Abstract
The purpose of this study was to assess whether an alternative treatment approach that targets angiogenesis, delivered through ligand-targeted nanotherapy, would ameliorate inflammatory arthritis. Arthritis was induced using the K/BxN mouse model of inflammatory arthritis. After arthritis was clearly established, mice received three consecutive daily doses of αvβ3-targeted fumagillin nanoparticles. Control groups received no treatment or αvβ3-targeted nanoparticles without drugs. Disease score and paw thickness were measured daily. Mice that received αvβ3-targeted fumagillin nanoparticles showed a significantly lower disease activity score (mean score of 1.4±0.4; P<0.001) and change in ankle thickness (mean increase of 0.17±0.05 mm; P<0.001) 7 d after arthritis induction, whereas the group that received αvβ3-targeted nanoparticles without drugs exhibited a mean arthritic score of 9.0 ± 0.3 and mean change in ankle thickness of 1.01 ± 0.09 mm. Meanwhile, the group that received no treatment showed a mean arthritic score of 9.8 ± 0.5 and mean change in ankle thickness of 1.05 ± 0.10 mm. Synovial tissues from animals treated with targeted fumagillin nanoparticles also showed significant decrease in inflammation and angiogenesis and preserved proteoglycan integrity. Ligand-targeted nanotherapy to deliver antiangiogenic agents may represent an effective way to treat inflammatory arthritis.—Zhou, H.-F., Chan, H. W., Wickline, S. A., Lanza, G. M., Pham, C. T. N. αvβ3-Targeted nanotherapy suppresses inflammatory arthritis in mice.
Keywords: angiogenesis, K/BxN mouse model, perfluorocarbon nanoparticles, systemic drug delivery
Rheumatoid arthritis (RA) is a chronic inflammatory arthropathy affecting ∼1% of the general population worldwide. Although the etiology of RA remains controversial, the hallmark of the disease is characterized by inflammation of the synovial lining of diarthrodial joints, leading to massive synovial proliferation and infiltration by inflammatory cells. This cellular proliferation, termed pannus, is the key factor leading to the destruction of connective tissue, cartilage, and subchondral bone of the affected joints.
The standard treatment of RA involves systemic use of nonsteroidal anti-inflammatory drugs in conjunction with one or more disease-modifying antirheumatic drugs. Recently, new therapeutic avenues, such as tumor necrosis factor (TNF) blockade, have been developed for RA and proven to be clinically beneficial for a large percentage of the patients. Yet, despite these advances in medical treatment over recent years, a significant number of patients with RA fail to respond to these new biological agents (1,2,3). In addition, studies (4) show that around half of the initial responders to anti-TNF therapy stop responding in the first year due to inefficacy or side effects. Although other biological therapies, such as B-lymphocyte stimulator (Blys) and anti-IL-17 antagonists, just to name a few, are beginning to emerge (5), efficacy and safety remain important issues. Thus, the continued development of new and more effective treatments is clearly warranted.
One potential alternative treatment approach in RA is to target new blood vessel growth or angiogenesis. Angiogenesis is one of the earliest pathological findings in RA and seems to be crucial for the development of the proliferating pannus (6). Moreover, endothelial cells are not just passive bystanders but play a critical role in inflammation, including the adhesion and recruitment of leukocytes to inflammatory sites and the production of inflammatory cytokines and chemokines (7). Therefore, suppression of new blood vessels could inhibit the progression of synovial hyperplasia and mitigate joint destruction by diminishing a major source of proinflammatory cytokines.
While angiogenesis-based therapy is making rapid advances in oncology, it has only begun to be explored for RA treatment (8, 9). The benefits of antiangiogenic agents have been demonstrated in some preclinical models of arthritis (10,11,12,13). These studies suggest that antagonism of the integrin αvβ3 effectively reduced synovial angiogenesis, inflammation, and cartilage erosions in animal models. To further explore its potential antiangiogenic activity, blockade of αvβ3 integrin with a humanized monoclonal antibody commenced as treatment for patients with RA. However, these studies were stopped in 2004 due to lack of efficacy (8, 9).
Inhibition of angiogenesis may also be achieved with antiangiogenic agents such as fumagillin or fumagillin derivatives. Fumagillin, a mycotoxin produced by Aspergillus fumagatus, suppresses angiogenesis by inhibition of methionine aminopeptidase 2 (14). The fumagillin derivative TNP-470 has been studied in various models of arthritis (15,16,17). Administration of TNP-470 before the onset of disease prevented arthritis and attenuated clinical severity in established disease. However, at dosages required for therapeutic effects, TNP-470 elicits severe symptoms of neurotoxicity (18,19,20). Although another fumagillin derivative, PPI2458, has improved neurotoxicity profile in animal models (16, 17), application to human diseases remains limited.
