Abstract
Objective
To estimate the risks of cervical intraepithelial neoplasia-3 among women aged 13 to 24 years of age who were referred for abnormal cytology while receiving care in a large health maintenance organization.
Methods
At the time of referral, women had a colposcopic examination and biopsy if needed. Histology was sent to a centralized laboratory. Women were interviewed for risk behaviors. Data analysis included multinomial logistic regression analysis to compare 3 groups: CIN-3 to CIN-1 or less CIN-3 to CIN-2, and CIN-2 to CIN-1 or benign.
Results
CIN-3 was found in 6.6% (95% CI = 4.6 - 8.6%) of the 622 women referred and no cancers were detected. Risk for CIN 3 compared to CIN 1 or less included HPV 16 or 18 (odds ratio [OR] 30.93 [95% confidence interval (CI); 6.95, 137.65]), high-risk, non-16/18 HPV (OR 6.3 [95% CI; 1.3, 29.4]), and time on oral contraceptives (OR 1.36 per year of use [95% CI ; 1.08, 1.71]).
Conclusion
Our data support conservative care for adolescents and young women with abnormal cytology since CIN-3 was rare, and cervical cancer was never found. HPV-16 or 18 were strongly associated with for CIN-3, and testing for these types may be warranted for triage of abnormal cytology in this age group.
Introduction
Although human papillomavirus (HPV) infections are quite common in adolescents, HPV-associated cancers are extremely rare. (1, 2) This discrepancy is thought to be due to the benign nature of HPV early after infection and the requirement of long-time persistence for the development of cancer. HPV is commonly acquired shortly after the onset of sexual activity and new infections are strongly associated with new sexual partners. (3) Hence, the high rate of sexual activity and reported number of partners in adolescents explains the high rate of HPV detection in this population. (3, 4) Most of these infections in young women, however, are transient with only 5-10% persisting. (5, 6) Since HPV can be cleared even after 1-3 years of persistence, most believe that the risk of developing cancer and its precursor, cervical intraepithelial neoplasia (CIN) 3, requires at least several years of viral persistence. This lag from initial infection to cancer is supported by the prevalence data which shows that the peak of CIN 3 occurs in women aged 25-29 years approximately 5-7 years after the peak of HPV and the peak of cancer by more than another decade later. (7) Cervical cancer is rarely diagnosed under the age of 20 years. (2) In contrast to cervical cancer, data on CIN 3, encompassing both severe dysplasia and carcinoma in-situ, is not systematically collected. Consequently, the rates of CIN 3 remain somewhat vague in this age group. In a recent study of women aged 18 to 20 years, Winer et al (8) observed that some women appeared to develop CIN 3 within a few months of an incident HPV infection. Why these young women develop CIN 3 so rapidly has not been well studied.
Other than HPV persistence specifically with HPV 16 and 18, few other risks have been specific to CIN 3 development. (7) The most convincing data regarding risk is for prolonged hormonal contraceptive use and years of cigarette smoking. (9-11) It is unclear whether young women who develop CIN 3 rapidly have different risks from women who appear to develop it within a longer time frame. The primary purpose of this study is to estimate the prevalence of and risks for CIN 3 among adolescents and young women under the age of 25 years who were referred for abnormal cytology while receiving care in a large health maintenance organization (HMO), Northern California Kaiser Permanente.
Materials and Methods
Study Population
The purpose of this study was to identify adolescents and young women with CIN 1, 2, and 3 among a referral population. . All adolescents aged 13-24 years who had abnormal cervical cytologic screening while attending one of the 12 participating clinics within Kaiser Permanente Northern California (KPNC). were eligible for recruitment. Recruitment took place from 9/2002 to 6/2006. Referral for abnormal cytology included high-grade squamous intraepithelial lesions (HSIL), low-grade squamous intraepithelial lesions (LSIL), and atypical squamous cells-undetermined significance (ASC-US)/high-risk HPV positive, ASC-H (cannot exclude HSIL), or repeated ASC-US. Atypical glandular cells were not eligible for this study.
Before colposcopic examination, attempts were made to contact the patient for recruitment into the study. Study coordinators at each respective KPNC clinic would be notified of abnormal cytology through the Regional Laboratory System. Once identified, the patient would then be approached by the Primary Care Physician or Dysplasia Nurse Coordinator, depending on site. The exact number of adolescents who were not reached or had the primary care physician refuse their participation is not known since we were not able to track this data for reasons of confidentiality. Of those contacted, 80% agreed to participate. Exclusion criteria included those with previous treatment for CIN or who were immunosuppressed, pregnant, or planning to leave the area within 3 years. No demographic information was available or collected on those individuals who refused or were ineligible. Women who were contacted successfully and agreed to participate were scheduled for consent, interview and colposcopic examination by a trained site coordinator (SC) and colposcopist. At the time of the appointment, adolescent females were consented according to the guidelines of the Committee on Human Subject Research at the University of California, San Francisco and the Institutional Review Board at KPNC. This study was approved by the Committee on Human Research at University of California, San Francisco and the Institutional Review Board at Kaiser Permanente Northern California.
