-mediated endocytosis facilitates the internalization of selectins (5). Although caveolar proteins were found associated with A1AT-treated endothelial cells (6), the precise mechanism required for A1AT internalization by lung endothelial cells remains unknown.
A1AT deficiency is one of the most common genetic causes of emphysema, which, when present, requires replacement therapy with purified A1AT pooled from donor plasma (7). This condition, which remains underdiagnosed (8), is associated with retention of mutant polymerized A1AT in hepatocytes and therefore low levels of circulating A1AT, allowing for unopposed action of neutrophil elastase to degrade elastin, a major component of the lung matrix. Moreover, cigarette smoking has been reported to oxidize A1AT, decreasing its inhibitory action against elastase (9) and, as we reported, caspase-3 (2). What effect these clinically relevant changes of polymerization and oxidation have on the A1AT uptake by the lung endothelium remains unknown. Furthermore, the effect of cigarette smoke exposure on the cellular endocytosis mechanism in general is underappreciated. In the present study, we investigated the mechanism by which A1AT is internalized by primary microvascular endothelial cells and the effect of cigarette smoke on this process in vitro and in vivo.
MATERIALS AND METHODS
Chemicals and reagents
Chemicals and reagents, including purified human A1AT, were obtained from Sigma (St. Louis, MO, USA), unless otherwise specified. Specific human A1AT antibody was from Bethyl (Montgomery, TX, USA), Filipin 111 was from Cayman Chemicals (Ann Arbor, MI, USA), and BODIPY FL C5-lactosylceramide and Alexa Fluor 488 transferrin were from Molecular Probes/Invitrogen (Carlsbad, CA, USA).
Cells and cell culture
Primary rat lung endothelial cells, kindly provided by Dr. Troy Stevens (University of South Alabama, Mobile, AL, USA), were maintained in Dulbecco’s modified Eagle’s high-glucose medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C in 5% CO2 and 95% air and utilized in culture up to passage 20. Human primary lung microvascular endothelial cells were obtained from Lonza (Walkersville, MD, USA) and maintained in EMB2 medium with the appropriate growth factor supplements. Primary mouse lung endothelial cells were a gift from Dr. Patty J. Lee (Yale University, New Haven, CT, USA), and were maintained in the same medium used for rat endothelial cells, except with 20% FBS. Human dermal microvascular cells and human umbilical vein endothelial cells were a gift from Dr. Matthias Clauss (Indiana University). Primary rat alveolar macrophages were obtained by bronchoalveolar lavage of Sprague-Dawley rats, and monocyte-derived macrophages were obtained from American Type Culture Collection (Manassas, VA, USA). Cells were maintained in complete culture medium consisting of Dulbecco’s modified Eagle’s high-glucose medium (Life Technologies, Grand Island, NY, USA) with 20% fetal bovine serum and 1% penicillin/streptomycin at 37°C in 5% CO2 and 95% air. For confocal microscopy, cells were grown on glass coverslips coated with 1% gelatin.
A1AT labeling and treatment
Human A1AT was labeled with Alexa Fluor 488 (Molecular Probes) with the excess dye being removed via gravity flow, using the resin provided by the manufacturer. The concentration of labeled A1AT was calculated using NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA). The labeled A1AT retained a potent antielastase activity after the labeling procedure (Supplemental Fig. 1). For several experiments, A1AT was also labeled using Dylight NHS ester (Pierce, Rockford, IL, USA). To generate A1AT polymers, native A1AT was heated at 60°C for 2 h (2).
Experiments in cultured primary lung endothelial cells were performed at 80–90% confluence. The FBS-containing medium was replaced with basal medium for 2 h prior to treatment with A1AT. At the conclusion of the experiment, cells were washed 3 times with ice-cold PBS, to remove any extracellular A1AT. The amount of A1AT internalized by cells was analyzed using microscopy, immunoblotting of the cytoplasm fractions, or flow cytometry. For flow cytometry studies, the extracellular fluorescence was quenched with Trypan blue (0.04%).
Cigarette smoke extract preparation and treatments
Aqueous cigarette smoke extract was prepared from filtered research-grade cigarettes (1R3F) from the Kentucky Tobacco Research and Development Center (University of Kentucky, Lexington, KY, USA). Cigarette smoke extract (100%) was prepared by bubbling smoke from 2 cigarettes into 20 ml of basal culture medium at a rate of 1 cigarette/min to 0.5 cm above the filter (10), followed by pH adjustment to 7.4 and filtration (0.2 μm, 25 mm Acrodisc; Pall, Ann Arbor, MI, USA). A similar procedure was followed for the air control extract preparation, replacing the bubbling of cigarette smoke with that of ambient air into the cell culture medium. For experiments involving exposure ex vivo, the extract was prepared in PBS followed by filtration and pH adjustment as above. The final concentrations of cigarette smoke extract used in cell culture experiments ranged from 0.1 to 7%, to avoid cell death (observed at >10%); whereas the concentrations used in ex vivo experiments of coincubation with A1AT purified protein to induce oxidation of A1AT (11) ranged from 0 to 50%.
Endocytosis inhibition studies
The uptake of lactosyl-ceramide (LacCer), known to exclusively enter cells by caveolin-mediated endocytosis (12), was studied using C5-BODIPY-LacCer, as described previously (13). Briefly cells were placed in basal medium, followed by addition of BODIPY-LacCer (1 μM; 10°C; 30 min). Cells were then washed with ice-cold minimum Eagle’s medium with HEPES (10 mM), then placed at 37°C (5 min) to induce endocytosis of attached LacCer. Fluorescent lipid on the cell surface was removed by incubating cells with defatted bovine serum albumin (5% in HMEM-G at 10°C) (14). Filipin (3 μg/ml or 5 μg/ml) and nystatin (25 μg/ml), inhibitors of caveoli-mediated endocytosis (13, 15), were employed in A1AT uptake studies at concentrations that effectively inhibited the endocytosis of LacCer into lung endothelial cells (at least 80% inhibition determined in pilot studies, not shown).
