Abstract
Targeted deletion of IA-2 and IA-2β, major autoantigens in type 1 diabetes and transmembrane secretory vesicle proteins, results in impaired secretion of hormones and neurotransmitters. In the present study, we evaluated the effect of these deletions on daily rhythms in blood pressure, heart rate, core body temperature, and spontaneous physical and neuronal activity. We found that deletion of both IA-2 and IA-2β profoundly disrupts the usual diurnal variation of each of these parameters, whereas the deletion of either IA-2 or IA-2β alone did not produce a major change. In situ hybridization revealed that IA-2 and IA-2β transcripts are highly but nonrhythmically expressed in the suprachiasmatic nuclei, the site of the brain’s master circadian oscillator. Electrophysiological studies on tissue slices from the suprachiasmatic nuclei showed that disruption of both IA-2 and IA-2β results in significant alterations in neuronal firing. From these studies, we concluded that deletion of IA-2 and IA-2β, structural proteins of secretory vesicles and modulators of neuroendocrine secretion, has a profound effect on the circadian system.—Kim, S. M., Power, A., Brown, T. M., Constance, C. M., Coon, S. L., Nishimura, T., Hirai, H., Cai, T., Eisner, C., Weaver, D. R., Piggins, H. D., Klein, D. C., Schnermann, J., Notkins, A. L. Deletion of the secretory vesicle proteins IA-2 and IA-2β disrupts circadian rhythms of cardiovascular and physical activity.
Keywords: blood pressure, heart rate, temperature, diabetes, behavior
Circadian rhythmicity is an essential feature of biology, functioning to coordinate daily biological rhythms to the light-dark cycle resulting from the rotation of the earth (1). Species from bacteria to plants to mammals have these endogenous, near-24-h biological rhythms. In mammals there are circadian rhythms in cardiovascular function, gastrointestinal physiology, body temperature, sleep/wake cycles, and physical activity. These rhythms are coordinated by output from the suprachiasmatic nuclei (SCN) of the hypothalamus, the master oscillator (2). SCN neurons and even peripheral fibroblasts possess cell-autonomous oscillators that are regulated by intracellular transcriptional-translational feedback loops (3, 4). For the SCN to function as the principal circadian oscillator, interaction between oscillators is required. This involves, in part, the release of one or more neurotransmitters within the SCN (5, 6). In addition, neural input to the SCN from the retina serves to reset the clock.
IA-2 and IA-2β are major autoantigens in type 1 diabetes (7, 8). Autoantibodies to these proteins appear years before the development of clinical disease, and their presence is a predictor of disease (9). Both are transmembrane proteins located in dense core and synaptic vesicles. In mice, targeted disruption of these genes results in impaired secretion of hormones and neurotransmitters, which in turn leads to a variety of abnormalities including impaired glucose tolerance, female infertility (10,11,12,13) and behavioral and learning disorders (14). In the current study, initiated to assess cardiovascular function in IA-2- and IA-2β-double-knockout (DKO) mice, we observed profoundly suppressed circadian rhythms in cardiovascular function, body temperature, and physical activity and significantly altered electrophysiological activity in the SCN.
MATERIALS AND METHODS
Telemetry
Blood pressure and temperature were monitored by radiotelemetry (Data Sciences International, St. Paul, MN, USA). In mice anesthetized with ketamine and xylazine (90 and 10 mg/kg, respectively), the blood pressure telemeter catheter was inserted into the carotid artery and advanced into the aortic arch, with the telemeter body positioned in a subcutaneous pocket on the right flank (15). Each transmitter (model TA11PA-C10) was magnetically activated >24 h before implantation. One day after surgery, animals were returned to their home cage and provided with ad libitum food and water for the duration of the study. To obtain mean arterial blood pressure, heart rate, and activity data, the telemeter signal was processed using a model RPC-1 receiver, a 20-channel data exchange matrix, an APT-1 ambient pressure monitor, and a Data Quest ART Silver 2.3 acquisition system (Data Sciences International). In most of the studies presented here, the mean systolic and diastolic blood pressure, pulse pressure, heart rate, and activity were sampled for 10-s periods at 2-min intervals. To record temperature, in separate experiments, radiotelemetry transmitters were implanted in the abdominal cavity, and animals were recorded as described above. The recording room was maintained at 21–22°C with a 12-h light-dark (LD) cycle (light intensity ∼60 lux at 1 m above floor level during the light phase) except as noted.
