Abstract
MicroRNAs provide a formidable tool not only in cancer research but also to investigate physiological mechanisms and to assess the effect of environmental exposures in healthy tissues. Collectively, cigarette smoke and sunlight have been estimated to account for 40% of all human cancers, and not only smoke but also, surprisingly, UV light induced genomic and postgenomic alterations in mouse lung. Here we evaluated by microarray the expression of 484 microRNAs in the lungs of CD-1 mice, including newborns, postweanling males and females, and their dams, either untreated or exposed to environmental cigarette smoke and/or UV-containing light. The results obtained highlighted age-related variations in microRNA profiles, especially during the weanling period, due to perinatal stress and postnatal maturation of the lung. UV light alone did not affect pulmonary microRNAs, whereas smoke produced dramatic changes, mostly in the sense of down-regulation, reflecting both adaptive mechanisms and activation of pathways involved in the pathogenesis of pulmonary diseases. Both gender and age affected smoke-related microRNA dysregulation in mice. The data presented provide supporting evidence that microRNAs play a fundamental role in both physiological and pathological changes occurring in mouse lung.—Izzotti, A., Calin, G. A., Vernon E. St., Croce, G. M., De Flora, S. Relationships of microRNA expression in mouse lung with age and exposure to cigarette smoke and light.
Keywords: lung cancer, molecular mechanisms
Micrornas (mirnas) are small, noncoding RNAs that regulate gene expression at the post-transcriptional level. MiRNAs affect a variety of developmental and physiological processes, such as stem cell differentiation, hematopoiesis, cardiac and skeletal muscle development, neurogenesis, insulin secretion, cholesterol metabolism, and the immune response (1). In addition, modulation of miRNA expression has been involved in the pathogenesis of cancer and other diseases (2), to such an extent that alterations in miRNA genes have been proposed to play a critical role in the pathogenesis of many, and perhaps all, human cancers (3). MiRNA expression profiles improve classification, diagnosis, and prognostic information in oncology (4), and miRNAs are important in cancer biology and therapy (5).
In lung cancer, which is the most common cause of cancer mortality worldwide, down-regulation of specific miRNAs has been observed in both mice and humans (6). Less attention has been paid to postgenomic changes occurring in apparently healthy respiratory tissues of subjects exposed to environmental agents. Cigarette smoke (CS), the most important human carcinogen, causes alterations of multigene expression in the lungs of mice (7, 8) and rats (9, 10) and in airway epithelial cells of humans who smoke (11). In parallel, a number of miRNAs have been shown to be dysregulated in the lungs of rats exposed to environmental cigarette smoke (ECS) (12) and in the bronchial epithelium from humans who smoke (13). Down-regulation of several miRNAs was also observed in the lungs of rats treated with typical CS components, such as the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (14). These alterations are crucial because the number of cloned miRNAs is limited (on the order of hundreds), but each of them regulates hundreds of genes by complementary binding to the 3′-untranslated region of target mRNAs (15).
The present study had several goals. The first one was to determine miRNA profiles in the lungs of variously aged mice, from birth to the end of the weanling period and then to adulthood, to explore the physiological mechanisms underlying the development of this organ. The other objective was to investigate possible alterations of miRNA expression in the lung as related to exposure of mice to environmental agents in different periods of life. In particular, we evaluated the effects of exposure of mice to ECS and UV-containing light, either individually or in combination. Collectively, CS and sunlight have been estimated to account for 40% of all human cancers (16). ECS produces a variety of molecular, biochemical, and cytogenetic alterations in rodents (17), some of which are more evident when exposure occurs early in life (18). Thus, it was of interest to evaluate whether the response to ECS in terms of modulation of miRNA expression varies depending on age and gender. Exposure to light was carried out because we had previously demonstrated that light not only produces a variety of alterations in mouse skin but also has systemic effects. Presumably, these effects are due to formation, in the irradiated skin, of unidentified genotoxic derivatives that can travel at a distance and affect other organs. In particular, studies in SKH-1 hairless mice demonstrated that exposure to light results in formation of bulky DNA adducts in both lung and bone marrow as well as in induction of cytogenetic damage in bone marrow and peripheral blood erythrocytes (19). Moreover, compared with exposure of mice to ECS only, the combined exposure to light and ECS significantly increased both lipid peroxidation products and DNA adduct levels in lungs as well as cytogenetic damage in pulmonary alveolar macrophages (19). These findings were further supported by postgenomic analyses. In fact, exposure of mice to light only resulted in overexpression of two genes in lung (catalase 1 and glutathione S-transferase Pi 1). Exposure to ECS plus light resulted in overexpression of 29 genes, compared with up-regulation of 24 genes in mice exposed to ECS only (7). The results obtained demonstrate that miRNAs play a fundamental role in the physiological changes occurring in the lung as a function of age as well in age-related dysregulation after exposure to ECS.
MATERIALS AND METHODS
Mice
A total of 26 pregnant Swiss CD-1 albino mice were purchased from Harlan Italy (San Pietro al Natisone, Udine, Italy). The mice were housed individually in Makrolon cages on sawdust bedding and maintained on standard rodent chow (MIL; Morini, S. Polo d’Enza, Italy) and tap water ad libitum. The cages were kept in a cabinet in which filtered air was circulated. The temperature of the animal room was 23 ± 2°C, with a relative humidity of 55%, ventilation accounting for 15 air renewal cycles/h, and a 12-h day-night cycle. The housing and treatments of mice were in accordance with our national and institutional guidelines and the National Institutes of Health guidelines and were approved by the Italian Ministry of Health.