The uptake of AF488 fluorescently labeled transferrin (5 μg/ml), known to exclusively enter cells by clathrin-mediated endocytosis (16), was studied in lung endothelial cells deprived of fetal bovine serum for 2 h (to up-regulate transferrin receptors) (13). After transferrin treatment, cells were first incubated at 10°C (30 min), washed with HMEM, and then incubated at 37°C (2 h). Chlorpromazine, a specific inhibitor of clathrin-mediated endocytosis (17), was employed at concentrations (10–20 μM) that effectively inhibited (at least by 80%) transferrin endocytosis in lung endothelial cells without inducing cell death (as determined in pilot experiments, not shown).
Cholesterol depletion, which inhibits both clathrin- and caveolin-mediated endocytosis, was accomplished by β-methyl-cyclodextrin (18).
Knockdown of rat clathrin heavy chain was achieved using siGENOME rCHC siRNA (0–100 nM), nontarget siRNA (0–100 nM), or siGlo (100 nM; Dharmacon, Lafayette, CO, USA) for 48 h following transfection using siPORT NeoFX (Applied Biosystems, Foster City, CA, USA).
Flow cytometry
After treatments, primary rat lung endothelial cells were washed with ice-cold PBS, and the extracellular fluorescent protein was quenched with 0.04% Trypan blue at room temperature for 1 min. Cells were then collected via scraping and were fixed with paraformaldehide (1%). The uptake of fluorescently labeled molecules was quantified by flow cytometry using a Beckman Coulter Cytomics FC500 cytofluorimeter (Beckman Coulter, Fullerton, CA, USA).
Western blot analysis
Cells were harvested and washed, and the cytosolic or membrane fractions were extracted with the Proteoextract kit (Calbiochem, San Diego, CA, USA),using the manufacturer’s protocol. Endothelial cell lysates were loaded in equal amounts (10 μg, unless otherwise specified), as determined by BCA protein analysis (Pierce). Proteins were separated by SDS-PAGE or native PAGE, followed by routine immunoblotting, as described previously (2). The chemiluminescent signals were detected using ECL or ECL-plus (Amersham; Piscataway, NJ, USA), quantified by densitometry, and normalized using specific vinculin (1:5000; Calbiochem) or actin (1:30,000; Sigma) antibody.
Microscopy and colocalization studies
After treatments, primary rat lung endothelial cells grown on coverslips were fixed with paraformaldehyde and mounted on slides using Slow Fade with DAPI (nuclear stain). Slides were visualized by conventional fluorescent microscopy using Olympus 1 × 70 fluorescence microscope (Olympus, Tokyo, Japan) and by confocal microscopy with a Zeiss model 510 laser scanning microscope (Carl Zeiss, Oberkochen, Germany). For quantitative studies, all photomicrographs in a given experiment were exposed and processed identically for a given fluorophore; images were captured in a blinded fashion, and the intensity of staining was quantified using Metamorph software (Molecular Devices, Downingtown, PA, USA).
Animal studies
DBA2 mice (males, 4 mo old, from Charles River Laboratories, Raleigh, NC, USA) were exposed to a cigarette smoke in a smoking chamber (TE-10Z; Teague Enterprises, Woodland, CA, USA) for 5 h/d, 5 d/wk, 2 wk (n=16) or to ambient air (n=11). Research-grade cigarettes (1R3F; Kentucky Tobacco Research and Development Center) were smoked at a rate that achieved 70–90 ng/m3 microparticles in the exposure chamber. Following smoke exposure for the indicated time, animals were injected in the tail vein with 20 mg/kg of either human unlabeled or Dylight-labeled A1AT (Sigma; n=19) or sterile PBS (Invitrogen; n=8). At the end of the experiment, the blood was collected, and the pulmonary circulatory system was washed with PBS (5× circulatory volume), followed by lung inflation and fixation in either formalin or optimal cutting temperature (OCT) medium (19).
Human samples
Human lung tissue sections from fixed, paraffin-embedded explanted lung tissue from nondiseased patients were generously provided by Dr. Rubin Tuder (Johns Hopkins University, Baltimore, MD, USA, and the University of Colorado, Denver, CO, USA). The specimen collection and storage were approved by the Johns Hopkins University Institutional Research Board.
Immunohistochemistry
Immunohistochemistry was performed on 4-μm sections of paraffin-embedded tissues or from frozen tissue. Briefly, after deparaffinization, antigen retrieval was performed (Antigen Unmasking Solution; Vector Laboratories, Burlingame, CA, USA), followed by blocking endogenous peroxidase activity with 3% H2O2 and nonspecific sites with 1% BSA (1 h). Sections were then incubated at room temperature with primary antibodies against human A1AT (A0012, 1:100 dilution; Dako, Carpinteria, CA, USA) for 1 h. After washing the sections, the appropriate secondary antibody was applied (Vector Universal Elite Kit; Vector Laboratories). Immunostaining was visualized with 3–3′-diaminobenzidine (Vector Laboratories). Notably, this protocol was optimized for the mouse lung tissue, in order to minimize background staining in the lungs of animals, which did not receive human A1AT; for this experiment, a human lung tissue section was stained simultaneously as a positive control. For the immunofluorescence colocalization studies in human lung tissue sections, the protocol was optimized for the human lung, in order to minimize the typical high green autofluorescence of the lung structures. After incubation with primary antibodies against human A1AT, sections were incubated with primary antibodies against CD31 (SC-1506; 1:50 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After the sections were washed, the appropriate fluorescently labeled secondary antibodies were applied (Invitrogen), and nuclei were counterstained with DAPI (Slow Fade Gold; Eugene, OR, USA).
Frozen sections from animals receiving fluorescently labeled A1AT were visualized under fluorescence microscope, without antibody staining.
