Abstract
Folate (vitamin B9) is utilized for synthesis of both S-adenosylmethionine (AdoMet) and deoxythymidine monophosphate (dTMP), which are required for methylation reactions and DNA synthesis, respectively. Folate depletion leads to an imbalance in both AdoMet and nucleotide pools, causing epigenetic and genetic damage capable of initiating tumorigenesis. Polyamine biosynthesis also utilizes AdoMet, but polyamine pools are not reduced under a regimen of folate depletion. We hypothesized that high polyamine biosynthesis, due to the high demand on AdoMet pools, might be a factor in determining sensitivity to folate depletion. We found a significant correlation (P<0.001) between polyamine biosynthesis and the amount of folate required to sustain cell line proliferation. We manipulated polyamine biosynthesis by genetic and pharmacological intervention and mechanistically demonstrated that we could thereby alter AdoMet pools and increase or decrease demand on folate availability needed to sustain cellular proliferation. Furthermore, growing a panel of cell lines with 100 nM folate led to imbalanced nucleotide and AdoMet pools only in cells with endogenously high polyamine biosynthesis. These data demonstrate that polyamine biosynthesis is a critical factor in determining sensitivity to folate depletion and may be particularly important in the prostate, where biosynthesis of polyamines is characteristically high due to its secretory function.—Bistulfi, G., Diegelman, P., Foster, B. A., Kramer, D. L., Porter, C. W., Smiraglia, D. J. Polyamine biosynthesis impacts cellular folate requirements necessary to maintain S-adenosylmethionine and nucleotide pools.
Keywords: one-carbon metabolism, prostate cancer
Once acquired from the diet, folate is essential for providing several substrates crucial for cell proliferation. These include deoxythymidine monophosphate (dTMP), which is required for DNA synthesis, and S-adenosylmethionine (AdoMet) (Fig. 1), the intracellular methyl donor for methyltransferase activities that modify DNA, RNA, histones, and other proteins (reviewed in refs. 1, 2). AdoMet is also required for the biosynthesis of polyamines (Fig. 1), small ubiquitous alkylamines important for multiple biochemical processes, including apoptosis and DNA stability (reviewed in refs. 2,3,4). In accordance with their important functions, polyamine intracellular concentrations are tightly regulated (3,4,5,6).
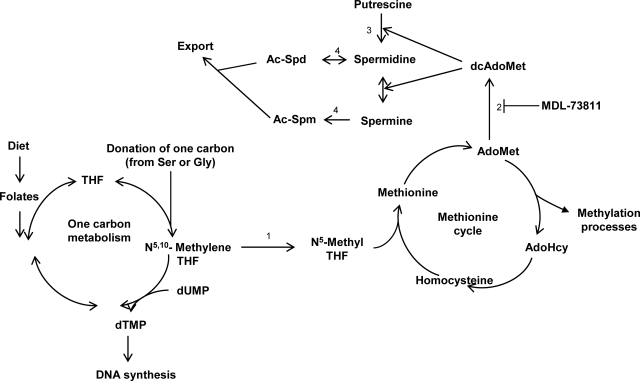
Figure 1.
Folate, 1-carbon metabolism, methionine cycle and polyamine biosynthesis. A low dietary folate intake (left) results in low dTMP and AdoMet. Polyamine (spermidine and spermine) biosynthesis (top) also draws on AdoMet through AdoMetDC (2), which converts AdoMet into dcAdoMet for the synthesis of Spd and Spm, starting with the enzyme ODC (3). MDL-73811 is a specific inhibitor of AdoMetDC (2) that prevents polyamine biosynthesis. MTHFR (1) decides the fate of 1-carbon molecules toward either dTMP or AdoMet synthesis. SSAT (4) acetylates polyamines for cellular secretion.
Dietary folate depletion in human cells in vitro and in rodent models in vivo, alone or combined with methionine/choline deficiency, strongly impacts the AdoMet and nucleotide pools, resulting in both epigenetic and genetic damage (1, 7,8,9) and in carcinogenesis of liver and colon (10, 11). Specifically, folate depletion results in lower levels of N-5,10-methylene tetrahydrofolate (THF) that is necessary to convert deoxyuridine monophosphate (dUMP) into dTMP (Fig. 1). This ultimately leads to an increase of deoxyuridine triphosphate (dUTP) and a decrease of deoxythymidine triphosphate (dTTP). An increased dUTP:dTTP ratio is characteristic of cells that are folate depleted and culminates in uracil misincorporation into the DNA and futile cycles of excision and repair to remove uracil from the DNA, ultimately leading to single-strand breaks and DNA damage (8, 12). N-5,10-methylene THF can also be converted to N-5-methyl THF, which is a substrate necessary for the conversion of homocysteine into methionine (Fig. 1). The enzyme responsible for the irreversible conversion of N-5,10-methylene THF into N-5-methyl THF is methylenetetrahydrofolate reductase (MTHFR), which acts as a switch for 1-carbon groups to go toward either thymidylate synthesis or AdoMet synthesis (Fig. 1).
S-adenosylhomocysteine (AdoHcy) is generated by demethylation of AdoMet in intracellular methylation reactions (Fig. 1). Low folate prevents recycling of homocysteine into AdoMet and leads to accumulation of AdoHcy and low levels of AdoMet. AdoHcy is a potent inhibitor of DNA methyltransferases, and the decrease of the AdoMet:AdoHcy ratio consequent to folate depletion is associated with global DNA hypomethylation and, in certain cases, specific CpG hypermethylation (7, 13, 14). Interestingly, epigenetic aberrations are found in the preneoplastic liver of rats fed a folate/methyl-deficient diet, but not in spleen, thymus, kidney, and pancreas (15). Although clinically relevant, it is currently not known why dietary folate depletion is oncogenic in certain organs but not others.
Folate is required for production of AdoMet through 1-carbon metabolism, and in turn AdoMet is required for polyamine biosynthesis; nonetheless, the interdependency of these pathways has not been studied. Several studies found that a folate-depleted diet in rodents results in the imbalance of AdoMet pools (14, 16, 17) and nucleotide pools (8, 12). Conversely, one study reported that a folate-depleted diet does not decrease polyamine levels, but surprisingly increases them in the blood and liver of rats, likely as a consequence of a compensatory response (18).
To the best of our knowledge, no study so far investigated the complex interplay among 1-carbon metabolism, methionine cycle, polyamine biosynthesis, and cell growth in response to folate depletion. We hypothesized that high polyamine biosynthesis, because of the high demand on AdoMet pools, might be a factor in determining sensitivity to folate depletion. Therefore, cells characterized by high levels of polyamine biosynthesis, like prostate, might suffer an imbalance in their nucleotide and AdoMet pools at levels of folate that are well tolerated by other tissues. The high levels of polyamines characteristic of prostate are consequent to the high activities of the 2 rate-limiting enzymes responsible for polyamine biosynthesis: ornithine decarboxylase (ODC) and AdoMet decarboxylase (AdoMetDC) that, being induced by androgens, are highly expressed in the prostate (19,20,21,22,23,24). Indeed, in addition to synthesizing polyamines for epithelial cell replacement, the prostate produces massive amounts of polyamines for export in the reproductive fluids (5, 19, 25). While liver and colon are known targets of hypomethylation and DNA damage consequent to folate depletion (7, 9, 12, 16, 26), no data are currently available on the effects of folate depletion on prostate cells in vivo or in vitro. This is relevant because prostate cancer is the most common and the second most lethal cancer in men in the United States (27), and because antifolate drugs are used in the clinic for their antiproliferative effects in the treatment of other types of cancer as well as opportunistic agent infections (reviewed in ref. 28).