Design of the study
Each dam generated an average of 10 pups/litter, accounting for a total of 260 newborns. Eight newborns were sacrificed after birth, and their lungs were collected. All treatments of the other neonatal mice and their dams started within 12 h after birth. After 5 wk, 40 postweanling mice and all dams were deeply anesthetized with diethyl ether and killed by cervical dislocation. All remaining mice were kept alive for a carcinogenicity study, which is still in progress.
In particular, in addition to the group of 8 untreated mixed-gender newborns, the lungs were collected from mice belonging to variously aged and variously treated mice, as follows: sham-exposed mice—10 postweanling mice (5 males and 5 females) and 7 dams, kept in filtered air; ECS-exposed mice—10 postweanling mice (5 males and 5 females) and 7 dams, exposed to ECS only; light-exposed mice—10 postweanling mice (5 males and 5 females) and 5 dams, exposed to light only; and ECS plus light-exposed mice—10 postweanling mice (5 males and 5 females) and 5 dams, exposed to both ECS and light.
Exposure of mice to ECS
Neonatal mice were exposed to ECS for 5 wk, corresponding to the weanling period. Their dams were kept in the same cages during the exposure periods. ECS was generated by burning Kentucky 2R4F reference cigarettes (Tobacco Research Institute, University of Kentucky, Lexington, KY, USA), having a declared content of 9.2 mg of tar and 0.8 mg of nicotine each. Whole-body exposure of mice to ECS was achieved by using a smoking machine (model TE-10; Teague Enterprises, Davis, CA, USA). The machine was adjusted to produce a combination of sidestream smoke (89%) and mainstream smoke (11%), mimicking a high-dose exposure to ECS. Burning 5 cigarettes at one time yielded on average a total suspended particulate of 63.3 mg/m3 in the exposure chambers. Exposure was daily, 6 h/d divided into two rounds with a 3-h interval.
Exposure of mice to UV-containing light
For the same period of time, the neonatal mice and their dams were exposed to the light emitted by halogen quartz bulbs, incorporated into dichroic spotlight lamps (12 V, 50 W), supplied by Leuci (File, Lecco, Italy). The lamps were equipped with filters cutting UV-C light (WG 280; Schott Optics Division, Mainz, Germany). The distance from the back of the mice was ∼50 cm and yielded an illuminance level of 10,000 lux. Exposure was daily, 6 h/d.
Combined exposure of mice to ECS and light
A combined exposure of mice to ECS and light was achieved by applying the following schedule: 3 h ECS, 3 h light, 3 h ECS, and 3 h light. Light and ECS were not simultaneously applied to avoid the risk of alterations of ECS components by light (20).
RNA extraction
The lungs collected from the 72 mice belonging to the 13 experimental groups described above were pooled to obtain 39 samples (3 pools/experimental group). RNA was extracted by a TRIzol Plus RNA Purification Kit (Invitrogen, Carlsbad, CA, USA). In brief, lung samples were homogenized in TRIzol (1 ml/50–100 mg of tissue), and then 200 μl of chloroform was added to 1 ml of TRIzol, and the mixture was shaken for 10 min using a tissue lyser (Qiagen, Hilden, Germany). Samples were centrifuged at 12,000 g, the pellet was collected, and an equal volume of 70% ethanol was added and mixed thoroughly. The mixture was dispensed into an RNA spin cartridge and centrifuged at 12,000 g for 15 min, the flow-through was discarded, and the cartridge retaining the RNA was washed twice and dried by centrifugation. The RNA was then eluted by adding 50 μl of RNase-free water to the cartridge followed by centrifugation for 2 min at 12,000 g.
RNA quantification and evaluation of integrity
RNA was quantified by fiberoptic spectrophotometry (ND-1000; NanoDrop Technologies, Wilmington, DE, USA). Sample absorbance was quantified at 260 and 280 nm, and the 260/280 ratio was calculated. A 260/280 ratio >1.9 was consistently observed, which was assumed to be an indicator of RNA purity. RNA structural integrity, confirmed by the detection of fragments in the 20- to 100-bp range, was determined by capillary electrophoresis (Bioanalyzer Agilent 2100; Agilent Technologies, Santa Clara, CA, USA).
Microarray analyses
MiRNA expression was evaluated by microarray following a previously reported protocol (21). RNA (5 μg) was hybridized on a custom miRNA microarray platform containing quadruplicates of 484 mouse miRNAs, 627 human miRNAs, and 6 Arabidopsis thaliana miRNAs. The list of miRNAs included in the microarray used is available in the Gene Expression Omnibus (GEO) database (GSE13260; http://www.ncbi.nlm. nih.gov/geo/query/acc.cgi?acc=GSE13260). Microarrays contain 40-mer oligonucleotide probes, spotted by contacting technology, and covalently attached to a polymeric matrix (21). The hybridized biotinylated transcripts were detected by streptavidin-Alexa647 conjugation, scanned on an Axon 4000B microarray scanner (Axon Instruments, Union City, CA, USA), and analyzed using Genepix Pro 6.0 (Axon Instruments).
Real-time quantitative PCR (qPCR)
To confirm microarray results, miRNA let-7f was also analyzed by real-time qPCR. The miRNAs contained in 200 ng of total RNA were polyadenylated, and the first-strand cDNA was obtained by using an NCode miRNA First-Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA). Each sample (5 μl) was diluted 1:10 in water, and then PCR amplification was performed using an SYBR Green-containing mix (22). Universal qPCR primers (Invitrogen) and a specific forward primer for let-7f (TIB Molbiol, Berlin, Germany) were used. The forward primer sequence was the entire mature miRNA sequence, as previously reported (http://microrna.sanger. ac.uk/sequences). Fluorescence intensity was acquired at the annealing step of each amplification cycle, which was performed in a rotating thermocycler (Rotor-Gene 6.1.81 software; Corbett Life Sciences, Mortlake, Australia).