Wheel-running
Mice were singly housed in polycarbonate cages (15×30×12 cm) containing a running wheel (8.5 cm) with ad libitum access to food (mouse chow) and water. Rotation of the running wheel was recorded using the Collect module of the Chronobiology Kit (Stanford Software Systems, San Jose, CA, USA) and visualized offline as double-plotted actograms. Animals were maintained under an LD cycle (light intensity during lights on ∼200 lux) for 14 d to observe synchronization to an LD cycle. The mice were then released into constant darkness for ∼20 d before again being returned to the LD cycle for the remainder of the experiment. Rhythm period and strength (Qp) were assessed by χ2 periodogram analysis (Chronobiology Kit).
Pupillary reflex
In a dimly lit room, a conscious mouse was placed sideways under a stereomicroscope equipped with an eyepiece micrometer. After focusing on the eye, the light was completely turned off for 1–2 min. A fiberoptic light source set at constant light intensity (1.2 mW cm−2) and fixed in a constant position was then turned on for 1 min, and the initial and final widths of the pupil were read off the scale of the micrometer.
Design of probes
To generate a construct for transcribing a riboprobe for IA-2, plasmid pMSCVpuro-mIA-2-f-EGFP (from Dr. Keiichi Saeki, University of Tokyo, Tokyo, Japan; Kobe University, Kobe, Japan) was cut with SacI, and the 703-bp fragment was cloned into a circularized version of pGEM-T Easy (Promega Corp., Madison, WI, USA) that had been digested with SacI. This fragment contains 682 bp of IA-2 corresponding to nucleotides 84-765 of the sequence given in GenBank accession number NM_008985, along with 21 bp of vector sequence. Similarly, to generate a construct for transcribing a riboprobe for IA-2β, plasmid pMSCVpuro-mIA-2β-f-EGFP (from Dr. Keiichi Saeki) was cut with SacI, and the 724-bp fragment corresponding to nucleotides 702-1425 of the sequence given in GenBank accession number NM_011215 was cloned into a circularized version of pGEM-T Easy (Promega Corp.) that had been digested with SacI. The plasmid used to detect mPer1 corresponds to nucleotides 340-761 of GenBank accession number AF022992 (16). Sense and antisense probes were generated from each plasmid.
In situ hybridization
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were euthanized by carbon dioxide asphyxiation at 4-h intervals on the first day in constant darkness (DD). Brains were removed, frozen in cooled 2-methylbutane, and stored at −80°C until sectioned on a cryostat at a 15-μm section thickness. Sections were distributed to slides as a 1-in-8 series so that sections through the SCN were examined at intervals of 120 μm with each probe. Probe hybridization, washes, and image analysis were performed as described previously (16). Briefly, sections were hybridized with probe (107 cpm/ml) overnight at 53°C, washed first in RNase A buffer and then washed to high stringency, dehydrated, and apposed to Kodak BioMax MR film (Eastman Kodak, Rochester, NY, USA). Films for semiquantitative image analysis of IA-2 and IA-2β were exposed for 2 d. Films used for figure preparation were exposed for 2 or 4 d. Per1 films were exposed for 10 d.
Electrophysiology
Slices containing the SCN were prepared during the subjective day and maintained using methods similar to those described earlier (3). Mice were culled by cervical dislocation and decapitation, and the brain was removed and placed in 4°C artificial cerebrospinal fluid (aCSF) (pH 7.4) of the following composition: 124 mM NaCl, 2.2 mM KC1, 1.2 mM KH2PO4, 2.5 mM CaC12, 1.0 mM MgSO4, 25.5 mM NaHCO3, 10 mM d-glucose, and 1.14 mM ascorbic acid. Coronal brain sections (350 μm thick) and the section corresponding to the middle region of the rostrocaudal axis of SCN were cut using a vibroslicer (Campden Instruments, Leicester, UK), transferred to the recording chamber, and equilibrated for ∼1 h before the start of electrophysiological experiments. Throughout the dissection procedure aCSF was bubbled with 95% O2/5% CO2.
Slices were maintained at 34 ± 1°C in a submerged recording changer (PDMI-2; Harvard Apparatus, Eddenbridge, UK) and continuously perfused with oxygenated aCSF at ∼1.5 ml/min. Extracellular multiunit activity (MUA) was recorded simultaneously from both SCN using two aCSF-filled suction electrodes constructed as described previously (6). Multiunit signals were differentially amplified (×20,000) and bandpass-filtered via a neurology system (Digitimer, Welwyn Garden City, UK), digitized (25,000 Hz) using a Micro 1401 mkII interface (Cambridge Electronic Design, Cambridge, UK) and recorded on a PC running Spike2 version 6 software (Cambridge Electronic Design). By using Spike2 software, single unit activity (SUA) was discriminated from MUA recordings off-line as described previously (6). Slices were transilluminated and, under visual inspection via stereozoom microscopy, electrodes were guided and placed on the central region of the coronal SCN. Single units were discriminated on the basis of waveform shape, principal component-based clustering, and the presence of a clear refractory period in an interspike interval histogram. Using these criteria we were able to successfully isolate up to four single units from each recording.