MATERIALS AND METHODS
Cell culture
The human prostate (PC-3 and DU145) and colon (HCT-116 and RKO-9) cell lines were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA), and the 3 clonal cell lines (TRAMPC-2D, TRAMPC-2G, and TRAMPC-2H) were generated from a primary prostate tumor in a transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse (29), where tumorigenesis is driven at puberty by the expression of large T antigen, specifically in prostate tissue. LNCaP/SSAT cells were generated by stable transfection of the human prostate cancer cell line LNCaP with a tetratcycline (Tet)-off expression construct for the spermidine/spermine N-1-acetyltransferase (SSAT) enzyme (30). LNCaP/SSAT cells were routinely grown on poly-d-lysine cellware (Becton Dickinson, Bedford, MA, USA). All cell lines were grown in folate-free RPMI 1640 (Invitrogen, Carlsbad, CA, USA) plus 10% dialyzed fetal bovine serum (Invitrogen) supplemented with the specified amount of folic acid (FA; Sigma-Aldrich, St. Louis, MO, USA). TRAMPC cell lines were also grown with 10−8 dihydrotestosterone (DHT). There was no significant change in the polyamine pools of TRAMPC cell lines grown with or without DHT, probably because of significant amount of androgens available from the serum and the medium. In the experiment growing cell lines for 5 population doublings (PD) with either 100 nM or 2 μM FA, all cell lines were grown in 10−8 DHT.
Growth curves
We established a reproducible method to study cell growth dependency on folate concentration in the medium. Cells were seeded in duplicate at 3000 cells/well in 6-well plates, and cell counts were carried out with trypan blue at 4, 8, and 12 PD in medium supplemented with different amounts of FA, as specified, to obtain growth curves. The growth rate was established as the difference in cell number between PD 12 and 8, when intracellular stores of folate were depleted (data not shown) and differences in cell number were more evident. The population doubling time (PDT) per cell line was assessed in control medium (2 μM FA) (TRAMPC-2D, 16.5 h; TRAMPC-2G, 15.8 h; TRAMPC-2H, 16.9 h; PC-3, 21.8 h; DU145, 23.6 h; HCT-116, 17.75 h; RKO-9, 11.8 h). We did not establish growth curves for LNCaP/SSAT because of the high-PDT characteristic of this cell line (48 h) and because of the necessity to grow these cells on poly-d-lysine cellware (see above).
Increasing polyamine flux in LNCaP/SSAT
LNCaP/SSAT cells were grown in RPMI + 10% dialyzed fetal bovine serum + tetracycline (Tet) (1 μg/ml) with or without folate (2 μM) for 2 PD (4 d), after which cells were split into samples either with or without Tet. Residual Tet was washed out after 24 h as described previously (30), and cells were allowed to grow for an additional 48 h before being harvested for cell counts and HPLC analyses.
Growth with pharmacological inhibition of polyamine biosynthesis
Cells were seeded with medium containing the specified concentrations of folate to establish growth curves as described above, with the only addition to the medium 1 mM aminoguanidine, which prevents the metabolism of exogenously added spermidine (Spd) and spermine (Spm) in the medium (31). After 24 h, cells were treated with 10 μM 5′-((-4-amino-2-butenyl)methylamino)-5′-deoxyadenosine (MDL-73811) to block the activity of AdoMetDC (32, 33) and 10 μM Spd to supply cells with enough polyamines to prevent any effect on cell growth despite the inhibition of AdoMetDC (34, 35). Treatments were performed in duplicate, and cells were counted after 12 PD of cells grown with control amounts of folate (2 μM). Percentage of growth was calculated as a percentage of cells grown with control amounts of folate, set as 100%.
HPLC analyses
HPLC analyses were carried out as described previously (36,37,38,39,40,41). Standards for AdoMet, acetyl-spermidine (Ac-Spd), acetyl-spermine (Ac-Spm), putrescine, Spd, Spm, dTTP, and dUTP were purchased from Sigma. Purified dcAdoMet was a kind gift of Dr. Janice Sufrin (Roswell Park Cancer Institute, Buffalo, NY, USA). All analyses were carried out on a reverse-phase Econosil (C18) column (5 μm particle size, 4.6×250 mm) (Grace Davison Discovery Sciences, Deerfield, IL, USA) with a C18 guard column assembled on the Waters 2796 Bioseparation module (Waters Corp., Milford, MA, USA) of the Biopolymer Facility at Roswell Park Cancer Institute (Buffalo, NY, USA).
Analysis of ODC activity
ODC activity was determined by a CO2 trap assay as described previously (40) and reported as nanomoles of radiolabeled CO2 per hour per milligram of protein.
Analysis of intracellular folates by Lactobacillus casei
Intracellular quantification of folate pools was carried out as described previously (42) with the following modification to the extraction protocol. Folate pools were extracted by sonication on a Fisher Scientific Sonic Dismembrator, model 100, on output 2, for 15 s on ice, starting from 50,000 cells in 600 μl extraction buffer (42). After centrifugation, 15 to 60 μl of the clarified lysate was analyzed in triplicate as described previously (42).
Statistical analysis
Statistical significance in the experiments evaluating intracellular concentration of folates (Figs. 2B, 3A, and 5A), polyamines (Fig. 3B), and AdoMet pools (Figs. 3C and 4A), and growth rates (Fig. 3D) was calculated with the standard Student’s t test. Because of biological variability within different experiments in the AdoMet pools, statistical significance in this experiment (Fig. 3C) was calculated by normalizing the results to the control sample (+Tet +FA), which was set as 100%. Correlation between either polyamine levels, ODC activity, or dUTP:dTTP ratio, and folate sensitivity (Figs. 2B, 2C, and 5D) was calculated with the 1-tailed Pearson correlation test. Statistical significance in the experiments evaluating difference in the growth curves (Figs. 2C, 4B, and 4C) was calculated by 2-way ANOVA.
Figure 2.