Using face-to-face interviews, demographic and detailed sexual behavior information was obtained. Charts at Kaiser Permanente were reviewed to collect laboratory confirmation on sexually transmitted infections (STIs). All subjects had a baseline clinic examination which included: vaginal samples for gram stain for bacterial vaginosis (12) and for normal saline and KOH wet mount examinations for evidence of trichomonas and yeast, endocervical and exocervical samples for cytology and HPV testing using liquid based media (PreservCyt™ (Cytyc Corp., Marlborough, MA); N. gonorrhoeae (NG) and C. trachomatis (CT) testing if not tested at the referral Pap visit. Routine colposcopy was performed; colposcopic lesions were biopsied and sent to each of the respective KPNC site pathology laboratory.
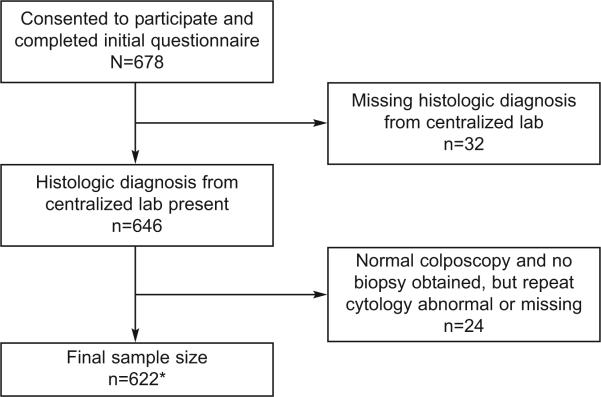
All cytology samples (PreservCyt™) at baseline were sent to the UCSF Department of Anatomic Pathology for interpretation using standard Bethesda nomenclature and criteria. Biopsies and endocervical curettage (ECC) samples were released from each of the respective KPNC site pathology laboratories and sent to the UCSF Department of Anatomic Pathology to be read by a single pathologist (Dr. Darragh). For purposes of distinguishing CIN-1, 2 and 3 lesions, histologic diagnosis were made according to the W.H.O. CIN classification. All CIN 3 diagnosis at UCSF were confirmed by a second pathologist (Dr. Ted Miller). Other diagnoses were not reviewed. For purposes of this analysis, if a woman had more than one biopsy, the most severe diagnosis was used. For women who had a normal colposcopy with no indication for biopsy or ECC and had normal cytology, the colposcopy visit was considered normal and they were included in the benign group. We excluded these women who had normal colposcopy (no biopsy) but had a repeat abnormal cytology at the time of the colposcopic examination since we lacked any histologic verification and misclassification was likely if we based their diagnosis on cytology alone (see Table 1 and Figure 1).
Table 1.
Referral diagnosis on cytology compared to histologic diagnosis
| Histology Diagnosis | ||||||
|---|---|---|---|---|---|---|
| Normal | CIN 1 | CIN 2 | CIN 3 | Total | ||
| Referral Cytology* | ||||||
| ASC-US | N | 138 | 56 | 22 | 15 | 231 |
| % | 59.74 | 24.24 | 9.52 | 6.49 | ||
| ASC-H | N | 13 | 8 | 6 | 3 | 30 |
| % | 43.33 | 26.67 | 20 | 10 | ||
| LSIL | N | 159 | 87 | 50 | 18 | 314 |
| % | 50.64 | 27.71 | 15.92 | 5.73 | ||
| HSIL | N | 5 | 2 | 1 | 3 | 11 |
| % | 45.45 | 18.18 | 9.09 | 27.27 | ||
36 missing referral diagnosis
ASC-US = atypical squamous cells of undetermined significance; includes high-risk HPV/ASCUS, repeat ASC-US, and ASC-US suggestive of LSIL.
ASC-H = ASC-US suggestive of HSIL.
LSIL = low grade squamous intra-epithelial lesion
HSIL = high grade squamous intra-epithelial lesion
CIN = cervical intra-epithelial lesion
Figure 1. Algorithm for inclusion into study.
• includes 19 subjects with normal colposcopy, no biopsy, and repeat cytology benign
The samples in PreservCyt™ media were sent to the UCSF laboratory, where 7 ml were removed for PCR under PCR sterile conditions and the remaining sent to the UCSF Anatomic Pathology laboratory for cytology processing. Testing for HPV types 6,11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54,55, 56, 58, 59, 61,62,64,66, 67,68, 69,70,71,72,73, 81,82, 83,84,IS39 and CP6108 was performed as previous described (Roche Molecular Diagnostics, Inc., Alameda, CA). (13, 14) PCR data were classified as: negative, positive for the types identified Samples, without beta-globin signal, or which were positive for 3 or more HPV types were re-prepped and retested. Samples with two negative beta-globin signals, were considered inadequate and excluded from analysis. In addition, 5% of samples chosen at random were tested induplicate.