Animal care and use
Targeted disruption of the individual IA-2 and IA-2β genes was described previously (10,11,12,13). Eighth-generation C57BL/6 IA-2+/− mice were mated with fourth-generation C57BL/6 IA-2β+/− mice, and the double-heterozygous offspring were interbred to generate IA-2+/+/IA-2β+/+, IA-2+/−/IA-2β−/−, and IA-2−/−/IA-2β−/− mice. Unless indicated otherwise, IA-2+/+/IA-2β+/+ wild-type (WT) mice served as controls. Because female IA-2−/−/IA-2β−/− mice are infertile, male IA-2−/−/IA-2β−/− mice were bred to female IA-2+/−/IA-2β−/− mice to generate IA-2−/−/ IA-2β−/− (DKO) mice. Animal studies were conducted under protocols approved by the institutional animal care and use committees of the U.S. National Institutes of Health (NIH), University of Massachusetts Medical School, and the University of Manchester.
RESULTS
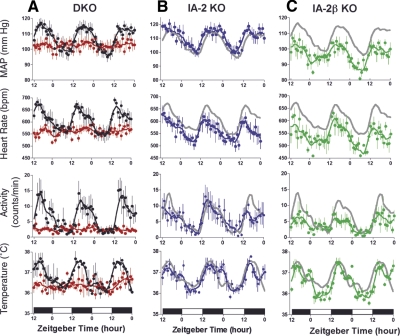
The demonstration that IA-2 and IA-2β are involved in neuroendocrine secretion prompted us to investigate their role in cardiovascular physiology. With use of telemetry to evaluate blood pressure, heart rate, physical activity, and body temperature, WT mice showed the expected robust diurnal variation in blood pressure and heart rate (Fig. 1A). Both parameters peaked during the dark phase of the LD lighting cycle. In marked contrast, there was little evidence of diurnal variation in IA-2- and IA-2β-DKO mice (Fig. 1A). Blood pressure and heart rate remained essentially stable and within the range of values seen in WT mice during the daytime. Similarly, WT mice showed the expected diurnal variation in body temperature and physical activity, but there was no diurnal variation in DKO mice. In the DKO mice, these parameters remained essentially flat and within the daylight range of the WT mice. When blood pressure, heart rate, and physical activity were evaluated in mice kept in constant darkness (DD), rhythmicity persisted in WT mice, demonstrating that these are circadian rhythms, whereas DKO mice lacked rhythmicity in DD (Supplemental Fig. 1). DKO mice thus have profound disruption of several circadian rhythms.
Figure 1.
Mean arterial pressure (MAP), heart rate, spontaneous physical activity, and body temperature in WT and KO mice. A) WT (black) and DKO mice (red). B) IA-2 KO mice (blue). C) IA-2β KO mice (green). Gray lines in B and C represent WT data from A. Data represent means ± se of 5 to 8 mice/group, where the value for each mouse is the hourly average of 10-s recordings taken every 2 min. Mice were maintained in a 12-h light-dark (LD) lighting cycle, and data were collected continuously for 60 h. Zeitgeber times 0 and 12 correspond to lights on at 6:00 AM and lights off at 6:00 PM, respectively. Lines represent data smoothing using the weighted average of the 9 nearest points.
Both IA-2 and IA-2β influence neurotransmitter secretion, but the impact of disruption of these two genes is not identical. Disruption of either gene alone does not produce the more severe abnormalities observed in DKO mice (12, 13), consistent with the view that they are functionally redundant. To determine whether the absence of rhythmicity in the DKO mice was due to the absence of IA-2, IA-2β, or both gene products, single-knockout (KO) mice were examined. As seen in Fig. 1B, IA-2-KO mice were found to have WT-like rhythms in diurnal blood pressure, heart rate, temperature, and physical activity patterns. The IA-2β-KO mice also showed essentially normal rhythmicity, although blood pressure, heart rate, and physical activity were reduced compared with those in WT mice (Fig. 1C). From these findings we concluded that the deletion of both IA-2 and IA-2β is required to produce the severe alterations in physiological rhythms observed in DKO mice.
