Abstract
We examine the potential for erythropoietin signaling to promote donor cell survival in a model of myoblast transplantation. Expression of a truncated erythropoietin receptor in hematopoietic stem cells has been shown to promote selective engraftment in mice. We previously demonstrated expression of endogenous erythropoietin receptor on murine myoblasts, and erythropoietin treatment can stimulate myoblast proliferation and delay differentiation. Here, we report that enhanced erythropoietin receptor expression, as well as exogenous erythropoietin treatment in myoblasts, provided a survival advantage and protection against apoptosis under serum-starvation conditions. When cultured in differentiation medium, expression of the myogenic regulatory proteins shifted toward early differentiation with increased erythropoietin receptor. Expression of early myogenic differentiation proteins Myf-5 and MyoD increased, while later stage protein myogenin decreased. Transplantation of C2C12 myoblasts overexpressing truncated erythropoietin receptor showed more transplanted cell incorporation into muscle fibers in muscular dystrophy mdx mice. These cells also restored dystrophin protein expression in mdx mice at 6 wk after cell treatment that was further increased with exogenous erythropoietin administration. In summary, enhanced erythropoietin receptor expression promotes transplanted cell survival in a mouse model for myoblast transplantation and provides dystrophin expression in mice with muscular dystrophy.—Jia, Y., Warin, R., Yu, X., Epstein, R., Noguchi, C. T. Erythropoietin signaling promotes transplanted progenitor cell survival.
Keywords: myoblast, dystrophin, transplantation, receptor, EpoR
Erythropoietin (EPO) is made principally in the kidney, is secreted into the circulation, and targets hematopoietic progenitor cells in the bone marrow to differentiate down the erythroid lineage. EPO acts as a survival factor for erythroid progenitor cells, and loss of EPO or its receptor (EpoR) results in death in utero due to severe anemia (1, 2). The expression of EpoR extends beyond hematopoietic tissue and includes the brain, cardiovascular system, retina, skeletal muscle, and gut system (3, 4). Other sources of EPO beyond the kidney and the hypoxia induction in the liver provide the potential for EPO production locally, such as the hypoxic induction of EPO in the brain and the estrogen-dependent EPO production in the uterus that promotes blood vessel formation in the endometrium in rodents (5, 6). Administration of exogenous EPO to elevate EPO in the circulation can activate EpoR in nonhematopoietic tissue to provide protection against apoptosis under select conditions, such as ischemic injury in the brain and heart in animal models (7, 8). In the bone marrow, EPO stimulates erythropoiesis and also contributes to mobilization of bone marrow endothelial progenitor cells (9). Elevated EPO signaling in hematopoietic stem cells provides for preferential survival in bone marrow transplantation in mice (10). Expression of endogenous EpoR in nonhematopoietic progenitor cells raises the possibility of preferential survival of transplanted nonhematopoietic cells via stimulated Epo signaling.
We previously reported that EPO stimulates the proliferation of mouse skeletal muscle progenitor cells and delays the differentiation and fusion of myoblasts into myotubes in culture (3). EPO functions through the interaction with its membrane receptor (EpoR) (11). Analogous to the down-regulation of EpoR during terminal erythroid differentiation and lack of EpoR expression on mature red blood cells, EpoR is down-regulated during myoblast differentiation to myotubes and is not detected on mature muscle fibers. EPO increases cytoplasmic calcium in myoblasts but not in myotubes that lack EpoR (3). These findings suggest that EPO may be useful as a candidate factor to promote donor-cell survival and facilitate myoblast transplantation in cellular therapy.
Duchenne muscular dystrophy (DMD) is a progressive genetic disease characterized by decreased muscle mass and loss of muscle function in male children (12). This disorder results from a genetic mutation within the X chromosome that encodes the dystrophin protein, a large peripheral membrane protein that stabilizes the muscle cell membrane and cytoskeleton in skeletal and cardiac muscle. Cell-based therapy suggests the transplantation into an affected host of normal donor cells or genetically modified host cells with the capacity to fuse with existing muscle fibers to restore dystrophin expression (13, 14). However, the survival of transplanted cells is one of the major limitations of such treatment (15). Murine and human hematopoietic stem cells expressing a truncated erythropoietin receptor (tEpoR) showed a competitive repopulation advantage in bone marrow transplantation in mice (10, 16). In this study, we report that expression of tEpoR in conjunction with EPO treatment increases cell survival both in cell culture and after cell transplantation, and restores dystrophin expression in dystrophin-deficient (mdx) mice.
The interaction of EPO with the extracellular domain of the EpoR homodimer induces signal transduction (3, 17, 18). EpoR is a polypeptide with a single transmembrane domain and is a member of the hematopoietic class I cytokine receptor superfamily. EpoR contains no intrinsic kinase activity, and EPO binding to EpoR activates tyrosine phosphorylation through JAK2 associated with the EpoR cytoplasmic domain, stimulating STAT activation and various other downstream cell signaling pathways (17, 19). The carboxyl terminus of the EpoR cytoplasmic domain negatively regulates EPO signaling (20, 21). Individuals with truncation of the EpoR carboxyl-terminal amino acids exhibit excessive erythrocytosis associated with enhanced sensitivity to EPO and increased EPO activity in erythroid progenitor cells (21, 22).
We used a dual-promoter lentivirus construct to drive expression of a reporter gene and of the mouse EpoR (mEpoR) or truncated EpoR (tmEpoR) lacking the carboxyl-terminal negative regulatory domain (23). We report that murine myoblasts (C2C12) with lentivirus transduction to increase EpoR expression showed a survival advantage compared to untreated cells when challenged with inflammatory cytokines or serum starvation to induce apoptosis. In differentiation medium, increased expression of mEpoR or tmEpoR in C2C12 cells altered expression of myogenic regulatory factor proteins but did not prevent or eliminate the capacity to change morphology or differentiate. After transplantation into the gastrocnemius muscle, C2C12 cells with overexpression of tmEpoR exhibited greater survival compared to the control cells in both mdx and wild-type (WT) mice. Interestingly, transplantation of C2C12 cells treated with exogenous EPO increased the contribution of dystrophin expression in mdx mice, and the greatest amount of dystrophin-expressing fibers was observed with the combination of tmEpoR C2C12 transplantation and EPO treatment. Thus, EPO stimulation with overexpression of EpoR may increase transplanted progenitor cell survival leading to greater muscle fiber incorporation of donor cells in mice and suggests that increased EPO signaling may be useful in cell-based therapy for the muscular dystrophy diseases.