Sample size estimates were based on HPV status. Comparison of CIN3 or CIN 2 and CIN1 or less by HPV status gave us an estimated power over 0.99 with α = 0.05 and two-sided test. Comparison of CIN3 and CIN2 gave us a lower estimated power over 0.64. For purposes of the risk analysis, the following outcome categories were used: CIN 1 or less, CIN 2 and CIN 3. Since CIN 1 is considered benign, CIN 1 and benign (referred to CIN 1 or less in the text) diagnoses were combined for analysis. Statistical analyses were performed on each of the pairs of outcomes (i.e., CIN 3 vs. CIN 1 or less, CIN 3 vs. CIN 2, CIN 2 vs. CIN 1 or less). Although CIN 3 was our primary outcome, we compared CIN 2 and CIN 1 or less and CIN 2 and CIN 3 to see if risk factors might differentiate CIN 3 vs. CIN 2. Predictor variables examined included those listed in Table 2. Reported history of STIs and vaginal infections was used instead of chart review since the chart review likely underestimated the number of infections. We report both in Table 2. We collapsed HPV status into three categories: 1) high-risk HPV with type other than HPV 16 and/or 18; 2) HPV 16/18 only; and 3) low-risk only and HPV negative. Low-risk HPV-positive and HPV negative results were pooled because they did not show any statistical significant differences between the CIN comparison groups (data not shown). High-risk HPV types included 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53,56, 58, 59, 66, 68, 73 and 82. (15)
Table2.
Unadjusted associations between selected predictors and CIN outcomes
| Variable (continuous) | CIN 1 or less* | CIN 2 | CIN 3 | CIN 3 vs. CIN 1 or less* | CIN 3 vs. CIN 2 | CIN 2 vs. CIN 1 or less* | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | p-value† | p-value† | p-value† | |
| Age | 20.2 | 2.1 | 19.8 | 2.2 | 19.9 | 1.6 | 0.37 | 0.72 | 0.07 |
| Age at first intercourse | 15.8 | 1.8 | 15.7 | 1.8 | 15.5 | 1.7 | 0.22 | 0.61 | 0.40 |
| Years sexually active | 4.4 | 2.1 | 4.1 | 2.4 | 4.4 | 2.2 | 0.95 | 0.48 | 0.30 |
| Months currently on OCs | 5.9 | 11.9 | 7.8 | 12.9 | 8.4 | 16.6 | 0.22 | 0.84 | 0.22 |
| Months currently on medroxyprogesteroneuse | 3.6 | 9.1 | 7.7 | 14.6 | 6.4 | 13.2 | 0.07 | 0.65 | 0.002 |
| Months of OC use (ever) | 13.2 | 17.4 | 14.2 | 18.7 | 18.2 | 20.7 | 0.09 | 0.29 | 0.62 |
| Number of pregnancies | 0.6 | 1.0 | 0.5 | 0.7 | 0.6 | 0.8 | 0.91 | 0.81 | 0.82 |
| Days since last sex | 24.9 | 73.6 | 22.3 | 53.3 | 8.5 | 12.9 | 0.14 | 0.16 | 0.76 |
| Lifetime sex partners | 8.0 | 8.9 | 7.8 | 7.1 | 8.0 | 8.2 | 0.90 | 0.91 | 0.99 |
| Years smoked | 1.8 | 3.0 | 2.2 | 3.8 | 1.8 | 2.5 | 0.94 | 0.48 | 0.28 |
| # of cigarettes smoked last 24 hrs | 1.4 | 3.3 | 1.5 | 3.5 | 2.5 | 4.4 | 0.05 | 0.18 | 0.75 |
| Variable (dichotomous) | N | % | N | % | N | % | |||
|---|---|---|---|---|---|---|---|---|---|
| Current OC use | 186 | 37.2 | 35 | 43.2 | 15 | 36.6 | 0.94 | 0.48 | 0.30 |
| Current condom use | 166 | 33.2 | 22 | 27.2 | 12 | 29.3 | 0.61 | 0.81 | 0.28 |
| Current smoker | 137 | 27.4 | 25 | 30.9 | 15 | 36.6 | 0.21 | 0.53 | 0.52 |
| History of anal intercourse (ever) | 196 | 39.2 | 27 | 33.3 | 19 | 46.3 | 0.37 | 0.16 | 0.31 |
| History of Chlamydia‡ | 122 | 24.4 | 29 | 35.8 | 9 | 22.0 | 0.73 | 0.12 | 0.03 |
| History of Gonorrhea‡ | 23 | 4.6 | 5 | 6.2 | 1 | 2.4 | 0.53 | 0.38 | 0.54 |
| History of Trichomonas‡ | 16 | 3.2 | 3 | 3.7 | 1 | 2.4 | 0.79 | 0.71 | 0.81 |
| History of Bacterial Vaginosis‡ | 97 | 19.4 | 14 | 17.3 | 6 | 14.6 | 0.46 | 0.71 | 0.65 |
| History of yeast‡ | 260 | 52.0 | 44 | 54.3 | 21 | 51.2 | 0.92 | 0.75 | 0.79 |
| History of Herpes‡ | 16 | 3.2 | 5 | 6.2 | 2 | 4.9 | 0.57 | 0.77 | 0.19 |
| One new sex partner in last 2 months§ | 369 | 73.0 | 60 | 74.1 | 31 | 75.6 | 0.10 | 0.12 | 0.95 |
| Two new sex partners in last 2 months§ | 77 | 15.4 | 12 | 14.8 | 9 | 22.0 | 0.05 | 0.07 | 0.89 |
| Ever pregnant | 185 | 37.0 | 34 | 42.0 | 17 | 41.5 | 0.57 | 0.96 | 0.39 |
| Smokes MJ at least once per week | 70 | 14.0 | 13 | 16.0 | 6 | 14.6 | 0.91 | 0.84 | 0.63 |
| Drinks alcohol at least once per week | 105 | 21.0 | 14 | 17.3 | 7 | 17.1 | 0.55 | 0.98 | 0.44 |
| Uses drugs (other than MJ) at least once per week | 2 | 0.4 | 1 | 1.2 | 0 | 0.0 | 0.40 | 0.27 | 0.36 |
CIN 1 or less includes Benign
P values reflect the association between a single predictor and CIN outcome categories from a multinomial logistic regression model.