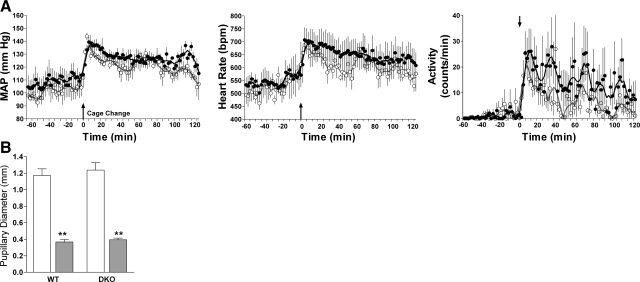
To rule out the possibility that the alterations in DKO mice were due to a basic defect in cardiovascular function or physical activity, WT and DKO mice were challenged by a mildly stressful event (i.e., a cage change). As seen in Fig. 2A, blood pressure, heart rate, and physical activity in DKO mice increased within minutes after initiation of the stressful event, similar to the responses of WT mice. To exclude the possibility that the absence of circadian rhythms was due to a gross abnormality in the visual system, the pupillary reflex of dark-adapted eyes to light in conscious animals was examined (Fig. 2B) and found to be similar in the WT and DKO mice. Retinal function was assessed by electroretinograms. Despite subtle differences, both WT and DKO mice responded to acute light pulses in a grossly normal manner (Supplemental Table 1). Thus, the alteration in rhythmicity in the DKO mice is not caused by an inherent limitation in cardiovascular or visual function but appears to reflect a defect in the circadian timekeeping system.
Figure 2.
A) Effect of acute stress induced by cage change on MAP, heart rate, and spontaneous physical activity in WT (•) and DKO (○) mice. At time 0, mice were transferred into cages previously occupied by different animals. Points represent 10-s data collections taken every 2 min. Vertical lines represent se of 3 to 5 mice/group. B) Effect of light exposure [transition from dark (□) to medium bright light (▒); 1.2 mW cm−2] on pupillary diameter of WT and DKO mice. Vertical lines represent se of 3 to 5 mice/group.
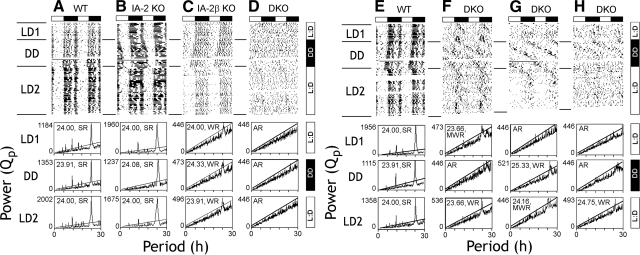
To further evaluate circadian competence, wheel-running activity was used to record locomotor behavioral rhythms in individual mice over a period of ∼50 d. Mice were initially exposed to an LD lighting cycle, were released into DD, and then were returned to an LD lighting cycle. As seen in Fig. 3A, E, WT mice characteristically synchronized the onset of wheel-running activity to lights off and expressed rhythms with a strong 24-h component. On release into DD, WT mice maintained this rhythmic component. Similarly, IA-2-KO mice (Fig. 3B) showed a WT-like rhythm and rhythm strength. The IA-2β-KO mice (Fig. 3C) displayed weaker wheel-running rhythms, and the rhythm strength was substantially reduced relative to the WT mice. In contrast, DKO mice showed poor synchronization of wheel-running activity to lights off and displayed a variety of behavioral phenotypes and variable rhythm strengths, ranging from single or multiple weak components to arrhythmicity (Fig. 3D, F–H). In all cases, DKO mice showed severe disruption of behavioral rhythmicity in both LD and DD, consistent with the results of the telemetry experiments (Fig. 1).
Figure 3.
Wheel-running rhythms of WT, IA-2-KO, IA-2β-KO, and DKO mice. Double-plotted actograms (top panels) and χ2 periodograms (bottom panels) show activity under light-dark (LD1), constant dark (DD), and LD2 conditions. A, E) WT mice. B) IA-2 KO mouse. C) IA-2β KO mouse. D, F–H) IA-2/IA-2β DKO mice. Actograms depict wheel rotations double-plotted over 48 h. Rhythm strength (Qp) is shown on the ordinate and period on the abscissa of the periodograms. Peak Qp value for each panel is shown. Within each panel, period estimate and strength of rhythmicity are indicated. Components traversing the solid diagonal line in each panel are significant at P < 0.05 (χ2 periodogram). DKO mice express a variety of behavioral phenotypes, with some evidence of a weak rhythmic component when released back into LD (LD2). SR, strong rhythm; WR, single weak rhythm; MWR, multiple weak rhythms; AR, arrhythmic.