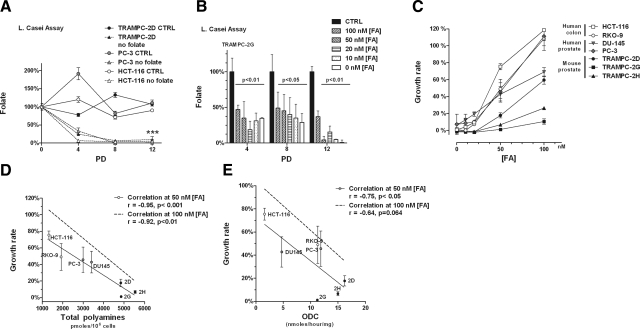
Requirement for folate correlates with polyamine biosynthesis. A) Intracellular folate stores are depleted in cell lines grown in zero-folate medium by PD 8. B) Intracellular folate levels are directly proportional to folate in the medium. The higher the folate concentration in medium, the slower the depletion of intracellular folate. C) Comparison of folate-dependent growth rates. Graphical representation of growth rates of the different cell lines grown with different amounts of FA. Data are averages + sd of 2 biological replicates per point. D) Inverse correlation between polyamine pools (intracellular plus secreted) and folate-dependent growth rates. Each cell line is represented according to its levels of polyamine pools (Table 1) on the x axis and its growth rate in limiting amounts of folate (50 nM) (as calculated in panel C) on the y axis. There is a significant (P<0.001) inverse correlation between polyamine pools and growth rates with 50 nM folate, as calculated by a 1-tailed Pearson correlation test. Significant correlation was also found when cells were grown at 100 nM folate (figure shows only the correlation as a dashed line, but not the actual experimental points, which can be inferred by Table 1 and panel C). E) Significant (P<0.05) inverse correlation between ODC activity and folate-dependent growth rates. Experimental points on graph correspond to growth rates of cells grown in 50 nM folate. Significant correlation was also found when cells were grown at 100 nM folate, shown as in panel D.
Figure 3.
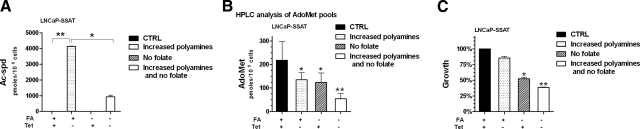
Folate depletion and polyamine biosynthesis affect AdoMet pools and cell growth additively. A) HPLC analysis of polyamines shows that SSAT overexpression (−Tet) increases Ac-Spd, but this increase is greatly reduced in the absence of folate. B) HPLC analysis of AdoMet pools shows that increasing polyamine biosynthesis (−Tet) and depleting folate from the medium draw additively on AdoMet pools. C) Cell growth mirrors trend observed in AdoMet pools. Results are presented as averages + sem of 2 independent experiments.
Figure 4.
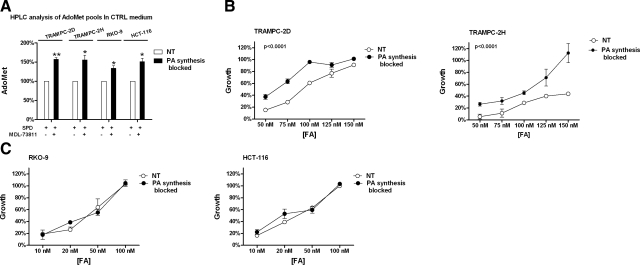
Blocking polyamine biosynthesis increases AdoMet availability and reduces sensitivity to folate depletion in prostate cell lines. A) Treatment with the AdoMetDC inhibitor MDL-73811 increases AdoMet availability in prostate and colon cell lines. Results are averages + sem of 2 independent experiments. B) Treatment with MDL-73811 in the presence of restrictive amounts of folate significantly (P<0.001) decreased sensitivity of both TRAMPC-2D (left panel) and TRAMPC-2H (right panel) to folate depletion. Data represent percentage of growth at 12 PD, relative to growth in control amount of folate (2 μM), which was set to 100%. Data are averages + sd of 2 biological replicates. C) Treatment with MDL-73811 in the presence of restrictive amounts of folate did not change sensitivity of colon cell lines to folate depletion. Data are averages + sd of 2 biological replicates.
Figure 5.
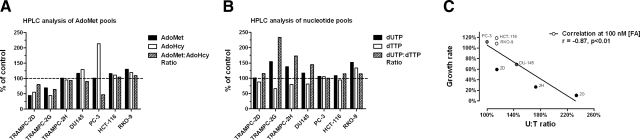
A, B) Growth for 5 PD in 100 nM FA affected the AdoMet (A) and nucleotide pools (B) only of those cell lines characterized by higher polyamine biosynthesis. C) Increase in dUTP:dTTP ratio in cells shows a significant, inverse correlation with folate-dependent growth rate.
RESULTS
Characterization of folate-dependent growth rates in a panel of prostate and colon cancer cell lines
To study cell growth in relation to folate concentration in the medium, we established a reproducible protocol to determine viable cell numbers in the presence of restrictive amounts of folate at different time points corresponding to 4, 8, and 12 PD in control medium in a panel of 7 cell lines including 3 murine prostate cell lines derived from the TRAMP model (29) (TRAMPC-2D, TRAMPC-2G, and TRAMPC-2H), 2 human prostate cancer cell lines (DU145 and PC-3), and 2 human colon cancer cell lines (RKO-9 and HCT-116) (see Materials and Methods). Using PD, whose time differs among all the cell lines, rather then a set period of time allowed us to compare cell dependency on folate, as all cells had the same amount of folate available per cell per duplication through the experiments. Intracellular folate levels of cells grown in control medium oscillated in a range likely defining the normal amount of intracellular folate when folate in the medium is available in excess (2 μM). All cell lines depleted their intracellular folate pools, when grown in zero-folate medium, by PD 8 (Fig. 2A). When cells were grown with limiting concentrations of folate in the medium (FA=[0–100 nM]), we found that the intracellular concentration of folate at each time point was correspondingly reduced (Fig. 2B).
We measured folate-dependent growth rates normalizing the growth rate of each cell line in folate-restricted medium (folate-free RPMI 1640+10% dialyzed FBS + specified amounts of FA) to growth rates in control medium (folate-free RPMI 1640+10% dialyzed FBS+2 μM FA) set as 100% growth. Using dialyzed serum also in the control medium allowed us to monitor those changes in growth rate that were exclusively consequent to the change in folate concentration in the medium by eliminating variables between normal and dialyzed serum. Plotting the growth rate of the cell lines at different concentrations of folate resulted in curves characterized by significantly different slopes (2-way ANOVA, P<0.001), indicating that the cells displayed different degrees of sensitivity to folate depletion (Fig. 2C).
Correlation between levels of polyamine biosynthesis and sensitivity to folate depletion
To determine whether polyamine biosynthesis could be a factor affecting sensitivity to folate depletion, we measured the intracellular polyamine pools (Spd and Spm) by HPLC analysis (Table 1). Because prostate is known to secrete significant amounts of polyamines, we also measured secreted polyamines (Ac-Spd and Ac-Spm) in the medium (Table 1). Overall, we observed that in this panel, prostate cell lines have higher polyamine pools, both intracellular and secreted, than colon cell lines.
TABLE 1.