MATERIALS AND METHODS
Cell culture
Primary myoblasts were isolated from embryonic day 16 (E16) C57/B6 mouse embryos. Limb muscles were dissected in phosphate-buffered saline, mechanically dissociated, and treated with 0.25% trypsin/0.5 mM EDTA for 1 h at 37°C. Cells were filtered through a Falcon 70-μm cell strainer (BD Biosciences, Bedford, MA, USA), centrifuged at 3000 g for 15 min, and seeded in growth medium on culture dishes.
The C2C12 cells (CRL-1772; American Type Culture Collection, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM), 10% fetal bovine serum (FBS), 0.5 U/ml penicillin/streptomycin, and 1 mM l-glutamine (Invitrogen, Carlsbad, CA, USA) (growth medium) at 37°C under a humidified atmosphere of 5% CO2. For cell survival experiments, 1 × 106 C2C12 cells were seeded onto 100-mm dishes in growth medium for 24 h to ∼80% confluence and then switched to growth medium with 0% FBS (serum starvation medium) and different concentrations of EPO (Epoetin-Alfa; Amgen, Thousand Oaks, CA, USA). Alternatively, to induce apoptosis, cells were treated with a cocktail of inflammatory cytokines consisting of interleukin 1β (IL-1β; 200 U/ml), tumor necrosis factor-α (TNF-α; 2000 U/ml), and interferon-γ (INF-γ; 2000 U/ml) for 48 h. Cell viability was determined by the luminescence ATP detection assay system (ATPlite-1-Step;, PerkinElmer, Boston, MA, USA) and measured by a Victor 3 Multilable Counter (PerkinElmer). In separate experiments, differentiation medium (DMEM with 2% FBS and penicillin/streptomycin/glutamine) was used for C2C12 cells after 24 h of culture in growth medium.
Construction, production, and transduction of lentiviral vectors
To increase EpoR expression, we used the dual-promoter HIV-EF1α-CMV lentiviral vector to drive expression of a reporter gene and mEpoR or tmEpoR with deletion from the coding region 91 aa of the carboxyl-terminal region that contains the negative regulatory domain (23). The EF-1α promoter drives the EpoR target gene expression. The immediately downstream CMV promoter drives enhanced green fluorescent protein (EGFP) to make LV-GFP (control), LV-mEpoR-GFP, and LV-tmEpoR-GFP or firefly luciferase (Luc) as the reporter gene to make LV-tmEpoR-Luc. As a target gene, the mEpoR or tmEpoR cDNA was inserted at the EcoRV restriction site of the plasmid HIV-EF1α-CMV, downstream of the EF-1α promoter. As a control construct, EGFP was used in place of EpoR with the CMV-firefly luciferase construct to make LV-GFP-Luc.
Recombinant lentivirus was produced by cotransfection of HIV-EF1a-CMV, pCMVDR8.91, and PVSV-G plasmids into 293T producer cells, as described previously (23). Human 293T cells were seeded at a density of 105/ml in 2 ml on poly-d-lysine-coated 6-well plates with DMEM and 10% FBS at d 0. Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used to transfect recombined lentiviral and two packaging plasmids into 293T cells at d 1. At d 3, the culture medium containing viral supernatants were concentrated to 1000:1 by Centricon Plus-20 filtration columns (Millipore, Bedford, MA, USA) and centrifugation. C2C12 cells and primary myoblasts were cultured at a density of 5 × 104/ml in 2 ml of media in 6-well plates for 24 h before the concentrated virus supernatants were added with 8 μg/ml Polybrene (Sigma, St. Louis, MO, USA) for transduction. After 48 h posttransduction, C2C12 cells and primary myoblasts were examined by fluorescence microscopy (Olympus, Center Valley, PA, USA) for GFP and EpoR expression.
Quantitative real-time RT-PCR
After 48 h of the lentiviral infection, C2C12 and control cells were harvested, and total RNA was isolated using Qiagen RNeasy (Qiagen, Germantown, MD, USA). First-strand cDNA was synthesized using murine leukemia virus reverse transcriptase and oligo (dT) 16 (PE Applied Biosystems, Foster City, CA, USA). Quantitative real-time RT-PCR analysis was performed with EpoR and S16 primers and fluorescently labeled TaqMan probes (Molecular Probes, Eugene, OR, USA) in a 7900HT fast real-time PCR system (PE Applied Biosystems) as described before (3).
TUNEL analysis
Plated cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) (Invitrogen) and analyzed with an in situ cell death detection system (TMR red; Roche Diagnostics, Mannheim, Germany). The TUNEL-positive cells were detected by fluorescence microscopy.
Western blot analysis
Cultured C2C12 cells were washed with cold PBS, scraped, and centrifuged at 8000 g for 10 min. Cell pellets were lysed with radioimmune precipitation assay buffer buffer (10 mM Tris-HCl, 1 mM EDTA, 0.1% SDS, 0.1% Na3VO4, and 1% Triton X-100) and protease inhibitor (Roche Diagnostics), incubated at 4°C for 30 min, and centrifuged at 17,000 g for 10 min. The supernatants containing proteins were collected and measured for protein concentration with an Eppendorf Biophotometer (Eppendorf AG, Hamburg, Germany). NuPAGE LDS sample buffer (Invitrogen) was added, and samples were heated at 70°C for 10 min. Protein samples were loaded onto a NuPAGE 4–12% BisTris gel (Invitrogen) and run for 1 h at 150 V. Proteins were transferred to nitrocellulose by iBot gel-transfer system (Invitrogen). The blots were blocked with 5% nonfat milk in Tween 20 Tris-buffered saline at room temperature for 1 h; probed with primary antibodies for Myf-5 (C-20), MyoD (M-318), myogenin (M-225), GATA-3 (H-48), and β-actin (SC-1615) (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight; probed with a corresponding secondary antibody and chemiluminescent reagents (Invitrogen); and detected by Biomax XAR imaging film (Kodak, Rochester, NY, USA).
Myoblast transplantation and animal experiments
Three-month-old mdx and WT mice were used for experiments and maintained in the National Institutes of Health animal facility. Age- and sex-matched mdx and WT mice were randomly assigned into treatment groups for transplantation of LV-tmEpoR-Luc-treated C2C12 cells, with and without EPO treatment, and transplantation of LV-GFP-Luc-treated C2C12 cells, with or without EPO treatment (n=6). Prior to transplanting, the hind limbs of mice that received transplants were shaved. C2C12 cells with LV-tmEpoR-Luc and C2C12 cells with LV-GFP-Luc (control cells) were collected and transplanted into anesthetized mice after 48 h of lentiviral treatment. About 2 × 105 cells were injected at 2-mm depth into the gastrocnemius muscle, ∼2 mm below the great saphenous vein. Erythropoietin was administered by intraperitoneal injection at 3000 U/kg to the EPO treatment groups on the same day (d 1) of transplantation.
