Abstract
Hookworms digest hemoglobin from erythrocytes via a proteolytic cascade that begins with the aspartic protease, APR-1. Ac-APR-1 from the dog hookworm, Ancylostoma caninum, protects dogs against hookworm infection via antibodies that neutralize enzymatic activity and interrupt blood-feeding. Toward developing a human hookworm vaccine, we expressed both wild-type (Na-APR-1wt) and mutant (Na-APR-1mut—mutagenesis of the catalytic aspartic acids) forms of Na-APR-1 from the human hookworm, Necator americanus. Refolded Na-APR-1wt was catalytically active, and Na-APR-1mut was catalytically inactive but still bound substrates. Vaccination of canines with Na-APR-1mut and heterologous challenge with A. caninum resulted in significantly reduced parasite egg burdens (P=0.034) and weight loss (P=0.022). Vaccinated dogs also had less gut pathology, fewer adult worms, and reduced blood loss compared to controls but these did not reach statistical significance. Vaccination with Na-APR-1mut induced antibodies that bound the native enzyme in the parasite gut and neutralized enzymatic activity of Na-APR-1wt and APR-1 orthologues from three other hookworm species that infect humans. IgG1 against Na-APR-1mut was the most prominently detected antibody in sera from people resident in high-transmission areas for N. americanus, indicating that natural boosting may occur in exposed humans. Na-APR-1mut is now a lead antigen for the development of an antihematophagy vaccine for human hookworm disease.—Pearson, M. S., Bethony, J. M., Pickering, D. A., de Oliveira, L. M., Jariwala, A., Santiago, H., Miles, A. P., Zhan, B., Jiang, D., Ranjit, N., Mulvenna, J., Tribolet, L., Plieskatt, J., Smith, T., Bottazzi, M. E., Jones, K., Keegan, B., Hotez, P. J., Loukas, A. An enzymatically inactivated hemoglobinase from Necator americanus induces neutralizing antibodies against multiple hookworm species and protects dogs against heterologous hookworm infection.
Keywords: vaccine, protease, hemoglobin, neglected tropical disease
The hookworms Necator americanus and Ancylostoma duodenale collectively infect 576–740 million people in impoverished nations (1) and cause one of the most debilitating neglected tropical diseases (2). These hematophagous parasites attach to the small intestine, where they feed on blood, and are a major cause of iron-deficiency anemia in people in developing countries (3, 4).
Hookworm infection can be treated effectively with chemotherapy, although reinfection rates are high and occur rapidly after treatment (5). Given the number of individuals infected, the widespread global distribution of infection, especially in areas of poverty, high rates of drug failures with mebendazole, and the emergence of anthelmintic resistance (6), periodic mass drug administration may not be a sustainable, long-term strategy for hookworm control (4, 7). These concerns have driven the development of a human hookworm vaccine (3).
Hookworms attach to the host intestinal mucosa and ingest the blood from ruptured capillaries. Red blood cells within the parasite gut are lysed by pore-forming proteins (8), and the liberated hemoglobin (Hb) is digested by a semiordered cascade of mechanistically distinct proteases, ultimately reducing the Hb to small peptides that can be absorbed across the gut lumen (9, 10). These gut enzymes, or hemoglobinases, have been the focus of vaccine development in recent years given the essential roles they play in the acquisition of life-sustaining nutrients (4). Indeed, numerous hookworm intestinal proteases have now been expressed in recombinant form and their vaccine efficacies have been tested in animal models of hookworm disease, resulting in significant reductions in the intensity of infection (11,12,13,14), and most importantly, protection against blood loss (12). Extracts enriched for hemoglobinases from the nematodes of livestock, Hemonchus contortus (15) and Ostertagia ostertagi (16), and recombinant cysteine hemoglobinases from H. contortus (17) and the liver fluke Fasciola hepatica (18), all confer varying levels of protection as vaccines.
The cathepsin-D-like aspartic proteases Na-APR-1 and Ac-APR-1 from N. americanus and the canine hookworm Ancylostoma caninum, respectively, are responsible for initiating the Hb digestion cascade (19). Moreover, further proteolysis of Hb by other proteases cannot occur until APR-1 has initiated the cleavage process (9). Silencing of mRNA expression for the APR-1 orthologue from the human blood fluke, Schistosoma mansoni, results in a lethal developmental phenotype when parasites are injected into mice after treatment with double-stranded RNA in vitro (20).
We previously showed a protective role for anti-APR-1 antibodies using the canine model of human hookworm disease (12). Dogs were immunized with recombinant Ac-APR-1 expressed in yeast, then challenged experimentally with A. caninum. Of the adult hookworm antigens tested to date (4), Ac-APR-1 has yielded the best and most consistent protection, giving the highest reductions in adult worm and fecal egg burdens after hookworm challenge. Most important, Hb levels of dogs vaccinated with Ac-APR-1 were significantly elevated compared with control dogs, probably due to the generation of IgG antibodies that bound to the worm’s intestine and neutralized the catalytic activity of the native enzyme, and consequently impaired blood-feeding by the parasite (12).
Like many proteases, catalytically active APR-1 is unstable and autodegrades rapidly. Moreover, immunization with an active protease is problematic for safety reasons. In light of the strong evidence supporting APR-1 as a vaccine candidate, we decided to assess the vaccine efficacy of Na-APR-1 from N. americanus as a human hookworm vaccine. Both Ac-APR-1 (12) and Na-APR-1 (not shown) can be produced in yeast, but the yields of secreted protein are relatively low, so we focused here on production of high-yield expression in a low-cost bacterial expression system. Both a catalytically active wild-type (Na-APR-1wt) and a catalytically inactive mutant (Na-APR-1mut) protein were expressed in order to compare their abilities to induce neutralizing antibodies against the native enzyme. Here, we show the expression, refolding, and biochemical characterization of Na-APR-1wt and Na-APR-1mut in Escherichia coli, and the ability of Na-APR-1mut formulated with Alhydrogel and a CpG oligodeoxynucleotide to protect dogs in a heterologous challenge infection with A. caninum compared with adjuvant alone. Antibodies to Na-APR-1mut neutralized the catalytic activity of APR-1 species from three additional hookworms that infect people, and infected individuals from Brazil mounted an IgG but not an IgE response to APR-1, implying that a vaccine based on Na-APR-1mut would utilize an already existing antibody response and likely rely on this response for continued boosting, extending the life of vaccine protection, which has been a major concern for parasite gut antigens.
MATERIALS AND METHODS
Cloning of cDNAs and molecular modeling
Full-length Na-apr-1wt was synthesized in codon-optimized form for expression in E. coli by GeneArt AG, using the Na-apr-1 cDNA sequence in GenBank (accession no. AJ245459). The mature form, excluding the proregion and stop codon—Ser-62–Phe-430 (numbering of amino acid residues is relative to the start of the proenzyme)—of the Na-APR-1wt ORF, was amplified by PCR using a proofreading DNA polymerase (Pfu Turbo; Stratagene, La Jolla, CA, USA), cloned into the NdeI and XhoI sites of the pET41a vector (thereby removing the GST fusion tag but retaining the 6×His tag and allowing for native N-terminal protein expression—the resulting plasmid after removal of the nucleotide encoding GST was denoted pET41aΔGST) and chemically transformed into E. coli TOP10 cells (Invitrogen, Carlsbad, CA, USA). Recombinant plasmid was then extracted and chemically transformed into E. coli BL21(DE3) cells (Invitrogen). Both of the active site residues of the ORF of Na-apr-1wt/pET41aΔGST (Asp-97 and Asp-284) were mutated to Ala by site-directed mutagenesis, using previously described methods (21). Nucleotide mutations were confirmed by sequencing and plasmid encoding the mutated form of Na-apr-1wt (Na-apr-1mut) was chemically transformed into E. coli BL21(DE3) cells. The proform, excluding the stop codon (Ser-1–Leu-430), of the ORF of Ay-APR-1 (GenBank accession no. AY181028) from Ancylostoma ceylanicum and the corresponding region (Ser-1–Leu-433) of Ac-APR-1 (GenBank accession no. U34888) from A. caninum were available in GenBank and were amplified from cDNA libraries in our laboratories for each respective parasite. The Ad-apr-1 cDNA sequence from A. duodenale had not been previously identified, so we amplified it from a cDNA library derived from mRNA from A. duodenale infective larvae using PCR and oligonucleotide primers complimentary to the 5′ and 3′ untranslated regions of Ay-apr-1. The amplicon generated was cloned into a T-ended vector (pGEM-T; Promega, Madison, WI, USA) and sequenced and used as template to reamplify the predicted proform (Ser-1–Leu-430) for protein expression. The cDNA sequence of Ad-apr-1 was deposited in GenBank under accession number FJ172357. cDNA sequences corresponding to the proenzymes of all three proteases (Ad-apr-1, Ac-apr-1 and Ay-apr-1) were then cloned into pET41aΔGST and transformed into E. coli BL21(DE3) cells, as described above for Na-apr-1wt. The 3-dimensional structures of mature Na-APR-1wt and Na-APR-1mut were predicted based on the structure of human pepsin [Protein Data Bank (PDB) code 1PSO] in complex with pepstatin using the program Modeler (22). Pepstatin was included in the model using block residues for IVA and STA corresponding to the CHARMM type undf. Fifty models were calculated, and the model with the lowest objective function score was selected. Alignments for the model were generated using the Modeler align function. Because of the high level of sequence identity (45% over 326 residues), no additional loop refinement was performed.