Analysis of polyamine pools and ODC enzymatic activity
| Cell line | Spd (pmol/106 cells) | Spm (pmol/106 cells) | Ac-Spd (pmol/106 cells) | Ac-Spm (pmol/106 cells) | ODC activity (nmol/h/mg protein) |
|---|---|---|---|---|---|
| TRAMPC-2D | 3087.2 ± 254.4 | 1141.7 ± 150.7 | 559.0 ± 4.5 | 55.9 ± 6.2 | 16.2 ± 2.6 |
| TRAMPC-2G | 3788.6 ± 527.3 | 1269.0 ± 104.8 | 291.9 ± 0.6 | 12.4 ± 2.2 | 11.2 ± 1.9 |
| TRAMPC-2H | 3636.4 ± 161.0 | 1413.7 ± 209.2 | 476.0 ± 1.8 | 17.7 ± 3.6 | 15.0 ± 2.1 |
| DU145 | 2027.2 ± 687.6 | 1120.6 ± 438.5 | 240.8 ± 6.2 | 23.5 ± 1.1 | 4.7 ± 0.8 |
| PC-3 | 1749.2 ± 403.3 | 1107.4 ± 304.4 | 121.5 ± 13.4 | 14.5 ± 2.7 | 11.9 ± 0.29 |
| HCT-116 | 734.0 ± 155.1 | 527.3 ± 63.3 | 59.6 ± 10.0 | 6.3 ± 0.6 | 1.6 ± 0.2 |
| RKO-9 | 970.9 ± 240.0 | 893.4 ± 126.5 | 50.6 ± 0.6 | 7.3 ± 1.1 | 11.3 ± 3.0 |
We plotted the folate-dependent growth rates of the different cell lines at either 50 or 100 nM FA against the polyamine levels to look for any correlation (Fig. 2D). We chose 50 and 100 nM FA because at these concentrations the growth of all cell lines was affected, even if to different extents, by folate restriction. Strikingly, we found a highly significant inverse correlation between the folate-dependent growth rates at both 50 nM FA (P<0.001) and 100 nM FA (P<0.01) and the total (intracellular+secreted) levels of polyamines. We analyzed the correlation between single components of the polyamine pools and folate-dependent growth rates (Table 2) and found significant correlations with Spd, Spm, and Ac-Spd. Interestingly, Spd, which is the output of the first reaction in polyamine biosynthesis catalyzed by ODC and utilizing AdoMet (Fig. 1), showed the most significant correlation (P<0.0001) to folate-dependent growth rates. Therefore, we measured ODC activity and found that indeed ODC activity significantly correlated with folate-dependent growth rates (Fig. 2E). To demonstrate a causal relation between polyamine biosynthesis and folate-dependent growth rates we next up-regulated and down-regulated polyamine biosynthesis and studied the consequent effects on AdoMet pools and folate demand in vitro.
TABLE 2.
Correlation analysis between folate-dependent growth rates and polyamine levels
| FA concentration | Spd | Spm | Ac-Spd | Ac-Spm |
|---|---|---|---|---|
| 50 nM | ||||
| rvalue | −0.97 | −0.90 | −0.78 | −0.39 |
| Pvalue | <0.0001 | <0.05 | <0.05 | 0.19 |
| 100 nM | ||||
| rvalue | −0.95 | −0.80 | −0.73 | −0.27 |
| Pvalue | <0.001 | <0.05 | 0.06 | 0.55 |
Table expresses statistical values for correlation between folate-dependent growth rates and polyamine levels, as calculated by the Pearson correlation test.
Folate restriction and polyamine biosynthesis affect AdoMet pools and cell growth, additively
Polyamine intracellular concentration is tightly regulated (3,4,5,6). To up-regulate polyamine biosynthesis in prostate cells, we took advantage of an experimental model previously described: the LNCaP/SSAT (30). The LNCaP/SSAT cells are human prostate cancer LNCaP cells stably transfected with a conditional (Tet-off) expression construct to induce expression of the SSAT enzyme (LNCaP/SSAT) (30). SSAT is the enzyme responsible for acetylating intracellular polyamines, promoting their secretion and export. When LNCaP/SSAT cells are grown in medium containing Tet, there is no detectable SSAT expression. However, on Tet removal for 24 h, there is a 20-fold increase in SSAT expression and a 50-fold increase in SSAT activity, with a concomitant increase in Ac-Spd and Ac-Spm (30, 43). As a consequence, polyamines are massively secreted into the medium. To maintain intracellular polyamine pools, cells overexpressing SSAT are characterized by a dramatic increase of polyamine biosynthesis via up-regulation of ODC and AdoMetDC activities (30, 43, 44).
We therefore studied the effects of increased polyamine biosynthesis via SSAT overexpression on AdoMet levels and cell growth in LNCaP prostate cells grown either in control medium, or in zero-folate medium. We grew LNCaP/SSAT cells for 2 PD in either control (2 μM FA) or folate-free medium prior to removal of Tet, in order to begin depleting the intracellular folate stores, and then with or without Tet for another 2 PD. Tet removal efficiently up-regulated SSAT, as demonstrated by a significant (P<0.01) accumulation of Ac-Spd (Fig. 3A, −Tet samples). As expected, increased biosynthesis of Spd and Spm successfully maintained polyamine pools in the samples where SSAT was overexpressed (data not shown). We observed a significant (P<0.05) decrease in the accumulation of Ac-Spd on overexpression of SSAT in the no-folate cells compared to cells grown in control medium (Fig. 3A). We hypothesized that this was due to decreased availability of AdoMet pools in the no-folate sample.
Analysis of AdoMet pools in LNCaP/SSAT cells revealed that indeed SSAT overexpression, inducing high polyamine flux, significantly (P<0.05) decreased AdoMet pools (Fig. 3B). Similarly, removing folate from the medium also significantly (P<0.05) decreased the availability of AdoMet (Fig. 3B). AdoMet pools were reduced under conditions of high polyamine flux in the presence of no folate (Fig. 3B). Thus, withdrawing folate from the medium compromises the AdoMet pools, and this effect can be enhanced by increased polyamine biosynthesis.
We then studied the effects of the interaction between increased polyamine biosynthesis and decreased folate availability on cell growth. As previously reported, increased polyamine biosynthesis decreased cell growth, likely because of the metabolic strain consequent to significant up-regulation of polyamine biosynthesis necessary to maintain intracellular polyamine levels on SSAT overexpression (30) (Fig. 3C). As expected, also folate depletion significantly (P<0.05) reduced cell growth, approximately by 50% (Fig. 3C). Increasing polyamine biosynthesis in the absence of folate had an additive effect on cell growth, which was reduced to 35% of control cells.