At the time of imaging, the substrate luciferin was injected intraperitoneally at a dose of 150 mg/kg body weight using a stock solution of 30 mg/ml in a volume less than 200 μl ∼5 min before imaging. Mice were anesthetized using isoflurane gas and placed in an IVIS100 in vivo imaging system using a cooled charge-coupled device (CCD) camera (Xenogen Corp., Alameda, CA, USA), dorsal side down. The photons emitted by the bioluminescent cells were then integrated and digitized. Regions of interest (ROI) from displayed images were identified around the transplant sites and quantified as total photon count/s using Living Image software (Caliper Life Sciences, Hopkinton, MA, USA). A pseudocolor bioluminescent image from blue (least intense) to red (most intense) representing the spatial distribution of the detected photons emitted within the animal allowed localization and measurement of the graft growth. Background photon flux was determined from an ROI of the same size placed in a nonluminescent area nearby and then subtracted from the measured luminescent signal intensity. All light measurements were performed under the same conditions. Images were collected and used to quantify the emitted photons. The pseudocolor image is merged with the grayscale reference image. Scans and bioluminescence signals data of luciferase activity were collected post-transplantation at d 1, 4, 7, 10, and 14 after transplantation. All animal studies were carried out according to guidelines of the National Institute of Diabetes and Digestive and Kidney Diseases Animal Care and Use Committee. Six weeks following the cell injection, mice were anesthetized and perfused with 4% paraformaldehyde in PBS. The gastrocnemius muscles were harvested, paraffin embedded, and sectioned for immunochemical staining.
Immunochemical staining
For cell culture plates, C2C12 cells and primary myoblasts cultures were fixed with 4% PBS paraformaldehyde in PBS at 4°C overnight. For muscle paraffin sections, slides were deparaffinized with xylene and washed with series concentrations (100, 90, 80, and 70%) of ethanol. After washing with Tris buffer saline (TBS) and blocking with 5% FBS in TBS for 1 h, cell culture plates or muscle sections were incubated with the primary antibody overnight at 4°C. Primary antibodies used were EpoR (M-20), CD34 (CBR-E8), M-cadherin (12G4), and desmin (RD-301) (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA); goat anti-luciferase (G7451; 1:100; Promega, Madison, WI, USA); and rabbit polyclonal to dystrophin (ab15277; 1:200; Abcam, Cambridge, MA, USA). After staining with fluorescence-conjugated secondary antibody, cells or sections were mounted using vector DAPI mounting medium (Vector Laboratories, Burlingame, CA, USA). Secondary antibodies used were bovine anti-goat IgG (SC-2786), bovine anti-rabbit IgG (SC-2365), goat anti-mouse IgG (SC-2039), and goat anti-rat IgG (SC-2041) (1:400; Santa Cruz Biotechnology). Cell culture plates and sections were then examined by fluorescent microscopy, and the images were captured.
Statistical analysis
Microsoft Excel (Microsoft, Redmond, WA, USA) was used to calculate sd and determine P values by the Student’s t test.
RESULTS
Forced EpoR expression in myoblasts
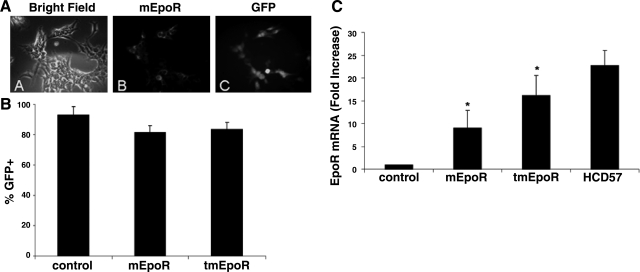
Lentiviral vectors driving expression of mEpoR and tmEpoR were constructed using a dual-promoter strategy with an EGFP reporter (23). Immunostaining indicated that GFP fluorescence coincides with EpoR expression after LV-mEpoR transduction in 293T producing cells (Fig. 1A). After lentiviral transduction into C2C12 cells, high levels of GFP-positive cells were detected by fluorescence microscopy. LV-mEpoR-GFP treatment (LV-mEpoR-GFP C2C12) yielded 81.7 ± 4% and LV-tmEpoR-GFP treatment (LV-tmEpoR-GFP C2C12) yielded 83.7 ± 4% GFP-positive cells (Fig. 1B). The coexpression of GFP and EpoR allows for the use of GFP as a marker for lentiviral-mediated EpoR expression in subsequent experiments using myoblasts.
Figure 1.
Lentiviral transduction of EpoR in myoblasts. A) Immunostaining of EpoR expression and GFP fluorescence in 293T cells after transduction of LV-mEpoR-GFP for 24 h. B) GFP-positive myoblasts were detected by fluorescence microscopy after lentiviral infection for 48 h and counted as a percentage of GFP-positive staining cells. C) Mouse EpoR mRNA expression in myoblasts after lentivirus infection by quantitative real-time RT-PCR. C2C12 cells treated with LV-mEpoR-GFP and LV-tmEpoR-GFP were harvested 48 h after lentivirus infection. Untreated C2C12 cells were used as negative control; and HCD57 cells were used as positive control. Mouse S16 mRNA expression was used as internal control. Mouse EpoR mRNA expression levels significantly increased in LV-mEpoR-GFP and LV-tmEpoR-GFP-infected C2C12 cells compared to control cells treated with LV-GFP. *P < 0.01.
Quantitative real-time RT-PCR using specific primers and TaqMan probe for the murine EpoR confirmed the induction of mEpoR and tmEpoR RNA, respectively, by lentivirus transduction in myoblasts (Fig. 1C). The EpoR mRNA expression level following lentivirus transduction was increased by 9.1 ± 3.8-fold (LV-mEpoR-GFP) and 16.2 ± 2.3-fold (LV-tmEpoR-GFP) compared to control myoblasts. The EPO-dependent erythroid cell line, HCD57, was used as a positive control, and S16 mRNA expression was used as the internal control. Primary myoblasts from WT mice were isolated from mouse skeletal muscle and assessed for CD34, M-cadherin, and desmin expression and costaining with EpoR antibody. We found that <50% of the CD34+ cells, ∼80% of the desmin-positive cells and >95% of the M-cadherin positive cells were also immunoreactive to the EpoR antibody, raising the possibility that EpoR increases with myoblast maturity. These primary myoblast cultures were also treated with lentivirus. Greater than 90% GFP-positive cells following 3 treatments with lentivirus could be achieved for both LV-mEpoR-GFP and LV-tmEpoR-GFP, as assessed by flow cytometry (data not shown).