RESULTS
Kinetics and mechanism of A1AT uptake by primary lung microvascular endothelial cells
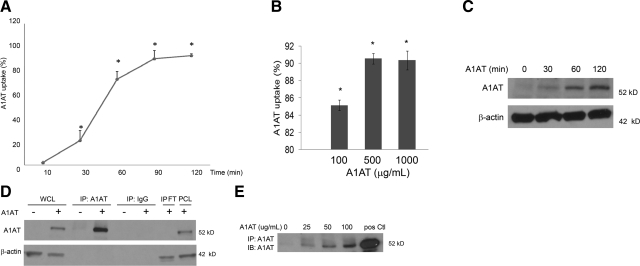
Primary rat lung microvascular endothelial cells were treated with purified A1AT labeled with Alexa Fluor 488. To avoid the effect of A1AT-containing serum, these experiments were performed in serum-free conditions. The cellular internalization was measured by flow cytometry, where the fluorescence of noninternalized A1AT was quenched, to ensure that only intracellular fluorescence was detected. Lung endothelial cells internalized A1AT in a time-dependent manner, beginning as early as 10 min following treatment (2% of cells demonstrated uptake), with a peak at 1.5 h (>90% of cells showed intracellular fluorescent signal) and a plateau after 2 h of treatment with A1AT (Fig. 1A). Similar results were obtained in both primary human lung microvascular cells (Fig. 1B) and primary mouse lung endothelial cells (not shown). There was a plateau in the uptake of A1AT after treating human lung microvascular endothelial cells with 500 μg/ml A1AT (Fig. 1B) and rat cells with 100 μg/ml (not shown). Kinetics of A1AT uptake by primary rat lung vascular endothelial cells were also studied by Western blot analysis, where cells were treated with nonlabeled purified human A1AT and then cell lysates were immunoblotted with a specific human A1AT antibody (Fig. 1C). Immunoprecipitation experiments with a specific human A1AT antibody confirmed the cellular uptake of A1AT (Fig. 1D) and documented the dose-response of the uptake with increasing concentration of A1AT treatment (Fig. 1E).
Figure 1.
Kinetics and dose response of A1AT uptake by primary lung endothelial cells. A) Time course of Alexa Fluor 488-tagged A1AT (100 μg/ml) internalization by primary rat lung microvascular endothelial cells, measured by flow cytometry. Data represent mean ± se percentage of cells that stained positive for A1AT. *P < 0.05 vs. nontreated cells; ANOVA. B) Quantification of Alexa Fluor 488-tagged A1AT (at indicated concentrations; 4 h) internalization by primary human lung microvascular endothelial cells, as measured by flow cytometry. Data represent mean ±se percentage of cells that stained positive for A1AT. *P < 0.05 vs. nontreated cells; ANOVA. C) Immunoblots of A1AT and actin (as loading control) of cells treated with A1AT (100 μg/ml) for indicated time. D) Immunoblots of A1AT and actin (as loading control) following immunoprecipitation with A1AT antibody (IP: A1AT) or with control IgG (IP: IgG) in cells treated with A1AT (100 μg/ml; 4 h; +) or with vehicle (PBS; −). WCL, whole cell lysate; IPFT, immunoprecipitation flow-through; PCL, lysate after bead preclearance prior to A1AT precipitation. E) Immunoblot of A1AT uptake following immunoprecipitation with A1AT antibody of cells treated with indicated concentrations of A1AT for 4 h. pos Ctl, purified A1AT (500 ng).
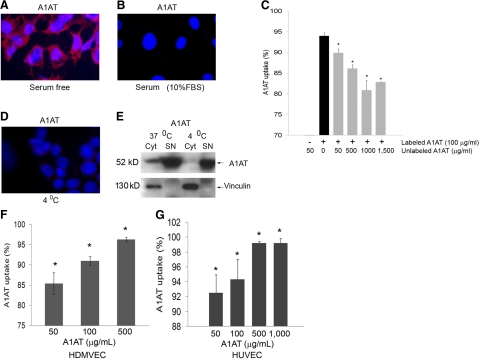
Interestingly, both fetal bovine serum (10–20% in the culture medium), which contains endogenous bovine A1AT (Fig. 2A, B) and increasing nonlabeled purified human A1AT concentrations (Fig. 2C) had significant dose-dependent inhibitory effect on the A1AT internalization. The internalization of A1AT was also profoundly decreased when cells were treated with A1AT at 4°C when compared to cells treated at 37°C, as demonstrated by confocal microscopy (Fig. 2D) and immunoblotting (Fig. 2E). These results suggested the process of A1AT internalization is a metabolically active and substrate-specific process. Furthermore, endothelial cells from other vascular beds, such as human dermal and umbilical vein endothelial cells, internalized labeled A1AT, as detected by flow cytometry (Fig. 2F, G, respectively), suggesting that this effect is not limited to the lung endothelium. In addition, macrophages (human monocyte-derived macrophages and primary rat alveolar macrophages) also exhibited A1AT internalization (Supplemental Fig. 1B).
Figure 2.
Specificity of A1AT uptake by primary lung endothelial cells. A, B) Representative micrographs of fluorescence microscopy studies of primary rat lung endothelial cells treated with labeled A1AT (red; arrow; 100 μg/ml; 2 h) in the absence (A) or presence of serum (10% FBS) (B). Nuclei were stained with DAPI. C) Effect of unlabeled A1AT (at indicated concentrations; 15 min pretreatment) on uptake of labeled A1AT (100 μg/ml; 2 h treatment) by primary rat lung endothelial cells, as measured by flow cytometry. Data represent mean ± se percentage labeled A1AT uptake. *P < 0.05 vs. uptake in absence of unlabeled A1AT. D) Effect of cold temperature of A1AT uptake in endothelial cells treated in serum-free conditions with A1AT in similar conditions as in A but placed at 4°C for the duration of uptake. Note absence of intracellular red staining compared to A. E) Immunoblots of A1AT and vinculin (as loading control) of cytoplasmic (Cyt) or supernatant (SN) fractions of cultured endothelial cells following treatment with A1AT (100 μg/ml; 2 h) at 37°C or 4°C. Note lack of uptake of A1AT into cytoplasm fraction of cells treated with A1AT at 4°C. F, G) Quantification of Alexa Fluor 488-tagged A1AT (at indicated concentrations; 4 h) internalization by primary human dermal microvascular endothelial cells (HDMVEC; F) or human umbilical vein endothelial cells (HUVEC; G), as measured by flow cytometry. Data represent mean ± se percentage of cells that stained positive for A1AT. *P < 0.05 vs. nontreated cells; ANOVA.