Blocking polyamine biosynthesis decreases the cellular demand for folate in prostate cells
We blocked polyamine biosynthesis through pharmacological inhibition of AdoMetDC, the enzyme that converts AdoMet into dcAdoMet, which is the substrate for polyamine biosynthesis (Fig. 1). The AdoMetDC inhibitor MDL-73811 prevents the decarboxylation of AdoMet into dcAdoMet and strongly affects the intracellular polyamine pools, with a dramatic decrease of Spm and Spd concomitant with an increase of putrescine (34, 35). We prevented the cytostatic effect of polyamine depletion by adding exogenous Spd to the medium, as described previously (34, 35). Treatment of TRAMPC cell lines with MDL-73811 alone resulted in an 80% inhibition of cell growth, which was completely prevented by 10 μM Spd supplementation (data not shown). We treated the 2 prostate cell lines TRAMPC-2D and TRAMPC-2H and the 2 colon cell lines RKO-9 and HCT-116 with MDL-73811 plus 10 μM Spd in control and folate-restricted (100 nM FA, data not shown) medium to see whether AdoMet levels were affected by blocking polyamine biosynthesis. As predicted, in response to MDL-73811, with both control and low levels of folate, AdoMet levels significantly (P<0.05) increased compared to nontreated cells (Fig. 4A). Treatment with Spd alone did not have an effect on AdoMet pools, even when folate was limiting (data not shown). We therefore asked whether blocking polyamine biosynthesis could restore growth rates of cells in the presence of restrictive amounts of folate. Figure 4B shows that, in the presence of exogenous Spd, the sensitivity of the 2 prostate cell lines to folate depletion was significantly (P<0.0001; 2-way ANOVA) decreased when polyamine biosynthesis was blocked by MDL-73811. Specifically, AdoMetDC inhibition increased the growth rate of TRAMPC-2D from 27 to 63% at 75 nM FA and from 60 to 100% (the level observed with nonlimiting amounts of folate) at 100 nM (Fig. 4B, left panel). This protective effect on folate-dependent growth was stronger in the TRAMPC-2H cells, whose growth rate shifted from 28 to 45% at 100 nM folate and from 43 to 100% at 150 nM; a growth rate comparable to cells grown in control medium (Fig. 4B, right panel). Notably, treatment with the AdoMetDC inhibitor had no effect on the growth rate of TRAMPC-2D cells at 150 nM FA, a concentration at which folate was no longer limiting for this cell line, confirming that the effect of the AdoMetDC inhibitor on cell growth was restricted to those cells presented with limiting amounts of folate in the medium. Interestingly, folate dependent growth rates were not increased as a consequence of polyamine blockade in the colon cell lines (Fig. 4C).
Thus, we have demonstrated that the range of FA concentrations that are restrictive to growth differ between the prostate cell lines (50–150 nM) and the colon cancer cell lines (0–50 nM), and that this difference is tightly correlated with polyamine production. Blocking polyamine production increased AdoMet pools in all cell lines, but reduced cellular folate demand only in the prostate lines. Therefore, the data suggest that the high polyamine production in the prostate lines is responsible for shifting the window of FA concentration demand. This further suggests that in the colon cancer lines, where polyamine synthesis is relatively low, the growth retardation observed at 0–50 nM FA has more to do with nucleotide pool imbalance than AdoMet pool insufficiency.
Nucleotide and AdoMet pool imbalances induced by low folate
Next, we grew all the cell lines in our panel at 100 nM FA for 5 PD and asked whether the reduced growth rates we observed in cells characterized by higher polyamine production was associated with an increase in the dUTP:dTTP ratio and a decrease in the AdoMet:AdoHcy ratio. These are the effects observed in vitro and in vivo in colon and liver cells (but not other cell types) grown in the absence of folate and are known to lead to genetic and epigenetic damage, respectively. Because the effects of folate depletion might differ based on the phenotype of target cells (17, 45), we included in our panel of cell lines 3 syngenic cell lines characterized by different phenotypes derived from the TRAMP model of prostate cancer: TRAMPC-2D is immortalized but untransformed, TRAMPC-2G is transformed but nonmetastatic, and TRAMPC-2H is transformed and metastatic. All 3 cell lines were susceptible to either nucleotide or AdoMet pools imbalance (or both) in the presence of 100 nM FA at 5 PD.
All cell lines grown in 100 nM FA showed significant depletion of intracellular folate at PD 5 (data not shown). The analysis of AdoMet pools revealed that indeed cells with higher polyamine biosynthesis tended to have lower AdoMet:AdoHcy ratios (Fig. 5A). Also, we observed that cells characterized by high polyamine biosynthesis and the lowest growth rates in folate-restricted conditions suffered the most severe nucleotide imbalance, having reduced dTTP pools and increased dUTP pools (Fig. 5B). Indeed, we found a significant correlation between dUTP:dTTP ratio and folate-dependent growth rates (r=−0.87, P<0.01), as cell lines with the highest dUTP:dTTP ratios had the most dramatically reduced growth rate at FA= [100 nM] (Fig. 5C).
DISCUSSION
Folate is essential to the production of AdoMet and dTMP through 1-carbon metabolism and the methionine cycle (Fig. 1). Folate depletion negatively affects AdoMet and nucleotide pools, inducing epigenetic and genetic damage and transformation of cells in specific organs. AdoMet is not only the universal intracellular methyl donor, but is also required for polyamine biosynthesis. Because polyamine intracellular levels are strictly maintained, we hypothesized that the high level of polyamine biosynthesis characteristic of organs such as prostate might increase the cellular demand for folate and render cells more prone to suffer nucleotide and AdoMet pools imbalance in the presence of amounts of folate that might be well tolerated by cells with lower levels of polyamine biosynthesis. Several studies have focused on the interactions between folate availability and methionine cycle (13, 16), others studied the relationship between folate availability and cell growth (16, 46), and one study assessed the effects of dietary folate depletion on polyamine levels in the blood and liver of rats (18). Altogether, these studies suggested that maintaining polyamine pools might be a priority over maintaining AdoMet and nucleotide pools, despite the negative effects consequent to long-term AdoMet and nucleotide pools imbalance. Although the connection between folate depletion and AdoMet pools has been studied before in colon and liver, it was never suggested that rates of polyamine biosynthesis might play a key role in this connection.
Here we show that sensitivity to folate depletion of 7 cell lines significantly correlates with their levels of polyamine biosynthesis (Fig. 2), and we mechanistically demonstrate that high polyamine biosynthesis increases the cellular demand for folate necessary to sustain cell growth. Specifically, we found that both polyamine biosynthesis and folate depletion affect AdoMet pools, and that they do so additively (Fig. 3). Further, by interrupting the connection between the methionine cycle and polyamine biosynthesis using a specific inhibitor of AdoMetDC, we increased AdoMet availability and reduced sensitivity to limiting amounts of folate in prostate cells. This was not the case in colon cells (Fig. 4), where polyamine blockade increased AdoMet pools but failed to increase folate-dependent growth rates, even at very low folate levels that were growth inhibitory in the colon cells. This result showed that high polyamine biosynthesis adds a layer of sensitivity to folate depletion, increasing the level of folate necessary to maintain AdoMet and dTTP pools and cell growth rates (Fig. 4). Replenishing AdoMet pools by blocking AdoMetDC could eliminate this enhanced sensitivity in prostate cells, which subsequently displayed sensitivity to folate depletion similar to that observed in colon cells. Conversely, blocking AdoMetDC activity in colon cell lines had minimal effect on folate-dependent growth rates, likely because the growth inhibition observed in these cells at FA = [0–50 nM] was mainly dictated by impaired nucleotide pools, which could not be significantly buttressed by replenished AdoMet, since MTHFR activity is not reversible (Fig. 1). Growing all cell lines with limited availability of folate (100 nM) had no effect on those cell lines in our panel that were characterized by lower polyamine biosynthesis, but had increasingly negative effects on the nucleotide and AdoMet pools of cells with higher polyamine biosynthesis. Interestingly, methionine is abundantly available (100 mM) in the medium used for our experiments, suggesting that increased methionine uptake is a compensatory mechanism that is either unavailable to the cells, or insufficient to compensate for folate restriction.