Nanotechnology is a multidisciplinary approach that uses a diverse array of tools and techniques aimed at the diagnosis of disease and delivery of therapeutic agents with the use of nanodevices. By encapsulating and targeting bioactive agents, nanosystems can permit controlled release over time and highly specific site-directed delivery. We have developed a perfluorocarbon (PFC) nanoparticle platform technology, with nominal sizes ∼250 nm, which can be modified to deliver antiangiogenic agents using αvβ3 integrin as a homing target (21,22,23). In previous studies, we have successfully used αvβ3-targeted nanoparticles to detect angiogenesis and deliver antiangiogenic drugs, such as fumagillin, at a total drug dose thousands of times lower than similar studies using conventional or nontargeted delivery systems (21, 24, 25). In this study, we showed that αvβ3-targeted PFC nanoparticles could specifically deliver fumagillin to the inflamed joints and suppress the progression of inflammatory arthritis in the K/BxN mouse model of arthritis.
MATERIALS AND METHODS
Preparation of nanoparticle platform
The nanoparticles were directed to the αvβ3-integrin with a peptidomimetic vitronectin antagonist developed by Bristol-Myers Squibb Medical Imaging (U.S. patent 6,511,648 and related patents), as described previously (21). In general, nanoparticles were comprised of 20% (v/v) perfluorooctylbromide (Exfluor Research Corp., Round Rock, TX, USA) and 2.0% (w/v) of a surfactant comixture, 1.7% (w/v) glycerin, and water for the balance. The surfactant comixtures included 60–70 mol% highly purified egg yolk lecithin (Avanti Polar Lipids, Inc., Alabaster, AL, USA), 0–1 mol% 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-4-(p-maleimidophenyl)butyramide (Avanti Polar Lipids) or amine-PEG2000-phosphatidylethanolamine (Avanti Polar Lipids) for ligand coupling, 2 mol% phosphatidylethanolamine, 0–20% phosphatidylglycerol, and 0–20 mol% cholesterol. The surfactant components were dissolved in chloroform/methanol and dried in a 50°C vacuum oven overnight. In some experiments, nanoparticles were modified to include a fluorescent lipid-conjugated marker, such as rhodamine (Avanti Polar Lipids) or fumagillin (a gift from the National Cancer Institute, Bethesda, MD, USA). Rhodamine and fumagillin were substituted to the surfactant mixture at the expense of lecithin on an equimolar basis, i.e., molecule for molecule. Nontargeted nanoparticles excluded the integrin-homing ligand, which was replaced in the surfactant mixture by an equivalent equimolar increase (1.5%) in dipalmitoyl phosphatidylethanolamine.
K/BxN mouse model of arthritis
All experiments with mice were performed in strict accordance with the guidelines established by the Division of Comparative Medicine at Washington University. Arthritis was induced using the K/BxN murine model of RA (26). Six- to 8-wk-old male C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME, USA) were injected intraperitoneally with 150 μl of serum from K/BxN mice (a generous gift from Dr. Fei Shih, Washington University) on d 0 to induce arthritis. The arthritis index was assessed on a scale of 0–3 (0=no swelling or erythema, 1=slight swelling or erythema, 2=moderate erythema and swelling in multiple digits or entire paw, 3=pronounced erythema and swelling of entire paw; maximum score of 12/mouse). The change from baseline in paw thickness was determined daily by dial calipers, and an average change in ankle thickness was determined for each mouse from the two hind paw measurements.
Systemic delivery of nanoparticles
Mice were allowed to develop a clinical score >1 in at least one paw before treatment. On d 2 after the serum transfer, mice were injected intravenously with 200 μl of αvβ3-targeted nanoparticles conjugated with 1.5 mol% fumagillin on 3 consecutive days. The control groups either received no treatment or 200 μl of αvβ3-targeted nanoparticles without drugs on 3 consecutive days as above. The arthritis index was assessed as above, and changes in paw thickness were monitored daily by dial calipers. On d 7 after K/BxN serum transfer, mice were sacrificed, and their paws were harvested for histology.
Histological analysis
Mouse paws were harvested, fixed in 10% formalin for 48 h, decalcified in EDTA solution, embedded in paraffin, and sectioned at 5 μm. The sections were stained with hematoxylin and eosin (H&E) or toluidine blue. Digital images of 5 random areas per H&E-stained paw section were acquired at ×400, and the numbers of exuded inflammatory cells were enumerated. Proteoglycan content, an indication of cartilage integrity, was graded on toluidine blue-stained sections on a scale of 0–4, as described previously (27) (0=fully stained cartilage, 1=<25% unstained, 2=25–50% unstained, 3=50–75% unstained, 4=>75% unstained cartilage).
Evaluation of angiogenesis
Sections of formalin-fixed mouse paws were deparaffined in xylene and rehydrated in ethanol. The sections were then blocked in blocking reagent for 30 min and incubated with a rabbit anti-von Willebrand factor (vWf) antibody (1:200 dilution; Chemicon International, Temecula, CA, USA) for 2 h at room temperature. After being washed, the sections were incubated with a biotinylated anti-rabbit antibody, followed by alkaline phosphatase conjugated strepavidin. Color was visualized using an alkaline phosphatase substrate kit (Vector Laboratories, Burlingame, CA, USA). The slides were couterstained with 1% methyl green, dehydrated in ethanol, cleared in xylene, and mounted in xylene-based mounting gel. Digital images of 5 random areas/paw were acquired at ×400, and the numbers of blood vessels were enumerated by a masked observer.