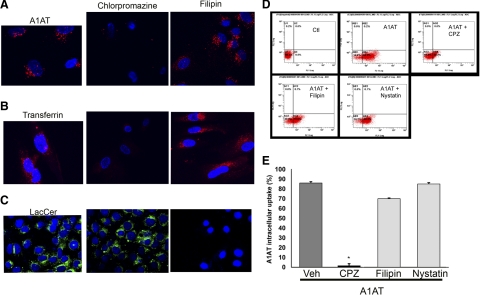
Next, we investigated whether A1AT is taken up by endothelial cells via endocytosis by using a nonspecific cholesterol-depleting agent, β-methyl-cyclodextrin (18). Pretreatment of rat lung endothelial cells with β-methyl-cyclodextrin effectively blocked A1AT endocytosis, measured by flow cytometry. Compared to A1AT-treated cells, of which 33% had positive fluorescence, indicating intracellular A1AT uptake, none of the cells pretreated with β-methyl-cyclodextrin (5 mM; 30 min) prior to A1AT (50 μg/ml; 30 min) manifested A1AT uptake (data not shown). Because the endothelial cell membrane is rich in caveoli, which are themselves located in cholesterol-rich microdomains, we examined their role in A1AT uptake. Sterol binding agents, such as filipin, bind to cholesterol, a major component of rafts and caveoli, and disrupt caveolar structure and function (15). Lung endothelial cells were pretreated with caveoli-mediated endocytosis inhibitors filipin or nystatin, followed by treatment with either fluorescently tagged A1AT or labeled LacCer, as a positive control (12). In contrast with the profound inhibitory effect of filipin or nystatin on LacCer endocytosis, their effect on A1AT uptake was only marginal (10% and 0 inhibition of uptake, respectively) (Fig. 3). Chlorpromazine is a cationic amphiphilic inhibitor of clathrin-dependent endocytosis by causing clathrin lattices to assemble on endosomal membranes, while simultaneously preventing coated pit formation at the plasma membrane (17). Unlike caveoli inhibitors, pretreatment with chlorpromazine at concentrations that inhibit transferrin uptake (Fig. 3B) markedly inhibited the A1AT internalization by primary rat lung endothelial cells in a dose-dependent manner (10 and 20 μg/ml inhibited 70 and 98% of A1AT uptake, respectively; Fig. 3). These results suggested that A1AT internalization in primary rat lung endothelial cells occurs predominantly via clathrin-mediated endocytosis.
Figure 3.
Effect of endocytosis inhibitors on A1AT uptake by lung endothelial cells. A–C) Representative fluorescence microscopy micrographs of primary rat lung microvascular endothelial cells treated with labeled A1AT (red; 100 μg/ml; 2 h; n=4 independent experiments) (A), labeled transferrin (red; 100 μg/ml; 2 h) (B), or labeled LacCer (green; 100 μg/ml; 2 h) (C) at baseline (left panels) or following pretreatment with filpin (3 μg/ml; 1 h) (right panels) or chlorpromazine (10 μg/ml; 1 h) (center panels). D) Representative panels of flow cytometry detection of labeled-A1AT uptake of lung endothelial cells. Internalized A1AT is observed in bottom right quadrant after A1AT treatment (100 μg/ml; 2 h; A1AT panel) compared to untreated cells (Ctl panel), or to cells treated with A1AT following pretreatment (1 h) with endocytosis inhibitors chlorpromazine (A1AT+CPZ; 10 μg/ml), filipin (3 μg/ml), or nystatin (25 μg/ml). E) Quantification of flow cytometry experiments described in D. Data represent mean ± se percentage of cells with labeled A1AT uptake; n = 3 independent experiments. *P < 0.05 vs. A1AT-treated cells; ANOVA.
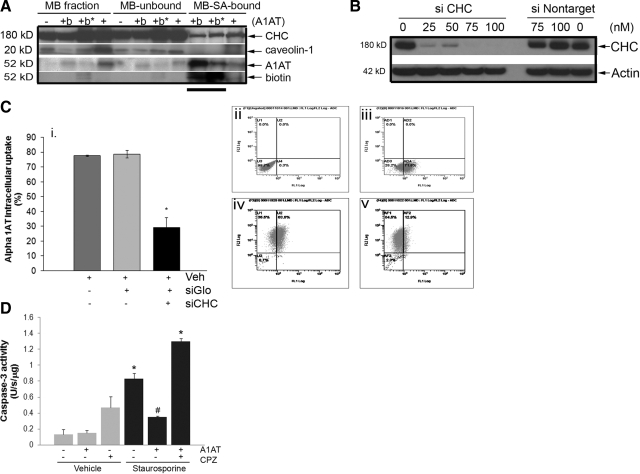
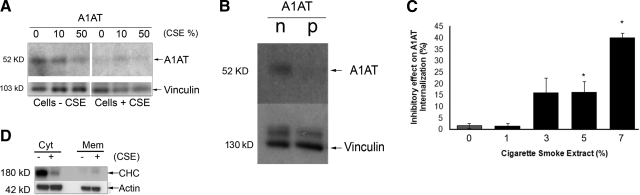
Clathrin-mediated endocytosis involves formation of “coated pits” on the plasma membrane by specialized proteins, the main assembly unit being clathrin. The 3-legged clathrin structure (triskeleton) comprises 3 clathrin heavy chains (CHCs) and 3 tightly associated light chains, which are crucial for driving the membrane invagination and vesicle formation. Immunoprecipitation with A1AT-specific antibody revealed an association of A1AT and CHC but not caveolin-1 in the plasma membrane fraction of rat lung endothelial cells following treatment with A1AT for 10 min (Fig. 4A). Knockdown of CHC expression in primary rat lung endothelial cells was achieved using CHC-specific siRNA (siCHC), which effectively impairs clathrin-mediated endocytosis (20). Transfection and internalization of siRNA were highly efficient, as judged by the presence of the cotransfected siRNA-Glo marker by flow cytometry (Fig. 4C), which was also used to identify cells with CHC knockdown for flow cytometric quantification of A1AT uptake in these particular cells. Under these conditions, CHC expression was reduced significantly and dose dependently in the siCHC-transfected cells relative to the untreated or nontargeted-siRNA-transfected cells (Fig. 4B). A1AT uptake was significantly decreased only in the siCHC-transfected cells compared to cells treated with a nontarget-labeled siRNA and to nontransfected rat lung endothelial cells (Fig. 4C). These results implicate clathrin-mediated endocytosis as the principal mechanism of A1AT uptake by primary lung endothelial cells.
Figure 4.
