Abstract
Understanding the complex cellular and tissue mechanisms and interactions resulting in periprosthetic osteolysis requires a number of experimental approaches, each of which has its own set of advantages and limitations. In vitro models allow for the isolation of individual cell populations and have furthered our understanding of particle-cell interactions; however, they are limited because they do not mimic the complex tissue environment in which multiple cell interactions occur. In vivo animal models investigate the tissue interactions associated with periprosthetic osteolysis, but the choice of species and whether the implant system is subjected to mechanical load or to unloaded conditions are critical in assessing whether these models can be extrapolated to the clinical condition. Rigid analysis of retrieved tissue from clinical cases of osteolysis offers a different approach to studying the biologic process of osteolysis, but it is limited in that the tissue analyzed represents the end-stage of this process and, thus, may not reflect this process adequately.
Osteolysis occurs in a complex tissue environment comprised of many cell types. The periprosthetic membrane is composed of a fibrovascular stroma that includes inflammatory cells in the bone microenvironment. Although macrophages have been studied for several decades as a key cell population, recent studies clearly define interactions with numerous other cell populations as critical in the process of osteolysis.
These populations include mesenchymal stem cells (MSCs) in the bone marrow and capsular tissues, synovial fibroblasts, osteoblasts, lymphocytes, and macrophages. Macrophages are essential in the process because they respond directly to particles and secrete inflammatory mediators. The macrophages are found within the fibroblastic inflammatory membrane. Although it was previously believed that the fibrovascular stroma provided a support function, it is now clear that the fibroblast population responds directly to particles and secretes inflammatory mediators such as interleukin (IL)-6. Similarly, osteoblasts also secrete inflammatory mediators, and both fibroblasts and osteoblasts express receptor activator of nuclear factor-κB ligand (RANKL)—the factor that is required for stimulation of osteoclast formation from macrophage precursors—in response to particles.
In Vitro Models
Much of the information regarding responsiveness of various cell populations involved in osteolysis comes from cell culture studies. Osteoblast cell culture models have used a variety of sources, both human and animal-derived cell populations, and include both primary cell cultures and cell lines. Osteoblast populations should undergo differentiation in cell culture with the expression of osteoblast markers such as osteocalcin, type I collagen, and alkaline phosphatase. Furthermore, these cultures should form ossification nodules in cell culture. In addition to their inflammatory and catabolic effects, particles reduce the maturation of osteoblasts. These effects tend to be through activation of specific signaling pathways. Bone marrow osteoprogenitor cells are also adversely affected by particles.
Fibroblast cultures to examine particle effects also have been derived from numerous sources, including human foreskin and synovium (from both animals and humans) and both cell lines and primary populations. Evidence exists suggesting that synovial fibroblasts that are derived from inflamed tissues have unique properties, including genetic alterations that target genes controlling proliferation, such as p53. All of the above populations have been responsive to particles and have been used to determine mechanisms involved in cell signaling.
Macrophage cultures have been used to determine mechanisms involved in the formation of osteoclasts. Macrophages stimulated with RANKL and membrane colony stimulating factor undergo fusion and develop into osteoclasts in cell cultures. Coculture experiments with fibroblasts, osteoblasts, and MSCs confirm that these cell populations can induce osteoclast formation. Of particular importance, pretreatment of fibroblasts with particles stimulates expression of RANKL and results in osteoclast formation in fibroblast-macrophage coculture experiments. These coculture experiments mimic the more complex tissue environment in which multiple cell interactions occur.
In Vivo Models
When considering in vivo models of osteolysis, several experimental variables must be established. The choice of animal species is critical. Species that have been used in the study of particle-induced osteolysis include mice, rats, rabbits, canines, and sheep. Many of the murine studies are nonimplant models with the higher species, using both nonloaded and loaded implants. As with all in vivo experiments, a power analysis is necessary as is determination of the bone density of the species used. Each in vivo model has advantages and disadvantages. Mice have the specific advantages of relatively low cost, established and homogenous genetic background allowing for knockout and transgenic strains, and potential high throughput, especially when addressing local and systemic pharmacologic interventions. However, their small size and limited cancellous bone restricts their usefulness, especially in studying implant fixation and periprosthetic osteolysis. Larger animal models allow for more clinically relevant implants that can be subjected to appropriate physiologic loads, but their cost is often prohibitive, especially when multiple therapeutic interventions are being considered.