Expression of aspartic proteases
For each aspartic protease, an overnight culture (10 ml) was grown from inoculation of a recombinant E. coli BL21(DE3) colony in Luria-Bertani (LB) medium containing 50 μg/ml kanamycin (LBkan) with shaking (225 rpm) overnight at 37°C. A 1-L expression culture was prepared by adding the overnight culture to 1 L of LBkan and shaking at 225 rpm for 24 h at 37°C. Isopropyl-β-d-thiogalactoside (IPTG; final concentration of 1.0 mM) was added after the first 3 h to induce recombinant protein expression. Bacteria were pelleted by centrifugation at 5000 g for 20 min at 4°C and resuspended in 30 ml of 0.1 M Tris (pH 8.0) and 0.5 M NaCl (resuspension buffer). Resuspended E. coli were then disrupted by 3 passes through a prechilled French pressure cell (KIN020; Sim Aminco, Urbana, IL, USA) at 16,000–18,000 psi. The homogenate was sonicated at 40% duty cycle for 30 s at 4°C. Triton X-100 was added to a final concentration of 3% and incubated for 1 h at 4°C with gentle shaking. The homogenate was then centrifuged at 20,000 g for 20 min at 4°C. Supernatant was discarded, and inclusion bodies were washed twice with 30 ml of resuspension buffer. Inclusion bodies were pelleted by centrifugation between and after washes, as described above, and resuspended in 20 ml of solubilization buffer (0.1 M Tris, pH 8.0; 0.5 M NaCl; 6 M urea; and 40 mM imidazole). Dithiothreitol (DTT) was added to the inclusion bodies at a final concentration of 100 mM and incubated at 4°C overnight with shaking. Inclusion bodies were then centrifuged at 20,000 g for 20 min at 4°C. Supernatant was decanted and dialyzed in a dialysis bag (Pierce, Rockford, IL, USA) with a cutoff size of 10 kDa against 3 changes of solubilization buffer (200 ml each) at 4°C. The first two dialyses were for 3 h, and the final dialysis was overnight.
Purification of denatured aspartic proteases
Each denatured aspartic protease was diluted 1:4 in additional solubilization buffer to a final volume of 80 ml and filtered through a 0.45-μm filter by vacuum. A prepacked 5.0-ml Hi-Trap IMAC-FF column (GE Healthcare, New York, NY) was charged with 0.1 M nickel sulfate and equilibrated with 10 column volumes of solubilization buffer. The solution containing the filtered inclusion bodies (80 ml) was passed through the column at a flow rate of 1.0 ml/min using an AKTA Prime UPC FPLC (GE Healthcare). Bound proteins were washed with 20 column volumes of wash buffer (0.1 M Tris, pH 8.0; 0.5 M NaCl; 60 mM imidazole; and 6 M urea) and eluted in 5 × 5.0-ml fractions in elution buffer (0.1 M Tris, pH 8.0; 0.5 M NaCl; 250 mM imidazole; and 6 M urea). Fractions containing recombinant protein (as determined by SDS-PAGE) were pooled, and the final concentration was adjusted to 1.0 mg/ml using Amicon Ultra-15 centrifugal concentration devices (Millipore, Beverley, MA, USA).
Refolding of purified Na-APR-1
Aliquots of purified, denatured Na-APR-1wt (1.0 mg/ml) were refolded at a concentration of 10 μg/ml in 0.1 M Tris (pH 8.0) with different concentrations (0–1.0 M) of l-arginine with and without 5.0 mM/0.5 mM reduced and oxidized glutathione (GSH/GSSG) for 16 h at 4°C with gentle stirring. After removal of urea, protein solutions were dialyzed against two changes of PBS (pH 7.4) at 4°C using 10-kDa cutoff dialysis tubing. The first dialysis was for 3 h, and the final dialysis was overnight. Refolded proteins were concentrated to volumes approximating the starting volume of denatured Na-APR-1wt using Amicon Ultra-15 centrifugal concentration devices, followed by centrifugation at 16,000 g for 10 min at 4°C to remove any precipitate. The yield of refolded protein was determined by comparative gel densitometry with bovine serum albumin (BSA) standards, and catalytic activity was assessed by cleavage of a fluorogenic peptide substrate (see below). The optimal refolding buffer (0.1 M Tris, pH 8.0, and 0.5 M l-arginine) was selected from a range of refolding conditions and buffers tested (not shown), and was determined by identifying the conditions that resulted in the greatest yield of catalytically active enzyme after refolding (see below). All subsequent experiments using Na-APR-1wt utilized material that was refolded in this manner. Refolding of Na-APR-1mut was explored using the same range of conditions that were utilized for Na-APR-1wt. The optimal refolding buffer (0.1 M Tris, pH 8.0, and 0.5 M l-arginine) was selected by identifying the conditions that resulted in the greatest yield of refolded protein, and this was used to refold Na-APR-1mut for use in all subsequent experiments. Immunological detection of Na-APR-1wt and Na-APR-1mut was conducted by Western blot analysis with anti-6×His (C-term) antibody (Invitrogen), using standard methods.
Refolding of purified Ad-APR-1, Ac-APR-1, and Ay-APR-1 proenzymes
Aliquots of purified, denatured proforms of (1.0 mg/ml) were refolded at a concentration of 10 μg/ml in 50 mM sodium acetate (NaAc) (pH 3.5) for 8 h at 4°C with gentle stirring. After refolding, protein solutions were dialyzed against two changes (2 h each) of 50 mM NaAc at 4°C using 10-kDa cutoff dialysis tubing. Refolded proteins were concentrated and assayed for protein concentration and catalytic activity as described for Na-APR-1wt.
Determination of pH optima and catalytic activity
To assess the pH at which Na-APR-1wt exhibited optimum activity, 1.0 μg of enzyme was added to 50 mM sodium acetate buffers of differing pH, increasing in half-unit increments from pH 2.0 to 6.0 in the presence of 7-methoxycoumarin-4-acetyl-GKPILFFRLK(DNP)-d-Arg-amide (MoCAc-GKPILFFRLK) (Sigma, St. Louis, MO, USA). MoCAc-GKPILFFRLK (1.0 mM in DMSO) was added to the reaction in a final concentration of 1.0 μM. The final volume of each reaction was 100 μl, and the assay was performed at 37°C. Every 10 min, the fluorescence generated (relative to substrate in buffer alone) by substrate hydrolysis was measured in relative fluorescence units (RFU) at excitation and emission wavelengths of 330 and 390 nm, respectively, in a Fluostar Optima microplate reader (BMG Labtech, Offenburg, Germany). The catalytic activity of Ad-APR-1, Ay-APR-1, and Ac-APR-1 was similarly determined, except that the buffer was kept constant at pH 3.5. Control reactions for each protein were similarly performed with the addition of the aspartic protease inhibitor, pepstatin A (Sigma), at a final concentration of 1.0 μM. Kinetic parameters were determined for each refolded enzyme by initial rates of hydrolysis of MoCAc-GKPILFFRLK, using a range of substrate concentrations (0.8–100 μM) and a fixed concentration (2.0 nM) of enzyme in 50 mM NaAc (pH 3.5) at 37°C. The experiment was repeated 3 times, and data presented are averages of 3 similar replicates. The ability of Na-APR-1wt (but not Na-APR-1mut) to cleave Hb was determined by incubation of 1.0 μg of each recombinant protein with 1.0 μg of human Hb in 50 mM NaAc (pH 3.5) at 37°C in a final reaction volume of 100 μl. Hydrolysates were collected at hourly intervals from 1 to 6 h and after 16 h, and analyzed by SDS-PAGE for evidence of Hb hydrolysis. Control reactions without enzyme and with the addition of 1.0 μM pepstatin A were also performed and analyzed after 16 h.