Association of A1AT with clathrin-mediated endocytosis in lung endothelial cells. A) Immunoblots with antibodies against clathrin heavy chain (CHC), caveolin-1, A1AT, or biotin of primary rat lung microvascular endothelial cell membrane (MB) fractions after treatment with A1AT (+), biotin-labeled A1AT (+b; 100 μg/ml; 15 min), or vehicle (−), before (MB fraction) or after streptavidin (SA) precipitation (MB-unbound and MB-SA-bound fractions, respectively). *Presence of a membrane crosslinker, BS3. Note presence of CHC but not caveolin-1 that coprecipitates with biotinylated-A1AT (horizontal line). B) Immunoblot of CHC (or actin used as loading control) of primary rat lung microvascular endothelial cells treated with siRNA against clathrin heavy chain (siCHC) or nontarget siRNA (siNontarget) at indicated concentrations for 48 h. C) Quantification of labeled A1AT uptake of rat lung endothelial cells by flow cytometry after treatment with A1AT (100 μg/ml; 2 h) in presence of vehicle (Veh), labeled-nontarget siRNA (siGlo; 100 nM; 48 h), or siGlo + siCHC (100 nM each; 48 h). i) Mean ± se percentage of cells with positive A1AT uptake. *P < 0.05 vs. Veh. ii) Untreated cells. iii) Labeled A1AT-treated cells. iv) siGlo-positive A1AT-treated cells. v) siGlo-positive siCHC-treated and A1AT-treated cells. D) Effect of chlorpromazine (CPZ) on the inhibitory effect of A1AT on staurosporine-induced caspase-3 activity in lung endothelial cells (mean±se). *P < 0.05 vs. vehicle only; #P < 0.05 vs. staurosporine only).
To determine whether the clathrin-mediated A1AT endocytosis is necessary for its intracellular function, we studied the effect of chlorpromazine on the A1AT antiapoptotic action. Primary rat lung microvascular endothelial cells were pretreated with chlorpromazine, followed by A1AT and then by apoptosis-inducing concentrations of staurosporine. Inhibition of clathrin-mediated A1AT intracellular uptake abolished the antiapoptotic effect of A1AT (Fig. 4D), suggesting that an active intracellular uptake of A1AT is required for its antiapoptotic function in lung endothelial cells.
Inhibitory effect of cigarette smoke exposure on A1AT internalization by primary lung endothelial cells
Interestingly, both the oxidized (via cigarette smoke extract coincubation) and the polymerized A1AT conformers exhibited decreased uptake by primary lung endothelial cells compared to the native A1AT (Fig. 5A, B). Although decreases in circulating levels of A1AT are associated with increases in the risk of cigarette smoke-induced lung damage, it remains unclear why normal levels of circulating A1AT are not sufficient to protect from cigarette smoke-induced injury. Besides a known direct oxidative effect on the serpin structure (11), we hypothesized that cigarette smoke may impair the entry of A1AT into endothelial cells, and thus deprive lung structural cells from the protective intracellular effects of A1AT. Primary rat lung endothelial cells were incubated with increasing concentrations of cigarette smoke extract (0.1, 1, 3, 5, and 7%) or ambient air extract (as control) prior to labeled-A1AT treatment. Compared to control cells, lung endothelial cells preincubated with cigarette smoke extract markedly decreased the intracellular A1AT content, measured by confocal microscopy (not shown) or flow cytometry (Fig. 5C). Compared to native A1AT, oxidized A1AT (from incubation of purified protein with cigarette smoke extract) had a decreased uptake in endothelial cells, but the inhibitory effect on uptake was most pronounced when both A1AT and endothelial cells were exposed to cigarette smoke extract (Fig. 5A).
Figure 5.
Effect of cigarette smoke extract on A1AT endocytosis in lung endothelial cells. A) Immunoblots of A1AT and vinculin (as loading control) of primary rat lung microvascular endothelial cells preexposed to cigarette smoke extract (5%, 2 h; Cells+CSE) or ambient air extract as vehicle control (5%; Cells−CSE) and then treated with A1AT (100 μg/ml; 4 h) that was preincubated with CSE at indicated concentrations (CSE %) prior to addition to cells in culture. B) Immunoblots of A1AT and vinculin (as loading control) in cell lysates after treatment with native (n) or polymerized (p) A1AT (100 μg/ml; 4 h). C) Quantification of inhibition effect of CSE (at indicated concentrations, 2 h) on uptake of A1AT, as measured by flow cytometry. Data represent mean ± se inhibition. *P < 0.05 vs. non-CSE-exposed control cells. D) Immunoblots of clathrin-heavy chain (CHC) and actin (as loading control) of endothelial cell lysate cytosolic (Cyt) and membrane (Mem) fractions in the absence (−) and presence (+) of CSE (7%; 2 h).
Next, we investigated whether cigarette smoke extract decreases the A1AT uptake by interfering with the process of clathrin-mediated endocytosis. Cigarette smoke exposure (5%; 2 h) decreased by 15% the uptake of transferrin but not LacCer (data not shown) and changed the distribution of clathrin heavy chains between the plasma membrane (increasing it) and the cytoplasm (decreasing it) of lung endothelial cells compared to that in cells exposed to ambient air extract (Fig. 5D).
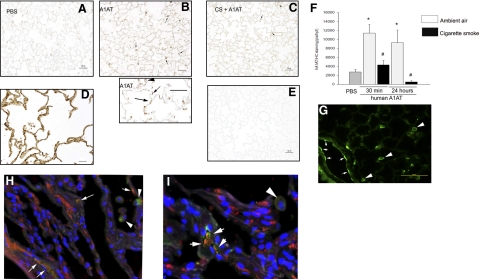
To investigate whether the inhibitory effect of cigarette smoking on A1AT uptake occurs in vivo, we exposed mice to cigarette smoke for 5 h/d, 5 d/wk, for 2 wk. Following cigarette smoke exposure, mice were administered human A1AT intravenously once. Mice were sacrificed either at 30 min or 24 h after this single A1AT injection, and the vasculature was flushed with saline (5× circulating volume) to remove any circulating A1AT. The presence and distribution of A1AT in the lungs were determined using immunohistochemistry with a specific human A1AT antibody. In nonexposed mice, human A1AT was taken up by lungs as early as 30 min and was still detectable at 24 h (Fig. 6A, B, F) in areas of alveolar walls and alveolar macrophages. In mice exposed to cigarette smoke, there was a dramatic and statistically significant decrease in the human A1AT staining in the lung after its injection, at both time points studied (Fig. 6C, F). Although appropriate controls (Fig. 6D, E) indicated specificity of the immunostaining for the exogenously administered A1AT, we complemented these studies with intravenous treatments with fluorescently labeled human A1AT followed by fluorescence microscopy of the frozen sections obtained from the mouse lung. These studies confirmed the pattern of intravenously administered A1AT uptake in mouse lung endothelial cells and alveolar macrophages (Fig. 6G). We next investigated whether lung endothelial cells in human lungs have evidence of intracytoplasmic A1AT in situ. Because human endothelial cells are not a source of A1AT, intracellular staining is likely to reflect A1AT taken up from circulation. Lung sections obtained from nondiseased individuals were coimmunostained for A1AT and the endothelial cell marker, CD31, and assessed by regular and confocal fluorescence microscopy. A1AT colocalized with endothelial cells lining vessels of various sizes, suggesting that A1AT is normally taken up in human lung endothelium (Fig. 6H, I).