obtainedby history; overall, 108 (68%) cases of C. trachomatis, 19 (73%)of N. gonorrhoeae, 12 (60%) of T. vaginalis, 110 (94%) of bacterial vaginosis, 128 (39%) of yeast vaginitis and 8 (35%) of HSV verified by chart review.
P-values are for comparisons with the group with no new sex partner in the last 2 months
OC = Oral Contraception
MJ = Marijuana
In this analysis, we used t-tests to evaluate the differences in average number of biopsies between CIN comparison groups. To compare the differences between included and excluded samples, we used t-tests for continuous variables, and chi-square and Fisher's exact tests for categorical variables. Because our outcome variable was polytomous, multinomial logistic regression method was used to investigate the associations between selected predictor variables and three CIN outcomes. (16) Initial models included candidate predictors singly, and all those with associations significant at the 10% level were considered further in models with multiple predictors. To adjust for possible years of exposure to HPV and site differences, years of sexual activity and clinical site were also included in the final model. The model estimated the odds ratios of having the more serious diagnoses in all three comparisons simultaneously. All the analyses were performed with SAS 9.1.
Results
Six hundred seventy-eight women were consented and completed a baseline questionnaire. Fifty-six women were excluded from the analysis based on either missing a cytology or biopsy diagnosis from the centralized laboratory (see Figure 1). There were 43 women who had normal colposcopy and no biopsy or ECC were indicated. Of these, 19 had a negative cytology interpretation at the screening visit and were included for analysis as “benign”. Those with abnormal Pap test results (ASC-US or worse) from the screening visit were excluded since a histologic diagnosis was not available in the face of persistent abnormal cytology. Demographic characteristics and selected behaviors of the 622 women by CIN status are given in Table 2. Overall, the group was racially/ethnically diverse; 32.5% were white, 17.5% black, 15% Latino, 6.9% Asian, and the remaining mixed or other. The cohort appeared to have relative high-risk sexual behaviors compared to national data (4, 17) defined by a high number of previous pregnancies (37.9%, 95% CI = [34.1-41.8%]), C. trachomatis infections (25.7%, 95% CI = [22.3-29.1%]), total number of lifetime sexual partners (mean = 8 [standard deviation (SD) = 8.6]), and history of anal intercourse (38.9%, 95% CI = [34.1-41.7%]). Comparison between the women who were included vs excluded (n = 56) for analysis showed that the excluded group had a fewer number of C. trachomatis infections (12.5%; 95% CI = [3.8-21.2%]; p = 0.03) and were less likely to report a history of anal intercourse (25.0%; 95% CI = [21.6-28.4%]; p = 0.04) than those included. No other differences were found for any of the variables listed in Table 2.
Of the 622 women, 41 (6.6%; 95% CI = [4.6 - 8.6%] ) had CIN 3, 81 (13%; 95% CI = [10.4-15.6%]) had CIN 2. 157 (25.2%; 95% CI = [21.8-28.6%]) had CIN 1 and 343 (55.1%; 95% CI = [51.2 - 59.0%]) were considered benign. Of the 622 women, 36 had missing referral diagnosis from KPNC. Table 1 compares the cytologic referral diagnosis from KPNC to the centralized histologic diagnosis. The majority of CIN 2 and 3 cases (95%; 95% CI = [91.1-98.8%]) were diagnosed from ASC-US or LSIL referral diagnosis. HSIL diagnoses were rare (1.9%; 95% CI = [0.1-2.9%]) and had a similar rate as reported by the cytology laboratories atKPNC for the year 1999-2000, near the time the study was initiated. Only 4 of the 11 HSIL diagnoses were confirmed by biopsy. The number of biopsies taken influenced final diagnosis. From the 622 women, there were 1,651 biopsies. Those with benign diagnosis including only those with biopsies had the least average number of biopsies (mean 2.3 [S.D. = 1.2] biopsies per women). This was significantly lower than those with CIN 1, CIN 2, and CIN 3 (mean number of biopsies per women was 3.1 [S.D. = 1.2], 2.9 [S.D. = 1.1], and 3.5 [S.D. = 1.1], respectively) with all the p-values < 0.001. In addition, the average number of biopsies of those with CIN 3 was also significantly different from those with CIN 2 (p < 0.01), but not with CIN 1.