Survival advantage of EPO treatment and mEpoR-lentivirus infection in myoblasts
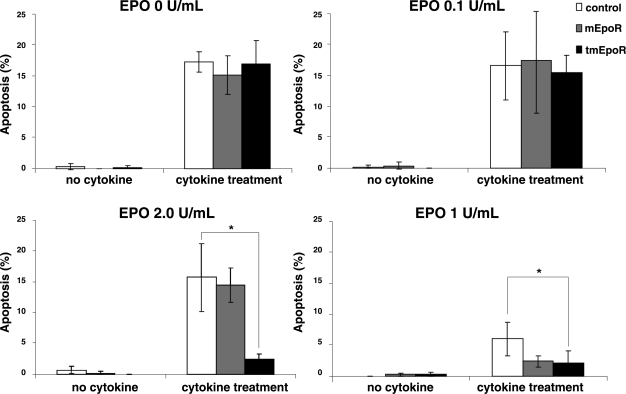
To assess the EPO-protective potential of EpoR-modified C2C12 cells, treatment with a cocktail of inflammatory cytokines (IL-1β, TNF-α, and INF-γ) under conditions that resulted in 15% apoptosis (TUNEL positive) was used (Fig. 2). At an EPO concentration of 0.1 U/ml, no difference in cell survival was observed for control C2C12 (LV-GFP), mEpoR C2C12 (LV-mEpoR-GFP), or tmEpoR C2C12 (LV-tmEpoR-GFP) cells. At 0.2 U/ml, only tmEpoR C2C12 cells exhibited EPO protection, with a reduction of 8-fold in apoptotic cells. At 1 U/ml, EPO protection was observed in all cell cultures, and apoptosis was reduced by 3-fold in control C2C12 cells, and by 8-fold in mEpoR and tmEpoR C2C12 cells. On the basis of these results, we selected an EPO concentration in the range of 1 to 5 U/ml for subsequent culture studies.
Figure 2.
EPO and EpoR protect myoblasts from inflammatory cytokine-induced apoptosis. C2C12 cells treated with LV-mEpoR-GFP, LV-tmEpoR-GFP, or LV-GFP control were cultured with a cocktail of inflammatory cytokines (IL-1β, TNF-α, and INF-γ) and exposed to different concentration of EPO (0, 0.1, 0.2, 1 U/ml) for 48 h. Percentage of apoptotic cells was measured by TUNEL-positive staining. *P < 0.01.
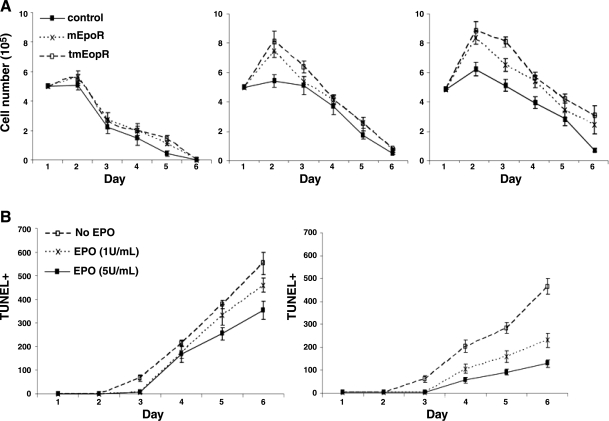
EPO supplementation also promoted cell survival under serum-starvation conditions (Fig. 3). No cells survived beyond 5 d under serum starvation without EPO treatment. EPO increased viability of serum-starved control C2C12 cells, as well as mEpoR and tmEpoR C2C12 cells, as measured by direct cell counting. Furthermore, mEpoR and tmEpoR C2C12 cells exhibited a survival advantage compared to control C2C12 cells in response to EPO treatment, and their cell numbers peaked at d 1, while no increase in cell numbers was observed in control cultures. The increase in cell survival by EPO had the greatest effect on tmEpoR C2C12 cell cultures, giving rise to cultures with the highest cell numbers (80% of the starting cell number) at d 5. TUNEL assay was used to measure the apoptotic C2C12 cells and confirm that increased EpoR expression protected murine myoblasts from apoptosis induced by serum starvation. The tmEpoR C2C12 cell cultures had fewer TUNEL-positive cells than the control C2C12 cultures throughout the culture period. EPO provided protection from apoptosis in a dose-dependent manner, and protection for tmEpoR C2C12 cells was greater than that for mEpoR C2C12 cells. Analogous but reduced EPO dose-dependent protection from apoptosis due to serum starvation was observed in mEpoR C2C12 cultures (data not shown). In the absence of EPO, we observed a slight but not significant protection with forced expression of EpoR in myoblasts. This may relate to the detection of endogenous EPO mRNA, raising the possibility of low levels of EPO production in muscle progenitor cells.
Figure 3.
EPO and EpoR protect myoblasts from apoptosis in serum-starvation condition. C2C12 cells treated with LV-mEpoR-GFP, LV-tmEpoR-GFP, or LV-GFP control were cultured in serum-free medium. A) Cell counts are indicated with EPO treatment at 0, 1, and 5 U/ml. B) Apoptotic myoblasts, LV-GFP C2C12 control cells, and LV-tmEpoR-GFP C2C12 cells were labeled with TUNEL-positive staining and counted from 20 random vision fields (×100) for cells treated with EPO at 0, 1, and 5 U/ml.