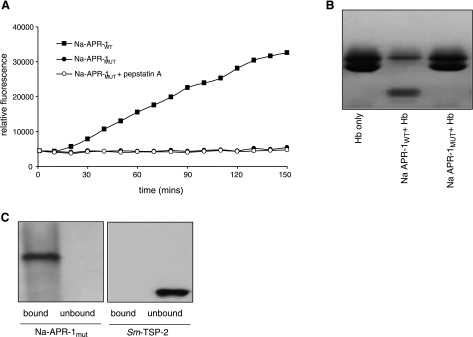
Binding of Na-APR-1mut to pepstatin A
Five micrograms of Na-APR-1mut in 500 μl of 50 mM NaAc (pH 3.5) was added to pepstatin A agarose (100 μl, Sigma) and rotated overnight at 4°C. The resin was pelleted by centrifugation at 5000 g for 5 min at room temperature, and the resultant supernatant (unbound fraction) was kept for analysis. Bound protein was eluted with 0.1 M sodium phosphate (pH 8.7) and 0.6 M NaCl, and both bound and unbound fractions were analyzed by Western blotting with an anti-6×His antibody. An equal amount of a nonproteolytic recombinant protein with a 6×His tag, Sm-TSP-2, was used as a negative control.
Generation of antibodies
Three BALB/c mice were subcutaneously immunized with an emulsion containing 25 μg of Na-APR-1mut (1.0 mg/ml) or Na-APR-1wt and an equal volume of Freund’s complete adjuvant (FCA). Two subsequent immunizations were administered 2 and 4 wk later, using Freund’s incomplete adjuvant (FIA) instead of FCA. Mice were bled 2 wk after the final immunization, and sera were collected by centrifugation. Polyclonal IgG was purified from pooled serum by binding to protein A Sepharose CL-4B (GE Healthcare). Bound antibodies were eluted with 0.1 M glycine (pH 3.0) and then equilibrated to neutral pH by adding 0.1 vol of 1.0 M Tris-HCl, pH 8.0. Preimmunization IgG was similarly purified from a pooled serum sample taken from each mouse prior to immunization.
Recognition of parasite-derived Na-APR-1 by anti-Na-APR-1mut IgG
N. americanus adult worms were recovered from hamsters, fixed, sectioned, and probed with anti-Na-APR-1mut IgG diluted 1:500 in PBS/0.1% Tween-20 (PBST), as described elsewhere (23). Secondary antibody was goat anti-mouse IgG-Alexa Fluor 568 (Invitrogen), diluted 1:500 in PBST. After washing twice with PBST, sections were viewed with a fluorescence microscope (Axioskop2; Zeiss, Jena, Germany).
Inhibition of proteolytic activity with anti-Na-APR-1mut IgG
Equal amounts (0.2 μg) of Na-APR-1wt, Ac-APR-1, Ad-APR-1, or Ay-APR-1 were incubated with anti-Na-APR-1mut IgG (1.0–5.0 μg) or anti-Na-APR-1wt in 50 mM NaAc, pH 5.5. MoCAc-GKPILFFRLK was added to a final concentration of 1.0 μM (200 μl final reaction volume), and the relative fluorescence generated for each reaction was measured as described earlier. Negative control reactions were similarly performed using control mouse IgG.
Canine vaccine trial
Male beagles were purchased from Ridglan Farms, Inc. (Mount Horeb, WI., USA). They were treated with fenbendazole prior to shipping and determined to be free of fecal helminth eggs by microscopy prior to the trial. Puppies were maintained at the George Washington University animal research facility for the duration of the study. Dogs were randomly assigned into groups of 9 each, which were identified only by group number, and all researchers but the study director were masked as to vaccine or control status until after necropsy and worm counts were conducted. All dogs were 62 ± 8 d old at the time of the first vaccination. All vaccine formulations appeared visually identical; test or control formulation was randomly assigned to a group by the study director. The dogs in the test group were each intramuscularly immunized with 0.55 ml of a formulation containing 100 μg of Na-APR-1mut and 0.5 mg of CpG 10103 (Coley Pharmaceutical Group, Ottawa, ON, Canada) adsorbed to Alhydrogel (1.6 mg/ml) in 50 mM NaAc (pH 6.0). Na-APR-1mut was completely adsorbed to the Alhydrogel prior to addition of CpG (not shown). The majority of the CpG was adsorbed, and a negligible amount of antigen was desorbed (not shown). Nine control dogs were similarly immunized with the adjuvant combination alone (no protein). Two identical immunizations were administered 21 and 42 d later. Blood was drawn just prior to each immunization, prior to infection, then once weekly until the end of the study (d 0, 21, 42, 56, 63, 70, 77, and 84). To determine Hb concentrations of each dog, 0.5–1.0-ml blood samples were collected into EDTA and analyzed using a Cell Dyn 3700 system (Abbott Laboratories, Abbott Park, IL, USA). Fourteen days after the final immunization, each dog was sedated by intramuscular injection with 3 mg acepromazine, and 500 individually counted A. caninum third-stage (L3) larvae suspended in PBS were loaded into the cap of an Eppendorf tube. The cap was applied directly to a shaved area of skin on the dorsal side at the nape of the neck, and held in place with a bandage layered with Parafilm (to prevent leakage into the bandage), medical tape, and vetwrap. An Elizabethan collar was placed around each dog’s neck to minimize tampering with the bandage. Dogs were then returned to their individual cages, and the bandages were removed after 1 h. Other clinical measurements, parasite recovery, and statistical analyses were performed as described previously (12), with some modifications. Two control dogs were removed from the study by the attending veterinarian for health concerns. The first was removed prior to challenge, and the second was euthanized 2 wk after challenge due to seizure activity. Both health concerns were deemed unrelated to the study by the attending veterinarian. After necropsy, each intestine was searched for worms by two separate operators, and counts from one operator were verified by another. After euthanasia, all animals were examined for gross pathology associated with hookworm infection.
Statistical analyses
The program GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA) was used to create graphs and perform statistical analyses. The small sample size did not provide sufficient data to differentiate between gaussian and nongaussian distribution, so a nonparametric test (Mann–Whitney U test) was used to determine statistical differences between medians of two groups. Student’s t test was used to compare the means of the groups for normally distributed data. Values of P (α) < 0.05 were considered statistically significant.
Human investigations
Patient sample and parasitological methods
A cross-sectional parasitological and serologic survey was conducted in a hookworm-endemic region of Minas Gerais, Brazil. A description of the testing site was reported previously (24,25,26,27). Ethics committee approval was obtained from the National Ethics Committee of Brazil, FIOCRUZ (protocol 003/2000), and the institutional review board (protocol 090303) of George Washington University. Written consent was obtained from all participants. Houses were assigned a unique household identification number, and each resident was given a unique personal identity number. Fecal samples and a blood sample were requested of all residents aged ≥6 yr. The presence of soil-transmitted helminth eggs was determined by ether sedimentation followed by Kato-Katz thick-smear technique. Blood was obtained by venipuncture. For persons whose fecal sample tested positive, three subsequent fecal samples were obtained over the course of 3 d. Two slides from each day’s fecal sample were prepared ≤24 h after receipt by use of the Kato-Katz thick-smear technique, and quantitative egg counts were obtained and expressed in eggs per gram of feces (EPG). Hookworm species (A. duodenale or N. americanus) was determined by morphological identification of L3 larvae reared from eggs by coproculture. After collection of fecal samples for egg counts and blood for serum, all infected subjects were treated with albendazole. Individuals who refused to participate in the study were offered a fecal examination and treatment for any diagnosed helminth infection.
Human indirect ELISA
Serum samples (10 ml) were obtained from whole blood collected into siliconized tubes. An indirect ELISA was then used to study isotype responses of participants to Na-APR-1mut. Maxisorb Surface 96-well plates (Nunc, Roskilde, Denmark) were coated with 5 μg/ml (100 μl/well) of antigen in 20 mM sodium bicarbonate/27 mM sodium carbonate (pH 9.6) and stored overnight at 4°C. For IgG2 assays, 96-well plates were adsorbed overnight at room temperature with 100 μl/well of poly-l-lysine (PLL) at 1 μg/ml in 50 mM sodium carbonate (pH 9.0). Plates were then washed with PBS, and Na-APR-1mut was added and incubated in the manner described above. Plates were washed with PBST and blocked with 1% fetal calf serum. Serum samples were diluted 1:100 in PBST, and 100 μl was added to each well in duplicate. The following dilutions of horseradish peroxidase-conjugated anti-human antibodies (Zymed Laboratories, Burlingame, CA, USA) were added to each well: 1:5000 of IgG1; 1:1000 of IgG2, IgG3, and IgG4. Plates were washed and developed for 30 min with o-phenylenediamine (Sigma) containing 0.03% hydrogen peroxide. For the IgE assay, other isotypes from serum were not preadsorbed, because they did not affect the detection of IgE in our ELISA (data not shown). Plates were prepared as above, with the exception of the serum concentration (1:25), the second antibody (1:500; murine monoclonal anti-human IgE Fc biotin, clone HP 6001B; Human Reagent Laboratory, Baltimore, MD) and incubation of the secondary antibody (3 h at 4°C). Plates were developed as above. Assays were standardized as described elsewhere (28, 29). A reactivity threshold was used to categorize a serum as to the presence or absence of human antibodies. The reactivity threshold of this assay was determined from the frequency distribution of log10 transformed OD values from a panel of sera from humans from a nonendemic area in Brazil (25 observations). The reactivity threshold was determined as the upper 95% confidence interval of the frequency distribution of the log10 transformed OD values of control sera tested at the dilutions indicated above.