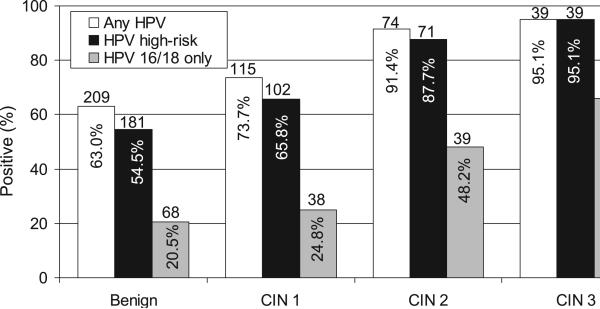
Twelve HPV tests were considered inadequate or missing. Figure 2 shows the number of HPV positive tests within each histologic category. HPV 16/18 was strongly associated with grade of CIN, with the observed percentage of women with HPV 16/18 gradually increasing with increasing severity of lesion. Women with benign examinations had the lowest rate of HPV 16/18 detection (20.5%, 95% CI = [16.2-24.8%]) and those with CIN 3 had the highest (65.9%, 95% CI = [51.2-80.1%]). Rates of HPV 16/18 were significantly higher in women with CIN 3 than in those with either CIN 1 (24.4%; 95% CI = [17.6-31.2%])or benign diagnoses (p<0.001 for both), and marginally higher than in those with CIN 2 (48.2%; 95% CI = [37.3-59.2%], p=0.06). Rates of HPV 16/18 in women with CIN 2 were also higher than in those with benign or CIN 1 diagnoses (p<0.001 for both). No differences for HPV 16/18 detection were seen between women with benign and CIN 1 outcomes. Only 2 women with CIN 3 were HPV negative and none had low risk HPV only.
Figure 2. CIN status by HPV status.
HPV 16/18 was significantly different between normal and CIN 2 and 3 (both P<0.001) and between CIN 1 and CIN 2 and 3 (both p<0.001). No difference was found for HPV 16/18 between normal vs CIN 1 and CIN 2 vs CIN 3.
Prevalence of any high-risk HPV was also higher in women with CIN 2 (87.7%, 95% CI = [80.5-94.9%]) or CIN 3 (95.1%, 95% CI = [88.4-100%]) than in those with CIN 1 (65.8%, 95% CI=[58.3-73.3%]) or benign diagnoses (54.5%, 95% CI = [49.1-59.9%], p <0.003 and p < 0.01, respectively).
Separate multinomial logistic regression models were fitted for each candidate predictor variable, with results summarized using p-values from tests of association for each of the following outcome comparisons: CIN 3 vs. CIN 1 or less, CIN 3 vs. CIN 2, and CIN 2 vs. CIN 1 or less. These results are summarized in Table 2. The first comparison (CIN 3 vs. CIN 1 and less) showed that the following variables associated with risk for CIN 3 at p < 0.1 level: total months of OCP use, total months on medroxyprogesterone, number of sex partners in last 2 months, and number of cigarettes smoked in the last 24 hours. The second comparison (CIN 3 vs CIN 2) found only number of sex partners in last 2 months significant at p < 0.1 level. The third comparison (CIN 2 vs. CIN 1 or less) showed that the risk of CIN 2 is associated with younger age, history of Chlamydia, and total months on medroxyprogesterone at the p < 0.1 level.
Predictor variables with associations significant at p< 0.1 level in Table 2 were included together in a final multinomial logistic model, with results summarized as odds ratios (and 95% confidence intervals) for the outcome comparisons introduced above. The model also adjusted for years of sexual activity and clinic site. The first comparison for CIN 3 vs. CIN 1 or less found total years of OCP use increased risk of CIN 3. For each one additional year on OCPs, the odds of having CIN 3 increases by 36% on average. Although not statistically significant, having 2 or more recent sex partners had an increased odds of CIN 3 (p=0.08)
The second comparison for CIN 2 vs. CIN 1 or less found that for each additional year on medroxyprogesterone increases the odds of CIN 2 by 46%. Both analyses found HPV 16 and/or 18 and other high-risk HPV detection significant: the former increases the odds of CIN 3 by almost 3000%, and CIN 2 by 600%; and the latter increases the odds of CIN 3 by 500%, and CIN 2 by 260%, comparing with those negative for HPV or low-risk HPV. No statistically significant differences were found for the comparison between CIN 3 and 2. The results are presented in Table 3.
Table 3.