Strikingly, we found that folate-dependent growth rates, which we showed to significantly (P<0.001) correlate with levels of polyamine biosynthesis, also showed a significant (P<0.01) inverse correlation with the dUTP:dTTP ratio, confirming that although the immediate biochemical link between polyamine biosynthesis and folate deficiency is through AdoMet pools, the reduction in the growth rates are likely also due to imbalanced nucleotide pools. In support of this finding, previous literature showed that the effect of folate depletion on cell growth seems to be mediated by a transcriptional modulation of genes involved in the cell cycle (46) including p53, which accumulates under conditions of nucleotide pool imbalance (47, 48) and DNA damage induced by folate depletion (7,8,9, 11, 12, 16), ultimately resulting in cell cycle arrest and apoptosis (8).
Our study is the first to attempt to systematically study the interplay between 1-carbon metabolism, methionine cycle, polyamine biosynthesis, and cell growth in response to folate depletion. The in vitro system enabled us to study different aspects of this complex metabolism at the same time, comparing results among different cell lines in strictly controlled experimental settings that allowed us to alter one component of the system at the time. Our panel comprised 7 cell lines, including colon cell lines, which were already known to be sensitive to folate depletion, and prostate cell lines, which we show to be exquisitely sensitive to folate depletion due to high polyamine biosynthesis. The majority of in vivo studies to date that contemplated the effects of folate depletion did so in the context of a low-methyl diet, which is characteristically low in folate, choline, and methionine (7, 9). A few studies demonstrated that at least in certain cell types, folate depletion by itself can be detrimental (11, 13). For example, it was previously shown that folate depletion affects the growth of immortalized, untransformed, human colon epithelial cells in vitro, and that growth inhibition is proportional to the concentration of FA in the medium at 0, 4, 9, 13, and 22 nM in one study (16) and at 25 and 50 nM in a second study (46). Consistent with our results, this latter study showed that 150 nM FA does not affect the growth of both transformed and untransformed colon cells in vitro (46). In this study, we show for the first time not only that prostate cells in vitro are sensitive to the negative effects of folate depletion, but that AdoMet and nucleotide pool imbalance can be induced in prostate cells in the presence of an amount of folate = [100 nM] that allows 2 colon cancer cell lines to grow with minimal effects on their AdoMet and nucleotide pools.
Interestingly, up-regulation of the enzymatic activities that control the polyamine pools has been known for many years to be associated with the process of transformation and, more specifically, with hyperproliferation (reviewed in ref. 3). In this regard, it is noteworthy that the prostate-specific membrane antigen (PSMA), which is highly expressed in human prostate (49, 50) and in the neovasculature of a range of tumors (51, 52), is in fact a folate hydrolase (53). It has been suggested that one of the roles of highly overexpressed PSMA in prostate tumors is to hydrolyze poly-γ-glutamated folates released by apoptotic cells, allowing internalization and recycling of folates (54). Such a mechanism may allow prostate cancer cells to maximize the intake of folate released by apoptotic/necrotic cells in order to sustain their characteristic high-polyamine biosynthesis. Still, prostate is a very slowly developing and growing cancer. It is intriguing to speculate that the slow growth characteristic of prostate cancer might be related to the extra strain put on the methionine cycle and 1-carbon metabolism by the high synthesis of polyamines. Indeed, a recent clinical trial designed to study the effects of folate supplementation on prevention of colon polyp formation resulted in a significant increase in the incidence of prostate cancer (55), suggesting that folate supplementation may have removed a limit on the growth rate of prostate carcinoma cells in men who already had indolent disease.
Epidemiological studies on the impact of dietary folate on the incidence of prostate cancer present us with contradictory results (55,56,57,58,59), suggesting that, similar to what has been postulated for colon (45), folate manipulation might act as a double-edged sword in the evolution of prostate cancer, with depletion inducing genetic and epigenetic damage, and supplementation sustaining cancer cell proliferation. These data, together with our study, suggest that folate intake might play a key role in prostate tumorigenesis in vivo. In vivo models of prostate carcinogenesis might help in clarifying the response of normal and tumor tissues to dietary folate manipulation.
Acknowledgments
This research was supported in part by an American Institute for Cancer Research Fellowship (G.B.); an American-Italian Cancer Foundation Fellowship (G.B.); National Cancer Institute (NCI) grants NCI R21 CA121216 (G.B. and D.J.S.), NCI CA022153 (C.W.P.), and NCI CA076428 (C.W.P.); and the NCI Cancer Center Support grant to the Roswell Park Cancer Institute (CA016056). We thank Dr. Sufrin (Roswell Park Cancer Institute, Buffalo, NY, USA), who kindly gave us purified dcAdoMet to use as an HPLC standard.