RESULTS
Na-APR-1wt is expressed in inclusion bodies in E. coli
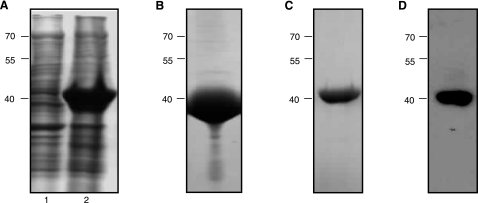
Recombinant Na-APR-1wt was expressed in E. coli as insoluble inclusion bodies at a concentration of ∼50 mg/L (Fig. 1A). After isolation of the inclusion bodies, denaturation and purification of denatured Na-APR-1wt by Ni2+ affinity chromatography, the protein yield was ∼40 mg/L (Fig. 1B). The final protein yield after optimal refolding (see below) was ∼15 mg/L (Fig. 1C). Western blot analysis of Na-APR-1wt with an anti-6×His (C-term) confirmed the identity of the refolded protein (Fig. 1D).
Figure 1.
Expression and refolding of recombinant Na-APR-1wt. A) SDS-PAGE gel stained with Coomassie Brilliant Blue showing insoluble protein profiles from E. coli BL21(DE3) cells before (lane 1) and 20 h after (lane 2) induction with 1.0 mM IPTG. B–D) Urea-solubilized inclusion bodies containing Na-APR-1wt were subsequently purified by immobilized metal ion affinity chromatography, then refolded and assessed by SDS-PAGE (C) and Western blotting (D) using anti-6×His(C-term)-HRP monoclonal antibody. Molecular mass (kDa) markers are shown at left.
Refolded Na-APR-1wt is catalytically active
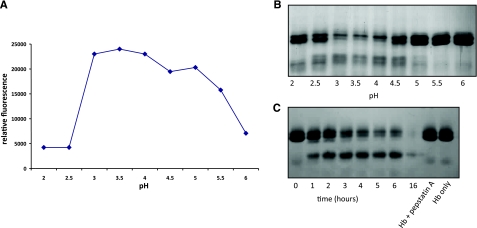
Refolded Na-APR-1wt had optimal activity against both the human cathepsin D peptide substrate MoCAc-GKPILFFRLK and Hb at pH 3.5 (Fig. 2A, B, respectively). Numerous refolding conditions were tested for Na-APR-1wt, resulting in differing rates of peptide hydrolysis (data not shown). Kinetic parameters (as determined by MoCAc-GKPILFFRLK hydrolysis) for the enzyme exhibiting the highest activity were as follows: km = 20.68 ± 4.55 μM, kcat = 0.09 ± 0.02 s−1, and kcat/km = 4352 M−1 s−1. The rate of Hb cleavage by Na-APR-1wt was also investigated (Fig. 2C). By 3 h, the majority of the Hb was digested, and by 16 h, there was no intact Hb visible on the gel. The addition of pepstatin A to the recombinant Na-APR-1wt ablated degradation of Hb at all time points assessed.
Figure 2.
Refolded Na-APR-1wt cleaves synthetic and natural substrates. A) Optimum pH profile of refolded Na-APR-1wt activity as determined by cleavage of the fluorogenic peptide 7-methoxycoumarin-4-acetyl-GKPILFFRLK (DNP)-d-Arg-Amide. Graph shows means of reproducible triplicate experiments. B) Cleavage of 1.0 μg of human Hb by Na-APR-1wt at different pH values in 50 mM sodium acetate for 1 h. C) Cleavage of 1.0 μg of human Hb over time by 1.0 μg of recombinant Na-APR-1wt at 37°C in 50 mM sodium acetate (pH 3.5).
E. coli-expressed Na-APR-1mut can be refolded to produce properly folded protein
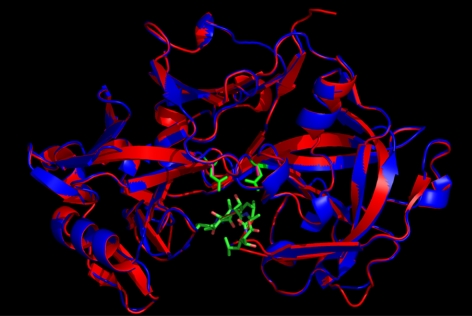
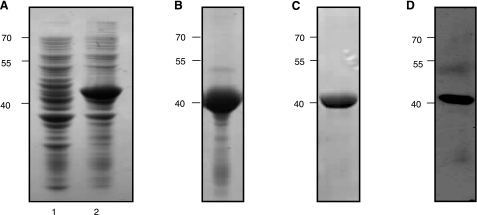
To ablate catalytic activity but potentially retain the correct fold, we mutated the two active site Asp residues to Ala. The predicted structures of Na-APR-1wt and APR-1mut based on the crystal structure of human pepsin docked with pepstatin A were determined by molecular modeling (Fig. 3). Na-APR-1mut was predicted to bind to substrates and inhibitors, but unlike APR-1wt, it would no longer cleave the scissile peptide bond due to the loss of general acid catalysis by substitution of the acidic Asp residues with alanines. Recombinant Na-APR-1mut was expressed in E. coli as insoluble inclusion bodies, denatured, purified, and optimally refolded at similar yields to Na-APR-1wt (Fig. 4A–C). Western blotting confirmed the identity of the refolded protein via recognition of the 6×His tag (Fig. 4D).
Figure 3.
Molecular model of Na-APR-1wt (red ribbon with green active-site Asp side chains) and Na-APR-1mut (red ribbon with fuschia active-site Ala side chains) superimposed on the template structure of human pepsin (blue ribbon) (PDB code 1PSO). Structure of 1PSO was solved in complex with pepstatin, shown in stick representation with carbon atoms in green, nitrogen atoms in blue, and oxygen atoms in pink. RMSD over the c-α atoms of the model and the template is 0.118, highlighting the overall similarity between the two molecules. Superimposition was produced using the program VMD (43).
Figure 4.
Expression and refolding of recombinant Na-APR-1mut. A) SDS-PAGE gel stained with Coomassie Brilliant Blue showing insoluble protein profiles from E. coli BL21(DE3) cells before (lane 1) and 20 h after (lane 2) induction with 1.0 mM IPTG. B–D) Urea-solubilized inclusion bodies containing Na-APR-1mut were subsequently purified by immobilized metal ion affinity chromatography, then refolded and assessed by SDS-PAGE (C) and Western blotting (D) using anti-6×His(C-term)-HRP monoclonal antibody. Molecular mass (kDa) markers are shown at left.
Na-APR-1mut did not cleave MoCAc-GKPILFFRLK or Hb (Figs. 5A and 5B, respectively), implying that proteolytic activity had been ablated by mutation of the active site residues of the protein. Na-APR-1mut did, however, bind to pepstatin A agarose (Fig. 5C), implying that although catalytically inactive, the mutant protein adopted proper conformation and still retained a correctly folded and accessible binding pocket.
Figure 5.
Refolded Na-APR-1mut is catalytically inactive and binds, but does not cleave, substrates. A, B)Incubation of 1.0 μg of refolded Na-APR-1mut with 7-methoxycoumarin-4-acetyl-GKPILFFRLK(DNP)-d-Arg-Amide at 37°C in 50 mM sodium acetate (pH 3.5) (A) and 1.0 μg of human hemoglobin (B). C) Western blot (using anti-6×His antibody) of refolded Na-APR-1mut after binding to and eluting from pepstatin A agarose. An equal amount of Sm-TSP-2, an irrelevant 6×His tagged protein, was used as a negative control.