Serum chemistry
Blood samples were drawn from the inferior vena cava after the mice were sacrificed on d 5 or 11 after the initiation of nanotherapy and analyzed by the Washington University Department of Comparative Medicine using routine clinical chemistry procedures. Mean and maximal values for liver enzymes in C57BL/6 mice were previously published (28).
Statistical analysis
Comparisons between two groups and multiple groups were made by t test and 2-way ANOVA, respectively, followed by Bonferroni post hoc test to compare all groups of data. Values of P < 0.05 were considered significant. Numerical results are reported as means ± se.
RESULTS
αvβ3-Targeted nanoparticles accumulate specifically in arthritic paws
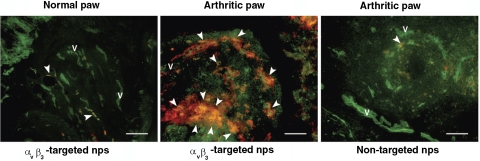
Administration of K/BxN serum leads to symmetrical polyarticular arthritis in the majority of C57BL/6 mice (29). Maximal arthritis develops by d 6 to 7 after serum injection and lasts for ∼2 wk (29). To determine whether αvβ3-targeted nanoparticles localized specifically to the inflamed paws of arthritic mice, we used rhodamine-conjugated nanoparticles that allowed the in situ visualization of nanoparticle deposition. At the peak of their disease, arthritic mice were injected with rhodamine-conjugated nontargeted or αvβ3-targeted nanoparticles. After sacrifice of the mice, examination of normal, nonarthritic paws revealed a rare accumulation of nanoparticles (Fig. 1, left panel). In contrast, examination of arthritic paws revealed extensive accumulation of rhodamine, indicating an increase in both the vascularity and αvβ3 integrin expression on these blood vessels (Fig. 1, middle panel). Administration of rhodamine-conjugated nontargeted nanoparticles to arthritic mice did not lead to significant accumulation of nanoparticles in the inflamed paws (Fig. 1, right panel). Taken together, these results suggest that αvβ3 integrin is a suitable target for specific delivery of nanoparticles to the inflamed joints.
Figure 1.
Rhodamine-conjugated αvβ3-targeted nanoparticles (nps) accumulate specifically in arthritic paws. Arthritis was induced by intraperitoneal injection of K/BxN serum as described in Materials and Methods. On d 7 after serum transfer, at the peak of disease, arthritic mice were injected intravenously with the indicated rhodamine-conjugated nps. Normal mice served as controls. Concomitant injection of FITC-conjugated lectin outlined blood vessels (v) in green, while nps fluoresced in red. Arrowheads indicate colocalization (yellow) of nps to areas of blood vessels. Scale bar = 0.1 mm.
αvβ3-Targeted nanotherapy suppresses arthritis induced by K/BxN serum transfer
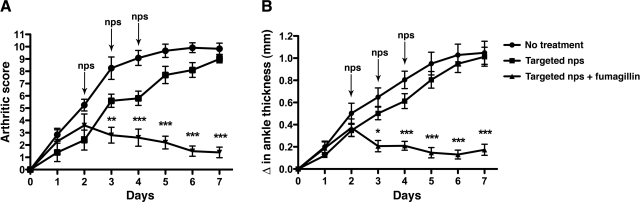
Next, we used the K/BxN model of arthritis (26) to determine the efficacy of targeted nanotherapy. Arthritis was induced as described in Materials and Methods. Arthritis development and change in paw thickness due to inflammation were monitored daily (Figs. 2A, B). Starting on d 2 after serum transfer, when arthritis was clearly established in all animals, αvβ3-targeted nanoparticles without or with fumagillin were administered intravenously into groups of mice. We found that a single dose of αvβ3-targeted fumagillin nanoparticles given on d 2 did not lead to sustained suppression of arthritis (data not shown). However, mice that received 3 doses of αvβ3-targeted fumagillin nanoparticles (d 2 through 4) showed significantly lower disease activity score (mean score of 1.4±0.4; P<0.001) and change in ankle thickness (mean increase of 0.17±0.05 mm; P<0.001) 7 d after K/BxN serum transfer (Fig. 2A, B). In contrast, joint inflammation was unabated in the group that received αvβ3-targeted nanoparticles without drugs (d 7 mean arthritic score of 9.0±0.3 and mean change in ankle thickness of 1.01±0.07 mm) and the group that received no treatment (mean arthritic score of 9.8±0.5 and mean change in ankle thickness of 1.04±0.10 mm; Fig. 2A, B). These results suggest that antiangiogenic therapy with αvβ3-targeted nanoparticles is effective in suppressing established arthritis.
Figure 2.