References
- Kim Y I. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr. 2005;135:2703–2709. doi: 10.1093/jn/135.11.2703. [DOI] [PubMed] [Google Scholar]
- Loenen W A. S-adenosylmethionine: jack of all trades and master of everything? Biochem Soc Trans. 2006;34:330–333. doi: 10.1042/BST20060330. [DOI] [PubMed] [Google Scholar]
- Gerner E W, Meyskens F L., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- Casero R A, Jr, Marton L J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6:373–390. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H G, Canellakis Z N. Polyamines in mammalian biology and medicine. Perspect Biol Med. 1979;22:421–453. doi: 10.1353/pbm.1979.0013. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun. 2000;271:559–564. doi: 10.1006/bbrc.2000.2601. [DOI] [PubMed] [Google Scholar]
- Pogribny I P, Basnakian A G, Miller B J, Lopatina N G, Poirier L A, James S J. Breaks in genomic DNA and within the p53 gene are associated with hypomethylation in livers of folate/methyl-deficient rats. Cancer Res. 1995;55:1894–1901. [PubMed] [Google Scholar]
- James S J, Miller B J, Basnakian A G, Pogribny I P, Pogribna M, Muskhelishvili L. Apoptosis and proliferation under conditions of deoxynucleotide pool imbalance in liver of folate/methyl deficient rats. Carcinogenesis. 1997;18:287–293. doi: 10.1093/carcin/18.2.287. [DOI] [PubMed] [Google Scholar]
- James S J, Pogribny I P, Pogribna M, Miller B J, Jernigan S, Melnyk S. Mechanisms of DNA damage, DNA hypomethylation, and tumor progression in the folate/methyl-deficient rat model of hepatocarcinogenesis. J Nutr. 2003;133:3740S–3747S. doi: 10.1093/jn/133.11.3740S. [DOI] [PubMed] [Google Scholar]
- Chandar N, Lombardi B. Liver cell proliferation and incidence of hepatocellular carcinomas in rats fed consecutively a choline-devoid and a choline-supplemented diet. Carcinogenesis. 1988;9:259–263. doi: 10.1093/carcin/9.2.259. [DOI] [PubMed] [Google Scholar]
- Knock E, Deng L, Wu Q, Leclerc D, Wang X L, Rozen R. Low dietary folate initiates intestinal tumors in mice, with altered expression of G2-M checkpoint regulators polo-like kinase 1 and cell division cycle 25c. Cancer Res. 2006;66:10349–10356. doi: 10.1158/0008-5472.CAN-06-2477. [DOI] [PubMed] [Google Scholar]
- Pogribny I P, Muskhelishvili L, Miller B J, James S J. Presence and consequence of uracil in preneoplastic DNA from folate/methyl-deficient rats. Carcinogenesis. 1997;18:2071–2076. doi: 10.1093/carcin/18.11.2071. [DOI] [PubMed] [Google Scholar]
- Kim Y I, Pogribny I P, Basnakian A G, Miller J W, Selhub J, James S J, Mason J B. Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. Am J Clin Nutr. 1997;65:46–52. doi: 10.1093/ajcn/65.1.46. [DOI] [PubMed] [Google Scholar]
- Sohn K J, Stempak J M, Reid S, Shirwadkar S, Mason J B, Kim Y I. The effect of dietary folate on genomic and p53-specific DNA methylation in rat colon. Carcinogenesis. 2003;24:81–90. doi: 10.1093/carcin/24.1.81. [DOI] [PubMed] [Google Scholar]
- Pogribny I P, James S J, Jernigan S, Pogribna M. Genomic hypomethylation is specific for preneoplastic liver in folate/methyl deficient rats and does not occur in non-target tissues. Mutat Res. 2004;548:53–59. doi: 10.1016/j.mrfmmm.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Duthie S J, Narayanan S, Blum S, Pirie L, Brand G M. Folate deficiency in vitro induces uracil misincorporation and DNA hypomethylation and inhibits DNA excision repair in immortalized normal human colon epithelial cells. Nutr Cancer. 2000;37:245–251. doi: 10.1207/S15327914NC372_18. [DOI] [PubMed] [Google Scholar]
- Stempak J M, Sohn K J, Chiang E P, Shane B, Kim Y I. Cell and stage of transformation-specific effects of folate deficiency on methionine cycle intermediates and DNA methylation in an in vitro model. Carcinogenesis. 2005;26:981–990. doi: 10.1093/carcin/bgi037. [DOI] [PubMed] [Google Scholar]
- Sun D, Wollin A, Stephen A M. Moderate folate deficiency influences polyamine synthesis in rats. J Nutr. 2002;132:2632–2637. doi: 10.1093/jn/132.9.2632. [DOI] [PubMed] [Google Scholar]
- Pegg A E, Lockwood D H, Williams-Ashman H G. Concentrations of putrescine and polyamines and their enzymic synthesis during androgen-induced prostatic growth. Biochem J. 1970;117:17–31. doi: 10.1042/bj1170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P J, Manzella J M, Stumpo D J, Wen L, Huang J K, Oyen O, Young W S., 3rd High level, cell-specific expression of ornithine decarboxylase transcripts in rat genitourinary tissues. Mol Endocrinol. 1989;3:68–78. doi: 10.1210/mend-3-1-68. [DOI] [PubMed] [Google Scholar]
- Janne O A, Crozat A, Palvimo J, Eisenberg L M. Androgen-regulation of ornithine decarboxylase and S-adenosylmethionine decarboxylase genes. J Steroid Biochem Mol Biol. 1991;40:307–315. doi: 10.1016/0960-0760(91)90196-c. [DOI] [PubMed] [Google Scholar]
- Fjosne H E, Strand H, Sunde A. Dose-dependent induction of ornithine decarboxylase and S-adenosyl-methionine decarboxylase activity by testosterone in the accessory sex organs of male rats. Prostate. 1992;21:239–245. doi: 10.1002/pros.2990210307. [DOI] [PubMed] [Google Scholar]
- Mohan R R, Challa A, Gupta S, Bostwick D G, Ahmad N, Agarwal R, Marengo S R, Amini S B, Paras F, MacLennan G T, Resnick M I, Mukhtar H. Overexpression of ornithine decarboxylase in prostate cancer and prostatic fluid in humans. Clin Cancer Res. 1999;5:143–147. [PubMed] [Google Scholar]
- Cyriac J, Haleem R, Cai X, Wang Z. Androgen regulation of spermidine synthase expression in the rat prostate. Prostate. 2002;50:252–261. doi: 10.1002/pros.10052. [DOI] [PubMed] [Google Scholar]
- Harrison G A. Spermine in human tissues. Biochem J. 1931;25:1885–1892. doi: 10.1042/bj0251885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y I, Salomon R N, Graeme-Cook F, Choi S W, Smith D E, Dallal G E, Mason J B. Dietary folate protects against the development of macroscopic colonic neoplasia in a dose responsive manner in rats. Gut. 1996;39:732–740. doi: 10.1136/gut.39.5.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun M J. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Gangjee A, Jain H D, Kurup S. Recent advances in classical and non-classical antifolates as antitumor and antiopportunistic infection agents: part I. Anticancer Agents Med Chem. 2007;7:524–542. doi: 10.2174/187152007781668724. [DOI] [PubMed] [Google Scholar]
- Foster B A, Gingrich J R, Kwon E D, Madias C, Greenberg N M. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- Kee K, Vujcic S, Merali S, Diegelman P, Kisiel N, Powell C T, Kramer D L, Porter C W. Metabolic and antiproliferative consequences of activated polyamine catabolism in LNCaP prostate carcinoma cells. J Biol Chem. 2004;279:27050–27058. doi: 10.1074/jbc.M403323200. [DOI] [PubMed] [Google Scholar]