Figure 6.
A1AT uptake in vivo. A–C) Representative micrographs of immunohistochemistry (IHC; brown staining, arrows) using specific human A1AT antibody on mouse lung sections from animals exposed to ambient air (A, B) or cigarette smoke (2 wk) and then injected intravenously with PBS (A) or human A1AT (20 mg/kg; 30 min) (B, C). D, E) Human lungs (D) and isotype IgG-stained mouse lungs (E) were utilized as positive and negative controls for IHC, respectively. Note intense brown staining (compared to nonspecific staining in A) in alveolar lung tissue of mice receiving A1AT (B, bottom panel, arrows), which suggested human A1AT presence in alveolar cells (arrows) and macrophages (arrowhead). F) Mean ± se intensity of A1AT IHC staining measured in a blinded fashion via image analysis software at 30 min or 24 h following intravenous injection of human A1AT in mice previously exposed to ambient air (gray bars) or cigarette smoke (black bars) for 2 wk. *P < 0.05 vs. control PBS treatment, #P < 0.05 vs. ambient air exposure; ANOVA. G) Confocal fluorescence microscopy of frozen sections from mouse lungs obtained 30 min following intravenous injection of fluorescently labeled-A1AT (20 mg/kg). Note intense green staining of endothelium (arrows) and alveolar macrophages (arrowheads). H, I) Coimmunofluorescence microscopy micrographs of paraffin-embedded sections from nondiseased human lungs stained with human A1AT-specific antibody (green); CD31 (endothelial cell marker-specific antibody; red) and DAPI (blue, nuclei). Note colocalization of A1AT with endothelial cell cytoplasm (white arrows) and A1AT staining of alveolar macrophages (arrowheads). Scale bars = 50 μm.
DISCUSSION
This study demonstrated that A1AT is taken up by lung endothelial cells predominantly by a clathrin-mediated endocytosis. The uptake is severely affected by exposure to cigarette smoke extract in vitro and in vivo, a process that may be due to a direct effect on clathrin-mediated endocytosis. Furthermore, polymerized A1AT, which mimics the post-translational changes in the protein that occur in the most common ZZ mutation accounting for A1AT deficiency, exhibited a marked decrease in lung endothelial cell uptake.
The kinetics of A1AT internalization and the blocking effect of cold temperature indicated an active process of intracellular uptake rather than diffusion, as had been previously suggested (21). The caveoli-mediated pathway is an important pathway of active transport in endothelial cells, since they make up 95% of cell surface vesicles and 20% of the endothelial cell volume (22). This pathway is responsible for the internalization of albumin and glycosphingolipids, such as lactosylceramide and is inhibited by filipin and nystatin. However, neither inhibitor of caveoli-mediated endocytosis had a major inhibitory effect on A1AT uptake. These results contrast with a recent report (6) that confirmed our previous observation that A1AT is internalized by lung endothelial cells, but invoked caveoli in its uptake, given the identification of A1AT in the caveolar fraction of the plasma membrane. Our study cannot rule out that A1AT localizes in this fraction of the plasma membrane, but our functional studies showed that the caveolin pathway inhibitor had no major blocking effect on A1AT uptake. While we cannot irrefutably rule out contributions of nonclathrin, noncaveoli-mediated endocytosis or macropinocytosis to the uptake of the protein, the profound effect of specific inhibitory strategies using chlorpromazine (23) and clathrin heavy-chain knockdown (20) indicated that A1AT was primarily internalized via clathrin-mediated endocytosis. Clathrin-mediated endocytosis occurs constitutively in all mammalian cells, and carries out the continuous uptake of essential nutrients, such as the cholesterol-laden low-density lipoprotein (LDL) particles that bind to the LDL receptor, and iron-laden transferrin that binds to transferrin receptor (24, 25).
A1AT is a prototypical serpin among the ∼30 other human serpin family members identified to date. Most serpins are extracellularly secreted, in the case of A1AT from sites of synthesis, primarily from hepatocytes, but also mononuclear phagocytes, and intestinal, renal, and lung epithelium (26, 27). Although little information exists regarding the cellular uptake of serpins after their release into the circulation, it has been shown that the complex formed when a serpin binds to its target enzyme is internalized by cells via endocytosis. A serpin-enzyme complex (SEC) receptor has been reported to be responsible for endocytosis of A1AT-elastase complex, A1AT-trypsin complex, and antichymotrypsin-cathepsin G complex in cultured hepatoma cells (28). The SEC receptor low-density-lipoprotein receptor-related protein does not, however, internalize free, uncomplexed A1AT (29), and indeed, we did not find the LDL receptor in the plasma A1AT immunoprecipitated with the membrane fraction containing the clathrin heavy chain (data not shown). To our knowledge, this is the first report to demonstrate a clathrin-mediated mechanism of intracellular uptake of a serpin. The fact that A1AT is internalized predominantly by clathrin-mediated endocytosis is not entirely surprising, in light of its rapid rate of internalization and of the finding that the mechanism by which particles enter nonphagocytic cells is strongly dependent on particle size (30). The native A1AT molecule is 7.8 × 4.9 × 2.2 nm (31) or <200 nm in diameter, and would be predicted to enter cells by a clathrin-mediated pathway, in contrast to larger-size particles, which were shown to utilize a caveoli-mediated pathway. A question still to be investigated is whether the internalized A1AT is further trafficked to the interstitium or remains retained within endothelial cells, where we and others have shown it exerts cytoprotective effects (2, 6, 32, 33).