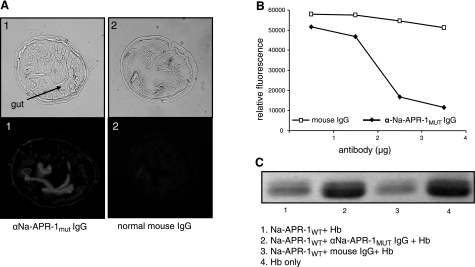
Anti-Na-APR-1mut antibody binds to parasite-derived Na-APR-1 and neutralizes Na-APR-1wt activity in vitro
The localization of Na-APR-1 to the intestinal brush border of the parasite has been previously documented (19). Antibodies to Na-APR-1mut bound to Na-APR-1 in the gut lumen of N. americanus, demonstrating that antibodies to the mutant protein recognized epitopes that are presented in the native protein in the worm gut (Fig. 6A). No fluorescence was observed with IgG from preimmunization serum. We then showed that anti-Na-APR-1mut IgG bound to and inhibited the catalytic activity of Na-APR-1wt against both MoCAc-GKPILFFRLK and Hb while control IgG had no effect. Five micrograms of polyclonal anti-Na-APR-1mut IgG completely inhibited the catalysis of peptide substrate generated by 0.2 μg of Na-APR-1wt (Fig. 6B). Five micrograms of polyclonal anti-Na-APR-1mut IgG also inhibited digestion of Hb by Na-APR-1wt by 69%, as determined by gel densitometry (Fig. 6C). Anti-Na-APR-1wt IgG displayed a similar neutralizing capacity per microgram of antibody (not shown).
Figure 6.
Anti-Na-APR-1mut antibodies bind to parasite-derived Na-APR-1 and neutralize catalytic activity of refolded Na-APR-1wt. A) Immunostaining of fixed Necator americanus sections with anti-Na-APR-1mut IgG and goat anti-mouse Alexa-Fluor 568. All images are shown at ×40, with corresponding brightfield images shown above fluorescence images. Sections in panel 1 were probed with anti-Na-APR-1mut IgG; sections in panel 2 were probed with normal mouse IgG. B, C)To test inhibition, refolded Na-APR-1wt was incubated with anti-Na-APR-1mut IgG or control IgG, and inhibition of activity was determined by a decrease in cleavage of methoxycoumarin-4-acetyl-GKPILFFRLK(DNP)-d-Arg-Amide (B) or human hemoglobin (Hb) (C). Lane 1, Hb + Na-APR-1wt; lane 2, Hb + Na-APR-1wt + anti-Na-APR-1mut IgG; lane 3, Hb + Na-APR-1wt + normal mouse IgG; lane 4, Hb only.
Proteolytic activity of APR-1 orthologues from other hookworms can be inhibited by anti-Na-APR-1mut IgG
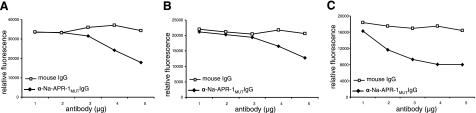
The proteolytic activity of recombinant forms of Na-APR-1 homologues from the 3 other major species of hookworm—A. caninum (Ac-APR-1wt), A. duodenale (Ad-APR-1wt) and A. ceylanicum (Ay-APR-1wt)—were also inhibited to varying degrees by IgG raised to Na-APR-1mut. Relative to control IgG, 5.0 μg of anti-Na-APR-1mut IgG neutralized the catalytic activities of Ac-APR-1wt (Fig. 7A), Ad-APR-1wt (Fig. 7B), and Ay-APR-1wt (Fig. 7C) by 58, 53, and 74%, respectively.
Figure 7.
Anti-Na-APR-1mut antibodies inhibit APR-1wt activity of other hookworm species. Refolded APR-1wt from A. caninum (A), A. duodenale (B), or A. ceylanicum (C) was incubated with anti-Na-APR-1mut IgG or control mouse IgG, and inhibition of activity was determined by a decrease in methoxycoumarin-4-acetyl-GKPILFFRLK(DNP)-d-Arg-amide cleavage.
Refolded Na-APR-1mut protects dogs against heterologous hookworm challenge
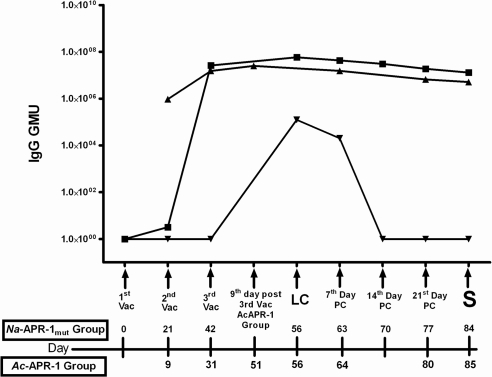
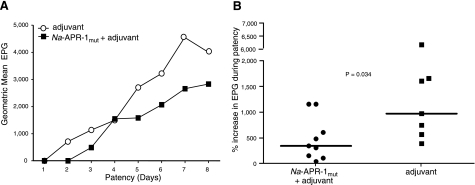
Na-APR-1mut formulated with Alhydrogel and CpG 10103 induced a strong IgG antibody response that was maintained until sacrifice (42 d after the third vaccination) (Fig. 8). Antibody responses were similar to but higher than those obtained when beagles were immunized with Ac-APR-1 expressed in Pichia pastoris and formulated with AS03 (12). An anti-Na-APR-1mut response was seen in control dogs after larval challenge, but the response was short lived, and levels returned to baseline 14 d later. Egg counts from the feces of vaccinated and control dogs were monitored from patency until necropsy. On d 28 postlarval challenge, the median EPG count of dogs vaccinated with Na-APR-1mut was 2800, compared to 8400 in dogs that received adjuvant alone, resulting in a 66.6% reduction in egg output. We also measured the effect of vaccination on egg output by female worms as a percentage increase in EPG during patency over an 8-d period (Fig. 9). Animals vaccinated with Na-APR-1mut consistently produced fewer eggs from patency (first egg count) to sacrifice than control dogs (P=0.034) on the fourth day. However, as previously noted with Ac-APR-1, dogs vaccinated with Na-APR-1mut and challenged with A. caninum did not have a significantly lower worm burden at the 0.05 level (12); the geometric mean worm burdens were 90 vs. 126 worms in vaccinated vs. control dogs, respectively, resulting in a 29% reduction in worm burdens in vaccinated dogs; these data were not statistically significant.
Figure 8.
Vaccination of dogs with Na-APR-1mut formulated with Alhydrogel + CpG 10103 induces a strong and persistent IgG response. Arbitrary geometric mean units (GMU) of IgG in beagles vaccinated with Na-APR-1mut + Alhydrogel + CPG 10103 (▪) (this study), Ac-APR-1+ASO3 (▴) (from ref. 12) and Alhydrogel + CPG 10103 (▾) (this study) at different time points. Vac, vaccination; LC, larval challenge; PC, post challenge, S, sacrifice.
Figure 9.
Vaccination of dogs with Na-APR-1mut results in decreased fecal egg counts after heterologous challenge with A. caninum. Reductions are shown in terms of geometric mean egg output during patency (A) and percentage increase in egg output during patency (B).
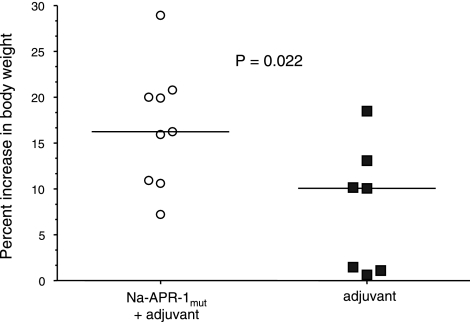
Vaccinated dogs are protected against weight and blood loss and generate neutralizing antibodies
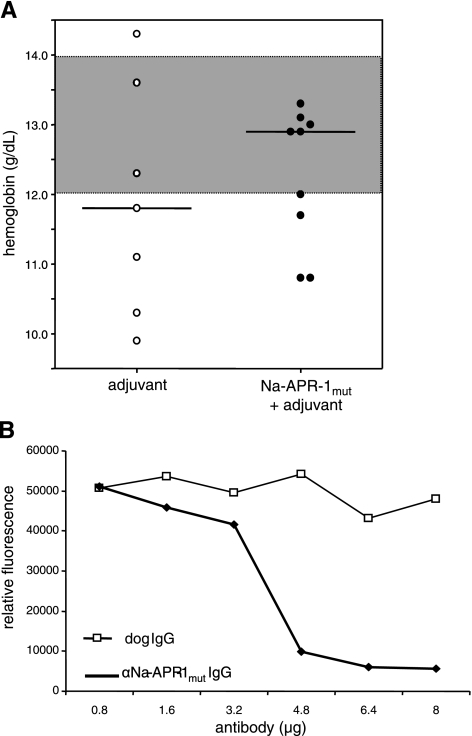
The clinical parameters of vaccinated and control dogs were measured throughout the trial. Vaccinated dogs gained weight more rapidly than control dogs, when measured as percentage increase in body weight from 8 separate measurements per dog between the beginning and the end of the trial (P=0.022; Fig. 10). Hb levels were assessed as a direct measure of anemia. At larval challenge, the median Hb levels were 12.8 and 12.5 g/dl for control and vaccinated dogs, respectively, vs. 11.8 and 12.9 g/dl for control and vaccinated dogs at the end of trial (Fig. 11A) but were not statistically significant.