Mouse Models
Air Pouch Models
Several types of air pouch models have been used in which bone tissue is implanted and then undergoes resorption.1,2 Polyethylene particles are then introduced into the pouch to promote inflammation and osteolysis. This model has been characterized and used to demonstrate that adeno-associated virus-mediated osteoprotegerin gene transfer protects against bone resorption.3
Calvarial Defect Models
The murine calvarial model has served as an important in vivo surrogate to understand the biologic effects of particles and the mechanisms involved in inflammatory bone resorption. Early studies demonstrated that IL-1 injection onto the mouse calvaria4 stimulated bone resorption, demonstrating the responsiveness of this system to inflammatory mediators. Merkel et al5 first adapted this model to study the effects of particles and showed that implantation of titanium particles onto the calvaria leads to profound inflammation, osteoclast formation, and bone resorption. Schwarz et al6 developed a method to measure the bone loss quantitatively, allowing assessment of the potential of various genetic approaches and biologic agents to prevent bone loss.
A variety of laboratories using this approach have shown that inhibitors of tumor necrosis factor (TNF)-α, RANKL, and cyclooxygenase-2 prevent bone loss. The model permits use of transgenic and knockout approaches in which the role of specific genes can be assessed, offering significant advantages over other approaches, including the use of larger animal models. Other strengths include the rapidity of the development of osteolysis (about 10 days), the relatively low cost, and the ability to screen a large number of compounds and doses of various agents. However, weaknesses are related to the fact that this model represents an acute (rather than chronic) effect, the lack of an implant, and the lack of other factors likely related to osteolysis, including oscillatory fluid pressures and mechanical forces. Recently, the use of micro computed tomography has provided an additional highly quantitative method to assess the degree of bone loss.
Tibial Hemiarthroplasty Model
The tibial hemiarthroplasty model has been developed by several groups as a method of studying osteolysis. Shi et al7 have characterized this model using histologic, immunohistochemical, and scanning Fourier transform infrared (FTIR) spectroscopic analyses. Although radiographic findings associated with osteolysis are not routinely demonstrated with this model, a significant infiltration of macrophages occurs in the periprosthetic tissue with an increase in osteoclasts and Howship's lacunae and a decrease in new bone formation in the surrounding tissue when polymethylmethacrylate (PMMA) particles are introduced into the periprosthetic tissue at the time of implantation.7 Further analysis with FTIR imaging has demonstrated that the mineral-to-matrix ratio of the surrounding tissue is diminished in implants subjected to particles. Other work demonstrated that IL-6 knockout mice have an enhanced macrophage and osteoclast response when exposed to PMMA particles.8 However, despite encouraging in vitro data, thalidomide, a potent TNF-α blocker, failed to demonstrate any inhibiting effect on the osteolytic process, although it did demonstrate inhibition of bone formation, probably secondary to its antiangiogenic properties. Yang et al9 recently reported the long-term effects of aseptic loosening with animals studied out to 6 months using a similar model.
Femoral Implant With Particles
Although tibial hemiarthroplasty in mice has the advantage of being a loaded implant model, size constraints limit the introduction of particles to the time of implantation, which clearly fails to mimic the clinical scenario of continuous exposure of the periprosthetic tissue to the particles. One method to avoid this limitation has been the use of an Alzet pump for continuous introduction of particles. Recently, Ortiz et al10 validated that particles can be introduced into the mouse femur using such an approach. Ongoing work will likely indicate that this model more accurately mimics the clinical phenotype seen in human osteolysis.
Rat Models
One of the earliest models of particle-induced osteolysis was developed in Cambridge by Allen et al.11 This model was then used to investigate the effects of alendronate on particle-induced osteolysis. Millet et al12 demonstrated that intra-articular injection of polyethylene particles caused substantial bone loss around a loaded implant and that alendronate effectively prevented the particle-induced periprosthetic bone loss.
In addition to intra-articular implants, others have used rodent models to study the effects of fluid pressure on bone in combination with wear debris particles.13 Surprisingly, fluid pressure alone was able to form osteolytic lesions displaying radiographic and histologic features similar to exposure to wear debris particles alone. Thus fluid pressure, or rather the hydrostatic pressures in the joint, must be considered in the pathophysiology of the osteolytic process.