Increased EpoR altered expression of the myogenic regulatory factor proteins
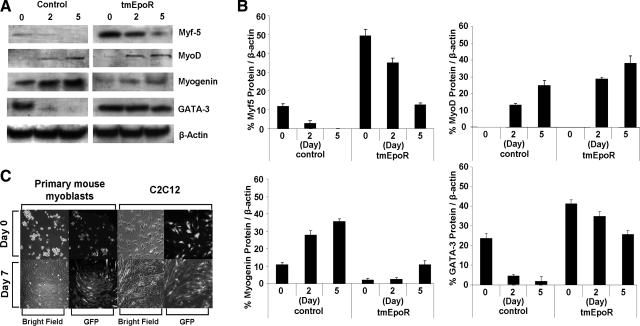
We previously reported that EPO exposure of cultured murine myoblasts leads to modification of mRNA expression for Myf-5, MyoD, and myogenin, and delayed differentiation and fusion into myoblasts (3). To determine whether lentiviral infection and/or overexpression of tmEpoR in myoblasts prevents differentiation of myoblasts, we cultured tmEpoR C2C12 (LV-tmEpoR-GFP) cells in differentiation medium. Compared with control cells, tmEpoR C2C12 cells in differentiation medium showed altered expression of the myogenic regulatory factor proteins. The tmEpoR C2C12 cells had significantly (P<0.01) higher protein expression of Myf-5 and MyoD, and lower protein expression of myogenin compared to control cells (Fig. 4). Expression of the early myogenic protein, Myf5, extended to d 5 in tmEpoR C2C12 cells cultured in differentiation medium. The induction of myogenin protein was reduced in tmEpoR C2C12 cells. The GATA-like transcription factor family protein, GATA-3, is also up-regulated in tmEpoR C2C12 cells. C2C12 cells with full-length mEpoR lentiviral infection indicated the similar pattern of myogenic regulatory factor proteins expression as the tmEpoR C2C12 cells (data not shown). These results suggest that EpoR overexpression in muscle progenitor cells further contributes to the delay in differentiation, as previously observed for EPO treatment of these cells (3).
Figure 4.
Myoblasts overexpressing LV-tmEpoR-GFP modified the expression of myogenic regulatory factor proteins. A) Western blot analysis of Myf-5, MyoD, myogenin, and GATA-3 was determined for C2C12 cells infected with LV-tmEpoR-GFP and LV-GFP control cultured in differentiation medium for 0, 2, and 5 d. β-Actin protein expression was used as internal control. B) Western blot analysis results were quantified and compared to β-actin. C) Primary myoblasts and C2C12 cells infected with LV-tmEpoR-GFP (top panels) were cultured in the differentiation medium for 7 d (bottom panels) and stained for GFP expression.
Overexpression of mEpoR or tmEpoR did not appear to modify cell morphology. For primary myoblasts harvested from mouse skeletal muscle treated with EpoR-expressing lentivirus, the morphology in culture medium (Fig. 4C, top panels) remained similar to control cultures (not shown). When placed in differentiation medium cultured for 7 d, the morphology change characteristic of myoblast differentiation was observed (Fig. 4C, bottom panels). Together, these results suggest that tmEpoR overexpression in myoblasts can delay cell differentiation and fusion to myotubes with the modifications in regulatory protein expression.
Transplant of tmEpoR myoblasts into mdx mice
We determined that tmEpoR (LV-tmEpoR-GFP) overexpression protected muscle progenitor cells from apoptosis in culture. To examine the ability of enhanced EPO signaling through increased expression of EpoR to promote donor cell survival, we used the mouse model of myoblast transplantation. To maximize EpoR expression, we chose LV-tmEpoR-GFP, which provides a higher increase of EpoR mRNA for a similar level of transduction compared to LV-mEpoR-GFP (Fig. 1C). For in vivo imaging of transplanted cells, the GFP reporter gene was replaced with a firefly luciferase reporter gene, and a GFP/luciferase lentiviral vector (LV-GFP-Luc) was used as a control. C2C12 myoblasts overexpressing tmEpoR (LV-tmEpoR-Luc) were transplanted in the right limb of the gastrocnemius muscle of WT and mdx mice. After transplantation, mice were imaged for luciferase activity to follow the population of transplanted cells at different time points (Fig. 5). On the first day after transplantation, donor cells expressing the luciferase reporter gene were visualized following injection of luciferin using the IVIS imaging system. In mice receiving C2C12 cells marked with the GFP/luciferase vector (LV-tmEpoR-Luc and LV-GFP-Luc C2C12), luciferase activity was readily detected in the hind limb at the site of injection. The signal remained localized, indicating that injected C2C12 cells did not readily migrate beyond the site of injection. The signal intensity decreased by about a log in 3 d, requiring an increase in acquisition time and sensitivity for imaging particularly between d 7 and 14. Treating animals with high dose of EPO (3000 U/kg) by intraperitoneal injection following transplantation did not markedly affect imaging of luciferase activity in LV-tmEpoR-Luc and LV-GFP-Luc C2C12 cells.
Figure 5.
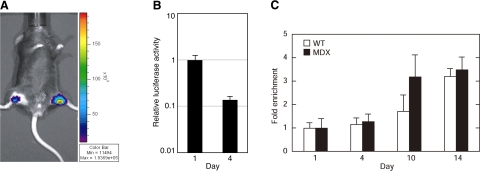
Myoblasts expressing LV-tmEpoR-Luc and LV-GFP-Luc C2C12 control cells were collected (2×105) and were injected at 2-mm depth into the gastrocnemius muscle of 3-mo-old mdx and WT mice. Donor cells expressing the luciferase reporter gene were imaged following injection of luciferin (150 mg/kg, i.p. injection) using the IVIS imaging system. Luciferin activity was monitored at d 1, 4, 7, 10, and 14 after transplantation. A) Sample bioluminescence image in a representative mdx mouse orthotopically transplanted with LV-GFP-Luc C2C12 control cells (left limb) and LV-tmEpoR-Luc C2C12 (right limb). B) Bioluminescent signal over time shows relative decrease in signal intensity. C) Fold enrichment in bioluminescent signal in mdx and WT mice transplanted with LV-tmEpoR-Luc and LV-GFP-Luc C2C12 control cells.
The limb injected with LV-tmEpoR-Luc C2C12 cells also readily exhibited luciferase activity that continued to decrease during the 2 wk following transplant similar to the limb injected with LV-GFP-Luc C2C12 control cells (data not shown). The relative luciferase signal at d 14 compared with d 1 was 3 times higher in the limb injected with the LV-tmEpoR-Luc C2C12 cells than in the limb injected with the LV-GFP-Luc C2C12 control cells. These data suggest increased survival of myoblasts with forced expression of tmEpoR. Administration of a high dose of EPO following transplantation did not markedly affect the decrease in luciferase activity from transplanted progenitor cells expressing tmEpoR, as observed for the LV-tmEpoR-Luc C2C12 cells.