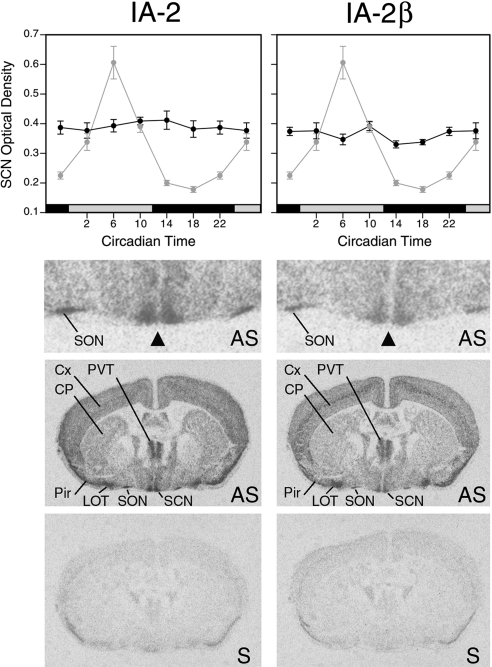
Disruption of signaling within the SCN disrupts circadian rhythms (3, 5, 6). The fact that deletion of IA-2/IA-2β impairs neuroendocrine secretion suggested that the marked disruption in circadian rhythms in the DKO mice might be due to the deletion of IA-2/IA-2β in the SCN. Although it is well established that both IA-2 and IA-2β are expressed in the brain, their presence in the SCN had not been assessed previously. In the present study, in situ hybridization experiments showed that both IA-2 and IA-2β are highly expressed in the SCN (Fig. 4). However, neither IA-2 nor IA-2β mRNA levels were rhythmic, whereas in adjacent sections Per1 mRNA showed the expected high-amplitude rhythmicity (4, 16).
Figure 4.
Expression of IA-2 (left) and IA-2β (right) (dark black lines) as determined by in situ hybridization in brain sections through the SCN from WT mice housed in constant darkness (top panels). IA-2 and IA-2β showed no evidence of rhythmicity (P>0.05; ANOVA,). Adjacent sections from the same animals revealed the expected high-amplitude rhythm of mPer1 expression (light gray symbols with same line plotted in both left and right panels). Values represent means ± se of 5 to 6 animals. Sections hybridized with antisense (AS) probes showed that both IA-2 and IA-2β genes are highly expressed in the SCN, with no obvious subregional localization. Top image is a higher magnification of the image below it. Triangle indicates the bilaterally symmetrical SCN. Sense (S) strand control probes showed the level of the background hybridization (bottom panels). Cx, cortex; CP, caudate-putamen; LOT, nucleus of the lateral olfactory tract; Pir, piriform cortex; SON, supraoptic nucleus; PVT, paraventricular nucleus of the thalamus.
We then examined the effect of the deletion of IA-2 and IA-2β on SCN function by electrophysiology. As expected, MUA recordings on SCN slices from WT mice (Fig. 5A) revealed high-amplitude 24-h oscillations. Periods of high activity occurred during the projected day, and low activity occurred during the projected night. From these MUA recordings, the SUA of 15 individual SCN neurons was successfully discriminated. Ten (66.7%) exhibited an overt circadian rhythm (Figs. 5B, C). Consistent with earlier reports, the amplitude of WT SCN MUA and SUA damped somewhat on the second 24-h cycle in vitro (17, 18). In contrast, MUA and SUA recordings from DKO neurons (Figs. 5D–F) exhibited very low-amplitude rhythmicity or were arrhythmic. In most cases, activity rapidly damped from the initial level to frequencies considerably lower than the WT nadir. Of the 22 individual SCN neurons that were discriminated from 8 DKO recordings, only 3 (13.6%) exhibited rhythmicity, significantly less than the percentage of WT neurons that were rhythmic (P < 0.001, χ2 test) (Fig. 5G). In addition, the peak firing rate of neurons from DKO mice was significantly lower than that of the WT mice (P<0.05) (Fig. 5H). These data reveal clear abnormalities in SCN function in vitro.
Figure 5.