Figure 10.
Dogs vaccinated with Na-APR-1mut gain weight more rapidly than control dogs after heterologous challenge with A. caninum. Data are presented as percentage increase in body weight over the duration of the trial after larval challenge.
Figure 11.
Dogs vaccinated with Na-APR-1mut generate antibodies that neutralize enzymatic activity of Na-APR-1wt and have higher mean Hb concentrations than control dogs after heterologous challenge with A. caninum. A) Hb concentrations of individual vaccinated and control dogs immediately prior to necropsy. Highlighted area between 12 and 14 g/dl Hb denotes normal range in a healthy dog. Horizontal lines denote median values. B) Na-APR-1wt (250 ng) was incubated with different amounts of dog anti-Na-APR-1mut IgG or control dog IgG for 2 h at 37°C in the presence of methoxycoumarin-4-acetyl-GKPILFFRLK(DNP)-d-Arg-Amide. Data are expressed as relative fluorescence generated by cleavage of the substrate in the presence of dog IgGs and are presented as means of 3 reproducible triplicate experiments.
IgG from dogs vaccinated with Na-APR-1mut neutralized the enzymatic activity of the wild-type enzyme (Fig. 11B) with approximately the same efficiency as IgG from vaccinated mice. Although 10- to 20-fold more IgG than protease was required to obtain >80% neutralization, it should be noted that the IgGs used in this experiment were polyclonal and that the entire IgG repertoires of the animals were represented in these purified immunoglobulins.
Vaccinated dogs have reduced gut pathology
Abnormal postmortem findings were mainly confined to the intestinal tract and included petechial and ecchymotic hemorrhages at sites of worm attachment in the jejunum, mucosal hemorrhage at the ileocecocolic valve, and in severe cases, frank blood in the intestinal contents. Overall, the pathology associated with hookworm infection was less severe in Na-APR-1mut-vaccinated animals compared to control animals (not shown). Note that while adult A. caninum were present in the small intestines of all experimentally infected animals, four of the animals vaccinated with Na-APR-1mut had minimal pathology with no mucosal hemorrhage at the sites of worm attachment in the jejunum (not shown). In addition, these animals had resolving hemorrhages at the ileocecocolic valve. These findings suggest that in addition to reducing adult worm burdens in the intestine, vaccination with Na-APR-1mut ameliorates the pathology associated with hookworm infection.
Na-APR-1mut is a major target of the IgG1 response in hookworm-infected individuals
To assess serologic recognition of Na-APR-1mut by humans resident in areas of high transmission for N. americanus, sera from 648 hookworm-infected individuals from Minas Gerais state, Brazil, were screened for anti-APR-1mut antibody responses. Sixty-two percent showed detectable IgG responses (42% had IgG1, 0.6% IgG2, 15% IgG3, 53% IgG4) and only 0.6% had IgE antibodies (Table 1). There was no association between the presence of antibody and current infection status (positive or negative), intensity of infection (EPG), or age. These data differ markedly from those of the larval antigen Na-ASP-2, where high levels of the Th2 antibodies IgG4 and IgE were detected, with IgE against Na-ASP-2 being associated with a decreased risk of heavy hookworm infection (24).
TABLE 1.
Recognition of Na-APR-1mut by IgG, IgG subclasses (IgG1–4), IgE, and IgA using quantitative ELISA
| ELISA method | IgG1 | IgG2 | IgG3 | IgG4 | Total IgG | IgE | IgA |
|---|---|---|---|---|---|---|---|
| Dichotomous, positive [% (n)]a | 42 (270) | 0.6 (4) | 15 (97) | 53 (343) | 62 (401) | 0.6 (4) | 3 (22) |
| Quantitative (μg/ml)b | 0.195 ± 0.104c | −d | 0.123 ± 0.066c | 0.203 ± 0.136c | 0.592 ± 0.518c | − | − |
Study sample was 648 individuals resident in an area of “high” N. americanus transmission (WHO) in Minas Gerais state, Brazil, 2005.
Positivity as indicated by a reactivity threshold, which was determined from a frequency distribution from the upper 95% CI of the frequency distribution from log10 transformed OD values from a panel of sera from Brazilian volunteers not resident in an N. americanustransmission area (n=56) and a panel of control sera from U.S. volunteers (24). Control sera were tested at the following dilutions: IgG subclasses 1:100; total IgG 1:1000; IgE 1:25, and serum IgA 1:50 (29).
Values are means ± sd. Amounts were not calculated for samples in which <25 individuals were positive for the antibody.
Homologous interpolation is not feasible for Ig with a low number of responders, as it requires large amounts of serum containing Ig to the antigen. Homologous interpolation of antibody levels to Na-APR-1mut refers to a calibration scheme in which the calibration curve is constructed using Ig against Na-APR-1mut affinity purified from human reference sera. Here, both the calibrator and sample are analyzed using identical reagents.
Heterologous interpolation of antibody levels to Na-APR-1mut refers to a calibration scheme in which the calibration curve uses reagents that have a different specificity from those used to measure the analyte of interest; in this case, Na-APR-1mut-specific antibody ELISA was simultaneously performed alongside a total Ig assay, with the mass values calibrating by interpolating the sample values by the standard curve of the total Ig assay.
DISCUSSION
The Ac-APR-1 aspartic hemoglobinase from the canine hookworm protects dogs against homologous challenge with the canine hookworm, A. caninum (12), so we reasoned that the orthologous enzyme from N. americanus (Na-APR-1) would be a lead candidate as a human hookworm vaccine. Na-APR-1 is a promiscuous protease that digests Hb and other host proteins (10, 19, 30). To compare the immunogenicity of the catalytically active enzyme with a catalytically inactive form of the protein, we expressed a mutagenized form of Na-APR-1mut that contained two alanine residues in place of the two canonical aspartic acid residues required for enzymatic activity. We showed that the recombinant mutagenized protein retained the same fold as Na-APR-1wt, and although it was no longer catalytically active, Na-APR-1mut bound to pepstatin A, which requires an intimate and complex association between enzyme and inhibitor (31). Further evidence of Na-APR-1mut displaying many or all of the same epitopes as the wild-type enzyme was demonstrated by the ability of anti-Na-APR-1mut IgG to bind to parasite-derived Na-APR-1 in hookworm gut sections and neutralize wild-type recombinant enzyme.
Aspartic proteases are protective antigens against other infectious organisms, including other blood-feeding parasites, such as schistosomes (32) and ticks (33), as well as the fungal pathogens Coccidioides (34) and Candida (35). Indeed, in the Candida albicans mouse model, passive transfer of anti-protease IgG significantly decreased the yeast burden in kidneys of mice infected with C. albicans, highlighting the role of anti-protease antibodies in protection. Our work with hookworms (12, 13) and studies on another blood-feeding helminth parasite, F. hepatica (36, 37), are unique, however, in showing that antibodies generated to hemoglobinases probably exert their efficacy by neutralizing the catalytic activity of their target proteases. To the best of our knowledge, this is the first report describing the use of antibodies raised against a catalytically inactive protease from a parasite to inhibit the catalytic activity of its wild-type counterpart. We postulate two ways in which this might occur: 1) antibodies bind to epitopes proximal to the active site and physically hinder access of substrates to the active site cleft; 2) antibodies bind to epitopes that are distant from the active site cleft but alter the enzyme’s conformation and render the active site pocket conformationally inaccessible to substrate. Presumably through one of these mechanisms, anti-Na-APR-1mut IgG inhibited the proteolytic activity of APR-1 homologues from three Ancylostoma species of hookworms, although to a lesser degree than it did Na-APR-1. Ac-APR-1, Ad-APR-1, and Ay-APR-1 from A. caninum, A. duodenale, and A. ceylanicum, respectively, are between 84 and 89% identical to Na-APR-1 at the amino acid level, so the different levels of inhibition are most probably reflective of the variability of antibody binding caused by the antigenic differences between the enzymes. The ability of Na-APR-1mut to induce such a response against APR-1 homologues from all of the medically important species of hookworm suggests that a vaccine based on Na-APR-1mut may reduce the intensity of all forms of hookworm disease, and not just necatoriasis.