- Fogel W A, Bieganski T, Ulatowska M. Oxidative deamination of 14C putrescine in mouse tissues in vivo. Arch Immunol Ther Exp (Wars) 1980;28:907–910. [PubMed] [Google Scholar]
- Danzin C, Marchal P, Casara P. Irreversible inhibition of rat S-adenosylmethionine decarboxylase by 5′-([(Z)-4-amino-2-butenyl]methylamino)-5′-deoxyadenosine. Biochem Pharmacol. 1990;40:1499–1503. doi: 10.1016/0006-2952(90)90446-r. [DOI] [PubMed] [Google Scholar]
- Kramer D L, Chang B D, Chen Y, Diegelman P, Alm K, Black A R, Roninson I B, Porter C W. Polyamine depletion in human melanoma cells leads to G1 arrest associated with induction of p21WAF1/CIP1/SDI1, changes in the expression of p21-regulated genes, and a senescence-like phenotype. Cancer Res. 2001;61:7754–7762. [PubMed] [Google Scholar]
- Heston W D. Prostatic polyamines and polyamine targeting as a new approach to therapy of prostatic cancer. Cancer Surv. 1991;11:217–238. [PubMed] [Google Scholar]
- Mi Z, Kramer D L, Miller J T, Bergeron R J, Bernacki R, Porter C W. Human prostatic carcinoma cell lines display altered regulation of polyamine transport in response to polyamine analogs and inhibitors. Prostate. 1998;34:51–60. doi: 10.1002/(sici)1097-0045(19980101)34:1<51::aid-pros7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Kabra P M, Lee H K, Lubich W P, Marton L J. Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J Chromatogr. 1986;380:19–32. doi: 10.1016/s0378-4347(00)83621-x. [DOI] [PubMed] [Google Scholar]
- Yarlett N, Bacchi C J. Effect of DL-alpha-difluoromethylornithine on methionine cycle intermediates in Trypanosoma brucei brucei. Mol Biochem Parasitol. 1988;27:1–10. doi: 10.1016/0166-6851(88)90019-9. [DOI] [PubMed] [Google Scholar]
- Cross D R, Miller B J, James S J. A simplified HPLC method for simultaneously quantifying ribonucleotides and deoxyribonucleotides in cell extracts or frozen tissues. Cell Prolif. 1993;26:327–336. doi: 10.1111/j.1365-2184.1993.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Kramer D, Stanek J, Diegelman P, Regenass U, Schneider P, Porter C W. Use of 4-fluoro-L-ornithine to monitor metabolic flux through the polyamine biosynthetic pathway. Biochem Pharmacol. 1995;50:1433–1443. doi: 10.1016/0006-2952(95)02037-3. [DOI] [PubMed] [Google Scholar]
- Kramer D, Mett H, Evans A, Regenass U, Diegelman P, Porter C W. Stable amplification of the S-adenosylmethionine decarboxylase gene in Chinese hamster ovary cells. J Biol Chem. 1995;270:2124–2132. doi: 10.1074/jbc.270.5.2124. [DOI] [PubMed] [Google Scholar]
- Chen Y, Kramer D L, Diegelman P, Vujcic S, Porter C W. Apoptotic signaling in polyamine analogue-treated SK-MEL-28 human melanoma cells. Cancer Res. 2001;61:6437–6444. [PubMed] [Google Scholar]
- Horne D W, Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin Chem. 1988;34:2357–2359. [PubMed] [Google Scholar]
- Kramer D L, Diegelman P, Jell J, Vujcic S, Merali S, Porter C W. Polyamine acetylation modulates polyamine metabolic flux, a prelude to broader metabolic consequences. J Biol Chem. 2008;283:4241–4251. doi: 10.1074/jbc.M706806200. [DOI] [PubMed] [Google Scholar]
- Kee K, Foster B A, Merali S, Kramer D L, Hensen M L, Diegelman P, Kisiel N, Vujcic S, Mazurchuk R V, Porter C W. Activated polyamine catabolism depletes acetyl-CoA pools and suppresses prostate tumor growth in TRAMP mice. J Biol Chem. 2004;279:40076–40083. doi: 10.1074/jbc.M406002200. [DOI] [PubMed] [Google Scholar]
- Kim Y I. Folate: a magic bullet or a double edged sword for colorectal cancer prevention? Gut. 2006;55:1387–1389. doi: 10.1136/gut.2006.095463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crott J W, Liu Z, Keyes M K, Choi S W, Jang H, Moyer M P, Mason J B. Moderate folate depletion modulates the expression of selected genes involved in cell cycle, intracellular signaling and folate uptake in human colonic epithelial cell lines. J Nutr Biochem. 2007;19:328–335. doi: 10.1016/j.jnutbio.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke S P, Clarkin K C, Di Leonardo A, Tsou A, Wahl G M. A reversible, p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev. 1996;10:934–947. doi: 10.1101/gad.10.8.934. [DOI] [PubMed] [Google Scholar]
- Bronder J L, Moran R G. A defect in the p53 response pathway induced by de novo purine synthesis inhibition. J Biol Chem. 2003;278:48861–48871. doi: 10.1074/jbc.M304844200. [DOI] [PubMed] [Google Scholar]
- Israeli R S, Powell C T, Fair W R, Heston W D. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993;53:227–230. [PubMed] [Google Scholar]
- Fair W R, Israeli R S, Heston W D. Prostate-specific membrane antigen. Prostate. 1997;32:140–148. doi: 10.1002/(sici)1097-0045(19970701)32:2<140::aid-pros9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V, Knudsen B, Bander N H. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997;57:3629–3634. [PubMed] [Google Scholar]
- Silver D A, Pellicer I, Fair W R, Heston W D, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- Pinto J T, Suffoletto B P, Berzin T M, Qiao C H, Lin S, Tong W P, May F, Mukherjee B, Heston W D. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res. 1996;2:1445–1451. [PubMed] [Google Scholar]
- Ghosh A, Heston W D. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer J. Cell Biochem. 2004;91:528–539. doi: 10.1002/jcb.10661. [DOI] [PubMed] [Google Scholar]
- Cole B F, Baron J A, Sandler R S, Haile R W, Ahnen D J, Bresalier R S, McKeown-Eyssen G, Summers R W, Rothstein R I, Burke C A, Snover D C, Church T R, Allen J I, Robertson D J, Beck G J, Bond J H, Byers T, Mandel J S, Mott L A, Pearson L H, Barry E L, Rees J R, Marcon N, Saibil F, Ueland P M, Greenberg E R. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- Rossi E, Hung J, Beilby J P, Knuiman M W, Divitini M L, Bartholomew H. Folate levels and cancer morbidity and mortality: prospective cohort study from Busselton, Western Australia. Ann Epidemiol. 2006;16:206–212. doi: 10.1016/j.annepidem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pelucchi C, Galeone C, Talamini R, Negri E, Parpinel M, Franceschi S, Montella M, La Vecchia C. Dietary folate and risk of prostate cancer in Italy. Cancer Epidemiol Biomarkers Prev. 2005;14:944–948. doi: 10.1158/1055-9965.EPI-04-0787. [DOI] [PubMed] [Google Scholar]
- Stevens V L, Rodriguez C, Pavluck A L, McCullough M L, Thun M J, Calle E E. Folate nutrition and prostate cancer incidence in a large cohort of US men. Am J Epidemiol. 2006;163:989–996. doi: 10.1093/aje/kwj126. [DOI] [PubMed] [Google Scholar]
- Hultdin J, Van Guelpen B, Bergh A, Hallmans G, Stattin P. Plasma folate, vitamin B12, and homocysteine and prostate cancer risk: a prospective study. Int J Cancer. 2005;113:819–824. doi: 10.1002/ijc.20646. [DOI] [PubMed] [Google Scholar]