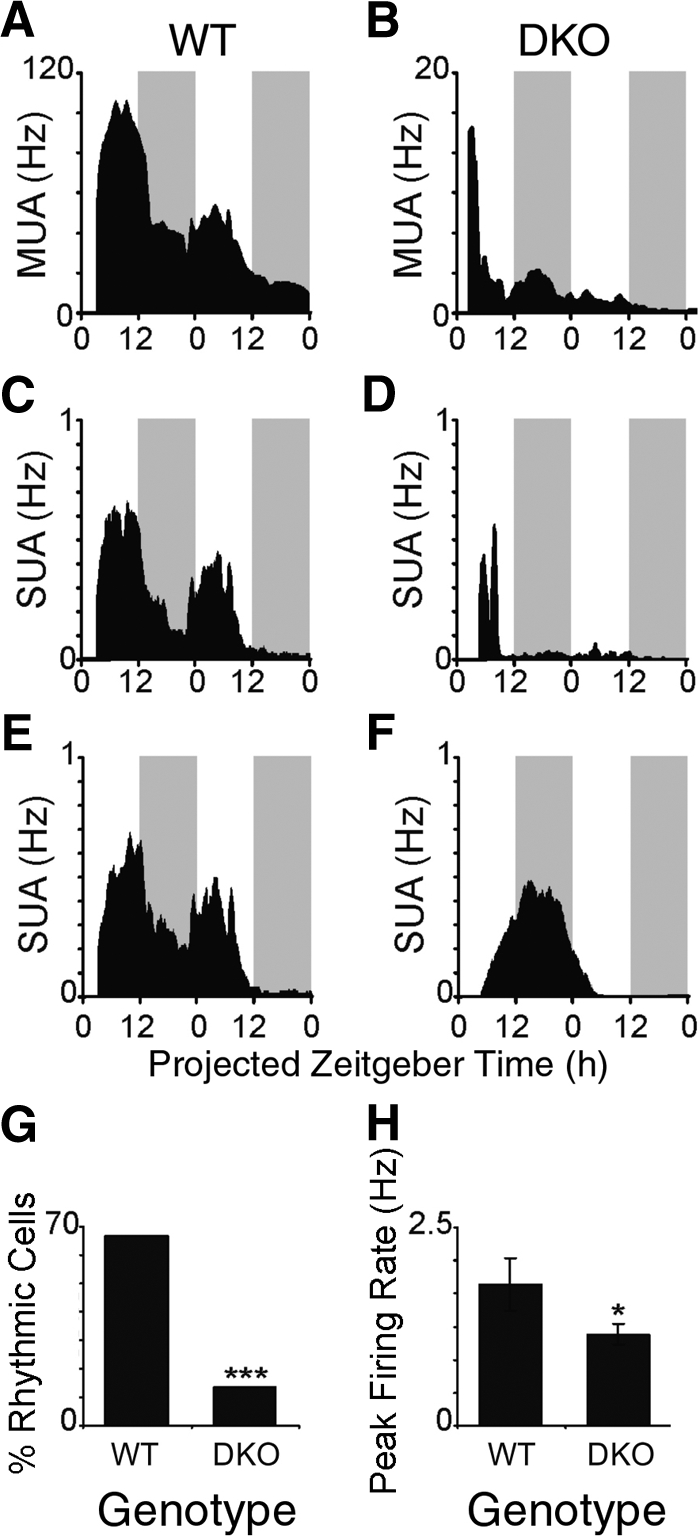
Neuronal firing rate rhythms are disrupted in the SCN of DKO mice. WT mice show clear rhythms in MUA (A) and SUA (B, C), whereas DKO mice show blunted or arrhythmic MUA (D) and SUA profiles (E, F). Firing rate traces show the average firing rate each minute from a representative slice, smoothed using a 1-h moving average. Shaded gray bars represent the projected lights-off period. In contrast to the neurons (n=15) from the WT mice, the percentage of rhythmic neurons (G) and the peak firing rate (H) of neurons (n=22) from DKO mice are significantly reduced. *P < 0.05; unpaired t test. ***P < 0.001; χ2 test.
DISCUSSION
Our experiments show that the targeted deletion of IA-2 and IA-2β disrupts the diurnal variations in blood pressure, heart rate, temperature, and physical activity. In DKO mice, all four endpoints remained essentially flat and at the daytime level. That this loss in diurnal rhythm was not due to a basic defect in cardiovascular function or a gross abnormality in the visual system was shown by the increase in blood pressure, heart rate, and physical activity after exposure to acute stress and the normal pupillary and electroretinogram responses to light, respectively. Thus, the loss of circadian rhythmicity in the DKO mice appears to be due to an abnormality in endogenous circadian signaling secondary to the deletion of IA-2 and IA-2β.
Our in situ hybridization studies showed that IA-2 and IA-2β are both highly expressed in the SCN of WT mice, whereas our electrophysiological studies show that the targeted deletion of both of these proteins results in loss in rhythmicity of firing and a decrease in the peak rate of firing of SCN neurons. These findings are consistent with the view that changes in the SCN are responsible for the loss of circadian rhythms in the DKO mice.
The synchronous firing of neurons in the SCN requires the release of different neurotransmitters such as vasoactive intestinal peptide (VIP) (3,4,5,6). IA-2 and IA-2β are transmembrane proteins on both dense core and synaptic vesicles. The targeted deletion of these proteins results in a decrease in the secretion of a variety of hormones (e.g., insulin, luteinizing hormone, and follicle-stimulating hormone) and neurotransmitters (e.g., epinephrine, dopamine, and serotonin) due to a decrease in the number and cargo content of secretory vesicles (19). These changes in number and content are thought to be the result of a decrease in the stability of secretory vesicles in the DKO mice (19). The decrease in hormone and neuroendocrine secretion in the DKO mice has been shown to produce a variety of phenotypic changes ranging from abnormal glucose tolerance tests to female infertility (12, 13), as well as behavioral and learning abnormalities (14). Based on these findings, a likely explanation for the alterations in SCN neuronal firing and the loss of physiological rhythmicity is that the deletion of IA-2/IA-2β impairs the secretion of neurotransmitters in the SCN that are required for cell-to-cell communication.