Analysis of data
Raw data were subjected to local background subtraction, log transformation, normalization, and analysis by using GeneSpring 7.2 software (Agilent Technologies). Expression data were median centered by using the GeneSpring normalization option, which normalizes both per gene and per array. Comparisons between treatments were done by evaluating the fold variation of the mean values of quadruplicate data generated for each miRNA. The statistical significance of the differences was evaluated by ANOVA after Bonferroni multiple testing correction. Differences with P < 0.05 were taken as statistically significant.
RESULTS
Baseline miRNA expression in mouse lung as related to age
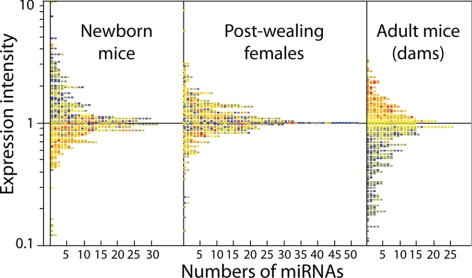
Baseline miRNA expression was compared by microarray in the lungs of mixed-gender newborns, postweanling females, and adult females. The box plot presented in Fig. 1 shows at a glance the evident changes of miRNA expression with age. In particular, Table 1 shows the fold variations of miRNA expression in newborns vs. postweanling females and in postweanling vs. adult females, along with the statistical significance level of the differences in intensity of miRNA expression recorded at different ages. Fourteen miRNAs varied to a statistically significant extent in variously aged mice.
Figure 1.
Horizontal box-plot analysis showing the distribution of the 484 miRNAs analyzed according to their intensity expression in the lung from untreated mice of various ages. Expression intensity in adult mice is on a scale color ranging from blue (lowest) to red (highest). Corresponding miRNAs keep the same color in newborn mice and postweanling females. It is apparent that miRNAs having a high expression intensity in adult mice were generally expressed at a lower level in newborns, whereas an intermediate situation was detected in postweanling females.
TABLE 1.
Fold variations of baseline miRNA expression in mouse lung, as related to age, and statistical significance of differences between either newborns vs. postweanling females or postweanling females vs. adult females
| MiRNA | Newborns/postweanling females | Postweanling females/adult females (dams) |
|---|---|---|
| miR-27b | 0.4** | 1.1 |
| miR-124a | 3.5* | 0.5* |
| miR-138 | 0.9 | 2.6** |
| miR-148 | 1.2 | 3.7* |
| miR-196 | 0.3* | 0.9 |
| miR-199a | 2.2** | 1.6 |
| miR-214 | 5.2* | 1.4 |
| miR-219 | 0.9 | 5.5** |
| miR-335 | 5.3** | 1.2 |
| miR-337 | 8.2* | 0.2* |
| miR-341 | 0.2** | 2.8** |
| miR-345 | 0.3* | 1.0 |
| miR-376 | 6.5* | 6.8** |
| miR-411 | 11.8*** | 0.9 |
Statistical significant notes indicate difference in miRNA expression between the two age groups reported in the column head.
P < 0.05;
P < 0.01;
P < 0.001.
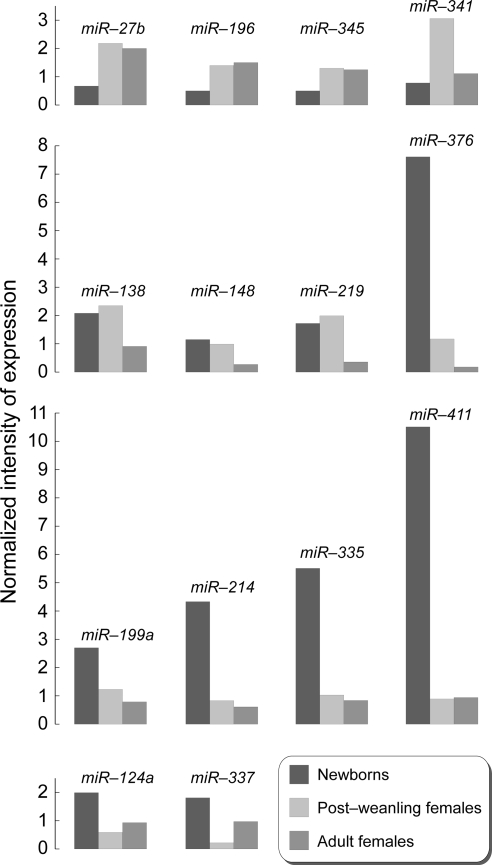
Distinctive age-related patterns can be envisaged from the observation of Table 1 and Fig. 2. Three miRNAs (miR-27b, miR-196, and miR-345) were less expressed in newborns compared with both postweanling and adult females. One miRNA (miR-341) was up-regulated during the weanling period but thereafter returned to the original levels of expression. Three miRNAs (miR-138, miR-148, and miR-219) had similar levels of expression in newborns and postweanling females but thereafter decreased in adult life. One miRNA only (miR-376) progressively decreased from the high levels recorded at birth to low levels in adulthood. Four miRNAs (miR-199a, miR-214, miR-335, and miR-411) were expressed at high levels only in newborns. Finally, two miRNAs (miR-124a and miR-337) were down-regulated during the weanling period but thereafter tended to increase again to a moderate extent.
Figure 2.