Targeted nanotherapy suppresses K/ BxN serum-induced arthritis. Arthritis was induced by intraperitoneal injection of K/BxN serum, and arthritis development (A) and changes in ankle thickness (B) were monitored daily by two independent observers. Starting on d 2 after serum transfer, when arthritis was clearly established in all animals, mice were given 3 daily intravenous doses of either αvβ3-targeted nps without drugs or αvβ3-targeted nps with 1.5 mol% fumagillin. Another control group received no treatment. Values are means ± se; n = 6–7 mice/group. *P < 0.05; **P < 0.01; ***P < 0.001.
αvβ3-Targeted nanotherapy limits inflammation, cartilage degradation, and angiogenesis
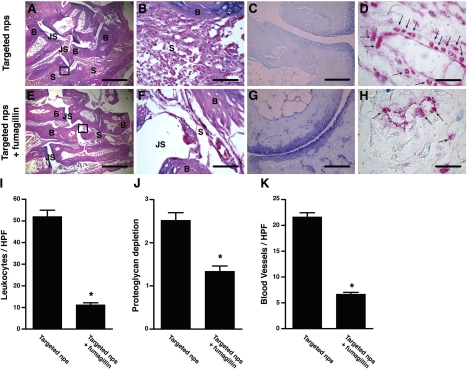
To assess how treatment with αvβ3-targeted nanotherapy affected inflammation in the joints, we examined sections of paws obtained from animals treated with targeted nanoparticles without drugs or targeted nanoparticles with fumagillin. Synovial tissues from animals treated with targeted fumagillin nanoparticles showed a significant decrease in the influx of inflammatory leukocytes [11.2±1.0 cells/high power field (HPF) in the fumagillin group vs. 52.0±3.0 cells/HPF in the no drug group; P<0.0001; Fig. 3E, F, I] and preserved cartilage integrity (shown as toluidine blue staining of cartilage proteoglycan content in Fig. 3G). In contrast, mice that received targeted nanoparticles without drugs showed extensive leukocyte infiltrates (Fig. 3A, B, I) and proteoglycan depletion (Fig. 3C, J). To examine the number of blood vessels in the inflamed tissues, we stained sections of arthritic paws harvested on d 7 after serum transfer for vWf, a molecule that is synthesized by endothelial cells and a marker for blood vessels (10). Examination of sections obtained from mice treated with fumagillin nanoparticles revealed significantly fewer blood vessels compared with sections obtained from animals that received nanoparticles without drugs (6.7±0.8 blood vessels/HPF in mice treated with fumagillin nanoparticles vs. 21.6±0.4 blood vessels/HPF in mice treated with targeted nanoparticles without drugs; P<0.0001; Fig. 3D, H, K). These results suggest that αvβ3-targeted nanotherapy ameliorates inflammatory arthritis by decreasing inflammatory cell influx, preserving cartilage integrity, and reducing angiogenesis.
Figure 3.
Targeted nanotherapy reduces inflammatory cell influx, proteoglycan depletion, and angiogenesis. On d 7 after K/BxN serum transfer, groups of mice that received αvβ3-targeted nps without drugs or αvβ3-targeted nps with fumagillin were sacrificed, their paws were harvested, and sections were stained with H&E (A, B, E, F) to assess degree of inflammation and inflammatory cell influx, with toluidine blue (C, G) to assess the proteoglycan content in cartilage, or with an antibody to vWF to visualize blood vessels (arrows, D, H). Insets from A and E are shown at higher magnification in B and F, respectively. Scale bars = 0.5 mm (A, E); 0.05 mm (B, F); 0.1 mm (C, G); 0.02 mm (E, H). Micrographs represent 10 paws/group. Number of leukocytes (I) per HPF, degree of proteoglycan depletion by toluidine blue staining (J), and number of vWf+ blood vessels (K) per HPF were assessed in a masked fashion. Values are means ± se; 5 random fields/paw, 10 paws/group. *P < 0.0001. B, bone; JS, joint space; S, subsynovial tissue.
Limitations of αvβ3-targeted nanotherapy in mice
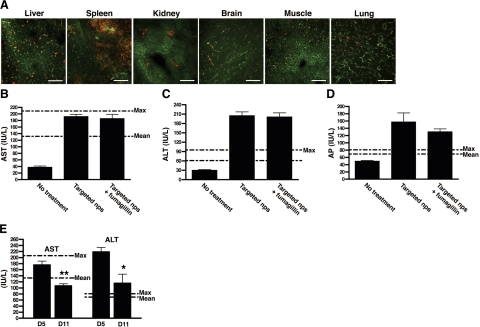
PFC nanoparticles are naturally constrained by size to the circulation, which minimizes unintended binding to extravascular, nontarget tissues expressing similar epitopes (30). Although the biodistribution of systemically administered PFC nanoparticles has been studied in rabbits and rodents, we wanted to examine the biodistribution of systemically administered PFC nanoparticles in arthritic mice. To this end, mice were injected with rhodamine-conjugated αvβ3-targeted nanoparticles at the peak of their disease (on d 7 after serum transfer), and the organs were harvested after sacrifice. Consistent with previous studies (31) and studies in our laboratory (23), αvβ3-targeted nanoparticles accumulated mainly in the reticuloendothelial system (liver and spleen), while accumulation in other organs, including brain, was relatively low (Fig. 4A).