Rabbit Models
Although not ideal in terms of size, rabbits have been used with a variety of implant systems including a tibial hemiarthroplasty.14 Rabbits are the smallest laboratory animal to have haversian systems in their bone. Using implant systems in the rabbit, several investigators have shown that placing ultra-high molecular weight polyethylene particles around an implant decreases bone formation; however, radiographic changes of osteolysis are not observed. Others have used implants to study the effect of hydroxyapatite seals around an implant in preventing polyethylene ingress into the bone-implant interface.15 The bone harvest chamber has been a useful model to delineate the effects of different particle types and concentrations on bone ingrowth and to explore the effects of different pharmacologic interventions16-21 (Figure 1).
Figure 1.
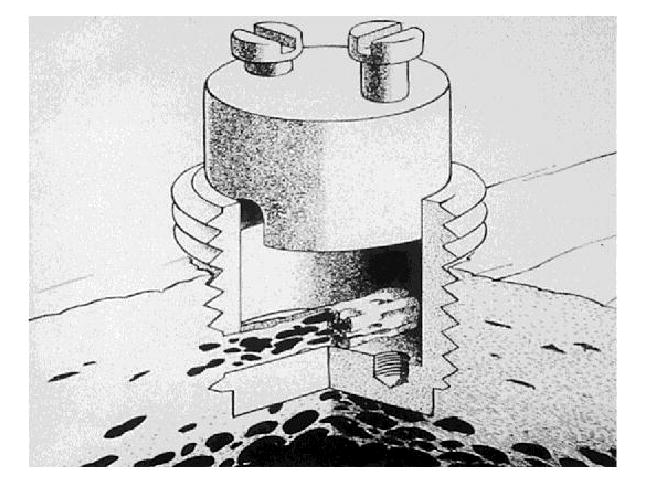
The bone harvest chamber with removable inner core and two 1-mm diameter holes allowing for continuous bone ingrowth through the inner chamber. (Reproduced with permission from Vonau RL, Bostrom MP, Aspenberg P, Sams AE: Combination of growth factors inhibits bone ingrowth in the bone harvest chamber. Clin Orthop Relat Res 2001;386:243-251.)
Canine Models
Shanbhag et al22 were some of first investigators to demonstrate inhibition of wear-induced osteolysis. In a canine total hip arthroplasty model, they demonstrated a significant reduction in implant loosening when dogs were treated with alendronate and wear debris compared to dogs treated with debris alone. However, reduction in inflammatory markers was not associated with alendronate treatment.
Another canine implant model used to study both implant fixation and osteolysis is that of Rahbek et al23 in Aarhus, Denmark. This group recently demonstrated that hydroxyapatite coatings prevent osteolysis associated with the injection of intra-articular particles.
Sheep Models
Several total hip replacement and nonloaded implant models have been developed in sheep. As with other animal models, most sheep studies use intra-articular injection of polyethylene particles.24 The advantage of this species is that the implants and the forces on the implants and surrounding bone more closely resemble that in humans.
Unfortunately, none of the animal models truly demonstrate the radiographic findings that the orthopaedic surgeon associates clinically with osteolysis. Despite this limitation, animal models remain useful because they display many of the histologic, cellular, molecular, biologic and mechanical features associated with the clinical phenotype. Furthermore, these features can often be quantified, providing useful approaches for assessing preventative or therapeutic interventions.
Tissue Retrieval Approaches
Because of limitations of both in vitro and in vivo approaches, the continued study of the biologic tissues obtained at retrieval of failed implants remains a useful adjunct to understand the mechanism of osteolysis. This approach also has limitations in that the tissue studied is from an end-stage process and as such may not accurately reflect the evolution of the biologic processes associated with osteolysis.
One of the earliest attempts at investigating the biologic effects of osteolysis was performed by Bostrom et al,25 who investigated the patterns of gene expression in fibroblasts and synovial tissues obtained from osteolytic sites of failed total hip arthroplasties. They found an upregulation of proinflammatory cytokines and destructive enzymes that correlated with monocyte infiltration and a pattern of gene activation similar to that seen in rheumatoid arthritis patients. Others have demonstrated the ability of wear debris to activate proinflammatory macrophage signaling, with macrophages and osteoclasts being the final common pathway for the bone destruction seen in osteolysis.26 More recent work using expression profiling has demonstrated the existence of an alternative activation of macrophages and impaired osteogenesis in osteolytic tissue.27
Future Directions for Research
No single experimental approach or model is capable of answering all of the biologic questions surrounding wear-induced osteolysis. However, all three approaches (in vitro, in vivo, and tissue retrieval) have proven useful and improved our understanding of this complex process. Because the clinical phenotype of osteolysis remains multifactorial and complex, it will be unlikely that any single approach will ever be able to truly replicate the clinical scenario. This does not invalidate the use of each of these models as basic principles and approaches to treatment can still employ these models and validate potential therapeutic approaches with the ultimate goal being to improve the treatment of patients with osteolysis.