Effect of age on the expression intensity of 14 miRNAs in the lung from untreated mice. All reported miRNAs varied at least 2-fold and to a statistically significant extent among the variously aged mice.
Variations in miRNA expression in the lungs of variously aged mice as related to exposure to ECS and/or light
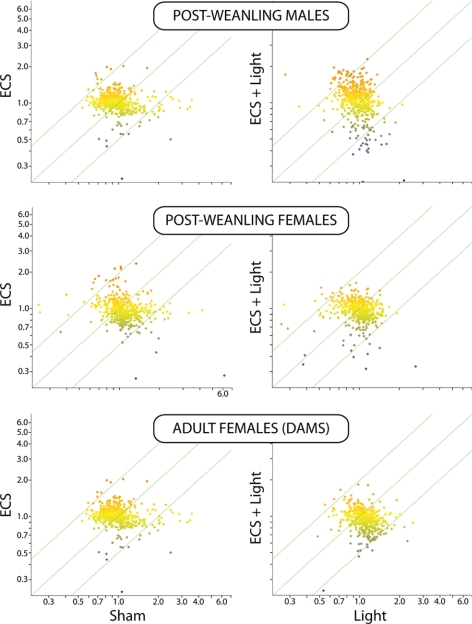
No differences in miRNA expression in the lung, as evaluated by microarray, were observed when light-exposed mice were compared with sham-exposed mice or ECS plus light-exposed mice were compared with ECS-exposed mice (data not shown). On the other hand, exposure of mice to either ECS or ECS plus light dysregulated miRNA expression compared with either sham or light. As shown in Fig. 3, miRNA alterations were prevailingly in the sense of down-regulation and were more evident in postweanling males and females exposed to ECS since birth than in adult females exposed to ECS for an equivalent period of time.
Figure 3.
Scatter plots relating the expression intensity of 484 miRNAs in the lung either of sham-exposed mice vs. ECS-exposed mice or of light-exposed mice vs. ECS plus light-exposed mice at different ages.
The general trend toward ECS-induced down-regulation of miRNA expression is supported by the finding that only down-regulated miRNAs modified their expression to a statistically significant extent. Identity and intensity of variation for the 15 miRNAs whose expression was significantly affected by exposure of mice to either ECS or ECS plus light are reported in Table 2. Although the ECS-related fold variations were statistically significant at all ages, almost invariably the effect of ECS was more pronounced in postweanling females than in their dams. Down-regulation was more pronounced in females than in males for miR-26a (P<0.01) and miR-140 (P<0.05).
TABLE 2.
MiRNAs that were down-regulated at least 2-fold and to a statistically significant extent in mouse lung
| MiRNA | Postweanling males
|
Postweanling females
|
Adult females (dams)
|
|||
|---|---|---|---|---|---|---|
| Sham/ECS | Light/ECS + light | Sham/ECS | Light/ECS + light | Sham/ECS | Light/ECS + light | |
| let-7a | 2.0** | 2.2** | 2.2* | 2.0* | 2.0* | 2.1* |
| let-7b | 2.0** | 1.9** | 2.4*** | 2.2* | 2.0* | 2.1* |
| let-7f | 2.7* | 2.6* | 3.0** | 2.8** | 2.3* | 2.0* |
| miR-26a | 2.6* | 2.8* | 4.3* | 3.8** | 2.8* | 2.8* |
| miR-30b | 2.0* | 2.2* | 2.1* | 2.3* | 2.0* | 2.0* |
| miR-30c | 2.0** | 2.3* | 2.4* | 2.2* | 1.7* | 1.9* |
| miR-34b | 1.9* | 1.9* | 1.9* | 2.2* | 1.7* | 1.9* |
| miR-99b | 2.0* | 2.0* | 2.2* | 2.0* | 1.8* | 1.6* |
| miR-122a | 2.0* | 1.9* | 2.1* | 1.9* | 2.1* | 2.0* |
| miR-124a | 1.9** | 2.2** | 2.3** | 2.4** | 1.6* | 1.7* |
| miR-125a prec | 2.1** | 2.0* | 2.2** | 2.2** | 2.0* | 1.8* |
| miR-125b | 2.1* | 2.3* | 2.1* | 2.1* | 2.0* | 2.1* |
| miR-140 | 3.1** | 2.6* | 3.3* | 3.4** | 2.1* | 2.4* |
| miR-192 | 2.1* | 1.9** | 2.2* | 2.0* | 2.0* | 1.9* |
| miR-431 | 2.2* | 2.1* | 2.1* | 2.0* | 2.1* | 1.8* |
Reported values are fold-variations of miRNA expression in mice of various age and gender, either sham-exposed or ECS-exposed or exposed to both ECS and light. Statistical significant notes indicate difference in miRNA expression between the two age groups reported in the column heading.
P < 0.05;
P < 0.01;
P < 0.001.
Analysis by qPCR of let-7f expression in mouse lung
To validate microarray data, we analyzed let-7f expression by qPCR in the lungs of postweanling males, either sham-exposed or ECS-exposed. This miRNA was selected for further analysis because it was one of the most potently down-regulated miRNAs by ECS, as assessed by microarray (Table 2) and because of its crucial role in regulating key biological functions involved in pulmonary carcinogenesis. qPCR analyses, run in 6 replicates, confirmed that exposure of mice to ECS induces a down-regulation of let-7f expression, as inferred from the increase of the positivity threshold of qPCR curves (data not shown). The sham/ECS fold variation in signal intensity averaged 2.7.