Figure 4.
αvβ3-Targeted nanoparticles accumulate in the reticuloendothelial system. A) Cryosections from different organs were examined for nanoparticle accumulation (red). Blood vessels were visualized with FITC-lectin (green). On d 5 after the initiation of nanoparticle injection, blood samples were drawn, and liver enzymes, including serum concentrations of aspartate aminotransferase (AST; B), alanine aminotransferase (ALT; C), and alkaline phosphatase (AP; D) were assessed. Increase in liver enzymes was transient, with levels falling toward normal on d 11 after initiation of nanotherapy (E). Values are means ± se; 3–4 animals/group. *P < 0.05; **P < 0.01.
To understand the effects of PFC nanoparticle accumulation in the reticuloendothelial system, we examined liver function tests in mice treated with different regimens of nanoparticles. Mice treated with targeted fumagillin nanoparticles showed elevated alanine aminotransferase and alkaline phosphatase levels to twice the upper limits of normal, while aspartate aminotransferase levels were at the upper limit of normal (Fig. 4C, D). However, these elevations were transient, as 6 d later the levels of transaminases returned to normal or near normal (Fig. 4E). The incorporation of fumagillin to the nanoparticles had no additional effects at these doses (Fig. 4B, D), suggesting that the high accumulation of nanoparticles themselves in the reticuloendothelial system likely caused the transient transaminitis.
DISCUSSION
In the present study, we demonstrated for the first time that systemic administration of αvβ3-targeted nanoparticles significantly suppressed the progression of inflammation in mice with established arthritis. Amelioration of arthritis was also accompanied by a concomitant reduction in inflammatory cell influx, proteoglycan depletion, and angiogenesis.
A survey of the literature suggests that the use of nanotherapeutics in the treatment of inflammatory arthritis remains largely unexplored. The short list of published studies using nanoparticles in animal models of arthritis indicates that nanoparticle/liposomal/microsphere formulations are aimed mainly at intra-articular delivery (32,33,34,35). However, nontargeted formulations are usually poorly retained within the articular space. Moreover, high intra-articular accumulation of nonbiodegradable nanoparticles is well known to incite foreign-body reactions and local inflammation (36). In contrast, the perfluorochemicals in PFC nanoparticles have seen use in varied human applications, including blood replacement (37). To date, no carcinogenic, mutagenic, or teratogenic effects or immunogenicity have been found with any recent, properly formulated PFC emulsions (38). Due to their hydrophobicity and size, these nanoparticles are constrained to the vascular space where they interact primarily with endothelial surface epitopes, in this case αvβ3 integrin, thus limiting unwanted accumulation in other organs (22, 30). The safety of αvβ3-targeted fumagillin nanoparticles has previously been shown in studies with rabbits, where systemic administration of nanoparticles inhibits angiogenesis in tumor-bearing rabbits and models of atherosclerosis without adversely affecting liver function (22, 39). However, the intensity and tempo of disease progression in the K/BxN arthritis model differed greatly from cancer and atherosclerosis and required significantly higher doses of nanoparticles to control disease activity, thus resulting in a transient and reversible transaminitis. It is worth pointing out that the effect on liver function is unrelated to the incorporation of fumagillin to the nanoparticles. More likely, the transaminitis was the result of increased intrahepatic pressure caused by the sequestration of PFC nanoparticles in the liver (and spleen) after repeated injections of high doses of nanoparticles. Over time, the PFC nanoparticles cleared through the reticuloendothelial system, and the liver enzymes quickly returned toward baseline. Reduction of the total dosage of PFC particles while maintaining the higher total level of fumagillin may be achieved by increasing membrane loading of fumagillin from 1.5–3.0 mol%, which would avoid the intrahepatic pressure effect observed with repeated doses. However, natural fumagillin, used in this study, is extremely photosensitive (40), requiring increased compensatory lipid membrane drug loading. New hydrophobic analogues of fumagillin that eliminate photoinstability, as previously achieved with TNP-470 (41), can be developed and would lower the total fumagillin requirement.
Angiogenesis is an important factor in the pathogenesis of RA (7). Neovascularization is thought to result partly from an imbalance between pro- and antiangiogenic factors (9), and this imbalance perpetuates pannus growth. It has been shown that antiangiogenic therapy in tumors reestablishes the balance between pro- and antiangiogenic factors, “normalizes” the tumor vasculature, and improves function (42). Even when this vasculature normalizing effect is transient, it has been shown to elicit a marked effect that subsequently augments the effectiveness of or synergizes with a second conventional agent to preserve the antiangiogenic effect (42). Indeed, we have shown that the combination of fumagillin nanoparticles followed by a conventional statin allowed a level of antiangiogenic effect in an atherosclerosis model that was sustained and not achievable with either agent alone (39). We may indeed find that the antiangiogenic effect afforded by nanotherapy in the arthritis model can only be maintained when combined with another conventional drug, as it is the case with atherosclerosis (39). The duration of the antiangiogenic effect and sequencing of nanotherapy with conventional drug treatment remain to be determined. The results presented herein provide proof-of-concept for this emerging technology and encourage further experimentation to discern the integration of nanotherapeutic formulations into current treatment strategies to achieve improved and lasting efficacy.