Footnotes
None of the following authors or the departments with which they are affiliated has received anything of value from or owns stock in a commercial company or institution related directly or indirectly to the subject of this article: Dr. Bostrom and Dr. O'Keefe.
Contributor Information
Mathias Bostrom, Professor of Orthopaedic Surgery, Weill Medical School of Cornell University, Hospital for Special Surgery, New York, NY
Regis O'Keefe, Professor of Orthopaedic Surgery, Chairman of the Department of Orthopaedics and Rehabilitation, and Director of the Center for Musculoskeletal Research, University of Rochester, Rochester, NY
References
- 1.Wooley PH, Morren R, Andary J, et al. Inflammatory responses to orthopaedic biomaterials in the murine air pouch. Biomaterials. 2002;23:517–526. doi: 10.1016/s0142-9612(01)00134-x. [DOI] [PubMed] [Google Scholar]
- 2.Ren W, Yang SY, Wooley PH. A novel murine model of orthopaedic wear-debris associated osteolysis. Scand J Rheumatol. 2004;33:349–357. doi: 10.1080/03009740410005944. [DOI] [PubMed] [Google Scholar]
- 3.Yang SY, Mayton L, Wu B, Goater JJ, Schwarz EM, Wooley PH. Adeno-associated virus-mediated osteoprotegerin gene transfer protects against particulate polyethylene-induced osteolysis in a murine model. Arthritis Rheum. 2002;46:2514–2523. doi: 10.1002/art.10527. [DOI] [PubMed] [Google Scholar]
- 4.Garcia C, Boyce BF, Gilles J, et al. Leukotriene B4 stimulates osteoclastic bone resorption both in vitro and in vivo. J Bone Miner Res. 1996;11:1619–1627. doi: 10.1002/jbmr.5650111105. [DOI] [PubMed] [Google Scholar]
- 5.Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol. 1999;154:203–210. doi: 10.1016/s0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz EM, Benz EB, Lu AP, et al. Quantitative small-animal surrogate to evaluate drug efficacy in preventing wear debris-induced osteolysis. J Orthop Res. 2000;18:849–855. doi: 10.1002/jor.1100180602. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Yang X, Nestor BJ, et al. Histologic and FTIR studies on long term effect of PMMA particle in a murine intramedullary osteolysis model. Trans Orthop Res Soc. 2007;53:217. [Google Scholar]
- 8.Shi Y, Yang X, Nestor BJ, et al. PMMA increase bone destruction in interleukin-6 deficiency mice. Tran Orthop Res Soc. 2006;52:692. [Google Scholar]
- 9.Yang X, Hernandez-Soria A, Grewal S, Demetrakopoulus D, Ricciardi BF, Bostrom MPG. Unexpected effect of thalidomide in a particulate induced periprosthetic osteolysis mouse model: Is 8 weeks long enough? Trans Orthop Res Soc. 2006;52:703. [Google Scholar]
- 10.Ortiz SG, Ma T, Epstein NJ, Smith RL, Goodman SB. Validation and quantification of an in vitro model of continuous infusion of submicron-sized particles. J Biomed Mater Res B Appl Biomater. 2008;84:328–333. doi: 10.1002/jbm.b.30875. [DOI] [PubMed] [Google Scholar]
- 11.Allen M, Brett F, Millett P, Rushton N. The effects of particulate polyethylene at a weight-bearing bone-implant interface: A study in rats. J Bone Joint Surg Br. 1996;78:32–37. [PubMed] [Google Scholar]
- 12.Millett PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84:236–249. doi: 10.2106/00004623-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Skoglund B, Aspenberg P. PMMA particles and pressure: A study of the osteolytic properties of two agents proposed to cause prosthetic loosening. J Orthop Res. 2003;21:196–201. doi: 10.1016/S0736-0266(02)00150-X. [DOI] [PubMed] [Google Scholar]