Identification of the main functions of miRNA affected either by age and/or exposure to ECS
The biological functions of the miRNAs whose expression was affected either by age and/or by exposure to ECS were scrutinized by taking into account the context of the present study. They were inferred by selecting miRNA target genes having a context score >0.31, as reported in Targetscan (http://www.targetscan.org). Miranda (http://www.microrna.org) and Sanger Institute (http://www.microrna.sanger.ac.uk) databases were also examined, along with the pertinent literature available in PubMed.
The results of these functional analyses are reported in Table 3. The biological roles of the affected miRNAs encompass a multitude of functions, both physiological and adaptive. They include morphogenesis, development of airway epithelial cells, extracellular matrix and formation of pulmonary surfactant, inflammation, modulation of oncogenes and tumor suppressor genes, signaling of growth factors, regulation of cell proliferation and differentiation, apoptosis, protein repair and transport, stress response, regulation of transcription, and angiogenesis.
TABLE 3.
Main functions regulated by miRNAs whose expression was affected either by age and/or by treatment with ECS or ECS + light
| MiRNA | Regulated functions |
|---|---|
| let-7a | Cell proliferation, oncogene (Ras) activation, angiogenesis |
| let-7b | Cell proliferation, oncogene (Ras) activation, angiogenesis |
| let-7f | Cell proliferation, oncogene (Ras) activation, angiogenesis |
| miR-26a | Transforming growth factor expression |
| miR-27b | Cell proliferation, embryogenesis, protein repair, stress response |
| miR-30b | Cell adhesion, protein repair, stress response (NF-κB activation) |
| miR-30c | Cell cycle, oncogene (EGF) activation |
| miR-34b | P53 effector |
| miR-99b | Apoptosis |
| miR-122a | Stress response |
| miR-124a | Stress response, cell growth and differentiation, oncogenic transformation |
| miR-125a prec | Oncogene (ERBB2) activation |
| miR-125b | Stress response |
| miR-138 | Transcriptional regulation, cell proliferation |
| miR-140 | P53 effector |
| miR-148 | Growth factor receptors, protein repair, apoptosis, transcriptional regulation |
| miR-192 | Oncogene (Ras) activation |
| miR-196 | Embryogenesis, cell proliferation |
| miR-199a | Morphogenesis, embryogenesis, stress response (NF-κB activation), protein repair |
| miR-214 | Apoptosis, airway epithelial cells proliferation, extracellular matrix remodelling, ATPase and calcium homeostasis, protein repair |
| miR-219 | Protein repair, transcriptional regulation, growth factor receptors |
| miR-335 | Cell proliferation and differentiation, airway epithelial cells proliferation, protein repair, stress response |
| miR-337 | Growth factor signaling, apoptosis, protein repair, embryogenesis, cell proliferation |
| miR-341 | Protein transport, Ras-related pathways activation |
| miR-345 | Cell proliferation, transcriptional regulation, angiogenesis |
| miR-376 | Inflammation, protein repair, embryogenesis, cell proliferation, formation of pulmonary surfactant |
| miR-411 | Protein repair, Ras-related pathways activation |
| miR-431 | Protein repair, oncogene (Ras) activation |
DISCUSSION
The results of the present study provide, for the first time, information regarding both the baseline miRNA expression in the mixed-cell population of mouse lung, as related to different developmental stages during postnatal life, from birth to adulthood, and the effects of exposure to environmental agents at different ages.
The major physiological age-related changes occurred during the weanling period. At birth, because of the sudden transition form the maternal-mediated respiration of the fetus to the autonomous pulmonary respiration of the newborn, there is tremendous oxidative stress in the lung. By comparing newborn lung with fetal lung in untreated mice, we demonstrated the occurrence of important changes at both genomic and postgenomic levels (23). In fact, in the very few hours elapsing from prenatal to postnatal life, a 5-fold increase in bulky DNA adduct levels and a 2-fold increase in oxidatively generated DNA damage occurred. DNA alterations were accompanied by overexpression of several genes, mainly involved in the stress response (23). Affymetrix analyses confirmed that a large number of genes are differently expressed in mouse fetal lung compared with postnatal lung (24).
The data reported herein show that the observed variations in miRNA expression are linked to perinatal stress in the lung and to the physiological maturation of this organ. In fact, the 11 miRNAs that varied significantly from birth to the end of the weanling period are involved in regulation of important physiological functions early in life. Five of them (miR-27b, miR-196, miR-199a, miR-337, and miR-376) are involved in embryogenesis and morphogenesis, thus reflecting the fact that development and maturation of the lung are completed after birth. Cell proliferation and differentiation contribute to lung maturation, as shown by changes in the expression of 7 miRNAs sharing this function (miR-27b, miR-124a, miR-196, miR-335, miR-337, miR-345, and miR-376). Some miRNAs specifically affect the proliferation of airway epithelial cells (miR-214 and miR-235), extracellular matrix remodeling (miR-214), and formation of pulmonary surfactant (miR-376), which is particularly rich in antioxidants, such as reduced glutathione, vitamin C, vitamin E, superoxide dismutase, catalase, albumin, ceruloplasmin, transferrin, lactoferrin, and other proteins (25). In parallel, the expression of miRNAs targeting oncogene transformation (miR-124a) and, in particular, Ras activation (miR-341 and miR-411), varied early in life. The response to stress is an important determinant in all of the observed changes, as supported by the finding that other altered miRNAs targeted stress response mechanisms (miR-124a and miR-335), with particular reference to NF-κB (miR-199a), as well as inflammation (miR-376), apoptosis (miR-214 and miR-337) and angiogenesis (miR-341). Thus, it appears that perinatal stress involves adaptive mechanisms that contribute to the full development of the lung in term of tissue growth and remodeling, cell differentiation, and physiological vascularization.