In the mdx mouse model, myoblast transplantation was assessed using LV-tmEpoR-Luc and LV-GFP-Luc C2C12 control cells. The luciferase activity in the limb receiving LV-GFP-Luc C2C12 cells exhibited a similar decrease during the 14 d following transplantation compared to that observed in WT mice (data not shown). Interestingly, transplantation of LV-tmEpoR-Luc C2C12 cells again showed a more gradual decrease in luciferase activity with time, and by d 14, the relative residual signal was 3 times greater than that of the limb injected with the LV-GFP-Luc C2C12 control cells (Fig. 5C). Treating the mdx or WT mice following transplantation with high dose of EPO did not appear to affect the decrease in luciferase activities in LV-tmEpoR-Luc or LV-GFP-Luc C2C12 cells that decreased by a factor of ∼3 × 10−3 to 10−4, respectively, over 14 d following transplantation.
To determine the extent to which donor cells successfully engrafted and fused with existing muscle fibers, sections were prepared from the injected muscle 6 wk after transplantation when the IVIS system was no longer sensitive enough to detect luciferase activity by whole body imaging. Muscle sections from mice treated with a high dose of exogenous EPO (3000 U/kg) following myoblast transplantation were also prepared and stained for luciferase. Expression of luciferase in muscle sections was determined by immunohistochemistry (Fig. 6A). Muscles injected with C2C12 cells marked with GFP/luciferase control showed no luciferase-positive fibers or luciferase staining cells without exogenous EPO treatment. In contrast, luciferase staining was present in some muscle sections that received either LV-tmEpoR-Luc C2C12 cell transplantation or exogenous EPO (Fig. 6B). The luciferase staining appeared to localize to the area of injection and was not uniformly distributed throughout the muscle, suggesting limited migration of myoblasts following direct injection into muscle, as suggested from visualization of whole-body luciferase activity during the initial 14 d following transplantation.
Figure 6.
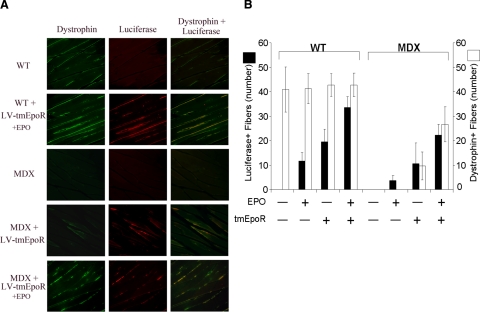
tmEpoR overexpression in grafted myoblasts favors formation of functional muscle fibers in mdx mice. C2C12 cells treated with LV-tmEpoR-Luc or LV-GFP-Luc control were injected at 2-mm depth into the gastrocnemius muscle of 3-mo-old mdx and WT mice. Erythropoietin (3000 U/kg) was administered by intraperitoneal injection to the EPO treatment groups on the same day of myoblast transplantation. After 6 wk of myoblast transplantation, mice were anesthetized and perfused with 4% paraformaldehyde in PBS. Gastrocnemius muscle was collected and sectioned for immunohistochemistry luciferase (red) and dystrophin (green) staining. A) Representative pictures of gastrocnemius muscle sections stained for dystrophin (left column), luciferase (center column), and an overlap of dystrophin/luciferase (right column) in WT and mdx mice grafted with LV-tmEpoR-Luc C2C12 cells and exposed to EPO. B) Quantification of luciferase and dystrophin-positive fibers in WT and mdx mice (positive-stained fibers/20 random field views at ×40).
EPO treatment significantly increased the number of luciferase-positive fibers in myoblast transplanted limbs under all conditions. However, the number of luciferase-positive fibers suggested that transplantation of LV-GFP-Luc C2C12 cells even with EPO treatment was lower compared with LV-tmEpoR-Luc C2C12 cells without EPO treatment. Examination of muscle from limbs transplanted with LV-tmEpoR-Luc C2C12 cells showed a significantly increased number of luciferase-positive fibers without EPO treatment compared with muscle from LV-GFP-Luc limbs, even with EPO treatment.
As demonstrated by EPO treatment in WT mice, mdx mice receiving LV-tmEpoR-Luc C2C12 transplants and supplemental EPO injections showed increased potential for donor cell engraftment and luciferase-marked muscle fibers (Fig. 6B). The greatest number of luciferase-positive fibers were observed for transplants of LV-tmEpoR-Luc C2C12 cells with administration of exogenous EPO, followed by LV-tmEpoR-Luc C2C12 cells without exogenous EPO. In comparison, luciferase-positive fibers were lower for transplants of LV-GFP-Luc C2C12 cells compared with LV-tmEpoR-Luc C2C12 cells and were only observed with exogenous EPO treatment.
Since the mdx mice lack the ability to express dystrophin, successful transplantation and engraftment of normal myoblasts should also provide muscle fibers that express dystrophin. Immunostaining for dystrophin demonstrated that muscle fibers expressing luciferase colocalized with fibers expressing dystrophin in LV-tmEpoR-Luc C2C12 cells transplants. With EPO treatment following transplantation, an increase in the dual-stained luciferase and dystrophin fibers was also observed. No dystrophin-positive fibers were detected in LV-GFP-Luc C2C12 cells with or without EPO treatment. Therefore, the maximal effect of myoblast transplant in the mdx mice was observed for LV-tmEpoR-Luc C2C12 cells transplanted into mdx mice followed by high-dose treatment of EPO.
DISCUSSION
The potential for EPO signaling to promote engraftment of transplanted cells has been previously investigated for hematopoietic stem cells. In mice expressing a truncated EpoR transgene, EPO treatment increased the number of multipotent, clonogenic hematopoietic cells in vivo, and long-term cultures of hematopoietic stem cells exhibited an exponential expansion of trilineage hematopoietic stem cells in the presence of Epo (24). In competitive bone marrow repopulation studies in mice, hematopoietic stem cells expressing the truncated EpoR transgene demonstrated a competitive advantage in engraftment in EPO-treated recipients. The ability of the truncated EpoR transgene to facilitate transplantation was maintained in secondary transplants without changes in hematopoietic stem cell depletion or excessive proliferation (10). Human cord blood CD34+ cells transduced with an EpoR-expressing vector showed an increase in proliferation when treated with EPO (25) and increased bone marrow repopulation capacity in xenotransplantation into NOD-SCID mice (16). We now show for the first time the ability to increase EpoR expression in myoblasts by lentiviral transduction, the increase in transplanted C2C12 cell survival by increased EPO signaling, and the resultant increase in muscle fibers expressing dystrophin in mdx mice. These findings indicate that increased expression of EpoR facilitates donor cell survival and engraftment beyond the hematopoietic lineage and may be useful in cell-based therapy for muscle diseases.