The rapidly diminishing MUA and SUA activity in the SCN of DKO mice is similar to that observed in mice with impaired VIP neurotransmission (6, 18), as well as that seen in the SCN of mice lacking expression of key molecular elements of the SCN clock, the cryptochrome genes (20). This rapid decrease in activity is unlikely to be due to deteriorating tissue, because robust SCN electrical and molecular activities can be reevoked by application of depolarizing stimuli (6, 21). Thus, the loss of overt MUA and SUA rhythms in the IA-2/IA-2β-deficient SCN is most probably due to rapid desynchronization among single SCN neurons, resulting in reduced amplitude oscillations at the population level, as well as a reduction in the rhythmicity of individual SCN neurons.
The severe alteration of rhythms in DKO mice when housed in an LD cycle suggests that these mice have additional deficits in circadian regulation. VIP-deficient mice respond to light-dark cycles, despite altered SCN rhythms (22). Furthermore, mice with targeted disruption of genes necessary for maintenance of circadian behavioral rhythms in constant darkness are nevertheless rhythmic when studied in a light-dark cycle (23,24,25,26,27,28,29). This imposed rhythmicity is primarily due to the acute effects of ambient lighting on behavior, a phenomenon referred to as masking. That the DKO mice do not exhibit rhythmicity even when housed in a light-dark cycle suggests an alteration in masking, which could involve either alterations in the input pathways from the retina to the SCN or alterations in output pathways downstream of the SCN. The severe alterations in circadian rhythms in DKO mice also might contribute to some of the previously reported secretory and behavioral impairments observed in the DKO mice.
The IA-2/IA-2β DKO mouse represents a useful model for the study of circadian rhythmicity. Circadian rhythms are cell-autonomous, but an important role is played by neurotransmitter-mediated intercellular communication within the SCN (5, 6). Because of the widespread distribution of IA-2 and IA-2β in neuroendocrine cells, the absence of these proteins in DKO mice may affect circadian pathways at more than one level and thereby make it possible to identify and evaluate the relative role of different neurotransmitters by in vivo and in vitro reconstitution experiments. The fact that deletion of the IA-2 and IA-2β genes, which are not rhythmically expressed, can so profoundly affect circadian rhythms raises the possibility that other proteins that regulate secretory vesicle secretion and trafficking also might have a major influence on rhythmicity. In this regard, it is noteworthy that mice with a deletion of chromogranin A, another constituent of secretory vesicles, also show absence of circadian blood pressure variation (30). These findings point to the possibility that in humans, mutations in one or more of the many noncircadian structural proteins of secretory vesicles may similarly affect circadian timing and, in turn, a variety of other physiological and behavioral functions.
Supplementary Material
Acknowledgments
This work was supported by the intramural research programs of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Dental and Craniofacial Research, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, and by project grants from the Biotechnology and Biological Sciences Research Council (UK) to H.D.P. and NIH grant NS056125 to D.R.W. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interest.