Less remarkable variations were observed from the end of the weanling period (∼35 d) to young adulthood (∼75 d) in female mice. The ontogeny of the 7 miRNAs that varied significantly from 35 to 75 d of age suggests that stress response no longer plays a major role as a driving force for further maturation of the lung. However, some changes in miRNA expression did still occur during this period, mainly regarding protein repair transport (miR-148, miR-219, miR-337, miR-341, and miR-376), cell proliferation, growth, and differentiation (miR-124a, miR-138, miR-148, miR-219, miR-337, and miR-376) and, to a more limited extent, inflammation (miR-376), apoptosis (miR-148 and miR-337), and activation of oncogenes (miR-124a and miR-341). It is noteworthy that 3 miRNAs involved in transcription regulation (miR-138, miR-148, and miR-219), all of which had the same expression intensity in newborns and postweanling females, were less expressed in young adult females, which suggests a late change in post-transcriptional regulation mechanisms.
Thus, on the whole, our results provide evidence that important variations in miRNA expression do occur physiologically in the lung during the early postnatal period of nutritional dependence from dams. These variations tend to become more attenuated during the shift to young adulthood. Interestingly, it has been reported that the expression of 256 miRNAs is unchanged in mouse lung during further transition from adulthood (180 d) to old age (540 d) (26).
Exposure to UV-containing light, either during the weanling period or for an equivalent period of time during adulthood, had no detectable effect on miRNA expression in lung. The observed lack of alterations of miRNA expression in the lung of light-exposed albino mice suggests that the genomic and postgenomic changes observed in previous studies in hairless mice (7, 19) do not result in dysregulation of post-transcriptional mechanisms, at least with the battery of miRNAs analyzed. It should be taken into account, however, that the skin of hairless mice is less protected from UV light compared with that of albino mice.
Conversely, exposure of mice either to ECS or ECS plus light resulted in dramatic changes in the miRNA domain, as indicated by a significant dysregulation of 15 miRNAs, which was consistently in the sense of down-regulation. Part of the observed changes in miRNA expression can be interpreted as adaptative mechanisms aimed to attenuate the effect of ECS, whereas other changes are likely to be involved in the pathogenesis of ECS-related pulmonary diseases. Some specific targets can be envisaged. For instance, several down-regulated miRNAs are involved in stress response (miR-122a, miR-124a, and miR-125b), with special reference to NF-κB (miR-30b), protein repair (miR-30b and miR-431), and apoptosis (miR-99b). Previous studies in rodents have demonstrated that ECS is a potent inducer of apoptosis in pulmonary alveolar macrophages and in the bronchial/bronchiolar epithelium (27, 28). Many other ECS-down-regulated miRNAs are involved in cell proliferation (let-7a, miR-30b, miR-30c, miR-124a, miR-219, and miR-376), which is known to be stimulated in the respiratory tract of ECS-exposed rodents (18, 27). Despite the fact that miRNA profiles were evaluated after only 4 wk of exposure to ECS, some mechanisms involved in the carcinogenetic process were found to be affected in mouse lung. These included miRNA down-regulation, resulting in activation of oncogenes and growth factors, such as Ras, ERBB2, EGF, and TGF (let-7b, miR-26a, miR-30c, miR-124a, miR-125a, miR-192, and miR-431) as well as effectors of oncosuppressor genes, such as P53 (miR-34b and miR-140). let-7 acts as an oncosuppressor gene in the lung and reduces tumor growth in mouse models of lung cancer (29). Previously, we had demonstrated that the expression of the K-ras gene was significantly increased in mouse lung after 4 wk of exposure to ECS (30) and that P53 inactivation plays a role in ECS-induced pulmonary carcinogenesis (8, 31). In addition, exposure of mice to ECS resulted in down-regulation of a miRNA (let-7f) involved in angiogenesis. This finding is consistent with our previous data indicating that ECS up-regulates the expression of genes encoding for angiogenin, angiopoietin, and vascular endothelial growth factors (VEGF-A and VEFG-B) in mouse lung (8) and increases the amounts of endothelin 1 receptor protein in rat lung (10).
In general, miRNA alterations were more evident in postweanling males and females exposed to ECS since birth than in their dams exposed to ECS during adulthood. The two miRNAs that were most strikingly down-regulated in postweanling females, compared with adult females, were miR-26a and miR-140, which regulate TGF expression and P53 pathways, respectively. These mechanisms contribute to explaining the high susceptibility of mice exposed to carcinogens early in life. Postweanling mice exposed to ECS since birth have an increased susceptibility to oxidatively generated DNA damage and show early histopathological alterations, such as bronchial and alveolar hyperplasias, which were not detectable in their ECS-exposed dams (18). It is noteworthy that the same two miRNAs affected by age (miR-26a and miR-140) were more effectively down-regulated in postweanling females than in postweanling males. This intergender difference is consistent with the finding that females are more susceptible than males to the induction of lung tumors, at least after exposure to mainstream CS since birth (32). A further contribution to the interindividual variability in response to ECS is likely to be related to miRNA gene polymorphisms. In fact, the human homologs of four ECS-down-regulated miRNAs (let-7a, miR-124a, miR-125a, and miR-140) are known to undergo genetic polymorphisms (33, 34).