Mutation of the dystrophin gene causing absence of dystrophin protein results in destabilization of the muscle cell membrane and progressive muscle degeneration, giving rise to diseases such as DMD (26, 27). Transplantation and engraftment of muscle progenitor cells that can divide, differentiate, and fuse into existing muscle fibers have the potential to retard or stop muscle degeneration associated with DMD (28, 29). However, early clinical myoblast implantation did not show therapeutic efficiency or restore significant dystrophin production in DMD muscle, which may have been limited due to donor-cell survival and immune rejection (15, 30). Rapid proliferation of the donor-cell population combined with enhanced donor-cell fusion may contribute to survival of myoblasts and provide some protection from host immune response. We observed that exogenous erythropoietin and the increased erythropoietin receptor expression can promote the survival of cultured C2C12 cells under the inflammatory cytokine-induced apoptosis.
The cell-based therapy approach for muscle dystrophy requires improved survival of donor myoblasts to restore dystrophin expression and to maintain the function of muscle fibers (15, 28). Recent studies reached high efficiency of myoblast transplantation and restored dystrophin expression in the mdx mouse model for DMD after using cell-surface markers to purify the myoblast pool (14, 29). For proof of concept, we used C2C12 cells overexpressing tmEpoR and marked with a luciferase reporter gene (LV-tmEpoR-Luc) for transplantation into mice. Our study suggests that increasing EpoR expression in myoblast transplantation in combination with exogenous EPO administration can promote donor cell survival in the mdx mouse. A contribution from increased hematocrit by exogenous EPO treatment can also be possible through enhanced oxygen delivery. We observed restoration of dystrophin expression in mice after transplantation of LV-tmEpoR-Luc C2C12 cells. Exogenous EPO administration further improved the dystrophin expression in the mdx mice that received the LV-tmEpoR-Luc C2C12 cells. The role of cytokine signaling in myoblast transplantation warrants further investigation.
The broad distribution of EpoR beyond hematopoietic tissue gives rise to EPO response in nonhematopoietic cells, including endothelial, neural, cardiac, and skeletal muscle progenitor cells. Studies in cell cultures and animal models indicate a protective effect of EPO in brain and spinal cord against ischemic damage and in the retina against UV light damage. In cultures of myoblasts from skeletal muscle, EPO treatment stimulated myoblast proliferation, promoted the expression of myogenic regulatory factors Myf 5 and MyoD during differentiation, and delayed expression of myogenin and fusion into myotubes (3). The myogenic regulatory factor family proteins have been used to define the early (Myf-5 and MyoD) and late (myogenin) stages of myogenic differentiation and myotube fusion in muscle cell development and differentiation (31). In this study, we showed that LV-tmEpoR-GFP C2C12 cells changed the expression of Myf-5, MyoD, and myogenin protein during differentiation, corresponding to the previously reported changes in mRNA expression by EPO treatment (3). Altered expression of myogenic regulatory factors in LV-tmEpoR-GFP C2C12 cells may originate from increased EPO signaling via the EPO produced by C2C12 cells. Quantitative RT-PCR indicated induction of EPO mRNA in C2C12 cells under low-oxygen conditions or treatment of EPO (data not shown). We demonstrated that EPO is protective for C2C12 cell cultures that are serum starved or treated with inflammatory cytokines to induce apoptosis and that the protective activity of EPO was enhanced with genetic modification of myoblasts to increase EpoR expression, resulting in increased cell survival in culture and in transplantation into the mdx mouse model.
EPO binds to EpoR on the cell surface, activates phosphorylation by JAK2, induces downstream signaling pathways, and, in myoblasts, modifies expression of the myogenic regulatory factor family. To enhance EPO activity and possibly prolong its effect, we used the dual-promoter lentiviral vector to genetically mark and increase EpoR expression in cultured C2C12 cells. Increased EpoR expression increased C2C12 cell proliferation in culture and survival in vitro and in vivo. Almost no C2C12 cells survived when cultured for 5 d without serum. In contrast, the maximum EPO-protective effect was observed in LV-tmEpoR-GFP C2C12 cells cultured with EPO, resulting in cell numbers of 80% of the starting value after 5 d of serum starvation. In erythroid progenitor cells, binding of the GATA-like transcription factor family member GATA-1 to the erythropoietin receptor promoter is important for high level of EpoR expression and erythroid development (32). We found that EPO stimulation of myoblast in differentiation medium results in increased GATA-3 protein expression in addition to induction of Myf-5 and MyoD. Interestingly, synergistic binding of Myf-5, MyoD, and GATA-3/4 to the enhancer element controls the expression of fibroblast growth factor protein, which is critical for myotome formation and limb growth (33). When cultured in differentiation medium for 7 d, forced expression of EpoR did not markedly affect cell morphology or inhibit myoblast fusion into myotubes, suggesting that EPO signaling can promote myoblast proliferation and survival, and delay differentiation into myotubes, but does not prevent myoblast differentiation. This latest characteristic is crucial to the success of a cell-based therapy for DMD and suggests activation of EPO signaling may be useful in facilitating myoblast transplantation. Therefore, increasing EPO signaling in the case of myoblast transplantation into mdx mice provides both an increased incorporation of donor cells into muscle fibers and the functional consequence of dystrophin expression. As a proof-of-concept study, these data suggest that in progenitor cells found to express endogenous erythropoietin receptor, increasing receptor expression and stimulation with erythropoietin after transplantation improves donor cell survival.