Multivariable logistic regression model* for risk of CIN 3 and 2
| CIN 3 vs. CIN 1 or less | CIN 3 vs. CIN 2 | CIN 2 vs. CIN 1 or less | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds | Lower | Upper | Odds | Lower | Upper | Odds | Lower | Upper | |
| Parameter | ratio | CI | CI | ratio | CI | CI | ratio | CI | CI |
| Age | 0.86 | 0.67 | 1.10 | 0.92 | 0.69 | 1.21 | 0.93 | 0.78 | 1.11 |
| Time on oral contraceptive use (per year) | 1.36 | 1.08 | 1.71† | 1.23 | 0.95 | 1.60 | 1.11 | 0.92 | 1.33 |
| Time on medroxyprogesterone (per year) | 1.14 | 0.78 | 1.65 | 0.78 | 0.53 | 1.15 | 1.46 | 1.12 | 1.90† |
| Ever had Chlamydia | 0.67 | 0.28 | 1.63 | 0.44 | 0.17 | 1.15 | 1.53 | 0.87 | 2.71 |
| Number of cigarettes last 24 hrs (per 20 cigarette) | 2.29 | 0.35 | 15.03 | 1.53 | 0.18 | 13.31 | 1.50 | 0.32 | 7.06 |
| New sex partners in last 2 months (1 vs 0) | 6.19 | 0.56 | 68.25 | 3.87 | 0.31 | 48.63 | 1.60 | 0.60 | 4.25 |
| New sex partners in last 2 months (≥ 2 vs. 0) | 9.80 | 0.78 | 123.67 | 6.02 | 0.40 | 89.70 | 1.63 | 0.50 | 5.33 |
| HPV 16/18 | 30.93 | 6.95 | 137.65† | 4.25 | 0.82 | 22.12 | 7.28 | 3.29 | 16.12† |
| Non 16/18 HR HPV | 6.26 | 1.33 | 29.38† | 1.72 | 0.32 | 9.34 | 3.63 | 1.65 | 7.96† |
Results from a single multinomial regression model including all the predictors with a p value <0.1 from Table 3, adjusting for clinic site and years sexually active.
p≤ 0.05
Discussion
Little is known about adolescents and young women who develop CIN 3 since most studies focus on older women. In this study of adolescents and young women attending a large HMO, less than 7% of the population referred for abnormal cytology was found to have CIN 3. Similar to adults, most of the study participants were referred because of ASC-US and LSIL. (18) In contrast, the rate of CIN 3 among study participants referred for ASC-US or LSIL was less than half of that reported for adults. It is estimated that 10-16 % of adult women with HPV-positive ASC-US/LSIL will have underlying CIN 3. (19-21) We found CIN 3 in 6.3% (95% CI = [4.3 - 8.3%]) of adolescent and young women with ASC-US or LSIL on referral Pap. This lower rate of CIN 3, along with data showing high rates of LSIL regression (13), support the new 2006 American Society for Colposcopy and Cervical Pathology (ASCCP) Consensus Guidelines which recommend following adolescents with ASC-US/LSIL by cytology rather than immediate referral to colposcopy. (18) Our data suggests that extending this conservative management to young women under the age of 25 years may be reasonable and that further analysis by guideline groups is warranted. The consensus guidelines also recommend continued follow-up of these young women with ASC-US/LSIL since the possibility of CIN 3 is not nil, as demonstrated in our study. However, the likelihood of progression to cancer is extremely low during this young age period. (2) This assumption was supported by the lack of finding a single cancer case in our study.
Although the rate of CIN 3 was relatively low, the risks for having CIN 3 were similar to those found in previous studies of adults. The strongest association with CIN 3 was that of having HPV 16/18 or other high-risk HPV infections. (22) Since ours was a sexually active group of adolescents and young women, it is not surprising that HPV DNA detection was high in all histology groups including those with benign histology. However, we noted that the rate of HPV 16 and 18 among those with CIN 3 was three fold that of those with benign/CIN 1 histology. The high rate found in CIN 3 (65%) is identical to the rates attributed to cervical cancer and CIN 3 by HPV 16 and 18 worldwide. (15, 23) Our findings suggest there may be clinical utility in separating these types for clinical testing in young women as well as older adults.
We found the risk for CIN 3 associated with time on hormonal contraceptives most interesting since most of the women had been on OCPs for a limited time. Although OCP use has been identified as a potential risk factor for both cervical cancer and CIN 3, the absolute attributable risk remains highly controversial and, when associated, the risk becomes appreciable only after some time period on OCPs ranging from 3 to 5 years. (10, 11, 24, 25) The significant association between CIN 3 and OCP use in our study, after controlling for HPV status, suggests that OCP use does contribute a small risk in some women. Given that these women had limited sexual exposure to HPV and OCPs, there may be some epigenetic event that occurs with exogenous hormones and HPV exposure explaining the occurrence of these CIN 3 lesions at this young age. (26) Several in vitro studies demonstrate the biologic plausibility for the association between estrogen and invasive cervical cancer. (27-30)
The inability of our analysis to discriminate between CIN 2 and 3, suggest that CIN 2 and 3 share some risks factors. On the other hand, risks that discriminated CIN 3 vs CIN 1 or less and CIN 2 vs CIN 1 or less were different underscoring the likely difference between these two lesions. It is worth noting that the prevalence of HPV 16 and 18 was greater in the CIN 3 than CIN 2 group; 66 vs 48%, respectively and the multinomial models showed a much greater risk for CIN 3 if positive for HPV 16/18 than for CIN 2. Only one risk factor was found that distinguished the CIN 2 and the CIN 1/benign group and this was association was between CIN 2 and the use of medroxyprogesterone. Some epidemiology studies have shown medroxyprogesterone use to be associated with cervical cancer. (31) The association with CIN 2 but not CIN 3 suggests to us that progesterone-only contraceptives may have other attributes which enhance the expression of CIN 2 type lesions, but lack the ability to cause sentinel events that lead to CIN 3 in young women. On the other hand, the atrophy induced by medroxyprogesterone may have led to the misdiagnosis of CIN 2 in this group. (32) CIN 2 remains a controversial diagnosis with some questioning its existence and reproducibility. We believe our results suggests that CIN 2 and CIN 3 are likely different biologically. (33) The lack of finding differences between CIN 2 and 3 may have been limited by our sample size for these groups. Examination of biologic markers is likely to be better suited to distinguish these lesions, if there is a true biologic distinction.