The influence of exposure to cigarette smoke on miRNA profiles in the respiratory tract had previously been investigated by us in the lungs of Sprague-Dawley rats exposed to ECS (12) and, in another laboratory, in the bronchial epithelium of humans who smoked (13). Exposure conditions to ECS and the battery of 484 miRNAs analyzed by microarray in rats were identical to those used in mice. Schembri et al. (13) compared miRNA expression in 10 smokers and 9 never smokers by using a battery of 467 miRNAs analyzed by microarray. All three studies came to the same conclusion: that the predominant effect of exposure to smoke is down-regulation of miRNAs, which was observed for 23 miRNAs (4.9%) in humans (13), 24 miRNAs (5.0%) in rats (12), and 15 miRNAs (3.1%) in mice (this study). In contrast, a smoke-related up-regulation was observed for just 5 miRNAs (1.1%) in humans (13), 1 miRNA (0.2%) in rats (12), and none in mice (this study). The results obtained largely overlap in mice and rats, although in general the ECS/sham fold variations were much more pronounced in rats than in mice. In particular, 13 miRNAs that were down-regulated in mouse lung were also down-regulated in rat lung. Two miRNAs (miR-30b and miR-431) were down-regulated in mice only, whereas 11 miRNAs (let-7c, miR-10a, miR-30a, miR-34c, miR-123, miR-145, miR-146, miR-191, miR-219, miR-222, and miR-223) were down-regulated in rats only (12). Three families of miRNAs that were down-regulated in both mice and rats (miR-30, miR-99, and miR-125) and two families of miRNAs that were down-regulated in rats only (miR-146 and miR-223) were also found to be down0regulated in humans (13). The most strongly down-regulated miRNA in humans who smoked was miR-218 (13). This miRNA was slightly, but significantly (1.8-fold, P<0.05), down-regulated in rats (12) and in postweanling female mice (1.7-fold, P<0.05), whereas the decrease in adult female mice (1.3-fold) was not statistically significant (this study).
In conclusion, the results obtained provide evidence that miRNAs play a fundamental role in both physiological and pathological changes occurring in mouse lung. Regulation of miRNAs appears to provide an essential contribution to the postnatal development and maturation of the lung. Moreover, pulmonary miRNAs react to environmental agents, and, in particular, to cigarette smoke, the most important human carcinogen, both by triggering adaptive mechanisms and by activating a variety of pathways involved in the pathogenesis of pulmonary diseases. The observed patterns highlight the role of both epigenetic and genotoxic mechanisms in the determinism of smoke-related diseases. Both gender and especially age affect the intensity of miRNA dysregulation in response to environmental agents.
Acknowledgments
This study was supported by the National Cancer Institute (contract N01-CN 53301).
References
- Williams A E. Functional aspects of animal microRNAs. Cell Mol Life Sci. 2008;65:545–562. doi: 10.1007/s00018-007-7355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin J. MicroRNAs make big impression in disease after disease. Science. 2008;319:1782–1784. doi: 10.1126/science.319.5871.1782. [DOI] [PubMed] [Google Scholar]
- Calin G A, Croce C M. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66:7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- Jay C, Nemunaitis J, Chen P, Fulgham P, Tong A W. MiRNA profiling for diagnosis and prognosis of human Cancer. DNA Cell Biol. 2007;26:293–300. doi: 10.1089/dna.2006.0554. [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack F J. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Liu X, Sempere L F, Galimberti F, Freemantle S J, Black C, Dragnev K H, Ma Y, Fiering S, Memoli V, Li H, DiRenzo J, Korc M, Cole C N, Bak M, Kauppinen S, Dmitrovsky E. Uncovering growth-suppressive microRNAs in lung cancer. Clin Cancer Res. 2009;15:1177–1183. doi: 10.1158/1078-0432.CCR-08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Cartiglia C, Longobardi M, Balansky RM, D'Agostini F, Lubet R A, De Flora S. Alterations of gene expression in skin and lung of mice exposed to light and cigarette smoke. FASEB J. 2004;18:1559–1561. doi: 10.1096/fj.04-1877fje. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Cartiglia C, Longobardi M, Bagnasco M, Merello A, You M, Lubet R A, De Flora S. Gene expression in the lung of p53 mutant mice exposed to cigarette smoke. Cancer Res. 2004;64:8566–8572. doi: 10.1158/0008-5472.CAN-04-1420. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Bagnasco M, Cartiglia C, Longobardi M, Camoirano A, Tampa E, Lubet R A, De Flora S. Modulation of multigene expression and proteome profiles by chemopreventive agents. Mutat Res. 2005;591:212–223. doi: 10.1016/j.mrfmmm.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Bagnasco M, Cartiglia C, Longobardi M, Balansky R M, Merello A, Lubet R A, De Flora S. Chemoprevention of genome, transcriptome, and proteome alterations induced by cigarette smoke in rat lung. Eur J Cancer. 2005;41:1864–1874. doi: 10.1016/j.ejca.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, Palma J, Brody J S. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci U S A. 2004;101:10143–10148. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Calin G A, Arrigo P, Steele V E, Croce C M, De Flora S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J. 2009;23:806–812. doi: 10.1096/fj.08-121384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schembri F, Sridhar S, Perdomo C, Gustafson A M, Zhang X, Ergun A, Lu J, Liu G, Zhang X, Bowers J, Vaziri C, Ott K, Sensinger K, Collins J J, Brody J S, Getts R, Lenburg M E, Spira A. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:2319–2324. doi: 10.1073/pnas.0806383106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalscheuer S, Zhang X, Zeng Y, Upadhyaya P. Differential expression of microRNAs in early-stage neoplastic transformation in the lungs of F344 rats chronically treated with the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis. 2008;29:2394–2399. doi: 10.1093/carcin/bgn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Higginson J, Muir C S. Environmental carcinogenesis: misconceptions and limitations to cancer control. J Natl Cancer Inst. 1979;63:1291–1298. [PubMed] [Google Scholar]
- De Flora S, D'Agostini F, Izzotti A, Zanesi N, Croce C M, Balansky R. Molecular and cytogenetical alterations induced by environmental cigarette smoke in mice heterozygous for Fhit. Cancer Res. 2007;67:1001–1006. doi: 10.1158/0008-5472.CAN-06-3882. [DOI] [PubMed] [Google Scholar]
- De Flora S, D'Agostini F, Balansky R, Camoirano A, Cartiglia C, Longobardi M, Travaini G, Steele V E, Pesce C, Izzotti A. High susceptibility of neonatal mice to molecular, biochemical and cytogenetic alterations induced by environmental cigarette smoke and light. Mutat Res. 2008;659:137–146. doi: 10.1016/j.mrrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Balansky R M, Izzotti A, D'Agostini F, Camoirano A, Bagnasco M, Lubet R A, De Flora S. Systemic genotoxic effects produced by light, and synergism with cigarette smoke in the respiratory tract of hairless mice. Carcinogenesis. 2003;24:1525–1532. doi: 10.1093/carcin/bgg108. [DOI] [PubMed] [Google Scholar]
- De Flora S, Camoirano A, Izzotti A, D'Agostini F, Bennicelli C. Photoactivation of mutagens. Carcinogenesis. 1989;10:1089–1097. doi: 10.1093/carcin/10.6.1089. [DOI] [PubMed] [Google Scholar]
- Liu C G, Calin G A, Meloon B, Gamliel N, Sevignani C, Ferracin M, Dumitru C D, Shimizu M, Zupo S, Dono M, Alder H, Bulrich F, Negrini M, Croce C M. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc Natl Acad Sci U S A. 2004;101:9740–9744. doi: 10.1073/pnas.0403293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Piana A, Cartiglia C, Longobardi M, De Flora S. Interplay between Helicobacter pylori and host gene polymorphisms in inducing oxidative DNA damage in the gastric mucosa. Carcinogenesis. 2007;28:892–898. doi: 10.1093/carcin/bgl208. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Balansky R M, Camoirano A, Cartiglia C, Longobardi M, Tampa E, De Flora S. Birth-related genomic and transcriptional changes in mouse lung: modulation by transplacental N-acetylcysteine. Mutat Res. 2003;544:441–449. doi: 10.1016/j.mrrev.2003.05.004. [DOI] [PubMed] [Google Scholar]
- Bonner A E, Lemon W J, Devereux T R, Lubet R A, You M. Molecular profiling of mouse lung tumors: association with tumor progression, lung development, and human lung adenocarcinomas. Oncogene. 2004;23:1166–1176. doi: 10.1038/sj.onc.1207234. [DOI] [PubMed] [Google Scholar]
- Davis WB, Pacht ER. Extracellular antioxidant defenses. Crystal R G, West J B, editors. New York: Raven Press; The LungScientific Foundations. :1821–1827. [Google Scholar]
- Williams A E, Perry M M, Moschos S A, Lindsay M A. MicroRNA expression in the aging mouse lung. BMC Genomics. 2007;8:172. doi: 10.1186/1471-2164-8-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostini F, Balansky R M, Izzotti A, Lubet R A, Kelloff G J, De Flora S. Modulation of apoptosis by cigarette smoke and cancer chemopreventive agents in the respiratory tract of rats. Carcinogenesis. 2001;22:375–380. doi: 10.1093/carcin/22.3.375. [DOI] [PubMed] [Google Scholar]
- D'Agostini F, Izzotti A, Balansky R M, Bennicelli C, De Flora S. Modulation of apoptosis by chemopreventive agents. Mutat Res. 2005;591:173–186. doi: 10.1016/j.mrfmmm.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Trang P, Wiggins J F, Patrawala L, Cheng A, Ford L, Weidhaas J B, Brown D, Bader A G, Slack F J. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–764. doi: 10.4161/cc.7.6.5834. [DOI] [PubMed] [Google Scholar]
- Yao R, Wang Y, Lubet R A, You M, D'Agostini F, Izzotti A, De Flora S. K-ras mutations in lung tumors from p53 mutant mice exposed to cigarette smoke. Exp Lung Res. 2005;31:271–281. doi: 10.1080/0190214059090386. [DOI] [PubMed] [Google Scholar]
- De Flora S, Balansky R M, D'Agostini F, Izzotti A, Camoirano A, Bennicelli C, Zhang Z, Wang Y, Lubet R A, You M. Molecular alterations and lung tumors in p53 mutant mice exposed to cigarette smoke. Cancer Res. 2003;63:793–800. [PubMed] [Google Scholar]
- Balansky R, Ganchev G, Iltcheva M, Steele V E, D'Agostini F, De Flora S. Potent carcinogenicity of cigarette smoke in mice exposed early in life. Carcinogenesis. 2007;28:2236–2243. doi: 10.1093/carcin/bgm122. [DOI] [PubMed] [Google Scholar]
- Saunders M A, Liang H, Li W-H. Human polymorphism at microRNAs and microRNA target sites. Proc Natl Acad Sci U S A. 2007;104:3300–3305. doi: 10.1073/pnas.0611347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R, Pak C, Jin P. Single nucleotide polymorphism associated with mature mirR-125a alters the processing of pri-miRNA. Human Mol Gen. 2007;16:1124–1131. doi: 10.1093/hmg/ddm062. [DOI] [PubMed] [Google Scholar]