In summary, our results suggest that nanotherapeutic approaches to RA may represent an efficient and specific approach for treating inflammatory arthritis. In addition, the 19F fluorocarbon core of these nanoparticles is acoustically active and has afforded concurrent opportunity for 19F imaging and spectroscopy in other animal models (43,44,45). Therefore, PFC nanoparticles may in the future serve as a platform technology that allows the delivery of specific therapy and the monitoring of effects simultaneously and accurately through noninvasive imaging modalities.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR-056468, CA-119342, and HL-78631). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. S.A.W. and G.M.L. are scientific cofounders of Kereos, Inc. and minority shareholders (<5%). We extend sincere appreciation to Ralph Fuhrhop for formulation chemistry. We thank Dr. Fei Shih (Washington University) for the generous gift of K/BxN serum.
References
- Gartlehner G, Hansen R A, Jonas B L, Thieda P, Lohr K N. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol. 2006;33:2398–2408. [PubMed] [Google Scholar]
- Zink A, Strangfeld A, Schneider M, Herzer P, Hierse F, Stoyanova-Scholz M, Wassenberg A, Kapelle A, Listing J. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum. 2006;54:3399–3407. doi: 10.1002/art.22193. [DOI] [PubMed] [Google Scholar]
- Siddiqui M A. The efficacy and tolerability of newer biologics in rheumatoid arthritis: best current evidence. Curr Opin Rheumatol. 2007;19:308–313s. doi: 10.1097/01.bor.0000265447.48722.04. [DOI] [PubMed] [Google Scholar]
- Buch M H, Bingham S J, Bryer D, Emery P. Long-term infliximab treatment in rheumatoid arthritis: subsequent outcome of initial responders. Rheumatology. 2007;46:1153–1156. doi: 10.1093/rheumatology/kem075. [DOI] [PubMed] [Google Scholar]
- Tarner I H, Muller-Ladner U, Gay S. Emerging targets of biologic therapies for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2007;3:336–345. doi: 10.1038/ncprheum0506. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z, Gaspar L, Koch A E. Angiogenesis in rheumatoid arthritis. Front Biosci. 2005;10:1739–1753. doi: 10.2741/1657. [DOI] [PubMed] [Google Scholar]
- Koch A E. Angiogenesis as a target in rheumatoid arthritis. Ann Rheum Dis. 2003;62:60–67. doi: 10.1136/ard.62.suppl_2.ii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainer D T, Brahn E. New antiangiogenic strategies for the treatment of proliferative synovitis. Expert Opin Investig Drugs. 2005;14:1–17. doi: 10.1517/13543784.14.1.1. [DOI] [PubMed] [Google Scholar]
- Lainer-Carr D, Brahn E. Angiogenesis inhibition as a therapeutic approach for inflammatory synovitis. Nat Clin Pract Rheumatol. 2007;3:434–442. doi: 10.1038/ncprheum0559. [DOI] [PubMed] [Google Scholar]
- Storgard C M, Stupack D G, Jonczyk A, Goodman S L, Fox R I, Cheresh D A. Decreased angiogenesis and arthritic disease in rabbits treated with an alphavbeta3 antagonist. J Clin Invest. 1999;103:47–54. doi: 10.1172/JCI3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlag D M, Borges E, Tak P P, Ellerby H M, Bredesen D E, Pasqualini R, Ruoslahti E, Firestein G S. Suppression of murine collagen-induced arthritis by targeted apoptosis of synovial neovasculature. Arthritis Res. 2001;3:357–361. doi: 10.1186/ar327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger A M, Blake S, Kapadia R, Sarkar S, Levin J, Swift B A, Hoffman S J, Stroup G B, Miller W H, Gowen M, Lark M W. Disease-modifying activity of SB 273005, an orally active, nonpeptide alphavbeta3 (vitronectin receptor) antagonist, in rat adjuvant-induced arthritis. Arthritis Rheum. 2001;44:128–137. doi: 10.1002/1529-0131(200101)44:1<128::AID-ANR17>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Koning G A, Schiffelers R M, Wauben M H, Kok R J, Mastrobattista E, Molema G, ten Hagen T L, Storm G. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate-containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheum. 2006;54:1198–1208. doi: 10.1002/art.21719. [DOI] [PubMed] [Google Scholar]
- Liu S, Widom J, Kemp C W, Crews C M, Clardy J. Structure of human methionine aminopeptidase-2 complexed with fumagillin. Science. 1998;282:1324–1327. doi: 10.1126/science.282.5392.1324. [DOI] [PubMed] [Google Scholar]