- 14.Sacomen D, Smith RL, Song Y, Fornasier V, Goodman SB. Effects of polyethylene particles on tissue surrounding knee arthroplasties in rabbits. J Biomed Mater Res. 1998;43:123–130. doi: 10.1002/(sici)1097-4636(199822)43:2<123::aid-jbm6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Sundfeldt M, Widmark M, Johansson CB, Campbell P, Carlsson LV. Effect of submicron polyethylene particles on an osseointegrated implant: An experimental study with a rabbit patello-femoral prosthesis. Acta Orthop Scand. 2002;73:416–424. doi: 10.1080/00016470216314. [DOI] [PubMed] [Google Scholar]
- 16.Goodman SB. The effects of micromotion and particulate materials on tissue differentiation: Bone chamber studies in rabbits. Acta Orthop Scand Suppl. 1994;258:1–43. doi: 10.3109/17453679409155227. [DOI] [PubMed] [Google Scholar]
- 17.Goodman S, Trindade M, Ma T, et al. Modulation of bone ingrowth by local infusion of IL-10 in the presence of UHMWPE wear particles. J Biomed Mater Res A. 2003;65:43–50. doi: 10.1002/jbm.a.10279. [DOI] [PubMed] [Google Scholar]
- 18.Goodman SB, Song Y, Yoo JY, et al. Local infusion of FGF-2 enhances bone ingrowth in rabbit chambers in the presence of polyethylene particles. J Biomed Mater Res A. 2003;65:454–461. doi: 10.1002/jbm.a.3000. [DOI] [PubMed] [Google Scholar]
- 19.Goodman SB, Ma T, Spanogle J, et al. Effects of a p38 MAP kinase inhibitor on bone ingrowth and tissue differentiation in rabbit chambers. J Biomed Mater Res A. 2007;81:310–316. doi: 10.1002/jbm.a.30983. [DOI] [PubMed] [Google Scholar]
- 20.Ma T, Nelson ER, Mawatari T, et al. Effects of local infusion of OP-1 on particle-induced and NSAID-induced inhibition of bone ingrowth in vivo. J Biomed Mater Res A. 2006;79:740–746. doi: 10.1002/jbm.a.30949. [DOI] [PubMed] [Google Scholar]
- 21.Trindade MC, Song Y, Aspenberg P, Smith RL, Goodman SB. Proinflammatory mediator release in response to particle challenge: Studies using the bone harvest chamber. J Biomed Mater Res. 1999;48:434–439. doi: 10.1002/(sici)1097-4636(1999)48:4<434::aid-jbm6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 22.Shanbhag AS, Hasselman CT, Rubash HE. The John Charnley Award: Inhibition of wear debris mediated osteolysis in a canine total hip arthroplasty model. Clin Orthop Relat Res. 1997;344:33–43. [PubMed] [Google Scholar]
- 23.Rahbek O, Kold S, Bendix K, Overgaard S, Soballe K. Superior sealing effect of hydroxyapatite in porous-coated implants: Experimental studies on the migration of polyethylene particles around stable and unstable implants in dogs. Acta Orthop. 2005;76:375–385. [PubMed] [Google Scholar]
- 24.Coathup MJ, Blackburn J, Goodship AE, Cunningham JL, Smith T, Blunn GW. Role of hydroxyapatite coating in resisting wear particle migration and osteolysis around acetabular components. Biomaterials. 2005;26:4161–4169. doi: 10.1016/j.biomaterials.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Bostrom MP, Gomez-Barrena E, Zhang H, et al. Patterns of gene expression in fibroblasts and synovial tissue obtained from osteolysis sites of failed total hip arthroplasties. Trans Orthop Res Soc. 1996;42:499. [Google Scholar]
- 26.Chun L, Yoon J, Song Y, Huie P, Regula D, Goodman S. The characterization of macrophages and osteoclasts in tissues harvested from revised total hip prostheses. J Biomed Mater Res. 1999;48:899–903. doi: 10.1002/(sici)1097-4636(1999)48:6<899::aid-jbm20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Koulouvaris P, Ly K, Ivashkiv LB, et al. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. J Orthop Res. 2008;26:106–116. doi: 10.1002/jor.20486. [DOI] [PubMed] [Google Scholar]