Acknowledgments
We thank Mingfeng Zhao for technical assistance in vector construction and Brenda Klaunberg for her expertise in animal imaging. Funding was provided by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- Yu X, Lin C S, Costantini F, Noguchi C T. The human erythropoietin receptor gene rescues erythropoiesis and developmental defects in the erythropoietin receptor null mouse. Blood. 2001;98:475–477. doi: 10.1182/blood.v98.2.475. [DOI] [PubMed] [Google Scholar]
- Wu H, Liu X, Jaenisch R, Lodish H F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- Ogilvie M, Yu X, Nicolas-Metral V, Pulido S M, Liu C, Ruegg U T, Noguchi C T. Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J Biol Chem. 2000;275:39754–39761. doi: 10.1074/jbc.M004999200. [DOI] [PubMed] [Google Scholar]
- Chen J, Connor K M, Aderman C M, Smith L E. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki R. Pleiotropic functions of erythropoietin. Int Med. 2003;42:142–149. doi: 10.2169/internalmedicine.42.142. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Masuda S, Chikuma M, Inoue K, Nagao M, Sasaki R. Estrogen-dependent production of erythropoietin in uterus and its implication in uterine angiogenesis. J Biol Chem. 1998;273:25381–25387. doi: 10.1074/jbc.273.39.25381. [DOI] [PubMed] [Google Scholar]
- Westenbrink B D, Lipsic E, van der Meer P, van der Harst P, Oeseburg H, Du Marchie Sarvaas G J, Koster J, Voors A A, van Veldhuisen D J, van Gilst W H, Schoemaker R G. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor-mediated neovascularization. Eur Heart J. 2007;28:2018–2027. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- Brines M L, Ghezzi P, Keenan S, Agnello D, de Lanerolle N C, Cerami C, Itri L M, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, Matsunaga S, Tateishi K, Nomura T, Takahashi T, Tatsumi T, Matsubara H. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–1413. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- Kirby S, Walton W, Smithies O. Hematopoietic stem cells with controllable tEpoR transgenes have a competitive advantage in bone marrow transplantation. Blood. 2000;95:3710–3715. [PubMed] [Google Scholar]
- Arcasoy M O. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- Cossu G, Sampaolesi M. New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol Med. 2007;13:520–526. doi: 10.1016/j.molmed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Cerletti M, Jurga S, Witczak C A, Hirshman M F, Shadrach J L, Goodyear L J, Wagers A J. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi R, Gehlbach K, Bachoo R M, Kamath S, Osawa M, Kamm K E, Kyba M, Perlingeiro R C. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med. 2008;14:134–143. doi: 10.1038/nm1705. [DOI] [PubMed] [Google Scholar]
- Peault B, Rudnicki M, Torrente Y, Cossu G, Tremblay J P, Partridge T, Gussoni E, Kunkel L M, Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Urbinati F, Lotti F, Facchini G, Montanari M, Ferrari G, Mavilio F, Grande A. Competitive engraftment of hematopoietic stem cells genetically modified with a truncated erythropoietin receptor. Hum Gene Ther. 2005;16:594–608. doi: 10.1089/hum.2005.16.594. [DOI] [PubMed] [Google Scholar]
- Pelletier S, Gingras S, Funakoshi-Tago M, Howell S, Ihle J N. Two domains of the erythropoietin receptor are sufficient for Jak2 binding/activation and function. Mol Cell Biol. 2006;26:8527–8538. doi: 10.1128/MCB.01035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livnah O, Stura E A, Middleton S A, Johnson D L, Jolliffe L K, Wilson I A. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science. 1999;283:987–990. doi: 10.1126/science.283.5404.987. [DOI] [PubMed] [Google Scholar]
- Zhao W, Kitidis C, Fleming M D, Lodish H F, Ghaffari S. Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway. Blood. 2006;107:907–915. doi: 10.1182/blood-2005-06-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A D, Yoshimura A, Youssoufian H, Zon L I, Koo J W, Lodish H F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991;11:1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer L, Deau B, Forejtnikova H, Dumenil D, Margottin-Goguet F, Lacombe C, Mayeux P, Verdier F. Beta-Trcp mediates ubiquitination and degradation of the erythropoietin receptor and controls cell proliferation. Blood. 2007;109:5215–5222. doi: 10.1182/blood-2006-10-055350. [DOI] [PubMed] [Google Scholar]
- Arcasoy M O, Harris K W, Forget B G. A human erythropoietin receptor gene mutant causing familial erythrocytosis is associated with deregulation of the rates of Jak2 and Stat5 inactivation. Exp Hematol. 1999;27:63–74. doi: 10.1016/s0301-472x(98)00003-4. [DOI] [PubMed] [Google Scholar]
- Yu X, Zhan X, D'Costa J, Tanavde V M, Ye Z, Peng T, Malehorn M T, Yang X, Civin C I, Cheng L. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol Ther. 2003;7:827–838. doi: 10.1016/s1525-0016(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Kirby S L, Cook D N, Walton W, Smithies O. Proliferation of multipotent hematopoietic cells controlled by a truncated erythropoietin receptor transgene. Proc Natl Acad Sci U S A. 1996;93:9402–9407. doi: 10.1073/pnas.93.18.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima T, Ueda Y, Hanazono Y, Kume A, Shibata H, Ageyama N, Terao K, Ozawa K, Hasegawa M. In vivo expansion of gene-modified hematopoietic cells by a novel selective amplifier gene utilizing the erythropoietin receptor as a molecular switch. J Gene Med. 2004;6:22–31. doi: 10.1002/jgm.467. [DOI] [PubMed] [Google Scholar]
- Shimizu R, Komatsu N, Miura Y. Dominant negative effect of a truncated erythropoietin receptor (EPOR-T) on erythropoietin-induced erythroid differentiation: possible involvement of EPOR-T in ineffective erythropoiesis of myelodysplastic syndrome. Exp Hematol. 1999;27:229–233. doi: 10.1016/s0301-472x(98)00048-4. [DOI] [PubMed] [Google Scholar]
- Judge L M, Haraguchiln M, Chamberlain J S. Dissecting the signaling and mechanical functions of the dystrophin-glycoprotein complex. J Cell Sci. 2006;119:1537–1546. doi: 10.1242/jcs.02857. [DOI] [PubMed] [Google Scholar]
- Partridge T. Myoblast transplantation. Neuromuscul Disord. 2002;12:S3–S6. doi: 10.1016/s0960-8966(02)00076-7. [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau H M. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R G, Sharma K R, Pavlath G K, Gussoni E, Mynhier M, Lanctot A M, Greco C M, Steinman L, Blau H M. Myoblast implantation in Duchenne muscular dystrophy: the San Francisco study. Muscle Nerve. 1997;20:469–478. doi: 10.1002/(sici)1097-4598(199704)20:4<469::aid-mus10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Gensch N, Borchardt T, Schneider A, Riethmacher D, Braun T. Different autonomous myogenic cell populations revealed by ablation of Myf5-expressing cells during mouse embryogenesis. Development. 2008;135:1597–1604. doi: 10.1242/dev.019331. [DOI] [PubMed] [Google Scholar]
- Chin K, Oda N, Shen K, Noguchi C T. Regulation of transcription of the human erythropoietin receptor gene by proteins binding to GATA-1 and Sp1 motifs. Nucleic Acids Res. 1995;23:3041–3049. doi: 10.1093/nar/23.15.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahori A, Fraidenraich D, Basilico C. A conserved enhancer element that drives FGF4 gene expression in the embryonic myotomes is synergistically activated by GATA and bHLH proteins. Dev Biol. 2004;270:525–537. doi: 10.1016/j.ydbio.2004.03.012. [DOI] [PubMed] [Google Scholar]