- De Bandt M, Grossin M, Weber A J, Chopin M, Elbim C, Pla M, Gougerot-Pocidalo M A, Gaudry M. Suppression of arthritis and protection from bone destruction by treatment with TNP-470/AGM-1470 in a transgenic mouse model of rheumatoid arthritis. Arthritis Rheum. 2000;43:2056–2063. doi: 10.1002/1529-0131(200009)43:9<2056::AID-ANR17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bernier S G, Lazarus D D, Clark E, Doyle B, Labenski M T, Thompson C D, Westlin W F, Hannig G. A methionine aminopeptidase-2 inhibitor, PPI-2458, for the treatment of rheumatoid arthritis. Proc Natl Acad Sci U S A. 2004;101:10768–10773. doi: 10.1073/pnas.0404105101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannig G, Bernier S G, Hoyt J G, Doyle B, Clark E, Karp R M, Lorusso J, Westlin W F. Suppression of inflammation and structural damage in experimental arthritis through molecular targeted therapy with PPI-2458. Arthritis Rheum. 2007;56:850–860. doi: 10.1002/art.22402. [DOI] [PubMed] [Google Scholar]
- Offodile R, Walton T, Lee M, Stiles A, Nguyen M. Regression of metastatic breast cancer in a patient treated with the anti-angiogenic drug TNP-470. Tumori. 1999;85:51–53. doi: 10.1177/030089169908500111. [DOI] [PubMed] [Google Scholar]
- Kudelka A P, Verschraegen C F, Loyer E. Complete remission of metastatic cervical cancer with the angiogenesis inhibitor TNP-470. N Engl J Med. 1998;338:991–992. doi: 10.1056/NEJM199804023381412. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Marshall J L, Rizvi N, Dahut W, Yoe J, Figuera M, Phipps K, Ong V S, Kato A, Hawkins M J. A Phase I and pharmacokinetic study of TNP-470 administered weekly to patients with advanced cancer. Clin Cancer Res. 1999;5:1989–1995. [PubMed] [Google Scholar]
- Winter PM, Morawski A M, Caruthers S D, Fuhrhop R W, Zhang H, Williams T A, Allen J S, Lacy E K, Robertson J D, Lanza G M, Wickline S A. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha (v) beta3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- Winter P M, Neubauer A M, Caruthers S D, Harris T D, Robertson J D, Williams T A, Schmieder A H, Hu G, Allen J S, Lacy E K, Zhang H, Wickline S A, Lanza G M. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- Hu G, Lijowski M, Zhang H, Partlow K C, Caruthers S D, Kiefer G, Gulyas G, Athey P, Scott M J, Wickline S A, Lanza G M. Imaging of Vx-2 rabbit tumors with alpha (v) beta3-integrin-targeted 111In nanoparticles. Int J Cancer. 2007;120:1951–1957. doi: 10.1002/ijc.22581. [DOI] [PubMed] [Google Scholar]
- Winter P M, Caruthers S D, Kassner A, Harris T D, Chinen L K, Allen J S, Lacy E K, Zhang H, Robertson J D, Wickline S A, Lanza G M. Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel alpha(v)beta3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res. 2003;63:5838–5843. [PubMed] [Google Scholar]
- Schmieder A H, Winter P M, Caruthers S D, Harris T D, Williams T A, Allen J S, Lacy E K, Zhang H, Scott M J, Hu G, Robertson J D, Wickline S A, Lanza G M. Molecular MR imaging of melanoma angiogenesis with alpha (v) beta3-targeted paramagnetic nanoparticles. Magn Reson Med. 2005;53:621–627. doi: 10.1002/mrm.20391. [DOI] [PubMed] [Google Scholar]
- Mandik-Nayak L, Allen P M. Initiation of an autoimmune response: insights from a transgenic model of rheumatoid arthritis. Immunol Res. 2005;32:5–13. doi: 10.1385/IR:32:1-3:005. [DOI] [PubMed] [Google Scholar]
- Adkison A M, Raptis S Z, Kelley D G, Pham C T. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109:363–371. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell M A, Hardy C, Hawley M, Propert K J, Wilson J M. Effect of blood collection technique in mice on clinical pathology parameters. Hum Gene Ther. 2002;13:155–161. doi: 10.1089/10430340152712700. [DOI] [PubMed] [Google Scholar]