As seen in previous studies with Ac-APR-1wt (12), the reduction in fecundity was more striking (and statistically significant) than the reduction in adult worm burden. We have suggested that an impaired ability to digest Hb affects nutrient acquisition (4, 12), manifesting most noticeably as adverse ova production, given the high nutritional requirement of egg-laying females of numerous helminth parasites (4, 15, 38). Impaired ability to digest Hb may not immediately affect patency (i.e., established adult worm infection) in the reduced time frame imposed by a laboratory trial (12 wk) as opposed to the months or years that a hookworm will reside in its human host. This is in marked contrast to larval hookworm vaccines, e.g., the irradiated larval vaccine (39) or Na-ASP-2 hookworm vaccine (24), which are expected to attenuate invading larvae at an earlier stage of infection, thereby reducing the number of worms that develop to sexual maturity in the gut. The full extent of the antibody-induced damage to worms would be best monitored in a clinical trial in humans in an endemic setting, where worm survival (and fecundity) can be monitored in vaccinated people over an extended period (months or years). Even in the absence of reduced worm burdens, significant reductions in egg output would lower the number of ova present in the environment and, possibly therefore, the risk of transmission of the infection.
It is noteworthy that the decreased parasite burden in vaccinated animals was achieved following a heterologous vaccination/challenge regime where dogs were vaccinated with an N. americanus protein (85% identical to the A. caninum orthologue) and challenged with A. caninum larvae. Dogs are natural hosts for A. caninum but not N. americanus, and the latter does not develop to maturity in the dog (40), so despite the differences in amino acid sequences of Na-APR-1 and Ac-APR-1, we chose to utilize the natural canine/A. caninum model of disease but vaccinate with Na-APR-1mut. We reasoned that any protection obtained in this model would be bettered in a homologous vaccination/parasite challenge scenario. Supporting this notion, anti-Na-APR-1mut antibodies were more effective at inhibiting Na-APR-1wt activity than they were at inhibiting the activity of APR-1 orthologues from any of the Ancylostoma species.
The presence of IgG1 to Na-APR-1 in sera from humans resident in hookworm endemic areas resolves one of the most critical obstacles in the use of gut antigens as viable targets for vaccines against blood-feeding helminths. Many consider antigens associated with or anchored in the gut membrane of blood-feeding nematodes as “hidden” (41), i.e., not continuously presented to the immune system, with the concomitant problem of rapid waning of the neutralizing antibody response due to the lack of a “natural boost.” The presence of IgG1 in the serum of individuals in endemic areas points to the fact that the human host recognizes Na-APR-1 during a natural infection. A vaccine could augment an already existing immune response, and the immune response to Na-APR-1 does not conform to the typical response (IgE and IgG4) seen with many other helminth antigens (42). The latter might be the result of location of Na-APR-1 in the gut, with its unique set of immunological circumstances compared to other organs through which hookworms pass, such as the skin or lung, and irrevocably bias the local immune response toward a T helper type 2 response. The abundance and high affinity of IgG1 make it the preferred immunoglobulin to function as a neutralizing antibody. These findings support the notion that antigens associated with or anchored in the gut membrane of blood-feeding nematodes have a limited and phenotypically distinct exposure to the host’s immune system during a natural infection. However, this may work to our advantage in terms of vaccine development, as such a protein is unlikely to elicit a typical IgG4 and/or IgE antibody response upon vaccination of individuals in high transmission areas, which have proven ineffective in the past and may raise safety concerns in the future.
We have shown here that a catalytically inactive form of the aspartic hemoglobinase from N. americanus, Na-APR-1, can be expressed at high yield and refolded to produce an antigen that protects animals against a heterologous hookworm challenge. Moreover, the antibodies raised to the mutagenized form of APR-1 are able to neutralize the enzymatic activity of wild-type APR-1 enzymes from four different hookworm species, highlighting the potential of this antigen as a component of a pan-hookworm vaccine. We are currently using these polyclonal (and recently generated monoclonal) neutralizing antibodies to identify the epitopes within APR-1 that are the target of neutralizing antibodies to further enhance the likelihood of incorporating APR-1 into an eventual human hookworm vaccine.
Acknowledgments
We thank Drs. Jane Halpern, Philip Russell, and Ami Shah-Brown for helpful discussions and Mark J. Hoenerhoff for conducting the canine clinical pathology. This work was funded by a grant awarded from the Bill and Melinda Gates Foundation to the Albert B. Sabin Vaccine Institute and a grant from the National Health and Medical Research Council of Australia (NHMRC). A.L. is the recipient of an NHMRC senior research fellowship. J.B. is the recipient of travel awards from the ARC/NHMRC Research Network for Parasitology and the Australian Centre for Vaccine Development.
References
- De Silva N R, Brooker S, Hotez P J, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hotez P J, Brindley P J, Bethony J M, King C H, Pearce E J, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P J. Hookworm and poverty. Ann N Y Acad Sci. 2007;1136:38–44. doi: 10.1196/annals.1425.000. [DOI] [PubMed] [Google Scholar]
- Loukas A, Bethony J, Brooker S, Hotez P. Hookworm vaccines: past, present, and future. Lancet Infect Dis. 2006;6:733–741. doi: 10.1016/S1473-3099(06)70630-2. [DOI] [PubMed] [Google Scholar]
- Albonico M, Smith P G, Ercole E, Hall A, Chwaya H M, Alawi K S, Savioli L. Rate of reinfection with intestinal nematodes after treatment of children with mebendazole or albendazole in a highly endemic area. Trans R Soc Trop Med Hyg. 1995;89:538–541. doi: 10.1016/0035-9203(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Albonico M, Engels D, Savioli L. Monitoring drug efficacy and early detection of drug resistance in human soil-transmitted nematodes: a pressing public health agenda for helminth control. Int J Parasitol. 2004;34:1205–1210. doi: 10.1016/j.ijpara.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kremer M, Miguel E. Harvard University: Center for Global Development; The illusion of sustainability. 2004 Working Paper No. 112 W10324, 55 pp. [Google Scholar]
- Don T A, Jones M K, Smyth D, O'Donoghue P, Hotez P, Loukas A. A pore-forming haemolysin from the hookworm, Ancylostoma caninum. Int J Parasitol. 2004;34:1029–1035. doi: 10.1016/j.ijpara.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Williamson A L, Lecchi P, Turk B E, Choe Y, Hotez P J, McKerrow J H, Cantley L C, Sajid M, Craik C S, Loukas A. A multi-enzyme cascade of hemoglobin proteolysis in the intestine of blood-feeding hookworms. J Biol Chem. 2004;279:35950–35957. doi: 10.1074/jbc.M405842200. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Zhan B, Hamilton B, Stenzel D, Lowther J, Pearson M, Gorman J, Hotez P, Loukas A. Proteolytic degradation of hemoglobin in the intestine of the human hookworm, Necator americanus [E-pub ahead of print] J Infect Dis. 2009;PMID:19191589. doi: 10.1086/597048. [DOI] [PubMed] [Google Scholar]
- Hotez P J, Ashcom J, Zhan B, Bethony J, Loukas A, Hawdon J M, Wang Y, Jin Q, Jones K C, Dobardzic A, Dobardzic R, Bolden J, Essiet-Gibson I, Brandt W, Russell P K, Zook B, Howard B, Chacon M. Effect of vaccination with a recombinant fusion protein encoding an astacin-like metalloprotease (MTP-1) secreted by host-stimulated Ancylostoma caninum third-stage infective larvae. J Parasitol. 2003;89:853–855. doi: 10.1645/GE-46R. [DOI] [PubMed] [Google Scholar]
- Loukas A, Bethony J M, Mendez S, Fujiwara R T, Goud G N, Ranjit N, Zhan B, Jones K, Bottazzi M E, Hotez P J. Vaccination with recombinant aspartic hemoglobinase reduces parasite load and blood loss after hookworm infection in dogs. PLoS Med. 2005;2:e295. doi: 10.1371/journal.pmed.0020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas A, Bethony J M, Williamson A L, Goud G N, Mendez S, Zhan B, Hawdon J M, Elena Bottazzi M, Brindley P J, Hotez P J. Vaccination of dogs with a recombinant cysteine protease from the intestine of canine hookworms diminishes the fecundity and growth of worms. J Infect Dis. 2004;189:1952–1961. doi: 10.1086/386346. [DOI] [PubMed] [Google Scholar]