References
- Dunlap J C, Loros J J, DeCoursey P J. Sunderland, MA, USA: Sinauer Associates; ChronobiologyBiological Timekeeping. 2004 [Google Scholar]
- Reppert S M, Moore R Y, Klein D C. New York.: Oxford University Press; Suprachiasmatic NucleusThe Mind’s Clock. 1991 [Google Scholar]
- Brown T M, Piggins H D. Electrophysiology of the suprachiasmatic circadian clock. Prog Neurobiol. 2007;82:229–255. doi: 10.1016/j.pneurobio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Reppert S M, Weaver D R. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Aton S J, Colwell C S, Harmar A J, Waschek J, Herzog E D. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T M, Hughes A T, Piggins H D. Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signaling. J Neurosci. 2005;25:11155–11164. doi: 10.1523/JNEUROSCI.3821-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan M S, Wasserfall C, Maclaren N K, Notkins A L. IA-2, a transmembrane protein of the protein tyrosine phosphatase family, is a major autoantigen in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1996;93:6367–6370. doi: 10.1073/pnas.93.13.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Li Q, Xie H, Chen Z J, Borovitskaya A E, Maclaren N K, Notkins A L, Lan M S. Identification of a second transmembrane protein tyrosine phosphatase, IA-2β, as an autoantigen in insulin-dependent diabetes mellitus: precursor of the 37-kDa tryptic fragment. Proc Natl Acad Sci U S A. 1996;93:2307–2311. doi: 10.1073/pnas.93.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins A L, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest. 2001;108:1247–1252. doi: 10.1172/JCI14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki K, Zhu M, Kubosaki A, Xie J, Lan M S, Notkins A L. Targeted disruption of the protein tyrosine phosphatase-like molecule IA-2 results in alterations in glucose tolerance tests and insulin secretion. Diabetes. 2002;51:1842–1850. doi: 10.2337/diabetes.51.6.1842. [DOI] [PubMed] [Google Scholar]
- Kubosaki A, Gross S, Miura J, Saeki K, Zhu M, Nakamura S, Hendriks W, Notkins A L. Targeted disruption of the IA-2β gene causes glucose intolerance and impairs insulin secretion but does not prevent the development of diabetes in NOD mice. Diabetes. 2004;53:1684–1691. doi: 10.2337/diabetes.53.7.1684. [DOI] [PubMed] [Google Scholar]
- Kubosaki A, Nakamura S, Notkins A L. Dense core vesicle proteins IA-2 and IA-2β: metabolic alterations in double knockout mice. Diabetes. 2005;54:S46–S51. doi: 10.2337/diabetes.54.suppl_2.s46. [DOI] [PubMed] [Google Scholar]
- Kubosaki A, Nakamura S, Clark A, Morris J F, Notkins A L. Disruption of the transmembrane dense core vesicle proteins IA-2 and IA-2β causes female infertility. Endocrinology. 2006;147:811–815. doi: 10.1210/en.2005-0638. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Kubosaki A, Ito Y, Notkins A L. Disturbances in the secretion of neurotransmitters in IA-2/IA-2β null mice: changes in behavior, learning and lifespan. Neuroscience. 2009;159:427–437. doi: 10.1016/j.neuroscience.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S M, Mizel D, Huang Y G, Briggs J P, Schnermann J. Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol. 2006;290:F1016–F1023. doi: 10.1152/ajprenal.00367.2005. [DOI] [PubMed] [Google Scholar]
- Shearman L P, Zylka M J, Weaver D R, Kolakowski L F, Jr, Reppert S M. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- Brown T M, Banks J R, Piggins H D. A novel suction electrode recording technique for monitoring circadian rhythms in single and multiunit discharge from brain slices. J Neurosci Methods. 2006;156:173–181. doi: 10.1016/j.jneumeth.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Brown T M, Colwell C S, Waschek J A, Piggins H D. Disrupted neuronal activity rhythms in the suprachiasmatic nuclei of vasoactive intestinal polypeptide-deficient mice. J Neurophysiol. 2007;97:2553–2558. doi: 10.1152/jn.01206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima S, Clark A, Christie M R, Notkins A L. The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. Proc Natl Acad Sci U S A. 2005;102:8704–8709. doi: 10.1073/pnas.0408887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albus H, Bonnefont X, Chaves I, Yasui A, Doczy J, van der Horst G T, Meijer J H. Cryptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Curr Biol. 2002;12:1130–1133. doi: 10.1016/s0960-9822(02)00923-5. [DOI] [PubMed] [Google Scholar]
- Maywood E S, Reddy A B, Wong G K, O'Neill J S, O'Brien J A, McMahon D G, Harmar A J, Okamura H, Hastings M H. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Hughes A T, Fahey B, Cutler D J, Coogan A N, Piggins H D. Aberrant gating of photic input to the suprachiasmatic circadian pacemaker of mice lacking the VPAC2 receptor. J Neurosci. 2004;24:3522–3526. doi: 10.1523/JNEUROSCI.5345-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst G T, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker A P, van Leenen D, Buijs R, Bootsma D, Hoeijmakers J H, Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- Vitaterna M H, Selby C P, Todo T, Niwa H, Thompson C, Fruechte E M, Hitomi K, Thresher R J, Ishikawa T, Miyazaki J, Takahashi J S, Sancar A. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci U S A. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger M K, Wilsbacher L D, Moran S M, Clendenin C, Radcliffe L A, Hogenesch J B, Simon M C, Takahashi J S, Bradfield C A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun Z S, Eichele G, Bradley A, Lee C C. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood E S, Hastings M H, Reppert S M, Weaver D R. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Bae K, Weaver D R. Transient, light-induced rhythmicity in mPer-deficient mice. J Biol Rhythms. 2007;22:85–88. doi: 10.1177/0748730406296718. [DOI] [PubMed] [Google Scholar]
- DeBruyne J P, Weaver D R, Reppert S M. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra N R, O'Connor D T, Vaingankar S M, Hikim A P, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy B P, Ziegler M G, Ross J, Mahata S K. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.