Our results revealed that cigarette smoke exposure, at concentrations that did not induce cell death or obvious cytotoxicity, decreased the ability of primary lung endothelial cells to internalize A1AT in a dose-dependent manner. The relevance of the inhibitory effect of smoke exposure on endocytosis in cultured primary lung endothelial cells was confirmed by similar findings in a model of cigarette smoke exposure in vivo. The mechanism by which cigarette smoke impairs phagocytosis is unknown, but it may involve direct effects on the clathrin machinery regulating endocytosis, as cigarette smoke also inhibited the internalization of transferrin but not LacCer in endothelial cells and altered the distribution of clathrin heavy chains between the cytoplasm and the plasma membrane in endothelial cells. To our knowledge, this is the first report of an inhibitory effect of cigarette smoke on clathrin-mediated endocytosis. Previous studies have shown that cigarette smoke can impair phagocytosis in specialized phagocytes, such as macrophages and PMNs, by a yet unclear mechanism. Because the increase in the membrane fraction of CHC did not associate with an increase in A1AT uptake, it is possible that the effects of CSE on clathrin-mediated endocytosis occur following the stage of CHC recruitment at the plasma membrane, a hypothesis that remains to be studied in future investigations.
In recent years, several previously unappreciated functions of A1AT have been discovered (34), including among others effects on atherogenesis (35), angiogenesis (36), fibroblast proliferation, procollagen synthesis (37), and inhibition of endothelial cell apoptosis (2, 3), in contrast to proapoptotic and proinflammatory effects exerted by the mutant Z-A1AT conformer (38, 39). The molecular mechanisms by which A1AT exerts these actions have not been elucidated. Our results suggest that circulating A1AT is endocytosed by lung endothelial cells via a clathrin-mediated process, which is a necessary step for A1AT to exert one of its noncanonical functions, that of caspase-3 inhibition (2). Although this mechanism is yet to be confirmed in humans, the presence of endothelial intracellular A1AT in human lung sections suggests that these results are relevant to human diseases induced by smoking. It is conceivable that intracellular protection by A1AT is required for the maintenance of alveolar structures in addition to inhibition of extracellular neutrophil elastase. Inhibition of A1AT uptake by cigarette smoke may further weaken the A1AT protective role in the lung. Optimizing the A1AT uptake by endothelial cells has, therefore, the potential to become a target for therapy in emphysema caused by both cigarette smoking and by A1AT deficiency and may prove useful for other systemic inflammatory diseases characterized by vascular dysfunction and endothelial cell apoptosis.
Supplementary Material
Acknowledgments
The authors thank Mary Van Demark and Jeremy Adamowicz for superb technical assistance with caspase-3 and elastase activity assays and mouse tail-vein injection, respectively. This study was supported by the Flight Attendent Medical Research Institute (FAMRI); the National Institutes of Health, National Heart, Lung, and Blood Institute grant 1P50 HL084945; and a VA Merit Award.
References
- Carrell R W. alpha 1-Antitrypsin: molecular pathology, leukocytes, and tissue damage. J Clin Invest. 1986;78:1427–1431. doi: 10.1172/JCI112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrache I, Fijalkowska I, Medler T R, Skirball J, Cruz P, Zhen L, Petrache H I, Flotte T R, Tuder R M. α-1 Antitrypsin inhibits cspase-3 ativity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155–1166. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrache I, Fijalkowska I, Zhen L, Medler T R, Brown E, Cruz P, Choe K H, Taraseviciene-Stewart L, Scerbavicius R, Shapiro L, Zhang B, Song S, Hicklin D, Voelkel N F, Flotte T, Tuder R M. A novel anti-apoptotic role for alpha-1 antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med. 2006;173:1222–1228. doi: 10.1164/rccm.200512-1842OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone E, Ding B S, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell Tissue Res. 2008;335:283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldonyte R, Hutchinson E T, Jin B, Brantly M, Block E, Patel J, Zhang J. Endothelial alpha-1-antitrypsin attenuates cigarette smoke induced apoptosis in vitro. COPD. 2008;5:153–162. doi: 10.1080/15412550802092936. [DOI] [PubMed] [Google Scholar]
- Gadek J E, Klein H G, Holland P V, Crystal R G. Replacement therapy of alpha 1-antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J Clin Invest. 1981;68:1158–1165. doi: 10.1172/JCI110360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos M A, Wanner A, Zhang G, Sandhaus R A. Trends in the diagnosis of symptomatic patients with α1-antitrypsin deficiency between 1968 and 2003. Chest. 2005;128:1179–1186. doi: 10.1378/chest.128.3.1179. [DOI] [PubMed] [Google Scholar]
- Janoff A, Carp H, Lee D K, Drew R T. Cigarette smoke inhalation decreases α1-antitrypsin activity in rat lung. Science. 1979;206:1313–1314. doi: 10.1126/science.316187. [DOI] [PubMed] [Google Scholar]
- Carp H, Janoff A. Inactivation of bronchial mucous proteinase inhibitor by cigarette smoke and phagocyte-derived oxidants. Exp Lung Res. 1980;1:225–237. doi: 10.3109/01902148009065462. [DOI] [PubMed] [Google Scholar]
- Taggart C, Cervantes-Laurean D, Kim G, McElvaney N G, Wehr N, Moss J, Levine R L. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem. 2000;275:27258–27265. doi: 10.1074/jbc.M004850200. [DOI] [PubMed] [Google Scholar]
- Singh R D, Puri V, Valiyaveettil J T, Marks D L, Bittman R, Pagano R E. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol Biol Cell. 2003;14:3254–3265. doi: 10.1091/mbc.E02-12-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D K, Choudhury A, Singh R D, Wheatley C L, Marks D L, Pagano R E. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J Biol Chem. 2003;278:7564–7572. doi: 10.1074/jbc.M210457200. [DOI] [PubMed] [Google Scholar]