- Xiao S, Zhan B, Xue J, Goud G N, Loukas A, Liu Y, Williamson A, Liu S, Deumic V, Hotez P. The evaluation of recombinant hookworm antigens as vaccines in hamsters (Mesocricetus auratus) challenged with human hookworm, Necator americanus. Exp Parasitol. 2008;118:32–40. doi: 10.1016/j.exppara.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Knox D P, Redmond D L, Newlands G F, Skuce P J, Pettit D, Smith W D. The nature and prospects for gut membrane proteins as vaccine candidates for Haemonchus contortus and other ruminant trichostrongyloids. Int J Parasitol. 2003;33:1129–1137. doi: 10.1016/s0020-7519(03)00167-x. [DOI] [PubMed] [Google Scholar]
- Geldhof P, Claerebout E, Knox D, Vercauteren I, Looszova A, Vercruysse J. Vaccination of calves against Ostertagia ostertagi with cysteine proteinase enriched protein fractions. Parasite Immunol. 2002;24:263–270. doi: 10.1046/j.1365-3024.2002.00461.x. [DOI] [PubMed] [Google Scholar]
- Redmond D L, Knox D P. Protection studies in sheep using affinity-purified and recombinant cysteine proteinases of adult Haemonchus contortus. Vaccine. 2004;22:4252–4261. doi: 10.1016/j.vaccine.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Dalton J P, Neill S O, Stack C, Collins P, Walshe A, Sekiya M, Doyle S, Mulcahy G, Hoyle D, Khaznadji E, Moire N, Brennan G, Mousley A, Kreshchenko N, Maule A G, Donnelly S M. Fasciola hepatica cathepsin L-like proteases: biology, function, and potential in the development of first generation liver fluke vaccines. Int J Parasitol. 2003;33:1173–1181. doi: 10.1016/s0020-7519(03)00171-1. [DOI] [PubMed] [Google Scholar]
- Williamson A L, Brindley P J, Abbenante G, Prociv P, Berry C, Girdwood K, Pritchard D I, Fairlie D P, Hotez P J, Dalton J P, Loukas A. Cleavage of hemoglobin by hookworm cathepsin D aspartic proteases and its potential contribution to host specificity. FASEB J. 2002;16:1458–1460. doi: 10.1096/fj.02-0181fje. [DOI] [PubMed] [Google Scholar]
- Morales M E, Rinaldi G, Gobert G N, Kines K J, Tort J F, Brindley P J. RNA interference of Schistosoma mansoni cathepsin D, the apical enzyme of the hemoglobin proteolysis cascade. Mol Biochem Parasitol. 2008;157:160–168. doi: 10.1016/j.molbiopara.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauer K, Buck M, Flanagan J, Belzer D, Sculley T. Identification of the nuclear localization signals within the Epstein-Barr virus EBNA-6 protein. J Gen Virol. 2004;85:165–172. doi: 10.1099/vir.0.19549-0. [DOI] [PubMed] [Google Scholar]
- Fiser A, Sali A. Modeller: generation and refinement of homology-based protein structure models. Methods Enzymol. 2003;374:461–491. doi: 10.1016/S0076-6879(03)74020-8. [DOI] [PubMed] [Google Scholar]
- Don T A, Bethony J M, Loukas A. Saposin-like proteins are expressed in the gastrodermis of Schistosoma mansoni and are immunogenic in natural infections. Int J Infect Dis. 2008;12:e39–e47. doi: 10.1016/j.ijid.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Bethony J M, Loukas A, Smout M J, Brooker S, Mendez S, Wang Y, Bottazzi M E, Zhan B, Williamson A L, Corrêa-Oliveira R, Shuhua X, Hotez P J. Antibodies against a secreted protein from hookworm larvae reduce the intensity of infection in humans and vaccinated laboratory animals. FASEB J. 2005;19:1743–1745. doi: 10.1096/fj.05-3936fje. [DOI] [PubMed] [Google Scholar]
- Brooker S, Alexander N, Geiger S, Moyeed R A, Stander J, Fleming F, Hotez P J, Correa-Oliveira R, Bethony J. Contrasting patterns in the small-scale heterogeneity of human helminth infections in urban and rural environments in Brazil. Int J Parasitol. 2006;36:1143–1151. doi: 10.1016/j.ijpara.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Jardim-Botelho A, Quinnell R J, Geiger S M, Caldas I R, Fleming F, Hotez P J, Correa-Oliveira R, Rodrigues L C, Bethony J M. Age-related changes in hookworm infection, anaemia and iron deficiency in an area of high Necator americanus hookworm transmission in south-eastern Brazil. Trans R Soc Trop Med Hyg. 2007;101:146–154. doi: 10.1016/j.trstmh.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Fleming F M, Brooker S, Geiger S M, Caldas I R, Correa-Oliveira R, Hotez P J, Bethony J M. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Trop Med Int Health. 2006;11:56–64. doi: 10.1111/j.1365-3156.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- Jacobson R H. Validation of serological assays for diagnosis of infectious diseases. Rev Sci Tech. 1998;17:469–526. doi: 10.20506/rst.17.2.1119. [DOI] [PubMed] [Google Scholar]
- Quinn C P, Semenova V A, Elie C M, Romero-Steiner S, Greene C, Li H, Stamey K, Steward-Clark E, Schmidt D S, Mothershed E, Pruckler J, Schwartz S, Benson R F, Helsel L O, Holder P F, Johnson S E, Kellum M, Messmer T, Thacker W L, Besser L, Plikaytis B D, Taylor T H, Jr, Freeman A E, Wallace K J, Dull P, Sejvar J, Bruce E, Moreno R, Schuchat A, Lingappa J R, Martin S K, Walls J, Bronsdon M, Carlone G M, Bajani-Ari M, Ashford D A, Stephens D S, Perkins B A. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg Infect Dis. 2002;8:1103–1110. doi: 10.3201/eid0810.020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A L, Brindley P J, Loukas A. Hookworm cathepsin D aspartic proteases: contributing roles in the host-specific degradation of serum proteins and skin macromolecules. Parasitology. 2003;126:179–185. doi: 10.1017/s0031182002002706. [DOI] [PubMed] [Google Scholar]
- Baldwin E T, Bhat T N, Gulnik S, Hosur M V, Sowder R C, 2nd, Cachau R E, Collins J, Silva A M, Erickson J W. Crystal structures of native and inhibited forms of human cathepsin D: implications for lysosomal targeting and drug design. Proc Natl Acad Sci U S A. 1993;90:6796–6800. doi: 10.1073/pnas.90.14.6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity C K, McManus D P, Brindley P J. Vaccine efficacy of recombinant cathepsin D aspartic protease from Schistosoma japonicum. Parasite Immunol. 2001;23:153–162. doi: 10.1046/j.1365-3024.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- Leal A T, Seixas A, Pohl P C, Ferreira C A, Logullo C, Oliveira P L, Farias S E, Termignoni C, da Silva Vaz I, Jr, Masuda A. Vaccination of bovines with recombinant Boophilus yolk pro-cathepsin. Vet Immunol Immunopathol. 2006;114:341–345. doi: 10.1016/j.vetimm.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Tarcha E J, Basrur V, Hung C Y, Gardner M J, Cole G T. A recombinant aspartyl protease of Coccidioides posadasii induces protection against pulmonary coccidioidomycosis in mice. Infect Immun. 2006;74:516–527. doi: 10.1128/IAI.74.1.516-527.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilanova M, Teixeira L, Caramalho I, Torrado E, Marques A, Madureira P, Ribeiro A, Ferreira P, Gama M, Demengeot J. Protection against systemic candidiasis in mice immunized with secreted aspartic proteinase 2. Immunology. 2004;111:334–342. doi: 10.1111/j.1365-2567.2004.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacenza L, Acosta D, Basmadjian I, Dalton J P, Carmona C. Vaccination with cathepsin L proteinases and with leucine aminopeptidase induces high levels of protection against fascioliasis in sheep. Infect Immun. 1999;67:1954–1961. doi: 10.1128/iai.67.4.1954-1961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacenza L, Acosta D, Dowd A, McGonicle S, Dalton J, Carmona C. Proteinases secreted by Fasciola hepatica: time course of the inhibitory effect of serum from experimentally infected rabbits demonstrated by gelatin-substrate polyacrylamide gel electrophoresis. J Helminthol. 1997;71:333–338. doi: 10.1017/s0022149x00016151. [DOI] [PubMed] [Google Scholar]
- McManus D P, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21:225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara R T, Loukas A, Mendez S, Williamson A L, Bueno L L, Wang Y, Samuel A, Zhan B, Bottazzi M E, Hotez P J, Bethony J M. Vaccination with irradiated Ancylostoma caninum third stage larvae induces a Th2-like response in dogs. Vaccine. 2005;24:501–509. doi: 10.1016/j.vaccine.2005.07.091. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Okamoto K, Higo A, Imai K. Studies on the development of Necator americanus in young dogs. Jpn J Parasitol. 1960;9:735–743. [Google Scholar]
- Munn E A. Rational design of nematode vaccines: hidden antigens. Int J Parasitol. 1997;27:359–366. doi: 10.1016/s0020-7519(97)00003-9. [DOI] [PubMed] [Google Scholar]
- Bethony J, Brooker S, Albonico M, Geiger S M, Loukas A, Diemert D, Hotez P J. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]