The main limitation to the study was lack of information on those not participating in the study. Although we examined differences between those included and excluded by our criteria, we were unable to interview the young women who we were not able to contact or get permission to contact, limiting our ability to generalize. However, this remains one of the largest studies to examine risk factors for CIN 3 of young women. Second, we did not verify all pathologic diagnosis using two reviewers; only CIN 3 diagnoses were confirmed by a second pathologist. The centralized readings for all cytology and histology allowed consistency among diagnoses. The tight association between HPV 16 and 18 infections and diagnosis suggest that the readings were consistent with other publications using two or more reviewers. (23) The lack of association with smoking was possibly due to the relative low nicotine exposure of smokers; few smoked more than 5 cigarettes a day.
In summary, our data show that CIN 3 is relatively rare in adolescents and young women referred for abnormal cytology. Those with CIN 3 appear to have an extremely low likelihood of progression to cancer since none were found in the time frame of this study. The primary risk for CIN 2 and 3 in this age group remains HPV 16 and 18 infections. Studies are warranted to examine the utility of using type-specific HPV DNA 16 and 18 assays for screening and evaluation in these young women. Our data also found that time on hormonal contraceptives contribute a small increased risk for the development of CIN 3 in young women, even with limited exposure to these exogenous hormones. Different associations were found with CIN 2 lesions than CIN 3, suggesting that CIN 2 is a different biologic lesion than CIN 3.
Acknowledgements
The authors thank the following staff of the Kaiser Permanente/University of California San Francisco CIN-2 Study for undergoing study training, completing study-specific forms and processing samples: Amber Dimick-Flores, MA, Cheryl Godwin de Medina, Wanda Griffin, RN, Debra Guisto, RN, Janet Jonte, NP, Katy Kurtzman, MD, Lesley Levine, MD, Anita Levine-Goldberg NP, Carol Lopez, LVN, Ellen McKnight, NP, Karen Milligan-Green, RN, Laura Minikel, MD, Heidi Olander, MD, Mary Phelps, MA, Diane Ragni, RN, Katy Ryan, MD, Debbie Russell, RN, Greg Sacher MD, Mark Seaver, MD, Carolyn Taylor, RN, and Nicole Zidenberg, MD.
The authors thank Dr. Ted Miller for his assistance in reviewing histology for the methods, Lisa Clayton for data entry and management, and Anthony Kung for data and site overview.
Supported by grant 3 R01 CA87905 and R37CA051323 from the National Institutes of Health. Roche Molecular Diagnostics (Pleasanton, CA) provided supplies for HPV DNA detection.
Footnotes
Financial Disclosure: The authors have no potential conflicts of interest to disclose.
Précis Cervical intraepithelial neoplasia-3 is not common in adolescents, and young women and risks include human papillomavirus 16/18 detection and oral contraceptive use.
References
- 1.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. Jama. 2007 Feb 28;297(8):813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al. SEER Cancer Statistics Review, 1975-2004. National Cancer Institute; Bethesda, MD: 2007. [Google Scholar]
- 3.Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285(23):2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 4.Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15-44 years of age, United States, 2002. Adv Data. 2005;362:1–55. [PubMed] [Google Scholar]
- 5.Moscicki AB, Shiboski S, Broering J, Powell K, Clayton L, Jay N, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–84. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 6.Munoz N, Mendez F, Posso H, Molano M, van den Brule AJ, Ronderos M, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004 Dec 15;190(12):2077–87. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 7.Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(Suppl 3):S42–51. doi: 10.1016/j.vaccine.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Winer RL, Kiviat NB, Hughes JP, Adam DE, Lee SK, Kuypers JM, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191(5):731–8. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 9.International Collaboration of Epidemiological Studies of Cervical Cancer. Appleby P, Beral V, Berrington de Gonzalez A, Colin D, Franceschi S, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007 Nov 10;370(9599):1609–21. doi: 10.1016/S0140-6736(07)61684-5. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre-Seltman K, Castle PE, Guido R, Schiffman M, Wheeler CM, The Alts Group Smoking is a risk factor for cervical intraepithelial neoplasia grade 3 among oncogenic human papillomavirus DNA-positive women with equivocal or mildly abnormal cytology. Cancer Epidemiol Biomarkers Prev. 2005;14:1165–70. doi: 10.1158/1055-9965.EPI-04-0918. [DOI] [PubMed] [Google Scholar]