- Ji H, Gauguier D, Ohmura K, Gonzalez A, Duchatelle V, Danoy P, Garchon H J, Degott C, Lathrop M, Benoist C, Mathis D. Genetic influences on the end-stage effector phase of arthritis. J Exp Med. 2001;194:321–330. doi: 10.1084/jem.194.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder A H, Caruthers S D, Zhang H, Williams T A, Robertson J D, Wickline S A, Lanza G M. Three-dimensional MR mapping of angiogenesis with α5β1(α[v]β3)-targeted theranostic nanoparticles in the MDA-MB-435 xenograft mouse model. FASEB J. 2008;22:179–189. doi: 10.1096/fj.08-112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. From clinical development of perfluorocarbon-based emulsions as red cell substitutes. Sjoblom J, editor. New York: Marcel Dekker; 1996:343–367. [Google Scholar]
- Van Lent P L, van den Bersselaar L, van den Hoek A E, van de Ende M, Dijkstra C D, van Rooijen N, van de Putte L B, van den Berg W B. Reversible depletion of synovial lining cells after intra-articular treatment with liposome-encapsulated dichloromethylene diphosphonate. Rheumatol Int. 1993;13:21–30. doi: 10.1007/BF00290330. [DOI] [PubMed] [Google Scholar]
- Ceponis A, Waris E, Monkkonen J, Laasonen L, Hyttinen M, Solovieva S A, Hanemaaijer R, Bitsch A, Konttinen Y T. Effects of low-dose, noncytotoxic, intraarticular liposomal clodronate on development of erosions and proteoglycan loss in established antigen-induced arthritis in rabbits. Arthritis Rheum. 2001;44:1908–1916. doi: 10.1002/1529-0131(200108)44:8<1908::AID-ART329>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Liggins R T, Cruz T, Min W, Liang L, Hunter W L, Burt H M. Intra-articular treatment of arthritis with microsphere formulations of paclitaxel: biocompatibility and efficacy determinations in rabbits. Inflamm Res. 2004;53:363–372. doi: 10.1007/s00011-004-1273-1. [DOI] [PubMed] [Google Scholar]
- Tsai C Y, Shiau A L, Chen S Y, Chen Y H, Cheng P C, Chang M Y, Chen D H, Chou C H, Wang C R, Wu C L. Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis Rheum. 2007;56:544–554. doi: 10.1002/art.22401. [DOI] [PubMed] [Google Scholar]
- Nahar M, Dutta T, Murugesan S, Asthana A, Mishra D, Rajkumar V, Tare M, Saraf S, Jain N K. Functional polymeric nanoparticles: an efficient and promising tool for active delivery of bioactives. Crit Rev Ther Drug Carrier Syst. 2006;23:259–318. doi: 10.1615/critrevtherdrugcarriersyst.v23.i4.10. [DOI] [PubMed] [Google Scholar]
- Riess J G. Oxygen carriers (“blood substitutes”)–raison d’etre, chemistry, and some physiology. Chem Rev. 2001;101:2797–2920. doi: 10.1021/cr970143c. [DOI] [PubMed] [Google Scholar]
- Krafft M P. Fluorocarbons and fluorinated amphiphiles in drug delivery and biomedical research. Adv Drug Deliv Rev. 2001;47:209–228. doi: 10.1016/s0169-409x(01)00107-7. [DOI] [PubMed] [Google Scholar]
- Winter P M, Schmieder A H, Caruthers S D, Keene J L, Zhang H, Wickline S A, Lanza G M. Minute dosages of alpha (v) beta3-targeted fumagillin nanoparticles impair Vx-2 tumor angiogenesis and development in rabbits. FASEB J. 2008;22:2758–2767. doi: 10.1096/fj.07-103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochansky J, Nasr M. Laboratory studies on the photostability of fumagillin, the active ingredient of Fumidil B1. Apidologie. 2004;36:301–310. [Google Scholar]
- Yadav J S, Sreedhar P, Srihari P, Sarma G D, Jagadeesh B. The concise synthesis of a key intermediate for the total synthesis of fumagillin, TNP-470, and ovalicin. Synthesis. 2008;9:1460–1466. [Google Scholar]
- Fukumura D, Jain R K. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza G M, Winter P M, Neubauer A M, Caruthers S D, Hockett F D, Wickline S A. (1)H/(19)F magnetic resonance molecular imaging with perfluorocarbon nanoparticles. Curr Top Dev Biol. 2005;70:57–76. doi: 10.1016/S0070-2153(05)70003-X. [DOI] [PubMed] [Google Scholar]
- Caruthers S D, Neubauer A M, Hockett F D, Lamerichs R, Winter P M, Scott M J, Gaffney P J, Wickline S A, Lanza G M. In vitro demonstration using 19F magnetic resonance to augment molecular imaging with paramagnetic perfluorocarbon nanoparticles at 1.5 Tesla. Invest Radiol. 2006;41:305–312. doi: 10.1097/01.rli.0000199281.60135.6a. [DOI] [PubMed] [Google Scholar]
- Neubauer A M, Caruthers S D, Hockett F D, Cyrus T, Robertson J D, Allen J S, Williams T D, Fuhrhop R W, Lanza G M, Wickline S A. Fluorine cardiovascular magnetic resonance angiography in vivo at 1.5 T with perfluorocarbon nanoparticle contrast agents. J Cardiovasc Magn Reson. 2007;9:565–573. doi: 10.1080/10976640600945481. [DOI] [PubMed] [Google Scholar]