- Martin O C, Pagano R E. Internalization and sorting of a fluorescent analogue of glucosylceramide to the Golgi apparatus of human skin fibroblasts: utilization of endocytic and nonendocytic transport mechanisms. J Cell Biol. 1994;125:769–781. doi: 10.1083/jcb.125.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K G, Ying Y S, Kamen B A, Anderson R G. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990;111:2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover J A, Willingham M C, Pastan I. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell. 1984;39:283–293. doi: 10.1016/0092-8674(84)90006-0. [DOI] [PubMed] [Google Scholar]
- Wang L H, Rothberg K G, Anderson R G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson M A, Keen J H, McGraw T E. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci U S A. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrache I, Medler T R, Richter A T, Kamocki K, Chukwueke U, Zhen L, Gu Y, Adamowicz J, Schweitzer K S, Hubbard W C, Berdyshev E V, Lungarella G, Tuder R M. Superoxide dismutase protects against apoptosis and alveolar enlargement induced by ceramide. Am J Physiol Lung Cell Mol Physiol. 2008;295:L44–L53. doi: 10.1152/ajplung.00448.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen L, Harborth J, Andrees L, Weber K, Ungewickell E J. Effect of clathrin heavy chain- and alpha-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J Biol Chem. 2003;278:45160–45170. doi: 10.1074/jbc.M307290200. [DOI] [PubMed] [Google Scholar]
- Hubbard R C, Crystal R G. Strategies for aerosol therapy of α1-antitrypsin deficiency by the aerosol route. Lung. 1990;168:565–578. doi: 10.1007/BF02718179. [DOI] [PubMed] [Google Scholar]
- Predescu D, Palade G E. Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium. Am J Physiol Heart Circ Physiol. 1993;265:H725–H733. doi: 10.1152/ajpheart.1993.265.2.H725. [DOI] [PubMed] [Google Scholar]
- Sofer A, Futerman A H. Cationic amphiphilic drugs inhibit the internalization of cholera toxin to the Golgi apparatus and the subsequent elevation of cyclic AMP. J Biol Chem. 1995;270:12117–12122. doi: 10.1074/jbc.270.20.12117. [DOI] [PubMed] [Google Scholar]
- Brodsky F M, Chen C Y, Knuehl C, Towler M C, Wakeham D E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- Schmid S L. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Carlson J A, Rogers B B, Sifers R N, Hawkins H K, Finegold M J, Woo S L. Multiple tissues express alpha 1-antitrypsin in transgenic mice and man. J Clin Invest. 1988;82:26–36. doi: 10.1172/JCI113580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molmenti E P, Perlmutter D H, Rubin D C. Cell-specific expression of α1-antitrypsin in human intestinal epithelium. J Clin Invest. 1993;92:2022–2034. doi: 10.1172/JCI116797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D H, Joslin G, Nelson P, Schasteen C, Adams S P, Fallon R J. Endocytosis and degradation of α1-antitrypsin-protease complexes is mediated by the serpin-enzyme complex (SEC) receptor. J Biol Chem. 1990;265:16713–16716. [PubMed] [Google Scholar]
- Kounnas M Z, Church F C, Argraves W S, Strickland D K. Cellular internalization and degradation of antithrombin III-thrombin, heparin cofactor II-thrombin, and alpha 1-antitrypsin-trypsin complexes is mediated by the low density lipoprotein receptor-related protein. J Biol Chem. 1996;271:6523–6529. doi: 10.1074/jbc.271.11.6523. [DOI] [PubMed] [Google Scholar]
- Rejman J, Oberle V, Zuhorn I S, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K F, Harrison R A, Perkins S J. Structural comparisons of the native and reactive-centre-cleaved forms of α1-antitrypsin by neutron- and X-ray-scattering in solution. Biochem J. 1990;267:203–212. doi: 10.1042/bj2670203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrache I, Fijalkowska I, Zhen L, Medler T R, Brown E, Cruz P, Choe K H, Taraseviciene-Stewart L, Scerbavicius R, Shapiro L, Zhang B, Song S, Hicklin D, Voelkel N F, Flotte T, Tuder R M. A novel antiapoptotic role for alpha1-antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med. 2006;173:1222–1228. doi: 10.1164/rccm.200512-1842OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniyam D, Virtala R, Pawlowski K, Clausen I G, Warkentin S, Stevens T, Janciauskiene S. TNF-α-induced self expression in human lung endothelial cells is inhibited by native and oxidized α1-antitrypsin. Int J Biochem Cell Biol. 2008;40:258–271. doi: 10.1016/j.biocel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Tuder R M, Petrache I. Molecular multitasking in the airspace: α1-antitrypsin takes on thrombin and plasmin. Am J Respir Cell Mol Biol. 2007;37:130–134. doi: 10.1165/rcmb.2007-0163TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmud P J, Martin S, Steiner G, Flavell D M, Whitehouse D B, Nagl S, Jackson R, Taskinen M R, Frick M H, Nieminen M S, Kesaniemi Y A, Pasternack A, Humphries S E, Syvanne M. Progression of atherosclerosis is associated with variation in the alpha1-antitrypsin gene. Arterioscler Thromb Vasc Biol. 2003;23:644–649. doi: 10.1161/01.ATV.0000065196.61663.8D. [DOI] [PubMed] [Google Scholar]
- Huang H, Campbell S C, Nelius T, Bedford D F, Veliceasa D, Bouck N P, Volpert O V. Alpha1-antitrypsin inhibits angiogenesis and tumor growth. Int J Cancer. 2004;112:1042–1048. doi: 10.1002/ijc.20494. [DOI] [PubMed] [Google Scholar]
- Dabbagh K, Laurent G J, Shock A, Leoni P, Papakrivopoulou J, Chambers R C. α-1-antitrypsin stimulates fibroblast proliferation and procollagen production and activates classical MAP kinase signalling pathways. J Cell Physiol. 2001;186:73–81. doi: 10.1002/1097-4652(200101)186:1<73::AID-JCP1002>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Mulgrew A T, Taggart C C, Lawless M W, Greene C M, Brantly M L, O'Neill S J, McElvaney N G. Z α1-antitrypsin polymerizes in the lung and acts as a neutrophil chemoattractant. Chest. 2004;125:1952–1957. doi: 10.1378/chest.125.5.1952. [DOI] [PubMed] [Google Scholar]
- Miller S D, Greene C M, McLean C, Lawless M W, Taggart C C, O'Neill S J, McElvaney N G. Tauroursodeoxycholic acid inhibits apoptosis induced by Z α-1 antitrypsin via inhibition of Bad. Hepatology. 2007;46:496–503. doi: 10.1002/hep.21689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.