- 11.Richardson H, Abrahamowicz M, Tellier PP, Kellsall G, du Berger R, Ferenczy A, et al. Modifiable risk factors associated with clearence of type-specific cervical papillomavirus infections in a cohort of university students. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1149–56. doi: 10.1158/1055-9965.EPI-04-0230. [DOI] [PubMed] [Google Scholar]
- 12.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moscicki AB, Shiboski S, Hills NK, Powell KJ, Jay N, Hanson EN, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364(9446):1678–83. doi: 10.1016/S0140-6736(04)17354-6. [DOI] [PubMed] [Google Scholar]
- 14.Gravitt P, Peyton CL, Alessi TQ, Wheeler C, Coutlée F, Hildesheim A, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muñoz N, Bosch FX, De Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiological Classification of Human Papillomavirus Types Associated with Cervical Cancer. NEJM. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 16.Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS system. Second Edition SAS Institute Inc; 1997. [Google Scholar]
- 17.Centers for Disease Control National and State-Specific Pregnancy Rates Among Adolescent - United States, 1995-1997. MMWR. 2000;49(27):605–11. [PubMed] [Google Scholar]
- 18.Wright TC, Jr., Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007 Oct;197(4):346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 19.Cox JT, Schiffman M, Solomon D, ASCUS-LSIL Triage Study (ALTS) Group Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188(6):1406–12. doi: 10.1067/mob.2003.461. [DOI] [PubMed] [Google Scholar]
- 20.ASCUS-LSIL Triage Study (ALTS) Group Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188(6):1383–92. doi: 10.1067/mob.2003.457. [DOI] [PubMed] [Google Scholar]
- 21.ASCUS-LSIL Triage Study (ALTS) Group A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am J Obstet Gynecol. 2003 Jun;188(6):1393–400. doi: 10.1067/mob.2003.462. [DOI] [PubMed] [Google Scholar]
- 22.Castle PE, Solomon D, Schiffman M, Wheeler CM. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst. 2005;97(14):1066–71. doi: 10.1093/jnci/dji186. [DOI] [PubMed] [Google Scholar]
- 23.Franceschi S, Clifford GM. Fraction of cervical neoplasias due to human papillomavirus 16 and 18 in vaccine trials. Int J Cancer. 2008 Feb 1;122(3):719–20. doi: 10.1002/ijc.23112. [DOI] [PubMed] [Google Scholar]
- 24.Shields TS, Brinton LA, Burk RD, Wang SS, Weinstein SJ, Ziegler RG, et al. A case-control study of risk factors for invasive cervical cancer among U.S. women exposed to oncogenic types of human papillomavirus. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1574–82. [PubMed] [Google Scholar]
- 25.Miller K, Blumenthal P, Blanchard K. Oral contraceptives and cervical cancer: critique of a recent review. Contraception. 2004;69(69):5. doi: 10.1016/j.contraception.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Couto E, Hemminki K. Heritable and environmental components in cervical tumors. Int J Cancer. 2006 Dec 1;119(11):2699–701. doi: 10.1002/ijc.22226. [DOI] [PubMed] [Google Scholar]
- 27.Ruutu M, Wahlroos N, Syrjanen K, Johansson B, Syrjanen S. Effects of 17beta-estradiol and progesterone on transcription of human papillomavirus 16 E6/E7 oncogenes in CaSki and SiHa cell lines. Int J Gynecol Cancer. 2006;16(3):1261–8. doi: 10.1111/j.1525-1438.2006.00563.x. [DOI] [PubMed] [Google Scholar]
- 28.Krusekopf S, Chauchereau A, Milgrom E, Henderson D, Cato AC. Co-operation of progestational steroids with epidermal growth factor in activation of gene expression in mammary tumor cells. J Steroid Biochem Mol Biol. 1991;40(13):239–45. doi: 10.1016/0960-0760(91)90188-b. [DOI] [PubMed] [Google Scholar]
- 29.Webster K, Taylor A, Gaston K. Oestrogen and progesterone increase the levels of apoptosis induced by the human papillomavirus type 16 E2 and E7 proteins. J Gen Virol. 2001;82(1):201–13. doi: 10.1099/0022-1317-82-1-201. [DOI] [PubMed] [Google Scholar]
- 30.Scott ME, Ma Y, Farhat S, Shiboski S, Moscicki AB. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26(3):222–32. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 31.WHO Collaborative Study Depot-medroxyprogesterone acetate (DMPA) and risk of invasive squamous cell cervical cancer. The WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Contraception. 1992;45(4):299–312. doi: 10.1016/0010-7824(92)90052-u. [DOI] [PubMed] [Google Scholar]
- 32.Valente PT, Schantz HD, Trabal JF. Cytologic changes in cervical smears associated with prolonged use of depot-medroxyprogesterone acetate. Cancer. 1998 Dec 25;84(6):328–34. doi: 10.1002/(sici)1097-0142(19981225)84:6<328::aid-cncr3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 33.Syrjanen K, Kataja V, Yliskoski, Chang F, Syrjanen S. Natural History of Cervical Human Papillomavirus Lesions Does Not Substantiate the Biologic Relevance of the Bethesda System. Obstet Gynecol. 1992;79:675–82. [PubMed] [Google Scholar]